Marine Algal Response to Cultural Eutrophication in a Tidal System in Argentina
Abstract
1. Introduction
2. Materials and Methods
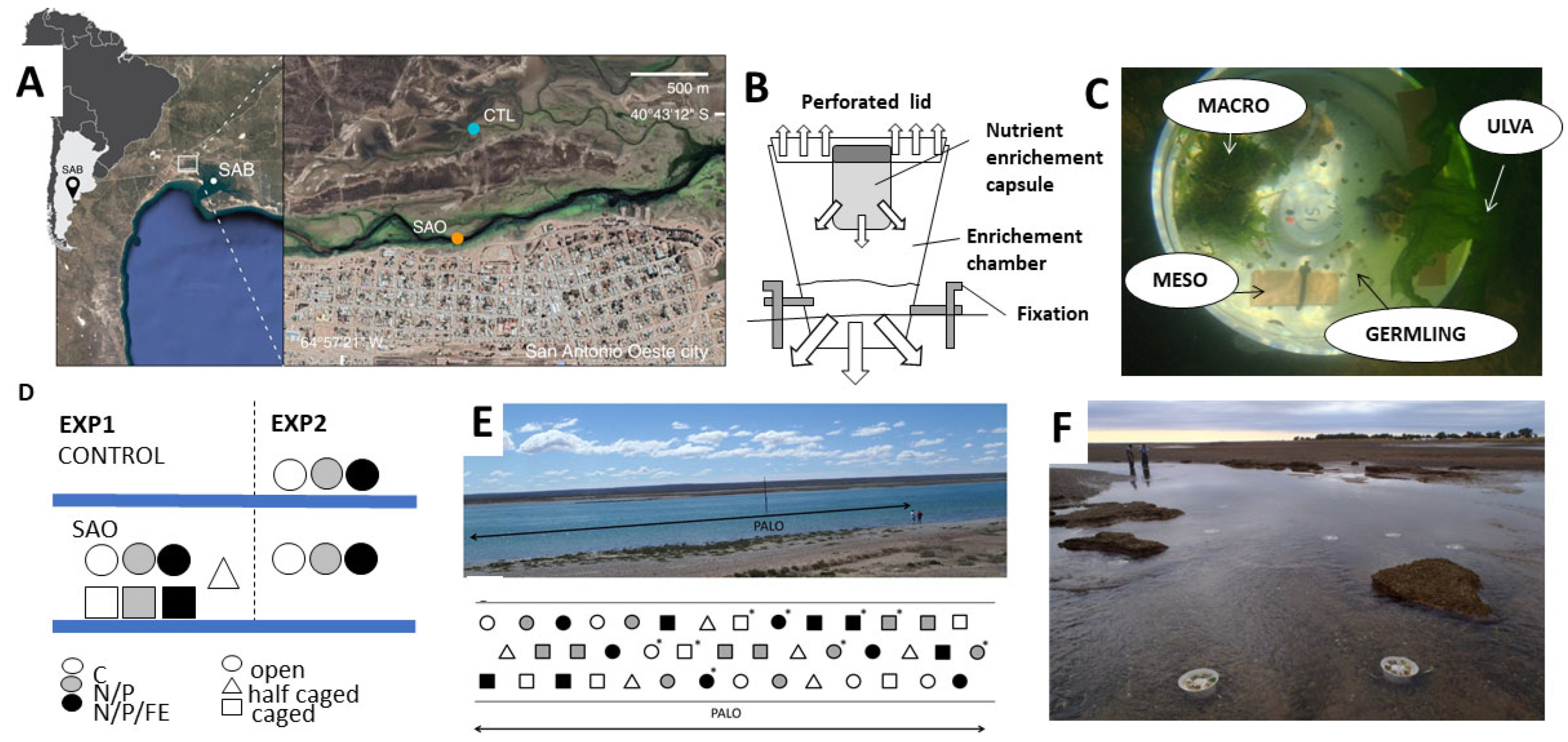
2.1. Research Area and Experimental Sites
2.2. Experimental Set-Up
2.2.1. Phytobenthic Units
2.2.2. Nutrient Enrichment Diffusers
2.2.3. Macrofauna Exclusion Treatment
2.2.4. Experimental Plan and Sampling Processing
EXP 1: Testing the Effect of Eutrophication and Grazer Exclusion in the SAO Channel
EXP2: Testing the Effect of Eutrophication in the SAO and CONTROL Channels
2.3. Sample Processing
2.4. Environmental Data
2.5. Laboratory Analyses
2.5.1. Composition and Biovolume of Macroalgal Recruits (GERMLING)
2.5.2. Biomass Accumulation and Biochemical Composition of Phytobenthic Communities (MESO, MACRO)
2.5.3. Composition of Macroalgal Communities (MACRO)
2.5.4. Growth Rates, Carbohydrate and Total Carbon Content of ULVA
2.6. Statistical Analyses
3. Results
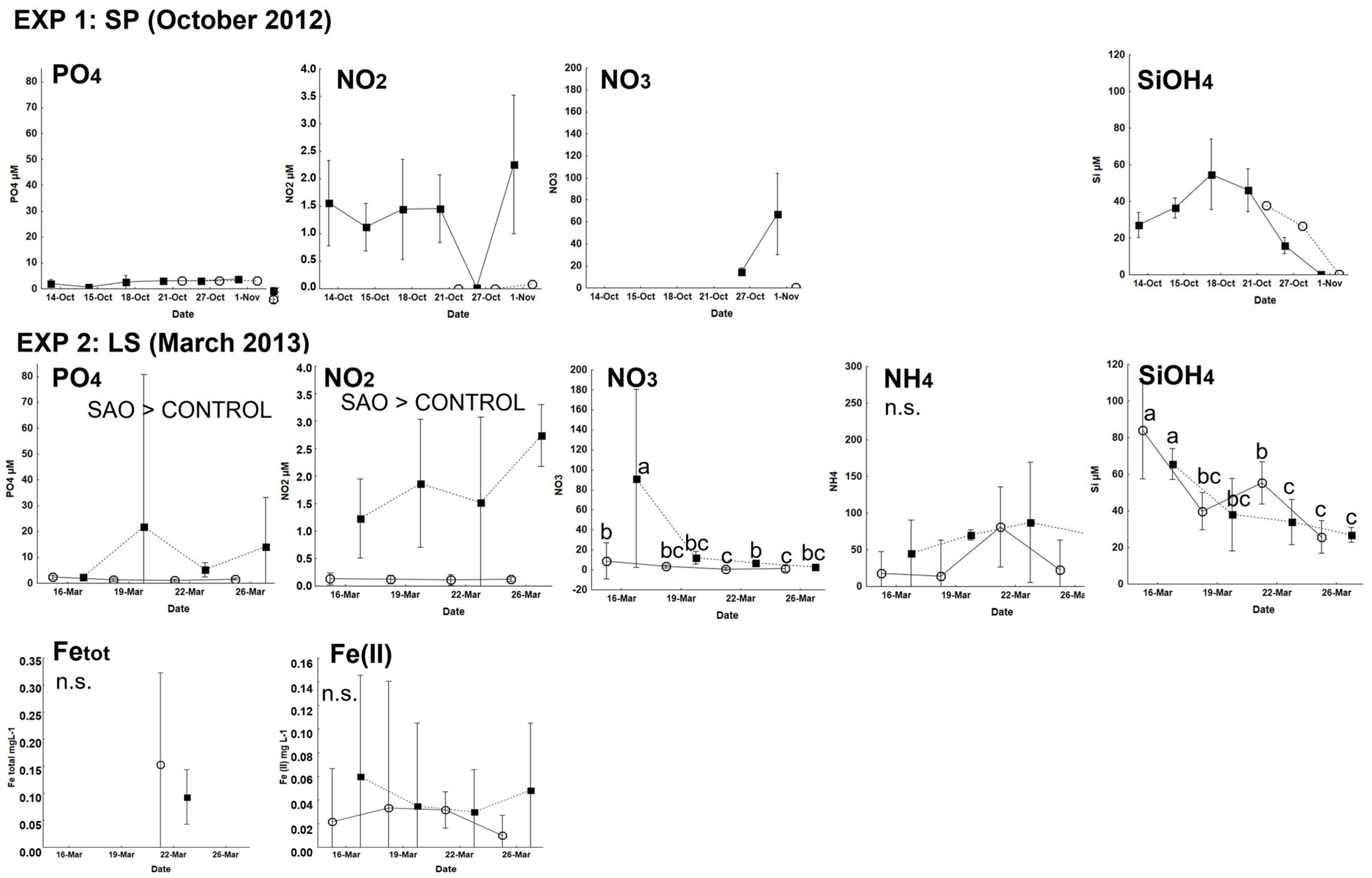
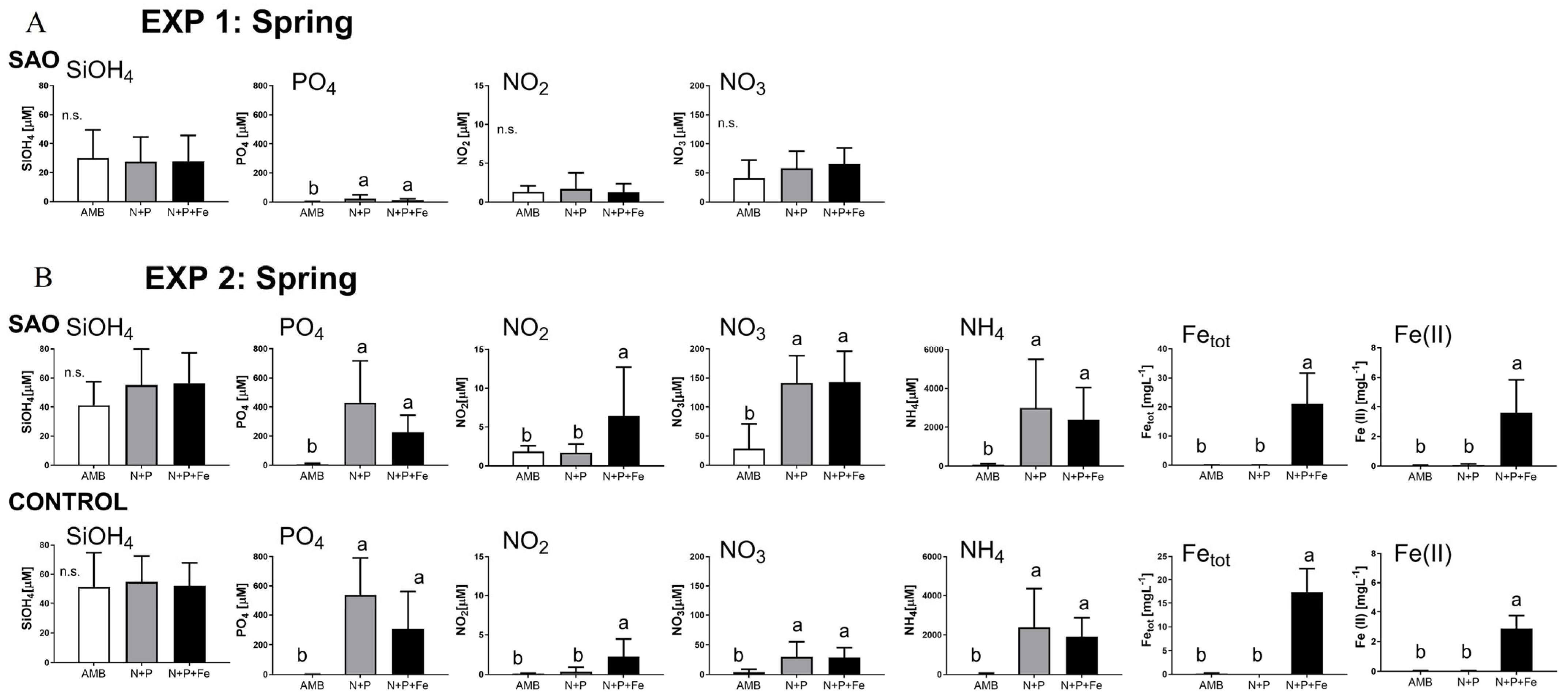
3.1. Physicochemical Characteristics of the Experiment
3.1.1. Natural Dynamics at the Experimental Sites
3.1.2. Measured Environmental Differences in the Experimental Set-Up
3.2. Responses of Different Phytobenthic Assemblages to Top-Down and Bottom-Up Controls
3.2.1. Response of Rhodophyte Germlings (GERMLINGs)
3.2.2. Responses of Mesoalgal Assemblages/Units (MESO)
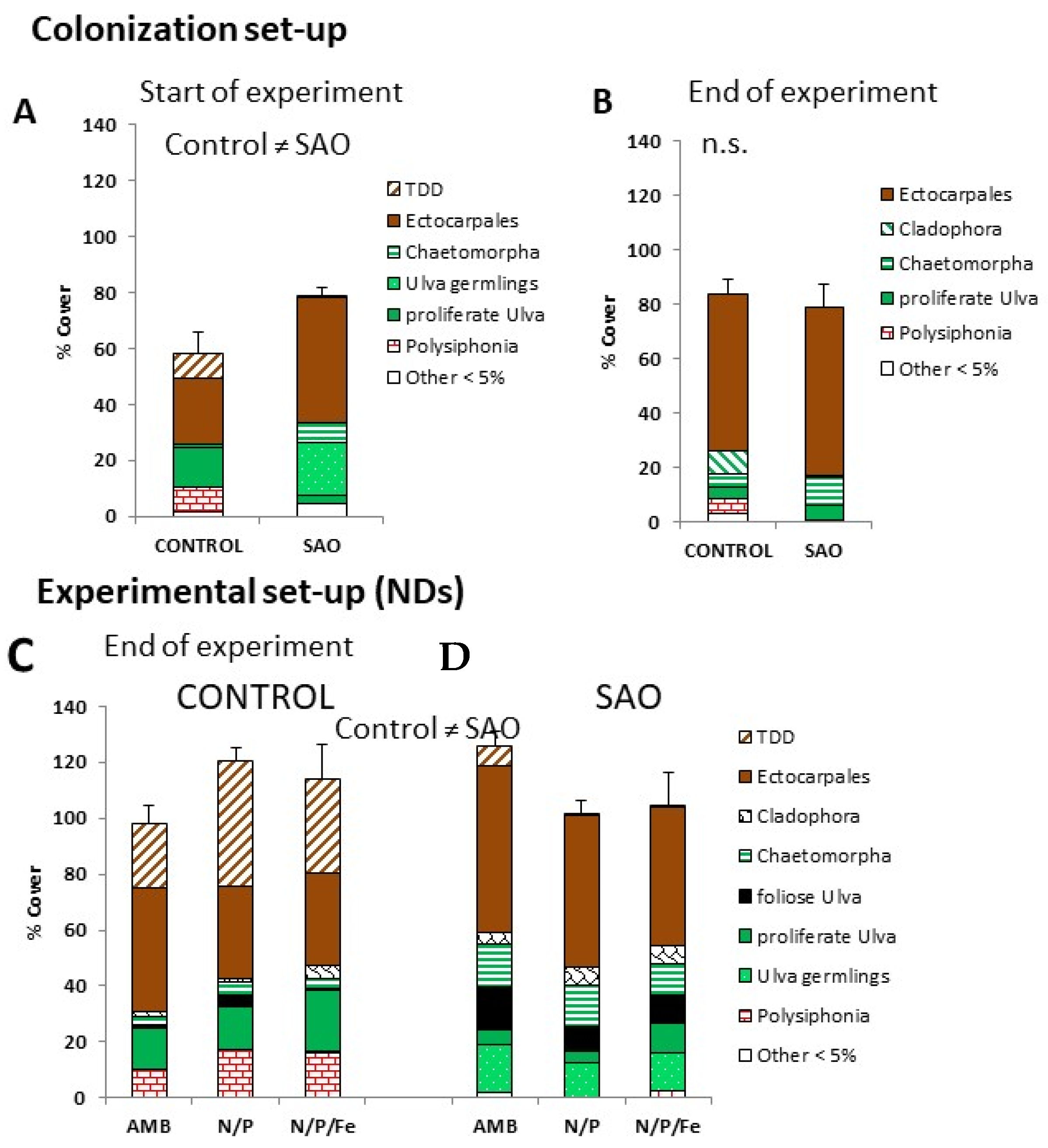
3.2.3. Responses in Macroalgal Assemblages (MACRO)
3.2.4. Responses in ULVA
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Factors | Df | MS | F | p |
|---|---|---|---|---|
| Water temperature | ||||
| EXP 1: Spring | ||||
| channels | 1 | 7.21 | 95,956 | <0.001 |
| conclusion | SAO < CONTROL | |||
| Water temperature | ||||
| EXP 2: Late summer | ||||
| channels | 1 | 46.346 | 5.883 | 0.015 |
| conclusion | SAO > CONTROL | |||
| Salinity | ||||
| EXP 1: Spring | ||||
| channels | 1 | 2.65 × 108 | 111,618 | <0.001 |
| conclusion | SAO < CONTROL | |||
| Water current | ||||
| EXP 2: Late summer | ||||
| channels | 1 | 0.001 | 0.988 | 0.349 |
| sites | 1 | 0.000 | 0.115 | 0.743 |
| channels × site | 1 | 0.000 | 0.303 | 0.597 |
| conclusion | No differences | |||
| Nutrients | PO4 | NO2 | NO3 | NH4 | SiOH4 | Fe(II) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Df | MS | F/H | P | MS | F/H | P | MS | F/H | P | MS | F/H | P | MS | F | P | MS | F/H | P |
| EXP1, EXP2: Ambient concentrations between seasons within the SAO channel | |||||||||||||||||||
| SEASON | 1 | 7.57 H | 0.006 | 1.995 | 3.48 | 0.07 | 2.53 H | 0.11 | 874.37 | 2.65 | 0.115 | ||||||||
| Conclusion | EXP 2 > EXP 1 | ||||||||||||||||||
| EXP1: Experimental conditions within site SAO | |||||||||||||||||||
| NUT | 2 | 22.5 H | <0.001 | 0.01 * | 0.13 * | 0.87 * | 0.12 | 1.07 | 0.37 | 38.8 | 0.12 | 0.89 | |||||||
| Conclusion | AMB < N/P, N/P/Fe | ||||||||||||||||||
| EXP2: Ambient concentrations (EXP 2) | |||||||||||||||||||
| SITE | 3 | 15.43 H | <0.001 | 17.29 H | <0.001 | 2.26 * | 57.03 * | <0.001 * | 7180 | 3.70 | 0.07 | 606.1 | 11.70 | 0.003 | 0.002 | 2.29 | 0.15 | ||
| TIME | 1 | 0.61 H | 0.89 | 9.14 H | 0.822 | 1.1 * | 27.7 * | <0.001 * | 3152 | 1.63 | 0.22 | 0.199 * | 42.2 * | <0.001 | 0.0001 | 0.17 | 0.92 | ||
| C × T | 3 | 0.16 * | 4.0 * | 0.03 * | 755.9 | 0.39 | 0.76 | 196.1 | 3.80 | 0.03 | 0.001 | 0.77 | 0.53 | ||||||
| Conclusion | SAO > CONTROL | SAO > CONTROL | SAO > CONTROL; D2 > all; D4 > D11; SAO > CONTROL at D7 und D11 | CONTROL > SAO; D2 > D4, D7 > D11; CONTROL > SAO at D7 | |||||||||||||||
| EXP2: Experimental conditions within site CONTROL | |||||||||||||||||||
| NUT | 2 | 25.5 H | <0.0001 | 23.9 H | <0.001 | 2.8 | 23.1 | <0.0001 | 23.4 H | <0.001 | 42.9 | 0.11 | 0.89 | 23.6 H | <0.001 | ||||
| Conclusion | AMB < N/P, N/P/Fe | AMB, N/P < N/P/Fe | AMB < N/P, N/P/Fe | AMB < N/P, N/P/Fe | AMB, N + P < N + P + Fe | ||||||||||||||
| EXP2: Experimental conditions within site SAO | |||||||||||||||||||
| NUT | 2 | 9.70 * | 93.4 * | <0.000 * | 10.33 H | 0.006 | 51,482 | 22.4 | <0.001 | 10.51 * | 86.23 * | <0.001 * | 846.9 | 1.94 | 0.16 | 23.56 H | <0.001 | ||
| Conclusion | AMB < N/P, N/P/Fe | AMB < N/P, N/P/Fe | AMB < N/P, N/P/Fe | AMB < N/P, N/P/Fe | AMB, N + P < N + P + Fe | ||||||||||||||
| Recruit Numbers | Biovolume | ||||||
|---|---|---|---|---|---|---|---|
| Factor | df | MS | F | P | MS | F | p |
| (a) Ambient Conditions: Succession and Cage | |||||||
| Cage (C) | 1 | 0.528 | 0.158 | 0.854 | 3 × 1011 | 0.608 | 0.551 |
| Time (T) | 2 | 13.444 | 4.033 | 0.054 | 5 × 1012 | 9.433 | 0.005 |
| C × T | 2 | 4.361 | 1.308 | 0.285 | 6 × 1011 | 1.172 | 0.324 |
| Conclusion | S2 > S1 | ||||||
| (b) S1: Cage and Nutrients | |||||||
| Nutrient (N) | 2 | 5583 | 1442 | 0.252 | 1 × 1010 | 0.859 | 0.434 |
| Cage (C) | 2 | 0.028 | 0.007 | 0.933 | 3 × 1010 | 2158 | 0.152 |
| N × C | 2 | 2694 | 0.696 | 0.507 | 5 × 1010 | 3047 | 0.062 |
| Conclusion | |||||||
| (c) S2: Cage and Nutrients | |||||||
| Nutrient (N) | 2 | 35,583 | 5369 | 0.010 | 4 × 1011 | 0.582 | 0.565 |
| Cage (C) | 2 | 26,694 | 4028 | 0.054 | 4 × 1012 | 5886 | 0.021 |
| N × C | 2 | 9028 | 1362 | 0.272 | 3 × 1011 | 0.442 | 0.647 |
| Conclusion | N/P/Fe > AMB | Open > Caged | |||||
| MESO Growth Rates | MACRO Stock | ||||||
|---|---|---|---|---|---|---|---|
| Factor | Df | MS | F | p | MS | F | p |
| (a) SEASONS in SAO channel | |||||||
| NUT | 2 | 0.102 | 5.582 | 0.008 | 0.05 | 0.752 | 0.482 |
| SEASON | 1 | 0.698 | 24.403 | <0.001 | 8.85 | 144.253 | <0.000 |
| N × S | 2 | 0.026 | 1.440 | 0.251 | 0.02 | 0.320 | 0.729 |
| Conclusion | AMB > N/P/Fe Spring > summer | Spring > summer | |||||
| (b) CHANNELs in EXP2 | |||||||
| CHANNEL | 1 | 0.788 | 24,416 | <0.000 | 0.284 | 3.930 | 0.058 |
| NUT | 2 | 0.024 | 0.734 | 0.490 | 0.048 | 0.663 | 0.524 |
| C × N | 2 | 0.114 | 3546 | 0.045 | 0.014 | 0.201 | 0.819 |
| Conclusion | SAO > CONTROL CONTROL: n.s.; SAO: N/P > N/P/FE; | ||||||
| (III) Pigment concentrations in MESO and MACRO communities. | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| chla | chlc | chlb | carotenoids | phaeo | ||||||||||||
| Factor | Df | MS | F/H | P | MS | F/H | P | MS | F/H | P | MS | F/H | P | MS | F/H | P |
| MESO | ||||||||||||||||
| CHANNEL | 1 | 2.91 | 232.6 | <0.000 | 2.68 | 234.59 | <0.000 | 0.81 | 12.5 | 0.002 | 2.72 | 288.4 | <0.000 | 7.43 | 36.8 | <0.000 |
| NUT | 2 | 0.14 | 11 | <0.000 | 0.06 | 5.57 | 0.01 | 0.05 | 0.77 | 0.473 | 0.1 | 11.09 | 0 | 0.19 | 0.93 | 0.409 |
| C × N | 2 | 0.07 | 5.27 | 0.013 | 0.03 | 2.5 | 0.103 | 0.05 | 0.77 | 0.473 | 0.05 | 5.39 | 0.012 | 0.19 | 0.93 | 0.409 |
| Conclusion | SAO > CONTROL CONTROL: AMB < N/P, N/P/Fe SAO: AMB < N/P/FE | SAO > CONTROL N/P/FE > AMB | CONTROL > SAO | SAO > CONTROL CONTROL: AMB < N/P, N/P/Fe SAO: AMB < N/P/FE | CONTROL > SAO | |||||||||||
| MACRO | ||||||||||||||||
| CHANNEL | 1 | 0.56 | 2.56 | 0.123 | 0.006 | 1.12 | 0.3 | 1.5 | 1.08 | 0.31 | 0.02 | 1.12 | 0.3 | 22.19 (H) | <0.000 | |
| NUT | 2 | 0.06 | 0.29 | 0.749 | 0 | 0.01 | 0.988 | 1.49 | 1.07 | 0.36 | 0 | 0.38 | 0.69 | 0.55 (H) | 0.756 | |
| C × N | 2 | 0.64 | 2.93 | 0.072 | 0.011 | 2.04 | 0.151 | 1.36 | 0.97 | 0.39 | 0.06 | 2.94 | 0.07 | |||
| Conclusion | CONTROL > SAO | |||||||||||||||
| Growth | Carbon | Carbohydrates | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Df | MS | F/H | P | MS | F/H | P | MS | F/H | P |
| NUT | 2 | 0.004 | 0.747 | 0.482 | 0.023 | 1.514 | 0.250 | 0.028 | 1.543 | 0.244 |
| CHANNEL | 1 | 0.013 | 2.621 | 0.116 | 0.067 | 4.451 | 0.051 | 0.083 | 4.520 | 0.049 |
| N × C | 2 | 0.004 | 0.813 | 0.453 | 0.015 | 0.513 | 0.608 | 0.010 | 0.551 | 0.587 |
| Conclusion | SAO > CONTROL | |||||||||
Appendix B
References
- Valiela, I.; Collins, G.; Kremer, J.; Lajtha, K.; Geist, M.; Seely, B.; Brawley, J.; Sham, C.H. Nitrogen loading from coastak watersheds to receiving estuaries: New method and application. Ecol. Appl. 1997, 7, 358–380. [Google Scholar] [CrossRef]
- Cloern, J.E.; Jassby, A.D. Drivers of change in estuarine-coastal ecosystems: Discoveries from four decades of study in San Francisco Bay. Rev. Geophys. 2012, 50. [Google Scholar] [CrossRef]
- Andersen, J.H.; Carstensen, J.; Holmer, M.; Krause-Jensen, D.; Richardson, K. Editorial: Research and Management of Eutrophication in Coastal Ecosystems. Front. Mar. Sci. 2019, 6, 768. [Google Scholar] [CrossRef]
- Nixon, S.W. Eutrophication and the macroscope. Hydrobiologia 2009, 629, 5–19. [Google Scholar] [CrossRef]
- Duarte, C.M. Submerged aquatic vegetation in relation to different nutrient regimes. Ophelia 1995, 41, 87–112. [Google Scholar] [CrossRef]
- Maúre, E.d.R.; Terauchi, G.; Ishizaka, J.; Clinton, N.; DeWitt, M. Globally consistent assessment of coastal eutrophication. Nat. Commun. 2021, 12, 6142. [Google Scholar] [CrossRef]
- Malone, T.C.; Newton, A. The Globalization of Cultural Eutrophication in the Coastal Ocean: Causes and Consequences. Front. Mar. Sci. 2020, 7, 670. [Google Scholar] [CrossRef]
- Chislock, M.F.; Doster, E.; Zitomer, R.; Wilson, A.E. Eutrophication: Causes, consequences, and controls in aquatic ecosystems. Nat. Educ. Knowl. 2013, 4, 10. [Google Scholar]
- Howarth, R.; Chan, F.; Conley, D.J.; Garnier, J.; Doney, S.C.; Marino, R.; Billen, G. Coupled biogeochemical cycles: Eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front. Ecol. Environ. 2011, 9, 18–26. [Google Scholar] [CrossRef]
- Anderson, D.; Glibert, P.; Burkholder, J. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Fricke, A.; Kopprio, G.A.; Alemany, D.; Gastaldi, M.; Narvarte, M.; Parodi, E.R.; Lara, R.J.; Hidalgo, F.; Martínez, A.; Sar, E.A.; et al. Changes in coastal benthic algae succession trajectories and assemblages under contrasting nutrient and grazer loads. Estuaries Coasts 2016, 39, 462–477. [Google Scholar] [CrossRef]
- Teichberg, M.; Fox, S.E.; Olsen, Y.S.; Valiela, I.; Martinetto, P.; Iribarne, O.; Muto, E.Y.; Petti, M.A.V.; Corbisier, T.N.; Soto-Jiminéz, M.; et al. Eutrophication and macroalgal blooms in temperate and tropical coastal waters: Nutrient enrichment experiments with Ulva spp. Glob. Change Biol. 2010, 16, 2624–2637. [Google Scholar] [CrossRef]
- Caniguan, M.A.; Becherucci, M.E.; Gastaldi, M.; Narvarte, M.A.; Saad, J.F. Seawater Temperature and Tidal Action as Modulators of Ulva spp. Micropropagules Density in a Eutrophicated Macrotidal Coastal System. Estuaries Coasts 2025, 48, 80. [Google Scholar] [CrossRef]
- Lüning, K. Seaweeds: Their Environment, Biogeography, and Ecophysiology; Revised Translation; Wiley: Hoboken, NJ, USA, 1991. [Google Scholar]
- Becherucci, M.; Alvarez, M.; Iribarne, O.; Martinetto, P. Eutrophication in a semi-desert coastal ecosystem promotes increases in N and C isotopic signatures and changes in primary sources. Mar. Environ. Res. 2019, 146, 71–79. [Google Scholar] [CrossRef]
- Fricke, A.; Biancalana, F.; Tonicelli, G.; Berasategui, A.A.; Kopprio, G.A.; Gauna, M.C.; Parodi, E.E. Insights into ecological and reproductive aspects of two cryptogenic peracarid crustaceans of the Argentinian coast. Braz. J. Oceanogr. 2015, 63, 195–206. [Google Scholar] [CrossRef]
- Gastaldi, M.; Firstater, F.; Narvarte, M.; Daleo, P. Context-dependent interaction between an intertidal sponge and a green macroalga in a variable temperate Patagonian bay. Mar. Ecol. Prog. Ser. 2017, 581, 21–32. [Google Scholar] [CrossRef]
- Fricke, A.; Kihara, T.C.; Kopprio, G.A.; Hoppenrath, M. Anthropogenically driven habitat formation by a tube dwelling diatom on the Northern Patagonian Atlantic coast. Ecol. Indic. 2017, 77, 8–13. [Google Scholar] [CrossRef]
- Venier, C.; Figueiredo da Silva, J.; McLelland, S.J.; Duck, R.W.; Lanzoni, S. Experimental investigation of the impact of macroalgal mats on flow dynamics and sediment stability in shallow tidal areas. Estuar. Coast. Shelf Sci. 2012, 112, 52–60. [Google Scholar] [CrossRef]
- Guiry, M.D. How many species of algae are there? A reprise. Four kingdoms, 14 phyla, 63 classes and still growing. J. Phycol. 2024, 60, 214–228. [Google Scholar] [CrossRef]
- Neveux, N.; Bolton, J.; Bruhn, A.; Roberts, D.; Ras, M. The Bioremediation Potential of Seaweeds: Recycling Nitrogen, Phosphorus, and Other Waste Products. In Blue Biotechnology: Production and Use of Marine Molecules; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar]
- Fricke, A.; Capuzzo, E.; Bermejo, R.; Hofmann, L.C.; Hernández, I.; Pereira, R.; Van den Burg, S.W.K.; Pereira, T.; Buschmann, A.H.; Cottier-Cook, E.J. Ecosystem Services Provided by Seaweed Cultivation: State of the Art, Knowledge Gaps, Constraints and Future Needs for Achieving Maximum Potential in Europe. Rev. Fish. Sci. Aquac. 2024, 33, 238–256. [Google Scholar] [CrossRef]
- Raven, J.A. Iron acquisition and allocation in stramenopile algae. J. Exp. Bot. 2013, 64, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Boyd, P.W.; Jickells, T.; Law, C.S.; Blain, S.; Boyle, E.A.; Buesseler, K.O.; Coale, K.H.; Cullen, J.J.; de Baar, H.J.W.; Follows, M.; et al. Mesoscale iron enrichment experiments 1993–2005: Synthesis and future directions. Science 2007, 315, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.S.; Van Donk, E.; Immers, A.K. Lake restoration by in-lake iron addition: A synopsis of iron impact on aquatic organisms and shallow lake ecosystems. Aquat. Ecol. 2016, 50, 121–135. [Google Scholar] [CrossRef]
- Hall, K.; Murphy, T.; Mawhinney, M.; Ashley, K. Iron Treatment for Eutrophication Control in Black Lake, British Columbia. Lake Reserv. Manag. 1994, 9, 114–117. [Google Scholar] [CrossRef]
- Roth, F.; Stuhldreier, I.; Sánchez-Noguera, C.; Morales-Ramírez, Á.; Wild, C. Effects of simulated overfishing on the succession of benthic algae and invertebrates in an upwelling-influenced coral reef of Pacific Costa Rica. J. Exp. Mar. Biol. Ecol. 2015, 468, 55–66. [Google Scholar] [CrossRef]
- Littler, M.; Littler, D.; Brooks, B. Harmful algae on tropical coral reefs: Bottom-up eutrophication and top-down herbivory. Harmful Algae 2006, 5, 565–585. [Google Scholar] [CrossRef]
- Alexander, T.J.; Vonlanthen, P.; Seehausen, O. Does eutrophication-driven evolution change aquatic ecosystems? Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160041. [Google Scholar] [CrossRef]
- Chase, J.M.; Knight, T.M. Effects of eutrophication and snails on Eurasian watermilfoil (Myriophyllum spicatum) invasion. Biol. Invasions 2006, 8, 1643. [Google Scholar] [CrossRef]
- Duran, A.; Collado-Vides, L.; Burkepile, D.E. Seasonal regulation of herbivory and nutrient effects on macroalgal recruitment and succession in a Florida coral reef. PeerJ 2016, 4, e2643. [Google Scholar] [CrossRef]
- Martinetto, P.; Daleo, P.; Escapa, M.; Alberti, J.; Isacch, J.; Fanjul, E.; Botto, F.; Piriz, M.; Ponce, G.; Casas, G.; et al. High abundance and diversity of consumers associated with eutrophic areas in a semi-desert macrotidal coastal ecosystem in Patagonia, Argentina. Estuar. Coast. Shelf Sci. 2010, 88, 357–364. [Google Scholar] [CrossRef]
- Piriz, M.L.; Eyras, M.C.; Rostagno, C.M. Changes in biomass and botanical composition of beach-cast seaweeds in a disturbed coastal area from Argentine Patagonia. J. Appl. Phycol. 2003, 15, 67–74. [Google Scholar] [CrossRef]
- Häder, D.-P.; Banaszak, A.T.; Villafañe, V.E.; Narvarte, M.A.; González, R.A.; Helbling, E.W. Anthropogenic pollution of aquatic ecosystems: Emerging problems with global implications. Sci. Total Environ. 2020, 713, 136586. [Google Scholar] [CrossRef] [PubMed]
- Saad, J.; Narvarte, M.; Abrameto, M.; Alder, V. Drivers of nano- and microplanktonic community structure in a Patagonian tidal flat ecosystem. J. Plankton Res. 2019, 41, 621–639. [Google Scholar] [CrossRef]
- Martinetto, P.; Teichberg, M.; Valiela, I.; Montemayor, D.; Iribarne, O. Top-down and bottom-up regulation in a high nutrient--high herbivory coastal ecosystem. Mar. Ecol. Prog. Ser. 2011, 432, 69–82. [Google Scholar] [CrossRef]
- Agosta, E.; Martin, P.; Serio, L. Persistent easterly winds leading to precipitation in the Atlantic Coast of Patagonia. Int. J. Climatol. 2019, 39, 5063–5090. [Google Scholar] [CrossRef]
- Martí Barclay, V.; Neill, S.P.; Angeloudis, A. Tidal range resource of the Patagonian shelf. Renew. Energy 2023, 209, 85–96. [Google Scholar] [CrossRef]
- Pessacg, N.; Blazquez, J.; Lancelotti, J.; Solman, S. Climate Changes in Coastal Areas of Patagonia: Observed Trends and Future Projections. In Global Change in Atlantic Coastal Patagonian Ecosystems: A Journey Through Time; Springer International Publishing: Cham, Switzerland, 2022; pp. 13–42. [Google Scholar]
- Marello Buch, M.; Gastaldi, M.; Abrameto, M.; Firstater, F. Relative Contribution of Top-Down and Bottom-Up Controls on the Regulation of the Sponge Hymeniacidon perlevis (Montagu, 1814) in Patagonia: An Experimental and Observational Approach. Estuaries Coasts 2024, 47, 1650–1667. [Google Scholar] [CrossRef]
- Fricke, A.; Kihara, T.C.; Hoppenrath, M. Studying mesoalgal structures: A non-destructive approach based on confocal laser scanning microscopy. Bot. Mar. 2017, 60, 181–195. [Google Scholar] [CrossRef]
- Fricke, A.; Molis, M.; Wiencke, C.; Valdivia, N.; Chapman, A.S. Natural succession of macroalgal-dominated epibenthic assemblages at different water depths and after transplantation from deep to shallow water on Spitsbergen. Polar Biol. 2008, 31, 1191–1203. [Google Scholar] [CrossRef]
- Fricke, A.; Teichberg, M.; Beilfuss, S.; Bischof, K. Succession patterns in algal turf vegetation on a Caribbean coral reef. Bot. Mar. 2011, 54, 111. [Google Scholar] [CrossRef]
- Fricke, A.; Bast, F.; Moreira-Saporiti, A.; Martins Bussanello, G.; Msuya, F.E.; Teichberg, M. Tropical bloom-forming mesoalgae Cladophoropsis sp. and Laurencia sp.—Responses to ammonium enrichment and a simulated heatwave. J. Phycol. 2024, 60, 554–573. [Google Scholar] [CrossRef]
- Barr, N.; Zeldis, J.; Scheuer, K.; Schiel, D. Macroalgal bioindicators of recovery from eutrophication in a tidal lagoon following wastewater diversion and earthquake disturbance. Estuaries Coasts 2020, 43, 240–255. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Littler, M.M.; Littler, D.S.; Brooks, B.L. The effects of nitrogen and phosphorus enrichment on algal community development: Artificial mini-reefs on the Belize Barrier Reef sedimentary lagoon. Harmful Algae 2010, 9, 255–263. [Google Scholar] [CrossRef]
- Nuñez, J.D.; Ribeiro, P.D.; Ocampo, E.H.; Luppi, T.A. Neohelice granulata burrow fidelity behaviour related to landscape heterogeneity. Helgol. Mar. Res. 2018, 72, 17. [Google Scholar] [CrossRef]
- Wentzky, V.C.; Tittel, J.; Jäger, C.G.; Bruggeman, J.; Rinke, K. Seasonal succession of functional traits in phytoplankton communities and their interaction with trophic state. J. Ecol. 2020, 108, 1649–1663. [Google Scholar] [CrossRef]
- Hillebrand, H.; Dürselen, C.-D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Arar, E.J. Method 446.0: In Vitro Determination of Chlorophylls a, b, c + c and Pheopigments in Marine and Freshwater Algae by Visible Spectrophotometry; U.S. Environmental Protection Agency: Washington, DC, USA, 1997. [Google Scholar]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Lorenzen, C.J. Determination of chlorophyll and phaeopigments; spectrometric equations. Limnol. Oceanogr. 1967, 12, 343–346. [Google Scholar] [CrossRef]
- Sand-Jensen, K.; Geertz-Hansen, O. Growth rates and photon yield of growth in natural populations of a marine macroalga Ulva lactuca. Mar. Ecol. Prog. Ser. 1992, 81, 179–183. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Tomio, Y.; Buosi, A.; Juhmani, A.-S.; Sfriso, A.; Santi, T.; Tarricone, M.; Sfriso, A. Update of Nutrient Concentrations in the Waters of the Venice Lagoon. 2020. Available online: https://www.researchgate.net/publication/349028415_Update_of_nutrient_concentrations_in_the_waters_of_the_Venice_lagoon#:~:text=Values%20above%20the%20imperative%20values,to%20the%20primary%20producer%20fluctuations (accessed on 6 August 2025).
- Topcu, D.; Brockmann, U. Consistency of thresholds for eutrophication assessments, examples and recommendations. Environ. Monit. Assess. 2021, 193, 677. [Google Scholar] [CrossRef] [PubMed]
- Kopprio, G.A.; Biancalana, F.; Fricke, A.; Garzón Cardona, J.E.; Martínez, A.; Lara, R.J. Global change effects on biogeochemical processes of Argentinian estuaries: An overview of vulnerabilities and ecohydrological adaptive outlooks. Mar. Pollut. Bull. 2015, 91, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Armijo, J.; Oerder, V.; Auger, P.-A.; Bravo, A.; Molina, E. The 2016 red tide crisis in southern Chile: Possible influence of the mass oceanic dumping of dead salmons. Mar. Pollut. Bull. 2020, 150, 110603. [Google Scholar] [CrossRef]
- Díaz, P.; López-Gappa, J.; Piriz, M. Symptoms of Eutrophication in Intertidal Macroalgal Assemblages of Nuevo Gulf (Patagonia, Argentina). Bot. Mar. 2002, 45, 267–273. [Google Scholar] [CrossRef]
- Funk, J.L. The physiology of invasive plants in low-resource environments. Conserv. Physiol. 2013, 1, cot026. [Google Scholar] [CrossRef]
- Radford, I.J. Fluctuating resources, disturbance and plant strategies: Diverse mechanisms underlying plant invasions. J. Arid. Land 2013, 5, 284–297. [Google Scholar] [CrossRef]
- Raffo, P.; Geoffroy, A.; Destombe, C.; Schwindt, E. First record of the invasive red alga Polysiphonia morrowii Harvey (Rhodomelaceae, Rhodophyta) on the Patagonian shores of the Southwestern Atlantic. Bot. Mar. 2014, 57, 21–26. [Google Scholar] [CrossRef]
- Geoffroy, A.; Destombe, C.; Kim, B.; Mauger, S.; Raffo, P.; Kim, M.; Gall, L. Patterns of genetic diversity of the cryptogenic red alga Polysiphonia morrowii (Ceramiales, Rhodophyta) suggest multiple origins of the Atlantic populations. Ecol. Evol. 2016, 6, 5635–5647. [Google Scholar] [CrossRef]
- Croce, M.E.; Parodi, E.R. The Japanese alga Polysiphonia morrowii (Rhodomelaceae, Rhodophyta) on the South Atlantic Ocean: First report of an invasive macroalga inhabiting oyster reefs. Helgol. Mar. Res. 2014, 68, 241–252. [Google Scholar] [CrossRef]
- Raven, J.A. The iron and molybdenum use efficiencies of plant growth with different energy, carbon and nitrogen sources. New Phytol. 1988, 109, 279–287. [Google Scholar] [CrossRef]
- Morimoto, S.; Masuda, T.; Sugihara, I.; Toyohara, H. Identification and characterization of a ferritin gene and its product from the multicellular green alga Ulva pertusa. Biosci. Biotechnol. Biochem. 2012, 76, 1913–1919. [Google Scholar] [CrossRef][Green Version]
- Ferreira, J.; Andersen, J.; Borja, A.; Bricker, S.; Camp, J.; Silva, M.; Garcés, E.; Heiskanen, A.-S.; Humborg, C.; Ignatiades, L.; et al. Overview of eutrophication indicators to assess environmental status within the European Marine Strategy Framework Directive. Estuar. Coast. Shelf Sci. 2011, 93, 117–131. [Google Scholar] [CrossRef]
- Stuart, V.; Head, E.J.H.; Mann, K.H. Seasonal changes in the digestive enzyme levels of the amphipod Corophium volutator (Pallas) in relation to diet. J. Exp. Mar. Biol. Ecol. 1985, 88, 243–256. [Google Scholar] [CrossRef]



| Site | Time (Days) | Unit | Factors | Levels | Replication | NDs |
|---|---|---|---|---|---|---|
| EXP1: Spring season | ||||||
| SAO | 5 | GERMLING | NUT X CAGE | 7: AMB (open, caged, cage control); N/P (open, caged); N/P/Fe (open, caged) | 6 | 42 |
| SAO | 20 | MESO, MACRO | NUT | 3: AMB, N/P, N/P/Fe | 8 | 24 |
| EXP2: Late summer season | ||||||
| SAO and CONTROL | 12 | MESO | NUT | 3: AMB, N/P, N/P/Fe | 5 | 15 |
| 14 | MACRO, ULVA | NUT | 3: AMB, N/P, N/P/Fe | 5 | 15 | |
| Parameter | TEMP | pH | Salinity | PO4 | NO2 | NO3 | NH4 | SiOH4 | Fe (II) | Fe tot |
|---|---|---|---|---|---|---|---|---|---|---|
| Unit | °C | ppt | µM | µM | µM | µM | µM | mgL−1 | mgL−1 | |
| EXP 1: Spring season | ||||||||||
| SAO | 13.0 ± 0.06 | 8.52 ± 0.06 | 35.4 ± 0.8 | 2.54 ± 1.12 | 1.31 ± 0.76 | 40.92 ± 31.04 | 30.12 ± 19.32 | |||
| CONTROL | 13.8 ± 0.01 * | 8.54 ± 0.07 | 39.1 ± 0.2 * | 3.10 ± 0.02 | 0.03 ± 0.04 | 0.38 s | 21.52 ± 19.20 | |||
| EXP 2: Late summer season | ||||||||||
| SAO | 16.53 ± 2.46 * | 6.86 ± 6.5 * | 1.83 ± 0.74 * | 28.35 ± 42.62 * | 68.42 ± 52.03 | 41.14 ± 16.23 | 0.04 ± 0.03 | 0.09 ± 0.03 | ||
| CONTROL | 16.15 ± 3.11 | 1.58 ± 0.58 | 0.12 ± 0.04 | 3.64 ± 5.21 | 34.05 ± 34.3 | 51.19 ± 23.63 * | 0.02 ± 0.02 | 0.15 ± 0.08 | ||
| GERMLING | |||||
| Season | Site | Number | Taxa | Volume | |
| TIME | SP | SAO | n.s. | T2 > T1 | |
| CAGE | SP | SAO | n.s. | T1: Cage control ≠ Caged | T2: open > caged |
| NUT | SP | SAO | T2: N/P/Fe > AMB | T2: N/P/Fe ≠ AMB | n.s. |
| MESO | |||||
| Season | Site | Growth | Pigments | ||
| SEASON | SP/LS | SAO | SP > LS | ||
| NUT | SP | SAO | N/P/Fe < Ambient | ||
| LS | SAO | N/P/Fe < N/P | N/P/Fe > AMB for Chla, Chlc, carotenoids | ||
| LS | CONTROL | n.s. | N/P/Fe, N/P > AMB for Chla, carotenoids N/P/Fe > AMB for Chlc | ||
| Site | LS | SAO/CONTROL | SAO > CONTROL | SAO > CONTROL for Chla, Chlc, carotenoids CONTROL > SAO for Chlb, Phaeo | |
| MACRO | |||||
| Season | Site | Comp | Standing Stock | Pigments | |
| SEASON | SP/LS | SAO | SP > LS | ||
| NUT | SP | SAO | n.s. | ||
| SITE | LS | SAO/CONTROL | CONTROL ≠ SAO | n.s. | CONTROL > SAO for Phaeo |
| NUT | LS | SAO | n.s. | n.s. | |
| NUT | LS | CONTROL | n.s. | n.s. | |
| GERMLINGS (EXP1: spring) | |||||
| Factors | Df | MS | Pseudo-F | p | Pair-wise/SIMPER |
| (a) Ambient Conditions: Succession Time and Grazer Exclosure | |||||
| TIME | 1 | 7771.6 | 3.0535 | 0.0283 | T1: Caged ≠ cage control [av.dis. 90%] 53% POLY (Caged) 27% CER (Caged) 20% INITIAL (Caged) HC: T1 ≠ T2 [av.dis. 92%] 74% POLY (S2) 16% ANO (S2) |
| CAGE | 2 | 5290.1 | 2.0785 | 0.0543 | |
| TIME × CAGE | 2 | 7989.5 | 3.1391 | 0.0131 | |
| Res | 30 | ||||
| (b) Germlings (3 days): Cage and nutrients | |||||
| CAGE | 2 | 9345.9 | 3.0941 | 0.016 | Caged ≠ HC [av.diss: 92%] 51% POLY (Caged) 39% INITIAL (Caged) 11% CER (Caged) |
| NUT | 2 | 5726.5 | 1.8958 | 0.1016 | |
| CAGE × NUT | 2 | 3443.8 | 1.1401 | 0.3397 | |
| Res | 35 | 3020.6 | |||
| (c) Germlings (6 days): Cage and Nutrients | |||||
| CAGE | 2 | 3911 | 1.8748 | 0.07 | N/P/Fe ≠ AMB [av.dis. 69%] 32% POLY (N/P/Fe) 26% INITIAL (N/P/Fe) 23% CER (N/P/Fe) |
| NUT | 2 | 4597.3 | 2.2038 | 0.037 | |
| CAGE × NUT | 2 | 2024.2 | 0.97032 | 0.449 | |
| Res | 35 | ||||
| MACRO taxa composition (EXP2: late summer) | |||||
| Factors | Df | MS | Pseudo-F | p | Pair-wise/SIMPER |
| (a) Differences between the experimental sites during late summer (colonization set-up) | |||||
| SITE | 1 | 2839.8 | 9.96 | 0.002 | T0: CONTROL ≠ SAO * [av.diss.:56%] 21% Ulva germlings (SAO) 14% Polysiphonia (CONTROL) 14% Turbular Ulva (CONTROL) 13% Tube-dwelling diatoms (CONTROL) 10% Chaetomorpha (SAO) 7% Cladophora (SAO) T1: CONTROL = SAO * |
| TIME | 1 | 1312.5 | 4.60 | 0.01 | |
| SITE × TIME | 1 | 2189.1 | 7.67 | 0.003 | |
| Res | 8 | ||||
| (b) Response to nutrient enrichment and sites (NDs) | |||||
| SITE | 1 | 12,249 | 28.08 | 0.001 | CONTROL ≠ SAO * [av.diss.:49%] 17% Tube-dwelling diatoms (CONTROL) 15% Ulva germlings (SAO) 14% Polysiphonia (CONTROL) 12% Foliose Ulva (SAO) 10% Turbular Ulva (CONTROL) |
| NUT | 2 | 381 | 0.87 | 0.55 | |
| SITE × NUT | 2 | 720 | 1.65 | 0.14 | |
| Res | 24 | ||||
| AMB | N/P | N/P/Fe | |
|---|---|---|---|
| MACRO: Fe (mgg−1 DW) | |||
| SAO | 7.11 ± 1.25 | 5.86 ± 2.29 | 8.55 ± 1.85 |
| CONTROL | 6.34 ± 1.80 | 7.16 ± 7.16 | 7.20 ± 1.37 |
| ULVA: Carbohydrates (µgmg−1 DW) | |||
| SAO | 12.9 ± 2.7 | 16.1 ± 8.9 | 17.0 ± 6.9 |
| CONTROL | 8.7 ± 1.3 | 9.8 ± 4.3 | 12.7 ± 2.5 |
| ULVA: Total carbon (µgmg−1 DW) | |||
| SAO | 5.2 ± 1.1 | 6.4 ± 3.5 | 6.8 ± 2.8 |
| CONTROL | 3.5 ± 0.5 | 3.9 ± 1.7 | 5.1 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fricke, A.; Kopprio, G.A.; Gastaldi, M.; Narvarte, M.; Alemany, D.; Martínez, A.M.; Biancalana, F.; Rodríguez Rendas, R.D.; Albano, M.J.; Hidalgo, F.J.; et al. Marine Algal Response to Cultural Eutrophication in a Tidal System in Argentina. Coasts 2025, 5, 38. https://doi.org/10.3390/coasts5040038
Fricke A, Kopprio GA, Gastaldi M, Narvarte M, Alemany D, Martínez AM, Biancalana F, Rodríguez Rendas RD, Albano MJ, Hidalgo FJ, et al. Marine Algal Response to Cultural Eutrophication in a Tidal System in Argentina. Coasts. 2025; 5(4):38. https://doi.org/10.3390/coasts5040038
Chicago/Turabian StyleFricke, Anna, Germán A. Kopprio, Marianela Gastaldi, Maite Narvarte, Daniela Alemany, Ana M. Martínez, Florencia Biancalana, R. David Rodríguez Rendas, Mariano J. Albano, Fernando J. Hidalgo, and et al. 2025. "Marine Algal Response to Cultural Eutrophication in a Tidal System in Argentina" Coasts 5, no. 4: 38. https://doi.org/10.3390/coasts5040038
APA StyleFricke, A., Kopprio, G. A., Gastaldi, M., Narvarte, M., Alemany, D., Martínez, A. M., Biancalana, F., Rodríguez Rendas, R. D., Albano, M. J., Hidalgo, F. J., Iribarne, O., Lara, R. J., & Martinetto, P. (2025). Marine Algal Response to Cultural Eutrophication in a Tidal System in Argentina. Coasts, 5(4), 38. https://doi.org/10.3390/coasts5040038







