Phosphorus-and-Silver-Doped Crystalline Oxide Coatings for Titanium Implant Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Anodization
2.3. Oxide Characterization
2.4. Oxide Photocatalytic Activity
2.5. Oxide Bacterial Attachment Efficiency
2.6. Pre-Osteoblast Cell Culture
2.7. Cell Viability
2.8. Statistical Analyses
3. Results
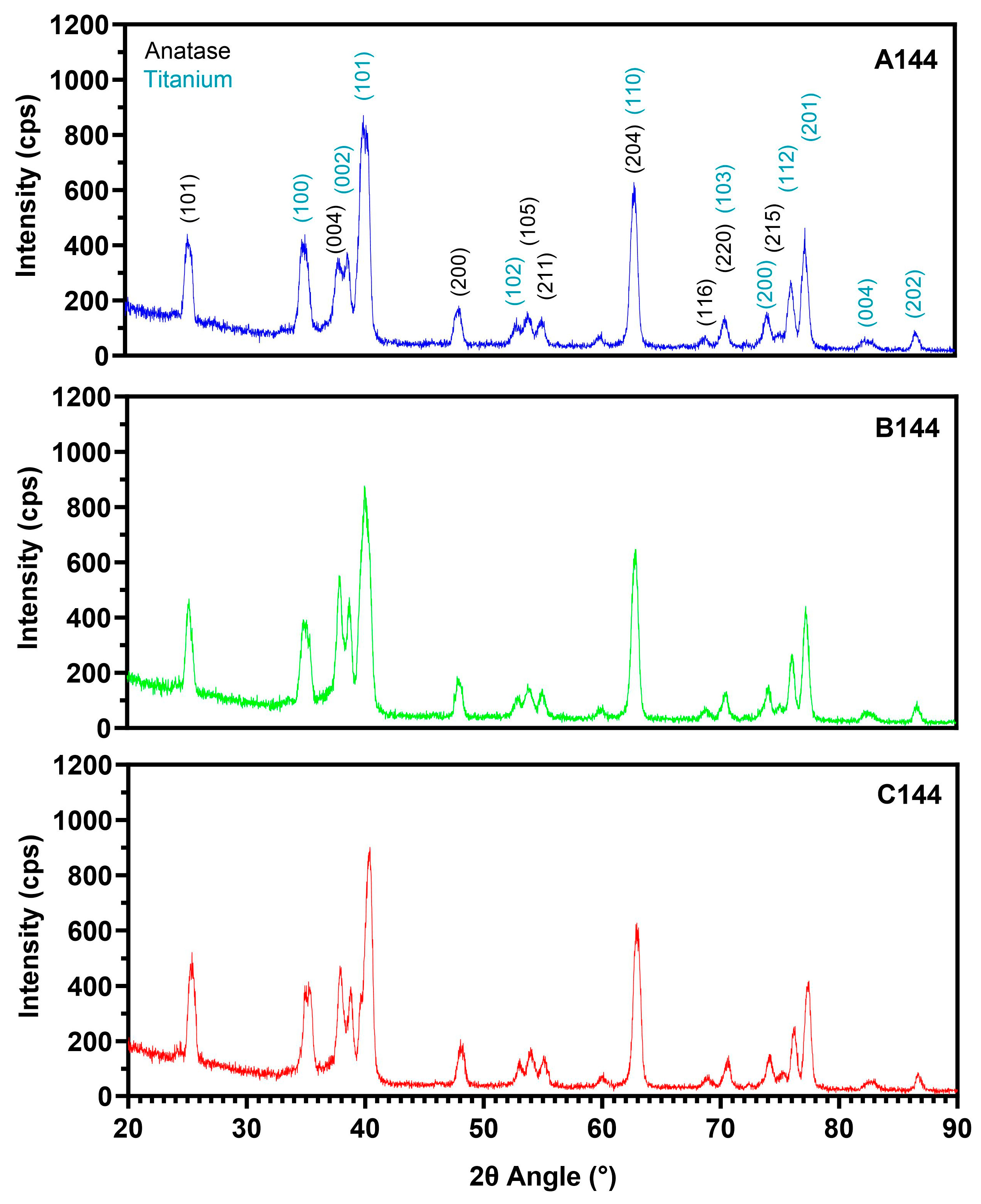
3.1. Oxide Characterization
3.2. Oxide Photocatalytic Activity
3.3. Oxide Bacteria Attachment
3.4. Pre-Osteoblast Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Dhanraj, M.; Sivagami, G. Status of surface treatment in endosseous implant: A literary overview. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2010, 21, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, J. Implants: Implications for general dentists. Dent. Econ. 1988, 78, 101. [Google Scholar] [PubMed]
- Graves, E.G.; Gillum, B.S. Detailed diagnoses and procedures, National Hospital Discharge Survey. Vital Health Stat. 1997, 130, 1–146. [Google Scholar] [PubMed]
- Pye, A.; Lockhart, D.; Dawson, M.; Murray, C.; Smith, A. A review of dental implants and infection. J. Hosp. Infect. 2009, 72, 104–110. [Google Scholar] [CrossRef]
- Sul, Y.-T. The significance of the surface properties of oxidized titanium to the bone response: Special emphasis on potential biochemical bonding of oxidized titanium implant. Biomaterials 2003, 24, 3893–3907. [Google Scholar] [CrossRef]
- Sul, Y.-T.; Johansson, C.B.; Jeong, Y.; Albrektsson, T. The electrochemical oxide growth behaviour on titanium in acid and alkaline electrolytes. Med. Eng. Phys. 2001, 23, 329–346. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Attarilar, S.; Wang, C.; Tamaddon, M.; Yang, C.; Xie, K.; Yao, J.; Wang, L.; Liu, C.; et al. Nano-Modified Titanium Implant Materials: A Way Toward Improved Antibacterial Properties. Front. Bioeng. Biotechnol. 2020, 8, 576969. [Google Scholar] [CrossRef]
- Williamson, R.; Disegi, J.; Janorkar, A.; Griggs, J.; Roach, M.D. Effect of duty cycle on the crystallinity, pore size, surface roughness and corrosion resistance of the anodized surface on titanium. Surf. Coat. Technol. 2015, 277, 278–288. [Google Scholar] [CrossRef]
- Williamson, R.S.; Disegi, J.; Griggs, J.A.; Roach, M.D. Nanopore formation on the surface oxide of commercially pure titanium grade 4 using a pulsed anodization method in sulfuric acid. J. Mater. Sci. Mater. Med. 2013, 24, 2327–2335. [Google Scholar] [CrossRef]
- Roach, M.D.; Williamson, R.; Blakely, I.; Didier, L. Tuning anatase and rutile phase ratios and nanoscale surface features by anodization processing onto titanium substrate surfaces. Mater. Sci. Eng. C 2016, 58, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Williamson, R.S.; Roach, M.D. Surface characterization, shear strength, and bioactivity of anodized titanium prepared in mixed-acid electrolytes. Surf. Coatings Technol. 2017, 325, 594–603. [Google Scholar] [CrossRef]
- Jain, S.; Williamson, R.S.; Janorkar, A.V.; Griggs, J.A.; Roach, M.D. Osteoblast response to nanostructured and phosphorus-enhanced titanium anodization surfaces. J. Biomater. Appl. 2019, 34, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Williamson, R.S.; Marquart, M.; Janorkar, A.V.; Griggs, J.A.; Roach, M.D. Photofunctionalization of anodized titanium surfaces using UVA or UVC light and its effects against Streptococcus sanguinis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 2284–2294. [Google Scholar] [CrossRef]
- Johnson, H.A.; Williamson, R.S.; Marquart, M.; Bumgardner, J.D.; Janorkar, A.V.; Roach, M.D. Photocatalytic activity and antibacterial efficacy of UVA-treated titanium oxides. J. Biomater. Appl. 2020, 35, 500–514. [Google Scholar] [CrossRef]
- Maness, P.-C.; Smolinski, S.; Blake, D.M.; Huang, Z.; Wolfrum, E.J.; Jacoby, W.A. Bactericidal Activity of Photocatalytic TiO 2 Reaction: Toward an Understanding of Its Killing Mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098. [Google Scholar] [CrossRef]
- Suketa, N.; Sawase, T.; Kitaura, H.; Naito, M.; Baba, K.; Nakayama, K.; Wennerberg, A.; Atsuta, M. An Antibacterial Surface on Dental Implants, Based on the Photocatalytic Bactericidal Effect. Clin. Implant. Dent. Relat. Res. 2005, 7, 105–111. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Choi, J.Y.; Chung, C.J.; Oh, K.T.; Choi, Y.J.; Kim, K.H. Photocatalytic Antibacterial Effect of TiO2 Film of TiAg on Streptococcus mutans. Angle Orthod. 2009, 79, 528–532. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Rodríguez-Hernández, A.G.; Delgado, L.M.; Manero, J.M.; Javier Gil, F.; Rodríguez, D. Silver deposition on titanium surface by electrochemical anodizing process reduces bacterial adhesion of Streptococcus sanguinis and Lactobacillus salivarius. Clin. Oral Impl. Res. 2015, 26, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, T.F.; Kuehl, R.; Coenye, T.; Metsemakers, W.-J.; Morgenstern, M.; Schwarz, E.M.; Riool, M.; Zaat, S.A.; Khana, N.; Kates, S.L.; et al. Orthopaedic device-related infection: Current and future interventions for improved prevention and treatment. EFORT Open Rev. 2016, 1, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Haupt, M.; Klostermann, H.; Kim, H.-S.; Eichler, M.; Peetsch, A.; Scheideler, L.; Doering, C.; Oehr, C.; Wendel, H.; et al. Multifunctional nature of UV-irradiated nanocrystalline anatase thin films for biomedical applications. Acta Biomater. 2010, 6, 4566–4577. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Al-Shamali, S.S. Photocatalytic Degradation of Methylene Blue in the Presence of TiO2 Catalyst Assisted Solar Radiation. Aust. J. Basic Appl. Sci. 2013, 7, 172–176. [Google Scholar]
- Pantaroto, H.N.; Ricomini-Filho, A.P.; Bertolini, M.M.; da Silva, J.H.D.; Neto, N.F.A.; Sukotjo, C.; Rangel, E.C.; Barão, V.A. Antibacterial photocatalytic activity of different crystalline TiO2 phases in oral multispecies biofilm. Dent. Mater. 2018, 34, e182–e195. [Google Scholar] [CrossRef]
- Zhu, J.; Zheng, W.; He, B.; Zhang, J.; Anpo, M. Characterization of Fe–TiO2 photocatalysts synthesized by hydrothermal method and their photocatalytic reactivity for photodegradation of XRG dye diluted in water. J. Mol. Catal. A Chem. 2004, 216, 35–43. [Google Scholar] [CrossRef]
- Tada, H.; Ishida, T.; Takao, A.; Ito, S. Drastic Enhancement of TiO2-Photocatalyzed Reduction of Nitrobenzene by Loading Ag Clusters. Langmuir 2004, 20, 7898–7900. [Google Scholar] [CrossRef]
- Dawson, A.; Kamat, P.V. Semiconductor−Metal Nanocomposites. Photoinduced Fusion and Photocatalysis of Gold-Capped TiO2 (TiO2/Gold) Nanoparticles. J. Phys. Chem. B 2001, 105, 960–966. [Google Scholar] [CrossRef]
- Nasirian, M.; Lin, Y.P.; Bustillo-Lecompte, C.F.; Mehrvar, M. Enhancement of photocatalytic activity of titanium dioxide using non-metal doping methods under visible light: A review. Int. J. Environ. Sci. Technol. 2018, 15, 2009–2032. [Google Scholar] [CrossRef]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91B, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B. Silver in health care: Antimicrobial effects and safety in use. Curr. Probl. Dermatol. 2006, 33, 17–34. [Google Scholar] [PubMed]
- van Hengel, I.; Putra, N.; Tierolf, M.; Minneboo, M.; Fluit, A.; Fratila-Apachitei, L.; Apachitei, I.; Zadpoor, A. Biofunctionalization of selective laser melted porous titanium using silver and zinc nanoparticles to prevent infections by antibiotic-resistant bacteria. Acta Biomater. 2020, 107, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.-Y.; Liu, C.-P.; Huang, H.-H.; Lee, S.-W. Both Enhanced Biocompatibility and Antibacterial Activity in Ag-Decorated TiO2 Nanotubes. PLoS ONE 2013, 8, e75364. [Google Scholar] [CrossRef] [PubMed]
- Ibukun, O.; Jeong, H.K. Tailoring titanium dioxide by silver particles for photocatalysis. Curr. Appl. Phys. 2020, 20, 23–28. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef]
- Croes, M.; Bakhshandeh, S.; van Hengel, I.; Lietaert, K.; van Kessel, K.; Pouran, B.; van der Wal, B.; Vogely, H.; Van Hecke, W.; Fluit, A.; et al. Antibacterial and immunogenic behavior of silver coatings on additively manufactured porous titanium. Acta Biomater. 2018, 81, 315–327. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Cheng, H.; Li, Y.; Huo, K.; Gao, B.; Xiong, W. Long-lasting in vivo and in vitro antibacterial ability of nanostructured titania coating incorporated with silver nanoparticles. J. Biomed. Mater. Res. Part A 2014, 102, 3488–3499. [Google Scholar] [CrossRef]
- Syrek, K.; Grudzień, J.; Sennik-Kubiec, A.; Brudzisz, A.; Sulka, G.D. Anodic Titanium Oxide Layers Modified with Gold, Silver, and Copper Nanoparticles. J. Nanomater. 2019, 2019, 9208734. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M. Photocatalytic degradation of methylene blue and inactivation of pathogenic bacteria using silver nanoparticles modified titanium dioxide thin films. World J. Microbiol. Biotechnol. 2015, 31, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Moma, J.; Baloyi, J. Modified Titanium Dioxide for Photocatalytic Applications. In Photocatalysts—Applications and Attributes; Sher Bahadar, K., Kalsoom, A., Eds.; IntechOpen: Rijeka, Croatia, 2019; Chapter 3; pp. 37–56. [Google Scholar] [CrossRef]
- Li, P.; Tong, Z.; Huo, L.; Yang, F.; Su, W. Antibacterial and biological properties of biofunctionalized nanocomposites on titanium for implant application. J. Biomater. Appl. 2016, 31, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Hardman, C.T.; Johnson, H.A.; Doukas, M.; Pettit, C.C.; Janorkar, A.V.; Williamson, R.S.; Roach, M.D. Photocatalytic, phosphorus-doped, anatase oxide layers applicable to titanium implant alloys. Surf. Interface Anal. 2022, 54, 619–630. [Google Scholar] [CrossRef]
- Joo, H.-C.; Lim, Y.-J.; Kim, M.-J.; Kwon, H.-B.; Han, J.-H. Characterization on titanium surfaces and its effect on photocatalytic bactericidal activity. Appl. Surf. Sci. 2010, 257, 741–746. [Google Scholar] [CrossRef]
- Loncar, E.; Radeka, M.; Petrovic, S.; Skapin, A.; Rudic, O.; Ranogajec, J. Determination of the photocatalytic activity of TiO2 coatings on clay roofing tile substrates methylene blue as model pollutant. Acta Period. Technol. 2009, 40, 125–133. [Google Scholar] [CrossRef]
- İzmir, M.; Ercan, B. Anodization of titanium alloys for orthopedic applications. Front. Chem. Sci. Eng. 2019, 13, 28–45. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Mo, S.-D.; Ching, W.Y. Electronic and optical properties of three phases of titanium dioxide: Rutile, anatase, and brookite. Phys. Rev. B 1995, 51, 13023–13032. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef]
- Gopal, N.O.; Lo, H.-H.; Ke, T.-F.; Lee, C.-H.; Chou, C.-C.; Wu, J.-D.; Sheu, S.-C.; Ke, S.-C. Visible Light Active Phosphorus-Doped TiO2 Nanoparticles: An EPR Evidence for the Enhanced Charge Separation. J. Phys. Chem. C 2012, 116, 16191–16197. [Google Scholar] [CrossRef]
- Iwase, M.; Yamada, K.; Kurisaki, T.; Prieto-Mahaney, O.O.; Ohtani, B.; Wakita, H. Visible-light photocatalysis with phosphorus-doped titanium(IV) oxide particles prepared using a phosphide compound. Appl. Catal. B Environ. 2013, 132–133, 39–44. [Google Scholar] [CrossRef]
- Barone, P.; Stranges, F.; Barberio, M.; Renzelli, D.; Bonanno, A.; Xu, F. Study of Band Gap of Silver Nanoparticles—Titanium Dioxide Nanocomposites. J. Chem. 2014, 2014, 589707. [Google Scholar] [CrossRef]
- Jiang, Z.; Ouyang, Q.; Peng, B.; Zhang, Y.; Zan, L. Ag size-dependent visible-light-responsive photoactivity of Ag–TiO2 nanostructure based on surface plasmon resonance. J. Mater. Chem. A 2014, 2, 19861–19866. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Mullani, S.B.; Koli, V.B.; Patil, S.M.; Kasabe, P.J.; Dandge, P.B.; Pawar, S.A.; Delekar, S.D. Ag Nanoparticles Connected to the Surface of TiO2 Electrostatically for Antibacterial Photoinactivation Studies. Photochem. Photobiol. 2018, 94, 1249–1262. [Google Scholar] [CrossRef]
- Chen, K.; Feng, X.; Tian, H.; Li, Y.; Xie, K.; Hu, R.; Cai, Y.; Gu, H. Silver-decorated titanium dioxide nanotube arrays with improved photocatalytic activity for visible light irradiation. J. Mater. Res. 2014, 29, 1302–1308. [Google Scholar] [CrossRef]
- Gopal, J.; George, R.; Muraleedharan, P.; Khatak, H. Photocatalytic Inhibition of Microbial Adhesion by Anodized Titanium. Biofouling 2004, 20, 167–175. [Google Scholar] [CrossRef]
- Ali, A.; Polepalli, L.; Chowdhury, S.; Carr, M.A.; Janorkar, A.V.; Marquart, M.E.; Griggs, J.A.; Bumgardner, J.D.; Roach, M.D. Silver-Doped Titanium Oxide Layers for Improved Photocatalytic Activity and Antibacterial Properties of Titanium Implants. J. Funct. Biomater. 2024, 15, 163. [Google Scholar] [CrossRef]
- Gao, A.; Hang, R.; Huang, X.; Zhao, L.; Zhang, X.; Wang, L.; Tang, B.; Ma, S.; Chu, P.K. The effects of titania nanotubes with embedded silver oxide nanoparticles on bacteria and osteoblasts. Biomaterials 2014, 35, 4223–4235. [Google Scholar] [CrossRef]









| Oxide Group | Sulfuric Acid (M) | Phosphoric Acid (M) | Oxalic Acid (M) | Hydrogen Peroxide (M) | Silver Acetate (M) | Silver Nitrate (M) |
|---|---|---|---|---|---|---|
| A144 | 3.5 | 0.19 | 0.25 | 0.75 | ||
| B144 | 3.5 | 0.19 | 0.25 | 0.75 | 0.05 | |
| C144 | 3.5 | 0.19 | 0.25 | 0.75 | 0.05 |
| Oxide Group | Total Pore Count 1 | Pore Density 1 | Percent Porosity 1 (%) | Pore Size Distribution (%) | ||
|---|---|---|---|---|---|---|
| <100 nm | 100 nm–1 µm | >1 µm | ||||
| A144 | 267 ± 21 A | 1.7 ± 0.1 AB | 9.9 ± 0.4 AB | 45.1 ± 3.9 | 54.9 ± 3.9 | - |
| B144 | 211 ± 20 B | 1.3 ± 0.1 B | 9.6 ± 0.5 B | 40.3 ± 2.6 | 59.0 ± 3.0 | 0.7 ± 0.9 |
| C144 | 285 ± 56 AB | 1.8 ± 0.3 A | 10.6 ± 0.6 A | 46.1 ± 3.1 | 53.5 ± 3.0 | 0.4 ± 0.4 |
| Oxide Group | Titanium (wt.%) | Oxygen (wt.%) | Phosphorus (wt.%) | Sulfur (wt.%) | Silver (wt.%) |
|---|---|---|---|---|---|
| A144 | 56.2 ± 0.3 | 38.6 ± 0.0 | 4.7 ± 0.2 | 0.5 ± 0.1 | - |
| B144 | 54.4 ± 0.6 | 39.6 ± 0.3 | 5.0 ± 0.3 | 0.4 ± 0.1 | 0.6 ± 0.1 |
| C144 | 54.6 ± 0.2 | 39.4 ± 0.2 | 4.9 ± 0.2 | 0.6 ± 0.1 | 0.6 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruni, C.L.; Johnson, H.A.; Ali, A.; Parekh, A.; Marquart, M.E.; Janorkar, A.V.; Roach, M.D. Phosphorus-and-Silver-Doped Crystalline Oxide Coatings for Titanium Implant Surfaces. Oxygen 2024, 4, 402-420. https://doi.org/10.3390/oxygen4040025
Bruni CL, Johnson HA, Ali A, Parekh A, Marquart ME, Janorkar AV, Roach MD. Phosphorus-and-Silver-Doped Crystalline Oxide Coatings for Titanium Implant Surfaces. Oxygen. 2024; 4(4):402-420. https://doi.org/10.3390/oxygen4040025
Chicago/Turabian StyleBruni, Catherine L., Haden A. Johnson, Aya Ali, Amisha Parekh, Mary E. Marquart, Amol V. Janorkar, and Michael D. Roach. 2024. "Phosphorus-and-Silver-Doped Crystalline Oxide Coatings for Titanium Implant Surfaces" Oxygen 4, no. 4: 402-420. https://doi.org/10.3390/oxygen4040025
APA StyleBruni, C. L., Johnson, H. A., Ali, A., Parekh, A., Marquart, M. E., Janorkar, A. V., & Roach, M. D. (2024). Phosphorus-and-Silver-Doped Crystalline Oxide Coatings for Titanium Implant Surfaces. Oxygen, 4(4), 402-420. https://doi.org/10.3390/oxygen4040025









