Abstract
This study explored the use of mandarin peels as an important source of health-promoting compounds by utilizing green methods (i.e., pulsed electric field and ultrasound-assisted extraction), along with conventional stirring. The impact of several extraction parameters, such as extraction duration, temperature, and solvent composition, on the recovery of bioactive compounds was evaluated through a response surface methodology. To identify the most effective conditions for all assays, a partial least-squares analysis was implemented. It was revealed that a combination of the above techniques was optimal at 80 °C for 30 min, with 75% v/v of ethanol in water as the extraction solvent. The concentration of bioactive compounds in the optimum extract had a total polyphenol content of 18.69 mg of gallic acid equivalents (GAE) per gram of dry weight (dw), and an ascorbic acid concentration of 18.25 mg/g dw. However, correlation analyses revealed a rather negative relationship between these bioactive compounds. The chromatographic analysis of optimum extracts supported this result by quantifying 20.53 mg/g dw of total individual polyphenols, with hesperidin being the dominant compound (13.98 mg/g dw). The antioxidant assays, including ferric-reducing antioxidant power and DPPH• inhibition activity, were measured at 123.21 and 65.12 μmol of ascorbic acid equivalents (AAE) per gram of dw, respectively. This research enhances the valorization of mandarin peels as a renewable source of bioactive compounds, providing the opportunity to generate high-added-value products from food waste in the food and pharmaceutical sectors.
1. Introduction
Citrus belongs to the Rutaceae family, and its fruits are recognized for their unique scent and refreshing properties [1]. Mandarins account for 22% of the world’s citrus production, making them second only to oranges in cultivation. Nearly 37,000 tons of this citrus fruit were produced worldwide in 2019 [2]. The medicinal properties are also known for mandarins. By lowering the risk of chronic illnesses and enhancing general health, they act effectively in reducing oxidative stress and mitigating free radicals [3]. Several flavonoids which are found mostly in pulp but also in peels are the prevalent flavonoids and contribute to health-promoting benefits [4]. Mandarin contains a significant concentration of flavonoids, particularly flavone glycosides. Mandarin primarily contains hesperidin, which is a flavone glycoside. Hesperetin and the disaccharide rutinose compose the structure of hesperidin [5]. Apart from its hypolipidemic and radical-scavenging effects, hesperidin has anti-inflammatory, anti-cancer, and anti-diabetic capabilities. It also provides analgesic relief [6,7,8]. Another essential antioxidant compound found in mandarin fruit peel is ascorbic acid, which functions by eliminating free radicals, including reactive oxygen species, thereby enhancing the antioxidant capacity of plant cells [9].
Due to their primary utilization in juice production, citrus fruits lose between 45 and 60% of their total weight. Mandarin peels are often discarded during juice production, even though they contribute to around 30% of the fruit mass [5]. Their flavedo- and albedo-containing peel is the primary waste product. Natural antioxidants, such as pectin, essential oils, carotenoids, and polyphenols exist in mandarin peel [10]. Unfortunately, the majority of mandarin peel waste is currently disposed of in landfills or marine environments worldwide, causing serious environmental issues [11]. Waste from mandarins is rich in rag and pulp, which increases chemical oxygen demand and has a serious negative impact on ecosystems [12]. The majority of several technologies, including ozonation, adsorption, and other membrane-based methods, on the other hand, are inadequate for mandarin waste due to the viscosity, biomacromolecule content, and increased chemical oxygen demand [13]. Alternative methods for addressing the environmental challenges linked to the disposal of mandarin peel waste, excluding combustion, include decomposition and animal feed [14]. Nevertheless, the biodegradation of mandarin peel waste is hindered by the presence of limonene and polyphenolic compounds, both of which impede the functionality of aerobic microorganisms [15].
Recent research has proposed valorization concepts as a solution to the agricultural waste disposal problem [16]. Prior to the development of environmentally sustainable extraction methods, the utilization of recyclable and green solvents in conformity to the principles of Green Chemistry should take place [17]. Conventional extraction in a hotplate often requires a high extraction duration or records low recovery yields [18].To this end, several green methods could be employed as pretreatment or as standalone extraction techniques [19]. Pulsed electric field (PEF) and ultrasounds (US) are among the green techniques that could be utilized for sustainable extraction. Due to their reduced extraction time, energy consumption requirements, and absence of petroleum-based solvents, these methods are ecologically friendly [20]. Besides being classified as a non-destructive and non-thermal technique, PEF also has the potential to reduce energy consumption and processing time [21]. PEF disrupts cell membranes through electroporation [22], while US promotes efficient extraction with ultrasound waves [23], in which both techniques ultimately enhance efficient compound extraction without high temperatures or energy requirements [24,25].
The objective of this research was to maximize the content of mandarin peel extracts in various bioactive compounds, including ascorbic acid, flavonoids, and other polyphenols, by employing various techniques/parameters, so as to promote the reuse of waste material to develop value-added products. To this end, green pretreatment techniques, such as PEF and US were examined prior to the stirring process. The optimal conditions, including extraction technique, time, temperature, and food-grade solvent mixtures of ethanol–water, were all determined through partial least-squares (PLS) analysis. The impact of the extraction parameters was evaluated through statistical analyses, whereas the optimum extract was evaluated for both its bioactive compound concentration and its antioxidant activity. Mandarin peel extracts were valued as an important source of health-promoting compounds, with a specific emphasis on their prospective use in the food and pharmaceutical sectors.
2. Materials and Methods
2.1. Chemicals and Reagents
Hydrochloric acid, methanol, L-ascorbic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH) 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), trichloroacetic acid, and all chemical standards for the HPLC determination of polyphenols were obtained from Sigma-Aldrich (Darmstadt, Germany). Ethanol, gallic acid, and the Folin–Ciocalteu reagent were bought from Panreac Co. (Barcelona, Spain). Iron (III) chloride was purchased from Merck (Darmstadt, Germany). Anhydrous sodium carbonate was purchased from Penta (Prague, Czech Republic). Deionized water was used for all conducted experiments.
2.2. Collection and Handling of Mandarin Peels
Mandarin (Citrus reticulata) fruits were bought from a store in Karditsa, Greece. They were dried with paper towels after being washed with tap water. Right after, they were peeled and hand-chopped. To produce mandarin powder of superior nutritional quality, the samples were lyophilized using a freeze-drying apparatus. Finally, they were ground to a fine powder (<400 μm diameter) and stored in the freezer at −40 °C.
2.3. Instrumentation
A Biobase BK-FD10P (Jinan, China) freeze-dryer was used to lyophilize the mandarin peels. A centrifuge from NEYA 16R (Remi Elektrotechnik Ltd., Palghar, India) was used to centrifuge and isolate the supernatant after extraction. A mode/arbitrary waveform generator (UPG100, ELV Elektronik AG, Leer, Germany), a digital oscilloscope (Rigol DS1052E, Beaverton, OR, USA), a high-voltage power generator (Leybold, LD Didactic GmbH, Huerth, Germany), and two custom stainless-steel chambers (Val-Electronic, Athens, Greece) were utilized for the PEF processing of the samples. In contrast, the Elmasonic P70H machine (Elma Schmidbauer GmbH, Singen, Germany) was used for the US pretreatment. A Shimadzu UV-1900i PharmaSpec Spectrophotometer (Kyoto, Japan) was used for spectrophotometric analyses. A Shimadzu CBM-20A liquid chromatograph and a Shimadzu SPD-M20A diode array detector (DAD) (Shimadzu Europa GmbH, Duisburg, Germany) were used for the quantification of individual polyphenols. The compounds were separated into a Phenomenex Luna C18(2) column from Phenomenex Inc. in Torrance, California, and kept at 40 °C (100 Å, 5 μm, 4.6 mm × 250 mm).
2.4. Mandarin Peel Extraction Procedure
The extraction procedure of mandarin peels involving a combination of PEF, US, and the stirring process stemmed from our previous research [26]. The extractions proceeded after 20 mL of the extraction solvent and 1 g of mandarin peel fine powder (<400 μm) were combined in a hermetically closed 50 mL Duran bottle and placed into a stirring plate. Detailed information about the green extraction procedure is listed in Table 1, whereas a graphical illustration of the procedure is depicted in Figure 1. The extraction solvent was several mixtures of ethanol in water (0–100% v/v). Stirring (ST) at 500 rpm at various temperatures (20–80 °C) and durations (30–150 min) was used to carry out the extraction process. Some design points underwent PEF and/or US pretreatment before extraction. Both pretreatment techniques were employed for 20 min each and under constant temperature in order to avoid polyphenol degradation, as per several studies’ reports [27,28,29]. The dried material was initially hydrated for 10 min by immersion in the solvent before treatment using either approach (ST, US, or PEF). Following extraction, the mixture was centrifuged for 10 min at 10,000 rpm in a centrifuge. The supernatant was then collected and kept at −40 °C until further analysis. For PEF pretreatment, the pulse period was set to 1 ms (frequency: 1 kHz), the pulse duration to 10 μs, and the electric field density to 1.0 kV/cm. For US pretreatment, the temperature was kept constant at 30 °C, with the frequency set at 37 kHz.

Table 1.
The actual and coded levels of the independent variables were used to optimize the process.
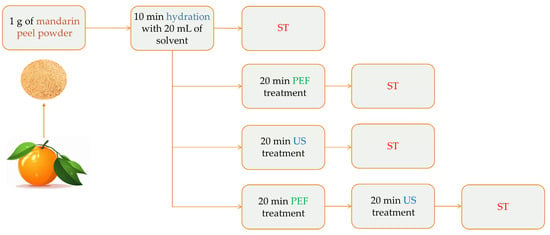
Figure 1.
The extraction procedure of mandarin peel powder using a stirring process and pretreatment techniques.
2.5. Response Surface Methodology (RSM) Optimization and Design of the Experiment
Quickly selecting the optimal parameters for an experiment with high-leveled factors is possible with the Main Effect Screening design [30]. When investigating factors with varying numbers of levels, factorial experiments benefit greatly from orthogonal arrays with mixed levels. Nevertheless, a drawback of mixed-level orthogonal arrays is the numerous runs that are necessary for many of them. In such situations, nearly orthogonal arrays could be a good fit since they reduce the number of experiments needed by slightly easing the requirement of orthogonality [31]. In addition, to evaluate the antioxidant capacity and bioactive compound concentration of mandarin peel extracts, the RSM approach was employed. This enhancement would also benefit the antioxidant capacity of the extracts. This was accomplished by optimizing the extraction technique, the ethanol-to-water concentration (C, % v/v), extraction time (t, min), and temperature (T, °C). The optimization was conducted through the Main Effect Screening design and 20 design points. The process variables were established in five levels in accordance with the experimental design. The overall model significance (R2, p-value) and the significance of the model (equations) coefficients were assessed at a minimum level of 95% using analysis of variance (ANOVA) and summary-of-fit tests. A second-order polynomial model, shown in the following equation, was also used to predict the response variable as a function of the examined independent factors:
where Xi and Xj are the independent variables; Yk is the anticipated response variable; and the intercept, regression coefficients of the linear, quadratic, and interaction terms of the model are, respectively, β0, βi, βii, and βij. The RSM was used to calculate the largest peak area, as well as the impact of a significant independent variable on the response. The model equation was visually represented by 3D surface response graphs.
2.6. Bioactive Compound Quantification
2.6.1. Total Polyphenol Content (TPC)
The quantification of TPC was evaluated as mg gallic acid equivalents (GAE) per g of dry weight (dw) and based on a prior spectrophotometric method [32]. The method relies on the formation of a phenolate ion, which is responsible for reducing the Folin–Ciocalteu reagent (i.e., from Mo+6 to Mo+5) when phenolic proton dissociates under alkaline conditions [33]. Briefly, 200 μL of diluted sample was mixed with 200 μL of the Folin–Ciocalteu reagent and after 2 min, 1600 μL of 5% w/v aqueous sodium carbonate solution was added in a 2 mL Eppendorf tube. The mixture was incubated at 40 °C for 20 min, and the absorbance was recorded at 740 nm. The total polyphenol concentration (CTP) was calculated from a gallic acid calibration curve (10–100 mg/L in methanol). Finally, TPC was determined using the following equation:
where the volume of the extraction medium is indicated with V (expressed in L), and the dried weight of the sample with w (expressed in g).
2.6.2. Individual Polyphenol Quantification
The quantification of individual polyphenols from the mandarin peel extracts was performed through high-performance liquid chromatography (HPLC), as established in our prior research [32]. The mobile phase included 0.5% aqueous formic acid (A) and 0.5% formic acid in acetonitrile/water (3:2) (B). The gradient program was as follows: initially from 0 to 40% B, then up to 50% B in 10 min, 70% B in another 10 min, and then constant for 10 min. The flow rate of the mobile phase was set at 1 mL/min. The compounds were identified by comparing the absorbance spectrum and retention time to those of pure standards, and then quantified through calibration curves (0–50 mg/L). The results were expressed both in mg/L and mg/g of dw.
2.6.3. Determination of Ascorbic Acid Content (AAC)
The determination of ascorbic acid content (AAC) was expressed as mg ascorbic acid per gram of dw and was performed based on a previously established method [26]. The method was introduced and validated by Jagota and Dani [34]. The authors observed that ascorbic acid, a potent reductant, could react with the Folin–Ciocalteu reagent and absorb at ~760 nm under acidic conditions without the need to convert to its dehydro form, thus providing a quick and effective method to determine the specific antioxidant compound. A quantity of 100 μL diluted sample extract along with 500 μL of 10% (v/v) the Folin–Ciocalteu reagent was mixed with 900 μL of 10% (w/v) trichloroacetic acid in an Eppendorf tube. The absorbance was measured at 760 nm after 10 min. Ascorbic acid was used as a calibration standard in a calibration curve ranging from 50 to 500 mg/L; however, the results are expressed as mg/g of dw.
2.7. Antioxidant Assays
2.7.1. Ferric-Reducing Antioxidant Power (FRAP) Assay
An established methodology by Shehata et al. [35] was used to evaluate antioxidant capacity through a FRAP assay. The method relies on the reduction of Fe+3-TPTZ to Fe+2-TPTZ, which results in a blue solvent and is initiated under acidic conditions to preserve iron solubility [33]. In a 2 mL Eppendorf tube, 100 μL of a properly diluted sample was mixed with 100 μL of an FeCl3 solution (4 mM in 0.05 M HCl). The mixture was incubated at 37 °C for 30 min, with 1800 μL of TPTZ solution (1 mM in 0.05 M HCl) being immediately added right after, and the absorbance was measured after 5 min at 620 nm. The ferric-reducing power (PR) was calculated using an ascorbic acid calibration curve (CAA) in 0.05 M HCl with ranging values (50–500 μM). The PR was calculated as μmol of ascorbic acid equivalents (AAE) per gram of dw, using Equation (3):
where V is represented (in L) as the volume of the extraction medium, and w (in g) represents the dried weight of the material.
2.7.2. DPPH• Antiradical Activity
To further evaluate the antioxidant activity from mandarin peel extracts through inhibition activity, a slightly adjusted DPPH• methodology by Shehata et al. [35] was used. The electron delocalization in an extended conjugated π system to form a stable, decolorized compound that does not polymerize describes the principle of the method [33]. In brief, 50 μL of a properly diluted sample was mixed with a quantity of 1950 μL of 100 μM of a DPPH• solution in methanol, with the solution being kept at room temperature for 30 min in the dark. Following that, the absorbance was measured at 515 nm. Moreover, a blank sample was used instead of the sample, including a DPPH• solution and methanol, with the absorbance immediately being measured. To calculate the percentage of inhibition, Equation (4) was employed:
An ascorbic acid calibration curve for Equation (5) was used to evaluate antiradical activity (AAR), which was expressed as μmol AAE per gram of dw:
where the volume of the extraction medium is indicated with V (expressed in L) and the dried weight of the sample with w (expressed in g).
2.8. Statistical Analysis
The statistical analysis was related to the response surface methodology and distribution analysis, which were applicable through JMP® Pro 16 software (SAS, Cary, NC, USA). The quantitative analysis was performed in triplicate, and the extraction procedures were repeated at least twice for each batch of lemon verbena extract. The results are represented in the form of the means and standard deviations. Principal component analysis (PCA), multivariate correlation analysis (MCA), and partial least-squares (PLS) analysis were conducted through JMP® Pro 16 software.
3. Results and Discussion
The primary goal of this project was to valorize mandarin peels into high-added-value extracts. To obtain high-quality mandarin powder, a lyophilizer was utilized, despite the costly expense of the process [36]. Future studies investigating a more sustainable way of extracting bioactive compounds could employ inexpensive alternative drying methods [37]. To this end, two advanced techniques (i.e., PEF and US) were employed in addition to the conventional stirring technique to enhance the extracts with bioactive compounds, such as polyphenols and ascorbic acid. Extracts were subjected to a screening process that involved conducting spectrophotometric analyses for the mentioned assays. The polyphenol evaluation was conducted using the Folin–Ciocalteu photometric method, a quick, inexpensive, and sensitive technique for quantifying total polyphenols [38]. This method is known to have a strong association with the liquid chromatographic method of quantification [39,40]. The partial least-square model identified the optimum and polyphenol-rich and highly antioxidant sample, which was then analyzed using liquid chromatography to quantify the individual polyphenols present in mandarin peel.
A range of extraction parameters were also used to ensure that the highest yield was reached. These included different solvent compositions of 0–100% v/v of ethanol, as well as different extraction times ranging from 30 to 150 min and temperatures from 20 to 80 °C. In addition, RSM was used to evaluate the impact of each parameter to improve the extraction efficiency. Its efficacy and model adequacy were assessed through ANOVA and summary-of-fit tests, in which the observed values were compared with the anticipated values.
3.1. Bioactive Compound Concentration and Antioxidant Activity of the Extracts
Regarding the compounds identified in natural products, polyphenols are among the most widely recognized classes. Multiple sectors, including the pharmaceutical and agricultural industries, have substantial potential for the application of polyphenols [41,42]. For each prepared extract, Table 2 contains the measured and predicted responses for bioactive compounds (TPC and AAC) and antioxidant assays (FRAP and DPPH). The minimal variance between the predicted and actual measurements across all assays is evident in Figures S1–S4, in addition to the desirability functions. The extracts showed a TPC value range of 10.77–20.57 mg GAE/g dw. According to Koraqi et al. [43], the observed fluctuation in TPC could be attributed to various extraction-related factors, such as temperature, duration, and extraction solvent. Comparable results were obtained in a study conducted by Singanusong et al. [44], polyphenols from mandarin Blanco peels were isolated through US extraction and several solvent combinations. By using 50% ethanol, an average of 15.48 mg GAE/g dw of polyphenols was quantified. Safdar et al. [45] also yielded high amounts of polyphenols while utilizing the US extraction technique and 50–80% ethanol as the extraction solvent. It was observed that ethanol at 80% v/v was the most preferable solvent, as it achieved ~30 mg GAE/g of polyphenols. By employing 50% or 100% v/v, it achieved ~26 and ~24 mg GAE/g polyphenols, respectively. The polarity of the extraction solvent which was a mixture of ethanol and water in a similar ratio to our study and the distribution coefficient of these polyphenols could be a possible explanation behind this trend. Regarding the AAC range between the design points, an almost ten-fold range (from 2.90 to 20.29 mg/g dw) was observed, which indicated the major impact of extraction conditions and pretreatment techniques. The impact of the extraction conditions is extensively discussed below (vide infra). In a study with Phlegrean mandarin by-products of different ripeness levels, Constanzo et al. [46] found that AAC in peels ranged from 2 to 7 μg/L. Anticona et al. [47] investigated the bioactive compound content in fresh mandarin peels (cv. Clemenvilla, Nadorcott, and Ortanique) when extracted using the US technique. The results were comparable to ours, as AAC ranged from 0.72 to 1.36 mg/g of fresh peels, whereas TPC ranged from 6.02 to 12.3 mg GAE/g of fresh peels. Regarding antioxidant activity assays, several concerns about directly determining DPPH• from the calibration curve occur, so it is recommended to use ascorbic acid as a standard when presenting antioxidant efficiency results [48]. While the DPPH• method is most commonly used to determine Trolox equivalents, it is common to normalize according to ascorbic acid, caffeic acid, catechin, gallic acid, or another antioxidant [49], especially in our case, where we studied an ascorbic acid-rich fruit. The data displayed similar variance compared to TPC. Specifically, the range for FRAP (57.34–129.52 μmol AAE/g dw) and DPPH (19.57–60.79 μmol AAE/g dw) assay revealed a two-fold difference.

Table 2.
Experimental results for the four investigated independent variables and the dependent variable responses.
The statistical parameters, including second-order polynomial equations (models) and especially coefficients (R2 > 0.95), indicate that the derived models fit the data well, and are presented in Table 3. Figure 2 illustrates the three-dimensional response plots for TPC, whilst Figures S5–S7 contain the three-dimensional response plots for the rest of the responses. It can be observed that the red area illustrates the most preferable conditions for each variable X1–X4. For instance, in Figure 2A, the most preferable conditions were X1: 1 and X2 between 3 and 4.

Table 3.
Mathematical models generated through RSM were used to optimize the extraction of mandarin peels. The models contained only significant terms.
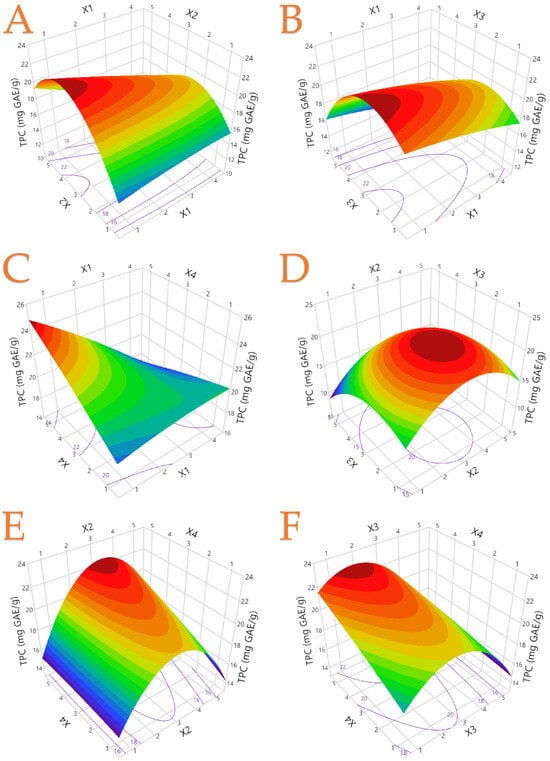
Figure 2.
The optimal extraction of mandarin peel extracts in 3D graphs shows the impact of the process variables considered in the response (total polyphenol content—TPC, mg GAE/g). Plot (A), covariation of X1 and X2; plot (B), covariation of X1 and X3; plot (C), covariation of X1 and X4; plot (D), covariation of X2 and X3; plot (E), covariation of X2 and X4; plot (F), covariation of X3 and X4.
3.2. Assessing Extraction Parameter Impact Through Pareto Plot
To assess the main effects and interactions from each extraction parameter, a standardized Pareto plot was employed, with statistical significance set at p < 0.05. The independent variables (technique, X1; % solvent concentration, X2; extraction duration, X3; extraction temperature, X4) and their impact on bioactive compounds and antioxidant capacity are displayed in Figure 3. Additionally, the orthogonal coded estimates are presented, which are obtained through the transformation that applies orthogonality to the estimates. It was possible to deduce from the Pareto plot that X1, X3, and predominantly X2, had an adverse effect on TPC in terms of the effects of extraction parameters. TPC recovery was found to be highly sensitive to changes in the concentration of ethanol in water, as evidenced by the X2 × X2 combination, producing the greatest negative contribution. For instance, it was previously discussed that a higher ethanol concentration was not preferable in our study, as well as in the study by Safdar et al. [45]. A similar trend occurred in both antioxidant capacity assays. This could indicate a strong association between extracted polyphenols and antioxidant capability. In addition, it was observed that the combination of X3 and X4 variables had a negative impact in these assays. A possible degradation of bioactive compounds under extensive duration and heat the explanation. Figure 2A also revealed that extraction temperature as a standalone parameter did not have a significant impact in total polyphenol extraction. This finding is also supported by Figure S1, in which the impact of each parameter is extensively provided. On the other hand, the X2 variable had a positive correlation in AAC recovery, which could be a matter of solvent polarity.
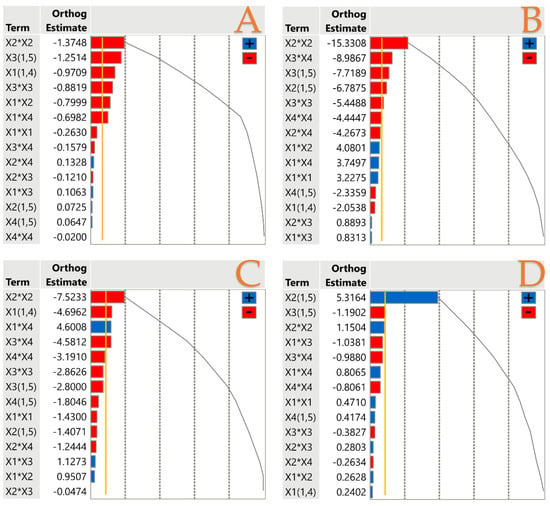
Figure 3.
Pareto plots of transformed estimates for TPC (A), FRAP (B), DPPH (C), and AAC (D) assays. A gold line is drawn on the plot as a reference to indicate the significance level (p < 0.05). The cumulative sum grey line in the plots sums the absolute values of the estimates.
3.3. Optimal Extraction Conditions
Conventional extraction methods face multiple challenges, including extended processing durations, reduced efficiency in extracting bioactive compounds, considerable solvent usage, potentially hazardous solvents, and the risk of thermolabile bioactive compounds deteriorating [50]. Significant progress has been made in recent years concerning the advancement in extraction techniques that minimize the need for hazardous solvents, ensure the safety of human health, and consume less energy [51]. US waves move through the extraction solvent by compression and expansion. At higher intensities, severe cavitation bubbles are formed and extract the compounds of interest thorough plant cell breakdown. It should be noted that the extraction solvent has a vast impact on these bubbles [52]. PEF operates as a technique that employs short electrical power pulses to non-thermally electroporate cell membranes across an array of electric field strengths, thereby facilitating the extraction of bioactive compounds [53]. Following this, the bioactive components of interest derived are transferred to the solvent used for extraction. The selection of solvent is of high importance, as its polarity could significantly impact the bioactive compound extraction. However, should the principles of green chemistry be followed, environmentally friendly solvents must be utilized [54]. Water is an extremely affordable and environmentally friendly solvent. By utilizing this solvent, the extraction of polar compounds is significantly improved. Although water is the most effective solvent for isolating polar bioactive compounds, other solvents like ethanol or methanol may be used to recover molecules with reduced polarity [55]. Given their potential for use in food-grade applications, a combination of water and ethanol is the most efficient binary solvent combination currently available [56].
To identify the maximum predicted values for bioactive compounds and antioxidant assays, the desirability function was used. The predicted responses were optimal when different extraction techniques and conditions were used for each assay. For instance, TPC demanded a sole stirring technique for 90 min at 50 °C, whereas FRAP required all techniques (PEF, US, and ST) for 60 min at 65 °C. However, it should be noted that a solvent of 50% ethanol v/v was the optimum in most cases. Additional information about the maximum predicted responses and optimal extraction conditions is provided in Table 4.

Table 4.
Maximum predicted responses and optimum extraction conditions for the dependent variables.
3.4. Principal Component Analysis (PCA) and Multivariate Correlation Analysis (MCA)
To facilitate a more comprehensive analysis of the data and gain further details from the variables under investigation, a PCA plot was implemented; the outcomes of which are depicted in Figure 4. The plot explained 83.4% of the variance. The point of the correlation analyses was to determine if any relationship between TPC, AAC, DPPH, and FRAP with variables was observed. The impact of independent variables on the analysis was also regarded as important. It was noted that TPC and two antioxidant capacity variables were plotted close to one another on the graph. Their shared influence on the extraction parameters could provide one possible explanation, revealing their high correlation. Conversely, the AAC was positioned at a considerable distance from the other variables, suggesting a weaker correlation among them. Additionally, it is important to acknowledge that AAC was positioned near variable X2 (i.e., solvent composition), indicating a strong association between the two variables. Previous results have shown a positive correlation between a higher ethanol concentration and AAC recovery.
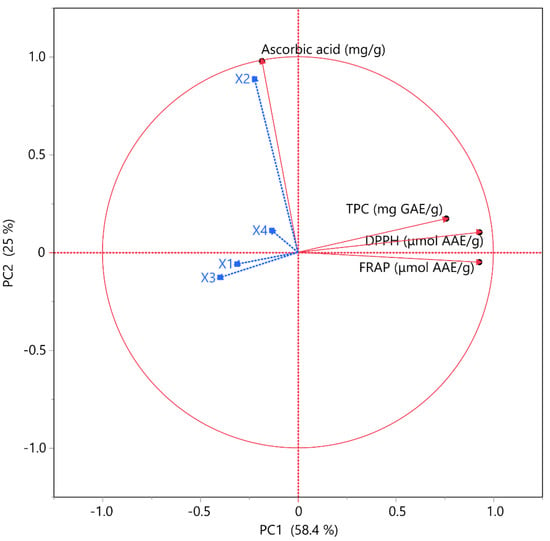
Figure 4.
Principal component analysis (PCA) for the investigated measured variables. Each X variable is highlighted with a blue color.
Additional information regarding the correlation between variables is offered in the MCA plot. The capability to determine the amount of positive or negative correlation among the investigated variables is a major advantage of this method. Table 5 shows the outcomes of this analysis. A good positive correlation (>0.5) between TPC and antioxidant assays was ascertained. A probable reason could lie beneath various procedures that resulted in multiple isolated polyphenols with diverse antioxidant capabilities, a trend that has previously been reported elsewhere [57]. In addition, it is now evident that AAC has little-to-no correlation with any measured variables.

Table 5.
Multivariate correlation analysis of the investigated measured variables.
3.5. Partial Least-Squares (PLS) Analysis
The impact of the extraction condition parameters (X1, X2, X3, and X4) was determined through the PLS model, a correlation loading plot of which is illustrated in Figure 5. It shows the effects of extraction conditions on mandarin peels. The goal was to optimize extraction efficiency through the mentioned parameters. Temperature, solvent composition, and extraction duration are some of the most important parameters to have a major impact on the extraction of bioactive compounds [58]. To start with, the variation in solubility and polarity of the extracted polyphenols could be a challenge for the extraction procedure [59]. It was observed that the X2 variable (i.e., solvent composition) had a vast contribution to yield maximum responses in most assays, reaching a plateau at level 4 (i.e., 75% v/v ethanol). Regarding the extraction techniques, it was observed that a sole ST process could not yield as high bioactive compounds as that with the assistance of both PEF and US pretreatment techniques. Similar results were obtained in our previous studies [26,60]. As revealed by the plot, the extraction technique variable (X1) was observed to reach the highest possible responses at 4, meaning that all extraction techniques (PEF, US, and ST) were necessary for optimal responses. Regarding the extraction duration parameter (X3), considering that previous studies have proven the efficacy of both short [47] and long [48] extraction durations, a comprehensive evaluation was required by partial least-squares to ensure the impact of time on extraction. As such, it was revealed that the higher the value, the lower the responses, so a low time extraction duration (i.e., 30 min) was selected. Finally, temperature is known to increase solubility and enhance the extraction of bioactive compounds; however, some thermolabile compounds could decompose at high temperatures [58]. The temperature variable (X4) was found to be optimal at high levels, which means that extraction at 80 °C was preferable.
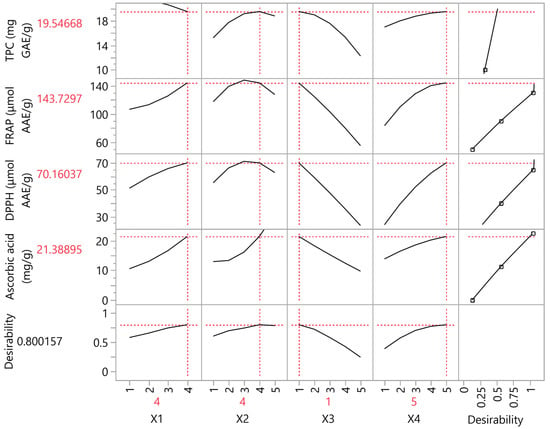
Figure 5.
Partial least-squares (PLS) prediction profiler of each variable and desirability function with extrapolation control for the optimization of mandarin peel extracts.
The comparison of the obtained results after experimental analysis with PLS model values showed that the correlation among them was found to be 0.9966, and they showed no deviations, with the p-value being <0.0001. Compared to other assays, the experimental TPC value showed the lowest deviation from the predicted value. Table 6 shows the predicted values by the PLS model with the corresponding values in bioactive compounds and antioxidant assays. The comprehensive details about the calibration curve of each assay are also shown. The most optimal extraction conditions were found to be PEF + US + ST, demanding 75% v/v of ethanol for 30 min at 80 °C for the latter process. No evaporation issues were detected in the closed system, as the mixture of ethanol/water required a higher temperature to evaporate. Figure 6 illustrates a representative HPLC chromatogram of the individual polyphenols under optimal conditions. Detailed information about the calibration values of each quantified polyphenol along with their measured concentrations is reported in Table 7.

Table 6.
Maximum desirability for all investigated variables using the partial least-squares (PLS) prediction profiler under optimal extraction conditions (X1: 4, X2: 4, X3: 1, and X4: 5). Calibration values of each assay are also included.
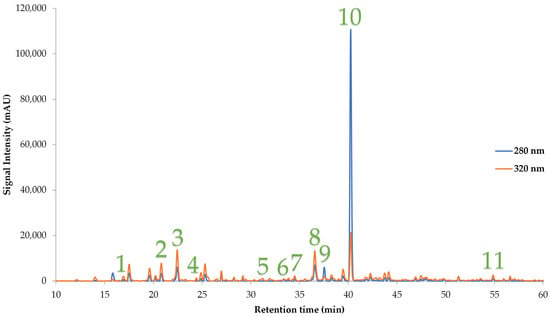
Figure 6.
A representative HPLC chromatogram of the optimal mandarin peel extract reveals the presence of identified polyphenolic compounds at 280 and 320 nm. 1: Neochlorogenic acid; 2: catechin; 3: chlorogenic acid; 4: vanillic acid; 5: Ferulic acid; 6: rutin; 7: quercetin 3-D-galactoside; 8: luteolin-7-glucoside; 9: narirutin; 10: hesperidin; 11: kaempferol.

Table 7.
Concentration of identified polyphenolic compounds under optimal extraction conditions (X1: 4, X2: 4, X3: 1, and X4: 5), along with calibration values.
The importance of the US technique compared to conventional extraction should be highlighted. In a study conducted by Ma et al. [61], satsuma mandarin (Citrus unshiu) peels yielded 1935.12 μg GAE/g dw by US-assisted extraction in only 10 min, achieving a six-fold increase when compared to the conventional maceration technique for 1 h. Under those conditions, hesperidin was quantified at 1124.97 mg/g, whereas ferulic acid was the major polyphenol, at 2264.83 mg/g dw. In addition, the US probe could also be used as an extraction technique with a short duration time, as per Kaur et al. [62]. By using a similar experimental design, they yielded 17–36 mg GAE/g dw. Satsuma mandarins were also investigated by Hwang et al. [63]. They found that at 3 kV/cm for 120 s of PEF treatment, hesperidin was measured at 46.96 mg/g dw. In the previous study by Anticona et al. [47], the concentration of several polyphenols was determined through HPLC, in which hesperidin was measured at ~57 μg/g fw and narirutin at ~29 μg/g fw, whereas catechin was not determined. When Safdar et al. [45] used 80% ethanol and the US extraction technique, they measured a total of 371.16 μg/g, with hesperidin being the major polyphenol (92.94 μg/g).
4. Conclusions
Mandarin peels are a major source of by-products in the food industry. According to the findings of this study, the waste peels can be effectively repurposed to produce extracts containing bioactive compounds in high amounts. To improve the extraction efficiency, PEF and US techniques were employed as pretreatment methods. The utilization of PEF and US prior to the stirring process proved to be the most efficient, maximizing the extraction yield. A stirring process with a medium–high polarity mixture consisting of 75% v/v ethanol for 30 min at 80 °C improved the efficiency of bioactive compound extraction. AAC was shown to have little correlation with other assays, while it was positively affected by ethanol concentration through correlation analyses. The optimal extract reached high values in bioactive compound concentration and considerable antioxidant activity. The use of health-promoting extracts has the potential to significantly enhance the products in the food, pharmaceutical, and cosmetic sectors, while sustainably reducing food waste.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/oxygen4030018/s1, The comparison between the predicted and actual responses for each parameter being investigated is depicted in Figures S1–S4, which also include the desirability functions. The three-dimensional response diagrams for the remaining responses are illustrated in Figures S5–S7.
Author Contributions
Conceptualization, V.A., T.C. and S.I.L.; methodology, V.A. and T.C.; software, V.A.; validation, T.C.; formal analysis, D.K., A.-I.I., E.B., V.A. and T.C.; investigation, E.B., K.K. and M.M.; resources, S.I.L.; data curation, D.K., M.M., K.K. and E.B.; writing—original draft preparation, D.K., A.-I.I. and E.B.; writing—review and editing, V.A., T.C., A.-I.I., D.K., K.K., E.B., M.M. and S.I.L.; visualization, D.K., K.K. and M.M; supervision, V.A., T.C. and S.I.L.; project administration, S.I.L.; funding acquisition, S.I.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All related data and methods are presented in this paper. Additional inquiries should be addressed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M.H. Citrus Waste Derived Nutra-/Pharmaceuticals for Health Benefits: Current Trends and Future Perspectives. J. Funct. Foods 2018, 40, 307–316. [Google Scholar] [CrossRef]
- FAO. Citrus Fruit Statistical Compendium 2020; FAO: Rome, Italy, 2021. [Google Scholar]
- Putnik, P.; Barba, F.J.; Lorenzo, J.M.; Gabrić, D.; Shpigelman, A.; Cravotto, G.; Bursać Kovačević, D. An Integrated Approach to Mandarin Processing: Food Safety and Nutritional Quality, Consumer Preference, and Nutrient Bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Sebghatollahi, Z.; Ghanadian, M.; Agarwal, P.; Ghaheh, H.S.; Mahato, N.; Yogesh, R.; Hejazi, S.H. Citrus Flavonoids: Biological Activities, Implementation in Skin Health, and Topical Applications: A Review. ACS Food Sci. Technol. 2022, 2, 1417–1432. [Google Scholar] [CrossRef]
- Cho, E.J.; Lee, Y.G.; Chang, J.; Bae, H.-J. A High-Yield Process for Production of Biosugars and Hesperidin from Mandarin Peel Wastes. Molecules 2020, 25, 4286. [Google Scholar] [CrossRef] [PubMed]
- Hajialyani, M.; Hosein Farzaei, M.; Echeverría, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef] [PubMed]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Molecular Mechanisms behind the Biological Effects of Hesperidin and Hesperetin for the Prevention of Cancer and Cardiovascular Diseases. Life Sci. 2015, 124, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Casagrande, R. Hesperidin Methyl Chalcone Inhibits Oxidative Stress and Inflammation in a Mouse Model of Ultraviolet B Irradiation-Induced Skin Damage. J. Photochem. Photobiol. B 2015, 148, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Kaul, R.; Sofi, S.A.; Bashir, N.; Nazir, F.; Ahmad Nayik, G. Citrus Peel as a Source of Functional Ingredient: A Review. J. Saudi Soc. Agric. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef]
- Çalışkan Eleren, S.; Öziş Altınçekiç, Ş.; Altınçekiç, E. Biofuel Potential of Fruit Juice Industry Waste. J. Hazard. Toxic Radioact. Waste 2018, 22, 05018002. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting Citrus Wastes into Value-Added Products: Economic and Environmently Friendly Approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Isolation of Polyphenols from Two Waste Streams of Clingstone Peach Canneries Utilizing the Cloud Point Extraction Method. Biomass 2023, 3, 291–305. [Google Scholar] [CrossRef]
- Yadav, V.; Sarker, A.; Yadav, A.; Miftah, A.O.; Bilal, M.; Iqbal, H.M.N. Integrated Biorefinery Approach to Valorize Citrus Waste: A Sustainable Solution for Resource Recovery and Environmental Management. Chemosphere 2022, 293, 133459. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L.; Astrup, T.F.; Asunis, F.; Clarke, W.P.; De Gioannis, G.; Dessì, P.; Lens, P.N.L.; Lavagnolo, M.C.; Lombardi, L.; Muntoni, A.; et al. Organic Waste Biorefineries: Looking towards Implementation. Waste Manag. 2020, 114, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Bhushan, B. An Overview of the Recent Trends on the Waste Valorization Techniques for Food Wastes. J. Environ. Manag. 2019, 233, 352–370. [Google Scholar] [CrossRef] [PubMed]
- Kalompatsios, D.; Athanasiadis, V.; Palaiogiannis, D.; Lalas, S.I.; Makris, D.P. Valorization of Waste Orange Peels: Aqueous Antioxidant Polyphenol Extraction as Affected by Organic Acid Addition. Beverages 2022, 8, 71. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F. Recent Advances and Comparisons of Conventional and Alternative Extraction Techniques of Phenolic Compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus Green Extraction Techniques—A Comparative Perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Rodríguez García, S.L.; Raghavan, V. Green Extraction Techniques from Fruit and Vegetable Waste to Obtain Bioactive Compounds—A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6446–6466. [Google Scholar] [CrossRef]
- Ghoshal, G. Comprehensive Review on Pulsed Electric Field in Food Preservation: Gaps in Current Studies for Potential Future Research. Heliyon 2023, 9, e17532. [Google Scholar] [CrossRef]
- Zhang, C.; Lyu, X.; Arshad, R.N.; Aadil, R.M.; Tong, Y.; Zhao, W.; Yang, R. Pulsed Electric Field as a Promising Technology for Solid Foods Processing: A Review. Food Chem. 2023, 403, 134367. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; You, S.; Xu, Z.; Li, Z.; Guo, J.; Ren, Z.; Fu, C. Novel Extraction Methods and Potential Applications of Polyphenols in Fruit Waste: A Review. J. Food Meas. Charact. 2021, 15, 3250–3261. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Comas-Serra, F.; Femenia, A.; Rosselló, C.; Simal, S. Effect of Power Ultrasound Application on Aqueous Extraction of Phenolic Compounds and Antioxidant Capacity from Grape Pomace (Vitis vinifera L.): Experimental Kinetics and Modeling. Ultrason. Sonochem. 2015, 22, 506–514. [Google Scholar] [CrossRef]
- Parniakov, O.; Lebovka, N.I.; Van Hecke, E.; Vorobiev, E. Pulsed Electric Field Assisted Pressure Extraction and Solvent Extraction from Mushroom (Agaricus bisporus). Food Bioprocess Technol. 2014, 7, 174–183. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Makrygiannis, I.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Antioxidant-Rich Extracts from Lemon Verbena (Aloysia citrodora L.) Leaves through Response Surface Methodology. Oxygen 2024, 4, 1–19. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, C.; Ma, Y.; Jia, M. Degradation Behavior of Polyphenols in Model Aqueous Extraction System Based on Mechanical and Sonochemical Effects Induced by Ultrasound. Sep. Purif. Technol. 2020, 247, 116967. [Google Scholar] [CrossRef]
- Cao, X.; Cai, C.; Wang, Y.; Zheng, X. The Inactivation Kinetics of Polyphenol Oxidase and Peroxidase in Bayberry Juice during Thermal and Ultrasound Treatments. Innov. Food Sci. Emerg. Technol. 2018, 45, 169–178. [Google Scholar] [CrossRef]
- Sun, J.; Bai, W.; Zhang, Y.; Liao, X.; Hu, X. Identification of Degradation Pathways and Products of Cyanidin-3-Sophoroside Exposed to Pulsed Electric Field. Food Chem. 2011, 126, 1203–1210. [Google Scholar] [CrossRef]
- Yilmaz, C.; Krishna, A.S.; Memon, A.; Porter, A.; Schmidt, D.C.; Gokhale, A.; Natarajan, B. Main Effects Screening: A Distributed Continuous Quality Assurance Process for Monitoring Performance Degradation in Evolving Software Systems. In Proceedings of the27th International Conference on Software Engineering-ICSE’05, St. Louis, MO, USA, 15–21 May 2005; ACM Press: St. Louis, MO, USA, 2005; p. 293. [Google Scholar]
- Lekivetz, R.; Sitter, R.; Bingham, D.; Hamada, M.S.; Moore, L.M.; Wendelberger, J.R. On Algorithms for Obtaining Orthogonal and Near-Orthogonal Arrays for Main-Effects Screening. J. Qual. Technol. 2015, 47, 2–13. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Mantiniotou, M.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Investigation of the Polyphenol Recovery of Overripe Banana Peel Extract Utilizing Cloud Point Extraction. Eng 2023, 4, 3026–3038. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef] [PubMed]
- Jagota, S.K.; Dani, H.M. A New Colorimetric Technique for the Estimation of Vitamin C Using Folin Phenol Reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction Optimisation Using Water/Glycerol for the Efficient Recovery of Polyphenolic Antioxidants from Two Artemisia Species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Kawasaki, H.; Shimanouchi, T.; Kimura, Y. Recent Development of Optimization of Lyophilization Process. J. Chem. 2019, 2019, 9502856. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, Á.A.; Figiel, A. Comparison of Traditional and Novel Drying Techniques and Its Effect on Quality of Fruits, Vegetables and Aromatic Herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef] [PubMed]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A Reproducible, Rapid and Inexpensive Folin–Ciocalteu Micro-Method in Determining Phenolics of Plant Methanol Extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Šeruga, M.; Novak, I.; Jakobek, L. Determination of Polyphenols Content and Antioxidant Activity of Some Red Wines by Differential Pulse Voltammetry, HPLC and Spectrophotometric Methods. Food Chem. 2011, 124, 1208–1216. [Google Scholar] [CrossRef]
- Alessandri, S.; Ieri, F.; Romani, A. Minor Polar Compounds in Extra Virgin Olive Oil: Correlation between HPLC-DAD-MS and the Folin-Ciocalteu Spectrophotometric Method. J. Agric. Food Chem. 2014, 62, 826–835. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, J.S.F.; de Souza, E.L.; Oliveira, J.R.; Gomes, A.C.A.; Kotzebue, L.R.V.; da Silva Agostini, D.L.; de Oliveira, D.L.V.; Mazzetto, S.E.; da Silva, A.L.; Cavalcanti, M.T. Microencapsulation of Sweet Orange Essential Oil (Citrus aurantium Var. Dulcis) by Liophylization Using Maltodextrin and Maltodextrin/Gelatin Mixtures: Preparation, Characterization, Antimicrobial and Antioxidant Activities. Int. J. Biol. Macromol. 2020, 143, 991–999. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef]
- Koraqi, H.; Petkoska, A.T.; Khalid, W.; Sehrish, A.; Ambreen, S.; Lorenzo, J.M. Optimization of the Extraction Conditions of Antioxidant Phenolic Compounds from Strawberry Fruits (Fragaria x ananassa Duch.) Using Response Surface Methodology. Food Anal. Methods 2023, 16, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Singanusong, R.; Nipornram, S.; Tochampa, W.; Rattanatraiwong, P. Low Power Ultrasound-Assisted Extraction of Phenolic Compounds from Mandarin (Citrus reticulata Blanco Cv. Sainampueng) and Lime (Citrus aurantifolia) Peels and the Antioxidant. Food Anal. Methods 2015, 8, 1112–1123. [Google Scholar] [CrossRef]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and Quantification of Polyphenols from Kinnow (Citrus reticulate L.) Peel Using Ultrasound and Maceration Techniques. J. Food Drug Anal. 2017, 25, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, G.; Vitale, E.; Iesce, M.R.; Naviglio, D.; Amoresano, A.; Fontanarosa, C.; Spinelli, M.; Ciaravolo, M.; Arena, C. Antioxidant Properties of Pulp, Peel and Seeds of Phlegrean Mandarin (Citrus reticulata Blanco) at Different Stages of Fruit Ripening. Antioxidants 2022, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Anticona, M.; Blesa, J.; Lopez-Malo, D.; Frigola, A.; Esteve, M.J. Effects of Ultrasound-Assisted Extraction on Physicochemical Properties, Bioactive Compounds, and Antioxidant Capacity for the Valorization of Hybrid Mandarin Peels. Food Biosci. 2021, 42, 101185. [Google Scholar] [CrossRef]
- Milardović, S.; Iveković, D.; Grabarić, B.S. A Novel Amperometric Method for Antioxidant Activity Determination Using DPPH Free Radical. Bioelectrochemistry 2006, 68, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Abramovič, H.; Grobin, B.; Poklar Ulrih, N.; Cigić, B. Relevance and Standardization of In Vitro Antioxidant Assays: ABTS, DPPH, and Folin–Ciocalteu. J. Chem. 2018, 2018, 4608405. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of Green Food Processing Techniques. Preservation, Transformation, and Extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Sik, B.; Hanczné, E.L.; Kapcsándi, V.; Ajtony, Z. Conventional and Nonconventional Extraction Techniques for Optimal Extraction Processes of Rosmarinic Acid from Six Lamiaceae Plants as Determined by HPLC-DAD Measurement. J. Pharm. Biomed. Anal. 2020, 184, 113173. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in Ultrasound Assisted Extraction of Bioactive Compounds from Cash Crops—A Review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kalompatsios, D.; Mantiniotou, M.; Bozinou, E.; Lalas, S.I. Pulsed Electric Field Applications for the Extraction of Bioactive Compounds from Food Waste and By-Products: A Critical Review. Biomass 2023, 3, 367–401. [Google Scholar] [CrossRef]
- Kalompatsios, D.; Palaiogiannis, D.; Makris, D.P. Optimized Production of a Hesperidin-Enriched Extract with Enhanced Antioxidant Activity from Waste Orange Peels Using a Glycerol/Sodium Butyrate Deep Eutectic Solvent. Horticulturae 2024, 10, 208. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New Insights of the Application of Water or Ethanol-Water Plant Extract Rich in Active Compounds in Food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef]
- Monroy, Y.M.; Rodrigues, R.A.F.; Sartoratto, A.; Cabral, F.A. Influence of Ethanol, Water, and Their Mixtures as Co-Solvents of the Supercritical Carbon Dioxide in the Extraction of Phenolics from Purple Corn Cob (Zea mays L.). J. Supercrit. Fluids 2016, 118, 11–18. [Google Scholar] [CrossRef]
- Segovia, F.J.; Luengo, E.; Corral-Pérez, J.J.; Raso, J.; Almajano, M.P. Improvements in the Aqueous Extraction of Polyphenols from Borage (Borago officinalis L.) Leaves by Pulsed Electric Fields: Pulsed Electric Fields (PEF) Applications. Ind. Crops Prod. 2015, 65, 390–396. [Google Scholar] [CrossRef]
- More, P.R.; Jambrak, A.R.; Arya, S.S. Green, Environment-Friendly and Sustainable Techniques for Extraction of Food Bioactive Compounds and Waste Valorization. Trends Food Sci. Technol. 2022, 128, 296–315. [Google Scholar] [CrossRef]
- Thoo, Y.; Ng, S.Y.; Khoo, M.; Mustapha, W.; Ho, C. A Binary Solvent Extraction System for Phenolic Antioxidants and Its Application to the Estimation of Antioxidant Capacity in Andrographis paniculata Extracts. Int. Food Res. J. 2013, 20, 1103. [Google Scholar]
- Kotsou, K.; Stoikou, M.; Athanasiadis, V.; Chatzimitakos, T.; Mantiniotou, M.; Sfougaris, A.I.; Lalas, S.I. Enhancing Antioxidant Properties of Prunus spinosa Fruit Extracts via Extraction Optimization. Horticulturae 2023, 9, 942. [Google Scholar] [CrossRef]
- Ma, Y.-Q.; Ye, X.-Q.; Fang, Z.-X.; Chen, J.-C.; Xu, G.-H.; Liu, D.-H. Phenolic Compounds and Antioxidant Activity of Extracts from Ultrasonic Treatment of Satsuma Mandarin (Citrus unshiu Marc.) Peels. J. Agric. Food Chem. 2008, 56, 5682–5690. [Google Scholar] [CrossRef]
- Kaur, S.; Panesar, P.S.; Chopra, H.K. Standardization of Ultrasound-Assisted Extraction of Bioactive Compounds from Kinnow Mandarin Peel. Biomass Convers. Biorefinery 2023, 13, 8853–8863. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Kim, H.-J.; Ko, M.-J.; Chung, M.-S. Recovery of Hesperidin and Narirutin from Waste Citrus unshiu Peel Using Subcritical Water Extraction Aided by Pulsed Electric Field Treatment. Food Sci. Biotechnol. 2021, 30, 217–226. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).