Clinical Demonstrations of Controlled-Release Tablets Constructed by the Combined Usage of Shellac and Hydroxypropyl Methylcellulose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Formulation of Controlled-Release Tablets and Study of Their Dissolution In Vitro
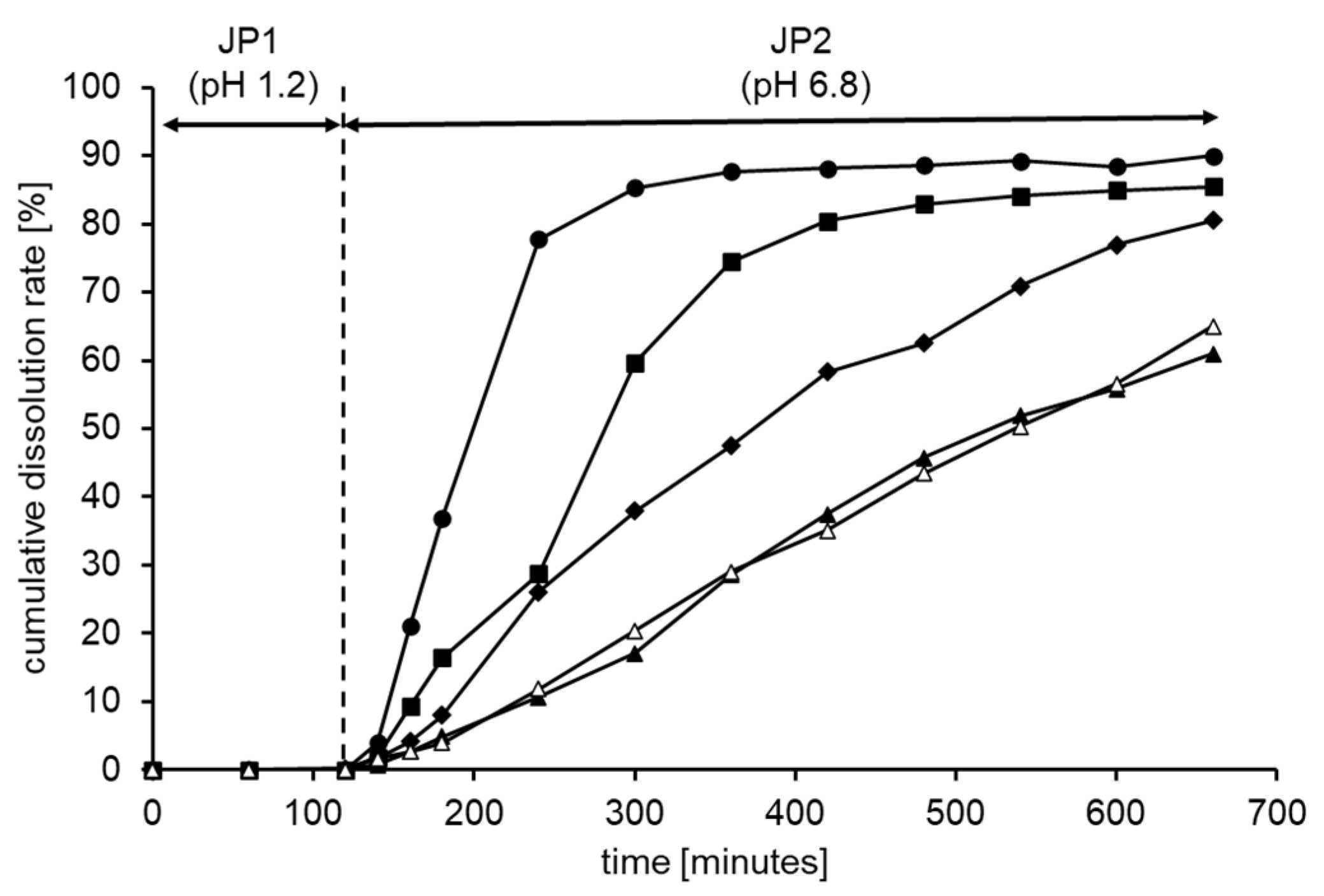
2.2.1. Preparation of X-ray Absorbable Controlled-Release Tablets with Barium Sulfate and Study of Their Dissolution In Vitro
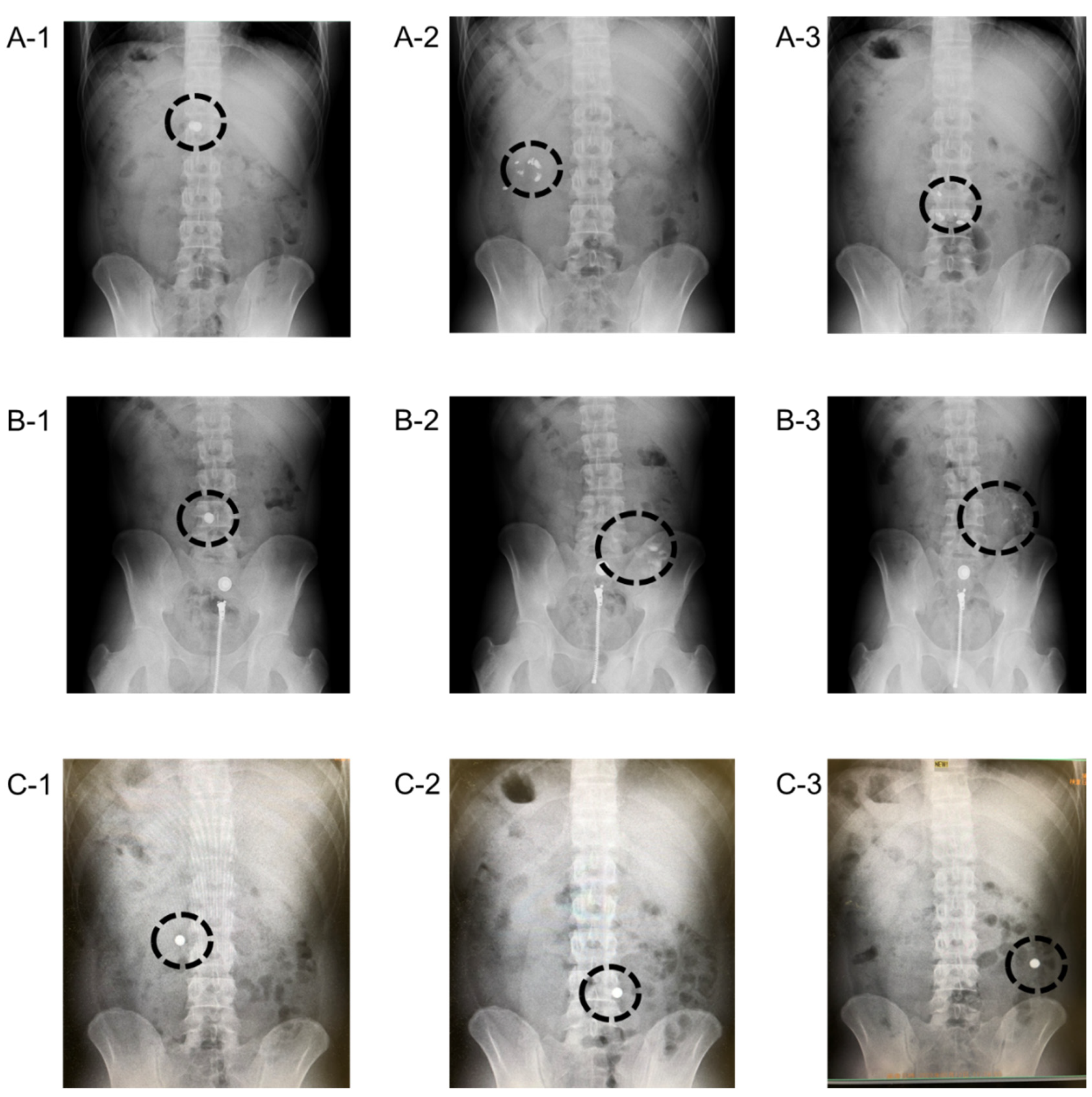
2.2.2. Human Volunteer Study with Barium-Containing Tablets by Means of X-ray Photography
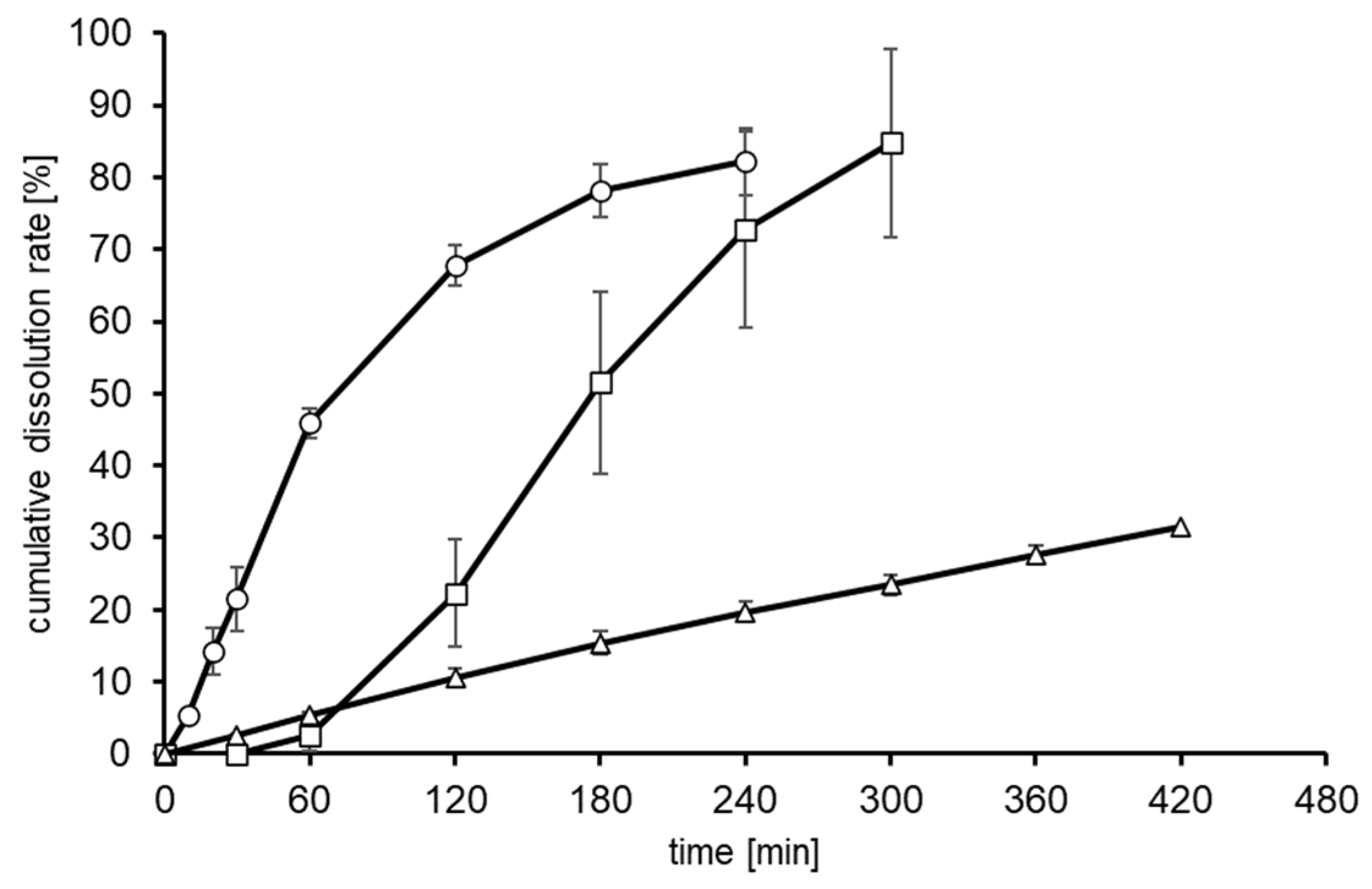
2.3. Preparation of Controlled-Release Tablets Containing Glucose and Quantitative Demonstration with Human Volunteers
2.4. Statistical Data Analysis
3. Results and Discussion
3.1. Formulation and Characterization of Controlled-Release Tablets Using HPMC and Shellac
3.2. Photographical Demonstration of Gastrointestinal Delivery System Using X-ray Absorbable Barium
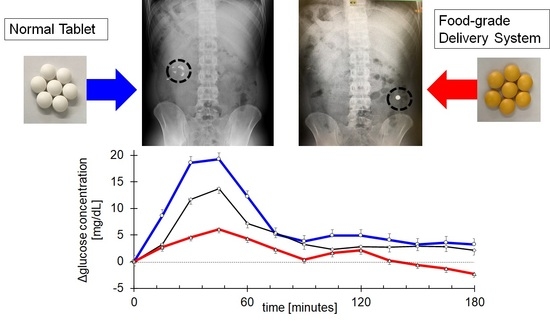
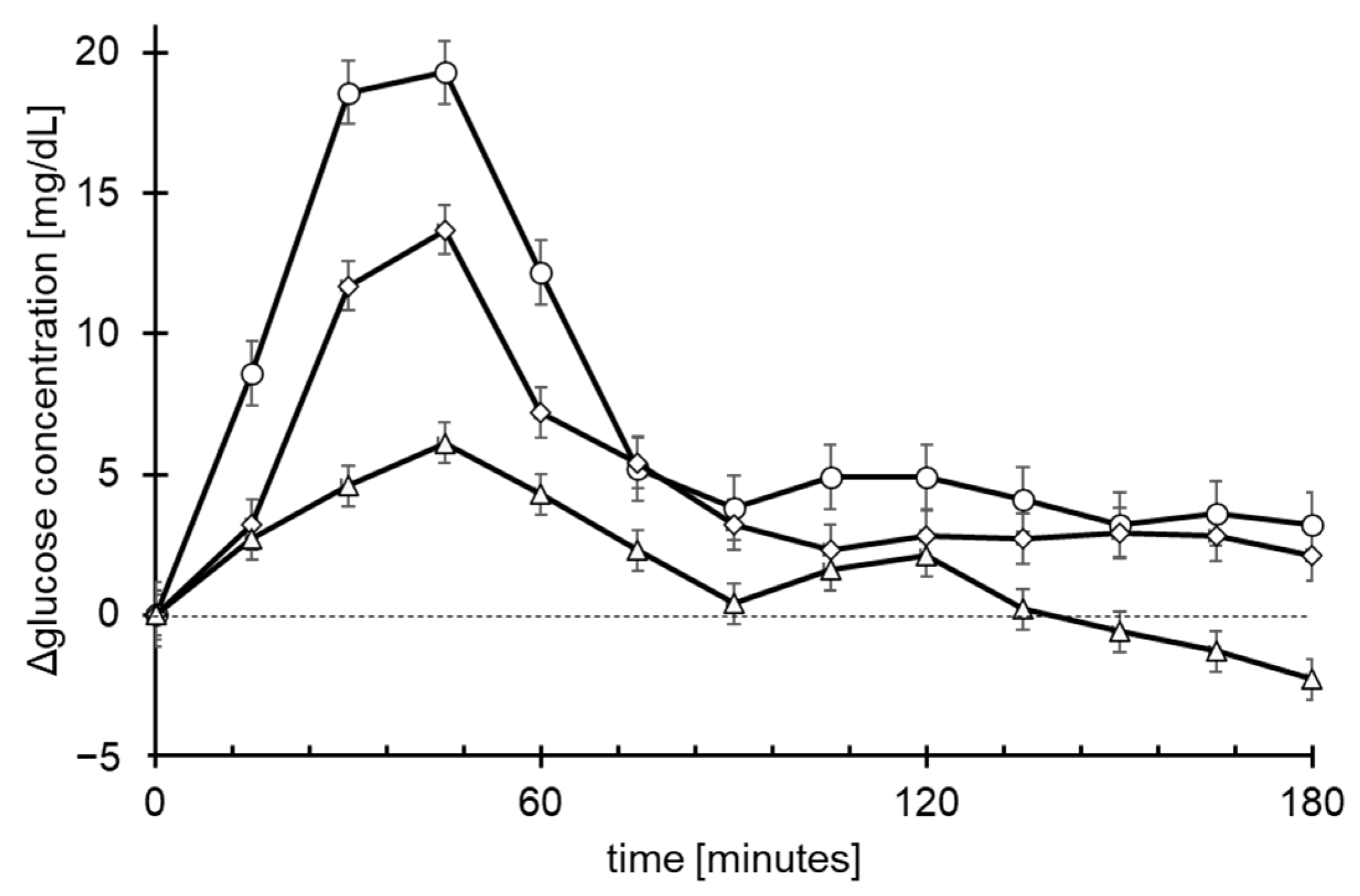
3.3. Quantitative Demonstration of Gastrointestinal Delivery System by Blood Glucose Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GRAND VIEW RESEARCH. Available online: https://www.grandviewresearch.com/industry-analysis/dietary-supplements-market (accessed on 30 June 2021).
- Fujii, T.; Jounai, K.; Horie, A.; Takahashi, H.; Suzuki, H.; Ohshio, K.; Fujiwara, D.; Yamamoto, N. Effects of heat-killed Lactococcus lactis subsp. lactis JCM 5805 on mucosal and systemic immune parameters, and antiviral reactions to influenza virus in healthy adults; a randomized controlled double-blind study. J. Funct. Foods 2017, 35, 513–521. [Google Scholar] [CrossRef]
- Sourav, D.; Sharat, S.; Lyndem, S.; Roy, A.S. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J. Biomol. Struct. Dyn. 2020, 39, 3347–3357. [Google Scholar]
- Piao, Z.-Z.; Lee, K.-H.; Kim, N.-J.; Lee, H.-G.; Lee, J.; Oh, K.T.; Lee, B.-J. Comparison of release-controlling efficiency of polymeric coating materials using matrix-type casted films and diffusion-controlled coated tablet. AAPS PharmSciTech 2010, 11, 630–636. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Kim, J.S.; Kang, S.H.; Yoo, Y.H.; Lee, S.; Park, J.S.; Woo, J.S.; Hwang, S.J. Influence of water soluble additives and HPMCP on drug release from surelease®-coated pellets containing tamsulosin hydrochloride. Arch. Pharm. Res. 2007, 30, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, G.; Chu, X.; Gao, C.; Wang, Y.; Gong, W.; Li, Z.; Yang, Y.; Yang, M.; Gao, C. Polymer distribution and mechanism conversion in multiple media of phase-separated controlled-release film-coating. Pharmaceutics 2019, 11, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obeidat, W.M.; Nokhodchi, A.; Alkhatib, H. Evaluation of matrix tablets based on eudragit®E100/carbopol®971P combinations for controlled release and improved compaction properties of water soluble model drug paracetamol. AAPS PharmSciTech 2015, 16, 1169–1179. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, J.; Murthy, P.N.; Biswal, S.; Sahoo, S.K.; Mahapatra, A.K. Comparative study of propranolol hydrochloride release from matrix tablets with Kollidon®SR or hydroxy propyl methyl cellulose. AAPS PharmSciTech 2008, 9, 577–582. [Google Scholar] [CrossRef]
- Cao, Q.-R.; Choi, Y.-W.; Cui, J.-H.; Lee, B.-J. Formulation, release characteristics and bioavailability of novel monolithic hydroxypropylmethylcellulose matrix tablets containing acetaminophen. J. Control. Release 2005, 108, 351–361. [Google Scholar] [CrossRef]
- Kibria, G.; Roni, M.A.; Absar, M.S.; Jalil, R.-U. Effect of plasticizer on release kinetics of diclofenac sodium pellets coated with eudragit RS 30 D. AAPS PharmSciTech 2008, 9, 1240–1246. [Google Scholar] [CrossRef] [Green Version]
- Phaechamud, T.; Ritthidej, G.C. Formulation variables influencing drug release from layered matrix system comprising chitosan and xanthan gum. AAPS PharmSciTech 2008, 9, 870–877. [Google Scholar] [CrossRef]
- Maity, S.; Sa, B. Compression-coated tablet for colon targeting: Impact of coating and core materials on drug release. AAPS PharmSciTech 2015, 17, 504–515. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.-J.; Ryu, S.-G.; Cui, J.-H. Controlled release of dual drug-loaded hydroxypropyl methylcellulose matrix tablet using drug-containing polymeric coatings. Int. J. Pharm. 1999, 188, 71–80. [Google Scholar] [CrossRef]
- He, W.; Huang, S.; Zhou, C.; Cao, L.; Yao, J.; Zhou, J.; Wang, G.; Yin, L. Bilayer matrix tablets for prolonged actions of metformin hydrochloride and repaglinide. AAPS PharmSciTech 2014, 16, 344–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EI Naggar, E.E.; Mohamed, E.A.; Borg, T.M.; EI-Sheakh, A.R.; Harmed, M.F. Colon targeting of naringin for enhanced cytoprotection against indomethacin-induced colitis in rabbits. Drug Des. Devel. Ther. 2020, 14, 677–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Gousous, J.; Penning, M.; Langguth, P. Molecular insights into shellac film coats from different aqueous shellac salt solutions and effect on disintegration of enteric-coated soft gelatin capsules. Int. J. Pharm. 2015, 484, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Farag, Y.; Leopold, C.S. Development of shellac-coated sustained release pellet formulations. Eur. J. Pharm. Sci. 2011, 42, 400–405. [Google Scholar] [CrossRef]
- Oehme, A.; Valotis, A.; Krammer, G.; Zimmermann, I.; Schreier, P. Preparation and characterization of shellac-coated anthocyanin pectin beads as dietary colonic delivery system. Mol. Nutr. Food Res. 2011, 55, S75–S85. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Limmatvapirat, S.; Limmatvapirat, C.; Puttipipatkhachorn, S.; Nuntanid, J.; Luangtana-Anan, M. Enhanced enteric properties and stability of shellac films through composite salts formation. Eur. J. Pharm. Biopharm. 2007, 67, 690–698. [Google Scholar] [CrossRef]
- Rujivipat, S.; Bodmeier, R. Modified release from hydroxypropyl methylcellulose compression-coated tablets. Int. J. Pharm. 2010, 402, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Koziolek, M.; Grimm, M.; Becker, D.; Lordanov, V.; Zou, H.; Shimizu, J.; Wanke, C.; Garbacz, G.; Weitschies, W. Investigation of pH and temperature profiles in the GI tract of fasted human subjects using the Intellicap® system. J. Pharm. Sci. 2015, 109, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.; Bode, B.W.; Christiansen, M.P.; Klaff, L.J.; Alva, S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol. Ther. 2015, 17, 787–794. [Google Scholar] [CrossRef]
- Wachters-Hagedoorn, R.E.; Priebe, M.G.; Heimweg, J.A.J.; Heiner, A.M.; Englyst, K.N.; Holst, J.J.; Stellaard, F.; Vonk, R.J. The rate of intestinal glucose absorption is correlated with plasma glucose-dependent insulinotropic polypeptide concentrations in healthy men. J. Nutr. 2006, 136, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
| Tablet 1 [%] | Tablet 2 [%] | Tablet 3 [%] | Tablet 4 [%] | Tablet 5 [%] | |
|---|---|---|---|---|---|
| maltitol | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| lactose | 33.5 | 28.5 | 23.5 | 18.5 | 13.5 |
| starch | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| HPMC | 0.0 | 5.0 | 10.0 | 15.0 | 20.0 |
| calcium stearate | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| coloring agent * | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| total | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Tablet 6a [%] | Tablet 6b [%] | Tablet 7a [%] | Tablet 7b [%] | Tablet 8a [%] | Tablet 8b [%] | |
|---|---|---|---|---|---|---|
| Barium sulphate | 65.0 | 65.0 | 65.0 | 65.0 | 65.0 | 65.0 |
| Crystalline cellulose | 28.5 | 32.0 | 23.5 | 27.0 | 18.5 | 22.0 |
| Agar | 0.0 | 0.0 | 5.0 | 5.0 | 0.0 | 0.0 |
| Psyllium husk powder | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.5 |
| HPMC | 0.0 | 0.0 | 0.0 | 0.0 | 9.5 | 9.5 |
| Silicon dioxide | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Calcium stearate | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Coloring agent * | 3.5 | 0.0 | 3.5 | 0.0 | 3.5 | 0.0 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Tablet 9 (mg) | Tablet 10 (mg) | Tablet 11 (mg) | |
|---|---|---|---|
| Glucose | 402.75 | 402.75 | 402.75 |
| Crystalline cellulose | 33.75 | 20.25 | 0.00 |
| Psyllium husk powder | 0.00 | 2.25 | 2.25 |
| HPMC | 0.00 | 13.50 | 33.75 |
| Calcium stearate | 13.50 | 13.50 | 13.50 |
| Total | 450.00 | 452.25 | 452.25 |
| Zero-Order | First-Order | Korsmeyer–Peppas | |||||
|---|---|---|---|---|---|---|---|
| K0 | R2 | K1 | R2 | n | Kkp | R2 | |
| Tablet 1 | 2.682 | 0.856 | 0.264 | 0.939 | 1.64 | 0.310 | 0.959 |
| Tablet 2 | 1.556 | 0.910 | 0.134 | 0.962 | 1.40 | 0.124 | 0.968 |
| Tablet 3 | 1.098 | 0.965 | 0.082 | 0.997 | 1.30 | 0.080 | 0.991 |
| Tablet 4 | 0.855 | 0.990 | 0.049 | 0.990 | 1.30 | 0.042 | 0.993 |
| Tablet 5 | 0.861 | 0.999 | 0.050 | 0.982 | 1.18 | 0.051 | 0.991 |
| Tablet 9 | Tablet 10 | Tablet 11 | |
|---|---|---|---|
| AUC (mg.h/L) | 276.1± 35.3 | 168.1± 33.9 * | 89 ± 30.7 ** |
| Cmax (mg/dL) | 24.5 ± 3.4 | 15.6 ± 2.5 # | 9 ± 2.7 ## |
| Tmax (hours) | 0.6 ± 0.1 | 1.1 ± 0.3 | 0.9 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakamatsu, J.; Sato, K.; Uryu, K.; Maru, I. Clinical Demonstrations of Controlled-Release Tablets Constructed by the Combined Usage of Shellac and Hydroxypropyl Methylcellulose. Future Pharmacol. 2021, 1, 48-59. https://doi.org/10.3390/futurepharmacol1010005
Wakamatsu J, Sato K, Uryu K, Maru I. Clinical Demonstrations of Controlled-Release Tablets Constructed by the Combined Usage of Shellac and Hydroxypropyl Methylcellulose. Future Pharmacology. 2021; 1(1):48-59. https://doi.org/10.3390/futurepharmacol1010005
Chicago/Turabian StyleWakamatsu, Junichiro, Kanae Sato, Keisuke Uryu, and Isafumi Maru. 2021. "Clinical Demonstrations of Controlled-Release Tablets Constructed by the Combined Usage of Shellac and Hydroxypropyl Methylcellulose" Future Pharmacology 1, no. 1: 48-59. https://doi.org/10.3390/futurepharmacol1010005
APA StyleWakamatsu, J., Sato, K., Uryu, K., & Maru, I. (2021). Clinical Demonstrations of Controlled-Release Tablets Constructed by the Combined Usage of Shellac and Hydroxypropyl Methylcellulose. Future Pharmacology, 1(1), 48-59. https://doi.org/10.3390/futurepharmacol1010005