Pre-Clinical Studies of MicroRNA-Based Therapies for Sepsis: A Scoping Review
Abstract
1. Introduction
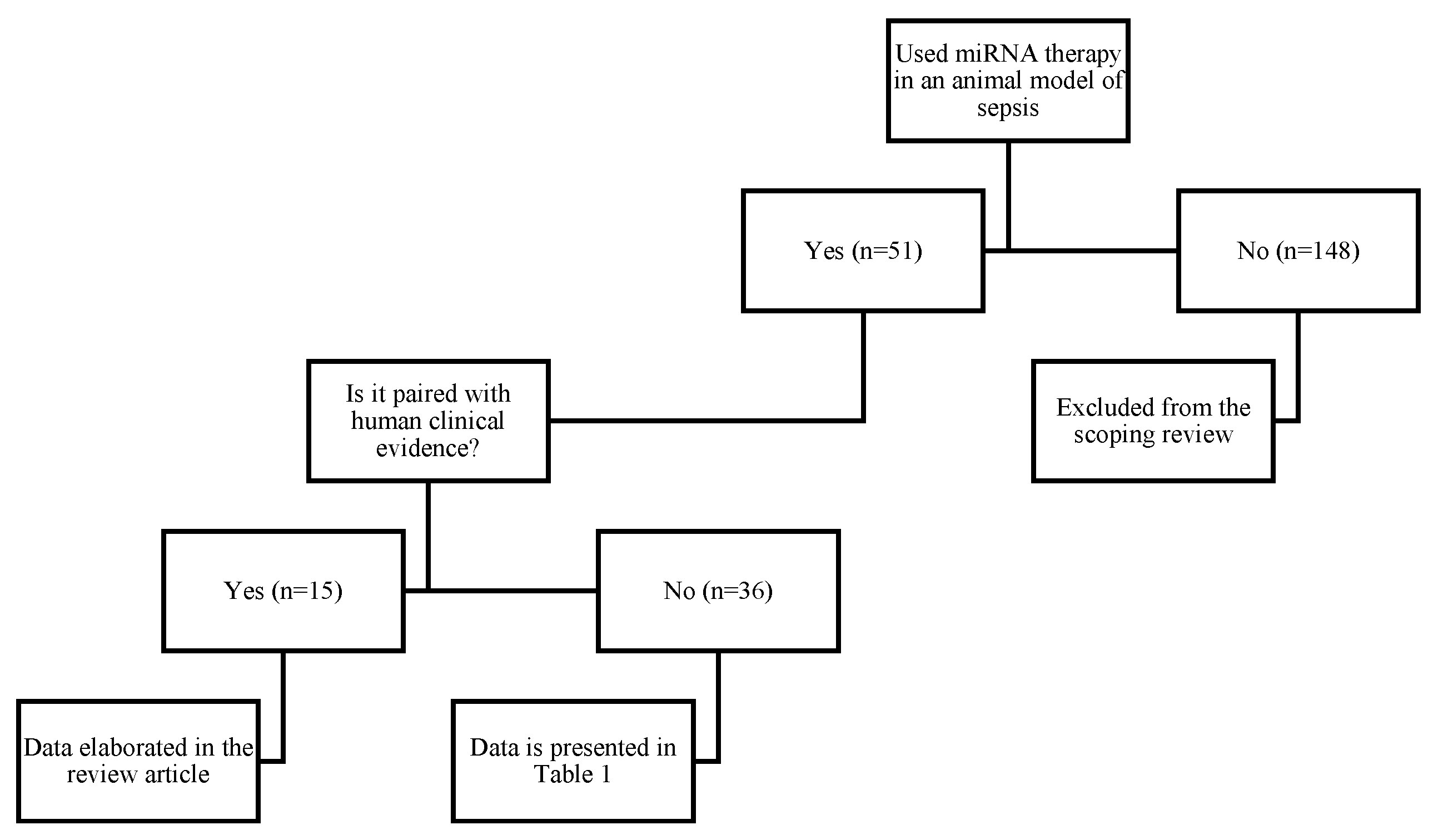
2. Methods
3. Results
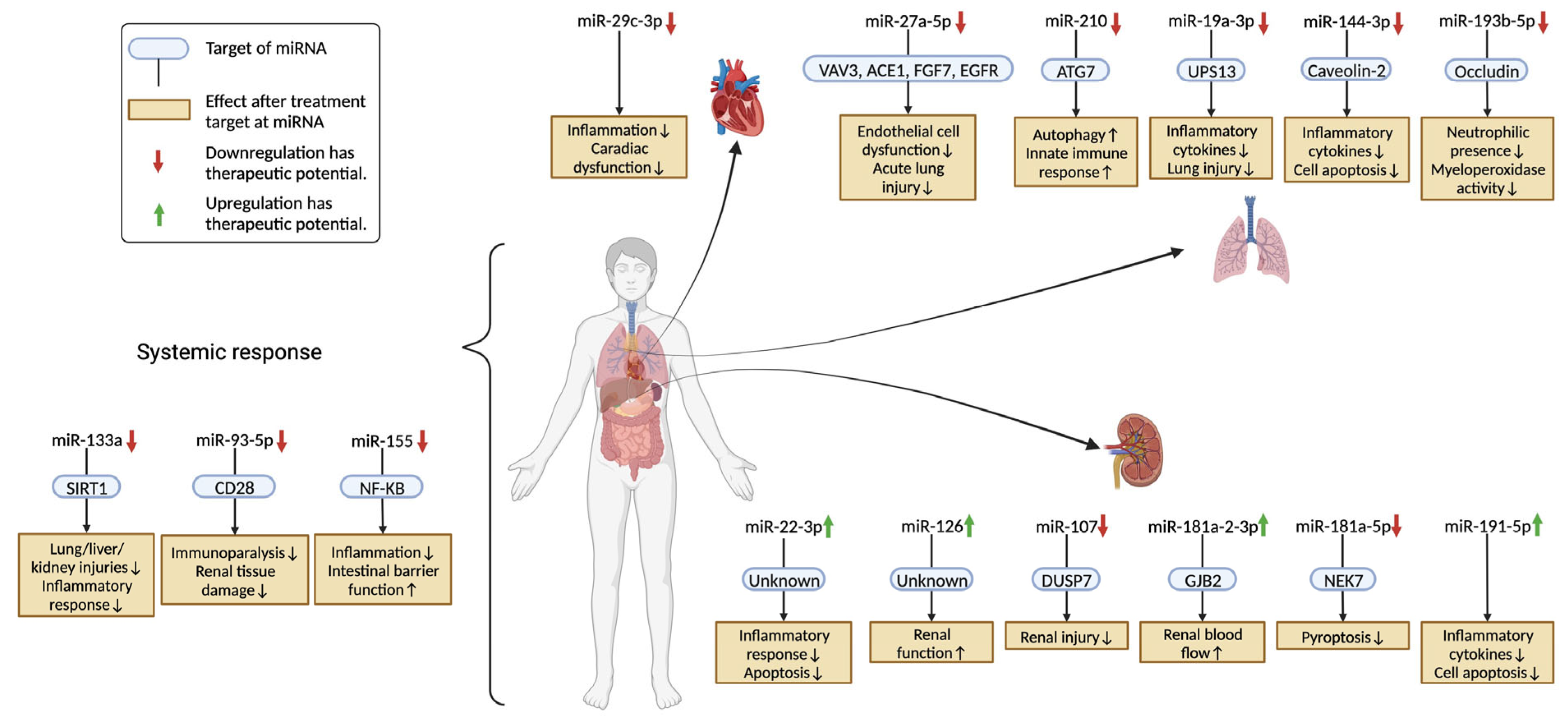
3.1. Acute Lung Injury (ALI)
3.1.1. miR-27a-5p [21]
3.1.2. miR-210 [62]
3.1.3. miR-19a-3p [33]
3.1.4. miR-144-3p [45]
3.1.5. miR-193b-5p [20]
3.2. Systemic Response
3.2.1. miR-133a [29]
3.2.2. miR-93-5p [30]
3.2.3. miR-155 [77]
3.3. Acute Kidney Injury (AKI)
3.3.1. miR-22-3p [35]
3.3.2. miR-126 [25]
3.3.3. miR-107 [61]
3.3.4. miR-181a-2-3p [49]
3.3.5. miR-181a-5p [90]
3.3.6. miR-191-5p [93]
3.4. Sepsis-Induced Cardiac Dysfunction
miR-29c-3p [37]
4. Discussion
Funding
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M.; Sepsis Definitions Task, F. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, M.; van Beuningen, F.E.; Ter Maaten, J.C.; Bouma, H.R. Hospital-related costs of sepsis around the world: A systematic review exploring the economic burden of sepsis. J. Crit. Care 2022, 71, 154096. [Google Scholar] [CrossRef] [PubMed]
- Prescott, H.C.; Angus, D.C. Postsepsis Morbidity. JAMA 2018, 319, 91. [Google Scholar] [CrossRef]
- Prescott, H.C.; Angus, D.C. Enhancing Recovery From Sepsis: A Review. JAMA 2018, 319, 62–75. [Google Scholar] [CrossRef]
- Cuthbertson, B.H.; Elders, A.; Hall, S.; Taylor, J.; MacLennan, G.; Mackirdy, F.; Mackenzie, S.J.; Scottish Critical Care Trials Group; Scottish Intensive Care Society Audit Group. Mortality and quality of life in the five years after severe sepsis. Crit. Care 2013, 17, R70. [Google Scholar] [CrossRef]
- Herridge, M.S.; Chu, L.M.; Matte, A.; Tomlinson, G.; Chan, L.; Thomas, C.; Friedrich, J.O.; Mehta, S.; Lamontagne, F.; Levasseur, M.; et al. The RECOVER Program: Disability Risk Groups and 1-Year Outcome after 7 or More Days of Mechanical Ventilation. Am. J. Respir. Crit. Care Med. 2016, 194, 831–844. [Google Scholar] [CrossRef]
- Ospina-Tascon, G.A.; Buchele, G.L.; Vincent, J.L. Multicenter, randomized, controlled trials evaluating mortality in intensive care: Doomed to fail? Crit. Care Med. 2008, 36, 1311–1322. [Google Scholar] [CrossRef]
- Kaukonen, K.M.; Bailey, M.; Suzuki, S.; Pilcher, D.; Bellomo, R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 2014, 311, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Ektesabi, A.M.; Mori, K.; Tsoporis, J.N.; Vaswani, C.M.; Gupta, S.; Walsh, C.; Varkouhi, A.K.; Mei, S.H.J.; Stewart, D.J.; Liles, W.C.; et al. Mesenchymal Stem/Stromal Cells Increase Cardiac miR-187-3p Expression in a Polymicrobial Animal Model of Sepsis. Shock 2021, 56, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Damase, T.R.; Sukhovershin, R.; Boada, C.; Taraballi, F.; Pettigrew, R.I.; Cooke, J.P. The Limitless Future of RNA Therapeutics. Front. Bioeng. Biotechnol. 2021, 9, 628137. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Song, Y.; Shaikh, Z.; Li, H.; Zhang, H.; Caudle, Y.; Zheng, S.; Yan, H.; Hu, D.; Stuart, C.; et al. MicroRNA-155 attenuates late sepsis-induced cardiac dysfunction through JNK and beta-arrestin 2. Oncotarget 2017, 8, 47317–47329. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, T.M.; Liu, X.R.; Bai, Y.P.; Li, J.; Tang, N.; Wang, X.B. MicroRNA-140 inhibits skeletal muscle glycolysis and atrophy in endotoxin-induced sepsis in mice via the WNT signaling pathway. Am. J. Physiol. Cell Physiol. 2019, 317, C189–C199. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Lu, H.T.; Wang, H.F.; Shen, M.J.; Zhang, H.B. MicroRNA-203 Acts as a Potent Suppressor in Septic Shock by Alleviating Lung Injury via Inhibition of VNN1. Kidney Blood Press Res. 2019, 44, 565–582. [Google Scholar] [CrossRef]
- He, S.Y.; Wang, G.; Pei, Y.H.; Zhu, H.P. miR-34b-3p protects against acute kidney injury in sepsis mice via targeting ubiquitin-like protein 4A. Kaohsiung J. Med. Sci. 2020, 36, 817–824. [Google Scholar] [CrossRef]
- Sang, Z.; Dong, S.; Zhang, P.; Wei, Y. miR-214 ameliorates sepsis-induced acute kidney injury via PTEN/AKT/mTOR-regulated autophagy. Mol. Med. Rep. 2021, 24, 683. [Google Scholar] [CrossRef]
- Wang, H.F.; Wang, Y.Q.; Dou, L.; Gao, H.M.; Wang, B.; Luo, N.; Li, Y. Influences of up-regulation of miR-126 on septic inflammation and prognosis through AKT/Rac1 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2132–2138. [Google Scholar] [CrossRef]
- Dos Santos, C.C.; Amatullah, H.; Vaswani, C.M.; Maron-Gutierrez, T.; Kim, M.; Mei, S.H.J.; Szaszi, K.; Monteiro, A.P.T.; Varkouhi, A.K.; Herreroz, R.; et al. Mesenchymal stromal (stem) cell therapy modulates miR-193b-5p expression to attenuate sepsis-induced acute lung injury. Eur. Respir. J. 2022, 59, 2004216. [Google Scholar] [CrossRef]
- Younes, N.; Zhou, L.; Amatullah, H.; Mei, S.H.J.; Herrero, R.; Lorente, J.A.; Stewart, D.J.; Marsden, P.; Liles, W.C.; Hu, P.; et al. Mesenchymal stromal/stem cells modulate response to experimental sepsis-induced lung injury via regulation of miR-27a-5p in recipient mice. Thorax 2020, 75, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lu, Q.; Deng, F.; Peng, S.; Yuan, J.; Liu, C.; Du, X. miR-103a-3p Could Attenuate Sepsis-Induced Liver Injury by Targeting HMGB1. Inflammation 2020, 43, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Li, L.B.; Sun, C.H. The effect of myocardial infarction-associated transcript 2 (Mirt2) and miR-101 on sepsis-induced myocardial injury in rats. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6299–6310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zeng, L.; Cai, G.; Zhu, Y.; Xiong, Y.; Zhan, H.; Yang, Z. miR-340-5p Alleviates Oxidative Stress Injury by Targeting MyD88 in Sepsis-Induced Cardiomyopathy. Oxid. Med. Cell Longev. 2022, 2022, 2939279. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Liu, C.; Hu, N.; Wang, W.; Wang, H. miR-126 ameliorates multiple organ dysfunction in septic rats by regulating the differentiation of Th17/Treg. Mol. Biol. Rep. 2022, 49, 2985–2998. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Hou, Y.; Cai, X. MiR-210-3p Enhances Cardiomyocyte Apoptosis and Mitochondrial Dysfunction by Targeting the NDUFA4 Gene in Sepsis-Induced Myocardial Dysfunction. Int. Heart J. 2021, 62, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Dong, J.; Li, P.; Tang, C.; Cheng, W.; Xu, Z.; Zhou, W.; Ge, J.; Xia, C.; Zhang, Z. MiRNA-21 has effects to protect kidney injury induced by sepsis. Biomed. Pharmacother. 2017, 94, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Li, Q.; Duan, Z.P.; Wang, Y.J.; Hu, B.Q.; Dai, X.G. LncRNA GAS5 inhibits miR-579-3p to activate SIRT1/PGC-1alpha/Nrf2 signaling pathway to reduce cell pyroptosis in sepsis-associated renal injury. Am. J. Physiol. Cell Physiol. 2021, 321, C117–C133. [Google Scholar] [CrossRef]
- Chen, L.; Xie, W.; Wang, L.; Zhang, X.; Liu, E.; Kou, Q. MiRNA-133a aggravates inflammatory responses in sepsis by targeting SIRT1. Int. Immunopharmacol. 2020, 88, 106848. [Google Scholar] [CrossRef]
- Dragomir, M.P.; Fuentes-Mattei, E.; Winkle, M.; Okubo, K.; Bayraktar, R.; Knutsen, E.; Qdaisat, A.; Chen, M.; Li, Y.; Shimizu, M.; et al. Anti-miR-93-5p therapy prolongs sepsis survival by restoring the peripheral immune response. J. Clin. Investig. 2023, 133, e158348. [Google Scholar] [CrossRef]
- Borjas, T.; Jacob, A.; Kobritz, M.; Ma, G.; Tan, C.; Patel, V.; Coppa, G.F.; Aziz, M.; Wang, P. An engineered miRNA PS-OMe miR130 inhibits acute lung injury by targeting eCIRP in sepsis. Mol. Med. 2023, 29, 21. [Google Scholar] [CrossRef]
- Ma, X.F.; Qin, J.; Guo, X.H. MiR-181-5p protects mice from sepsis via repressing HMGB1 in an experimental model. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9712–9720. [Google Scholar] [CrossRef]
- Ren, H.; Mu, W.; Xu, Q. miR-19a-3p inhibition alleviates sepsis-induced lung injury via enhancing USP13 expression. Acta Biochim. Pol. 2021, 68, 201–206. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Liu, L. Hyperoside prevents sepsis-associated cardiac dysfunction through regulating cardiomyocyte viability and inflammation via inhibiting miR-21. Biomed. Pharmacother. 2021, 138, 111524. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Kong, M.; Yang, J. MiR-22-3p suppresses sepsis-induced acute kidney injury by targeting PTEN. Biosci. Rep. 2020, 40, BSR20200527. [Google Scholar] [CrossRef]
- Funahashi, Y.; Kato, N.; Masuda, T.; Nishio, F.; Kitai, H.; Ishimoto, T.; Kosugi, T.; Tsuboi, N.; Matsuda, N.; Maruyama, S.; et al. miR-146a targeted to splenic macrophages prevents sepsis-induced multiple organ injury. Lab. Investig. 2019, 99, 1130–1142. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, L.; Sheng, Y. Clinical value and role of microRNA-29c-3p in sepsis-induced inflammation and cardiac dysfunction. Eur. J. Med. Res. 2021, 26, 90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, F.; Wu, J.; Yang, A.X.; Zhang, Y.Y.; Zhao, H.; Tao, W.Y. MiR-205 influences renal injury in sepsis rats through HMGB1-PTEN signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10950–10956. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wei, J.; Tian, D.; Wu, M.; Yan, C.; Hu, P.; Wu, X.; Yang, W.; Yin, T. miR-182-5p contributes to intestinal injury in a murine model of Staphylococcus aureus pneumonia-induced sepsis via targeting surfactant protein D. J. Cell. Physiol. 2020, 235, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, Z. MicroRNA-23a-3p ameliorates acute kidney injury by targeting FKBP5 and NF-kappaB signaling in sepsis. Cytokine 2022, 155, 155898. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, Z.; Cao, J.; Kong, X.; Gong, G. A TGFBR2/SMAD2/DNMT1/miR-145 negative regulatory loop is responsible for LPS-induced sepsis. Biomed. Pharmacother. 2019, 112, 108626. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Zhang, J. Protective role of matrine in sepsis-associated cardiac dysfunction through regulating the lncRNA PTENP1/miR-106b-5p axis. Biomed. Pharmacother. 2021, 134, 111112. [Google Scholar] [CrossRef]
- You, Q.; Wang, J.; Jia, D.; Jiang, L.; Chang, Y.; Li, W. MiR-802 alleviates lipopolysaccharide-induced acute lung injury by targeting Peli2. Inflamm. Res. 2020, 69, 75–85. [Google Scholar] [CrossRef]
- Yan, F.; Wang, Q.; Yang, H.; Lv, H.; Qin, W. miR-926-3p influences myocardial injury in septic mice through regulation of mTOR signaling pathway by targeting TSC1. Aging 2023, 15, 3826–3838. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Shao, Z.; Cao, Q. MicroRNA-144-3p enhances LPS induced septic acute lung injury in mice through downregulating Caveolin-2. Immunol. Lett. 2021, 231, 18–25. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Li, F.; Yuan, K.; Li, M.; Zhang, J.; Li, B.; Liang, W. MiR-130b attenuates vascular inflammation via negatively regulating tumor progression locus 2 (Tpl2) expression. Int. Immunopharmacol. 2017, 51, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Xiong, W.; Chen, X.; Liu, J.; Ye, Z. Overexpression of miR-129-5p Mitigates Sepsis-Induced Acute Lung Injury by Targeting High Mobility Group Box 1. J. Surg. Res. 2020, 256, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guan, H.; Zhang, J.L.; Zheng, Z.; Wang, H.T.; Tao, K.; Han, S.C.; Su, L.L.; Hu, D. Acute downregulation of miR-199a attenuates sepsis-induced acute lung injury by targeting SIRT1. Am. J. Physiol. Cell Physiol. 2018, 314, C449–C455. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.X.; Jiang, S.Y.; Yu, L.H.; Chen, K.; Yang, Z.X.; Wu, Q. MicroRNA 181a-2-3p Alleviates the Apoptosis of Renal Tubular Epithelial Cells via Targeting GJB2 in Sepsis-Induced Acute Kidney Injury. Mol. Cell Biol. 2021, 41, e0001621. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Huang, Y.; He, J.; Zhang, X.; Zhou, Y.; Wei, Y.; Tang, Y.; Liu, L. Upregulation of miR-335 exerts protective effects against sepsis-induced myocardial injury. Mol. Med. Rep. 2021, 24, 806. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ding, R.; Hu, Z.; Yin, X.; Xiao, F.; Zhang, W.; Yan, S.; Lv, C. MicroRNA-34a Inhibition Alleviates Lung Injury in Cecal Ligation and Puncture Induced Septic Mice. Front. Immunol. 2020, 11, 1829. [Google Scholar] [CrossRef]
- McClure, C.; Ali, E.; Youssef, D.; Yao, Z.Q.; McCall, C.E.; El Gazzar, M. NFI-A disrupts myeloid cell differentiation and maturation in septic mice. J. Leukoc. Biol. 2016, 99, 201–211. [Google Scholar] [CrossRef]
- Huang, X.; Hou, X.; Chuan, L.; Wei, S.; Wang, J.; Yang, X.; Ru, J. miR-129-5p alleviates LPS-induced acute kidney injury via targeting HMGB1/TLRs/NF-kappaB pathway. Int. Immunopharmacol. 2020, 89 Pt A, 107016. [Google Scholar] [CrossRef]
- Diao, X.; Sun, S. PMicroRNA-124a regulates LPS-induced septic cardiac dysfunction by targeting STX2. Biotechnol. Lett. 2017, 39, 1335–1342. [Google Scholar] [CrossRef]
- Yan, J.; Yang, F.; Wang, D.; Lu, Y.; Liu, L.; Wang, Z. MicroRNA-217 modulates inflammation, oxidative stress, and lung injury in septic mice via SIRT1. Free Radic. Res. 2021, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, X.; Ha, T.; Gao, M.; Liu, L.; Wang, R.; Yu, K.; Kalbfleisch, J.H.; Kao, R.L.; Williams, D.L.; et al. MicroRNA-125b Prevents Cardiac Dysfunction in Polymicrobial Sepsis by Targeting TRAF6-Mediated Nuclear Factor kappaB Activation and p53-Mediated Apoptotic Signaling. J. Infect. Dis. 2016, 214, 1773–1783. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhu, G.; Jiao, T.; Shao, F. Effects of circular RNA Ttc3/miR-148a/Rcan2 axis on inflammation and oxidative stress in rats with acute kidney injury induced by sepsis. Life Sci. 2021, 272, 119233. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; An, R.; Wang, H.; Chen, L.; Shen, Y.; Cai, W.; Zhu, W. Oxidative stress-related circulating miRNA-27a is a potential biomarker for diagnosis and prognosis in patients with sepsis. BMC Immunol. 2022, 23, 14. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, H.; Yang, Z.; Lin, X.; Zhao, F.; Huang, Y.; Wang, Y.; Yang, X.; Li, H.; Wang, L.; et al. Long non-coding RNA MALAT1 silencing elevates microRNA-26a-5p to ameliorate myocardial injury in sepsis by reducing regulator of calcineurin 2. Arch. Biochem. Biophys. 2022, 715, 109047. [Google Scholar] [CrossRef]
- He, Z.; Wang, H.; Yue, L. Endothelial progenitor cells-secreted extracellular vesicles containing microRNA-93-5p confer protection against sepsis-induced acute kidney injury via the KDM6B/H3K27me3/TNF-alpha axis. Exp. Cell Res. 2020, 395, 112173. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Wang, J.; Miao, H. MiR-107 induces TNF-alpha secretion in endothelial cells causing tubular cell injury in patients with septic acute kidney injury. Biochem. Biophys. Res. Commun. 2017, 483, 45–51. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Ding, X.; Zhang, X.; Tang, J.; Lin, H. Plasma extracellular vesicle delivery of miR-210-3p by targeting ATG7 to promote sepsis-induced acute lung injury by regulating autophagy and activating inflammation. Exp. Mol. Med. 2021, 53, 1180–1191. [Google Scholar] [CrossRef]
- Xu, J.; Feng, Y.; Jeyaram, A.; Jay, S.M.; Zou, L.; Chao, W. Circulating Plasma Extracellular Vesicles from Septic Mice Induce Inflammation via MicroRNA- and TLR7-Dependent Mechanisms. J. Immunol. 2018, 201, 3392–3400. [Google Scholar] [CrossRef] [PubMed]
- Mandelbaum, J.; Rollins, N.; Shah, P.; Bowman, D.; Lee, J.Y.; Tayber, O.; Bernard, H.; LeRoy, P.; Li, P.; Koenig, E.; et al. Identification of a lung cancer cell line deficient in atg7-dependent autophagy. Autophagy 2015. [Google Scholar] [CrossRef] [PubMed]
- Avila-Bonilla, R.G.; Yocupicio-Monroy, M.; Marchat, L.A.; De Nova-Ocampo, M.A.; Del Angel, R.M.; Salas-Benito, J.S. Analysis of the miRNA profile in C6/36 cells persistently infected with dengue virus type 2. Virus Res. 2017, 232, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, M.; Zhang, S. Identification of key miRNA-mRNA pairs in septic mice by bioinformatics analysis. Mol. Med. Rep. 2019, 20, 3858–3866. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, Y.; Jiang, R.; Nie, C.; Zeng, Z.; Zhao, N.; Huang, C.; Shao, Q.; Ding, C.; Qing, C.; et al. miR-132 inhibits lipopolysaccharide-induced inflammation in alveolar macrophages by the cholinergic anti-inflammatory pathway. Exp. Lung Res. 2015, 41, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sun, Z.; Yan, W.; Zhu, Y.; Lin, Y.; Chen, J.; Shen, B.; Wang, J. Identification of microRNA as sepsis biomarker based on miRNAs regulatory network analysis. Biomed. Res. Int. 2014, 2014, 594350. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.J.; Guo, C.Y.; Yin, H.J.; Liu, Y.; Shi, D.Z. [Effect of activating blood circulation or activating blood circulation and detoxication on platelet activation, inflammation, and coagulation status in acute myocardial infarction rats]. Zhongguo Zhong Xi Yi Jie He Za Zhi 2014, 34, 1329–1334. [Google Scholar] [PubMed]
- Wu, J.; Li, X. Plasma Tumor Necrosis Factor-alpha (TNF-alpha) Levels Correlate with Disease Severity in Spastic Diplegia, Triplegia, and Quadriplegia in Children with Cerebral Palsy. Med. Sci. Monit. 2015, 21, 3868–3874. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, P.; Wei, Y.; Piao, H.L.; Wang, W.; Maddika, S.; Wang, M.; Chen, D.; Sun, Y.; Hung, M.C.; et al. Deubiquitylation and stabilization of PTEN by USP13. Nat. Cell Biol. 2013, 15, 1486–1494. [Google Scholar] [CrossRef]
- Lu, C.; Zhou, D.; Wang, Q.; Liu, W.; Yu, F.; Wu, F.; Chen, C. Crosstalk of MicroRNAs and Oxidative Stress in the Pathogenesis of Cancer. Oxid. Med. Cell Longev. 2020, 2020, 2415324. [Google Scholar] [CrossRef]
- Xiang, C.; Cui, S.P.; Ke, Y. MiR-144 inhibits cell proliferation of renal cell carcinoma by targeting MTOR. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Han, X.; Qi, X.; Jin, X.; Li, X. TUG1 promotes osteosarcoma tumorigenesis by upregulating EZH2 expression via miR-144-3p. Int. J. Oncol. 2017, 51, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Mitchelson, K.R.; Qin, W.Y. Roles of the canonical myomiRs miR-1, -133 and -206 in cell development and disease. World J. Biol. Chem. 2015, 6, 162–208. [Google Scholar] [CrossRef] [PubMed]
- Essandoh, K.; Li, Y.; Huo, J.; Fan, G.C. MiRNA-Mediated Macrophage Polarization and its Potential Role in the Regulation of Inflammatory Response. Shock 2016, 46, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Y.; Wang, Z.; Wang, Z.H.; Jiang, X.G.; Lu, W.H. Inhibition of miR-155 alleviates sepsis-induced inflammation and intestinal barrier dysfunction by inactivating NF-kappaB signaling. Int. Immunopharmacol. 2021, 90, 107218. [Google Scholar] [CrossRef]
- Pandey, R.K.; Sundar, S.; Prajapati, V.K. Differential Expression of miRNA Regulates T Cell Differentiation and Plasticity During Visceral Leishmaniasis Infection. Front. Microbiol. 2016, 7, 206. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, L.; Huang, H.; Liu, S.; Liang, Y.; Xu, L.; Li, S.; Cheng, Y.; Tang, W. Serum miR-126-3p level is down-regulated in sepsis patients. Int. J. Clin. Exp. Pathol. 2018, 11, 2605–2612. [Google Scholar]
- Su, J.; Ding, L. Upregulation of miR-126 inhibits podocyte injury in sepsis via EGFL6/DKC1 signaling pathway. Mol. Med. Rep. 2021, 23, 373. [Google Scholar] [CrossRef]
- Nong, A.; Li, Q.; Huang, Z.; Xu, Y.; He, K.; Jia, Y.; Cen, Z.; Liao, L.; Huang, Y. MicroRNA miR-126 attenuates brain injury in septic rats via NF-kappaB signaling pathway. Bioengineered 2021, 12, 2639–2648. [Google Scholar] [CrossRef]
- Luo, H.; Chen, D.; Li, R.; Li, R.; Teng, Y.; Cao, Y.; Zou, X.; Wang, W.; Zhou, C. Genetically engineered CXCR4-modified exosomes for delivery of miR-126 mimics to macrophages alleviate periodontitis. J. Nanobiotechnol. 2023, 21, 116. [Google Scholar] [CrossRef] [PubMed]
- Shafei, S.; Khanmohammadi, M.; Ghanbari, H.; Nooshabadi, V.T.; Tafti, S.H.A.; Rabbani, S.; Kasaiyan, M.; Basiri, M.; Tavoosidana, G. Effectiveness of exosome mediated miR-126 and miR-146a delivery on cardiac tissue regeneration. Cell Tissue Res. 2022, 390, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Endo-Takahashi, Y.; Negishi, Y.; Nakamura, A.; Ukai, S.; Ooaku, K.; Oda, Y.; Sugimoto, K.; Moriyasu, F.; Takagi, N.; Suzuki, R.; et al. Systemic delivery of miR-126 by miRNA-loaded Bubble liposomes for the treatment of hindlimb ischemia. Sci. Rep. 2014, 4, 3883. [Google Scholar] [CrossRef] [PubMed]
- Jones Buie, J.N.; Zhou, Y.; Goodwin, A.J.; Cook, J.A.; Vournakis, J.; Demcheva, M.; Broome, A.M.; Dixit, S.; Halushka, P.V.; Fan, H. Application of Deacetylated Poly-N-Acetyl Glucosamine Nanoparticles for the Delivery of miR-126 for the Treatment of Cecal Ligation and Puncture-Induced Sepsis. Inflammation 2019, 42, 170–184. [Google Scholar] [CrossRef]
- Dumitru, C.D.; Ceci, J.D.; Tsatsanis, C.; Kontoyiannis, D.; Stamatakis, K.; Lin, J.H.; Patriotis, C.; Jenkins, N.A.; Copeland, N.G.; Kollias, G.; et al. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 2000, 103, 1071–1083. [Google Scholar] [CrossRef]
- Owens, D.M.; Keyse, S.M. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 2007, 26, 3203–3213. [Google Scholar] [CrossRef]
- Klein, J.D.; Wang, X.H. Electrically stimulated acupuncture increases renal blood flow through exosome-carried miR-181. Am. J. Physiol. Renal Physiol. 2018, 315, F1542–F1549. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shi, X.; Li, M.; Chen, S.; Gu, Q.; Zheng, J.; Li, D.; Wu, S.; Yang, H.; Li, X. MicroRNA-181a-2-3p shuttled by mesenchymal stem cell-secreted extracellular vesicles inhibits oxidative stress in Parkinson’s disease by inhibiting EGR1 and NOX4. Cell Death Discov. 2022, 8, 33. [Google Scholar] [CrossRef]
- Wang, J.; Song, J.; Li, Y.; Shao, J.; Xie, Z.; Sun, K. Down-regulation of LncRNA CRNDE aggravates kidney injury via increasing MiR-181a-5p in sepsis. Int. Immunopharmacol. 2020, 79, 105933. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, H. MicroRNA-181a-5p Regulates Inflammatory Response of Macrophages in Sepsis. Open Med. 2019, 14, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhi, D.; Lin, J.; Liu, P.; Wang, Y.; Duan, M. miR-181a-5p Inhibits Pyroptosis in Sepsis-Induced Acute Kidney Injury through Downregulation of NEK7. J. Immunol. Res. 2022, 2022, 1825490. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, G.; Peng, Z. MicroRNA-191-5p diminished sepsis-induced acute kidney injury through targeting oxidative stress responsive 1 in rat models. Biosci. Rep. 2019, 39, BSR20190548. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hu, Q.C.; Wang, J.P.; Ren, Q.Q.; Wang, X.P.; Luoreng, Z.M.; Wei, D.W.; Ma, Y. RNA-Seq Reveals the Role of miR-29c in Regulating Inflammation and Oxidative Stress of Bovine Mammary Epithelial Cells. Front. Vet. Sci. 2022, 9, 865415. [Google Scholar] [CrossRef]
- Abplanalp, W.T.; Fischer, A.; John, D.; Zeiher, A.M.; Gosgnach, W.; Darville, H.; Montgomery, R.; Pestano, L.; Allee, G.; Paty, I.; et al. Efficiency and Target Derepression of Anti-miR-92a: Results of a First in Human Study. Nucleic Acid Ther. 2020, 30, 335–345. [Google Scholar] [CrossRef]
| MiRNA Treatment of Interest | Species | Model | Effect | Change in Mortality | Clinical Relevance | Ref |
|---|---|---|---|---|---|---|
| MiR-155 mimic | Mouse (C57BL/6J) | CLP | Protected against cardiac dysfunction in late sepsis | Improved survival | Yes | [14] |
| MiR-140 siRNA | Mouse (BALB/c) | LPS (15 mg/kg) | Restored Wnt11 expression | Did not report | No evidence | [15] |
| MiR-203 mimic or inhibitor | Mouse (Kunming) | CLP | Reduced VNN1 expression | Did not report | Shown computationally | [16] |
| MiR-34b-3p agomiR | Mouse (C57BL/6N) | CLP | Reduced TNF-α, IL-1β, and IL-6 | Improved survival | No evidence | [17] |
| MiR-214 mimic or inhibitor adenovirus | Mouse (Kunming) | CLP | Mimic reduced GLP-1R, AMPK, oxidative stress, and inflammation | Did not report | No evidence | [18] |
| MiR-126 overexpression vector | Mouse (C57BL/6J) | CLP, LPS (40 mg/kg) | Reduced proinflammatory mediators | Improved survival | Yes | [19] |
| MiR-193b-5p inhibitor | Mouse (C57BL/6) | LPS (10 mg/kg) | Attenuated decreased occludin expression | Improved survival | Yes | [20] |
| MiR-27a-5p inhibitor | Mouse (C57BL/6) | LPS (dose unspecified) | Mitigated cellular infiltration but not protein and IgM leakage into alveolar space | Improved survival | No evidence | [21] |
| MiR-103a-3p agomiR lentivirus | Mouse (C57BL/6) | LPS (1 mg/kg) | Downregulated HMGB1, leading to attenuation of inflammatory response | Improved survival | Yes | [22] |
| MiR-101 agomiR | Rat (Sprague Dawley) | CLP | Improved left ventricle ejection fraction | Did not report | Yes | [23] |
| MiR-340-5p overexpression AAV | Mouse (C57BL/6J) | LPS (10 mg/kg) | Reduced myocardial oxidative stress injury and MyD88 expression | Did not report | No evidence | [24] |
| MiR-126 mimic or inhibitor | Rat (Sprague Dawley) | CLP | Mimic suppressed adhesion molecule expression and reduced immune cell accumulation in the myocardium | Mimic improved survival, inhibitor worsened survival | Shown in another study | [25] |
| MiR-210-3p inhibitor adenovirus | Mouse (strain unspecified) | CLP | Improved vascular density and autophagosome formation, increased ATG7 expression | Improved survival | Yes | [26] |
| MiR-21 mimic | Rat (Wistar) | CLP | Reduced sepsis-induced kidney cell apoptosis via the PTEN/PI3K/AKT signaling pathway | Did not report | No evidence | [27] |
| MiR-579-3p inhibitor lentivirus | Mouse (C57BL/6) | CLP | Enhanced SIRT1 expression, leading to reduced weight loss and renal injuries | Improved survival | No evidence | [28] |
| MiR-133a antagomiR | Mouse (C57BL/6J) | CLP | Reduced organ injury and inflammation, potentially through SIRT1 rescue | Did not report | Yes | [29] |
| MiR-93-5p antagomiR | Mouse (C57BL/6) | CLP | Reduced inflammatory monocytes and increased circulating effector memory T cells, especially of the CD4+ subset | Improved survival | Yes | [30] |
| MiR-130-3p mimic | Mouse (C57BL/6) | CLP | Reduced eCIRP-induced TNF-α and IL-6 proteins | Improved survival | No evidence | [31] |
| MiR-181-5p agomiR lentivirus | Mouse (C57BL/6J) | CLP | Alleviated sepsis-induced systemic inflammatory disease, may function as an HMGB1 antagonist | Improved survival | No evidence | [32] |
| MiR-19a-3p antagomiR | Mouse (C57BL/6) | LPS (1 mg/kg) | Reduced lung damage | Did not report | Reported in Chen et al. (2019) | [33] |
| MiR-21 mimic | Mouse (C57BL/6) | CLP | Ameliorated hyperoside-induced negative cardiac effects | Did not report | No evidence | [34] |
| MiR-22-3p mimic adenovirus | Rat (Sprague Dawley) | CLP | Protected against sepsis-induced acute kidney injury, possibly by repressing PTEN | Did not report | Yes | [35] |
| MiR-146a-expressing plasmid | Mouse (C57BL/6) | CLP | Prevented excessive inflammation and sepsis-induced multiple organ injury | Improved survival | No evidence | [36] |
| MiR-29c-3p antagomiR | Rat (Sprague Dawley) | CLP | Reduced sepsis-induced cardiac dysfunction and inflammatory response | Did not report | Yes | [37] |
| MiR-205 agonist | Rat (Sprague Dawley) | CLP | Reduced kidney injury and protein expression of HMGB1 and PTEN | Did not report | No evidence | [38] |
| MiR-182-5p inhibitor lentivirus | Mouse (C57BL/6J) | S. aureus pneumonia | Repressed intestinal epithelial cell apoptosis and rescued the cell viability | Did not report | No evidence | [39] |
| MiR-23a-3p overexpression AAV | Mouse (C57BL/6) | CLP | Ameliorated sepsis-induced acute kidney injury, targeted FKBP5 | Did not report | Yes | [40] |
| MiR-145 agomiR | Mouse (strain unspecified) | LPS (4 mL/kg) | Attenuated LPS-induced sepsis | Improved survival | No evidence | [41] |
| MiR-106b-5p inhibitor vector | Mouse (C57BL/6) | CLP | Reduced the cardioprotective effects of matrine administration | Did not report | No evidence | [42] |
| MiR-802 mimic | Mouse (BALB/c) | LPS (50 mg/kg) | Protected against LPS-induced acute lung injury by downregulating Peli2 | Did not report | No evidence | [43] |
| MiR-926-3p inhibitor | Mouse (C57BL/6) | CLP | Enhanced autophagy through regulation of the mTOR signaling pathway | Did not report | Yes | [44] |
| MiR-144-3p agomiR or antagomiR | Mouse (BALB/c) | LPS (10 mg/kg) | AntagomiR alleviated inflammation and cell apoptosis induced by LPS | Did not report | Yes | [45] |
| MiR-130b agomiR | Mouse (C57BL/6J) | LPS (40 mg/kg) | Attenuated LPS-induced vascular inflammation | Did not report | No evidence | [46] |
| MiR-129-5p agomiR | Mouse (C57BL/6) | CLP | Attenuated inflammatory response, apoptosis, lung wet/dry weight ratio, and myeloperoxidase activity induced by CLP | Did not report | No evidence | [47] |
| MiR-199a antagomiR | Mouse (C57BL/6) | Pseudomonas aeruginosa burn | Protected lung tissue against sepsis-induced ARDS by upregulating SIRT1 | Did not report | No evidence | [48] |
| MiR-181a-2-3p agomiR or antagomiR | Mouse (C57BL/6J) | CLP | AgomiR alleviated the inflammatory response and cell apoptosis by upregulating GJB2 expression | Did not report | Shown computationally | [49] |
| MiR-335 precursor or inhibitor | Mouse (Kunming) | CLP | Ameliorated myocardial injury following sepsis | Did not report | No evidence | [50] |
| MiR-34a agomiR or antagomiR | Mouse (C57BL/6) | CLP | AntagomiR reduced lung injury, inflammation, and oxidative stress, potentially through SIRT1 and ATG4B rescue | AgomiR worsened survival, antagomiR improved survival | No evidence | [51] |
| MiR-21 and miR181b antagomiR combination therapy | Mouse (BALB/c) | CLP | Restored Gr1+ CD11b+ cell differentiation and maturation | Improved survival | No evidence | [52] |
| MiR-146a overexpression lentivirus | Mouse (C57BL/6) | CLP | Attenuated sepsis-induced cardiac dysfunction by preventing NF-κB activation, inflammatory cell infiltration, and inflammatory cytokine production via targeting of IRAK and TRAF6 | Improved survival | No evidence | [36] |
| MiR-129-5p agomiR | Mouse (C57BL/6J) | LPS (20 mg/kg) | Reduced podocyte damage, inflammation, and apoptosis; targets the HMGB1/TLR2/TLR4/NF-κB axis | Improved survival | No evidence | [53] |
| MiR-124a agomiR or antagomiR | Mouse (BALB/c) | LPS (5 mg/kg) | AgomiR reduced LPS-induced cardiac dysfunction and apoptosis; targets STX2 | Did not report | No evidence | [54] |
| MiR-217 agomiR or antagomiR | Mouse (C57BL/6) | CLP | AntagomiR demonstrated anti-inflammatory and anti-oxidant effects, beneficial effects reversed with SIRT1 inhibition | AgomiR worsened survival, antagomiR improved survival | No evidence | [55] |
| MiR-125b overexpression lentivirus | Mouse (C57BL/6) | CLP | Attenuated sepsis-induced cardiac dysfunction | Improved survival | No evidence | [56] |
| MiR-148a agomiR or antagomiR | Rat (Sprague Dawley) | CLP | AgomiR rescued CIRC-Ttc3 in sepsis-induced acute kidney injury | Did not report | No evidence | [57] |
| MiR-27a antagomiR | Mouse (C57BL/6) | LPS (5 mg/kg) | Reduced the beneficial effects of paclitaxel in reducing sepsis-induced liver injury | Worsened survival advantage conferred by paclitaxel | No evidence | [58] |
| MiR-26a-5p agomiR or antagomiR | Mouse (C57BL/6J) | LPS (10 mg/kg) | AgomiR suppressed LPS-induced inflammation and apoptosis of cardiomyocytes | Did not report | No evidence | [59] |
| MiR-93-5p mimic or inhibitor EVs | Mouse (C57BL/6) | CLP | Mimic EVs attenuated multiple organ injury, vascular leakage, inflammation, and apoptosis | Inhibitor worsened survival | Shown in previous work | [60] |
| MiR-107 inhibitor | Mouse (C57BL/6J) | LPS (8 mg/kg) | Reduced sepsis-induced acute kidney injury, potentially through DUSP7 rescue | Did not report | Yes | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ektesabi, A.M.; Simone, J.; Vaswani, C.; Tan, G.W.; Wang, Y.; Pavelick, J.L.; Wu, X.; Tai, J.; Gupta, S.; Tsoporis, J.N.; et al. Pre-Clinical Studies of MicroRNA-Based Therapies for Sepsis: A Scoping Review. Oxygen 2024, 4, 20-36. https://doi.org/10.3390/oxygen4010002
Ektesabi AM, Simone J, Vaswani C, Tan GW, Wang Y, Pavelick JL, Wu X, Tai J, Gupta S, Tsoporis JN, et al. Pre-Clinical Studies of MicroRNA-Based Therapies for Sepsis: A Scoping Review. Oxygen. 2024; 4(1):20-36. https://doi.org/10.3390/oxygen4010002
Chicago/Turabian StyleEktesabi, Amin M., Julia Simone, Chirag Vaswani, Greaton W. Tan, Yanbo Wang, Jacqueline L. Pavelick, Xiao Wu, Janice Tai, Sahil Gupta, James N. Tsoporis, and et al. 2024. "Pre-Clinical Studies of MicroRNA-Based Therapies for Sepsis: A Scoping Review" Oxygen 4, no. 1: 20-36. https://doi.org/10.3390/oxygen4010002
APA StyleEktesabi, A. M., Simone, J., Vaswani, C., Tan, G. W., Wang, Y., Pavelick, J. L., Wu, X., Tai, J., Gupta, S., Tsoporis, J. N., & dos Santos, C. C. (2024). Pre-Clinical Studies of MicroRNA-Based Therapies for Sepsis: A Scoping Review. Oxygen, 4(1), 20-36. https://doi.org/10.3390/oxygen4010002








