Antioxidant and Antifungal Activity of the Cynophalla flexuosa (L.) J. Presl (Capparaceae) against Opportunistic Fungal Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of the Cynophalla flexuosa Hydroethanolic Extract (CFHEE)
2.3. Chemical Analyses
2.3.1. Qualitative Chemical Prospection
2.3.2. Total Phenol Quantification
2.3.3. Ultra-Performance Liquid Chromatography—ElectroSpray Ionization—Quadrupole-Time-of-Flight—Tandem Mass Spectrometry (UPLC-ESI-qTOF-MS/MS)
2.4. Antioxidant Activity
2.5. Antifungal Activity
2.5.1. Strains, Culture Media Used and Solution Preparation
2.5.2. Determination of the 50% Fungal Inhibitory Concentration IC50
2.5.3. Determination of the Minimum Fungicidal Concentration (MFC)
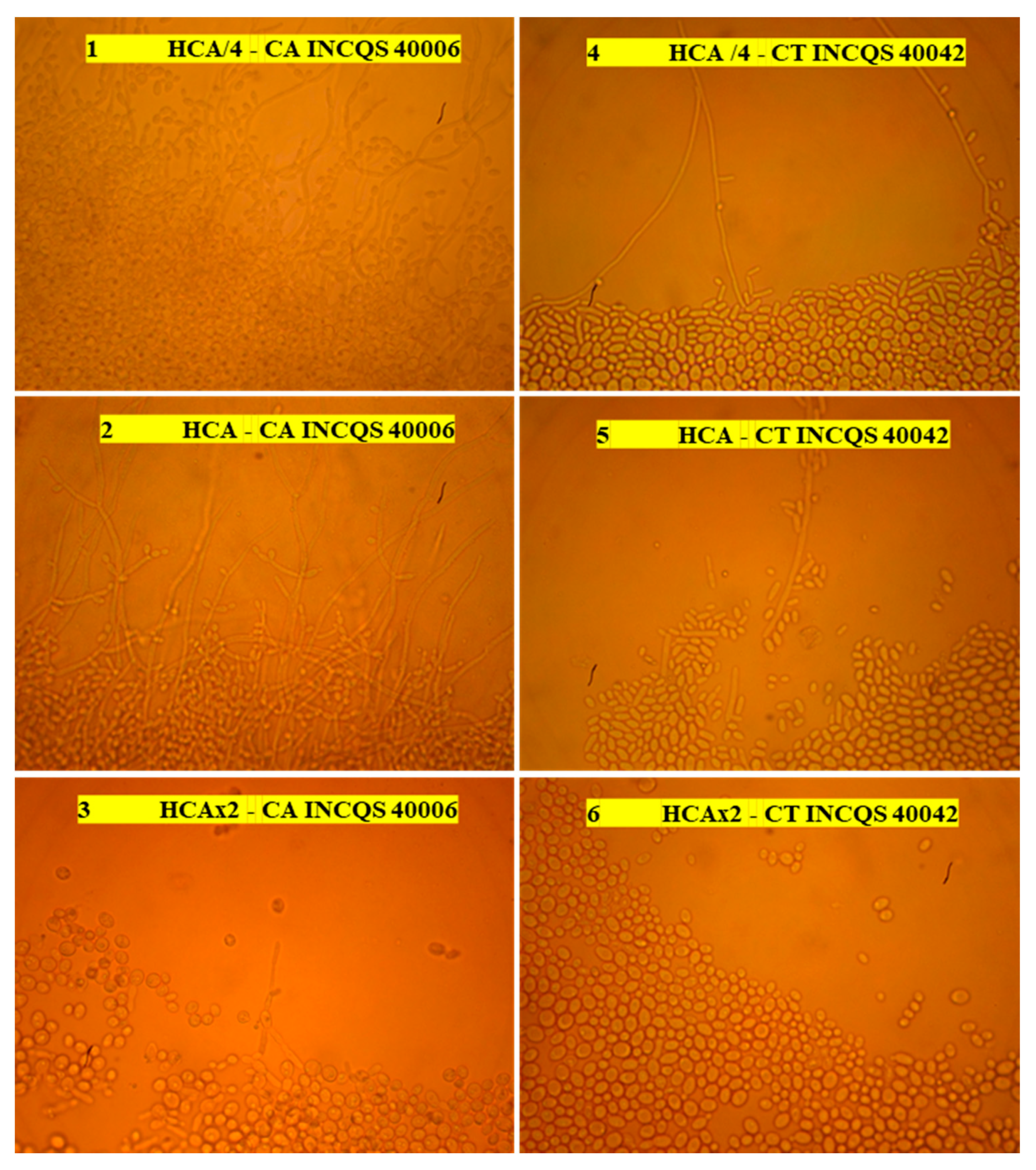
2.5.4. Extract Effect on Fungal Morphology
2.6. Statistical Analysis
3. Results
3.1. Extraction of Plant Material, Chemical Prospection and Total Phenols
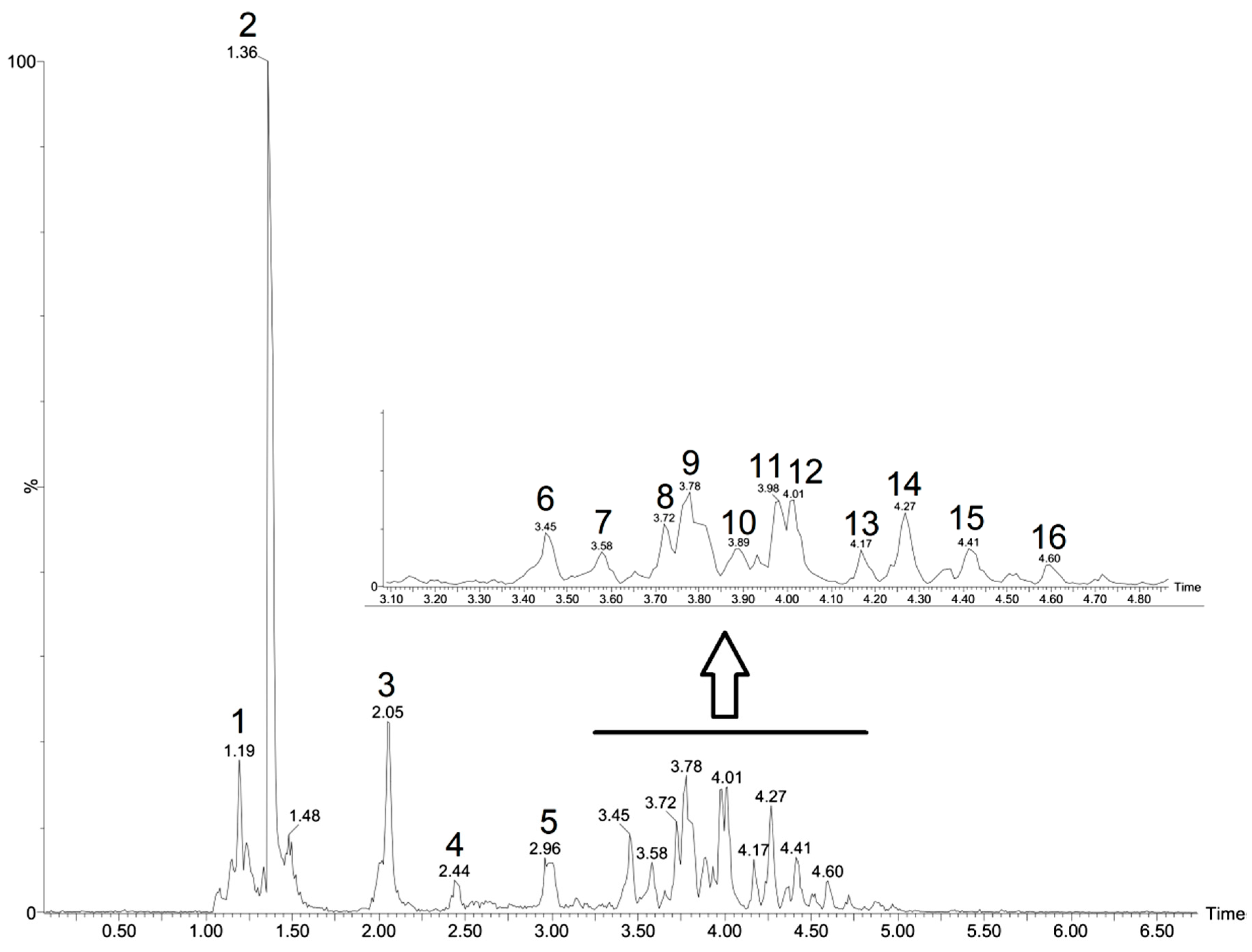
3.2. Chemical Composition by UPLC-ESI-qTOF-MS/MS
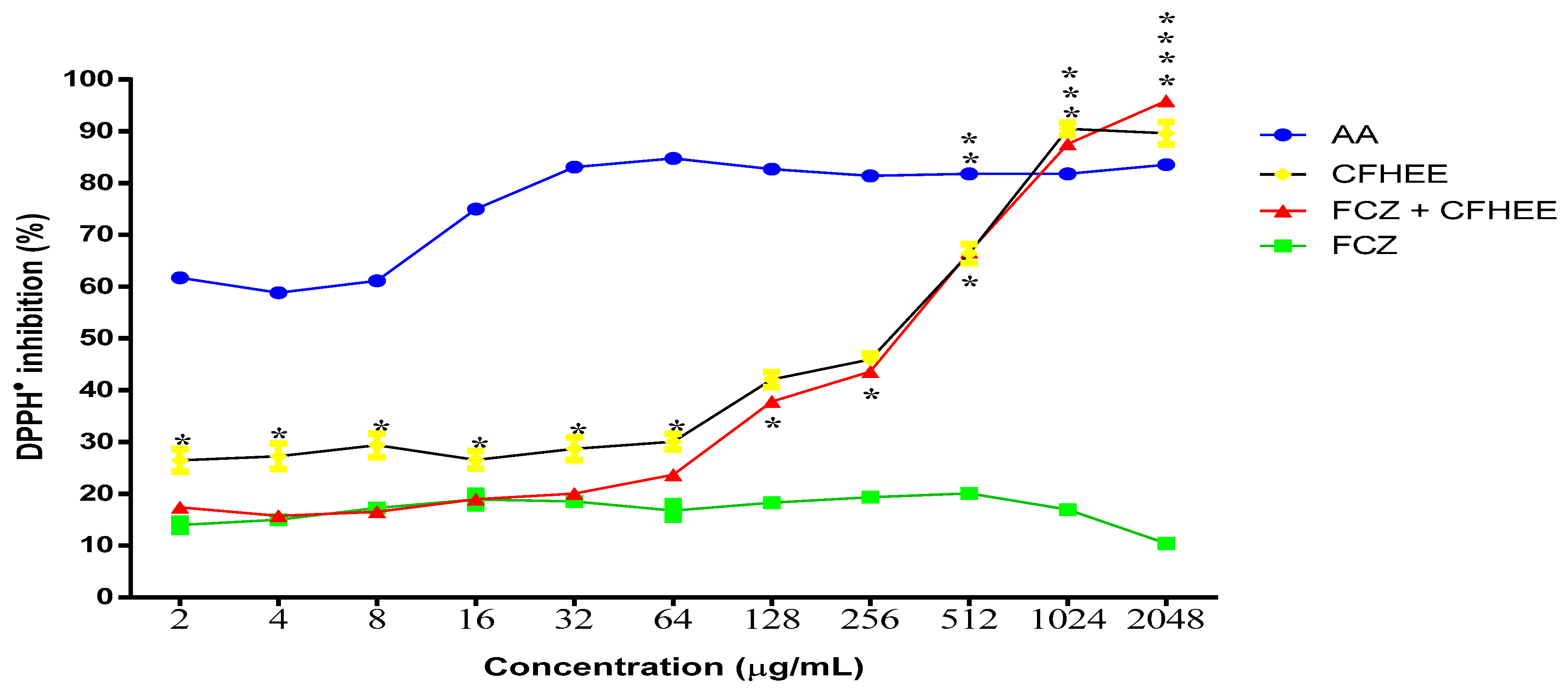
3.3. Antioxidant Activity
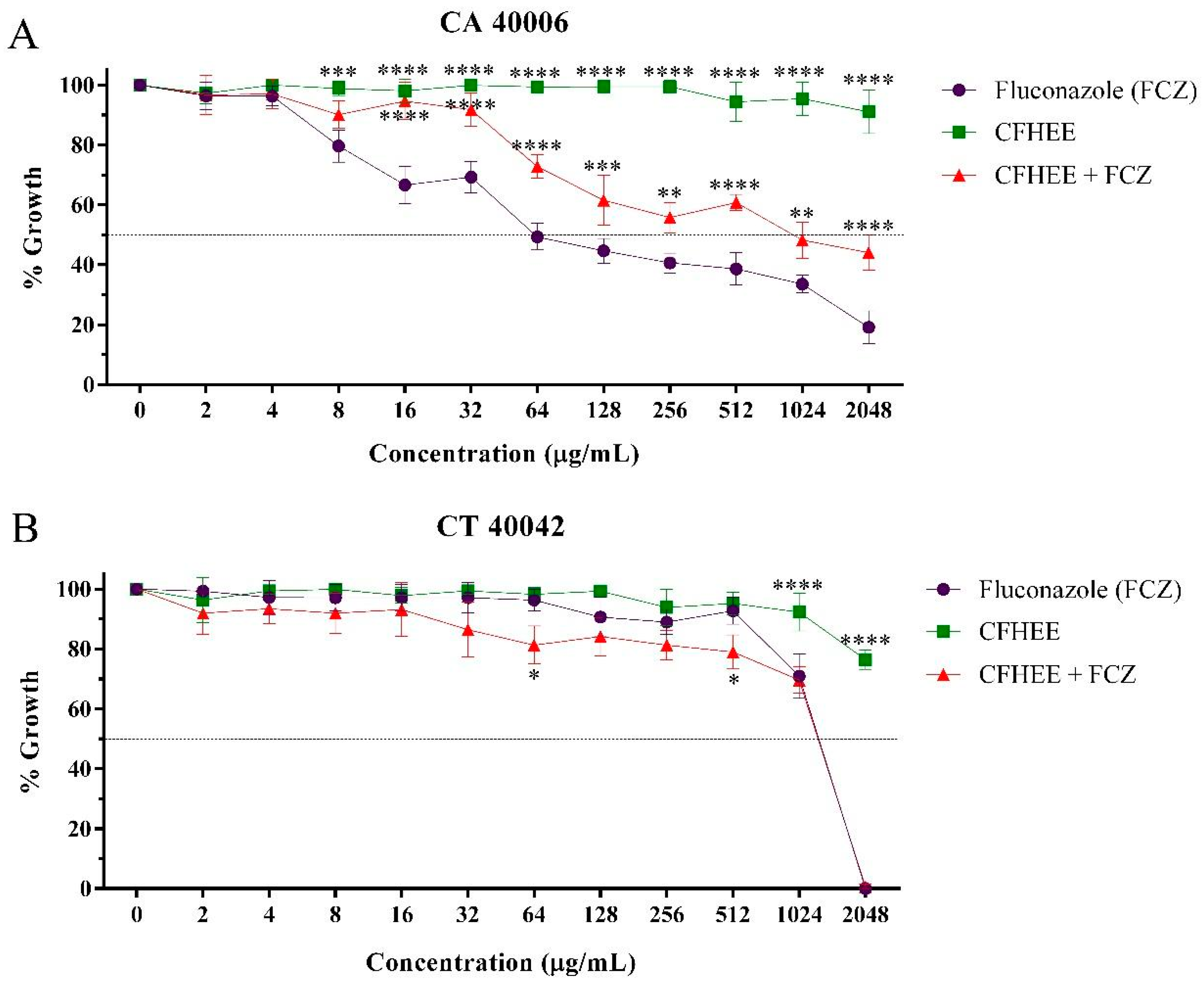
3.4. Antifungal Activity
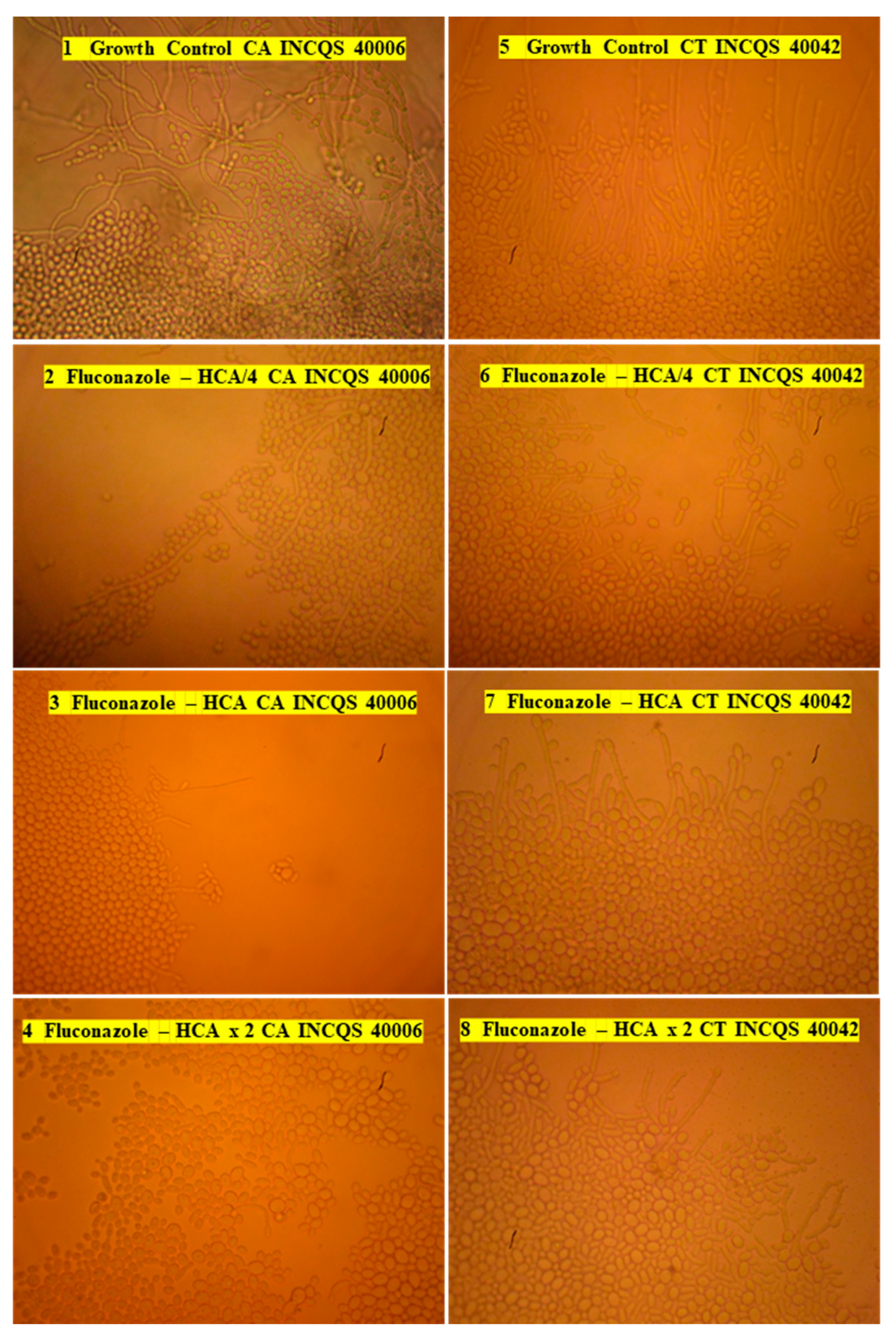
3.5. Control of Virulence of Candida Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arendrup, M.C.; Patterson, T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216, S445–S451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romo, J.A.; Kumamoto, C.A. On Commensalism of Candida. J. Fungi 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sustr, V.; Foessleitner, P.; Kiss, H.; Farr, A. Vulvovaginal Candidosis: Current Concepts, Challenges and Perspectives. J. Fungi. 2020, 6, 267. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Y.; Chuang, Y.-C.; Wu, U.-I.; Sun, H.-Y.; Wang, J.-T.; Sheng, W.-H.; Chen, Y.-C.; Chang, S.-C. Mechanisms of Azole Resistance and Trailing in Candida Tropicalis Bloodstream Isolates. J. Fungi. 2021, 7, 612. [Google Scholar] [CrossRef]
- Liu, W.-L.; Huang, Y.-T.; Hsieh, M.-H.; Hii, M.; Lee, Y.-L.; Ho, M.-W.; Liu, C.-E.; Chen, Y.-H.; Wang, F.-D. Clinical Characteristics of Candida tropicalis Fungaemia with Reduced Triazole Susceptibility in Taiwan: A Multicentre Study. Int. J. Antimicrob. Agents 2019, 53, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Nami, S.; Aghebati-Maleki, A.; Morovati, H.; Aghebati-Maleki, L. Current Antifungal Drugs and Immunotherapeutic Approaches as Promising Strategies to Treatment of Fungal Diseases. Biomed. Pharmacother. 2019, 110, 857–868. [Google Scholar] [CrossRef]
- Bezerra, J.W.A.; Costa, A.R.; de Freitas, M.A.; Rodrigues, F.C.; de Souza, M.A.; da Silva, A.R.P.; Dos Santos, A.T.L.; Linhares, K.V.; Coutinho, H.D.M.; de Lima, J.R.S.; et al. Chemical Composition, Antimicrobial, Modulator and Antioxidant Activity of Essential Oil of Dysphania Ambrosioides (L.) Mosyakin & Clemants. Comp. Immunol. Microb. 2019, 65, 58–64. [Google Scholar]
- Costa, A.R.; de Lima Silva, J.R.; de Oliveira, T.J.S.; da Silva, T.G.; Pereira, P.S.; de Oliveira Borba, E.F.; de Brito, E.S.; Ribeiro, P.R.V.; Almeida-Bezerra, J.W.; Júnior, J.T.C.; et al. Phytochemical Profile of Anacardium Occidentale L. (Cashew Tree) and the Cytotoxic and Toxicological Evaluation of Its Bark and Leaf Extracts. S. Afr. J. Bot. 2020, 135, 355–364. [Google Scholar] [CrossRef]
- Zida, A.; Bamba, S.; Yacouba, A.; Ouedraogo-Traore, R.; Guiguemdé, R.T. Anti-Candida Albicans Natural Products, Sources of New Antifungal Drugs: A Review. J. Mycol. Med. 2017, 27, 1–19. [Google Scholar] [CrossRef]
- Bezerra, C.F.; de Alencar Júnior, J.G.; Honorato, R.L.; dos Santos, A.T.L.; da Silva, J.C.P.; da Silva, T.G.; de Freitas, T.S.; Vieira, T.A.T.; Bezerra, M.C.F.; Sales, D.L.; et al. Antifungal Effect of Liposomal α-Bisabolol and When Associated with Fluconazole. Cosmetics 2021, 8, 28. [Google Scholar] [CrossRef]
- Ncube, B.; Finnie, J.F.; Van Staden, J. In Vitro Antimicrobial Synergism within Plant Extract Combinations from Three South African Medicinal Bulbs. J. Ethnopharmacol. 2012, 139, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.L.; Pereira, P.S.; Oliveira, C.V.B.; Freitas, M.A.; Silva, J.R.L.; Costa, A.R.; Oliveira-Tintino, C.D.M.; Morais-Braga, F.B.M.; Duarte, A.E.; Coutinho, H.D.M.; et al. Study of the Capacity of the Essential Oil of Lantana Montevidensis to Modulate the Action of Fluconazole on Candida Albicans and Candida Tropicalis Strains. J. Med. Mycol. 2021, 31, 101171. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. 2019, 2019, e6175804. [Google Scholar] [CrossRef] [PubMed]
- Agra, M.F.; Baracho, G.S.; Nurit, K.; Basílio, I.J.L.D.; Coelho, V.P.M. Medicinal and Poisonous Diversity of the Flora of “Cariri Paraibano”, Brazil. J. Ethnopharmacol. 2007, 111, 383–395. [Google Scholar] [CrossRef]
- Inocencio, C.; Rivera, D.; Alcaraz, F.; Tomás-Barberán, F.A. Flavonoid Content of Commercial Capers (Capparis Spinosa, C. Sicula and C. Orientalis) Produced in Mediterranean Countries. Eur. Food. Res. Technol. 2000, 212, 70–74. [Google Scholar] [CrossRef]
- Kiddle, G.; Bennett, R.N.; Botting, N.P.; Davidson, N.E.; Robertson, A.A.B.; Wallsgrove, R.M. High-Performance Liquid Chromatographic Separation of Natural and Synthetic Desulphoglucosinolates and Their Chemical Validation by UV, NMR and Chemical Ionisation-MS Methods. Phytochem. Analysis. 2001, 12, 226–242. [Google Scholar] [CrossRef]
- Almeida Neto, J.X.; Andrade, A.P.; Lacerda, A.V.; Félix, L.P.; Silva, D.S. Crescimento e Bromatologia Do Feijão-Bravo (Capparis flexuosa L.) Em Área de Caatinga No Curimataú Paraibano, Brasil. Rev. Cienc. Agron. 2011, 42, 488–494. [Google Scholar] [CrossRef]
- Ribeiro, D.A.; Macêdo, D.G.; Oliveira, L.G.S.; Saraiva, M.E.; Oliveira, S.F.; Souza, M.M.A.; Menezes, I.R.A. Potencial Terapêutico e Uso de Plantas Medicinais Em Uma Área de Caatinga No Estado Do Ceará, Nordeste Do Brasil. Rev. Bras. Plantas Med. 2014, 16, 912–930. [Google Scholar] [CrossRef] [Green Version]
- Yazbek, P.B.; Tezoto, J.; Cassas, F.; Rodrigues, E. Plants Used during Maternity, Menstrual Cycle and Other Women’s Health Conditions among Brazilian Cultures. J. Ethnopharmacol. 2016, 179, 310–331. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.P.; Pedrosa, K.M.; Lucena, C.M.; Kelly, T.; Félix, L.P.; Lucena, R.F.P. Uso e conhecimento de espécies vegetais úteis em uma comunidade rural no Vale do Piancó (Paraíba, Nordeste, Brasil). Rev. Biol. Farm. 2012, 5, 133–157. [Google Scholar]
- Matos, F.J.A. Introduction to Experimental Phytochemistry, 3rd ed.; UFC: Fortaleza, Brasil, 2009. [Google Scholar]
- Campbell, M.K.; Farrell, S.O. Biochemistry, 4th ed.; Thomson Asia Pte Ltd.: Singapore, 2005. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1996, 16, 144–158. [Google Scholar]
- Nuutila, A.M.; Puupponen-Pimiä, R.; Aarni, M.; Oksman-Caldentey, K.-M. Comparison of Antioxidant Activities of Onion and Garlic Extracts by Inhibition of Lipid Peroxidation and Radical Scavenging Activity. Food Chem. 2003, 81, 485–493. [Google Scholar] [CrossRef]
- Silva, V.B.; Almeida-Bezerra, J.W.; Brito, E.S.; Ribeiro, P.R.V.; Cordeiro, L.S.; Júnior, J.T.C.; Costa, J.G.M.; Silva, M.A.P. Effect of Decomposition of Leaves of Azadirachta Indica A. Juss. on Germination and Growth of Myracrodruon Urundeuva Allemão. S. Afr. J. Bot. 2021, 142, 42–52. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, H.J.; Kang, S.S. Alatemin, Cassiaside and Rubrofusarin Gentiobioside, Radical Scavenging Principles from the Seeds of Cassia tora on 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Radical. Arch. Pharm. Res. 1994, 17, 462–466. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Aprovada, N. Método de Referência para Testes de Diluicão em Caldo para Determinacão da Sensibilidade à Terapia Antifúngica das leveduras; Norma Aprovada; NCCLS Norma M27-A2, 2ª ed.; NCCLS: Wayne, PA, USA, 2002; pp. 19087–19898. ISBN 1-56238-469-4. [Google Scholar]
- Stoppa, M.A.; Casemiro, L.A.; Vinholis, A.H.C.; Cunha, W.R.; Silva, M.L.A.; Martins, C.H.G.; Furtado, N.A.J.C. Comparative study of the recommended CLSI and EUCAST methodologies for evaluation of antifungal activity. Quím. Nova 2009, 32, 498–502. [Google Scholar] [CrossRef] [Green Version]
- Javadpour, M.M.; Juban, M.M.; Lo, W.-C.J.; Bishop, S.M.; Alberty, J.B.; Cowell, S.M.; Becker, C.L.; McLaughlin, M.L. De Novo Antimicrobial Peptides with Low Mammalian Cell Toxicity. J. Med. Chem. 1996, 39, 3107–3113. [Google Scholar] [CrossRef]
- Morais-Braga, M.F.B.; Carneiro, J.N.; Machado, A.J.; Sales, D.L.; Brito, D.I.; Albuquerque, R.S.; Boligon, A.A.; Athayde, M.L.; Calixto Junior, J.T.; Souza, D.S.; et al. High-Performance Liquid Chromatography-Diodic Array Detector, Fungistatic, and Anti-Morphogenical Analysis of Extracts from Psidium Brownianum Mart. Ex DC. against Yeasts of the Genus Candida. Int. J. Food. Prop. 2016, 19, 1837–1851. [Google Scholar] [CrossRef] [Green Version]
- Ernst, E.J.; Klepser, M.E.; Ernst, M.E.; Messer, S.A.; Pfaller, M.A. In Vitro Pharmacodynamic Properties of MK-0991 Determined by Time-Kill Methods. Diagn. Microbiol. Infect. Dis. 1999, 33, 75–80. [Google Scholar] [CrossRef]
- Sidrin, J.J.C.; Rocha, M.F.G. Medical Mycology in the Light of Contemporary Authors, 1st ed.; Guanabara Koogan: Rio de Janeiro, Brasil, 2010. [Google Scholar]
- Silva, G.S.; Canuto, K.M.; Ribeiro, P.R.V.; de Brito, E.S.; Nascimento, M.M.; Zocolo, G.J.; Coutinho, J.P.; de Jesus, R.M. Chemical Profiling of Guarana Seeds (Paullinia cupana) from Different Geographical Origins Using UPLC-QTOF-MS Combined with Chemometrics. Food Res. Int. 2017, 102, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Bianco, G.; Lelario, F.; Battista, F.G.; Bufo, S.A.; Cataldi, T.R.I. Identification of Glucosinolates in Capers by LC-ESI-Hybrid Linear Ion Trap with Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (LC-ESI-LTQ-FTICR MS) and Infrared Multiphoton Dissociation. J. Mass Spectrom. 2012, 47, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Višnjevec, A.M.; Tamayo Tenorio, A.; Steenkjær Hastrup, A.C.; Hansen, N.M.L.; Peeters, K.; Schwarzkopf, M. Glucosinolates and Isothiocyantes in Processed Rapeseed Determined by HPLC-DAD-QTOF. Plants 2021, 10, 2548. [Google Scholar] [CrossRef] [PubMed]
- Kruszka, D.; Sawikowska, A.; Selvakesavan, R.K.; Krajewski, P.; Kachlicki, P.; Franklin, G. Silver Nanoparticles Affect Phenolic and Phytoalexin Composition of Arabidopsis thaliana. Sci. Total Environ. 2020, 716, 135361. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Hoyos, M.; Arnáez-Serrano, E.; Quesada-Mora, S.; Azofeifa-Cordero, G.; Wilhelm-Romero, K.; Quirós-Fallas, M.I.; Alvarado-Corella, D.; Vargas-Huertas, F.; Sánchez-Kopper, A. HRMS Characterization, Antioxidant and Cytotoxic Activities of Polyphenols in Malus domestica Cultivars from Costa Rica. Molecules 2021, 26, 7367. [Google Scholar] [CrossRef]
- Choucry, M.A.; Khalil, M.N.; El Awdan, S.A. Protective Action of Crateva nurvala Buch. Ham Extracts against Renal Ischaemia Reperfusion Injury in Rats via Antioxidant and Anti-Inflammatory Activities. J. Ethnopharmacol. 2018, 214, 47–57. [Google Scholar] [CrossRef]
- Pandey, R.; Chandra, P.; Arya, K.R.; Kumar, B. Development and Validation of an Ultra High Performance Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry Method for the Simultaneous Determination of Selected Flavonoids in Ginkgo Biloba. J. Sep. Sci. 2014, 37, 3610–3618. [Google Scholar] [CrossRef]
- Mosić, M.; Trifković, J.; Vovk, I.; Gašić, U.; Tešić, Ž.; Šikoparija, B.; Milojković-Opsenica, D. Phenolic Composition Influences the Health-Promoting Potential of Bee-Pollen. Biomolecules 2019, 9, 783. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, J.L.; de Almeida, T.S.; Lopes Neto, J.J.; Almeida Filho, L.C.P.; Ribeiro, P.R.V.; Brito, E.S.; Morgano, M.A.; da Silva, M.G.; Farias, D.F.; Carvalho, A.F.U. Chemical Composition, Nutritional Properties, and Antioxidant Activity of Licania tomentosa (Benth.) Fruit. Food Chem. 2020, 313, 126117. [Google Scholar] [CrossRef]
- Bor, M.; Ozkur, O.; Ozdemir, F.; Turkan, I. Identification and Characterization of the Glucosinolate–Myrosinase System in Caper (Capparis Ovata Desf.). Plant Mol. Biol. Rep. 2009, 27, 518–525. [Google Scholar] [CrossRef]
- Matthäus, B.; Özcan, M. Glucosinolate Composition of Young Shoots and Flower Buds of Capers (Capparis Species) Growing Wild in Turkey. J. Agric. Food Chem. 2002, 50, 7323–7325. [Google Scholar] [CrossRef]
- Montoro, P.; Braca, A.; Pizza, C.; De Tommasi, N. Structure–Antioxidant Activity Relationships of Flavonoids Isolated from Different Plant Species. Food Chem. 2005, 92, 349–355. [Google Scholar] [CrossRef]
- Rösch, D.; Bergmann, M.; Knorr, D.; Kroh, L.W. Structure—Antioxidant Efficiency Relationships of Phenolic Compounds and Their Contribution to the Antioxidant Activity of Sea Buckthorn Juice. J. Agric. Food Chem. 2003, 51, 4233–4239. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In Vitro and in Vivo Antioxidant Properties of Chlorogenic Acid and Caffeic Acid. Int. J. Pharmaceut. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of Phenolic Acids: Metabolites versus Parent Compounds: A Review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Embuscado, M.E. Spices and Herbs: Natural Sources of Antioxidants—A Mini Review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Fica, A. Tratamiento de Infecciones Fúngicas Sistémicas Primera Parte: Fluconazol, Itraconazol y Voriconazol. Rev. Chil. Infectol. 2004, 21, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Goel, N.; Gagneja, D.; Chaudhary, U.; Agarwal, R. Fluconazole Resistance among Candida Species Causing Vulvovaginitis. Res. Rev. A J. Microbiol. Virol. 2012, 2, 1–12. [Google Scholar]
- Costa, L.C.; Alves, S.F.; Nogueira, S.A.; Carvalho, G.K. Determination of fluconazole content of magistral and industrial capsules. Rev. Eletron. Fac. Montes Belos 2014, 7, 47–56. [Google Scholar]
- Odiba, J.; Musa, A.; Hassan, H.; Yahay, S.; Okolo, E. Antimicrobial Activity of Isolated Stigmast-5-En-3-β-Ol (β-Sitosterol) from Honeybee Propolis from North-Western, Nigeria. Int. J. Pharm. Sci. Res. 2014, 5, 908–918. [Google Scholar]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakr, R.O.; Bishbishy, E.; Helmy, M. Profile of Bioactive Compounds of Capparis Spinosa Var. Aegyptiaca Growing in Egypt. Rev. Bras. Farmacogn. 2016, 26, 514–520. [Google Scholar] [CrossRef] [Green Version]
- Ali-Shtayeh, M.S.; Abu Ghdeib, S.I. Antifungal Activity of Plant Extracts against Dermatophytes. Mycoses 1999, 42, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Su, C.; Liu, H. Candida albicans Hyphal Initiation and Elongation. Trends. Microbiol. 2014, 22, 707–714. [Google Scholar] [CrossRef] [Green Version]
- Morais-Braga, M.F.; Carneiro, J.N.; Machado, A.J.; Sales, D.L.; Dos Santos, A.T.; Boligon, A.A.; Athayde, M.L.; Menezes, I.R.; Souza, D.S.; Costa, J.G.; et al. Phenolic Composition and Medicinal Usage of Psidium Guajava Linn.: Antifungal Activity or Inhibition of Virulence? Saudi J. Biol. Sci. 2017, 24, 302–313. [Google Scholar] [CrossRef] [Green Version]
- Silva-Dias, A.; Miranda, I.M.; Branco, J.; Monteiro-Soares, M.; Pina-Vaz, C.; Rodrigues, A.G. Adhesion, Biofilm Formation, Cell Surface Hydrophobicity, and Antifungal Planktonic Susceptibility: Relationship among Candida spp. Front. Microbiol. 2015, 6, 205. [Google Scholar] [CrossRef] [Green Version]
- Deorukhkar, S.C.; Saini, S.; Mathew, S. Virulence Factors Contributing to Pathogenicity of Candida Tropicalis and Its Antifungal Susceptibility Profile. Int. J. Food Microbiol. 2014, 2014, 456878. [Google Scholar]
- Freitas, M.A.; Santos, A.T.; Machado, A.J.; Silva, A.R.P.; Campina, F.F.; Costa, M.S.; Martins, G.M.; Morais-Braga, M.F.B.; Tintino, S.R.; Menezes, I.R.; et al. Fern Extracts Potentiate Fluconazole Activity and Inhibit Morphological Changes in Candida Species. Asian Pac. J. Trop. Biomed. 2017, 7, 1025–1030. [Google Scholar] [CrossRef]
| Metabolites | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| + | − | + | + | + | + | + | + | + | + | + | + | + | − | + |
| N° Peak | Rt Min | [M-H]− Observed | [M-H]− Calculated | Product Ions (MS/MS) m/z | Empirical Formula [M-H]− | Mass Error (ppm) | Putative Name | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.19 | 341.1091 | 341.1084 | 179 | C12H21O11 | 2.1 | Sucrose | [35] |
| 2 | 1.36 | 360.0249 | 360.0245 | 96, 74 | C10H18NO9S2 | 1.1 | Isopropyl/n-Propyl-GLS isomer | [36] |
| 3 | 2.05 | 360.0252 | 360.0410 | 96, 74 | C10H18NO9S2 | 1.9 | Isopropyl/n-Propyl-GLS isomer | [36] |
| 4 | 2.44 | 374.0619 | 374.0600 | 259, 195 | C9H20N5O5S3 | 2.1 | Methylpropyl-GLS/butyl-GLS | [37] |
| 5 | 2.96 | 388.0750 | 388.0736 | 96, 74 | C12H22NO9S2 | 3.6 | Methylbutyl-GLS | [38] |
| 6 | 3.45 | 451.2181 | 451.2179 | 405, 193, 161 | C20H35O11 | 0.4 | Unknown | - |
| 7 | 3.58 | 625.1401 | 625.1405 | 301, 300 | C27H29O17 | 0.6 | Quercetin O-di-hexoside | [39] |
| 8 | 3.72 | 595.1258 | 595.1240 | 301, 300 | C26H27O16 | −2.4 | Quercetin-pentosyl-hexoside | [39] |
| 9 | 3.78 | 609.1382 | 609.1362 | 301, 300 | C27H29O16 | 3.3 | Rutin | [40,41] |
| 10 | 3.89 | 463.0888 | 463.0877 | 301, 300 | C21H19O12 | 2.4 | Quercetin O-glucoside | [39] |
| 11 | 3.98 | 593.1509 | 593.1506 | 447, 285 | C27H29O15 | 0.5 | Kaempferol O-rhamnosyl-hexoside | [42] |
| 12 | 4.01 | 579.1343 | 579.1350 | 447, 285 | C26H27O15 | 1.7 | Kaempferol O-pentosyl-hexoside | [42] |
| 13 | 4.17 | 581.2229 | 581.2234 | 401 | C28H37O13 | −0.5 | Unknown | - |
| 14 | 4.27 | 521.2040 | 521.2023 | 359, 329 | C26H33O11 | 2.1 | Lariciresinol hexoside | [43] |
| 15 | 4.41 | 331.1226 | 331.1232 | 179 | C20H19O5 | −1.8 | Unknown | - |
| 16 | 4.60 | 745.2650 | 745.2649 | 195, 179 | C44H41O11 | 0.1 | Unknown | - |
| IC50 DPPH● (μg/mL) | FCZ | AA | CFHEE | CFHEE + FCZ |
| >2048 | <2 | 124 | 196.6 |
| Tested Product | Strains | |
|---|---|---|
| CA INCQS 40006 | CT INCQS 40042 | |
| FCZ | 83.121 | 1544.197 |
| CFHEE | >2048 | >2408 |
| CFHEE + FCZ | 764.03 | 1339.706 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar, G.J.T.; Carneiro, J.N.P.; da Silva, A.C.A.; Cruz, B.G.; da Silva, R.O.M.; da Costa, J.G.M.; da Cruz, R.P.; da Silva, J.C.P.; Ferreira, M.H.; dos Santos, A.T.L.; et al. Antioxidant and Antifungal Activity of the Cynophalla flexuosa (L.) J. Presl (Capparaceae) against Opportunistic Fungal Pathogens. Future Pharmacol. 2022, 2, 16-30. https://doi.org/10.3390/futurepharmacol2010002
Salazar GJT, Carneiro JNP, da Silva ACA, Cruz BG, da Silva ROM, da Costa JGM, da Cruz RP, da Silva JCP, Ferreira MH, dos Santos ATL, et al. Antioxidant and Antifungal Activity of the Cynophalla flexuosa (L.) J. Presl (Capparaceae) against Opportunistic Fungal Pathogens. Future Pharmacology. 2022; 2(1):16-30. https://doi.org/10.3390/futurepharmacol2010002
Chicago/Turabian StyleSalazar, Gerson Javier Torres, Joara Nalyda Pereira Carneiro, Ana Cristina Albuquerque da Silva, Beatriz Gonçalves Cruz, Rakel Olinda Macedo da Silva, José Galberto Martins da Costa, Rafael Pereira da Cruz, Josefa Carolaine Pereira da Silva, Maciel Horácio Ferreira, Antonia Thassya Lucas dos Santos, and et al. 2022. "Antioxidant and Antifungal Activity of the Cynophalla flexuosa (L.) J. Presl (Capparaceae) against Opportunistic Fungal Pathogens" Future Pharmacology 2, no. 1: 16-30. https://doi.org/10.3390/futurepharmacol2010002
APA StyleSalazar, G. J. T., Carneiro, J. N. P., da Silva, A. C. A., Cruz, B. G., da Silva, R. O. M., da Costa, J. G. M., da Cruz, R. P., da Silva, J. C. P., Ferreira, M. H., dos Santos, A. T. L., Almeida-Bezerra, J. W., da Silva, V. B., Linhares, K. V., Coutinho, H. D. M., Andrade, J. C., de Brito, E. S., Ribeiro, P. R. V., Sales, D. L., & Morais-Braga, M. F. B. (2022). Antioxidant and Antifungal Activity of the Cynophalla flexuosa (L.) J. Presl (Capparaceae) against Opportunistic Fungal Pathogens. Future Pharmacology, 2(1), 16-30. https://doi.org/10.3390/futurepharmacol2010002









