Antioxidant-Rich Extracts from Lemon Verbena (Aloysia citrodora L.) Leaves through Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Lemon Verbena Leaves Material
2.3. Extraction Process
2.4. Optimization with Response Surface Methodology (RSM) and Experimental Design
2.5. Bioactive Compounds Determination
2.5.1. Total Polyphenol Content (TPC)
2.5.2. HPLC Quantification of Polyphenolic Compounds
2.5.3. Ascorbic Acid (AA) Content
2.5.4. Total Carotenoids (TC) Determination
2.6. Antioxidant Capacity of the Extracts
2.6.1. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.6.2. DPPH• Antiradical Activity Assay
2.6.3. Hydrogen Peroxide (H2O2) Scavenging Assay
2.7. Color Determination of the Extracts
2.8. Statistical Analysis
3. Results and Discussion
3.1. Total Polyphenol Content and Antioxidant Activity of the Extracts
3.2. Other Bioactive Compounds, Biological and Physicochemical Determination of Extracts
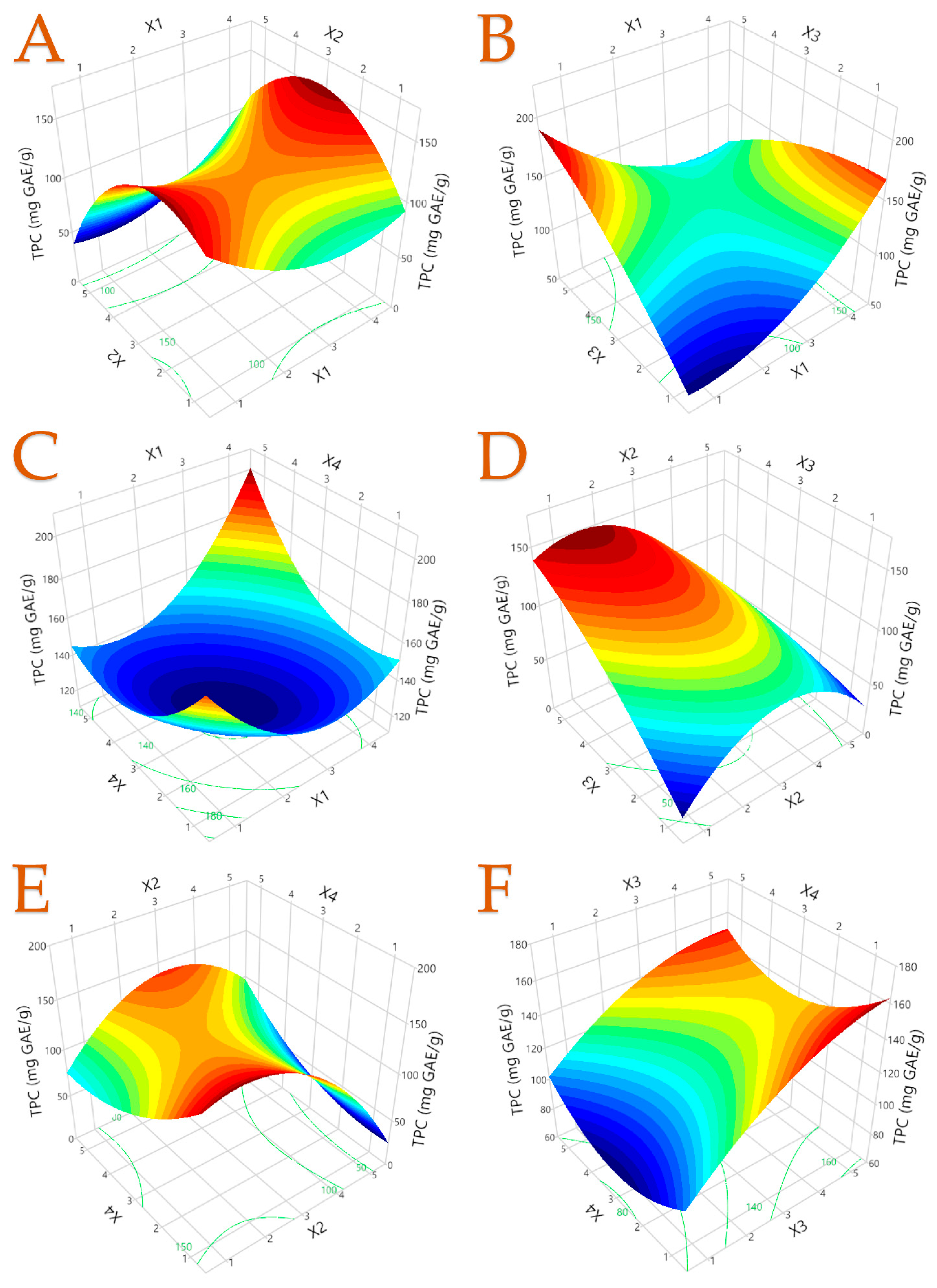
3.3. Optimal Extraction Conditions
3.4. Principal Component Analysis (PCA) and Multivariate Correlation Analysis (MCA)
3.5. Partial Least Squares (PLS) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Saini, R.; Suthar, P.; Kumar, V.; Sharma, R. Plant Secondary Metabolites: Their Food and Therapeutic Importance. In Plant Secondary Metabolites: Physico-Chemical Properties and Therapeutic Applications; Sharma, A.K., Sharma, A., Eds.; Springer Nature: Singapore, 2022; pp. 371–413. ISBN 9789811647796. [Google Scholar]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Characterization, Antioxidant Potential, and Pharmacokinetics Properties of Phenolic Compounds from Native Australian Herbs and Fruits. Plants 2023, 12, 993. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Sousa, M.J.; Ferreira, I.C.F.R. Contribution of Essential Oils and Phenolics to the Antioxidant Properties of Aromatic Plants. Ind. Crops Prod. 2010, 32, 152–156. [Google Scholar] [CrossRef]
- Liu, W.; Yin, D.; Li, N.; Hou, X.; Wang, D.; Li, D.; Liu, J. Influence of Environmental Factors on the Active Substance Production and Antioxidant Activity in Potentilla Fruticosa L. and Its Quality Assessment. Sci. Rep. 2016, 6, 28591. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Yadav, A.; Yadav, M.; Yadav, J.P. Effect of Climate Change on Phytochemical Diversity, Total Phenolic Content and in Vitro Antioxidant Activity of Aloe Vera (L.) Burm.f. BMC Res. Notes 2017, 10, 60. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The Effect of Developmental and Environmental Factors on Secondary Metabolites in Medicinal Plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Isolation of Polyphenols from Two Waste Streams of Clingstone Peach Canneries Utilizing the Cloud Point Extraction Method. Biomass 2023, 3, 291–305. [Google Scholar] [CrossRef]
- Belgacem, I.; Li Destri Nicosia, M.G.; Pangallo, S.; Abdelfattah, A.; Benuzzi, M.; Agosteo, G.E.; Schena, L. Pomegranate Peel Extracts as Safe Natural Treatments to Control Plant Diseases and Increase the Shelf-Life and Safety of Fresh Fruits and Vegetables. Plants 2021, 10, 453. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Colizzi, C. The Protective Effects of Polyphenols on Alzheimer’s Disease: A Systematic Review. Alzheimers Dement. Transl. Res. Clin. Interv. 2018, 5, 184–196. [Google Scholar] [CrossRef]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Identification and Characterization of Anthocyanins and Non-Anthocyanin Phenolics from Australian Native Fruits and Their Antioxidant, Antidiabetic, and Anti-Alzheimer Potential. Food Res. Int. 2022, 162, 111951. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as Antimicrobial Agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Aguiar Campolina, G.; das Graças Cardoso, M.; Rodrigues-Silva-Caetano, A.; Lee Nelson, D.; Mendes Ramos, E. Essential Oil and Plant Extracts as Preservatives and Natural Antioxidants Applied to Meat and Meat Products: A Review. Food Technol. Biotechnol. 2023, 61, 212–225. [Google Scholar] [CrossRef]
- Amer, S.A.; Rizk, A.E. Production and Evaluation of Novel Functional Extruded Corn Snacks Fortified with Ginger, Bay Leaves and Turmeric Powder. Food Prod. Process. Nutr. 2022, 4, 4. [Google Scholar] [CrossRef]
- Polumackanycz, M.; Petropoulos, S.A.; Añibarro-Ortega, M.; Pinela, J.; Barros, L.; Plenis, A.; Viapiana, A. Chemical Composition and Antioxidant Properties of Common and Lemon Verbena. Antioxidants 2022, 11, 2247. [Google Scholar] [CrossRef]
- Miara, M.D.; Bendif, H.; Rebbas, K.; Rabah, B.; Hammou, M.A.; Maggi, F. Medicinal Plants and Their Traditional Uses in the Highland Region of Bordj Bou Arreridj (Northeast Algeria). J. Herb. Med. 2019, 16, 100262. [Google Scholar] [CrossRef]
- Gil, A.; Van Baren, C.M.; Di Leo Lira, P.M.; Bandoni, A.L. Identification of the Genotype from the Content and Composition of the Essential Oil of Lemon Verbena (Aloysia Citriodora Palau). J. Agric. Food Chem. 2007, 55, 8664–8669. [Google Scholar] [CrossRef]
- Tammar, S.; Salem, N.; Aidi Wannes, W.; Limam, H.; Bourgou, S.; Fares, N.; Dakhlaoui, S.; Hammami, M.; Khammassi, S.; Re, G.D.; et al. Chemometric Profiling and Bioactivity of Verbena (Aloysia Citrodora) Methanolic Extract from Four Localities in Tunisia. Foods 2021, 10, 2912. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Samsampour, D.; Zahedi, S.M.; Zamanian, K.; Rahman, M.M.; Mostofa, M.G.; Tran, L.-S.P. Melatonin Alleviates Drought Impact on Growth and Essential Oil Yield of Lemon Verbena by Enhancing Antioxidant Responses, Mineral Balance, and Abscisic Acid Content. Physiol. Plant. 2021, 172, 1363–1375. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Rostamiasrabadi, P.; Shahpiri, Z.; Marques, A.M.; Rahimi, R.; Farzaei, M.H. Aloysia Citrodora Paláu (Lemon Verbena): A Review of Phytochemistry and Pharmacology. J. Ethnopharmacol. 2018, 222, 34–51. [Google Scholar] [CrossRef]
- Peixoto, J.A.B.; Álvarez-Rivera, G.; Costa, A.S.G.; Machado, S.; Cifuentes, A.; Ibáñez, E.; Oliveira, M.B.P.P.; Alves, R.C. Contribution of Phenolics and Free Amino Acids on the Antioxidant Profile of Commercial Lemon Verbena Infusions. Antioxidants 2023, 12, 251. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodríguez, A.; Martínez-Olcina, M.; Mora, J.; Navarro, P.; Caturla, N.; Jones, J. Anxiolytic Effect and Improved Sleep Quality in Individuals Taking Lippia Citriodora Extract. Nutrients 2022, 14, 218. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Lozano-Sánchez, J.; Fernández-Ochoa, Á.; de la Cádiz-Gurrea, M.L.; Arráez-Román, D.; Segura-Carretero, A. Optimized Extraction of Phenylpropanoids and Flavonoids from Lemon Verbena Leaves by Supercritical Fluid System Using Response Surface Methodology. Foods 2020, 9, 931. [Google Scholar] [CrossRef] [PubMed]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging Green Techniques for the Extraction of Antioxidants from Agri-Food By-Products as Promising Ingredients for the Food Industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kalompatsios, D.; Kotsou, K.; Mantiniotou, M.; Bozinou, E.; Lalas, S.I. Sustainable Valorization of Sour Cherry (Prunus Cerasus) By-Products: Extraction of Antioxidant Compounds. Sustainability 2024, 16, 32. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Makrygiannis, I.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. An Investigation into Crithmum Maritimum L. Leaves as a Source of Antioxidant Polyphenols. Compounds 2023, 3, 532–551. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Bozinou, E.; Lalas, S.I. Response Surface Optimization for the Enhancement of the Extraction of Bioactive Compounds from Citrus Limon Peel. Antioxidants 2023, 12, 1605. [Google Scholar] [CrossRef]
- Ayour, J.; Alahyane, A.; Harrak, H.; Neffa, M.; Taourirte, M.; Benichou, M. Assessment of Nutritional, Technological, and Commercial Apricot Quality Criteria of the Moroccan Cultivar “Maoui” Compared to Introduced Spanish Cultivars “Canino” and “Delpatriarca” towards Suitable Valorization. J. Food Qual. 2021, 2021, e6679128. [Google Scholar] [CrossRef]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction Optimisation Using Water/Glycerol for the Efficient Recovery of Polyphenolic Antioxidants from Two Artemisia Species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Cesa, S.; Carradori, S.; Bellagamba, G.; Locatelli, M.; Casadei, M.A.; Masci, A.; Paolicelli, P. Evaluation of Processing Effects on Anthocyanin Content and Colour Modifications of Blueberry (Vaccinium Spp.) Extracts: Comparison between HPLC-DAD and CIELAB Analyses. Food Chem. 2017, 232, 114–123. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Frosi, I.; Montagna, I.; Colombo, R.; Milanese, C.; Papetti, A. Recovery of Chlorogenic Acids from Agri-Food Wastes: Updates on Green Extraction Techniques. Molecules 2021, 26, 4515. [Google Scholar] [CrossRef]
- Dirar, A.I.; Alsaadi, D.H.M.; Wada, M.; Mohamed, M.A.; Watanabe, T.; Devkota, H.P. Effects of Extraction Solvents on Total Phenolic and Flavonoid Contents and Biological Activities of Extracts from Sudanese Medicinal Plants. S. Afr. J. Bot. 2019, 120, 261–267. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of Green Food Processing Techniques. Preservation, Transformation, and Extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kalompatsios, D.; Mantiniotou, M.; Bozinou, E.; Lalas, S.I. Pulsed Electric Field Applications for the Extraction of Bioactive Compounds from Food Waste and By-Products: A Critical Review. Biomass 2023, 3, 367–401. [Google Scholar] [CrossRef]
- Jintawiwat, R.; Punamorntarakul, N.; Hirunyasiri, R.; Jarupoom, P.; Pankasemsuk, T.; Supasin, S.; Kawee-ai, A. Testing the Efficacy of a Prototype That Combines Ultrasound and Pulsed Electric Field for Extracting Valuable Compounds from Mitragyna Speciosa Leaves. AgriEngineering 2023, 5, 1879–1892. [Google Scholar] [CrossRef]
- Rashid, H.M.; Mahmod, A.I.; Afifi, F.U.; Talib, W.H. Antioxidant and Antiproliferation Activities of Lemon Verbena (Aloysia Citrodora): An In Vitro and In Vivo Study. Plants 2022, 11, 785. [Google Scholar] [CrossRef]
- Zargoosh, Z.; Ghavam, M.; Bacchetta, G.; Tavili, A. Effects of Ecological Factors on the Antioxidant Potential and Total Phenol Content of Scrophularia Striata Boiss. Sci. Rep. 2019, 9, 16021. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the Extraction Method on the Recovery of Bioactive Phenolic Compounds from Food Industry By-Products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef]
- Boo, Y.C. P-Coumaric Acid as An Active Ingredient in Cosmetics: A Review Focusing on Its Antimelanogenic Effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef]
- Boz, H. P-Coumaric Acid in Cereals: Presence, Antioxidant and Antimicrobial Effects. Int. J. Food Sci. Technol. 2015, 50, 2323–2328. [Google Scholar] [CrossRef]
- Lei, P.; Lü, J.; Yao, T.; Zhang, P.; Chai, X.; Wang, Y.; Jiang, M. Verbascoside Exerts an Anti-Atherosclerotic Effect by Regulating Liver Glycerophospholipid Metabolism. Food Sci. Hum. Wellness 2023, 12, 2314–2323. [Google Scholar] [CrossRef]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital Roles of Carotenoids in Plants and Humans to Deteriorate Stress with Its Structure, Biosynthesis, Metabolic Engineering and Functional Aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Thoo, Y.; Ng, S.Y.; Khoo, M.; Mustapha, W.; Ho, C. A Binary Solvent Extraction System for Phenolic Antioxidants and Its Application to the Estimation of Antioxidant Capacity in Andrographis Paniculata Extracts. Int. Food Res. J. 2013, 20, 1103. [Google Scholar]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab Aziz, M.F.; Sazili, A.Q. Green Extraction of Bioactive Compounds from Plant Biomass and Their Application in Meat as Natural Antioxidant. Antioxidants 2021, 10, 1465. [Google Scholar] [CrossRef]
- Sik, B.; Hanczné, E.L.; Kapcsándi, V.; Ajtony, Z. Conventional and Nonconventional Extraction Techniques for Optimal Extraction Processes of Rosmarinic Acid from Six Lamiaceae Plants as Determined by HPLC-DAD Measurement. J. Pharm. Biomed. Anal. 2020, 184, 113173. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New Insights of the Application of Water or Ethanol-Water Plant Extract Rich in Active Compounds in Food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef]
- Monroy, Y.M.; Rodrigues, R.A.F.; Sartoratto, A.; Cabral, F.A. Influence of Ethanol, Water, and Their Mixtures as Co-Solvents of the Supercritical Carbon Dioxide in the Extraction of Phenolics from Purple Corn Cob (Zea Mays L.). J. Supercrit. Fluids 2016, 118, 11–18. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Oxygen Radical Absorbance Capacities of Grape/Wine Industry Byproducts and Effect of Solvent Type on Extraction of Grape Seed Polyphenols. J. Food Compos. Anal. 2006, 19, 41–48. [Google Scholar] [CrossRef]
- Lapornik, B.; Prošek, M.; Golc Wondra, A. Comparison of Extracts Prepared from Plant By-Products Using Different Solvents and Extraction Time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Ali Redha, A.; Salauddin, M.; Harahap, I.A.; Rupasinghe, H.P.V. Factors Affecting the Extraction of (Poly)Phenols from Natural Resources Using Deep Eutectic Solvents Combined with Ultrasound-Assisted Extraction. Crit. Rev. Anal. Chem. 2023, 1–22. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F. Recent Advances and Comparisons of Conventional and Alternative Extraction Techniques of Phenolic Compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B.; et al. Multiple Pharmacognostic Characterization on Hemp Commercial Cultivars: Focus on Inflorescence Water Extract Activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef]
- Guimarães, R.; Barreira, J.C.M.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Effects of Oral Dosage Form and Storage Period on the Antioxidant Properties of Four Species Used in Traditional Herbal Medicine. Phytother. Res. 2011, 25, 484–492. [Google Scholar] [CrossRef]
- Portmann, E.; Nigro, M.M.L.; Reides, C.G.; Llesuy, S.; Ricco, R.A.; Wagner, M.L.; Gurni, A.A.; Carballo, M.A. Aqueous Extracts of Lippia Turbinata and Aloysia Citriodora (Verbenaceae): Assessment of Antioxidant Capacity and DNA Damage. Int. J. Toxicol. 2012, 31, 192–202. [Google Scholar] [CrossRef]




| Independent Variables | Code Units | Coded Variable Level | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Technique | X1 | ST 1 | PEF 2 + ST | US 3 + ST | PEF + US + ST | – |
| C (% ethanol in water, v/v) | X2 | 0 | 25 | 50 | 75 | 100 |
| t (min) | X3 | 30 | 60 | 90 | 120 | 150 |
| T (°C) | X4 | 20 | 35 | 50 | 65 | 80 |
| Design Point | Independent Variables | Responses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC (mg GAE/g) | FRAP (μmol AAE/g) | DPPH (μmol AAE/g) | Hydrogen Peroxide (μmol AAE/g) | |||||||||
| X1 | X2 | X3 | X4 | Actual | Predicted | Actual | Predicted | Actual | Predicted | Actual | Predicted | |
| 1 | 3 | 1 | 3 | 4 | 90.79 | 93.72 | 1006.24 | 979.75 | 644.16 | 614.36 | 464.60 | 455.58 |
| 2 | 3 | 2 | 1 | 3 | 104.69 | 96.84 | 1145.26 | 1134.95 | 818.78 | 796.75 | 537.46 | 560.86 |
| 3 | 2 | 3 | 4 | 3 | 137.66 | 128.44 | 1145.77 | 1158.92 | 876.98 | 872.69 | 1110.67 | 1079.76 |
| 4 | 2 | 4 | 5 | 4 | 112.12 | 111.65 | 1102.59 | 1082.75 | 811.47 | 821.07 | 814.02 | 785.12 |
| 5 | 3 | 5 | 4 | 2 | 52.95 | 56.58 | 576.19 | 616.29 | 438.94 | 467.04 | 441.47 | 395.83 |
| 6 | 4 | 1 | 4 | 5 | 113.29 | 109.05 | 1062.39 | 1064.93 | 797.22 | 804.01 | 819.28 | 829.54 |
| 7 | 4 | 2 | 3 | 1 | 145.38 | 141.77 | 1131.38 | 1114.51 | 823.26 | 814.26 | 1425.10 | 1398.20 |
| 8 | 1 | 3 | 3 | 2 | 146.63 | 140.00 | 1103.47 | 1095.68 | 916.73 | 883.51 | 1445.65 | 1425.62 |
| 9 | 1 | 4 | 4 | 1 | 132.12 | 132.55 | 989.12 | 970.74 | 747.02 | 741.83 | 1103.30 | 1119.77 |
| 10 | 1 | 5 | 1 | 4 | 40.66 | 44.28 | 630.85 | 639.32 | 477.72 | 484.86 | 411.12 | 392.76 |
| 11 | 1 | 1 | 2 | 3 | 104.86 | 99.95 | 886.66 | 881.56 | 533.61 | 547.39 | 718.37 | 717.05 |
| 12 | 1 | 2 | 5 | 5 | 163.71 | 168.85 | 1326.45 | 1342.51 | 958.98 | 962.97 | 1579.52 | 1581.80 |
| 13 | 4 | 3 | 2 | 4 | 139.61 | 155.73 | 1265.94 | 1332.61 | 896.30 | 948.87 | 1418.19 | 1416.07 |
| 14 | 3 | 4 | 2 | 5 | 150.96 | 143.15 | 1272.40 | 1234.30 | 937.16 | 910.02 | 1320.31 | 1261.63 |
| 15 | 2 | 5 | 3 | 5 | 100.25 | 99.85 | 834.32 | 843.55 | 617.24 | 620.13 | 755.40 | 828.63 |
| 16 | 2 | 1 | 1 | 1 | 97.19 | 98.06 | 864.21 | 886.87 | 577.51 | 582.23 | 350.44 | 311.01 |
| 17 | 2 | 2 | 2 | 2 | 98.45 | 114.71 | 1068.68 | 1063.70 | 790.31 | 817.87 | 841.65 | 930.57 |
| 18 | 3 | 3 | 5 | 1 | 126.37 | 128.41 | 1121.96 | 1136.56 | 770.65 | 781.03 | 837.94 | 864.99 |
| 19 | 4 | 4 | 1 | 2 | 138.85 | 133.65 | 1161.42 | 1138.35 | 767.30 | 755.60 | 1140.69 | 1158.81 |
| 20 | 4 | 5 | 5 | 3 | 66.76 | 66.03 | 712.20 | 689.67 | 530.45 | 505.30 | 459.21 | 480.83 |
| Design Point | Independent Variables | Responses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | VA | p-CA | FA | RT | Q3G | VB | NRT | KG | |
| 1 | 3 | 1 | 3 | 4 | 0.53 | 5.65 | 1.86 | 1.64 | 1.90 | 36.03 | 6.36 | 1.84 |
| 2 | 3 | 2 | 1 | 3 | 0.53 | 5.90 | 0.48 | 1.43 | 1.82 | 59.22 | 3.05 | 1.72 |
| 3 | 2 | 3 | 4 | 3 | 0.22 | 5.25 | 1.79 | 1.43 | 1.77 | 115.78 | 3.28 | 1.79 |
| 4 | 2 | 4 | 5 | 4 | 0.17 | 0.07 | 0.53 | 1.46 | 1.76 | 88.67 | 3.12 | 2.89 |
| 5 | 3 | 5 | 4 | 2 | 0.01 | 0.01 | 0.50 | 1.43 | 0.01 | 21.77 | 0.58 | 1.86 |
| 6 | 4 | 1 | 4 | 5 | 0.24 | 5.56 | 1.83 | 1.99 | 2.07 | 98.21 | 0.91 | 2.59 |
| 7 | 4 | 2 | 3 | 1 | 0.21 | 6.25 | 1.76 | 3.25 | 1.78 | 122.68 | 0.23 | 2.10 |
| 8 | 1 | 3 | 3 | 2 | 0.20 | 5.75 | 1.79 | 3.41 | 1.79 | 130.98 | 1.01 | 1.81 |
| 9 | 1 | 4 | 4 | 1 | 0.14 | 1.00 | 0.92 | 1.44 | 1.75 | 119.80 | 1.90 | 3.48 |
| 10 | 1 | 5 | 1 | 4 | 0.01 | 0.07 | 0.51 | 0.01 | 1.76 | 21.02 | 0.68 | 1.87 |
| 11 | 1 | 1 | 2 | 3 | 0.28 | 5.13 | 1.90 | 1.48 | 1.76 | 60.13 | 0.88 | 1.92 |
| 12 | 1 | 2 | 5 | 5 | 0.27 | 5.45 | 2.13 | 1.95 | 2.17 | 147.41 | 0.52 | 2.23 |
| 13 | 4 | 3 | 2 | 4 | 0.44 | 5.15 | 1.86 | 1.37 | 1.79 | 125.21 | 0.31 | 1.81 |
| 14 | 3 | 4 | 2 | 5 | 0.33 | 1.95 | 0.72 | 1.44 | 1.80 | 125.14 | 0.43 | 2.12 |
| 15 | 2 | 5 | 3 | 5 | 0.01 | 0.02 | 0.52 | 1.46 | 1.77 | 58.09 | 1.31 | 1.95 |
| 16 | 2 | 1 | 1 | 1 | 0.23 | 5.12 | 1.81 | 1.41 | 2.36 | 84.58 | 0.29 | 1.90 |
| 17 | 2 | 2 | 2 | 2 | 0.25 | 5.44 | 1.77 | 1.43 | 2.72 | 81.25 | 0.01 | 1.90 |
| 18 | 3 | 3 | 5 | 1 | 0.19 | 5.51 | 1.84 | 1.45 | 1.78 | 85.42 | 0.21 | 1.83 |
| 19 | 4 | 4 | 1 | 2 | 0.56 | 1.28 | 1.04 | 1.25 | 1.78 | 128.25 | 0.05 | 2.11 |
| 20 | 4 | 5 | 5 | 3 | 0.05 | 0.01 | 0.52 | 0.01 | 0.01 | 29.15 | 0.01 | 1.91 |
| Design Point | Independent Variables | Responses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Carotenoids (μg CtE/g) | Ascorbic Acid (mg/g) | L* | C* | Hue | |
| 1 | 3 | 1 | 3 | 4 | 599.33 | 7.41 | 34.9 | 4.0 | 34.6 |
| 2 | 3 | 2 | 1 | 3 | 379.60 | 7.30 | 40.6 | 19.3 | 48.8 |
| 3 | 2 | 3 | 4 | 3 | 506.48 | 7.01 | 35.8 | 9.7 | 43.7 |
| 4 | 2 | 4 | 5 | 4 | 540.31 | 5.92 | 36.1 | 10.9 | 124.4 |
| 5 | 3 | 5 | 4 | 2 | 451.57 | 4.52 | 44.6 | 29.2 | 100.1 |
| 6 | 4 | 1 | 4 | 5 | 557.95 | 9.41 | 35.6 | 7.9 | 54.6 |
| 7 | 4 | 2 | 3 | 1 | 327.25 | 7.32 | 39.5 | 17.7 | 36.4 |
| 8 | 1 | 3 | 3 | 2 | 316.52 | 7.33 | 39.3 | 13.9 | 43.6 |
| 9 | 1 | 4 | 4 | 1 | 403.37 | 5.85 | 41.3 | 20.5 | 102.1 |
| 10 | 1 | 5 | 1 | 4 | 383.59 | 4.26 | 47.6 | 26.5 | 121.2 |
| 11 | 1 | 1 | 2 | 3 | 472.51 | 6.85 | 32.6 | 9.9 | 14.5 |
| 12 | 1 | 2 | 5 | 5 | 593.25 | 9.47 | 34.4 | 9.4 | 50.7 |
| 13 | 4 | 3 | 2 | 4 | 443.83 | 6.24 | 37.7 | 12.7 | 52.5 |
| 14 | 3 | 4 | 2 | 5 | 602.56 | 7.68 | 37.2 | 8.9 | 116.9 |
| 15 | 2 | 5 | 3 | 5 | 473.67 | 4.95 | 41.0 | 23.3 | 124.5 |
| 16 | 2 | 1 | 1 | 1 | 287.89 | 6.43 | 44.6 | 20.4 | 53.2 |
| 17 | 2 | 2 | 2 | 2 | 334.44 | 7.45 | 43.4 | 23.3 | 40.9 |
| 18 | 3 | 3 | 5 | 1 | 543.60 | 6.96 | 33.6 | 13.0 | 28.4 |
| 19 | 4 | 4 | 1 | 2 | 452.69 | 6.71 | 40.9 | 16.9 | 104.7 |
| 20 | 4 | 5 | 5 | 3 | 441.58 | 4.54 | 45.1 | 23.0 | 123.4 |
| Responses | Second-Order Polynomial Equations (Models) | R2 Predicted | R2 Adjusted | p-Value | Eq. |
|---|---|---|---|---|---|
| TPC | Y = 115.38 − 37.27X1 + 22.92X2 + 57.33X3 − 48.54X4 + 7.14X12 − 8.47X22 − 2.11X32 + 3.58X42 + 5.88X1X2 − 8.87X1X3 + 4.46X1X4 − 4.67X2X3 + 6.25X2X4 − 0.59X3X4 | 0.9558 | 0.8322 | 0.0169 | (10) |
| FRAP | Y = 555.9 − 3.4X1 + 347.79X2 + 127.21X3 − 106.27X4 + 9.4X12 − 77.88X22 + 7.66X32 + 10.63X42 + 16.88X1X2 − 36.84X1X3 + 16.43X1X4 − 16.72X2X3 + 19.82X2X4 − 5.13X3X4 | 0.9858 | 0.9460 | 0.0011 | (11) |
| DPPH | Y = 300.72 − 54.29X1 + 628.91X2 − 76.72X3 − 131.07X4 + 1.98X12 − 103.17X22 − 11.73X32 + 24.91X42 − 1.41X1X2 + 18.3X1X3 + 0.29X1X4 + 7.88X2X3 − 12.61X2X4 + 21.45X3X4 | 0.9815 | 0.9296 | 0.0022 | (12) |
| Hydrogen Peroxide | Y = 321.24 − 776.65X1 + 1041.24X2 + 479.02X3 − 397.71X4 + 136.8X12 − 184.79X22 − 89.51X32 + 69.27X42 + 54.16X1X2 + 0.57X1X3 − 23.9X1X4 − 15.65X2X3 − 0.58X2X4 + 33.12X3X4 | 0.9912 | 0.9665 | 0.0004 | (13) |
| Responses | Optimal Conditions | ||||
|---|---|---|---|---|---|
| Maximum Predicted Response | Technique (X1) | C (%, v/v) (X2) | t (min) (X3) | T (°C) (X4) | |
| TPC (mg GAE/g) | 168.85 ± 33.33 | ST (1) | 25 (2) | 150 (5) | 80 (5) |
| FRAP (μmol AAE/g) | 1342.51 ± 119.04 | ST (1) | 25 (2) | 150 (5) | 80 (5) |
| DPPH (μmol AAE/g) | 996.11 ± 83.47 | PEF + US + ST (4) | 50 (3) | 90 (3) | 65 (4) |
| Hydrogen Peroxide (μmol AAE/g) | 1581.8 ± 177.18 | ST (1) | 25 (2) | 150 (5) | 80 (5) |
| Variables | PLS Model Values | Experimental Values |
|---|---|---|
| TPC (mg GAE/g) | 174.83 | 175.03 ± 11.9 |
| FRAP (μmol AAE/g) | 1436.93 | 1462.17 ± 30.71 |
| DPPH (μmol AAE/g) | 1048.19 | 1108.91 ± 42.14 |
| Hydrogen Peroxide (μmol AAE/g) | 1610.24 | 1662.93 ± 68.18 |
| Parameters | Optimal Extract |
|---|---|
| Carotenoids (μg CtE/g) | 499.61 ± 32.47 |
| Ascorbic acid (mg/g) | 8.36 ± 0.28 |
| L* | 36.67 ± 0.2 |
| C* | 12.9 ± 0.6 |
| Hue | 55.1 ± 2.6 |
| Polyphenolic compounds (mg/g) | |
| Vanillic acid | 0.37 ± 0.02 |
| p-Coumaric acid | 5.27 ± 0.11 |
| Ferulic acid | 1.88 ± 0.04 |
| Rutin | 2.27 ± 0.13 |
| Quercetin 3-D-galactoside | 2.23 ± 0.07 |
| Verbascoside | 132.61 ± 9.81 |
| Narirutin | 1.49 ± 0.03 |
| Kaempferol 3-glucoside | 2.3 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athanasiadis, V.; Chatzimitakos, T.; Makrygiannis, I.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Antioxidant-Rich Extracts from Lemon Verbena (Aloysia citrodora L.) Leaves through Response Surface Methodology. Oxygen 2024, 4, 1-19. https://doi.org/10.3390/oxygen4010001
Athanasiadis V, Chatzimitakos T, Makrygiannis I, Kalompatsios D, Bozinou E, Lalas SI. Antioxidant-Rich Extracts from Lemon Verbena (Aloysia citrodora L.) Leaves through Response Surface Methodology. Oxygen. 2024; 4(1):1-19. https://doi.org/10.3390/oxygen4010001
Chicago/Turabian StyleAthanasiadis, Vassilis, Theodoros Chatzimitakos, Ioannis Makrygiannis, Dimitrios Kalompatsios, Eleni Bozinou, and Stavros I. Lalas. 2024. "Antioxidant-Rich Extracts from Lemon Verbena (Aloysia citrodora L.) Leaves through Response Surface Methodology" Oxygen 4, no. 1: 1-19. https://doi.org/10.3390/oxygen4010001
APA StyleAthanasiadis, V., Chatzimitakos, T., Makrygiannis, I., Kalompatsios, D., Bozinou, E., & Lalas, S. I. (2024). Antioxidant-Rich Extracts from Lemon Verbena (Aloysia citrodora L.) Leaves through Response Surface Methodology. Oxygen, 4(1), 1-19. https://doi.org/10.3390/oxygen4010001











