Root Pruning Enhances Leaf Oxidative Stress and Anthocyanin Accumulation in Hydroponically Grown Red Leaf Lettuce
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Measurement of Leaf Redness
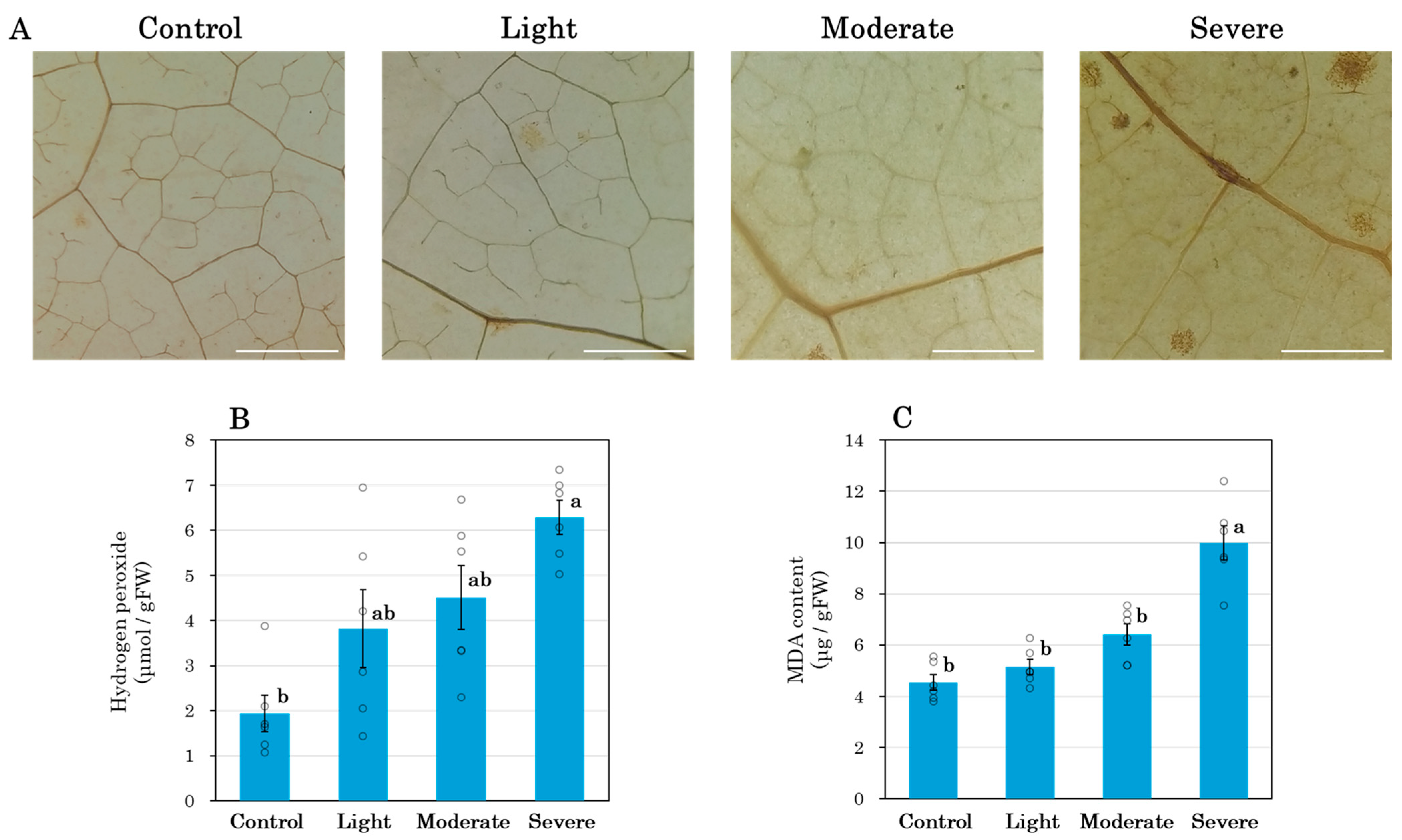
2.3. Histochemical Detection of Hydrogen Peroxide
2.4. Determination of Anthocyanin Content
2.5. Determination of Total Phenolic Content
2.6. Determination of Hydrogen Peroxide
2.7. Determination of Lipid Peroxidation
2.8. Determination of Nitrate Content
2.9. Data Analysis
3. Results
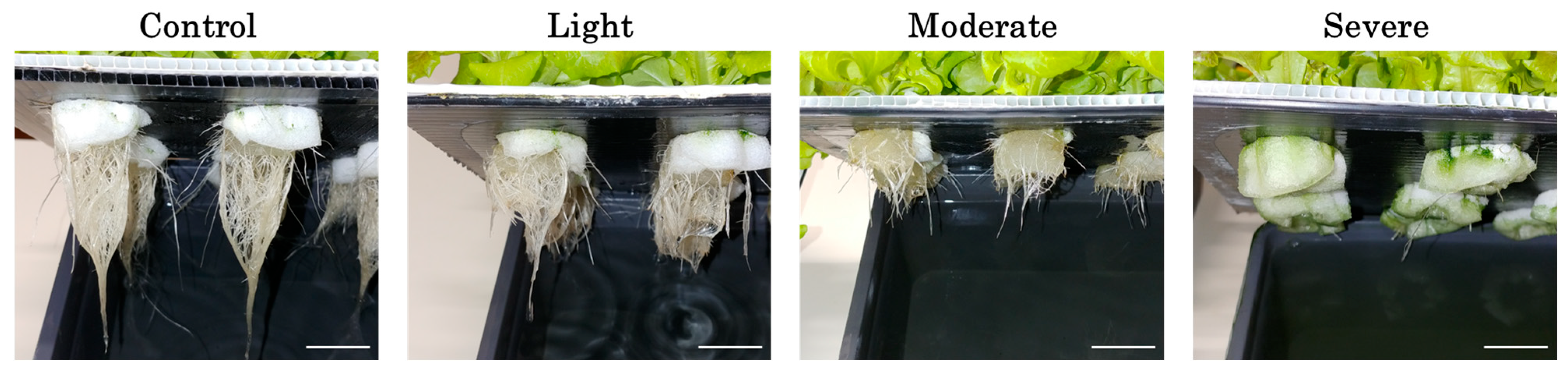
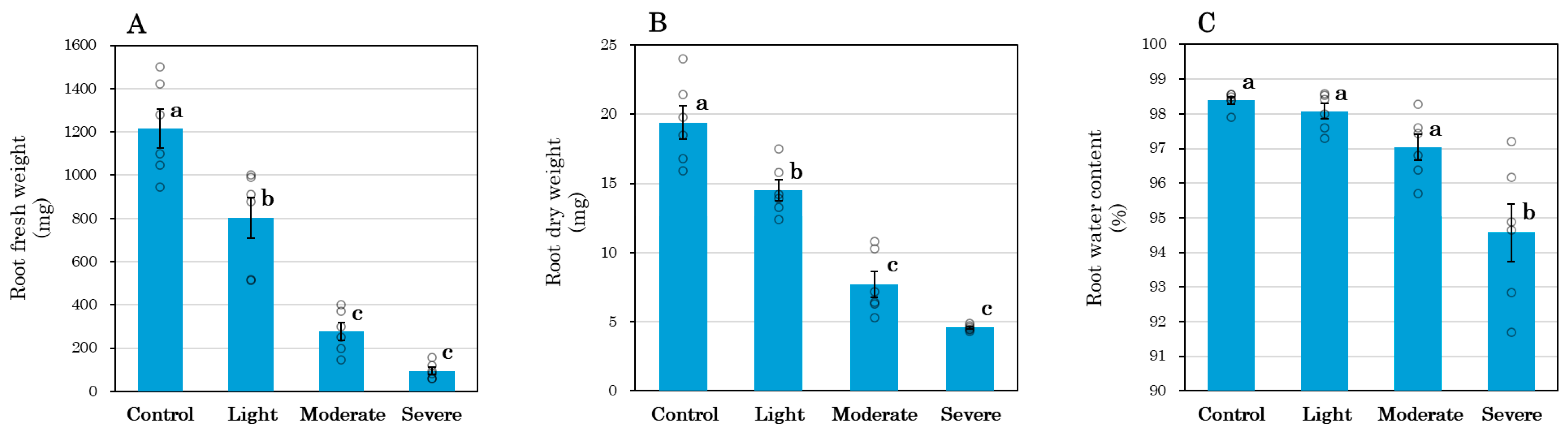
3.1. Root Morphological and Physiological Changes
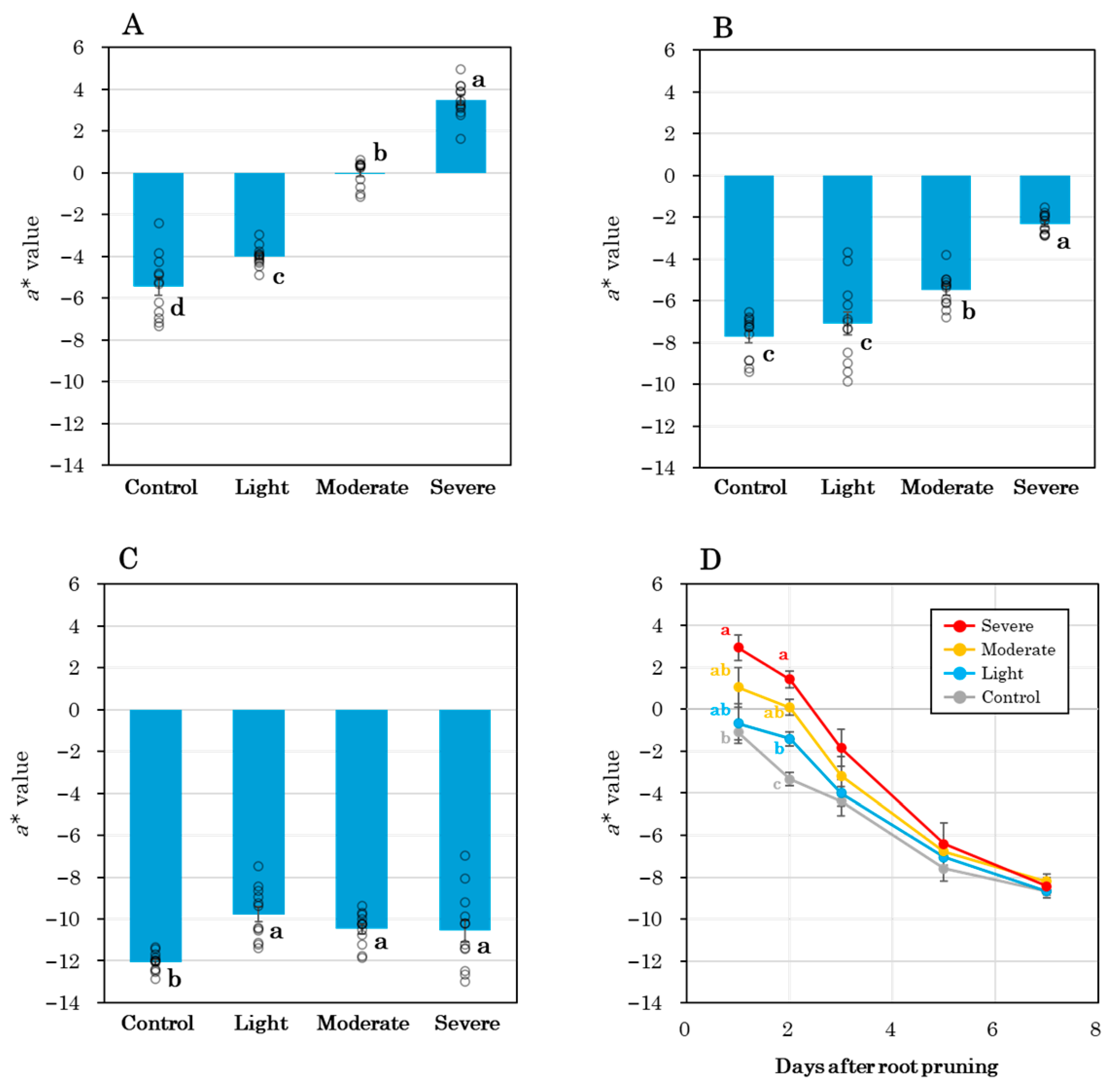
3.2. Shoot Morphological Response and Leaf Coloration
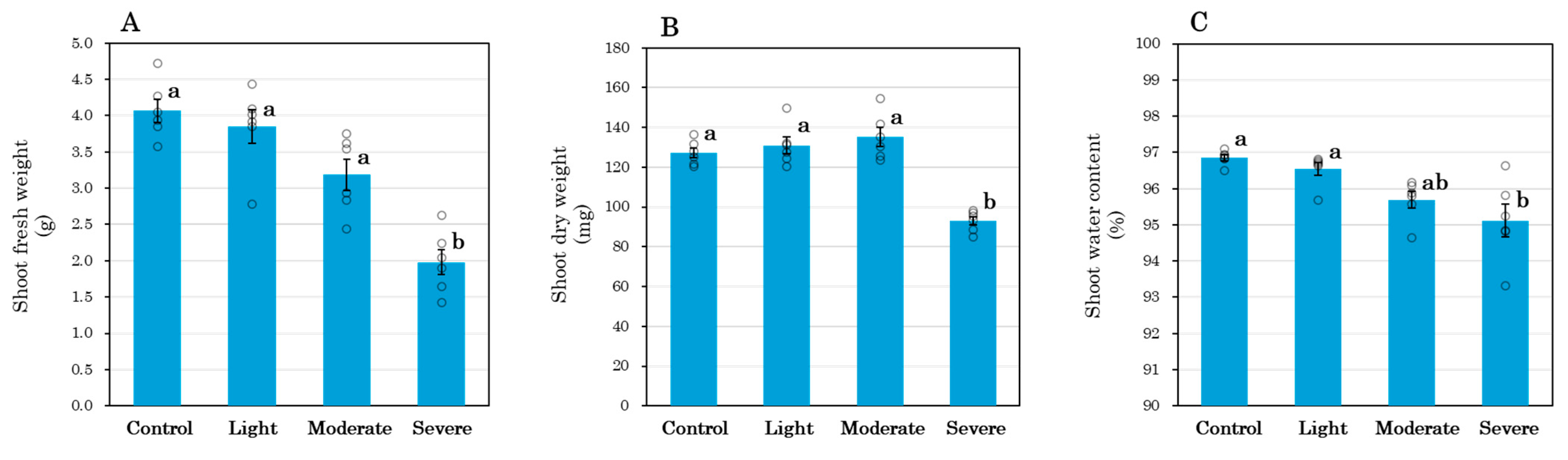
3.3. Shoot Biomass and Water Status
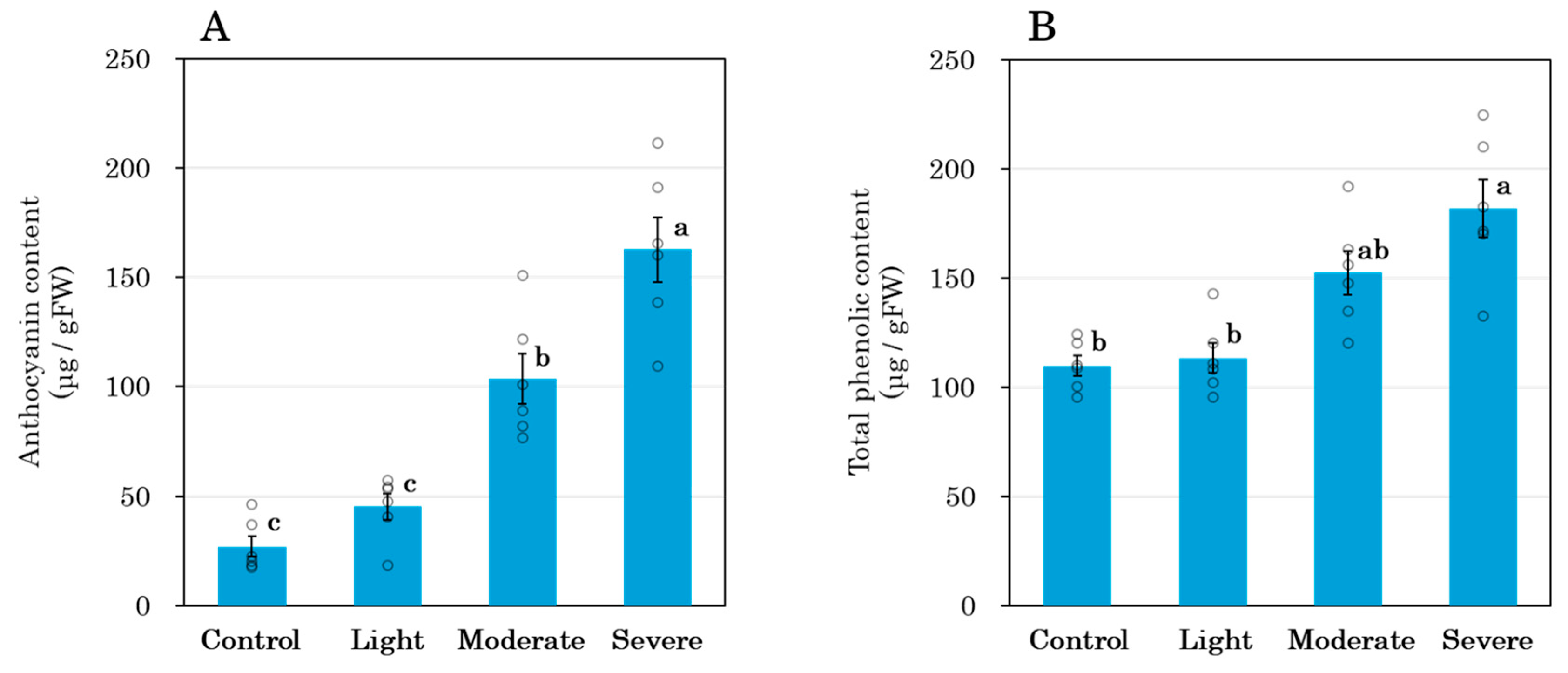
3.4. Shoot Anthocyanin Accumulation and Total Phenolic Content
3.5. Shoot Oxidative Stress
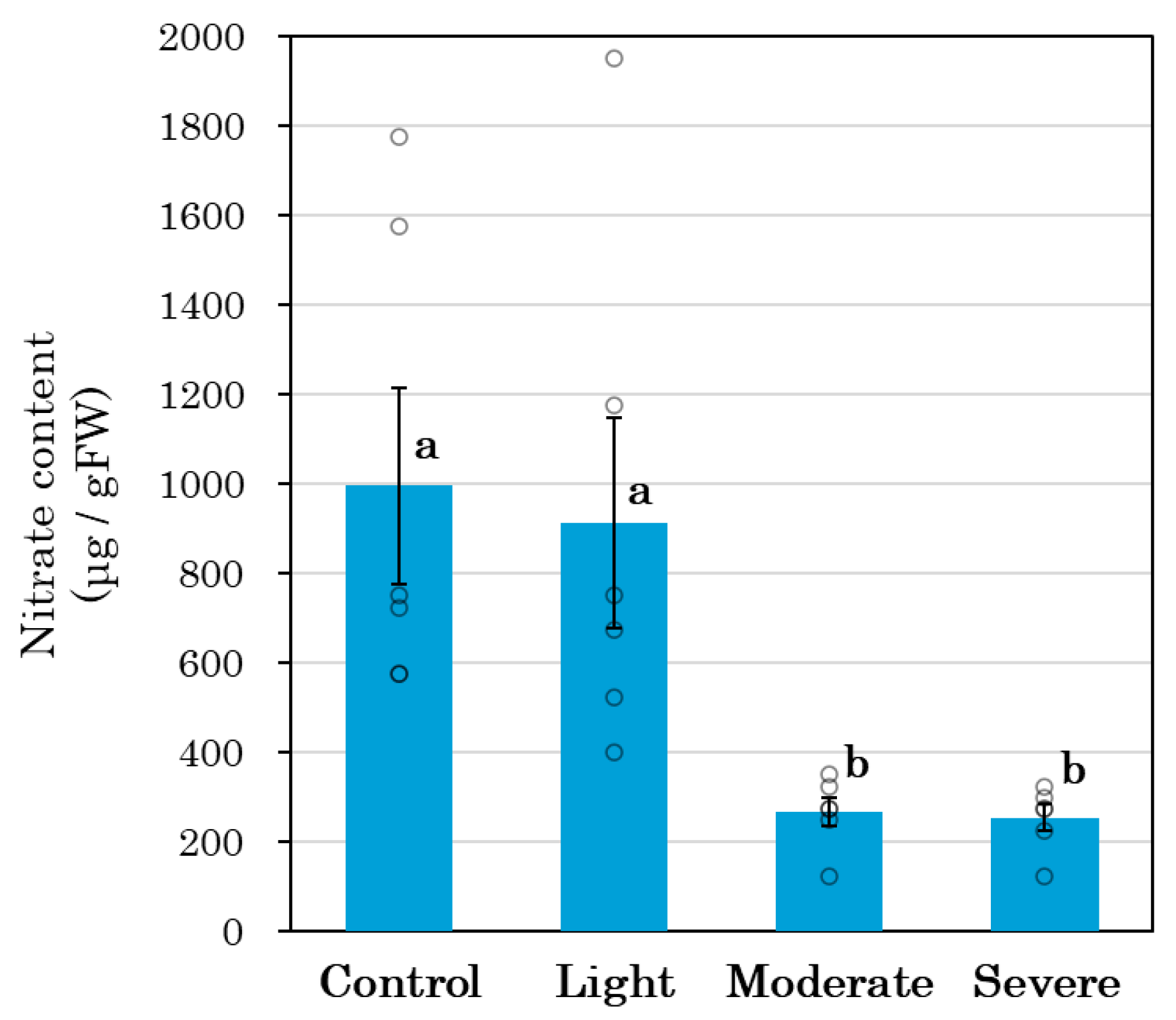
3.6. Shoot Nitrate Content
4. Discussion
4.1. Root Pruning and Drought-like Stress
4.2. Root Pruning and Plant Growth
4.3. Root Pruning and Leaf Metabolites
4.4. Pre-Harvest Strategies for Component Regulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anjum, S.A.; Ashraf, U.; Zohaib, A.; Tanveer, M.; Naeem, M.; Ali, I.; Tabassum, T.; Nazir, U. Growth and Developmental Responses of Crop Plants under Drought Stress: A Review. Zemdirb. Agric. 2017, 104, 267–276. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Song, W. Physiological and Growth Characteristics of Tomato Seedlings in Response to Low Root-Zone Temperature. HortScience 2023, 58, 442–448. [Google Scholar] [CrossRef]
- Daniel, K.; Hartman, S. How Plant Roots Respond to Waterlogging. J. Exp. Bot. 2024, 75, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of Abscisic Acid-Mediated Drought Stress Responses in Plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H. Effect of Drought Stress on Total Phenolic, Lipid Peroxidation, and Antioxidant Activity of Achillea Species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef]
- Rahimi, M.; Kordrostami, M.; Mohamadhasani, F.; Chaeikar, S.S. Antioxidant Gene Expression Analysis and Evaluation of Total Phenol Content and Oxygen-Scavenging System in Tea Accessions under Normal and Drought Stress Conditions. BMC Plant Biol. 2021, 21, 494. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. Interaction of Proline, Sugars, and Anthocyanins during Photosynthetic Acclimation of Arabidopsis thaliana to Drought Stress. J. Plant Physiol. 2012, 169, 577–585. [Google Scholar] [CrossRef]
- Jan, R.; Asif, S.; Asaf, S.; Lubna; Khan, Z.; Kim, K.-M. Unveiling the Protective Role of Anthocyanin in Rice: Insights into Drought-Induced Oxidative Stress and Metabolic Regulation. Front. Plant Sci. 2024, 15, 1397817. [Google Scholar] [CrossRef]
- Medina-Lozano, I.; Bertolín, J.R.; Díaz, A. Impact of Drought Stress on Vitamin C and Anthocyanin Content in Cultivated Lettuces (Lactuca sativa L.) and Wild Relatives (Lactuca spp.). Front. Plant Sci. 2024, 15, 1369658. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of Polyphenols and Antioxidant Properties of Five Lettuce Varieties and Escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-E.; Shang, X.; Assefa, A.D.; Keum, Y.-S.; Saini, R.K. Metabolite Profiling of Green, Green/Red, and Red Lettuce Cultivars: Variation in Health Beneficial Compounds and Antioxidant Potential. Food Res. Int. 2018, 105, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, H.; Liu, Y.; Zhang, L.; Li, D.; Zhao, X.; Zhang, J.; Sui, Y. Promoting Anthocyanin Biosynthesis in Purple Lettuce through Sucrose Supplementation under Nitrogen Limitation. Horticulturae 2024, 10, 838. [Google Scholar] [CrossRef]
- Liang, Y.; Dong, Y.; Yang, Q.; Urano, D.; Wang, Z. Interactive Effects of Light Quality and Nitrate Supply on Growth and Metabolic Processes in Two Lettuce Cultivars (Lactuca sativa L.). Environ. Exp. Bot. 2023, 213, 105443. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Effect of Root-Zone Temperature on Growth and Quality of Hydroponically Grown Red Leaf Lettuce (Lactuca sativa L. cv. Red Wave). Am. J. Plant Sci. 2015, 06, 2350. [Google Scholar] [CrossRef]
- Wittayathanarattana, T.; Wanichananan, P.; Supaibulwatana, K.; Goto, E. A Short-Term Cooling of Root-Zone Temperature Increases Bioactive Compounds in Baby Leaf Amaranthus tricolor L. Front. Plant Sci. 2022, 13, 944716. [Google Scholar] [CrossRef]
- Sakamoto, M.; Funaki, A.; Sakagami, F.; Kaida, T.; Suzuki, T. Unveiling the Impact of LED Light on Growing Carrot Taproots: A Novel Hydroponic Cultivation System. Eng 2025, 6, 87. [Google Scholar] [CrossRef]
- Caramanico, L.; Rustioni, L.; De Lorenzis, G. Iron Deficiency Stimulates Anthocyanin Accumulation in Grapevine Apical Leaves. Plant Physiol. Biochem. 2017, 119, 286–293. [Google Scholar] [CrossRef]
- Shi, K.; Ding, X.-T.; Dong, D.-K.; Zhou, Y.-H.; Yu, J.-Q. Root Restriction-Induced Limitation to Photosynthesis in Tomato (Lycopersicon esculentum Mill.) Leaves. Sci. Hortic. 2008, 117, 197–202. [Google Scholar] [CrossRef]
- Jing, D.; Du, Z.; Wang, M.; Wang, Q.; Ma, H.; Liu, F.; Ma, B.; Dong, Y. Regulatory Effects of Root Pruning on Leaf Nutrients, Photosynthesis, and Growth of Trees in a Closed-Canopy Poplar Plantation. PLoS ONE 2018, 13, e0197515. [Google Scholar] [CrossRef]
- Budiarto, R.; Poerwanto, R.; Santosa, E.; Efendi, D. A Review of Root Pruning to Regulate Citrus Growth. J. Trop. Crop Sci. 2019, 6, 1–7. [Google Scholar] [CrossRef]
- Fang, C.; Zhou, K.; Zhang, Y.; Li, B.; Han, M. Effect of Root Pruning and Nitrogen Fertilization on Growth of Young ‘Fuji’ Apple (Malus domestica Borkh.) Trees. J. Plant Nutr. 2017, 40, 1538–1546. [Google Scholar] [CrossRef]
- Kwon, J.K.; Kang, S.W.; Paek, Y.; Moon, J.P.; Jang, J.K.; Oh, S.S. Effects of Local Cooling and Root Pruning on Budding and Local Heating on Heating Energy Consumption in Forcing Cultivation of Strawberry. J. Bio-Environ. Control 2019, 28, 46–54. [Google Scholar] [CrossRef]
- Mathiyazhagan, K.; Subash, M.; Bose, C. Root Pruning—A Growth Regulation Practice in Fruit Crops. CABI Rev. 2021, 16, 1–12. [Google Scholar] [CrossRef]
- Salachas, G.; Savvas, D.; Argyropoulou, K.; Tarantillis, P.; Kapotis, G. Yield and Nutritional Quality of Aeroponically Cultivated Basil as Affected by the Available Root-Zone Volume. Emir. J. Food Agric. 2015, 27, 911. [Google Scholar] [CrossRef]
- Kharkina, T.G.; Ottosen, C.-O.; Rosenqvist, E. Effects of Root Restriction on the Growth and Physiology of Cucumber Plants. Physiol. Plant. 1999, 105, 434–441. [Google Scholar] [CrossRef]
- Nishizawa, T.; Saito, K. Effects of Rooting Volume Restriction on the Growth and Carbohydrate Concentration in Tomato Plants. J. Am. Soc. Hortic. Sci. 1998, 123, 581–585. [Google Scholar] [CrossRef]
- Al-Debei, H.; Mugnai, S. Starch Accumulation in the Leaves of Root-Restricted Pepper Affects Plant Growth by a Feedback-Inhibition of the Photosynthesis. Adv. Hortic. Sci. 2011, 25, 253–259. [Google Scholar] [CrossRef]
- Lam, V.P.; Kim, S.J.; Lee, H.J.; Park, J.S. Root Pruning Increased Bioactive Compounds of Hydroponically-Grown Agastache rugosa in a Greenhouse. Hortic. Environ. Biotechnol. 2019, 60, 647–657. [Google Scholar] [CrossRef]
- Biddington, N.L.; Dearman, A.S. Shoot and Root Growth of Lettuce Seedlings Following Root Pruning. Ann. Bot. 1984, 53, 663–668. [Google Scholar] [CrossRef]
- Suo, R.; Wang, W.; Ma, Y.; Fu, L.; Cui, Y. Effect of Different Root Lengths for Retaining Freshness of Hydroponic Lettuce. J. Agric. Food Res. 2021, 4, 100151. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Effect of Nutrient Solution Concentration on the Growth of Hydroponic Sweetpotato. Agronomy 2020, 10, 1708. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Methyl Jasmonate and Salinity Increase Anthocyanin Accumulation in Radish Sprouts. Horticulturae 2019, 5, 62. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. N-Acetylcysteine Mitigates Oxidative Stress Induced by Transplanting Lettuce Seedlings into a DFT Hydroponic System. Agronomy 2024, 14, 2112. [Google Scholar] [CrossRef]
- Poni, S.; Tagliavini, M.; Neri, D.; Scudellari, D.; Toselli, M. Influence of Root Pruning and Water Stress on Growth and Physiological Factors of Potted Apple, Grape, Peach and Pear Trees. Sci. Hortic. 1992, 52, 223–236. [Google Scholar] [CrossRef]
- Wang, Y.; Bertelsen, M.G.; Petersen, K.K.; Andersen, M.N.; Liu, F. Effect of Root Pruning and Irrigation Regimes on Leaf Water Relations and Xylem ABA and Ionic Concentrations in Pear Trees. Agric. Water Manag. 2014, 135, 84–89. [Google Scholar] [CrossRef]
- Patanè, C.; Cosentino, S.L.; Romano, D.; Toscano, S. Relative Water Content, Proline, and Antioxidant Enzymes in Leaves of Long Shelf-Life Tomatoes under Drought Stress and Rewatering. Plants 2022, 11, 3045. [Google Scholar] [CrossRef]
- Yan, S.; Weng, B.; Jing, L.; Bi, W. Effects of Drought Stress on Water Content and Biomass Distribution in Summer Maize (Zea mays L.). Front. Plant Sci. 2023, 14, 1118131. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, C.; Li, H.; Zhang, L.; Ren, Y.; Chen, Y.; Cai, H.; Zhang, S. Root Pruning Improves Maize Water-Use Efficiency by Root Water Absorption. Front. Plant Sci. 2023, 13, 1023088. [Google Scholar] [CrossRef]
- Smith, P.G.; Dale, J.E. The Effects of Root Cooling and Excision Treatments on the Growth of Primary Leaves of Phaseolus vulgar is L. New Phytol. 1988, 110, 293–300. [Google Scholar] [CrossRef]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A Review on Drought Stress in Plants: Implications, Mitigation and the Role of Plant Growth Promoting Rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Haghpanah, M.; Hashemipetroudi, S.; Arzani, A.; Araniti, F. Drought Tolerance in Plants: Physiological and Molecular Responses. Plants 2024, 13, 2962. [Google Scholar] [CrossRef] [PubMed]
- Huseynova, I.M.; Aliyeva, D.R.; Mammadov, A.C.; Aliyev, J.A. Hydrogen Peroxide Generation and Antioxidant Enzyme Activities in the Leaves and Roots of Wheat Cultivars Subjected to Long-Term Soil Drought Stress. Photosynth. Res. 2015, 125, 279–289. [Google Scholar] [CrossRef]
- Xia, L.; Yang, L.; Sun, N.; Li, J.; Fang, Y.; Wang, Y. Physiological and Antioxidant Enzyme Gene Expression Analysis Reveals the Improved Tolerance to Drought Stress of the Somatic Hybrid Offspring of Brassica napus and Sinapis alba at Vegetative Stage. Acta Physiol. Plant 2016, 38, 88. [Google Scholar] [CrossRef]
- Sezgin, A.; Altuntaş, C.; Sağlam, A.; Terzi, R.; Demiralay, M.; Kadıoğlu, A. Abscisic Acid Cross-Talking with Hydrogen Peroxide and Osmolyte Compounds May Regulate the Leaf Rolling Mechanism under Drought. Acta Physiol. Plant 2018, 40, 141. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, P.; Lu, Y.; Bai, Y.; Wei, Y.; Liu, G.; Shi, H. MeRAV5 Promotes Drought Stress Resistance in Cassava by Modulating Hydrogen Peroxide and Lignin Accumulation. Plant J. 2021, 107, 847–860. [Google Scholar] [CrossRef]
- Jing, D.-W.; Liu, F.-C.; Wang, M.-Y.; Ma, H.-L.; Du, Z.-Y.; Ma, B.-Y.; Dong, Y.-F. Effects of Root Pruning on the Physicochemical Properties and Microbial Activities of Poplar Rhizosphere Soil. PLoS ONE 2017, 12, e0187685. [Google Scholar] [CrossRef]
- Yang, S.; Xing, S.; Liu, C.; Du, Z.; Wang, H.; Xu, Y. Effects of Root Pruning on the Vegetative Growth and Fruit Quality of Zhanhuadongzao Trees. Hortic. Sci. 2010, 37, 14–21. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Deng, J.; Li, R.; Fan, X.; Dao, J.; Quan, Y.; Bukhari, S.A.H. Effect of Cutting Depth during Sugarcane (Saccharum spp. Hybrid) Harvest on Root Characteristics and Yield. PLoS ONE 2021, 16, e0238085. [Google Scholar] [CrossRef]
- Sakamoto, M.; Wada, M.; Suzuki, T. Effect of Partial Excision of Early Taproots on Growth and Components of Hydroponic Carrots. Horticulturae 2020, 6, 5. [Google Scholar] [CrossRef]
- Lei, C.; Engeseth, N.J. Comparison of Growth Characteristics, Functional Qualities, and Texture of Hydroponically Grown and Soil-Grown Lettuce. LWT 2021, 150, 111931. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, Z.; Lee, J.; Li, X.; Sun, W. Proteomic Analysis of Tea Plants (Camellia sinensis) with Purple Young Shoots during Leaf Development. PLoS ONE 2017, 12, e0177816. [Google Scholar] [CrossRef]
- Lee, J.-H.; Goto, E. Ozone Control as a Novel Method to Improve Health-Promoting Bioactive Compounds in Red Leaf Lettuce (Lactuca sativa L.). Front. Plant Sci. 2022, 13, 1045239. [Google Scholar] [CrossRef] [PubMed]
- Nagano, S.; Mori, N.; Tomari, Y.; Mitsugi, N.; Deguchi, A.; Kashima, M.; Tezuka, A.; Nagano, A.J.; Usami, H.; Tanabata, T.; et al. Effect of Differences in Light Source Environment on Transcriptome of Leaf Lettuce (Lactuca sativa L.) to Optimize Cultivation Conditions. PLoS ONE 2022, 17, e0265994. [Google Scholar] [CrossRef] [PubMed]
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. High Salinity Induces Different Oxidative Stress and Antioxidant Responses in Maize Seedlings Organs. Front. Plant Sci. 2016, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- AbdElgawad, H.; Zinta, G.; Hamed, B.A.; Selim, S.; Beemster, G.; Hozzein, W.N.; Wadaan, M.A.M.; Asard, H.; Abuelsoud, W. Maize Roots and Shoots Show Distinct Profiles of Oxidative Stress and Antioxidant Defense under Heavy Metal Toxicity. Environ. Pollut. 2020, 258, 113705. [Google Scholar] [CrossRef]
- Xu, Z.; Mahmood, K.; Rothstein, S.J. ROS Induces Anthocyanin Production Via Late Biosynthetic Genes and Anthocyanin Deficiency Confers the Hypersensitivity to ROS-Generating Stresses in Arabidopsis. Plant Cell Physiol. 2017, 58, 1364–1377. [Google Scholar] [CrossRef]
- Bykova, A.V.; Meleshin, A.A.; Shchennikova, A.V.; Kochieva, E.Z. Effect of Cold Stress on Anthocyanin Content and Anthocyanin Biosynthesis Pathway Gene Expression in Potato Solanum tuberosum L. Leaves. Russ. J. Genet. 2025, 61, 809–819. [Google Scholar] [CrossRef]
- Schäfer, E.D.; Owen, M.R.; Band, L.R.; Farcot, E.; Bennett, M.J.; Lynch, J.P. Modeling Root Loss Reveals Impacts on Nutrient Uptake and Crop Development. Plant Physiol. 2022, 190, 2260–2278. [Google Scholar] [CrossRef]
- Yu, X.; Wang, B.; Zhang, C.; Xu, W.; He, J.; Zhu, L.; Wang, S. Effect of Root Restriction on Nitrogen Levels and Glutamine Synthetase Activity in ‘Kyoho’ Grapevines. Sci. Hortic. 2012, 137, 156–163. [Google Scholar] [CrossRef]
- Yu, X.; Li, J.; Zhu, L.; Wang, B.; Wang, L.; Bai, Y.; Zhang, C.; Xu, W.; Wang, S. Effects of Root Restriction on Nitrogen and Gene Expression Levels in Nitrogen Metabolism in Jumeigui Grapevines (Vitis vinifera L. × Vitis labrusca L.). J. Integr. Agric. 2015, 14, 67–79. [Google Scholar] [CrossRef]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of Preharvest Abiotic Stresses on the Accumulation of Bioactive Compounds in Horticultural Produce. Front. Plant Sci. 2019, 10, 1212. [Google Scholar] [CrossRef]
- Shao, C.; Zhang, C.; Lv, Z.; Shen, C. Pre- and Post-Harvest Exposure to Stress Influence Quality-Related Metabolites in Fresh Tea Leaves (Camellia sinensis). Sci. Hortic. 2021, 281, 109984. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, J.; Zhang, L.; Yang, X.; Qiu, Y.; Cai, C.; Hu, J.; Huang, T.; Liang, Y.; Li, Z.; et al. Optimizing the Quality of Horticultural Crop: Insights into Pre-Harvest Practices in Controlled Environment Agriculture. Front. Plant Sci. 2024, 15, 1427471. [Google Scholar] [CrossRef]
- Sonjaroon, W.; Tepkaew, T.; Kupia, M.; Tongkok, P.; Boonkorkaew, P.; Thussagunpanit, J. Pre-Harvest UV-A Supplementation in Plant Factory with Artificial Lighting Improves Growth, Photosynthesis, and Phytonutrients in Kale. Horticulturae 2024, 10, 701. [Google Scholar] [CrossRef]
- Sakamoto, M.; Komatsu, Y.; Suzuki, T. Nutrient Deficiency Affects the Growth and Nitrate Concentration of Hydroponic Radish. Horticulturae 2021, 7, 525. [Google Scholar] [CrossRef]
- Balliu, A.; Zheng, Y.; Sallaku, G.; Fernández, J.A.; Gruda, N.S.; Tuzel, Y. Environmental and Cultivation Factors Affect the Morphology, Architecture and Performance of Root Systems in Soilless Grown Plants. Horticulturae 2021, 7, 243. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakamoto, M.; Suzuki, T. Root Pruning Enhances Leaf Oxidative Stress and Anthocyanin Accumulation in Hydroponically Grown Red Leaf Lettuce. Oxygen 2025, 5, 24. https://doi.org/10.3390/oxygen5040024
Sakamoto M, Suzuki T. Root Pruning Enhances Leaf Oxidative Stress and Anthocyanin Accumulation in Hydroponically Grown Red Leaf Lettuce. Oxygen. 2025; 5(4):24. https://doi.org/10.3390/oxygen5040024
Chicago/Turabian StyleSakamoto, Masaru, and Takahiro Suzuki. 2025. "Root Pruning Enhances Leaf Oxidative Stress and Anthocyanin Accumulation in Hydroponically Grown Red Leaf Lettuce" Oxygen 5, no. 4: 24. https://doi.org/10.3390/oxygen5040024
APA StyleSakamoto, M., & Suzuki, T. (2025). Root Pruning Enhances Leaf Oxidative Stress and Anthocyanin Accumulation in Hydroponically Grown Red Leaf Lettuce. Oxygen, 5(4), 24. https://doi.org/10.3390/oxygen5040024





