Aragonite Saturation State as an Indicator for Oyster Habitat Health in the Delaware Inland Bays
Abstract
1. Introduction
2. Materials and Methods
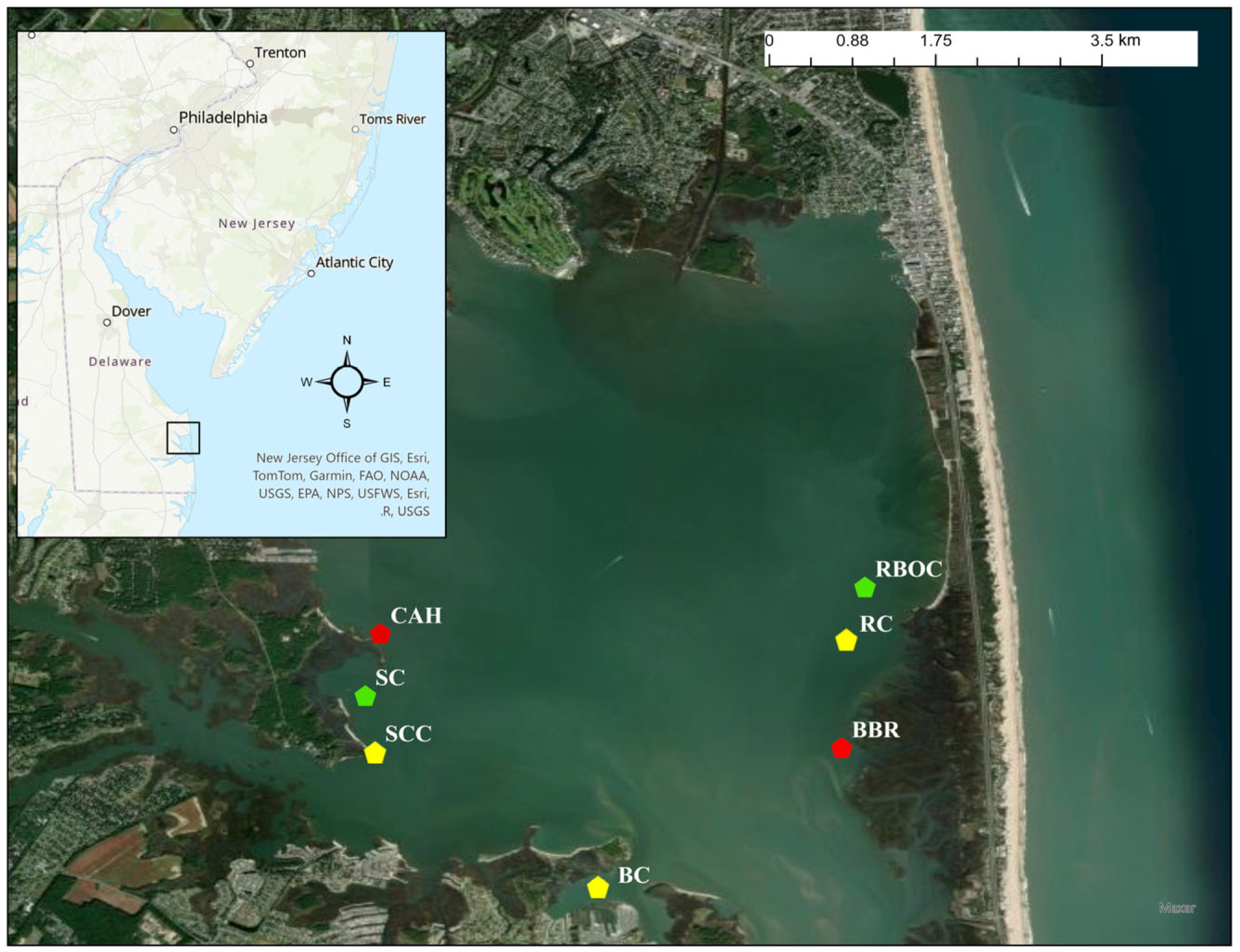
2.1. Study Sites
2.2. Water Quality Monitoring
2.3. Calculation of Aragonite Saturation State
3. Results
3.1. Physiochemical Water Quality Monitoring
3.2. Calculated Aragonite–Calcite Saturation States
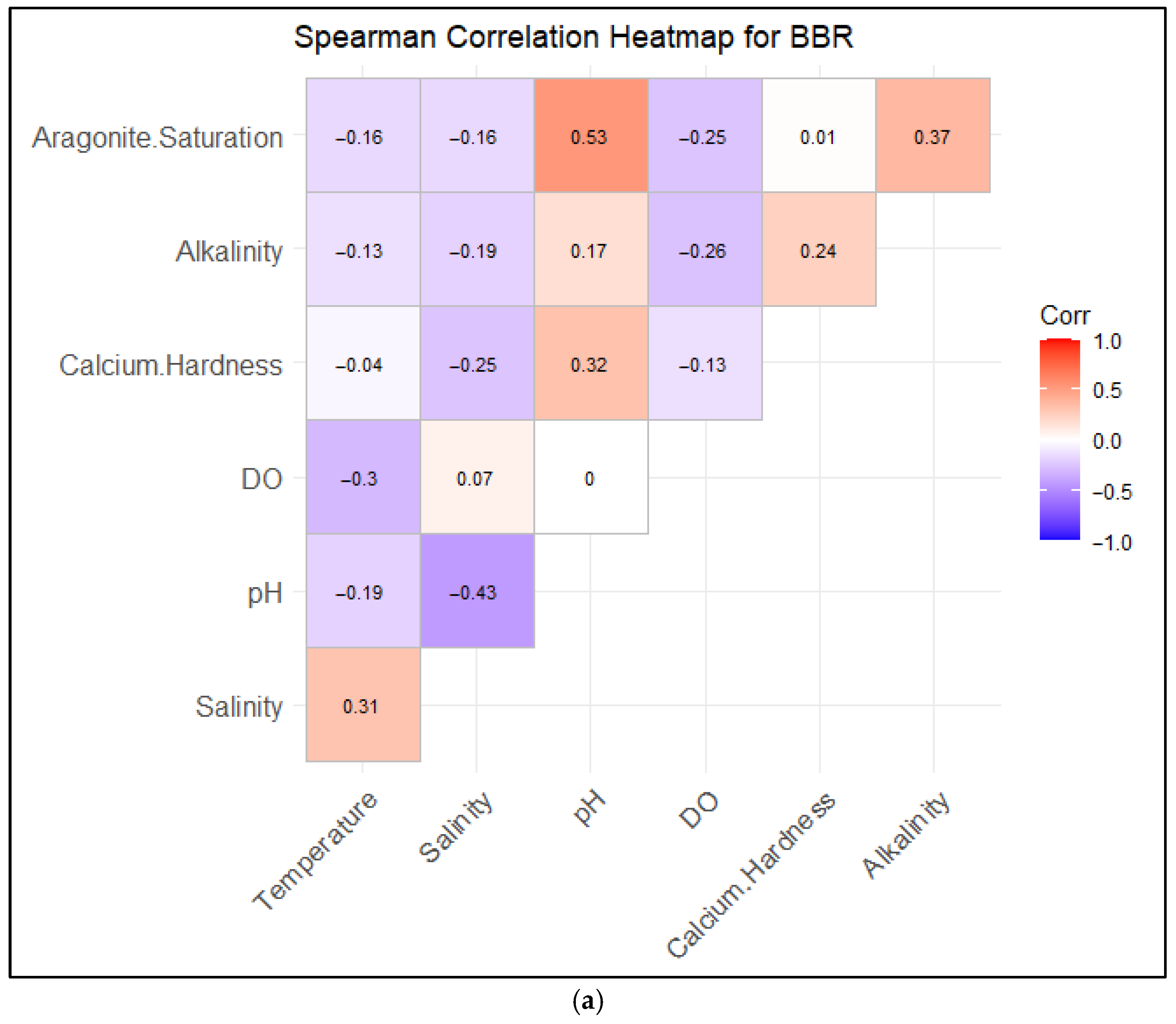
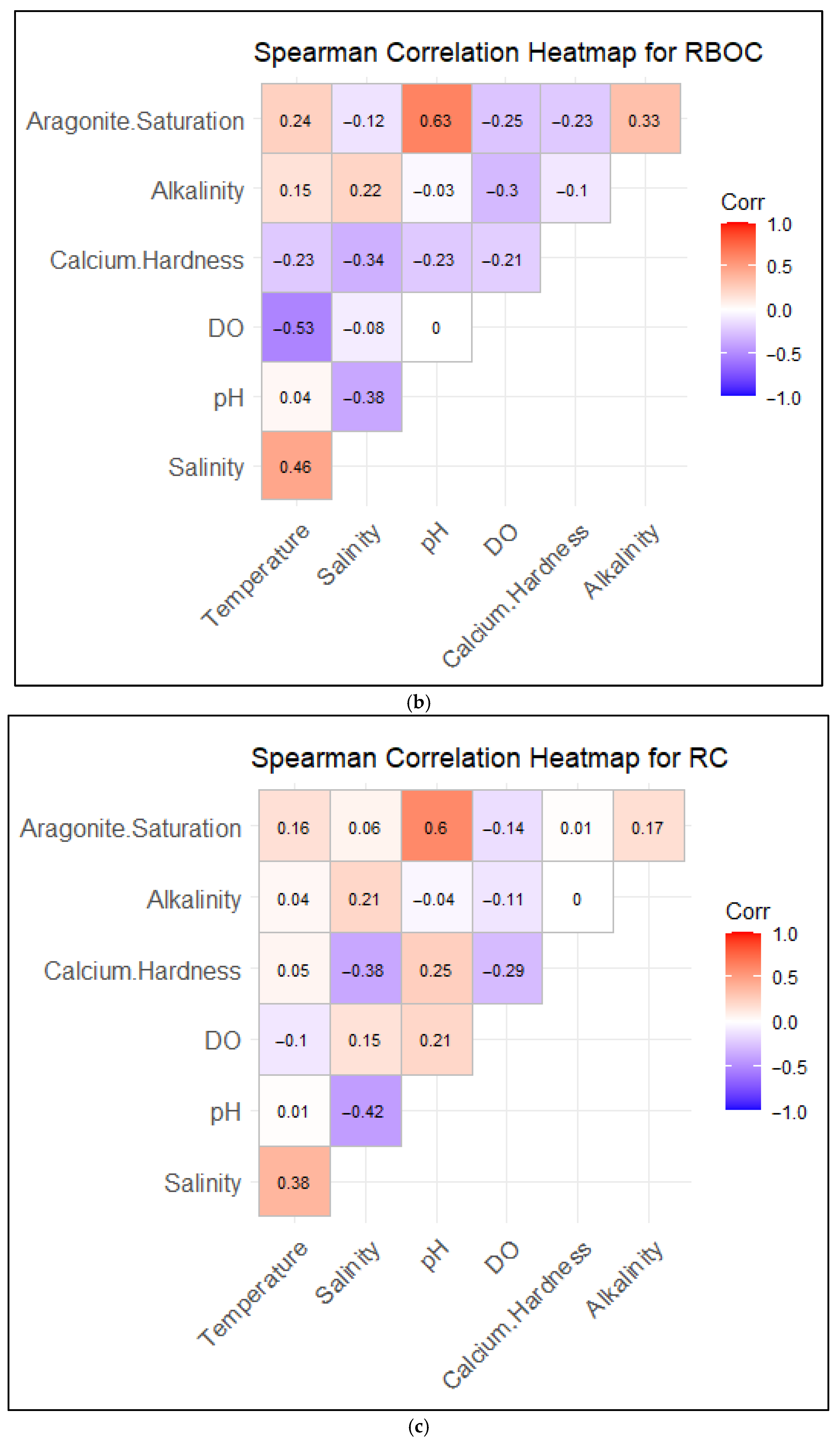
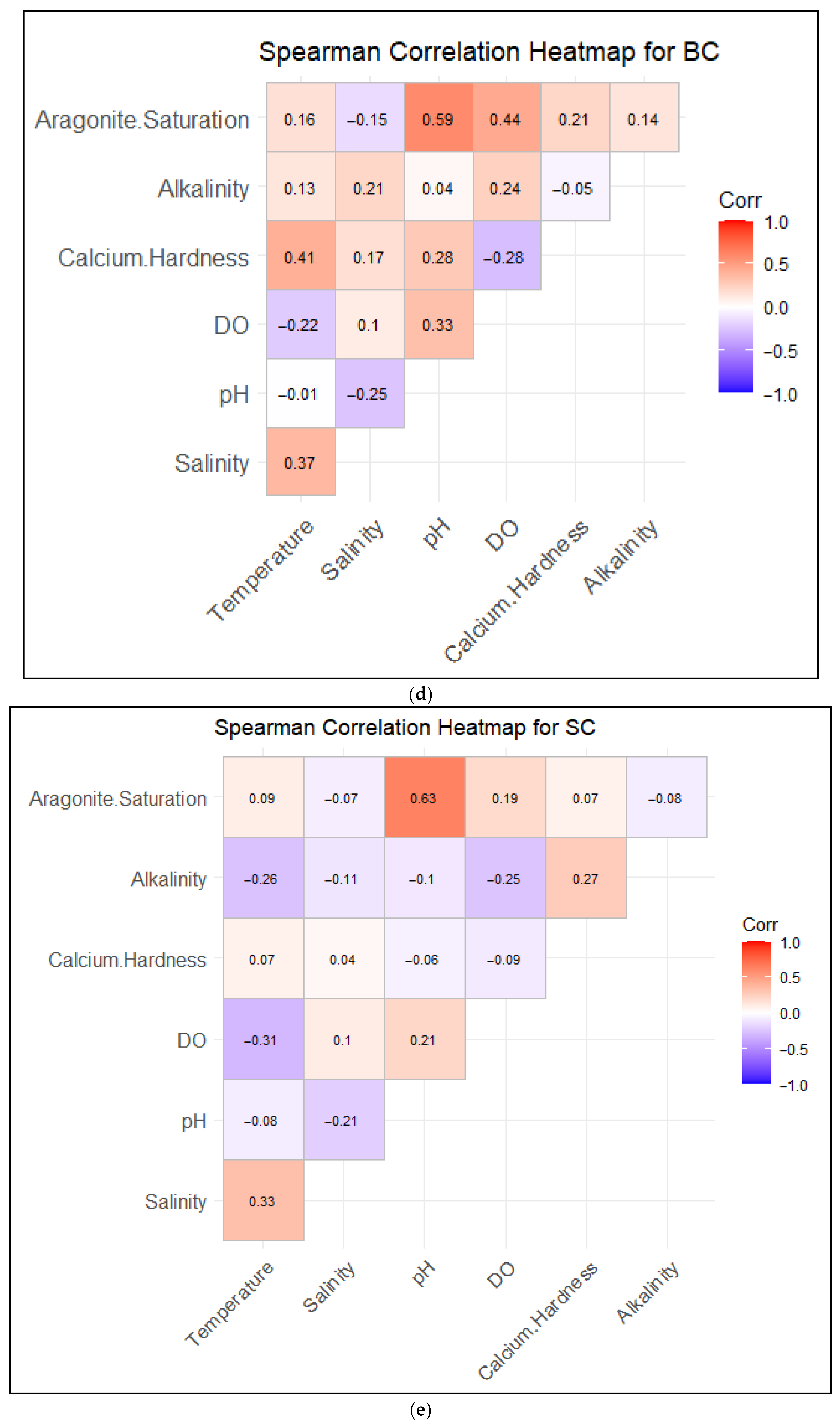
3.3. Correlations Between Water Quality and Saturation States
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waldbusser, G.G.; Voigt, E.P.; Bergschneider, H.; Green, M.A.; Newell, R.I. Biocalcification in the Eastern Oyster (Crassostrea virginica) in Relation to Long-term Trends in Chesapeake Bay pH. Estuaries Coasts 2011, 34, 221–231. [Google Scholar] [CrossRef]
- Walch, M.A.; McGowan, L.; Swanger, C.C.; Goss, M. State of the Delaware Inland Bays, 2021; Delaware Center for the Inland Bays Press: Sussex, TX, USA, 2023; 104p. [Google Scholar]
- Nunes, L.J.R. The Rising Threat of Atmospheric CO2: A Review on the Causes, Impacts, and Mitigation Strategies. Environments 2023, 10, 66. [Google Scholar] [CrossRef]
- Freely, R.A.; Doney, S.C.; Cooley, S.R. Ocean acidification: Present conditions and future changes in a high-CO2 world. Oceanography 2009, 22, 36–47. [Google Scholar] [CrossRef]
- Jiang, L.-Q.; Feely, R.A.; Carter, B.R.; Greeley, D.J.; Gledhill, D.K.; Arzayus, K.M. Climatological distribution of aragonite saturation state in the global oceans. Glob. Biogeochem. Cycles 2015, 29, 1656–1673. [Google Scholar] [CrossRef]
- Babb, R. Eastern Oysters of the Delaware Bay. New Jersey Department of Environmental Protection. Marine Issue. 2018. Available online: https://dep.nj.gov/wp-content/uploads/njfw/digest-marine-2018-eastern-oysters-of-the-delaware-bay-russ-babb.pdf (accessed on 13 July 2025).
- Sanjeeva Raj, P.J. Oysters in a new classification of keystone species. Resonance 2008, 13, 648–654. [Google Scholar] [CrossRef]
- Coen, L.D.; Brumbaugh, R.D.; Bushek, D.; Grizzle, R.; Luckenbach, M.W.; Posey, M.H.; Powers, S.P.; Tolley, S.G. Ecosystem Services related to oyster restoration. Mar. Ecol. Progress. Ser. 2007, 341, 303–307. [Google Scholar] [CrossRef]
- Grabowski, J.H.; Brumbaugh, R.D.; Conrad, R.F.; Keeler, A.G.; Opaluch, J.J.; Peterson, C.H.; Piehler, M.F.; Powers, S.P.; Smyth, A.R. Economic Valuation of Ecosystem Services Provided by Oyster Reefs. BioScience 2012, 62, 900–909. [Google Scholar] [CrossRef]
- Hauser, C.A.; Bason, C.W. The Economic Value of the Delaware Inland Bays. DE Center for the Inland Bays. 2020. Available online: https://www.inlandbays.org/about-the-bays/economic-value-of-the-inland-bays/ (accessed on 20 April 2023).
- Ewart, W.J. Shellfish Aquaculture in Delaware’s Inland Bays: Status Opportunities, and Constraints. National Oceanic and Atmospheric Administration. 2013. Available online: https://repository.library.noaa.gov/view/noaa/38095 (accessed on 13 July 2025).
- Callier, M.D.; Byron, C.J.; Bengtson, D.A.; Cranford, P.J.; Cross, S.F.; Focken, U.; Jansen, H.M.; Kamermans, P.; Kiessling, A.; Landry, T.; et al. Attraction and repulsion of mobile wild organisms to finfish and shellfish aquaculture: A review. Rev. Aquac. 2018, 10, 924–949. [Google Scholar] [CrossRef]
- Marshall, D.A.; Coxe, N.C.; La Peyre, M.K.; Walton, W.C.; Rikard, S.F.; Pollack, J.B.; Kelly, M.W.; La Peyre, J.F. Tolerance of northern Gulf of Mexico eastern oysters to chronic warming at extreme salinities. J. Therm. Biol. 2021, 100, 103072. [Google Scholar] [CrossRef]
- Hijuelos, A.C.; Sable, S.E.; O’Connell, A.M.; Geaghan, J.P. Coastal Master Plan: Attachment C3-12: Eastern Oyster, Crassostrea virginica, Habitat Suitability Index Model. Version Final; Coastal Protection and Restoration Authority Press: Baton Rouge, LA, USA, 2017; pp. 1–23. [Google Scholar]
- Barnes, T.K.; Volety, A.K.; Chartier, K.; Mazzotti, F.J.; Pearlstine, L. A habitat suitability index model for the eastern oyster (Crassostrea virginica), a tool for restoration of the Caloosahatchee Estuary, Florida. J. Shellfish Res. 2007, 26, 949–959. [Google Scholar] [CrossRef]
- EOBRT (Eastern Oyster Biological Review Team). Status Review of the Eastern Oyster (Crassostrea virginica), p. 7. Report to the National Marine Fisheries Service, Northeast Regional Office. Memo. NMFS F/SPO-88. 2007. Available online: https://repository.library.noaa.gov/view/noaa/3972 (accessed on 13 July 2025).
- Patterson, H.K.; Boettcher, A.; Carmichael, R.H. Biomarkers of dissolved oxygen stress in oysters: A tool for restoration and management efforts. PLoS ONE 2014, 9, 104440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gobler, C.J.; DePasquale, E.L.; Griffith, A.W.; Baumann, H. Hypoxia and acidification have additive and synergistic negative effects on the growth, survival, and metamorphosis of early life stage bivalves. PLoS ONE 2014, 9, 83648. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moreira, A.; Figueira, E.; Pecora, I.L.; Soares, A.M.V.M.; Freitas, R. Native and exotic oysters in Brazil: Comparative tolerance to hypercapnia. Environ. Res. 2018, 161, 202–211. [Google Scholar] [CrossRef]
- Gaines, E.; Sturmer, L.; Anderson, N.; Laramore, S.; Baker, S. The Role of pH, Alkalinity, and Calcium Carbonate in Shellfish Hatcheries. University of Florida. Available online: https://shellfish.ifas.ufl.edu/wp-content/uploads/pH-and-Alkalinity-Fact-Sheet-for-Hatcheries-Final-Draft.pdf (accessed on 13 July 2025).
- Turley, C.M.; Roberts, J.M.; Guinotte, J.M. Corals in deep water: Will the unseen hand of ocean acidification destroy cold-water ecosystems? Coral Reefs 2007, 26, 445–448. [Google Scholar] [CrossRef]
- Jan, N.; Terrie, K. Ocean Acidification in Pacific Northwest Coastal Waters: What Do We Know? College of the Environment Press: Washington, WA, USA, 2015. [Google Scholar]
- Miller, A.W.; Reynolds, A.C.; Sobrino, C.; Riedel, G.F. Shellfish face uncertain future in high CO2 World: Influence of acidification on oyster larvae calcification and growth in estuaries. PLoS ONE 2009, 4, 5661. [Google Scholar] [CrossRef]
- Shen, C.; Testa, J.M.; Li, M.; Cai, W.-J. Understanding anthropogenic impacts on pH and aragonite saturation state in Chesapeake Bay: Insights from a 30-year model study. J. Geophys. Res. Biogeosci. 2020, 125, e2019JG005620. [Google Scholar] [CrossRef]
- Talmage, S.C.; Gobler, C.J. Effects of past, present, and future ocean carbon dioxide concentrations on the growth and survival of larval shellfish. Proc. Natl. Acad. Sci. USA 2010, 107, 17246–17251. [Google Scholar] [CrossRef]
- Xue, L.; Yang, X.; Li, Y.; Li, L.; Jiang, L.-Q.; Xin, M.; Wang, Z.; Wei, Q.-X. Processes controlling sea surface pH and aragonite saturation state in a large northern temperate bay: Contrasting temperature effects. J. Geophys. Res. Biogeosci. 2020, 125, e2020JG005805. [Google Scholar] [CrossRef]
- Lemasson, A.J.; Fletcher, S.; Hall-Spencer, J.M.; Knights, A.M. Linking the biological impacts of ocean acidification on oysters to changes in ecosystem services: A review. J. Exp. Mar. Biol. Ecol. 2017, 492, 49–62. [Google Scholar] [CrossRef]
- NOAA (National Oceanic and Atmospheric Association). Eastern Oyster. NOAA Fisheries. 2023. Available online: https://www.fisheries.noaa.gov/species/eastern-oyster#oyster-management-and-restoration (accessed on 13 July 2025).
- Xu, X.; Hu, Y.; He, Z.; Wang, X.; Chen, H.; Han, J. Processes controlling the aragonite saturation state in the North Yellow Sea near the Yalu River estuary: Contrasting river input effects. Front. Mar. Sci. 2023, 10, 2296–7745. Available online: https://www.frontiersin.org/journals/marinescience/articles/10.3389/fmars.2023.1158896 (accessed on 13 July 2025). [CrossRef]
- Liberti, C.M.; Gray, M.W.; Mayer, L.M.; Testa, J.M.; Liu, W.; Brady, D.C. The impact of oyster aquaculture on the estuarine carbonate system. Elem. Sci. Anthr. 2022, 10, 00057. [Google Scholar] [CrossRef]

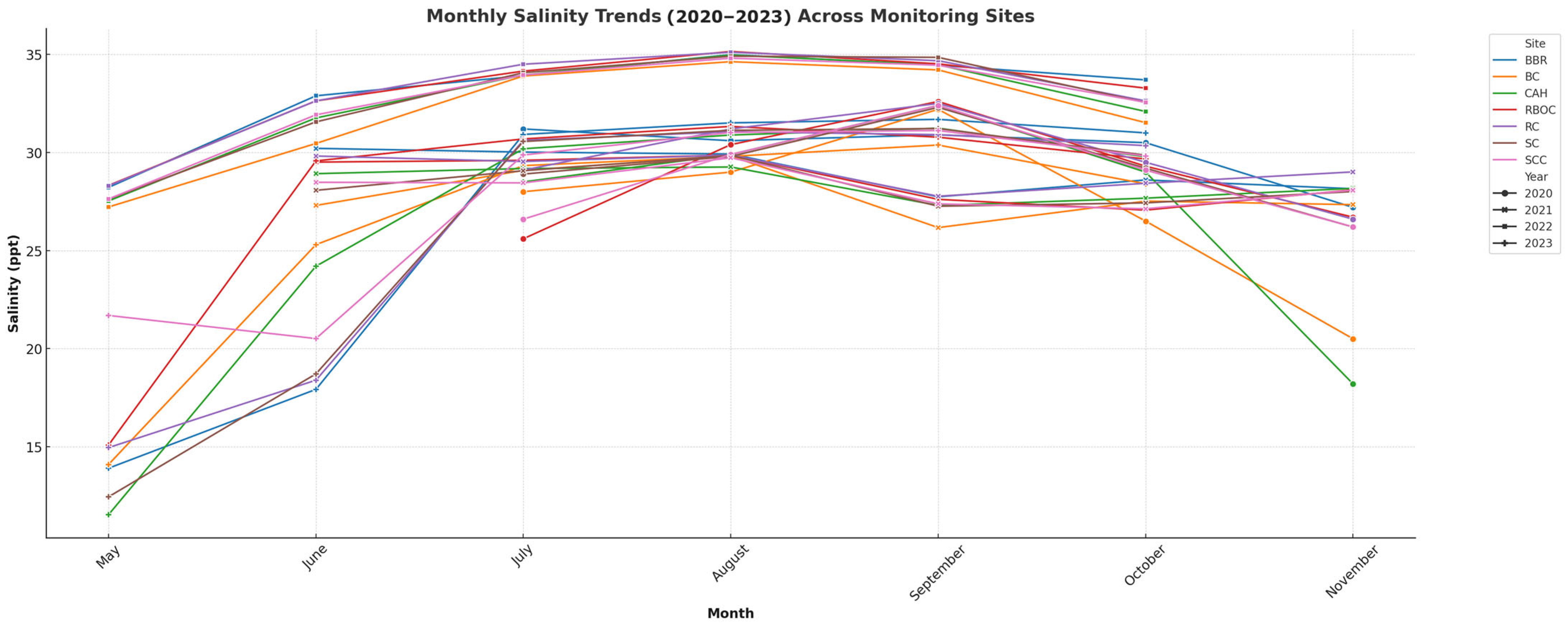
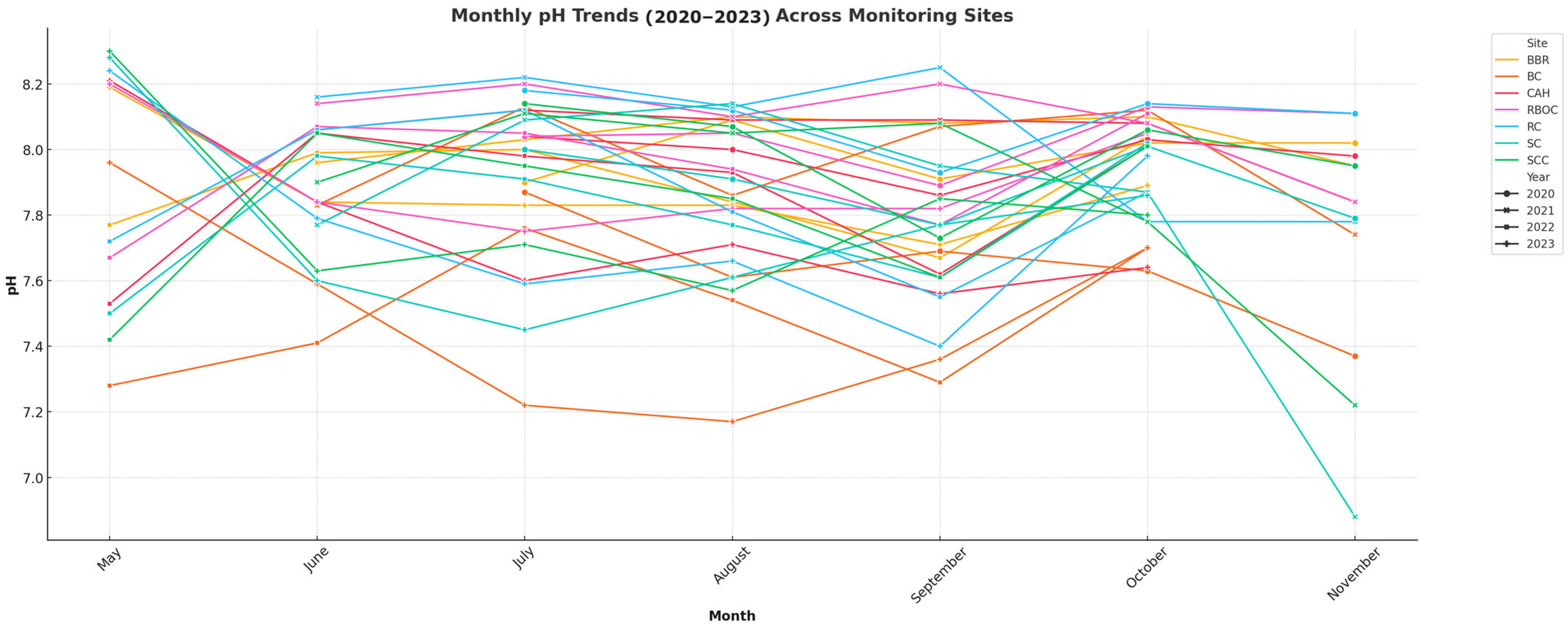



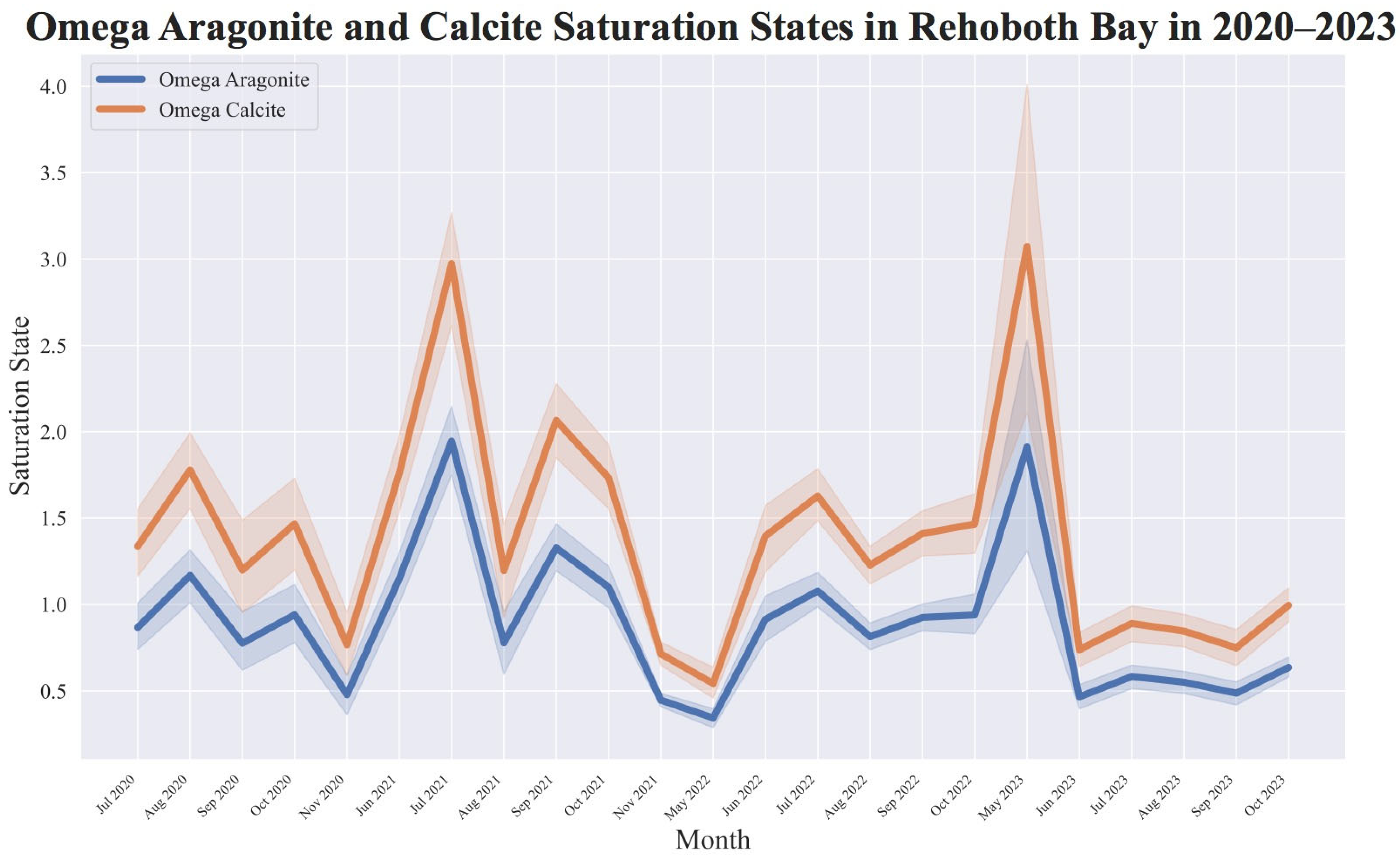

| Year | Temperature (°C) | Salinity (ppt) | pH | DO (mg/L) | Alkalinity (mg/L CaCO3) | Calcium Hardness (mg/L CaCO3) |
|---|---|---|---|---|---|---|
| 2020 | 23.0 ± 4.8 | 29.0 ± 3.0 | 7.96 ± 0.2 | 8.61 ± 1.6 | 82.0 ± 27.4 | 254.41 ± 79.8 |
| 2021 | 21.6 ± 5.6 | 28.5 ± 1.2 | 8.04 ± 0.1 | 5.84 ± 1.4 | 92.3 ± 27.7 | 261.51 ± 59.4 |
| 2022 | 23.0 ± 4.8 | 33.56 ± 2.0 | 7.83 ± 0.35 | 7.52 ± 1.7 | 87.9 ± 25.7 | 223.64 ± 60.7 |
| 2023 | 23.1 ± 3.6 | 26.50 ± 8.7 | 7.77 ± 0.46 | 6.87 ± 1.5 | 86.0 ± 20.5 | 221.31 ± 58.0 |
| Sites | Year | Omega Aragonite | Omega Calcite |
|---|---|---|---|
| BBR | 2020 | 0.72 ± 0.2 | 1.10 ± 0.3 |
| 2021 | 1.10 ± 0.5 | 1.70 ± 0.7 | |
| 2022 | 0.77 ± 0.1 | 1.19 ± 0.2 | |
| 2023 | 0.90 ± 0.5 | 1.40 ± 0.8 | |
| BC | 2020 | 1.31 ± 0.4 | 1.80 ± 0.7 |
| 2021 | 1.05 ± 0.6 | 1.60 ± 1.0 | |
| 2022 | 0.58 ± 0.4 | 0.88 ± 0.6 | |
| 2023 | 0.49 ± 0.3 | 0.77 ± 0.5 | |
| CAH | 2020 | 1.19 ± 0.5 | 1.80 ± 0.8 |
| 2021 | 1.16 ± 0.5 | 1.80 ± 0.8 | |
| 2022 | 0.86 ± 0.3 | 1.31 ± 0.4 | |
| 2023 | 0.75 ± 0.4 | 1.20 ± 0.7 | |
| RBOC | 2020 | 0.99 ± 0.5 | 1.50 ± 0.7 |
| 2021 | 1.20 ± 0.6 | 1.80 ± 0.9 | |
| 2022 | 1.02 ± 0.2 | 1.56 ± 0.3 | |
| 2023 | 0.97 ± 0.4 | 1.51 ± 0.6 | |
| RC | 2020 | 0.49 ± 0.2 | 0.80 ± 0.3 |
| 2021 | 1.38 ± 0.6 | 2.10 ± 0.9 | |
| 2022 | 0.92 ± 0.4 | 1.41 ± 0.6 | |
| 2023 | 0.87 ± 0.5 | 1.37 ± 0.8 | |
| SC | 2020 | 1.34 ± 0.4 | 2.10 ± 0.7 |
| 2021 | 1.10 ± 0.4 | 1.70 ± 0.6 | |
| 2022 | 0.80 ± 0.2 | 1.22 ± 0.4 | |
| 2023 | 0.67 ± 0.3 | 1.05 ± 0.5 | |
| SCC | 2020 | 0.48 ± 0.3 | 0.80 ± 0.4 |
| 2021 | 0.88 ± 0.6 | 1.40 ± 0.9 | |
| 2022 | 0.90 ± 0.3 | 1.38 ± 0.5 | |
| 2023 | 0.77 ± 0.5 | 1.21 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attarwala, T.; Boukari, A.; Ozbay, G. Aragonite Saturation State as an Indicator for Oyster Habitat Health in the Delaware Inland Bays. Coasts 2025, 5, 30. https://doi.org/10.3390/coasts5030030
Attarwala T, Boukari A, Ozbay G. Aragonite Saturation State as an Indicator for Oyster Habitat Health in the Delaware Inland Bays. Coasts. 2025; 5(3):30. https://doi.org/10.3390/coasts5030030
Chicago/Turabian StyleAttarwala, Tahera, Amin Boukari, and Gulnihal Ozbay. 2025. "Aragonite Saturation State as an Indicator for Oyster Habitat Health in the Delaware Inland Bays" Coasts 5, no. 3: 30. https://doi.org/10.3390/coasts5030030
APA StyleAttarwala, T., Boukari, A., & Ozbay, G. (2025). Aragonite Saturation State as an Indicator for Oyster Habitat Health in the Delaware Inland Bays. Coasts, 5(3), 30. https://doi.org/10.3390/coasts5030030







