El Niño-Driven Changes in Zooplankton Community Structure in an Amazonian Tropical Estuarine Ecosystem (Taperaçu, Northern Brazil)
Abstract
1. Introduction
2. Materials and Methods
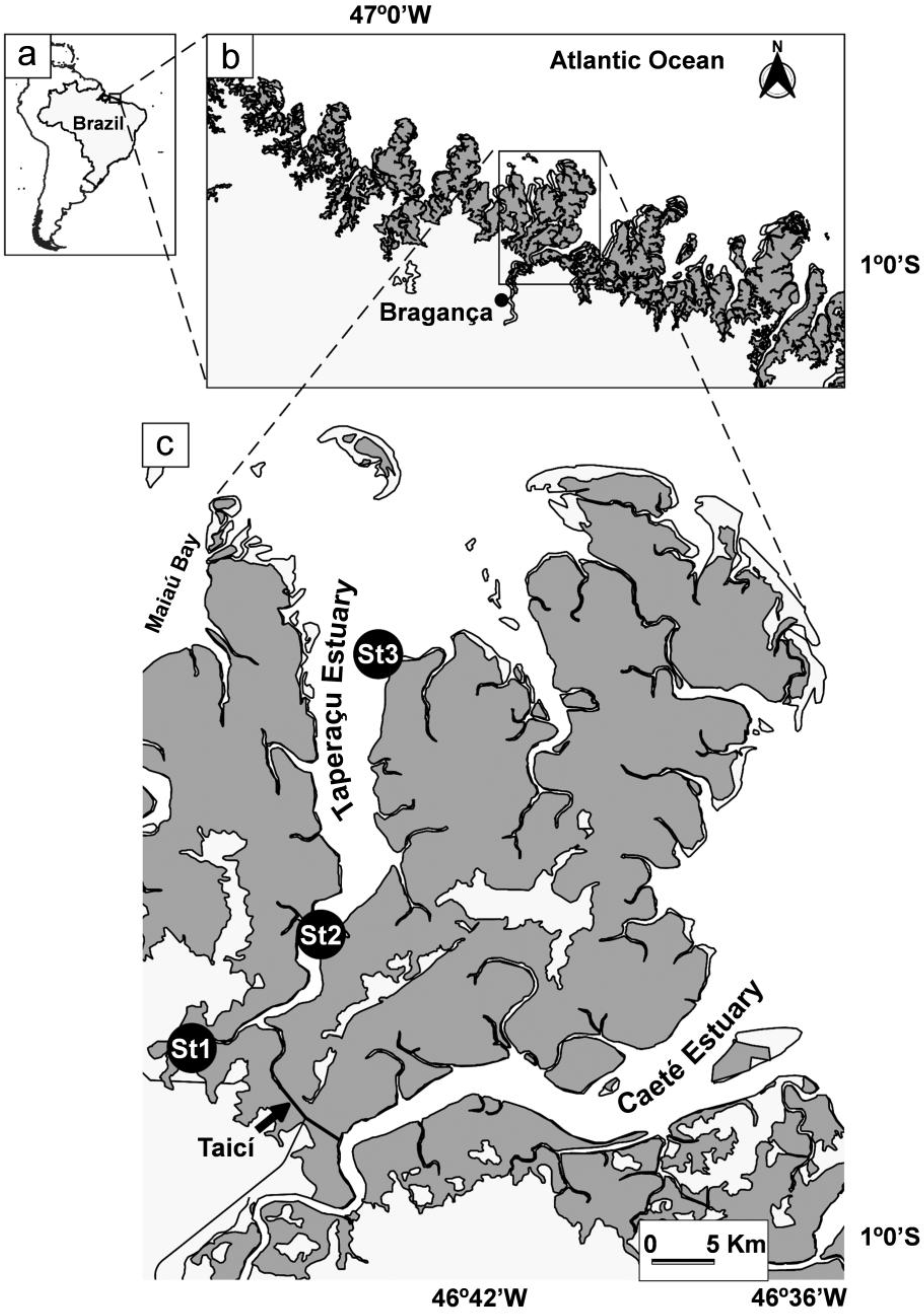
2.1. Study Area
2.2. Sampling and Study Design
2.3. Laboratory Methods
2.4. Statistical Analyses
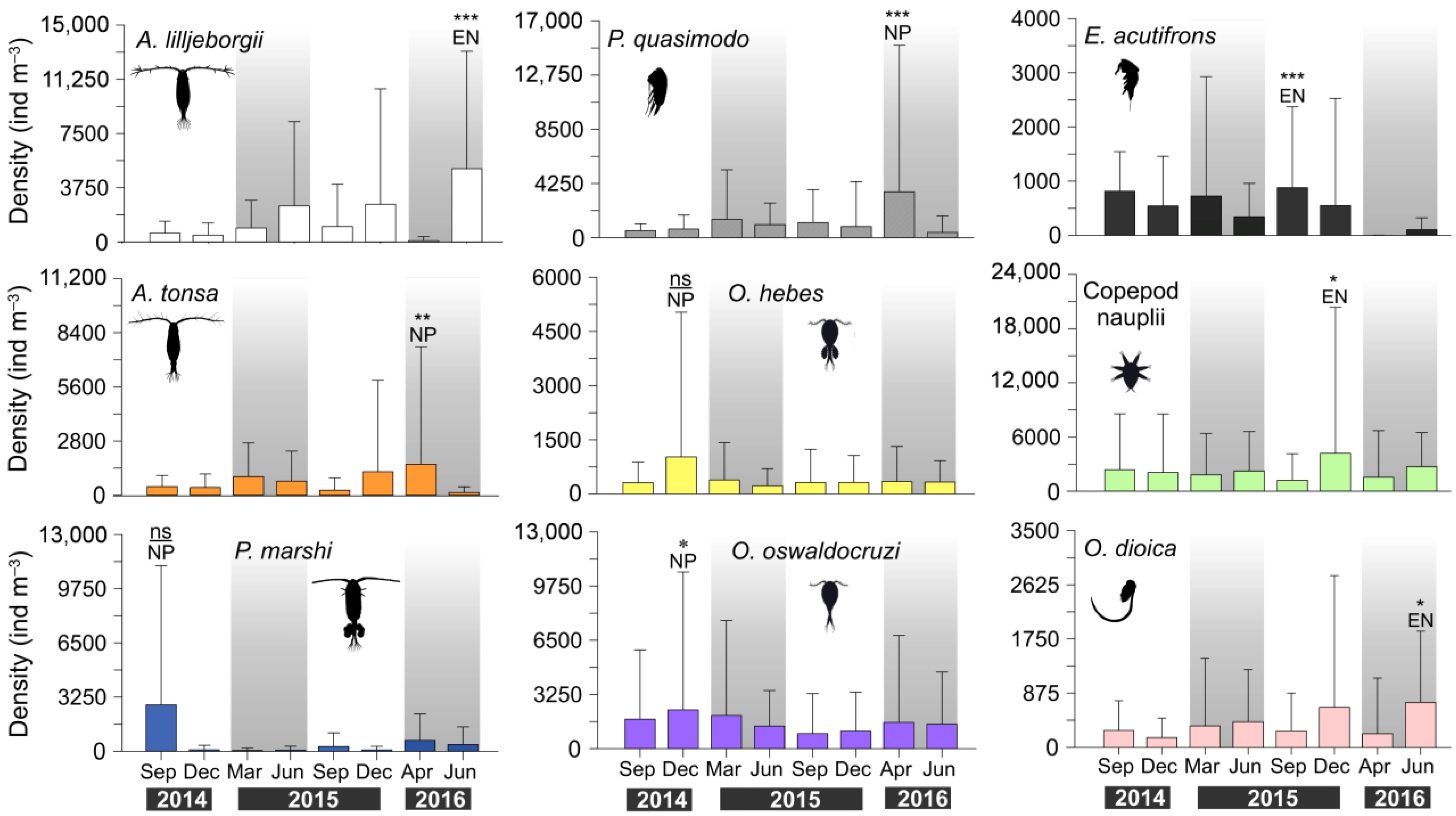
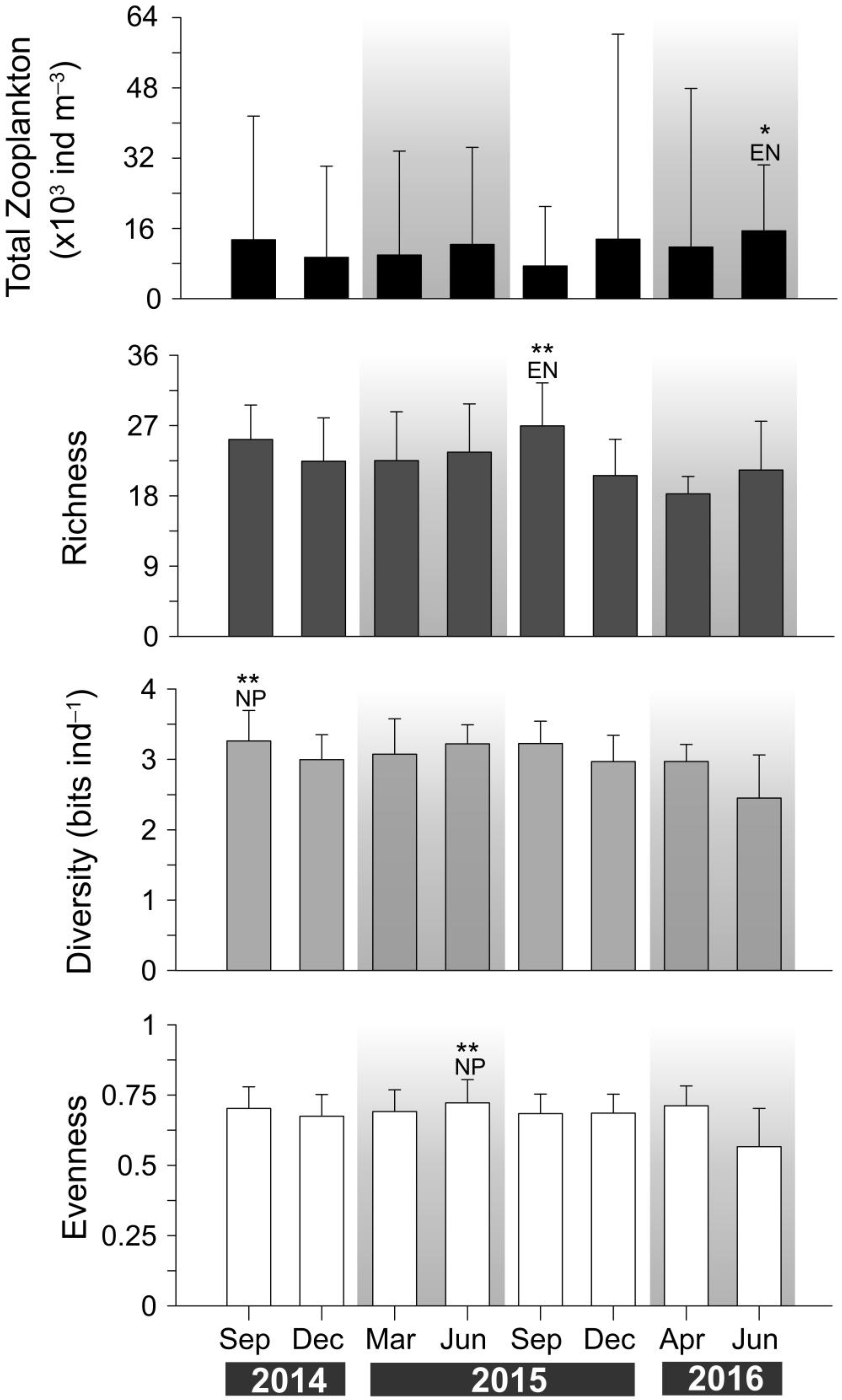
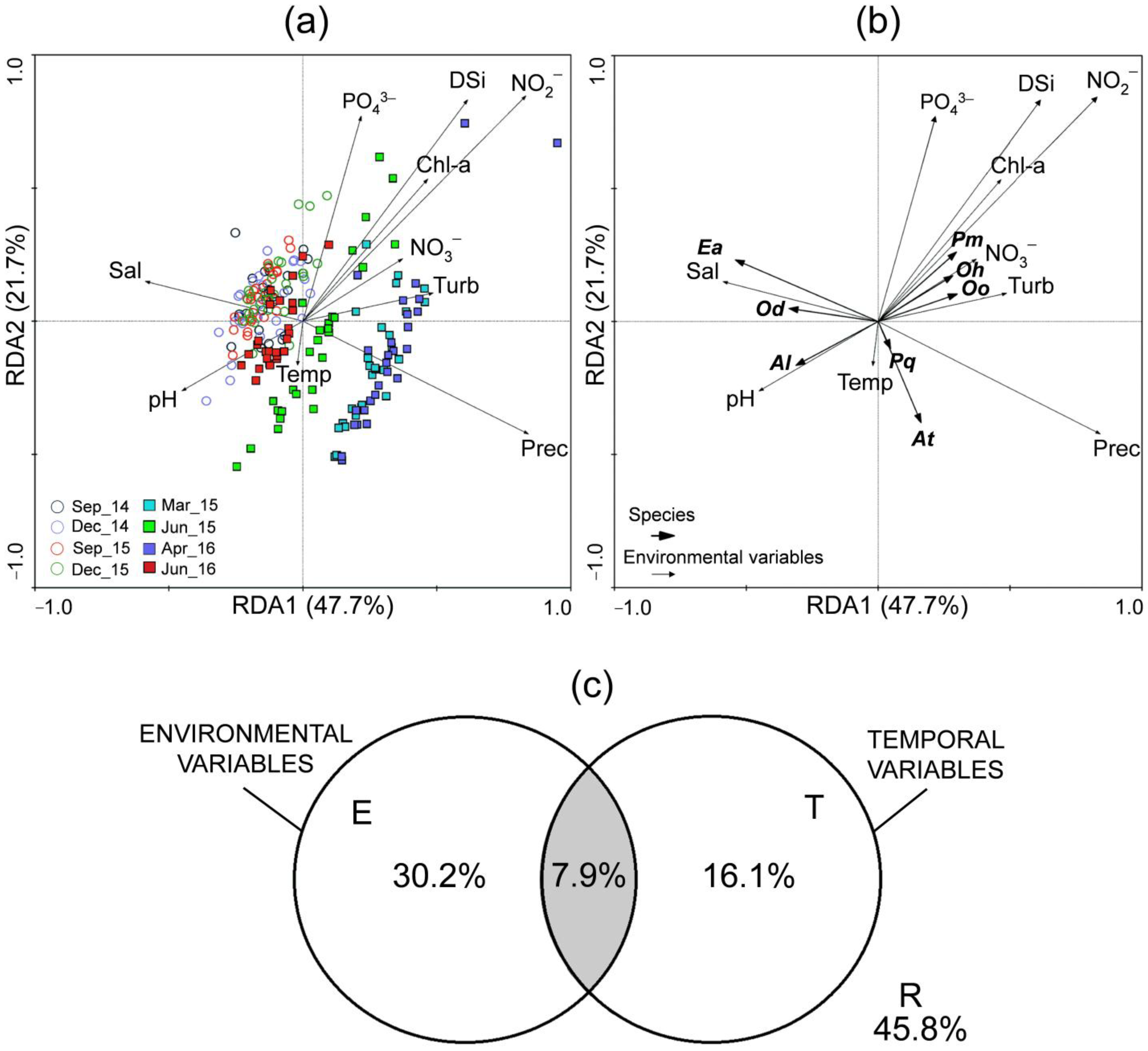
3. Results
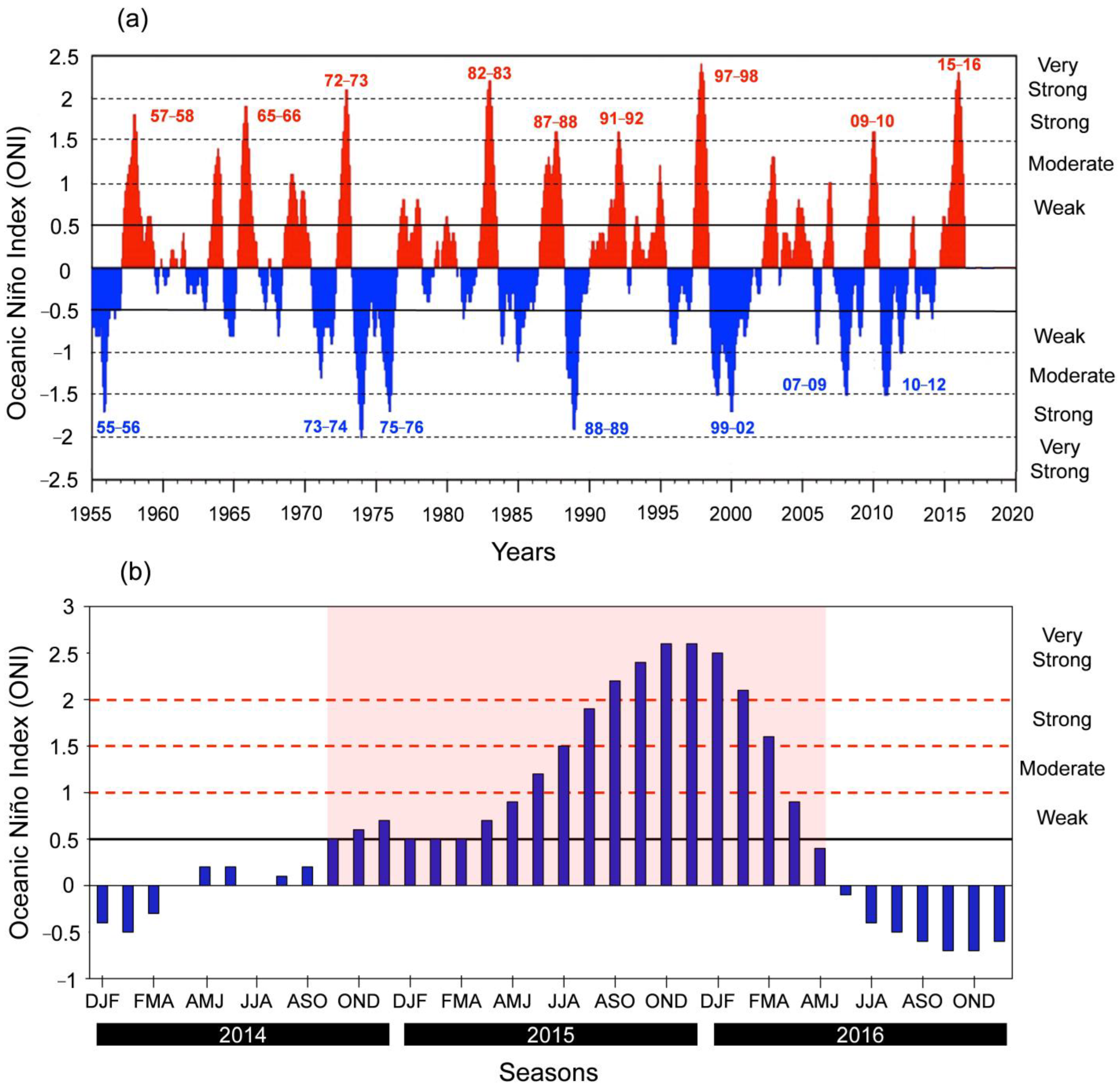
3.1. Changes in Rainfall Levels and Their Effects on Salinity and Chlorophyll-A Concentrations During Normal and El Niño Periods
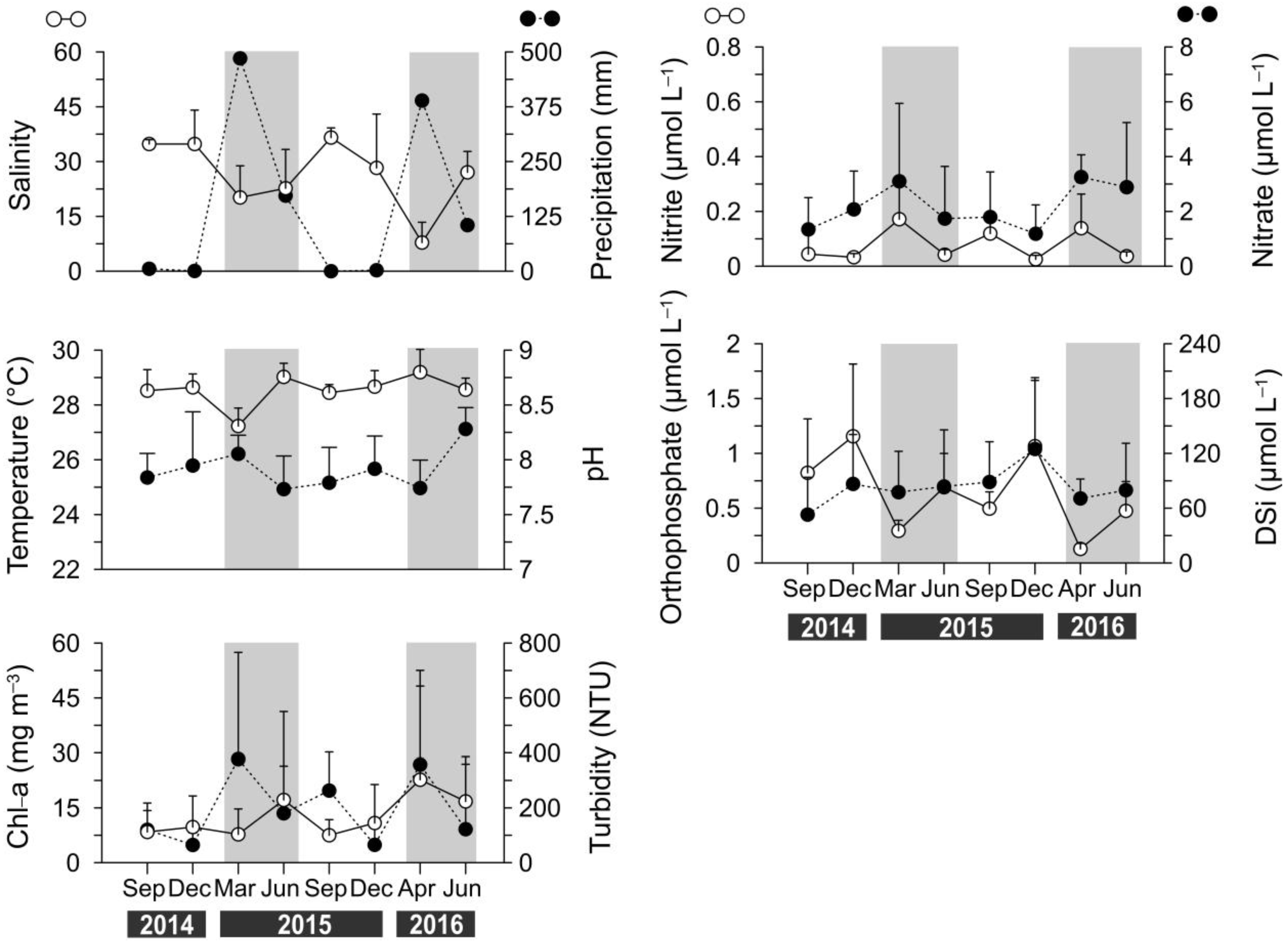
3.2. Hydrological Data
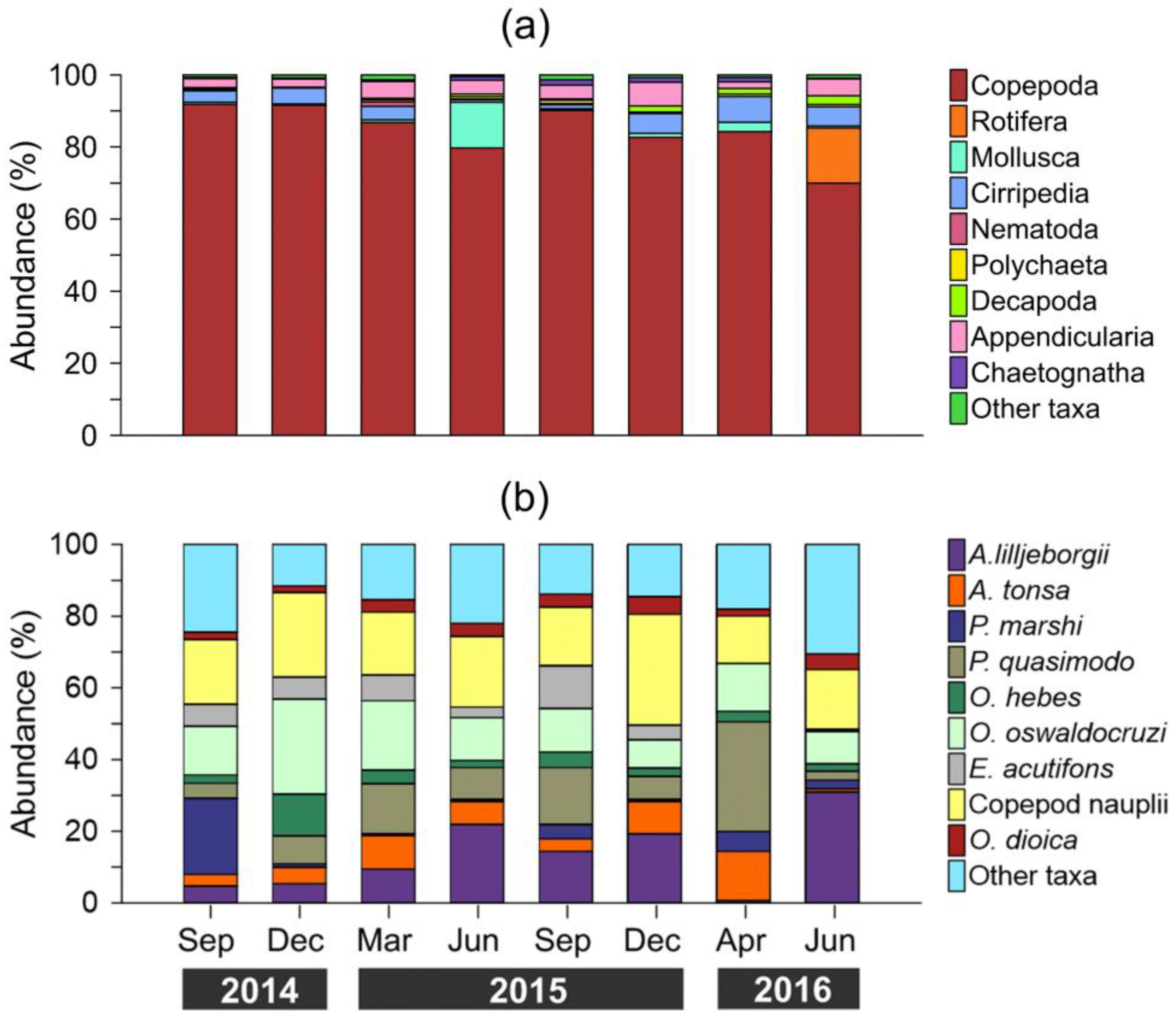
3.3. Zooplankton Composition and Temporal Variability
3.4. Multivariate Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, W.; Santoso, A.; Wang, G.; Yeh, S.-W.; An, S.-I.; Cobb, K.M.; Collins, M.; Guilyardi, E.; Jin, F.-F.; Kug, J.-S.; et al. ENSO and greenhouse warming. Nat. Clim. Chang. 2015, 5, 849–859. [Google Scholar] [CrossRef]
- Harari, O.; Garfinkel, C.I.; Ziskin Ziv, S.; Morgenstern, O.; Zeng, G.; Tilmes, S.; Kinnison, D.; Deushi, M.; Jöckel, P.; Pozzer, A.; et al. Influence of Arctic stratospheric ozone on surface climate in CCMI models. Atmos. Chem. Phys. 2019, 19, 9253–9268. [Google Scholar] [CrossRef]
- Capotondi, A.; Wittenberg, A.T.; Newman, M.; Di Lorenzo, E.; Yu, J.-Y.; Braconnot, P.; Cole, J.; Dewitte, B.; Giese, B.; Guilyardi, E.; et al. Understanding ENSO diversity. Bull. Am. Meteorol. Soc. 2015, 96, 921–938. [Google Scholar] [CrossRef]
- Marengo, J.A.; Alves, L.M.; Soares, W.R.; Rodriguez, D.A.; Camargo, H.; Riveros, M.P.; Pabló, A.D. Two contrasting severe seasonal extremes in tropical South America in 2012: Flood in Amazonia and drought in northeast Brazil. J. Clim. 2013, 26, 9137–9154. [Google Scholar] [CrossRef]
- dos Santos, L.O.F.; Machado, N.G.; Querino, C.A.S.; Biudes, M.S. Trends of Climate Extremes and Their Relationships with Tropical Ocean Temperatures in South America. Earth 2024, 5, 844–872. [Google Scholar] [CrossRef]
- Marengo, J.A.; Espinoza, J.C. Extreme seasonal droughts and floods in Amazonia: Causes, trends and impacts. Int. J. Climatol. 2016, 36, 1033–1050. [Google Scholar] [CrossRef]
- Gloor, M.; Barichivich, J.; Ziv, G.; Brienen, R.; Schöngart, J.; Peylin, P.; Cintra, B.B.L.; Feldpausch, T.; Phillips, O.; Baker, J. Recent Amazon climate as background for possible ongoing and future changes of Amazon humid forests. Glob. Biogeochem. Cycles 2015, 29, 1384–1399. [Google Scholar] [CrossRef]
- Pastana, D.N.B.; Modena, É.S.; Wadt, L.H.O.; Neves, E.S.; Martorano, L.G.; Lira-Guedes, A.C.; Souza, R.L.F.; Costa, F.F.; Batista, A.P.B.; Guedes, M.C. Strong El Niño reduces fruit production of Brazil-nut trees in the eastern Amazon. Acta Amaz. 2021, 51, 270–279. [Google Scholar] [CrossRef]
- Marengo, J.A.; Cunha, A.P.; Espinoza, J.C.; Fu, R.; Schöngart, J.; Jimenez, J.C.; Zhao, S. The drought of Amazonia in 2023–2024. Am. J. Clim. Chang. 2024, 13, 567–597. [Google Scholar] [CrossRef]
- Phillips, O.L.; Aragão, L.E.O.C.; Lewis, S.L.; Fisher, J.B.; Lloyd, J.; López-González, G.; Malhi, Y.; Monteagudo, A.; Peacock, J.; Quesada, C.A.; et al. Drought sensitivity of the Amazon rainforest. Science 2009, 323, 1344–1347. [Google Scholar] [CrossRef]
- Lewis, S.L.; Brando, P.M.; Phillips, O.L.; van der Heijden, G.M.F.; Nepstad, D. The 2010 Amazon drought. Science 2011, 331, 554. [Google Scholar] [CrossRef]
- Ribeiro, G.G.; Anderson, L.O.; Barretos, N.J.C.; Abreu, R.; Alves, L.; Dong, B.; Lott, F.C.; Tett, S.F.B. Tett. Attributing the 2015/2016 Amazon basin drought to anthropogenic influence. Clim. Resil. Sustain. 2022, 1, e25. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration: NOAA. National Weather Service: Climate Prediction Center. Available online: https://www.cpc.ncep.noaa.gov (accessed on 23 April 2023).
- Espinoza, J.C.; Ronchail, J.; Guyot, J.L.; Junquas, C.; Vauchel, P.; Lavado, W.; Drapeau, G.; Pombosa, R. Climate variability and extremes drought in the upper Solimões River (western Amazon Basin): Understanding the exceptional 2010 drought. Geophys. Res. Lett. 2011, 38, L13406. [Google Scholar] [CrossRef]
- Andreoli, R.V.; Souza, R.A.F.; Kayano, M.T.; Candido, L.A. Seasonal anomalous rainfall in the central and eastern Amazon and associated anomalous oceanic and atmospheric patterns. Int. J. Clim. 2012, 32, 1193–1205. [Google Scholar] [CrossRef]
- Marengo, J.A.; Torres, R.R.; Alves, L.M. Drought in Northeast Brazil-past, present, and future. Theor. Appl. Clim. 2017, 129, 1189–1200. [Google Scholar] [CrossRef]
- Marengo, J.A.; Alves, L.M.; Alvala, R.C.S.; Cunha, A.P.; Brito, S.; Moraes, O.L.L. Climatic characteristics of the 2010–2016 drought in the semiarid Northeast Brazil region. Acad. Bras. Cienc. 2018, 90, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.C.C.; Costa, Á.K.R.; Costa, R.M.; Magalhães, A.; Flores-Montes, M.J.; Jiménez, J.A. Influence of a drought event on hydrological characteristics of a small estuary on the Amazon mangrove coast. Estuar. Coast. 2018, 41, 676–689. [Google Scholar] [CrossRef]
- Procópio, A.D.; Costa, R.M.; Magalhaes, A.; Silva, D.C.; Silva, T.R.C.; Fernandes, F.D.S.; Pereira, L.C.C. Effects of the El Niño 2015/2016 event on Acartia tonsa, A. lilljeborgii (Copepoda) production in a Brazilian Amazon estuary. Ecohydrol. Hydrobiol. 2024, 25, 303–314. [Google Scholar] [CrossRef]
- de Andrade, M.P.; Magalhães, A.; Sousa, N.D.S.D.S.; Pereira, L.C.C.; da Costa, R.M. Impact of Drought—Induced Hydrological Changes on Copepod Communities in an Amazonian Estuary. Mar. Ecol. 2025, 46, e70027. [Google Scholar] [CrossRef]
- Arcos, D.F.; Cubillos, L.A.; Núñez, S.P. The jack mackerel fishery and El Niño 1997–1998 effects off Chile. Prog. Oceanogr. 2001, 49, 597–617. [Google Scholar] [CrossRef]
- Jiménez-Quiroz, M.d.C.; Cervantes-Duarte, R.; Funes-Rodríguez, R.; Barón-Campis, S.A.; García-Romero, F.d.J.; Hernández-Trujillo, S.; Hernández-Becerril, D.U.; González-Armas, R.; Martell-Dubois, R.; Cerdeira-Estrada, S.; et al. Impact of “The Blob” and “El Niño” in the SW Baja California Peninsula: Plankton and environmental variability of Bahia Magdalena. Front. Mar. Sci. 2019, 6, 25. [Google Scholar] [CrossRef]
- McClatchie, S.; Goericke, R.; Leising, A.; Auth, T.D. State of the California current 2015-16: Comparisons with the 1997-98 El Niño. CalCOFI Rep. 2016, 57, 1–57. [Google Scholar]
- Chavez, F.P.; Pennington, J.T.; Castro, C.G.; Ryan, J.P.; Michisaki, R.P.; Schlining, B.; Walz, P.; Buck, K.R.; McFadyen, A.; Collins, C.A. Biological and chemical consequences of the 1997–1998 El Niño in central California waters. Prog. Oceanogr. 2002, 54, 205–232. [Google Scholar] [CrossRef]
- Bertrand, A.; Lengaigne, M.; Takahashi, K.; Avadí, A.; Poulain, F.; Harrod, C. El Niño Southern Oscillation (ENSO) Effects on Fisheries and Aquaculture; FAO Fisheries and Aquaculture Technical Paper No. 660; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Romero, F.P. Centro Soberania e Clima. “Sovereignty and Climate Dialogue: Special Issue No. 01—January 2024.” Dialogos-Soberania-e-Clima 3, No. 1 (2024). Available online: https://soberaniaeclima.org.br/wp-content/uploads/2025/04/Dialogos-Soberania-e-Clima-Especial-No_01-Janeiro-2024-English-03-73-84.pdf. (accessed on 28 September 2025).
- Mackas, D.L.; Pepin, P.; Verheye, H. Interannual variability of marine zooplankton and their environments: Within- and between-region comparisons. Prog. Oceanogr. 2012, 97, 1–14. [Google Scholar] [CrossRef]
- Steinberg, D.K.; Landry, M.R. Zooplankton and the ocean carbon cycle. Ann. Rev. Mar. Sci. 2017, 9, 413–444. [Google Scholar] [CrossRef] [PubMed]
- Böckmann, S.; Koch, F.; Meyer, B.; Pausch, F.; Iversen, M.; Driscoll, R.; Laglera, L.M.; Hassler, C.; Trimborn, S. Salp fecal pellets release more bioavailable iron to Southern Ocean phytoplankton than krill fecal pellets. Curr. Biol. 2021, 31, 2737–2746. [Google Scholar] [CrossRef]
- Beaugrand, G.; Conversi, A.; Atkinson, A.; Cloern, J.; Chiba, S.; Fonda-Umani, S.; Kirby, R.R.; Greene, C.H.; Goberville, E.; Otto, S.A.; et al. Prediction of unprecedented biological shifts in the global ocean. Nat. Clim. Chang. 2019, 9, 237–243. [Google Scholar] [CrossRef]
- Heneghan, R.F.; Everett, J.D.; Blanchard, J.L.; Sykes, P.; Richardson, A.J. Climate-driven zooplankton shifts cause large-scale declines in food quality for fish. Nat. Clim. Chang. 2023, 13, 470–477. [Google Scholar] [CrossRef]
- Dam, H.G.; Baumann, H. Climate Change, Zooplankton and Fisheries. In Climate Change Impacts on Fisheries and Aquaculture: A Global Analysis; Phillips, B.F., Pérez-Ramírez, M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 851–874. [Google Scholar] [CrossRef]
- Ratnarajah, L.; Abu-Alhaija, R.; Atkinson, A.; Batten, S.; Bax, N.J.; Bernard, K.S.; Canonico, G.; Cornils, A.; Everett, J.D.; Grigoratou, M.; et al. Monitoring and modelling marine zooplankton in a changing climate. Nat. Commun. 2023, 14, 564. [Google Scholar] [CrossRef]
- Costa, A.K.R.; Pereira, L.C.C.; Jiménez, J.A.; Oliveira, A.R.G.; Flores-Montes, M.J.; Costa, R.M. Effects of Extreme Climatic Events on the Hydrological Parameters of the Estuarine Waters of the Amazon Coast. Estuar. Coast. 2022, 45, 1517–1533. [Google Scholar] [CrossRef]
- Asp, N.E.; Schettini, C.A.F.; Siegle, E.; Silva, M.S.; Brito, R.N.B. The dynamics of a frictionally-dominated Amazonic estuary. Braz. J. Oceanogr. 2012, 60, 391–403. [Google Scholar] [CrossRef]
- Araújo, W.P.; Asp, N.E. Hydrodynamic connectivity between two macrotidal Amazonian estuaries. J. Coast. Res. Spec. Issue 2013, 65, 1086–1091. [Google Scholar] [CrossRef]
- Diretoria de Hidrografia e Navegação: DHN. Tidal Predictions. Available online: http://www.dhn.mar.mil.br/chm/tabuas. (accessed on 28 September 2022).
- Instituto Nacional de Meteorologia: INMET. Meteorological Database. Available online: https://portal.inmet.gov.br. (accessed on 21 September 2021).
- Jiménez-Muñoz, J.C.; Mattar, C.; Barichivich, J.; Santamaría-Artigas, A.; Takahashi, K.; Malhi, Y.; Sobrino, J.A.; van der Schrier, G. Record-breaking warming and extreme drought in the Amazon rainforest during the course of El Niño 2015–2016. Sci. Rep. 2016, 6, 33130. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.K.; Rajagopalan, B.; Hoerling, M.; Bates, G.; Cane, M. Unraveling the mystery of Indian monsoon failure during El Niño. Science 2006, 314, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Hendon, H.H. Sensitivity of Australian rainfall to inter–El Niño variations. J. Clim. 2007, 20, 4211–4226. [Google Scholar] [CrossRef]
- Sekhon, N.; David, C.P.C.; Geronia, M.C.M.; Custado, M.J.G.; Ibarra, D.E. Investigating the response of hydrological processes to El Niño events using a 100-year dataset from the western Pacific Ocean. J. Hydrol. Reg. Stud. 2022, 42, 101174. [Google Scholar] [CrossRef]
- Magalhães, A.; Pereira, L.C.C.; Costa, R.M. Relationships between copepod community structure, rainfall regimes, and hydrological variables in a tropical mangrove estuary (Amazon coast, Brazil). Helgol. Mar. Res. 2015, 69, 123–136. [Google Scholar] [CrossRef]
- Andrade, M.P.; Magalhães, A.; Pereira, L.C.C.; Flores-Montes, M.J.; Pardal, E.C.; Andrade, T.P.; Costa, R.M. Effects of a La Niña event on hydrological patterns and copepod community structure in a shallow tropical estuary (Taperaçu, Northern Brazil). J. Mar. Syst. 2016, 164, 128–143. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1972. [Google Scholar]
- Grasshoff, K.; Ehrhardt, M.; Kremling, K. Methods of Seawater Analysis; Verlag Chemie: Weinheim, Germany, 1983. [Google Scholar]
- Parsons, T.R.; Strickland, J.D.H. Discussion of spectrophotometric determination of marine-plant pigments, with revised equations of ascertaining chlorophylls and carotenoids. J. Mar. Res. 1963, 21, 155–163. [Google Scholar] [CrossRef]
- United Nations Educational, Scientific and Cultural Organization (UNESCO). Monographs on Oceanographic Methodology, I. Determination of Photosynthetic Pigments in Sea-Water; UNESCO: Paris, France, 1966. [Google Scholar]
- Smith, D.L. A Guide to Marine Coastal Plankton and Marine Invertebrate Larvae; Kendall Hunt Publishing Company: Dubuque, IA, USA, 1977. [Google Scholar]
- Boltovskoy, D. Atlas del Zooplancton del Atlántico Sudoccidental y Métodos de Trabajo con el Zooplancton Marino; Instituto Nacional de Investigación y Desarrollo Pesquero (INIDEP): Mar del Plata, Argentina, 1981. [Google Scholar]
- Bradford-Grieve, J.M.; Markhaseva, E.L.; Rocha, C.E.F.; Abiahy, B. Copepoda. In South Atlantic Zooplankton; Boltovskoy, D., Ed.; Backhuys Publishers: Leiden, The Netherlands, 1999; pp. 869–1109. [Google Scholar]
- World Register of Marine Species: WoRMS. Marine Organisms Database. Available online: https://www.marinespecies.org. (accessed on 7 January 2023).
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Pielou, E.C. Mathematical Ecology; John Wiley & Sons: New York, NY, USA, 1977. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; Plymouth Marine Laboratory: Plymouth, UK, 1994. [Google Scholar]
- Zar, J.H. Biostatistical Analysis: Pearson New International Edition; Pearson Education: Harlow, UK, 2013. [Google Scholar]
- ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Legendre, P. Studying beta diversity: Ecological variation partitioning by multiple regression and canonical analysis. J. Plant Ecol. 2008, 1, 3–8. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P.; Drapeau, P. Partialling out the spatial component of ecological variation. Ecology 1992, 73, 1045–1055. [Google Scholar] [CrossRef]
- Leite, N.d.R.; Andrade, T.; Silva, L.; da Costa, Á.K.; Oliveira, A.R.; Magalhães, A.; Flores-Montes, M.; Pereira, L.C.; da Costa, R.M. Secondary production of Acartia tonsa Dana, 1.8.4.9.; A lilljeborgii Giesbrecht, 1889 in an Amazonian estuary (Brazil). Mar. Environ. Res. 2023, 191, 106148. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.A.L.; Andrade, M.P.; Silva, T.R.C.; Pereira, L.C.C.; Costa, R.M. Composition and spatio-temporal changes in mesozooplankton diversity and density in an Amazonian estuary (Emboraí Velho, Pará, Brazil). Bol. Mus. Para. Emílio Goeldi-Ciências Nat. 2019, 14, 307–330. [Google Scholar] [CrossRef]
- Brugnoli-Olivera, E.; Morales-Ramírez, A. Trophic planktonic dynamics in a tropical estuary, Gulf of Nicoya, Pacific coast of Costa Rica during El Niño 1997 event. Rev. Biol. Mar. Oceanogr. 2008, 43, 75–89. [Google Scholar] [CrossRef]
- Leite, N.R.; Magalhães, A.; Silva, L.M.; Andrade, T.P.; Matos, J.B.; Costa, A.K.; Pereira, L.C.C.; Flores-Montes, M.J.; Costa, R.M. Short and medium term variation in the dynamics of the mesozooplankton community of an Amazonian estuary. J. Coast. Res. Spec. Issue 2016, 75, 218–222. [Google Scholar] [CrossRef]
- Menéndez, M.C.; Dutto, M.S.; Piccolo, M.C.; Hoffmeyer, M.S. The role of the seasonal and semi-diurnal tidal cycle on mesozooplankton variability in a shallow mixed estuary (Bahía Blanca, Argentina). ICES J. Mar. Sci. 2012, 69, 389–398. [Google Scholar] [CrossRef]
- Primo, A.L.; Azeiteiro, U.M.; Marques, S.C.; Martinho, F.; Pardal, M.A. Changes in zooplankton diversity and distribution pattern under varying precipitation regimes in a southern temperate estuary. Estuar. Coast. Shelf Sci. 2009, 82, 341–347. [Google Scholar] [CrossRef]
- Sterza, J.M.; Fernandes, L.L. Zooplankton community of the Vitória Bay estuarine system (Southeastern Brazil): Characterization during a three-year study. Braz. J. Oceanogr. 2006, 54, 95–105. [Google Scholar] [CrossRef]
- Andrade, M.P.; Magalhães, A.; Pereira, L.C.C.; Costa, R.M. Effects of environmental variables on mesozooplankton dynamics in an Amazonian estuary. Ecohydrol. Hydrobiol. 2022, 22, 511–529. [Google Scholar] [CrossRef]
- Rakhesh, M.; Raman, A.V.; Kalavati, C.; Subramanian, B.R.; Sharma, V.S.; Babu, E.S.; Sateesh, N. Zooplankton community structure across an eddy-generated upwelling band close to a tropical bay-mangrove ecosystem. Mar. Biol. 2008, 154, 953–972. [Google Scholar] [CrossRef]
- Chew, L.L.; Chong, V.C. Copepod community structure and abundance in a tropical mangrove estuary, with comparisons to coastal waters. Hydrobiologia 2011, 666, 127–143. [Google Scholar] [CrossRef]
- Carrasco, N.K.; Perissinotto, R.; Pillay, D. Zooplankton of the St. Lucia Estuary during the current drought cycle: A comparison between open- and closed-mouth conditions. Mar. Ecol. Prog. Ser. 2010, 399, 157–171. [Google Scholar] [CrossRef]
- Srichandan, S.; Baliarsingh, S.K.; Lotliker, A.A.; Sahu, B.K.; Roy, R.; Nair, T.B. Unravelling tidal effect on zooplankton community structure in a tropical estuary. Environ. Monit. Assess. 2021, 193, 362. [Google Scholar] [CrossRef]
- Rosas, R.S.; Azevedo-Cutrim, A.C.G.; Cutrim, M.V.J.; da Cruz, Q.S.; Souza, D.S.C.; dos Santos Sá, A.K.D.; Santos, T.P. Spatial heterogeneity of zooplankton community in an eutrophicated tropical estuary. Aquat. Sci. 2024, 86, 102. [Google Scholar] [CrossRef]
- Huang, B.; L’Heureux, M.; Hu, Z.-Z.; Zhang, H.-M. Ranking the strongest ENSO events while incorporating SST uncertainty. Geophys. Res. Lett. 2016, 43, 9165–9172. [Google Scholar] [CrossRef]
- Zhai, P.; Yu, R.; Guo, Y.; Li, Q.; Ren, X.; Wang, Y.; Ding, Y. The strong El Niño of 2015/16 and its dominant impacts on global and China’s climate. J. Meteorol. Res. 2016, 30, 283–297. [Google Scholar] [CrossRef]
- Malhi, Y.; Román-Cuesta, R.M. Analysis of lacunarity and scales of spatial homogeneity in IKONOS images of Amazonian tropical forest canopies. Remote Sens. Environ. 2008, 112, 2074–2087. [Google Scholar] [CrossRef]
- Morales-Ramírez, A.; Brugnoli-Olivera, E. El Niño 1997–1998 impact on the plankton dynamics in the Gulf of Nicoya, Pacific coast of Costa Rica. Rev. Biol. Trop. 2001, 49, 103–114. [Google Scholar]
- Hopcroft, R.R.; Clarke, C.; Chavez, F.P. Copepod communities in Monterey bay during the 1997–1999 El Niño and La Niña. Prog. Oceanogr. 2002, 54, 251–264. [Google Scholar] [CrossRef]
- Harfmann, J.; Kurobe, T.; Bergamaschi, B.; Teh, S.; Hernes, P. Plant detritus is selectively consumed by estuarine copepods and can augment their survival. Sci. Rep. 2019, 9, 9076. [Google Scholar] [CrossRef]
- Chen, M.; Kim, D.; Liu, H.; Kang, C.-K. Variability in copepod trophic levels and feeding selectivity based on stable isotope analysis in Gwangyang Bay of the southern coast of the Korean Peninsula. Biogeosciences 2018, 15, 2055–2073. [Google Scholar] [CrossRef]
- Ara, K. Daily egg production rate of the planktonic calanoid copepod Acartia lilljeborgi Giesbrecht in the Cananéia Lagoon estuarine system, São Paulo, Brazil. Hydrobiologia 2001, 445, 205–215. [Google Scholar] [CrossRef]
- Ara, K. Temporal variability and production of Euterpina acutifrons (Copepoda: Harpacticoida) in the Cananéia Lagoon estuarine system, São Paulo, Brazil. Hydrobiologia 2001, 453, 177–187. [Google Scholar] [CrossRef]
- Dias, C.d.O.; Bonecker, S.L.C. Inter-annual variability of planktonic copepods in a Tropical Bay in Southeastern Brazil. Braz. Arch. Biol. Technol. 2008, 51, 731–742. [Google Scholar] [CrossRef]
- Figueirêdo, L.G.P.; Meloa, P.A.M.C.; Melo Júnior, M.; Silva, T.A.; Moura, R.L.; Thompson, F.L.; Neumann-Leitão, S. Summer micro- and mesozooplankton from the largest reef system of the South Atlantic Ocean (Abrolhos, Brazil): Responses to coast proximity. J. Sea Res. 2018, 141, 37–46. [Google Scholar] [CrossRef]
- Sant’anna, E.E.; Björnberg, T.K.S. Conhecimento atual sobre o comportamento alimentar de copépodos Calanoida em ambientes marinhos. Trab. Ocean. Univ. Fed. Pernamb. 2000, 28, 11–20. [Google Scholar] [CrossRef][Green Version]
- Sato, R.; Ishibashi, Y.; Tanaka, Y.; Ishimaru, T.; Dagg, M.J. Productivity and grazing impact of Oikopleura dioica (Tunicata, Appendicularia) in Tokio Bay. J. Plankton Res. 2008, 30, 299–309. [Google Scholar] [CrossRef][Green Version]
- Medeiros, G.F.; Medeiros, L.S.; Henriques, D.M.F.; Carlos, M.T.L.; Faustino, G.V.B.S.; Lopez, R.M. Current distribution of the exotic copepod Pseudodiaptomus trihamatus Wright, 1937 along the northeastern coast of Brazil. Braz. J. Oceanogr. 2006, 54, 241–245. [Google Scholar] [CrossRef]
- Tundisi, J.; Tundisi, T.M. Plankton studies in a mangrove environment, V. Salinity tolerances of some planktonic crustaceans. Bol. Inst. Ocean. 1968, 17, 57–65. [Google Scholar] [CrossRef]
- Costa, K.G.; Pereira, L.C.C.; Costa, R.M. Short and long-term temporal variation of the zooplankton in a tropical estuary (Amazon region, Brazil). Bol. Mus. Para. Emílio Goeldi Ciências Nat. 2008, 3, 127–141. [Google Scholar] [CrossRef]
- Ren, Z.; Jia, X.; Zhang, Y.; Ma, K.; Zhang, C.; Li, X. Biogeography and environmental drivers of zooplankton communities in permafrost-affected lakes on the Qinghai-Tibet Plateau. Glob. Ecol. Conserv. 2022, 38, e02191. [Google Scholar] [CrossRef]
- Lucena-Moya, P.; Duggan, I.C. Correspondence between zooplankton assemblages and the Estuary Environment Classification system. Estuar. Coast. Shelf Sci. 2017, 184, 1–9. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, K.; Zhang, S.; Xu, H.; Liu, H. Spatial distributions of mesozooplankton biomass, community composition and grazing impact in association with hypoxia in the Pearl River Estuary. Estuar. Coast. Shelf Sci. 2019, 225, 106237. [Google Scholar] [CrossRef]
- Bashevkin, S.M.; Hartman, R.; Thomas, M.; Barros, A.; Burdi, C.E.; Hennessy, A.; Tempel, T.; Kayfetz, K. Five decades (1972–2020) of zooplankton monitoring in the upper San Francisco Estuary. PLoS ONE 2022, 17, e0265402. [Google Scholar] [CrossRef]
- Escribano, R.; Daneri, G.; Farías, L.; Gallardo, V.A.; González, H.E.; Gutiérrez, D.; Lange, C.B.; Morales, C.E.; Pizarro, O.; Ulloa, O.; et al. Biological and chemical consequences of the 1997–1998 El Niño in the Chilean coastal upwelling system: A synthesis. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2004, 51, 2389–2411. [Google Scholar] [CrossRef]
- Checkley, D.M., Jr.; Barth, J.A. Patterns and processes in the California Current System. Prog. Oceanogr. 2009, 83, 49–64. [Google Scholar] [CrossRef]
- Darnis, G.; Geoffroy, M.; Daase, M.; Lalande, C.; Søreide, J.E.; Leu, E.; Renaud, P.R.; Berge, J. Zooplankton fecal pellet flux drives the biological carbon pump during the winter–spring transition in a high-Arctic system. Limnol. Oceanogr. 2024, 69, 1481–1493. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Kitchell, J.F.; Hodgson, J.R. Cascading Trophic Interactions and Lake Productivity. BioScience 1985, 35, 634–639. [Google Scholar] [CrossRef]
- Rogers, T.L.; Bashevkin, S.M.; Burdi, C.E.; Colombano, D.D.; Dudley, P.N.; Mahardja, B.; Mitchell, L.; Perry, S.; Saffarinia, P. Evaluating top-down, bottom-up, and environmental drivers of pelagic food web dynamics along an estuarine gradient. Ecology 2024, 105, e4274. [Google Scholar] [CrossRef]
- Marceniuk, A.P.; Caires, R.A.; Rotundo, M.M.; de Alcântara, R.A.K.; Wosiacki, W.B. The icthyofauna (Teleostei) of the Rio Caeté estuary, northeast Pará, Brazil, with a species identification key from northern Brazilian coast. Pan Am. J. Aquat. Sci. 2017, 12, 31–79. [Google Scholar]
- Camargo, M.; Isaac, V. Food categories reconstruction and feeding consumption estimates for the Sciaenid Macrodon ancylodon (Bloch & Schneider), and the congeneric fishes Stellifer rastrifer (Jordan) and Stellifer naso (Jordan) (Pisces, Perciformes) in the Caeté Estuary, Northern Coast of Brazil. Rev. Bras. Zool. 2004, 21, 85–89. [Google Scholar] [CrossRef]
- Zhong, Y.; Laws, E.A.; Zhuang, J.; Wang, J.; Wang, P.; Zhang, C.; Liu, X.; Huang, B. Responses of phytoplankton communities driven by differences of source water intrusions in the El Niño and La Niña events in the Taiwan Strait during the early spring. Front. Mar. Sci. 2022, 9, 997591. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; He, Y.; Zheng, J. The Relationship between Chlorophyll Concentration and ENSO Events and Possible Mechanisms off the Changjiang River Estuary. Remote Sens. 2023, 15, 2384. [Google Scholar] [CrossRef]
- Belarmino, E.; de Nobrega, M.F.; Grimm, A.M.; da Silva Copertino, M.; Vieira, J.P.; Garcia, A.M. Long-term trends in the abundance of an estuarine fish and relationships with El Niño climatic impacts and seagrass meadows reduction. Estuar. Coast. Shelf Sci. 2021, 261, 107565. [Google Scholar] [CrossRef]
- Wernberg, T.; Thomsen, M.S.; Baum, J.K.; Bishop, M.J.; Bruno, J.F.; Coleman, M.A.; Filbee-Dexter, K.; Gagnon, K.; He, Q.; Murdiyarso, D.; et al. Impacts of climate change on marine foundation species. Annu. Rev. Mar. Sci. 2024, 16, 247–282. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Albano, P.G.; Steger, J.; Bošnjak, M.; Dunne, B.; Guifarro, Z.; Turapova, E.; Hua, Q.; Kaufman, D.S.; Rilov, G.; Zuschin, M. Native biodiversity collapse in the eastern Mediterranean. Proc. R. Soc. B Biol. Sci. 2021, 288, 20202469. [Google Scholar] [CrossRef]
- Garrabou, J.; Gómez-Gras, D.; Medrano, A.; Cerrano, C.; Ponti, M.; Schlegel, R.; Bensoussan, N.; Turicchia, E.; Sini, M.; Gerovasileiou, V.; et al. Marine heatwaves drive recurrent mass mortalities in the Mediterranean Sea. Glob. Change Biol. 2022, 28, 5708–5725. [Google Scholar] [CrossRef]
- Cordero-Quirós, N.; Miller, A.J.; Pan, Y.; Balitaan, L.; Curchitser, E.; Dussin, R. Physical-Ecological Response of the California Current System to ENSOevents in, ROMS-NEMURO. Ocean Dyn. 2022, 72, 21–36. [Google Scholar] [CrossRef]
- Winans, A.K.; Herrmann, B.; Keister, J.E. Spatio-temporal variation in zooplankton community composition in the southern Salish Sea: Changes during the 2015–2016 Pacific marine heatwave. Prog. Oceanogr. 2023, 214, 103022. [Google Scholar] [CrossRef]
- Menezes, B.S.; Becker, É.C.; Agnelli, F.B.; Macedo-Soares, L.C.P.D.; Dias, C.D.O.; Freire, A.S. How is copepod functional diversity shaped by 2015–2016 El Niño and seasonal water masses in a coastal ecosystem of Southwest Atlantic? Ocean Coast. Res. 2024, 72 (Suppl. S1), e24006. [Google Scholar] [CrossRef]
- Alemany-Rodríguez, E.A.; Hernández-Trujillo, S. Non-predatory mortality assessment of zooplankton in Magdalena Bay, Mexico, during El Niño 2015. Environ. Sci. Pollut. Res. 2025, 32, 3227–3237. [Google Scholar] [CrossRef]
- Bates-Góngora, K.V.; Funes-Rodríguez, R.; Saldierna-Martínez, R.J.; Silva-Segundo, C.A.; De Silva-Dávila, R.; González-Rodríguez, E.; Aburto-Oropeza, O.; Ahern, A.L.M.; Gómez-Gutiérrez, J. Ichthyoplankton species assemblages during the 2015–2016 El Niño in the southwest Gulf of California. J. Plankton Res. 2025, 47, fbaf011. [Google Scholar] [CrossRef]
- Broughton, J.M.; Codding, B.F.; Faith, J.T.; Mohlenhoff, K.A.; Gruhn, R.; Brenner-Coltrain, J.; Hart, I.A. El Niño frequency threshold controls coastal biotic communities. Science 2022, 377, 1202–1205. [Google Scholar] [CrossRef]
- Wang, W.; Chen, G.; Zhao, R.; Gao, C.; Wu, J.; Han, D. Response of estuary ecosystem to El Niño event: A case study in the Yangtze River Estuary. J. Mar. Syst. 2025, 251, 104120. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa Silva, T.R.; Magalhães, A.; Procópio, A.D.; de Andrade, M.P.; Pereira, L.C.C.; Costa, R.M.d. El Niño-Driven Changes in Zooplankton Community Structure in an Amazonian Tropical Estuarine Ecosystem (Taperaçu, Northern Brazil). Coasts 2025, 5, 39. https://doi.org/10.3390/coasts5040039
da Costa Silva TR, Magalhães A, Procópio AD, de Andrade MP, Pereira LCC, Costa RMd. El Niño-Driven Changes in Zooplankton Community Structure in an Amazonian Tropical Estuarine Ecosystem (Taperaçu, Northern Brazil). Coasts. 2025; 5(4):39. https://doi.org/10.3390/coasts5040039
Chicago/Turabian Styleda Costa Silva, Thaynara Raelly, André Magalhães, Adria Davis Procópio, Marcela Pimentel de Andrade, Luci Cajueiro Carneiro Pereira, and Rauquírio Marinho da Costa. 2025. "El Niño-Driven Changes in Zooplankton Community Structure in an Amazonian Tropical Estuarine Ecosystem (Taperaçu, Northern Brazil)" Coasts 5, no. 4: 39. https://doi.org/10.3390/coasts5040039
APA Styleda Costa Silva, T. R., Magalhães, A., Procópio, A. D., de Andrade, M. P., Pereira, L. C. C., & Costa, R. M. d. (2025). El Niño-Driven Changes in Zooplankton Community Structure in an Amazonian Tropical Estuarine Ecosystem (Taperaçu, Northern Brazil). Coasts, 5(4), 39. https://doi.org/10.3390/coasts5040039




