Abstract
This review deals with the production of oxygen by photo-oxidation of water, which is a topic fitting a journal devoted to oxygen. Most of the present biosphere, including mankind, depends on oxygen. Elucidating the mechanism is of importance for solving the present energy crisis. Photosynthesis evolved in bacteria, first in a form that did not produce oxygen. The oxygen-producing version arose with the advent of cyanobacteria about three billion years ago. The production of oxygen by photo-oxidation of water requires the co-operative action of four photons. These are harvested from daylight by chlorophyll and other pigments (e.g., phycobiliproteins) and are channeled to photosystem II and photosystem I. The oxygen-evolving complex resides in photosystem II, surrounded by protein subunits, and contains one ion of calcium, four ions of manganese, and a number of oxygen atoms. For each quantum of energy it receives from absorbed light, it proceeds one step through a cycle of states known as the Kok–Joliot cycle. For each turn of the cycle, one molecule of oxygen (O2) is produced.
1. Introduction
Life arose on our planet in a reducing environment. The first kind of photosynthesis probably did not involve the evolution of molecular oxygen, but this complicated process evolved at least 3 billion years ago, as evidenced in a number of ways. Analysis of genomes indicates that cyanobacteria had already evolved at that time [1]. The lack of variability in the ratio of uranium isotopes in pre-3.0 Ga iron formation samples suggests minimal presence of dissolved U(VI) in rivers and seawater (indicating anoxic conditions); however, 2.95 billion years ago, this started to change [2]. By comparing the abundances of iron and other metals in sediments of ancient shallow oceans, Satkoski et al. [3] came to the conclusion that they contained oxygen 3.2 billion years ago.
Why do I say that evolution of molecular oxygen is a complicated process? Oxidation of water to O2 is not only energy-demanding; it also requires the co-operative action of four photons, arriving at random times, to remove four electrons from two water molecules. How this can happen and how it evolved is not clear. Let me first describe what we think we know about the mechanism and then speculate about how it has come to be.
In plants and algae, photosynthesis takes place in cell parts called chloroplasts, which are a few micrometers in size. They were derived from cyanobacteria more than a billion years ago, and the photosynthetic structures in chloroplasts and cyanobacteria are quite similar. They contain chlorophylls and carotenoids, as well as phycobiliproteins in cyanobacteria, which absorb the light that drives oxidation of water to molecular oxygen coupled with a reduction of carbon dioxide to carbohydrate. The light energy is absorbed by two large protein complexes with associated pigment molecules, called photosystem I and photosystem II (Figure 1). They reside in so-called thylakoid membranes, which are closed structures (thylakoids) with different inside and an outside structures. Here, we shall concentrate on photosystem II (PSII), which is the that which carries out the oxidation of water to oxygen molecules.
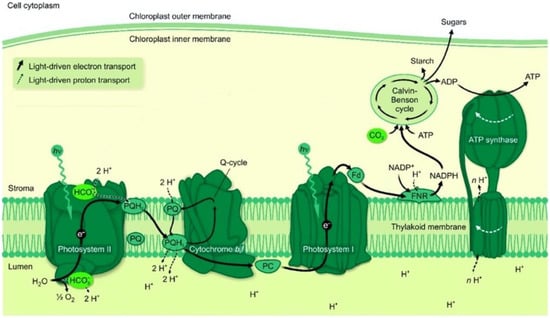
Figure 1.
Thylakoid membrane with photosynthetic protein complexes and sites of bicarbonate action. Slightly modified from Shevela et al. [4]. Reprinted with permission from [4]. © 2020 American Chemical Society. Reproduced under the Creative Commons Attribution (CC-BY) License (https://pubs.acs.org/page/policy/authorchoice_ccby_termsofuse.html, accessed on 27 July 2022).
The evolution of cyanobacteria and the availability of molecular oxygen have been necessary for the continued rich evolution of life on our planet.
2. Photosystem II
A breakthrough in the elucidation of the function of photosystem II was made, independently, by Pierre Joliot et al. [5] and Bessel Kok et al. [6]. When they exposed Chlorella algae or spinach chloroplasts that had been in darkness for some time to repeated very short and intense light flashes, they observed no oxygen after a single flash, a maximum of oxygen (O2) production after the third flash, and thereafter on every fourth flash (Figure 2). The sequence of flashes resulted in approach toward a constant oxygen yield per flash. The gradual levelling out is due to the fact that even these very short flashes often result in more than one step forward (in some cases, there is no hit at all) in what has come to be known as the “Kok cycle” or the “Joliot–Kok clock” (Figure 3B and Figure 6). Structures of the intermediates in this “clock” have been described by Umena et al. [7], Suga et al. [8], and Kern et al. [9]. All details in this cycle are not yet clarified, and various possible versions are discussed by Li et al. in [10].
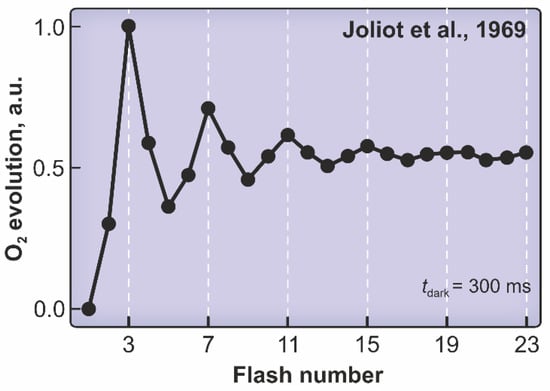
Figure 2.
Yield of molecular oxygen from Chlorella cells as a function of flash number. The first flash does not result in any oxygen; the first maximum comes on the third flash. Thereafter, maxima occur on every fourth flash, and the oscillation gradually decreases towards a flat line (steady state). Redrawn and modified from Joliot et al. [4]. Kok et al. [5] obtained very results with spinach chloroplasts. (See also Joliot & Kok [11]).
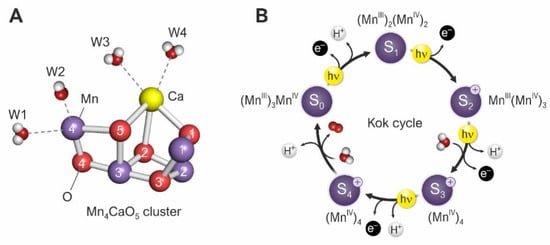
Figure 3.
(A) The oxygen-evolving complex (OEC). The four manganese ions are colored violet, the six oxygen atoms are indicated in red, and the calcium atom is indicated in yellow. W1 to W4 are water molecules. (B) The Kok cycle (also called the Kok–Joliot cycle). S0 to S4 are the OECs in various states, without or with a positive charge, with oxidation states of the manganese indicated. Yellow circles labelled hν indicate quanta from light. When S2+ changes to S3+, a water molecule is taken up, and an electron and a proton are released. When S3+ changes to S0, a water molecule is taken up, and a proton and an oxygen molecule are released. In darkness, the equilibrium state is mainly S1. Graphics by D. Shevela (SciGrafik, Sweden).
Because photons arrive at random times and four photons are needed for the production of one oxygen molecule, there must be some mechanism for storing energy and positive charge until enough is available for oxidation of the water molecules. This is achieved by the four manganese atoms of the Kok cycle (Figure 3B), in steps proceeding from MnIII3MnIV to MnIV4+. A minimum of four more photons are needed for complete photosynthesis, including assimilation of one CO2 molecule.
The molecular structure in which the oxidation of water to molecular oxygen takes place is called the Mn4CaO5 cluster (Figure 3A). The structure was first reported by Shen’s group, Umena et al. [6] and Suga et al. [7].
Figure 4 shows a schematically of how the Mn4CaO5 cluster forms part of the large photosystem II complex, which is one of the proteins in the thylakoid membranes in cyanobacteria and in the chloroplasts of algae and other plants.
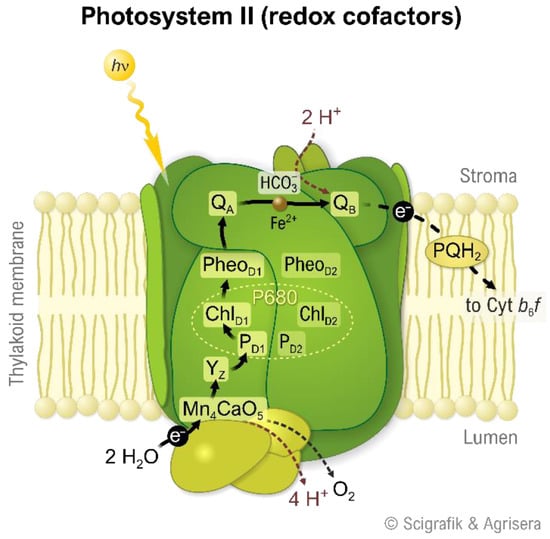
Figure 4.
Photosystem II: The water-splitting enzyme of photosynthesis. D1 and D2 are the central polypeptides in a very large complex in the thylakoid membranes of cyanobacteria and chloroplasts. Electrons from water are transferred via the “Mn4CaO5” cluster, the tyrosine Yz in D1, P680 (an ensemble of chlorophyll a molecules), Pheo (pheophytin), and QA (a molecule of plastoquinone attached to D2) to QB, another molecule of plastoquinone, which, after receiving two electrons, dissolves in the membrane and will be used as reductant in further reactions (as PQH2). Reproduced with permission of SciGrafik (Sweden) and Agrisera (Sweden).
In experiments with the extremophilic red alga Cyanidioschyzon merolae and comparing with results from spinach, Pham et al. [12] explained, in detail, patterns such as that shown in Figure 2 and arrived at the conclusion that the S2-to-S3 transition is the least efficient step during the oxidation of water to O2. It is also the step that has been most thoroughly investigated and divided into substeps. Thus, Klauss et al. [13] found that the proton transfer in this step precedes the electron transfer. They observed an exceptionally high activation energy of 540 ± 30 meV for the proton transfer. However, Amin et al. [14] concluded that even prior to the proton transfer step, oxidation of one of the manganese ions and a change of spin takes place, resulting in a change in the EPR signal from g = 2 to g = 4.1. The structural changes taking place during the S2-to-S3 transition were studied by Ibrahim et al. [15].
It is easy to forget that chlorine is one of the essential elements for plants, as deficiency seldom occurs under natural or agricultural conditions. However, as Mandal et al. [16] showed, chloride ions are necessary for the transition from state S2 to state S3 of the oxygen-evolving complex (Figure 5).
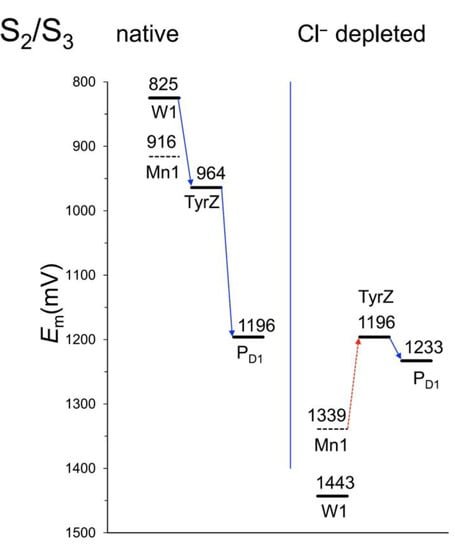
Figure 5.
When a chloride ion (Cl–) is present in the OEC, the transfer of an electron from water, via manganese ion 1 (Mn1) (and change between S2 and S3 states of the OEC) and tyrosine Z, to the plastoquinone B (QB) on the D1 polypeptide goes downhill, i.e., toward higher redox potential (Em). In the absence of Cl–, the redox potential of Mn1 is increased beyond that of tyrosine Z, and Mn1 cannot be oxidized; the electron transfer to tyrosine Z is blocked. From Mandal et al. [16]. Reproduced with permission from the authors. A detailed energy diagram of the S0–S1–S2 transitions in the native state was published by Siegbahn [19].
Using photoacoustics and a method called “photothermal beam deflection”, which, with high time resolution, monitors volume changes in the system, Klauss et al. [17,18] were able to separate and time several steps of electron and proton transfer in the Kok cycle for particles from spinach from S2+ to S0. State S4, originally proposed by Kok, has never been directly identified.
3. The Role of Manganese
As shown in Figure 3B, the molecular oxygen (two red spheres) is released during the transition from the hypothetical (never observed) state S4+ to S0. Figure 6 shows the changes in the OEC during this transition. In the S4 state, oxygen atoms 5 and 6 (labelled O5 and O6) form part of the distorted OEC cube. In the next structure, with lower internal energy, they are released, and the “cube” is opened. One of the oxygen atoms is then replaced by oxygen, with a hydrogen atom attached, from the water molecule labelled W3 in Figure 3 to close the structure again. The transition from S4 to S0 can take place without energy input from light because the internal energy is lower for the S0 structure than that for the S4 structure. It is important to note that Figure 6 is tentative, showing one of several possible mechanisms; many other possible versions of the cycle have been published [13,20,21,22,23,24]. It is too early to draw a final version of this cycle.
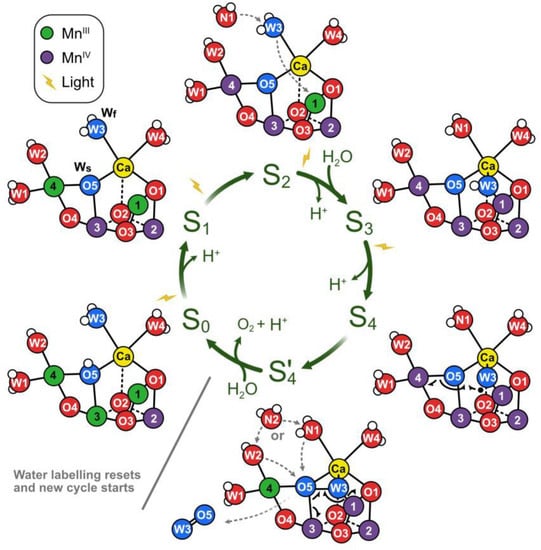
Figure 6.
Molecular details of the Kok cycle (cf. Figure 3). Red and blue circles, oxygen; white, hydrogen; green and violet, manganese; yellow, calcium. In the transition from state S0 to state S1, the proton on oxygen O5 is released, and Mn3 is oxidized. In dark-adapted PSII, the reaction cycle starts with the S1 state with two MnIII and two MnIV ions and in which all bridges are deprotonated. During the S1/S2 transition, Mn4 is oxidized. State S2 involves several conformations (not shown). In the transition from S2 to S3, water W3 is inserted into the binding site between Ca2+ and Mn1, concomitant with Mn1 oxidation and the binding of a new water molecule (N1) to the W3 site (dashed grey arrows). Only after rearrangements within S3 (not shown) can the Mn4CaO6 cluster be oxidized to S4. Instead of Mn oxidation, S4 state formation involves the oxidation of the fast substrate water, indicated by a black dot on W3. By rearranging the electrons of the chemical bonds (black half-arrows), the S4 state rapidly converts into the S4′ state, which contains a complexed peroxide. The further conversion of S4′ into S0 + O2 requires the binding of one water molecule and the release of a proton. It is suggested that a prebound water ligand (W2 or W3) fills the empty O5 binding site and that this ligand is concomitantly replaced by a new water molecule (N2; dashed grey arrows). In the S0 state, the O5 bridge is protonated, in line with the faster exchange of Ws and spectroscopic data. From de Lichtenberg et al. [25], https://creativecommons.org/licenses/by/3.0/, accessed on 27 July 2022. Many other versions of this cycle have been published (see the main text).
Manganese is remarkable in that it can attain all oxidation states from 0 (as metal) to +7 (as in potassium permanganate). However, for the Kok cycle, an alteration between +3 and +4 is sufficient. Zhang and Sun [26] proposed that the manganese atom (number 4 in Figure 3A) “dangling” outside the cubical structure would be oxidized all the way to oxidation number 7 (VII). However, computations by Li et al. [27] indicate that with this oxidation state, there would be a high-energy barrier to prevent the system from reaching the S4 state.
4. The Role of Calcium
The calcium ion is essential for the function of the Mn4CaO5 cluster. It can be removed by various methods and added back with restoration of function, and function can also be restored to some extent by strontium (Sr2+) [28] (see also Gates et al. (2016) [29]). Based on EPR signals, vanadyl ions (VO2+) have an effect similar to that of strontium ions [30]. All the transitions of the Kok–Joliot cycle, except the S0-to-S1 transition, are inhibited in the absence of a calcium ion [31]. Removal of the calcium ion reveals that it is not essential for the OEC structure [32,33], although some changes in the hydrogen bonding of water molecules take place [34]. So, what is the function of Ca2+? Nagashima et al. [35] arrived at the conclusion that Ca2+ maintain the hydrogen bond network near the Ca2+ site and provides of an electron transfer pathway to the manganese cluster. Yao et al. [36] have shown, in model experiments with synthetic clusters mimicking Mn4CaO4, that gadolinium and yttrium provide almost the same structure and electrical properties as calcium. Tsui and Agapie [37] demonstrated, with inorganic manganese-oxido-cubane complexes, how the redox potential is modified by various redox-inactive metals and that strontium and calcium have very similar effects.
An interesting observation was made by Bang et al. [38]. They found that Ca2+ ions, as well as Sr2+ ions, could cause the release of O2 from non-haem iron(III)–peroxo complexes in the presence of an electron acceptor (e.g., 1-benzyl-1,4-dihydronicotinamide dimer). The authors attributed this effect to the ion acting as a Lewis acid, i.e., accepting an electron pair. It is likely that Ca2+ in the OEC functions in the same way. Other examples of Ca2+ acting as a Lewis acid, facilitating various reactions, were reviewed by Begouin and Niggemann [39] and Hill et al. [40].
5. The Role of Bicarbonate in PSII Function
Boyle [41] noted an “apparent necessity of minute quantities of CO2 to bring about oxygen evolution” in a Hill reaction with p-benzoquinone as the electron acceptor. Warburg and Krippahl [42] confirmed the effect. A number of other researchers later repeated and extended these experiments, showing that the effect is due to bicarbonate ions rather than carbon dioxide and that it is quite separate from the role of carbon dioxide as a substrate for carbon assimilation. Wydrzynski and Govindjee [43] observed an effect of bicarbonate on the fluorescence from PSII. They concluded that “the bicarbonate affects the primary reactions in photosynthesis” and that it “is specific for Photosystem II”. Brinkert et al. [44] found that the removal of bicarbonate shifts the midpoint redox potential of the couple QA/QA−• from ∼−145 mV to −70 mV. Based on EPR measurements, Vermaas and Rutherford [45] concluded that the bicarbonate influences the conformation of the QA–• Fe2+ complex. Shevela et al. [46] found that when spinach thylakoids are depleted of carbon dioxide and bicarbonate, the miss probability in the Kok cycle is higher than under ambient conditions and that the addition of 5 mM bicarbonate to thylakoids depleted of inorganic carbon largely restores the original miss parameter. Shevela et al. [4] found that bicarbonate affects not only the reducing side but also the oxidizing side of PSII (Figure 6). It is unlikely that bicarbonate is tightly bound to the oxygen-evolving complex [47,48,49].
One bicarbonate ion per photosystem II becomes firmly bound, i.e., the one bound to the iron ion between QA and QB, whereas other bicarbonate ions help to shuffle protons around [50,51].
An interesting observation is that the purple anoxygenic bacterium Rhodovulum iodosum is able to oxidize Mn2+ to Mn3+ in the presence of bicarbonate, although not in its absence [52]. Rhodovulum iodosum has a type-2 photosystem. The mechanism by which carbonate and bicarbonate facilitate water oxidation in artificial systems was reviewed by Mizrahi and Meyerstein [53]. Bicarbonate has been directly shown to shift the midpoint redox potential of the Mn2+/Mn3+ couple in modified reaction centers of Rhodobacter sphaeroides mutants from 625 mV to 535 mV [54]. With pea chloroplasts, Kozlov et al. [55] identified two Mn-bicarbonate complexes with three bicarbonate ions per complex and midpoint redox potentials of 610 mV and 520 mV, respectively.
6. Assembly of the Oxygen-Evolving Complex
For an overview of photosystem II assembly, see [56]. Here, we shall focus on the OEC. Manganese is imported into the OEC protein as Mn2+, and oxidation to Mn3+ is achieved by Yz• generated by light-induced charge separation in the same way as in photosynthesis [57,58]). Only one Mn ion can be imported until structural changes in the protein have taken place. A small soluble protein in the lumen, Pbs27, aids the process by transiently binding to PSII (see [58] for details). The quantum yield of the process is low—much lower than the quantum yield for photosynthesis [59]—although to some degree, it is increased by the presence of Ca2+ [60,61,62]. Chloride, in association with the OEC, facilitates the oxidation of Mn2+ [63], with two chloride ions in PSII near the OEC [6]. The low quantum yield was explained by Sato et al. [64], with back reactions proceeding faster than forward reactions (Figure 7). The calcium ion protects the system from photoinactivation [62].
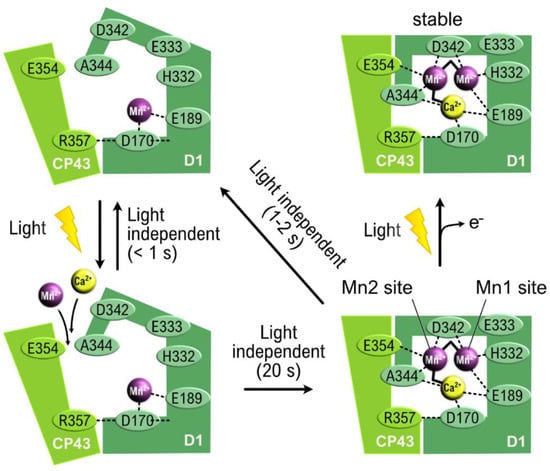
Figure 7.
The initial stages of photoassembly of the OEC; redrawn and modified from Sato et al. [64]. The first light reaction is counteracted by a dark reaction that is much faster than incorporation of calcium ions, a second manganese ion, and rearrangement of the protein scaffolding. This leads to an overall low quantum yield.
Complete photoactivation of the OEC requires a secondary photon absorption [65] (Figure 7).
7. Evolution of Oxygen Evolution
Oxygenic photosynthesis is a complicated process. How could it arise so early in the evolution of life on Earth? Nothing is known with certainty, but various explanations have been proposed. Sauer and Yachandra [66] point to structural similarities between the OEC and certain minerals. Dismukes et al. [67] suggest that the first kind of oxygenic photosynthesis to evolve produced molecular oxygen from bicarbonate ions rather than from water. One theory is that the evolution of water-oxidizing photosynthesis was preceded by a form in which mineral manganese (Mn2+) was oxidized. Chernev et al. [68] created a model of this system by removing the Mn4CaO5 cluster from purified spinach PSII, which also resulted in the loss of three extrinsic proteins (PsbQ, PsbP, and PsbO). When Mn2+ ions were supplied at a low concentration, only a minimal electron transport to an added artificial electron acceptor was observed. The results with a higher Mn2+ concentration (240 µM) are interpreted as rapid oxidation of manganese and formation of Mn(III/IV) oxide particles, similar to the mineral birnessite (confirmed by X-ray spectroscopy). Chernev et al. [68] suggest that “in the evolution of PSII, there may have been a transition from extended Mn-oxide nanoparticles towards the Mn4CaO5 cluster of today’s PSII”. They further point out that “a Mn oxide denoted as ranciéite is isostructural to birnessite and contains Mn and Ca ions at approximately the same 4:1 stoichiometry as present in the Mn4CaO5 cluster of PSII”.
Funding
No funding (except for free access to the digital library of the University of Lund).
Acknowledgments
The author is greatly indebted to Dmitry Shevela of SciGrafik and Umeå University (Sweden) not only for drawing some of the figures but also for suggesting many improvements in the text. Martijn van Praagh has improved the language.
Conflicts of Interest
The author declares no conflict of interest.
References
- Dvorák, P.; Casamatta, D.A.; Poulícková, A.; Hasler, P.; Ondrej, V.; Sanges, R. Synechococcus: 3 billion years of global dominance. Molec. Ecol. 2014, 23, 5538–5551. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Planavsky, N.J.; Hofmann, A.; Saupe, E.E.; De Corte, B.P.; Philippot, P.; LaLonde, S.V.; Jemison, N.E.; Zou, H.; Ossa, F.O.; et al. A Mesoarchean shift in uranium isotope systematics. Geochim. Cosmochim. Acta 2018, 238, 438–452. [Google Scholar] [CrossRef]
- Satkoski, A.; Beukes, N.J.; Li, W.; Beard, B.L.; Johnson, C.M. A redox-stratified ocean 3.2 billion years ago. Earth Planet. Sci. Lett. 2015, 430, 43–53. [Google Scholar]
- Shevela, D.; Do, H.-N.; Fantuzzi, A.; Rutherford, A.W.; Messinger, J. Bicarbonate-mediated CO2 formation on both sides of photosystem II. Biochemistry 2020, 59, 2442–2449. [Google Scholar] [PubMed]
- Joliot, P.; Barbieri, G.; Chabaud, R. Un nouveau modèle des centres photochimiques du système II. Photochem. Photobiol. 1969, 10, 309–329. [Google Scholar]
- Kok, B.; Forbush, B.; McGloin, M. Cooperation of charges in photosynthetic O2 evolution—I. A linear four step mechanism. Photochem. Photobiol. 1970, 11, 457–475. [Google Scholar]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9A. Nature 2011, 273, 55–60. [Google Scholar]
- Suga, M.; Akita, F.; Hirata, K.; Ueno, G.; Murakami, H.; Nakajima, Y.; Shimizu, T.; Yamashita, K.; Yamamoto, M.; Ago, H.; et al. Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature 2015, 517, 99–103. [Google Scholar]
- Kern, J.; Chatterjee, R.; Young, I.D.; Fuller, F.D.; Lassalle, L.; Ibrahim, M.; Gul, S.; Fransson, T.; Brewster, A.S.; Alonso-Mori, R.; et al. Structures of the Intermediates of Kok’s Photosynthetic Water Oxidation Clock. Nature 2018, 563, 421–425. [Google Scholar]
- Li, Y.; Yao, R.; Chen, Y.; Xu, B.; Chen, C.; Zhang, C. Mimicking the catalytic center for the water-Splitting reaction in photosystem II. Catalysts 2020, 10, 185. [Google Scholar] [CrossRef]
- Joliot, P.; Kok, B. Oxygen evolution in photosynthesis. In Bioenergetics of Photosynthesis; Govindjee, Ed.; Academic Press: New York, NY, USA, 1975; pp. 387–412. [Google Scholar]
- Pham, L.V.; Olmos, J.D.J.; Chernev, P.; Kargul, J.; Messinger, J. Unequal misses during the flash-induced advancement of photosystem II: Effects of the S state and acceptor side cycles. Photosynth. Res. 2019, 139, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Klauss, A.; Haumann, M.; Dau, H. Alternating electron and proton transfer steps in photosynthetic water oxidation. Proc. Natl. Acad. Sci. USA 2012, 109, 16035–16040. [Google Scholar] [PubMed]
- Amin, M.; Kaur, D.; Yang, K.R.; Wang, J.; Mohamed, Z.; Brudvig, G.W.; Gunner, M.R.; Batista, V. Thermodynamics of the S2-to-S3 state transition of the oxygen-evolving complex of photosystem II. Phys. Chem. Chem. Phys. 2019, 37, 20840–20848. [Google Scholar]
- Ibrahim, M.; Fransson, T.; Chatterjee, R.; Cheah, M.H.; Hussein, R.; Lassalle, L.; Sutherlin, K.D.; Young, I.D.; Fuller, F.D.; Gul, S.; et al. Untangling the sequence of events during the S2 → S3 transition in photosystem II and implications for the water oxidation mechanism. Proc. Natl. Acad. Sci. USA 2020, 117, 12624–12635. [Google Scholar]
- Mandal, M.; Saito, K.; Ishikita, H. Requirement of chloride for the downhill electron transfer pathway from the water-splitting center in natural photosynthesis. J. Phys. Chem. B 2022, 126, 123–131. [Google Scholar] [CrossRef]
- Klauss, A.; Sikora, T.; Süss, B.; Dau, H. Fast structural changes (200–900 ns) may prepare the photosynthetic manganese complex for oxidation by the adjacent tyrosine radical. Biochim. Biophys. Acta 2012, 1817, 1196–1207. [Google Scholar] [CrossRef]
- Klauss, A.; Haumann, M.; Dau, H. Seven steps of alternating electron and proton transfer in photosystem II water oxidation traced by time-resolved photothermal beam deflection at improved sensitivity. J. Phys. Chem. B 2015, 119, 2677–2689. [Google Scholar] [CrossRef]
- Siegbahn, P.E.M. Water oxidation mechanism in photosystem II, including oxidations, proton release pathways, O–O bond formation and O2 release. Biochim. Biophys. Acta 2013, 1827, 1003–1019. [Google Scholar]
- Barber, J. A mechanism for water splitting and oxygen production in photosynthesis. Nat. Plants 2017, 3, 17041. [Google Scholar]
- Pushkar, Y.; Davis, K.M.; Palenik, M.C. Model of the oxygen evolving complex which is highly predisposed to O–O bond formation. J. Phys. Chem. Lett. 2018, 9, 3525–3531. [Google Scholar]
- Guo, Y.; Messinger, J.; Kloo, L.; Sun, L. Reversible structural isomerization of Nature’s water oxidation catalyst prior to O–O bond formation. J. Am. Chem. Soc. 2022, 144, 11736–11747. [Google Scholar] [PubMed]
- Allgöwer, F.; Gamiz-Hernandez, A.P.; Rutherford, A.W.; Kaila, V.R.I. Molecular principles of redox-coupled protonation dynamics in photosystem II. J. Am. Chem. Soc. 2022, 144, 7171–7180. [Google Scholar] [PubMed]
- Han, G.; Chernev, P.; Styring, S.; Messinger, J.; Mamedov, F. Molecular basis for turnover inefficiencies (misses) during water oxidation in photosystem II. Chem. Sci. 2022, 13, 8667–8678. [Google Scholar] [PubMed]
- De Lichtenberg, C.; Kim, C.J.; Chernev, P.; Debus, R.J.; Messinger, J. The exchange of the fast substrate water in the S2 state of photosystem II is limited by diffusion of bulk water through channels–implications for the water oxidation mechanism. Chem. Sci. 2021, 12, 12763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, L. Why Nature chose the Mn4CaO5 cluster as water-splitting catalyst in photosystem II: A new hypothesis for the mechanism of O–O bond formation. Dalton Trans. 2018, 47, 14381–14387. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-C.; Li, J.; Siegbahn, P.E.M. A theoretical study of the recently suggested MnVII mechanism for O–O bond formation in photosystem II. J. Phys. Chem. A 2020, 124, 8011–8018. [Google Scholar]
- Ghanotakis, D.F.; Babcock, G.T.; Yocum, C.F. Calcium reconstitutes high rates of oxygen evolution in polypeptide depleted Photosystem II preparations. FEBS 1984, 167, 127–130. [Google Scholar]
- Gates, C.; Ananyev, G.; Dismukes, G.C. The strontium inorganic mutant of the water oxidizing center (CaMn4O5) of PSII improves WOC efficiency but slows electron flux through the terminal acceptors. Biochim. Biophys. Acta 2016, 1857, 1550–1560. [Google Scholar]
- Lockett, C.J.; Demetriou, C.; Simon, J.; Bowden, S.J.; Nugent, J.H.A. Studies on calcium depletion of PS II by pH 8.3 treatment. Biochim. Biophys. Acta 1990, 1016, 213–218. [Google Scholar]
- Miqyass, M.; Marosvolgyi, M.A.; Nagel, Z.; Yocum, C.F.; van Gorkom, H.J. S-state dependence of the calcium requirement and binding characteristics in the oxygen-evolving complex of photosystem II. Biochemistry 2008, 47, 7915–7924. [Google Scholar]
- Latimer, M.J. The Role of Calcium in the Oxygen Evolving Center of Photosystem II. Ph.D. Thesis, Lawrence Berkeley Laboratory, University of California, Berkeley, CA, USA, May 1995. [Google Scholar]
- Yachandra, V.K.; Yano, J. Calcium in the oxygen-evolving complex: Structural and mechanistic role determined by X-ray spectroscopy. J. Photochem. Photobiol. B Biol. 2011, 104, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, H.; Mino, H. Local Structural Modification of Ca2+-Depleted Photosystem II Detected by Proton Matrix ENDOR. Appl. Magn. Reson. 2018, 49, 803–812. [Google Scholar] [CrossRef]
- Nagashima, H.; Nakajima, Y.; Shen, J.-R.; Mino, H. Proton matrix ENDOR studies on Ca2+-depleted and Sr2+-substituted manganese cluster in photosystem II. J. Biol. Chem. 2015, 290, 28166–28174. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Li, Y.; Chen, Y.; Xu, B.; Chen, C.; Zhang, C. Rare-earth elements can structurally and energetically replace the calcium in a synthetic Mn4CaO4-cluster mimicking the oxygen-evolving center in photosynthesis. J. Am. Chem. Soc. 2021, 143, 17360–17365. [Google Scholar] [CrossRef]
- Tsui, E.Y.; Agapie, T. Reduction potentials of heterometallic manganese–oxido cubane complexes modulated by redox-inactive metals. Proc. Natl. Acad. Sci. USA 2013, 110, 10084–10088. [Google Scholar] [CrossRef]
- Bang, S.; Lee, Y.-M.; Hong, S.; Cho, K.-B.; Nishida, Y.; Seo, M.S.; Sarangi, R.; Fukuzumi, S.; Nam, W. Redox-inactive metal ions modulate the reactivity and oxygen release of mononuclear non-haem iron(III)–peroxo complexes. Nat. Chem. 2014, 6, 394–939. [Google Scholar] [CrossRef] [Green Version]
- Begouin, J.-M.; Niggemann, M. Calcium-based lewis acid catalysts. Chem. Eur. J. 2013, 19, 8030–8041. [Google Scholar] [CrossRef]
- Hill, M.S.; Liptrot, D.J.; Weetman, C. Alkaline earths as main group reagents in molecular catalysis. Chem. Soc. Rev. 2016, 45, 972–988. [Google Scholar] [CrossRef]
- Boyle, F.P. Some Factors involved in oxygen evolution from triturated spinach leaves. Science 1948, 108, 359–360. [Google Scholar] [CrossRef]
- Warburg, O.; Krippahl, G. Notwendigkeit der Kohlensäure für die Chinon- und Ferricyanid-Reaktionen in grünen Grana. Zschr. Naturforsch. 1960, 15, 367–369. [Google Scholar] [CrossRef]
- Govindjee, W.T. A new site of bicarbonate effect in photosystem II of photosynthesis: Evidence from chlorophyll fluorescence transients in spinach chloroplasts. Biochim. Biophys. Acta 1975, 387, 403–408. [Google Scholar]
- Brinkert, K.; De Causmaecker, S.; Krieger-Liszkay, A.; Fantuzzia, A.; Rutherford, A.W. Bicarbonate-induced redox tuning in Photosystem II for regulation and protection. Proc. Natl. Acad. Sci. USA 2016, 113, 12144–12149. [Google Scholar] [CrossRef] [PubMed]
- Vermaas, W.F.J.; Rutherford, A.W. EPR measurements on the effects of bicarbonate and triazine resistance on the acceptor side of Photosystem II. FEBS 1984, 175, 243–248. [Google Scholar] [CrossRef]
- Shevela, D.; Nöring, B.; Koroidov, S.; Shutova, T.; Samuelsson, G.; Messinger, J.J. Efficiency of photosynthetic water oxidation at ambient and depleted levels of inorganic carbon. Photosynth. Res. 2013, 117, 401–412. [Google Scholar] [CrossRef]
- Aoyama, C.; Suzuki, H.; Sugiura, M.; Noguchi, T. Flash-induced FTIR difference spectroscopy shows no evidence for the structural coupling of bicarbonate to the oxygen-evolving Mn cluster in photosystem II. Biochemistry 2008, 47, 2760–2765. [Google Scholar] [CrossRef]
- Shevela, D.; Su, J.H.; Klimov, V.; Messinger, J. Hydrogencarbonate is not a tightly bound constituent of the water-oxidizing complex in photosystem II. Biochim. Biophys. Acta 2008, 1777, 532–539. [Google Scholar] [CrossRef]
- Ulas, G.; Olack, G.; Brudvig, G.W. Evidence against bicarbonate bound in the O2-evolving complex of photosystem II. Biochemistry 2008, 47, 3073–3075. [Google Scholar] [CrossRef]
- Koroidov, S.; Shevela, D.; Shutova, T.; Samuelsson, G.; Messinger, J. Mobile hydrogen carbonate acts as proton acceptor in photosynthetic water oxidation. Proc. Natl. Acad. Sci. USA 2014, 111, 6299–6304. [Google Scholar] [CrossRef]
- Tikhonov, K.; Shevela, D.; Klimov, V.V.; Messinger, J. Quantification of bound bicarbonate in photosystem II. Photosynthetica 2018, 56, 210–216. [Google Scholar] [CrossRef]
- Khorobrykh, A.; Dasgupta, J.; Kolling, D.R.J.; Terentyev, V.; Klimov, V.V.; Dismukes, C.C. Evolutionary origins of the photosynthetic water oxidation cluster: Bicarbonate permits Mn2+ photooxidation by anoxygenic bacterial reaction centers. ChemBioChem 2013, 14, 1725–1731. [Google Scholar] [CrossRef]
- Mizrahi, A.; Meyerstein, D. Plausible roles of carbonate in catalytic water oxidation. Adv. Inorg. Chem. 2013, 74, 343–360. [Google Scholar] [CrossRef]
- Kálmán, L.; Williams, J.C.; Allen, J.P. Energetics for oxidation of a bound manganese cofactor in modified bacterial reaction centers. Biochemistry 2011, 50, 3310–3320. [Google Scholar] [CrossRef]
- Kozlov, Y.N.; Zharmukhamedov, S.K.; Tikhonov, K.G.; Dasgupta, J.; Kazakova, A.A.; Dismukes, G.C.; Klimov, V.V. Oxidation potentials and electron donation to photosystem II of manganese complexes containing bicarbonate and carboxylate ligands. Phys. Chem. Chem. Phys. 2004, 6, 4905–4911. [Google Scholar] [CrossRef]
- Nickelsen, J.; Rengstl, B. Photosystem II assembly: From cyanobacteria to plants. Annu. Rev. Plant Biol. 2013, 64, 609–635. [Google Scholar] [CrossRef] [PubMed]
- Ono, T. Metallo-radical hypothesis for photoassembly of (Mn)4-cluster of photosynthetic oxygen evolving complex. Biochim. Biophys. Acta 2001, 1503, 40–51. [Google Scholar] [CrossRef]
- Burnap, R.L. D1 protein processing and Mn cluster assembly in light of the emerging photosystem II structure. Phys. Chem. Chem. Phys. 2004, 6, 4803–4809. [Google Scholar] [CrossRef]
- Huang, G.; Xiao, Y.; Pi, X.; Zhao, L.; Zhu, Q.; Wang, W.; Kuang, T.; Han, G.; Sui, S.-F.; Shen, J.-R. Structural insights into a dimeric Psb27-photosystem II complex from a cyanobacterium Thermosynechococcus vulcanus. Proc. Natl. Acad. Sci. USA 2021, 118, e2018053118. [Google Scholar] [CrossRef]
- Baranov, S.V.; Tyryshkin, A.M.; Katz, D.; Dismukes, G.C.; Ananyev, G.M.; Klimov, V.V. Bicarbonate is a native cofactor for assembly of the manganese cluster of the photosynthetic water oxidizing complex. Kinetics of reconstitution of O2 evolution by photoactivation. Biochemistry 2004, 43, 2070–2079. [Google Scholar] [CrossRef]
- Hwang, H.J.; Burnap, R.L. Multiflash experiments reveal a new kinetic phase of photosystem II manganese cluster assembly in Synechocystis sp. PCC6803 in vivo. Biochemistry 2005, 44, 9766–9774. [Google Scholar] [CrossRef]
- Avramov, A.P.; Hwanga, H.J.; Burnap, R.L. The role of Ca2+ and protein scaffolding in the formation of nature’s water oxidizing complex. Proc. Natl. Acad. Sci. USA 2020, 117, 28036–28045. [Google Scholar] [CrossRef]
- Russell, B.P.; Vinyard, D.J. Chloride facilitates Mn(III) formation during photoassembly of the photosystem II oxygen-evolving complex. Photosynth. Res. 2021, 1–6. [Google Scholar] [CrossRef]
- Sato, A.; Nakano, Y.; Nakamura, S.; Noguchi, T. Rapid-scan time-resolved ATR-FTIR study on the photoassembly of the water-oxidizing Mn4CaO5 cluster in photosystem II. J. Phys. Chem. B 2021, 125, 4031–4045. [Google Scholar] [CrossRef] [PubMed]
- Cheniae, G.M.; Martin, I.F. Photoactivation of the manganese catalyst of O2 evolution. I. Biochemical and kinetic aspects. Biochim. Biophys. Acta 1971, 253, 167–181. [Google Scholar] [CrossRef]
- Sauer, K.; Yachandra, V.K. A possible evolutionary origin for the Mn4 cluster of the photosynthetic water oxidation complex from natural MnO2 precipitates in the early ocean. Proc. Natl. Acad. Sci. USA 2002, 99, 8631–8636. [Google Scholar] [CrossRef] [PubMed]
- Dismukes, G.C.; Klimov, V.V.; Baranov, S.V.; Kozlov, Y.N.; DasGupta, J.; Tyryshkin, A.; Dismukes, G.C. The origin of atmospheric oxygen on Earth: The innovation of oxygenic photosynthesis. Proc. Natl Acad. Sci. USA 2001, 98, 2170–2175. [Google Scholar] [CrossRef] [PubMed]
- Chernev, P.; Fischer, S.; Hoffmann, J.; Oliver, N.; Assunção, R.; Yu, B.; Burnap, R.L.; Zaharieva, I.; Nürnberg, D.J.; Haumann, M.; et al. Light-driven formation of manganese oxide by today’s photosystem II supports evolutionarily ancient manganese-oxidizing photosynthesis. Nat. Commun. 2020, 11, 6110. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).