An Interplay of Gases: Oxygen and Hydrogen in Biological Systems
Abstract
1. Introduction
2. Solubility of Oxygen and Hydrogen in Water
3. Oxyhydrogen
4. Is there an Effect of H2 on Haem Groups and a Disruption of Function?
5. Direct Interaction of H2 with Proteins
6. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hancock, J.T. A brief history of oxygen: 250 years on. Oxygen 2022, 2, 31–39. [Google Scholar] [CrossRef]
- Renger, G.; Hanssum, B. Oxygen detection in biological systems. Photosynth. Res. 2009, 102, 487–498. [Google Scholar] [CrossRef]
- Diguiseppi, J.; Fridovich, I.; McCord, J.M. The toxicology of molecular oxygen. CRC Crit. Rev. Toxicol. 1984, 12, 315–342. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Venditti, P. Evolution of the knowledge of free radicals and other oxidants. Oxid. Med. Cell. Longev. 2020, 2020, 9829176. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.T.; LeBaron, T.W. The early history of hydrogen and other gases in respiration and biological systems: Revisiting Beddoes, Cavallo, and Davy. Oxygen 2023, 3, 102–119. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Ohno, K.; Hancock, J.T. The on/off history of hydrogen in medicine: Will the interest persist this time around? Oxygen 2023, 3, 143–162. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688. [Google Scholar] [CrossRef] [PubMed]
- Danilova, D.A.; Brichkin, Y.D.; Medvedev, A.P.; Pichugin, V.V.; Fedorov, S.A.; Taranov, E.V.; Nazarov, E.I.; Ryazanov, M.V.; Bolshukhin, G.V.; Deryugina, A.V. Application of molecular hydrogen in heart surgery under cardiopulmonary bypass. Mod. Med. Technol. 2021, 13, 71–75. [Google Scholar] [CrossRef]
- Kalocayova, B.; Kura, B.; Vlkovicova, J.; Snurikova, D.; Vrbjar, N.; Frimmel, K.; Hudec, V.; Ondrusek, M.; Gasparovic, I.; Sramaty, R.; et al. Molecular hydrogen: Prospective treatment strategy of kidney damage after cardiac surgery. Can. J. Physiol. Pharmacol. 2023, 101, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Zhang, J.; Zhang, Y. Role of molecular hydrogen in ageing and ageing-related diseases. Oxid. Med. Cell. Longev. 2022, 2022, 2249749. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Su, N.; Cai, J.; Shen, Z.; Cui, J. Hydrogen-rich water enhances cadmium tolerance in Chinese cabbage by reducing cadmium uptake and increasing antioxidant capacities. J. Plant Physiol. 2015, 175, 174–182. [Google Scholar] [CrossRef]
- Cui, W.; Yao, P.; Pan, J.; Dai, C.; Cao, H.; Chen, Z.; Zhang, S.; Xu, S.; Shen, W. Transcriptome analysis reveals insight into molecular hydrogen-induced cadmium tolerance in alfalfa: The prominent role of sulfur and (homo) glutathione metabolism. BMC Plant Biol. 2020, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Ding, X.W.; Jiang, R.; Ouyang, P.L.; Gui, J.; Feng, L.; Yang, L.; Song, L.H. Effects of hydrogen-rich water on the nutrient composition and antioxidative characteristics of sprouted black barley. Food Chem. 2019, 299, 125095. [Google Scholar] [CrossRef] [PubMed]
- Alwazeer, D.; Özkan, N. Incorporation of hydrogen into the packaging atmosphere protects the nutritional, textural and sensorial freshness notes of strawberries and extends shelf life. J. Food Sci. Technol. 2022, 59, 3951–3964. [Google Scholar] [CrossRef] [PubMed]
- Yun, Z.; Gao, H.; Chen, X.; Duan, X.; Jiang, Y. The role of hydrogen water in delaying ripening of banana fruit during postharvest storage. Food Chem. 2022, 373, 131590. [Google Scholar] [CrossRef]
- Shin, D.; Cho, E.S.R.; Bang, H.T.; Shim, K.S. Effects of oxygenated or hydrogenated water on growth performance, blood parameters, and antioxidant enzyme activity of broiler chickens. Poult. Sci. 2016, 95, 2679–2684. [Google Scholar] [CrossRef]
- Qi, D.D.; Ding, M.Y.; Wang, T.; Hayat, M.A.; Liu, T.; Zhang, J.T. The therapeutic effects of oral intake of hydrogen rich water on cutaneous wound healing in dogs. Vet. Sci. 2021, 8, 264. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, M.; Li, Y.; Zhang, Z.; Chen, M.; Liu, T.; Zhang, J.; Shan, A. Therapeutic effect of hydrogen injected subcutaneously on onion poisoned dogs. J. Vet. Res. 2017, 61, 527. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Zhang, X.; Ungerfeld, E.M.; Long, D.; Mao, H.; Jiao, J.; Beauchemin, K.A.; Tan, Z. Molecular hydrogen generated by elemental magnesium supplementation alters rumen fermentation and microbiota in goats. Br. J. Nutr. 2017, 118, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Kashio, A.; Sakamoto, T.; Suzukawa, K.; Kakigi, A.; Yamasoba, T. Hydrogen in drinking water attenuates noise-induced hearing loss in guinea pigs. Neurosci. Lett. 2011, 487, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhao, C.; Che, N.; Jing, L.; Ge, R. Hydrogen-rich saline attenuates eosinophil activation in a guinea pig model of allergic rhinitis via reducing oxidative stress. J. Inflamm. 2017, 14, 1–12. [Google Scholar] [CrossRef]
- Tsubone, H.; Hanafusa, M.; Endo, M.; Manabe, N.; Hiraga, A.; Ohmura, H.; Aida, H. Effect of treadmill exercise and hydrogen-rich water intake on serum oxidative and anti-oxidative metabolites in serum of thoroughbred horses. J. Equine Sci. 2013, 24, 1–8. [Google Scholar] [CrossRef]
- Yamazaki, M.; Kusano, K.; Ishibashi, T.; Kiuchi, M.; Koyama, K. Intravenous infusion of H2-saline suppresses oxidative stress and elevates antioxidant potential in Thoroughbred horses after racing exercise. Sci. Rep. 2015, 5, 15514. [Google Scholar] [CrossRef]
- Varga, V.; Németh, J.; Oláh, O.; Tóth-Szűki, V.; Kovács, V.; Remzső, G.; Domoki, F. Molecular hydrogen alleviates asphyxia-induced neuronal cyclooxygenase-2 expression in newborn pigs. Acta Pharmacol. Sin. 2018, 39, 1273–1283. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, Q.; Zheng, W.; Yao, W. Morphological and molecular response of small intestine to lactulose and hydrogen-rich water in female piglets fed Fusarium mycotoxins contaminated diet. J. Anim. Sci. Biotechnol. 2019, 10, 9. [Google Scholar] [CrossRef]
- Su, J.; Yang, X.; Shao, Y.; Chen, Z.; Shen, W. Molecular hydrogen–induced salinity tolerance requires melatonin signalling in Arabidopsis thaliana. Plant Cell Environ. 2021, 44, 476–490. [Google Scholar] [CrossRef]
- Xie, Y.; Mao, Y.; Lai, D.; Zhang, W.; Shen, W. H2 enhances Arabidopsis salt tolerance by manipulating ZAT10/12-mediated antioxidant defence and controlling sodium exclusion. PLoS ONE 2012, 7, e49800. [Google Scholar] [CrossRef]
- Jiang, K.; Kuang, Y.; Feng, L.; Liu, Y.; Wang, S.; Du, H.; Shen, W. Molecular hydrogen maintains the storage quality of Chinese chive through improving antioxidant capacity. Plants 2021, 10, 1095. [Google Scholar] [CrossRef]
- Liu, S.; Zha, Z.; Chen, S.; Tang, R.; Zhao, Y.; Lin, Q.; Duan, Y.; Wang, K. Hydrogen-rich water alleviates chilling injury-induced lignification of kiwifruit by inhibiting peroxidase activity and improving antioxidant system. J. Sci. Food Agric. 2023, 103, 2675–2680. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, J.; Zhao, Z.; Kong, L.; Lou, W.; Zhang, T.; Jing, D.; Yu, J.; Shu, Z.; Huang, L.; et al. Molecular hydrogen increases quantitative and qualitative traits of rice grain in field trials. Plants 2021, 10, 2331. [Google Scholar] [CrossRef]
- Russell, G.; Zulfiqar, F.; Hancock, J.T. Hydrogenases and the role of molecular hydrogen in plants. Plants 2020, 9, 1136. [Google Scholar] [CrossRef]
- Marreiros, B.C.; Batista, A.P.; Duarte, A.M.; Pereira, M.M. A missing link between complex I and group 4 membrane-bound [NiFe] hydrogenases. Biochim. Biophys. Acta (BBA) Bioenerg. 2013, 1827, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Greening, C.; Biswas, A.; Carere, C.R.; Jackson, C.J.; Taylor, M.C.; Stott, M.B.; Cook, G.M.; Morales, S.E. Genomic and metagenomic surveys of hydrogenase distribution indicate H2 is a widely utilised energy source for microbial growth and survival. ISME J. 2016, 10, 761–777. [Google Scholar] [CrossRef]
- Petersen, J.M.; Zielinski, F.U.; Pape, T.; Seifert, R.; Moraru, C.; Amann, R.; Hourdez, S.; Girguis, P.R.; Wankel, S.D.; Barbe, V.; et al. Hydrogen is an energy source for hydrothermal vent symbioses. Nature 2011, 476, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Zgonnik, V. The occurrence and geoscience of natural hydrogen: A comprehensive review. Earth-Sci. Rev. 2020, 203, 103140. [Google Scholar] [CrossRef]
- Jafta, N.; Magagula, S.; Lebelo, K.; Nkokha, D.; Mochane, M.J. The production and role of hydrogen-rich water in medical applications. In Applied Water Science Volume 1: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2021; pp. 273–298. [Google Scholar]
- Liu, S.; Oshita, S.; Thuyet, D.Q.; Saito, M.; Yoshimoto, T. Antioxidant activity of hydrogen nanobubbles in water with different reactive oxygen species both in vivo and in vitro. Langmuir 2018, 34, 11878–11885. [Google Scholar] [CrossRef]
- Kim, S.A.; Jong, Y.C.; Kang, M.S.; Yu, C.J. Antioxidation activity of molecular hydrogen via protoheme catalysis in vivo: An insight from ab initio calculations. J. Mol. Model. 2022, 28, 287. [Google Scholar] [CrossRef]
- Jin, Z.; Zhao, P.; Gong, W.; Ding, W.; He, Q. Fe-porphyrin: A redox-related biosensor of hydrogen molecule. Nano Res. 2023, 16, 2020–2025. [Google Scholar] [CrossRef]
- Ohta, S. Molecular hydrogen may activate the transcription factor Nrf2 to alleviate oxidative stress through the hydrogen-targeted porphyrin. Aging Pathobiol. Ther. 2023, 5, 25–32. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, W.; Qi, F.; Cui, W.; Xie, Y.; Shen, W. Hydrogen-rich water regulates cucumber adventitious root development in a heme oxygenase-1/carbon monoxide-dependent manner. J. Plant Physiol. 2014, 171, 1–8. [Google Scholar] [CrossRef]
- Peck, H.D. The ATP-dependent reduction of sulfate with hydrogen in extracts of Desulfovibrio desulfuricans. Proc. Natl. Acad. Sci. USA 1959, 45, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.T.; LeBaron, T.W.; Russell, G. Molecular hydrogen: Redox reactions and possible biological interactions. React. Oxyg. Species 2021, 11, m17–m25. [Google Scholar] [CrossRef]
- Hancock, J.T.; Hancock, T.H. Hydrogen gas, ROS metabolism, and cell signaling: Are hydrogen spin states important? React. Oxyg. Species 2018, 6, 389–395. [Google Scholar] [CrossRef]
- Hancock, J.T.; Russell, G.; Craig, T.J.; May, J.; Morse, H.R.; Stamler, J.S. Understanding Hydrogen: Lessons to be learned from physical interactions between the inert gases and the globin superfamily. Oxygen 2022, 2, 578–590. [Google Scholar] [CrossRef]
- Hancock, J.T. Are protein cavities and pockets commonly used by redox active signalling molecules? Plants 2023, 12, 2594. [Google Scholar] [CrossRef]
- Westall, F.; Brack, A. The importance of water for life. Space Sci. Rev. 2018, 214, 50. [Google Scholar] [CrossRef]
- Russell, G.; Nenov, A.; Hancock, J. Oxy-hydrogen gas: The rationale behind its use as a novel and sustainable treatment for COVID-19 and other respiratory diseases. Eur. Med. J. 2021, 21-00027. [Google Scholar] [CrossRef]
- Xing, W.; Yin, M.; Lv, Q.; Hu, Y.; Liu, C.; Zhang, J. Oxygen solubility, diffusion coefficient, and solution viscosity. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–31. [Google Scholar]
- Dmitry Petrov, D.; Panina, E. Characteristics of hydrogen rich water at different stages of electrolysis. BIO Web Conf. 2024, 82, 01006. [Google Scholar]
- Bjurstedt, H.; Severin, G. The prevention of decompression sickness and nitrogen narcosis by the use of hydrogen as a substitute for nitrogen, the Arne Zetterstrom method for deep-sea diving. Mil. Surg. 1948, 103, 107–116. [Google Scholar] [CrossRef]
- WorldData.info. Available online: https://www.worlddata.info/average-bodyheight.php (accessed on 11 December 2023).
- Guan, W.J.; Wei, C.H.; Chen, A.L.; Sun, X.C.; Guo, G.Y.; Zou, X.; Shi, J.D.; Lai, P.Z.; Zheng, Z.G.; Zhong, N.S. Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with Coronavirus disease 2019 in a recent multicenter, open-label clinical trial. J. Thorac. Dis. 2020, 12, 3448. [Google Scholar] [CrossRef]
- Zheng, Z.G.; Sun, W.Z.; Hu, J.Y.; Jie, Z.J.; Xu, J.F.; Cao, J.; Song, Y.L.; Wang, C.H.; Wang, J.; Zhao, H.; et al. Hydrogen/oxygen therapy for the treatment of an acute exacerbation of chronic obstructive pulmonary disease: Results of a multicenter, randomized, double-blind, parallel-group controlled trial. Respir. Res. 2021, 22, 149. [Google Scholar] [CrossRef]
- Zhou, Z.Q.; Zhong, C.H.; Su, Z.Q.; Li, X.Y.; Chen, Y.; Chen, X.B.; Tang, C.L.; Zhou, L.Q.; Li, S.Y. Breathing hydrogen-oxygen mixture decreases inspiratory effort in patients with tracheal stenosis. Respiration 2018, 97, 42–51. [Google Scholar] [CrossRef]
- Handajani, Y.S.; Tenggara, R.; Suyatna, F.D.; Surjadi, C.; Widjaja, N.T. The effect of oxygenated water in Diabetes Mellitus. Med. J. Indones. 2009, 18, 102–107. [Google Scholar] [CrossRef]
- Gruber, R.; Axmann, S.; Schoenberg, M.H. The influence of oxygenated water on the immune status, liver enzymes, and the generation of oxygen radicals: A prospective, randomised, blinded clinical study. Clin. Nutr. 2005, 24, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Kiyoi, T.; Liu, S.; Takemasa, E.; Nakaoka, H.; Hato, N.; Mogi, M. Constitutive hydrogen inhalation prevents vascular remodeling via reduction of oxidative stress. PLoS ONE 2020, 15, 0227582. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D.; Neish, A.S. Nox enzymes and new thinking on reactive oxygen: A double-edged sword revisited. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.; Fieschi, F. NADPH oxidases (NOX): An overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Piantadosi, C.A. “Oxygenated” water and athletic performance. Br. J. Sports Med. 2006, 40, 740–741. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Yu, L.; Quinn, M.T.; Cross, A.R.; Dinauer, M.C. Gp91 phox is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc. Natl. Acad. Sci. USA 1998, 95, 7993–7998. [Google Scholar] [CrossRef]
- Singh, R.B.; Halabi, G.; Fatima, G.; Rai, R.H.; Tarnava, A.T.; LeBaron, T.W. Molecular hydrogen as an adjuvant therapy may be associated with increased oxygen saturation and improved exercise tolerance in a COVID-19 patient. Clin. Case Rep. 2021, 9, e05039. [Google Scholar] [CrossRef]
- Iolascon, A.; Bianchi, P.; Andolfo, I.; Russo, R.; Barcellini, W.; Fermo, E.; Toldi, G.; Ghirardello, S.; Rees, D.; Van Wijk, R.; et al. Recommendations for diagnosis and treatment of methemoglobinemia. Am. J. Hematol. 2021, 96, 1666–1678. [Google Scholar] [CrossRef]
- Kawamura, T.; Wakabayashi, N.; Shigemura, N.; Huang, C.S.; Masutani, K.; Tanaka, Y.; Noda, K.; Peng, X.; Takahashi, T.; Billiar, T.R.; et al. Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L646–L656. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xiao, J.H. The Keap1-Nrf2 system: A mediator between oxidative stress and aging. Oxid. Med. Cell. Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, H.S.; Moosavi-Movahedi, A.A. Catalase and its mysteries. Prog. Biophys. Mol. Biol. 2018, 140, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Zhang, M.; Sun, X. Molecular hydrogen is involved in phytohormone signaling and stress responses in plants. PLoS ONE 2013, 8, e71038. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, Y.; Yang, M.; Wang, C.; Xie, K.; Yu, Y. Hydrogen gas reduces HMGB1 release in lung tissues of septic mice in an Nrf2/HO-1-dependent pathway. Int. Immunopharmacol. 2019, 69, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Roose, B.W.; Zemerov, S.D.; Dmochowski, I.J. Xenon–protein interactions: Characterization by X-ray crystallography and Hyper-CEST NMR. Methods Enzymol. 2018, 602, 249–272. [Google Scholar]
- Prangé, T.; Schiltz, M.; Pernot, L.; Colloc’h, N.; Longhi, S.; Bourguet, W.; Fourme, R. Exploring hydrophobic sites in proteins with xenon or krypton. Proteins Struct. Funct. Bioinform. 1998, 30, 61–73. [Google Scholar] [CrossRef]
- Abraini, J.H.; Marassio, G.; David, H.N.; Vallone, B.; Prangé, T.; Colloc’h, N. Crystallographic studies with xenon and nitrous oxide provide evidence for protein-dependent processes in the mechanisms of general anesthesia. Anesthesiology 2014, 121, 1018–1027. [Google Scholar] [CrossRef]
- Geyer, M.; Gutierrez, R.; Mujica, V.; Silva, J.F.; Dianat, A.; Cuniberti, G. The contribution of intermolecular spin interactions to the London dispersion forces between chiral molecules. J. Chem. Phys. 2022, 156, 234106. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, R.; Sun, X. Bustling argon: Biological effect. Med. Gas Res. 2013, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Nespoli, F.; Redaelli, S.; Ruggeri, L.; Fumagalli, F.; Olivari, D.; Ristagno, G. A complete review of preclinical and clinical uses of the noble gas argon: Evidence of safety and protection. Ann. Card. Anaesth. 2019, 22, 122. [Google Scholar] [PubMed]
- Perov, A.Y.; Ovchinnikov, B.M.; Parusov, V.V.; Bobrovnikov, A.V.; Safronova, V.G.; Holodilin, Y.D. The method of inhalation therapy with micro-doses of mixtures noble gases with oxygen. arXiv 2021, arXiv:2111.10231. [Google Scholar]
- Lawrence, J.H.; Loomis, W.F.; Tobias, C.A.; Turpin, F.H. Preliminary observations on the narcotic effect of xenon with a review of values for solubilities of gases in water and oils. J. Physiol. 1946, 105, 197–204. [Google Scholar] [CrossRef]
- Maze, M.; Laitio, T. Neuroprotective properties of xenon. Mol. Neurobiol. 2020, 57, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Sykes, W.S.; Lawrence, R.C. Helium in anaesthesia. Br. Med. J. 1938, 2, 448. [Google Scholar] [CrossRef]
- Manuilov, V.M.; Suvorov, A.V.; Kurkin, S.V.; Olenev, Y.O.; Pavlov, N.B.; Logunov, A.T.; Anikeev, D.A.; Orlov, O.I. Evaluation of the efficiency of oxygen–helium therapy for patients with Covid-19-associated pneumonia. Hum. Physiol. 2022, 48, 863–870. [Google Scholar] [CrossRef]
- Tilton, R.F., Jr.; Kuntz, I.D., Jr.; Petsko, G.A. Cavities in proteins: Structure of a metmyoglobin xenon complex solved to 1.9. ANG. Biochemistry 1984, 23, 2849–2857. [Google Scholar] [CrossRef]
- Cazade, P.A.; Meuwly, M. Oxygen migration pathways in NO-bound truncated hemoglobin. ChemPhysChem 2012, 13, 4276–4286. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves Structure, Chemistry and Use; John Wiley and Sons: New York, NY, USA, 1974; pp. 492–493. [Google Scholar]
- Freude, D. Size, mass and kinetics of molecules. In Molecular Physics; Leipzig University (Universität Leipzig): Leipzig, Germany, 2004; Available online: https://home.uni-leipzig.de/energy/freume.html (accessed on 31 January 2024).
- Matteucci, S.; Yampolskii, Y.; Freeman, B.D.; Pinnau, I. Transport of gases and vapors in glassy and rubbery polymers. In Materials Science of Membranes for Gas and Vapor Separation; Yampolskii, Y., Pinnau, I., Freeman, B., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 1–47. [Google Scholar]
- Fauzi, A.; Kailash, I.; Khulbe, C.; Matsuura, T. Gas Separation Membranes: Polymeric and Inorganic; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Almasalmeh, A.; Krenc, D.; Wu, B.; Beitz, E. Structural determinants of the hydrogen peroxide permeability of aquaporins. FEBS J. 2014, 281, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Zadeh Haghighi, H.; Salahub, D.; Simon, C. Radical pairs may play a role in xenon-induced general anesthesia. Sci. Rep. 2021, 11, 6287. [Google Scholar] [CrossRef] [PubMed]
- Barach, A.L. Use of helium as a new therapeutic gas. Proc. Soc. Exp. Biol. Med. 1934, 32, 462–464. [Google Scholar] [CrossRef]
- Oei, G.T.; Weber, N.C.; Hollmann, M.W.; Preckel, B. Cellular effects of helium in different organs. J. Am. Soc. Anesthesiol. 2010, 112, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Roos, D. Chronic granulomatous disease. Br. Med. Bull. 2016, 118, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Liu, C.; Zhou, L.; Qu, K.; Wang, R.; Tai, M.H.; Lei, J.C.W.L.; Wu, Q.F.; Wang, Z.X. A review of hydrogen as a new medical therapy. Hepato Gastroenterol. Curr. Med. Surg. Trends 2012, 59, 1026. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lou, W.; Kong, L.; Shen, W. Hydrogen commonly applicable from medicine to agriculture: From molecular mechanisms to the field. Curr. Pharm. Des. 2021, 27, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Hebelstrup, K.H.; Mur, L.A.; Igamberdiev, A.U. Plant hemoglobins: Important players at the crossroads between oxygen and nitric oxide. FEBS Lett. 2011, 585, 3843–3849. [Google Scholar] [CrossRef] [PubMed]



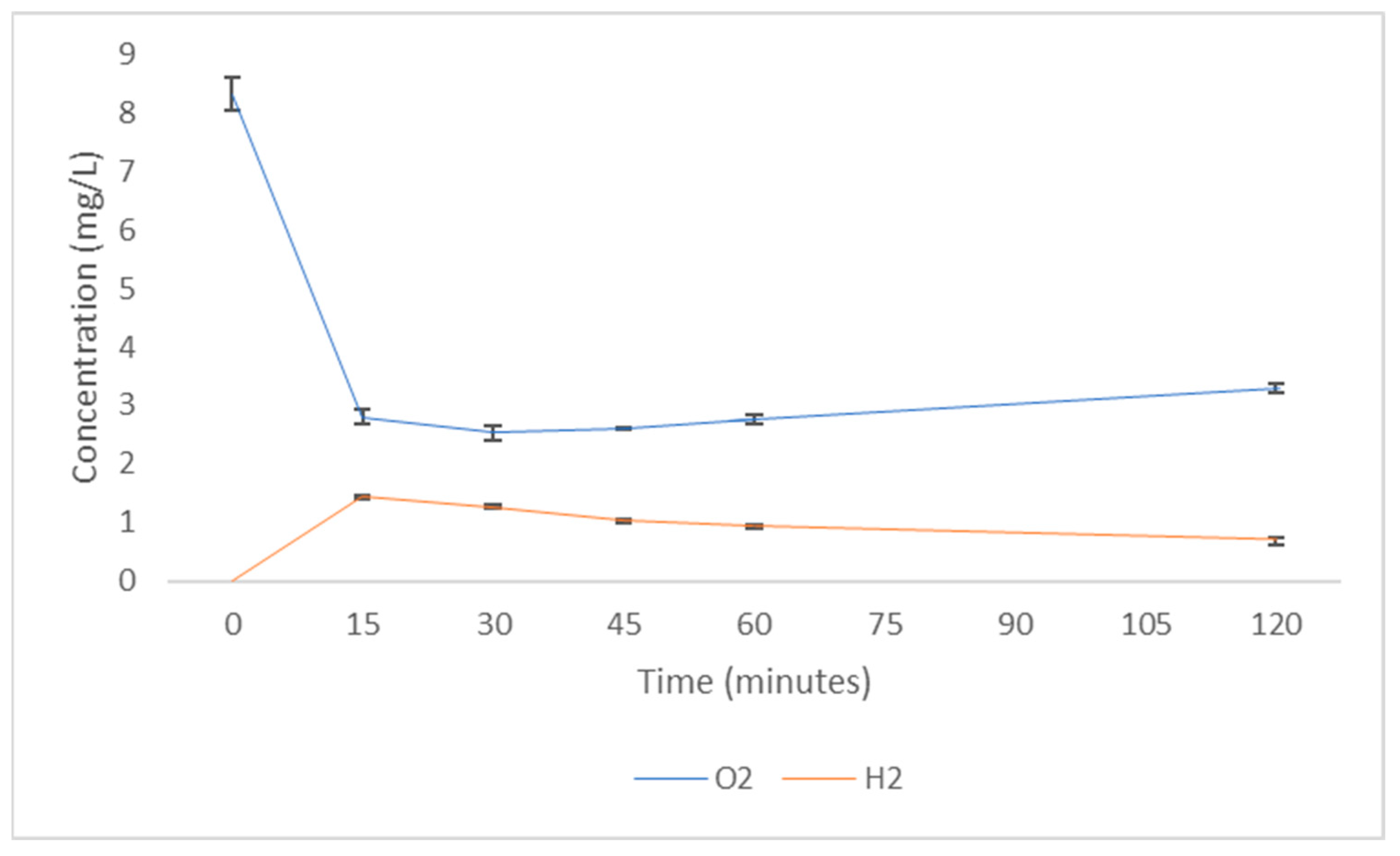
| Animal | Method of Treatment | Effect Reported | Reference |
|---|---|---|---|
| Chickens | Oral HRW: H2 (1–1.5 mg/L) (ad libitum) | Increased IgG and IgM antibodies | [18] |
| Dogs | Oral HRW: H2 (1.6 mg/L) (800 mL/day) | Improved angiogenesis and dermal thickness Increased SOD, Nrf-2, HO-1, NQO-1, VEGF, and PDGF Reduced MDA | [19] |
| Dogs | Injected H2 gas (0.2 mL/kg) | Increased RBC count, Haemoglobin and Haematocrit Reduced haemolysis and WBC infiltration | [20] |
| Goats | Mg supplement: either Mg(OH)2 or elemental Mg (Mg(s) + 2H2O(l) → Mg(OH)2(s) + H2(g)) | Altered rumen microflora and fermentation process (increased propionate metabolism); increased methanogenesis | [21] |
| Guinea Pigs | Oral HRW: H2 (>1.6 mg/L) (ad libitum) | Attenuated noise-induced hearing loss | [22] |
| Guinea Pigs | HRS: H2 (~1.2 mg/L) 10 mL/kg intraperitoneal + 20 µg intranasal | Increased SOD Decreased eosinophils, IgE antibodies mucous production and MDA | [23] |
| Horses | Nasogastric HRW: H2 (>1 mg/L) (2L) | Reduced accumulation of reactive oxygen metabolites | [24] |
| Horses | Intravenous HRS: H2 (0.6 mg/L) (2 L) | Elevated antioxidant potential Suppressed oxidative stress | [25] |
| Pigs | H2 gas (2.1% in air/4 h) | Reduced COX-2 expression and 8-oxo-dG | [26] |
| Pigs | HRW gavage: H2 (1.6 mg/L) (10 mL/kg) | Reduced intestinal leakage and toxin-induced apoptosis Restored CLDN3 expression | [27] |
| Plants | Method of Treatment | Effect Reported | Reference |
|---|---|---|---|
| Alfalfa (M. sativa L. victoria) | Seedlings incubated HRW/12 h: H2 (0.16 mg/L) | Regulated expression of genes relevant to sulphur and glutathione metabolism; enhanced glutathione metabolism | [14] |
| Arabidopsis thaliana | HRW and H2-infused growth medium: H2 (1.6 mg/L) | Enhanced salinity tolerance via modulation of redox homeostasis and upregulation of serotonin N-acetyltransferase and melatonin expression | [28] |
| Arabidopsis thaliana | HRW and H2-infused growth medium: H2 (0.16, 0.4, 0.8, 1.2 and 1.6 mg/L) | Enhanced salinity tolerance via modulation of redox homeostasis and reduced ion influx through modulation of H+ pump and antiporter activity | [29] |
| Bananas (Musa spp. AAA cv. Baxijiao) | Soaked in HRW/10 min: H2 (0.8 mg/L) | Delayed ripening as a result of repressed ethylene signalling and respiration | [17] |
| Barley (Hordeum distichum L.) | Soaked in HRW/6 h: H2 (2 mg/L) | Increased concentration of vanillic acid, coumaric acid, sinapic acid, Ca and Fe, significantly increasing the germination and growth rates | [15] |
| Cabbage (Brassica campestris spp. Chinensis L.) | Seeds soaked in HRW/3 h before germination: H2 (0.8 mg/L) | Improved Cd tolerance, associated with reduced Cd uptake and increased antioxidant defences | [13] |
| Chives (Allium tuberosum Rottler ex Spreng.) | H2 gas in packaging: H2 (1%, 2%, 3%) | Here, 3% H2 significantly delayed post-harvest ripening via enhancing antioxidant capacity (APX, CAT, POD, SOD) | [30] |
| Kiwi fruit (Actinidia deliciosa, cv. Hayward) | Soaked in HRW/5 min: H2 (1.2 mg/L) | Alleviated chilling injury through enhanced sugar production and repressed peroxidation | [31] |
| Rice (Oryza sativa L.) | HNW in irrigation system: H2 (1 mg/L) | Increased grain size, weight and yield; decreased amylose content; significantly reduced Cd accumulation | [32] |
| Strawberries (variety unknown) | H2 gas in the packaging atmosphere (10% CO2, 4% H2, 86% N2) | Extended the shelf-life (300–500%) by moderating antioxidant responses | [16] |
| Proposed Mechanism | Comment | Reference(s) |
|---|---|---|
| Direct scavenging of •OH | •OH will be derived from O2. Little evidence of other ROS scavenged | [9] |
| Scavenging of other ROS, e.g., H2O2 | Evidence suggests that this is not a possible mechanism | [9] |
| Action of nanobubbles | Could remove •OH, hypochlorite (ClO–), ONOO– and O2•− | [39] |
| Scavenging of reactive nitrogen species | Evidence suggests that this is not a possible mechanism, e.g., no reaction with nitric oxide (NO) for example. | [9] |
| Scavenging •OH in a mechanism that involves haem | Would explain some of the effects reported | [40,41,42] |
| Effects mediated by haem oxidase, e.g., HO-1 | May involve carbon monoxide (CO)-mediated signalling | [43] |
| Effects mediated by the redox poise of the H2 couple | Suggested as a widely used mechanism, but no evidence | [44,45] |
| H2 spin state | Spin states mediate interactions with other biomolecules. No direct evidence for this | [46] |
| Acting through protein hydrophobic polypeptide pockets | Globin proteins are a model system, where several inert gases are known to interact | [47,48] |
| H2 and O2 interacting | May account for some of the effects seen? | Discussed here |
| Compound | Measured Results (%) | Inferred Output Percentage (%) |
|---|---|---|
| H2 | 60.16% | 66% |
| O2 | 29.50% | 33% |
| N2 | 10.32% | Not expected |
| Methane (CH4) | 0.01% | 0.01% |
| Carbon dioxide (CO2) | 0.01% | 0.01% |
| Carbon monoxide (CO) | N/D | N/D |
| Air (250 mL/s) | Oxyhydrogen (450 mL/min) | H2-Only (300 mL/min) | ||||
|---|---|---|---|---|---|---|
| (% inhaled) | O2 | H2 | O2 | H2 | O2 | H2 |
| Female | 21% | <0.0001% | 22.27% | 2.5% | ~19% | 2.5% |
| Male | 21% | <0.0001% | 22.01% | 2% | ~19.5% | 2% |
| Treatment Given | Characteristic Measured | Effect Seen |
|---|---|---|
| Oxygenated water | Weight | Significantly increased |
| Body mass index | Significantly reduced abdominal fat accumulation | |
| Triacylglyceride | Reduced | |
| Total cholesterol | Reduced | |
| LDL cholesterol | Reduced | |
| IgG antibodies | Significantly increased | |
| IgM antibodies | Significantly increased | |
| Hydrogenated water | Weight | Increased |
| Body mass index | Non-significant reduction | |
| Triacylglyceride | Reduced | |
| Total cholesterol | Reduced | |
| LDL cholesterol | Reduced | |
| IgG antibodies | Increased | |
| IgM antibodies | Increased |
| Substance | Chemical Symbol | Molecular Mass (g/mol) | Kinetic Diameter (nm) | Reference |
|---|---|---|---|---|
| Argon | Ar | 40 | 0.34 | [87] |
| Carbon dioxide | CO2 | 44 | 0.33 | [88] |
| Carbon monoxide | CO | 28 | 0.37 | [89] |
| Helium | He | 4 | 0.26 | [89] |
| Hydrogen | H2 | 2 | 0.28 | [88] |
| Krypton | Kr | 84 | 0.36 | [87] |
| Neon | Ne | 20 | 0.28 | [87] |
| Nitrogen | N2 | 28 | 0.36 | [90] |
| Nitric oxide | NO | 30 | 0.32 | [89] |
| Oxygen | O2 | 32 | 0.35 | [88] |
| Xenon | Xe | 131 | 0.40 | [87] |
| Water | H2O | 18 | 0.20 | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russell, G.; May, J.; Hancock, J.T. An Interplay of Gases: Oxygen and Hydrogen in Biological Systems. Oxygen 2024, 4, 37-52. https://doi.org/10.3390/oxygen4010003
Russell G, May J, Hancock JT. An Interplay of Gases: Oxygen and Hydrogen in Biological Systems. Oxygen. 2024; 4(1):37-52. https://doi.org/10.3390/oxygen4010003
Chicago/Turabian StyleRussell, Grace, Jennifer May, and John T. Hancock. 2024. "An Interplay of Gases: Oxygen and Hydrogen in Biological Systems" Oxygen 4, no. 1: 37-52. https://doi.org/10.3390/oxygen4010003
APA StyleRussell, G., May, J., & Hancock, J. T. (2024). An Interplay of Gases: Oxygen and Hydrogen in Biological Systems. Oxygen, 4(1), 37-52. https://doi.org/10.3390/oxygen4010003







