Augmenting Coral Growth on Breakwaters: A Shelter-Based Approach
Abstract
1. Introduction
1.1. Coastal Defense Structures and Breakwaters
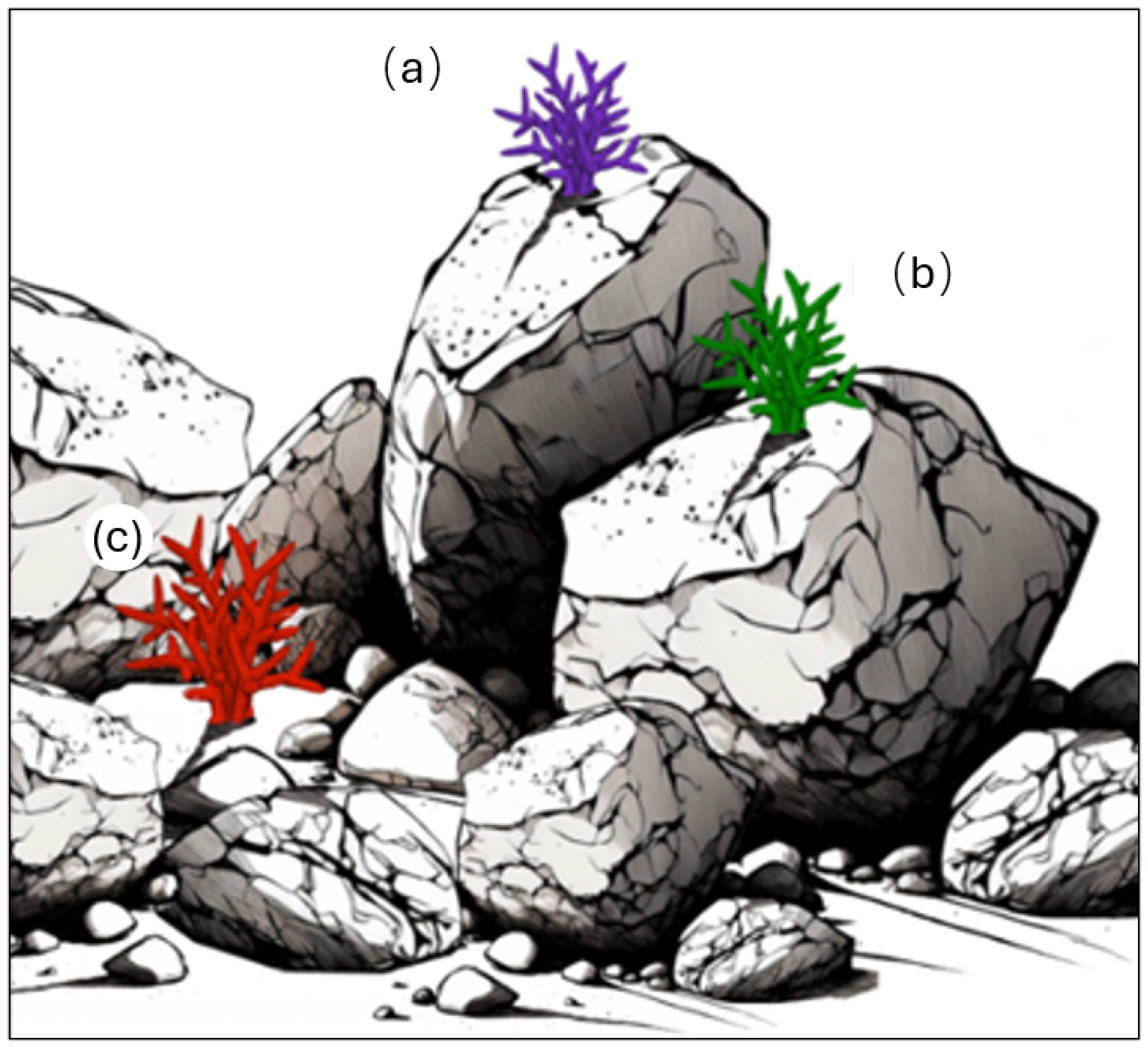
1.2. Diversity in Sheltered Niches in a Breakwater
- (a)
- Exposed—no protection for the coral.
- (b)
- Semi-sheltered—one to two sides of the coral are protected.
- (c)
- Sheltered—three to four sides of the coral are protected.
2. Materials and Methods
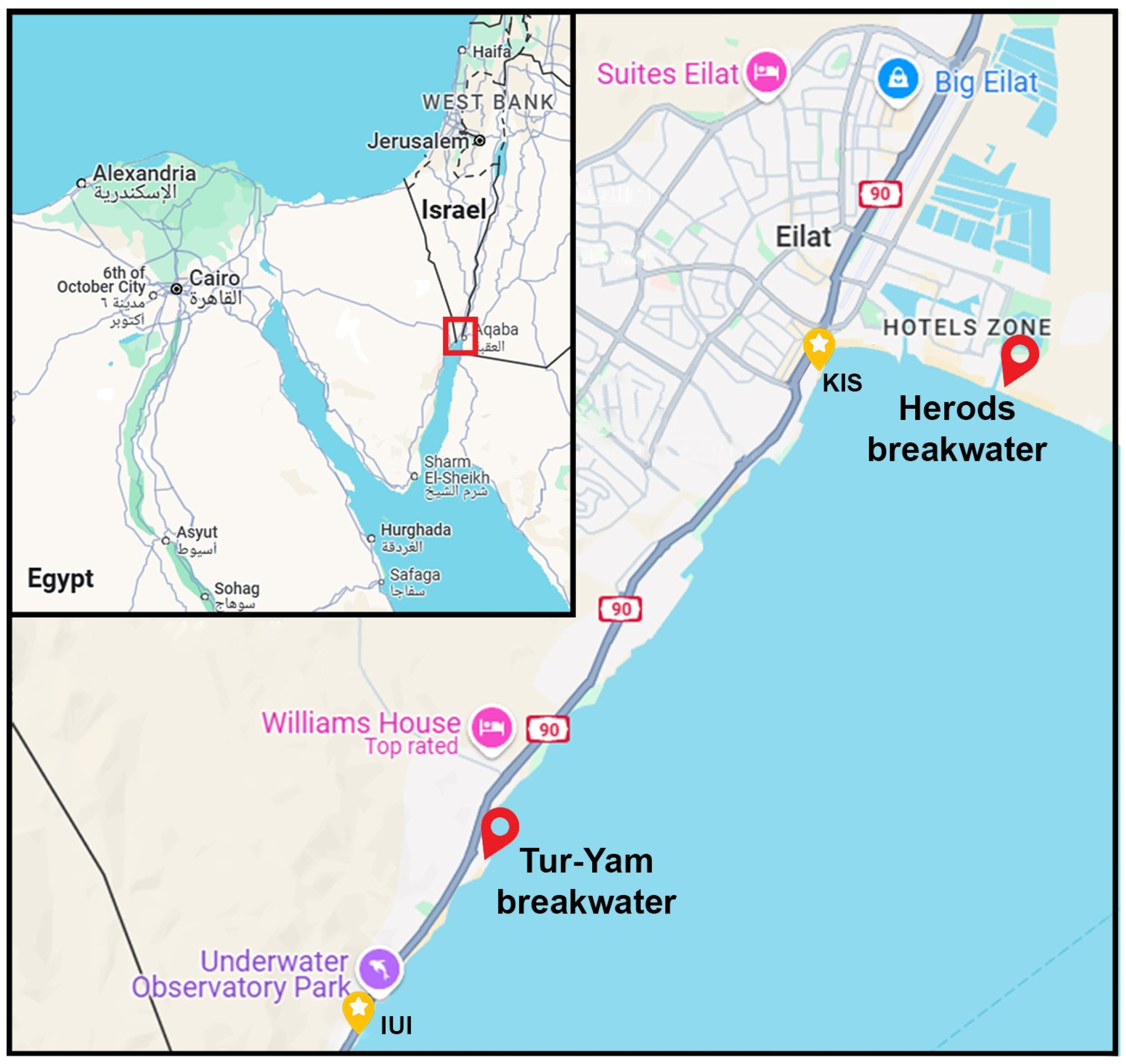
2.1. Study Site
- Tur-Yam breakwater at Eilat’s south marina, located at Almog’s beach (29.515122, 34.926736);
- Herod’s breakwater on Eilat’s north beach, located in front of Herod’s hotel (29.546614, 34.966668).
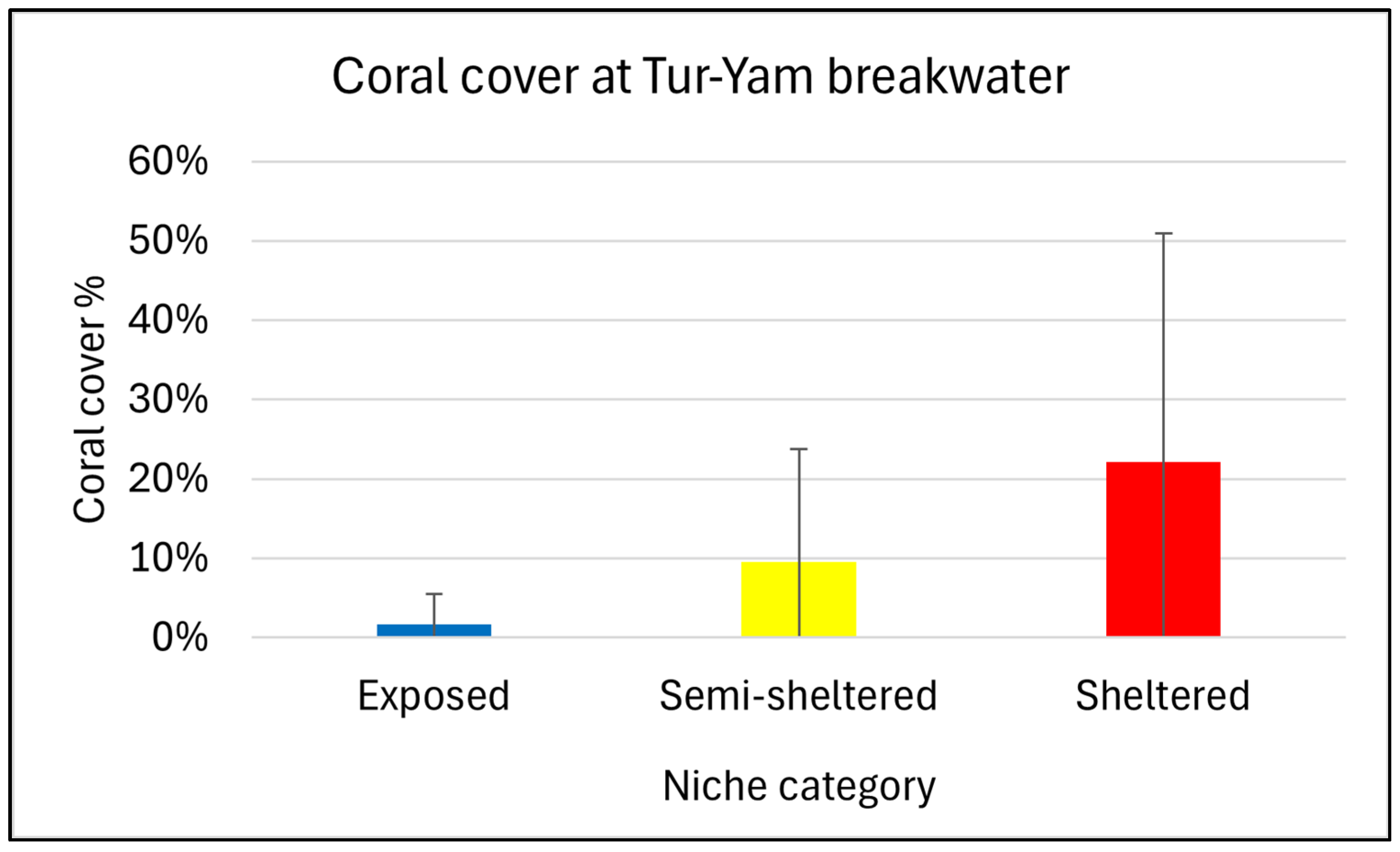
2.2. Spatial Coral Distribution at Different Niches of a Breakwater
2.3. Coral Recruitment to Breakwaters
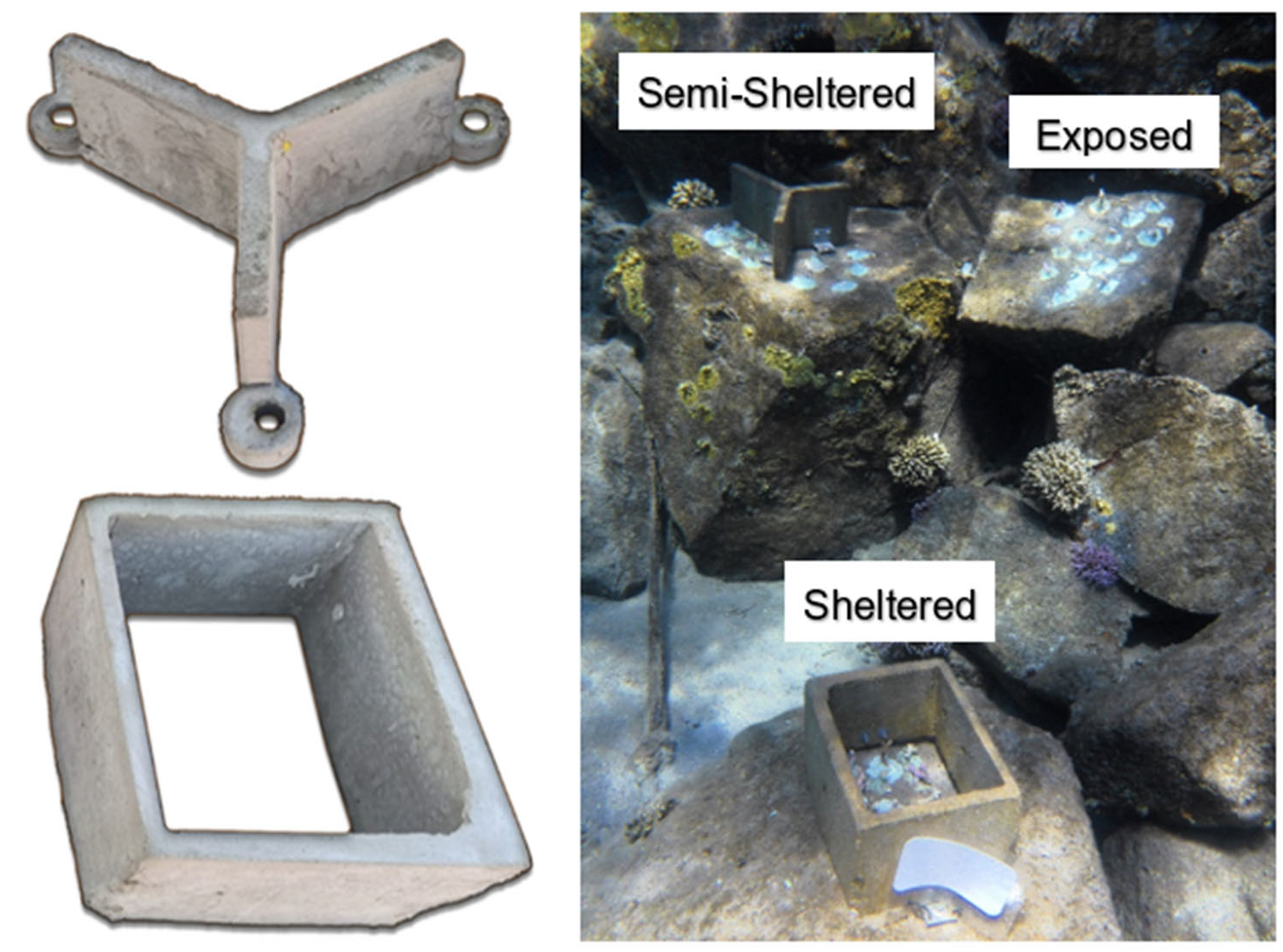
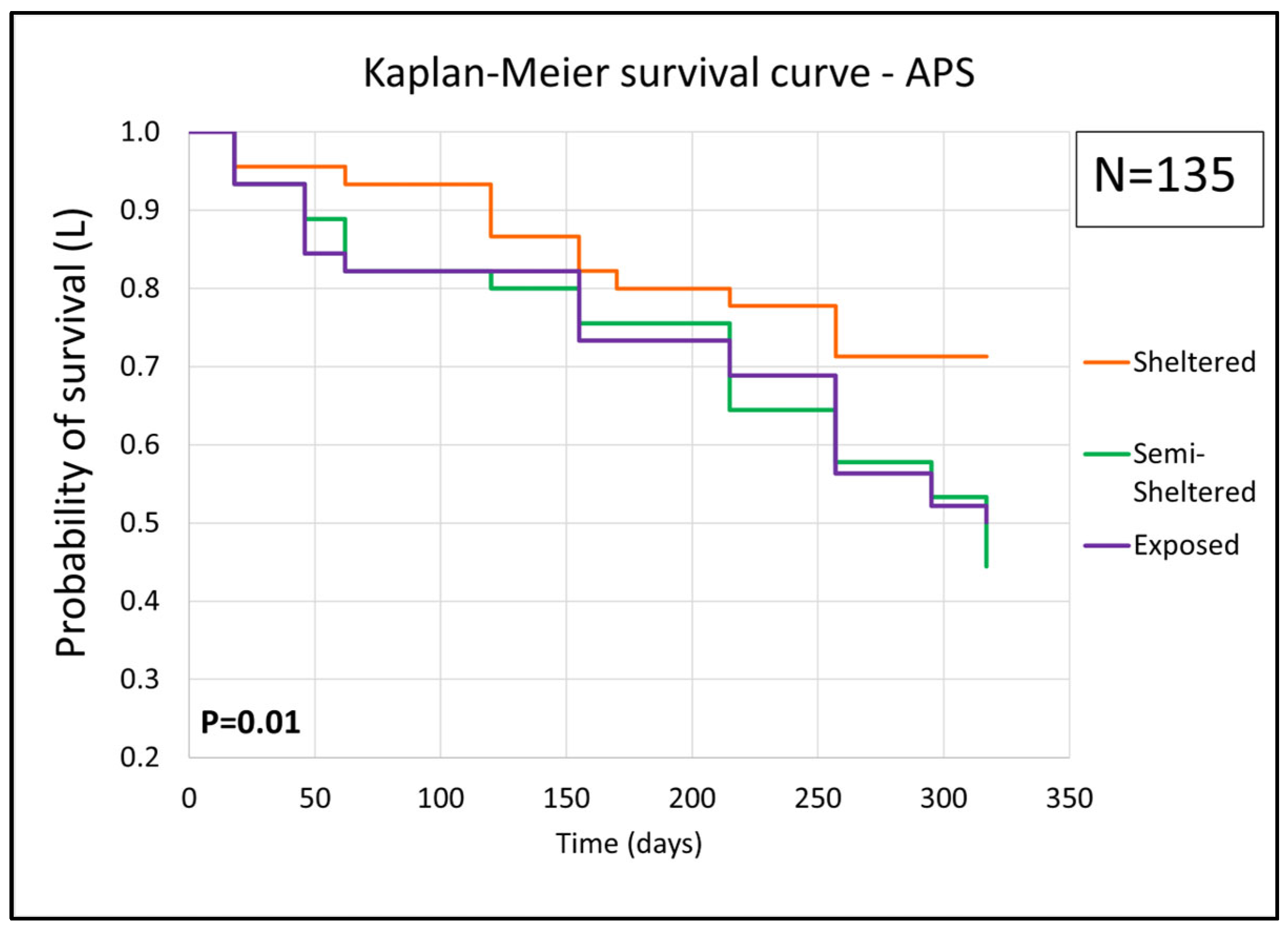
2.4. Coral Survivability at Breakwaters With and Without Artificial Protection Structures
Coral Survivability on a Breakwater
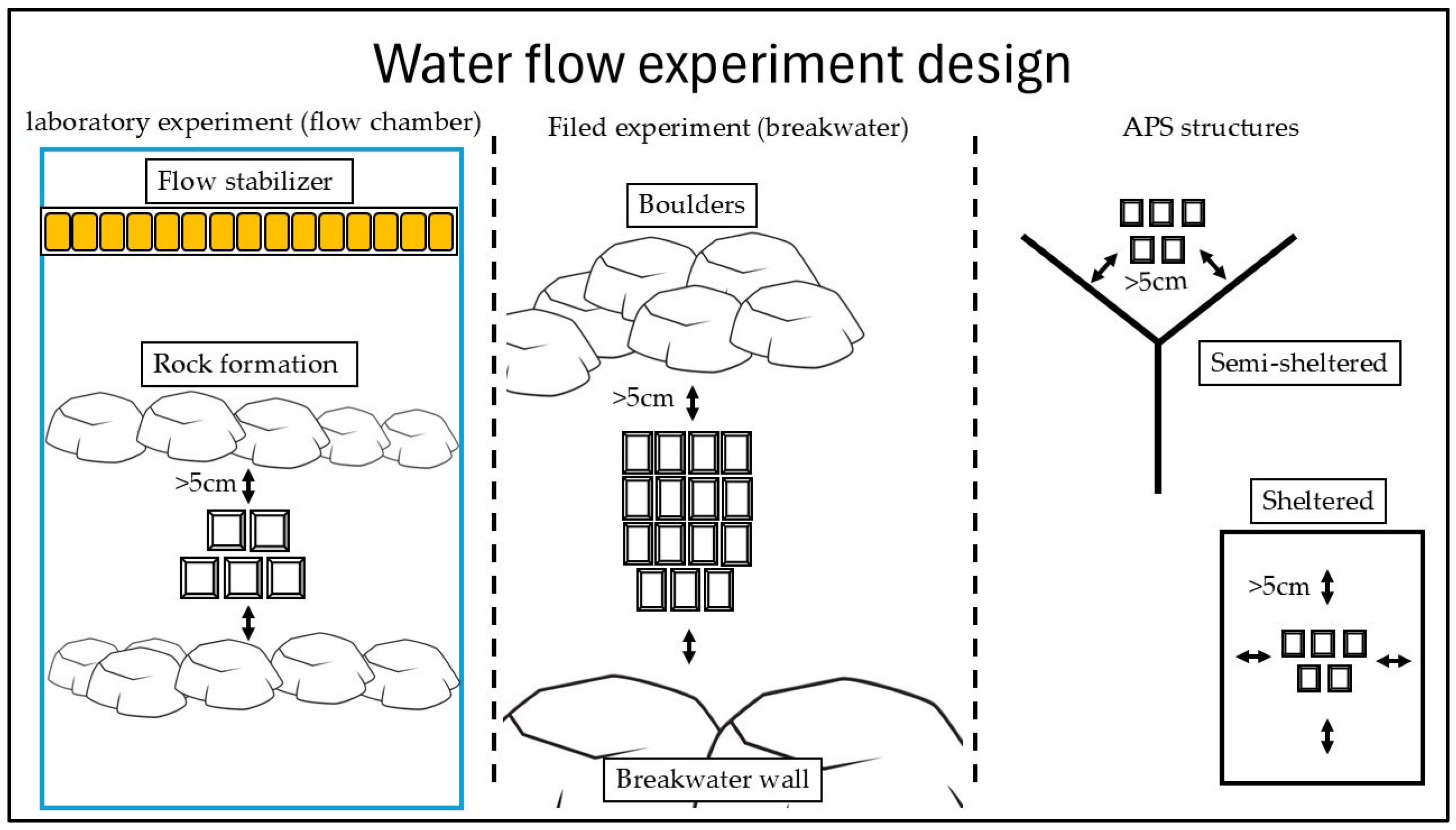
2.5. Water Flows at Different Niches in the Field (Breakwaters) and in Laboratory Experiments
2.5.1. Water Motion Measurements
2.5.2. Calibration Curve (Flow Tank)
2.5.3. Water Flow in Defined Niches on a Breakwater (Field Experiment)
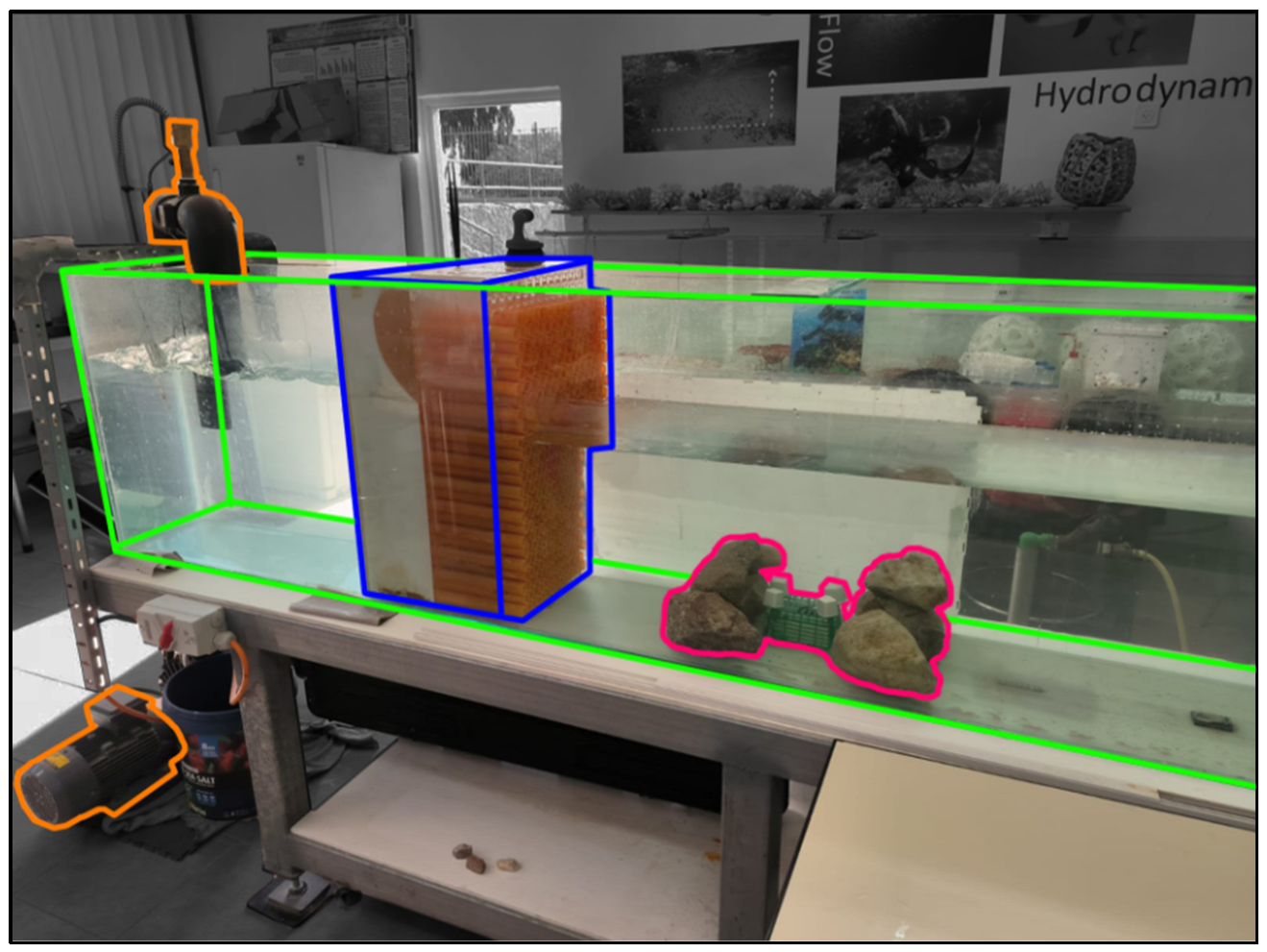
2.5.4. Water Flow in Laboratory Conditions (Laboratory Experiment)
2.5.5. Water Motion Inside APSs
2.5.6. Data Analysis
2.6. Sedimentation at Different Niches of Breakwater
3. Results
3.1. Coral Distribution
3.2. Coral Recruitment
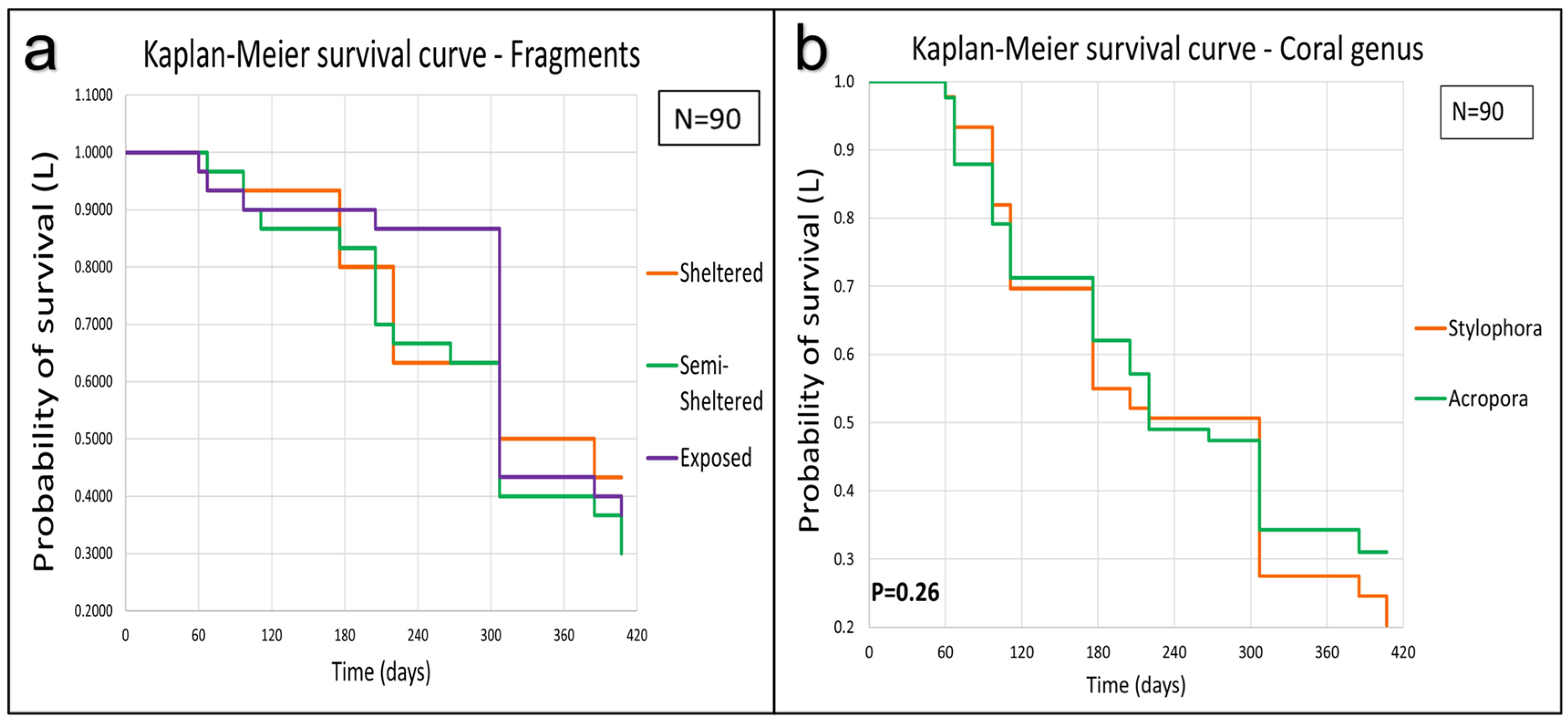
3.3. Coral Survival
Survival Rate
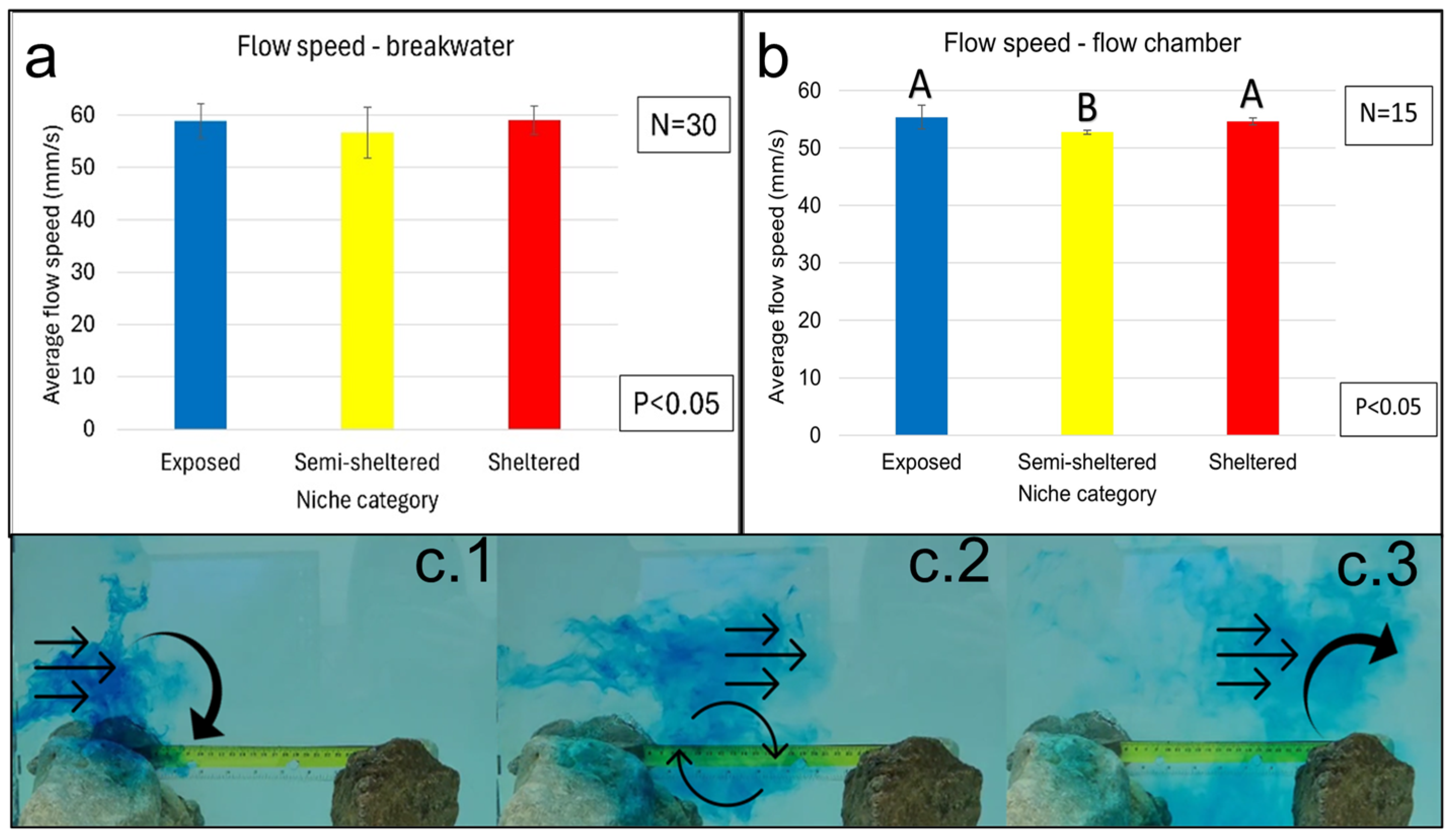
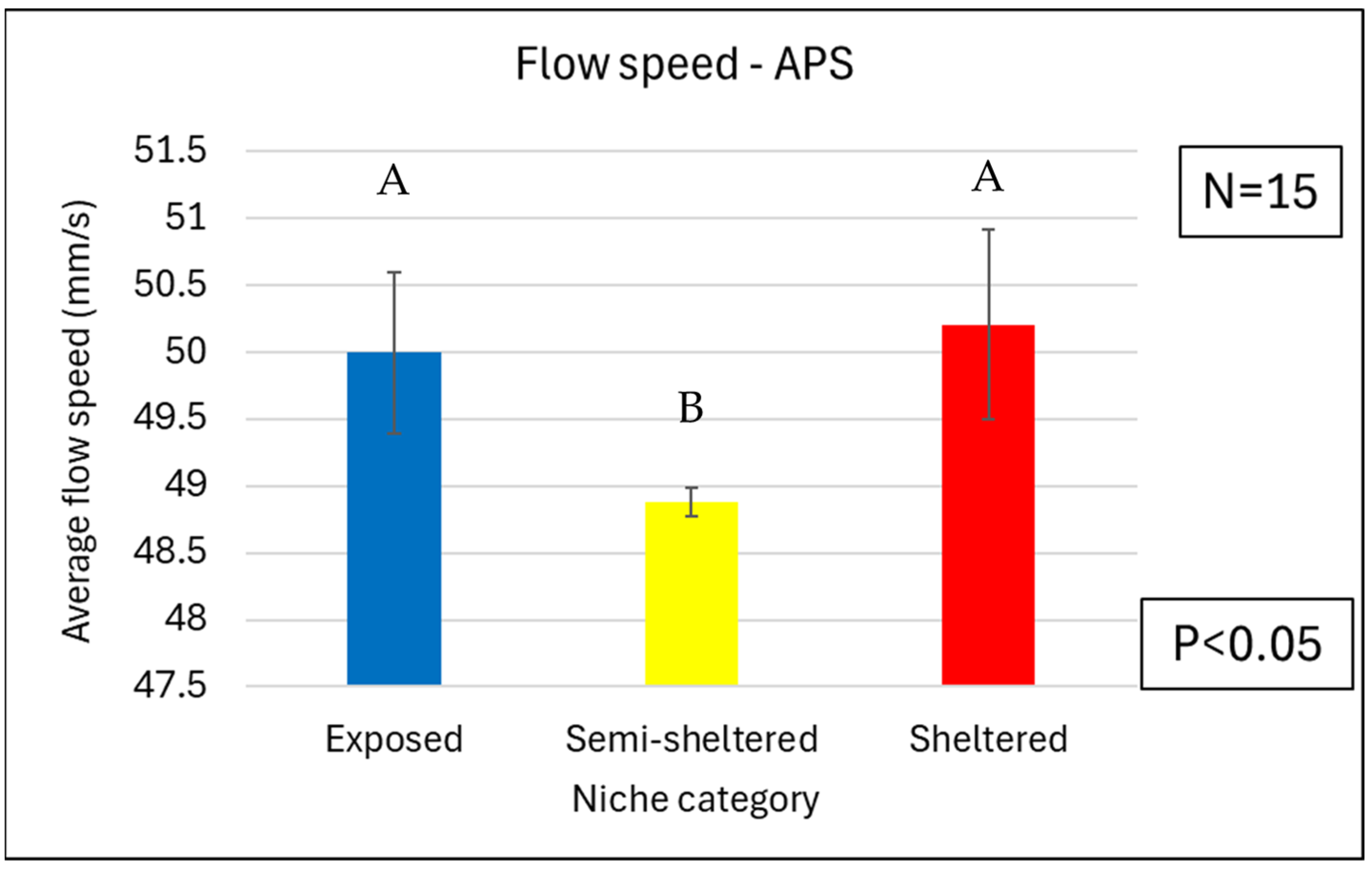
3.4. Water Flow
3.4.1. Water Flow in Protection Niches (Field and Laboratory)
3.4.2. Water Flows near the APSs
3.5. Sedimentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Levy, M. Percentage of Total Population Living in Coastal Areas. 2011. Available online: https://www.un.org/esa/sustdev/natlinfo/indicators/methodology_sheets/oceans_seas_coasts/pop_coastal_areas.pdf (accessed on 15 June 2007).
- Rao, N.S.; Ghermandi, A.; Portela, R.; Wang, X. Global values of coastal ecosystem services: A spatial economic analysis of shoreline protection values. Ecosyst. Serv. 2015, 11, 95–105. [Google Scholar] [CrossRef]
- Hauer, M.E.; Hardy, D.; Kulp, S.A.; Mueller, V.; Wrathall, D.J.; Clark, P.U. Assessing population exposure to coastal flooding due to sea level rise. Nat. Commun. 2021, 12, 6900. [Google Scholar] [CrossRef] [PubMed]
- Foti, E.; Musumeci, R.E.; Stagnitti, M. Coastal defence techniques and climate change: A review. Rend. Fis. Acc. Lincei 2020, 31, 123–138. [Google Scholar] [CrossRef]
- Schoonees, T.; Gijón Mancheño, A.; Scheres, B.; Bouma, T.J.; Silva, R.; Schlurmann, T.; Schüttrumpf, H. Hard structures for coastal protection, towards greener designs. Estuaries Coasts 2019, 42, 1709–1729. [Google Scholar] [CrossRef]
- De Graauw, A. Ancient Port Structures. Parallels between the ancient and the modern. Méditerranée Rev. Géographique Pays Méditerranéens/J. Mediterr. Geogr. 2022, 134. [Google Scholar] [CrossRef]
- Bakker, P.; van den Berge, A.; Hakenberg, R.; Klaboratorybers, M.; Muttray, M.; Reedijk, B.; Rovers, I. Development of concrete breakwater Armour Units. In Proceedings of the 1st Coastal Estuary and Offshore Engineering Specialty Conference of the Canadian Society for Civil Engineering, Moncton, NB, Canada, 4–7 June 2003. [Google Scholar]
- Muttray, M.; Reedijk, B. Design of concrete armour layers. Hansa Int. Marit. J. 2009, 6, 111–118. [Google Scholar]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Barbier, E.B. Valuing ecosystem services for coastal wetland protection and restoration: Progress and challenges. Resources 2013, 2, 213–230. [Google Scholar] [CrossRef]
- Nicholls, R.J.; Cazenave, A. Sea-level rise and its impact on coastal zones. Science 2010, 328, 1517–1520. [Google Scholar] [CrossRef]
- Sherrard, T.R.; Hawkins, S.J.; Barfield, P.; Kitou, M.; Bray, S.; Osborne, P.E. Hidden biodiversity in cryptic habitats provided by porous coastal defence structures. Coast. Eng. 2016, 118, 12–20. [Google Scholar] [CrossRef]
- Connell, S.D.; Glasby, T.M. Do urban structures influence local abundance and diversity of subtidal epibiota? A case study from Sydney Harbour, Australia. Mar. Environ. Res. 1999, 47, 373–387. [Google Scholar] [CrossRef]
- Masucci, G.D.; Acierno, A.; Reimer, J.D. Eroding diversity away: Impacts of a tetrapod breakwater on a subtropical coral reef. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 290–302. [Google Scholar] [CrossRef]
- Fadli, N.; Muchlisin, Z.A.; Sofyan, H.; El-Rahimi, S.A.; Dewiyanti, I.; Pratama, F.O.; Siti-Azizah, M.N.; Rahman, A.; Yusuf, M.; Ibrahim, S. The composition of reef-associated fishes in Ulee Lheue breakwater Banda Aceh, Aceh, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2018, 216, 012021. [Google Scholar] [CrossRef]
- Burt, J.A. The environmental costs of coastal urbanization in the Arabian Gulf. City 2014, 18, 760–770. [Google Scholar] [CrossRef]
- Wen, C.K.C.; Chen, K.S.; Hsieh, H.J.; Hsu, C.M.; Chen, C.A. High coral cover and subsequent high fish richness on mature breakwaters in Taiwan. Mar. Pollut. Bull. 2013, 72, 55–63. [Google Scholar] [CrossRef]
- Tan, Y.Z.; Ng, C.S.L.; Chou, L.M. Natural colonisation of a marina seawall by scleractinian corals along Singapore’s east coast. Nat. Singap. 2012, 5, 177–183. [Google Scholar]
- Balqadi, A.A.; Salama, A.J.; Satheesh, S. Microfouling development on artificial substrates deployed in the central Red Sea. Oceanologia 2018, 60, 219–231. [Google Scholar] [CrossRef]
- Toledo, M.I.; Torres, P.; Díaz, C.; Zamora, V.; López, J.; Olivares, G. Ecological succession of benthic organisms on niche-type artificial reefs. Ecol. Process. 2020, 9, 38. [Google Scholar] [CrossRef]
- Chee, S.Y.; Yee, J.C.; Cheah, C.B.; Evans, A.J.; Firth, L.B.; Hawkins, S.J.; Strain, E.M.A. Habitat complexity affects the structure but not the diversity of sessile communities on tropical coastal infrastructure. Front. Ecol. Evol. 2021, 9, 673227. [Google Scholar] [CrossRef]
- González-Duarte, M.M.; Fernández-Montblanc, T.; Bethencourt, M.; Izquierdo, A. Effects of substrata and environmental conditions on ecological succession on historic shipwrecks. Estuar. Coast. Shelf Sci. 2018, 200, 301–310. [Google Scholar] [CrossRef]
- Vaselli, S.; Bulleri, F.; Benedetti-Cecchi, L. Hard coastal-defence structures as habitats for native and exotic rocky-bottom species. Mar. Environ. Res. 2008, 66, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Dugan, J.E.; Airoldi, L.; Chapman, M.G.; Walker, S.J.; Schlacher, T.; Wolanski, E.; McLusky, D. 8.02-Estuarine and coastal structures: Environmental effects, a focus on shore and nearshore structures. Treatise Estuar. Coast. Sci. 2011, 8, 17–41. [Google Scholar] [CrossRef]
- Hall, A.E.; Herbert, R.J.; Britton, J.R.; Hull, S.L. Ecological enhancement techniques to improve habitat heterogeneity on coastal defence structures. Estuar. Coast. Shelf Sci. 2018, 210, 68–78. [Google Scholar] [CrossRef]
- Sedano, F.; Navarro-Barranco, C.; Guerra-García, J.M.; Espinosa, F. Understanding the effects of coastal defence structures on marine biota: The role of substrate composition and roughness in structuring sessile, macro-and meiofaunal communities. Mar. Pollut. Bull. 2020, 157, 111334. [Google Scholar] [CrossRef]
- Köhler, J.; Hansen, P.D.; Wahl, M. Colonization patterns at the substratum-water interface: How does surface microtopography influence recruitment patterns of sessile organisms? Biofouling 1999, 14, 237–248. [Google Scholar] [CrossRef]
- Ben-Natan, A.; Shashar, N. A Short Review of Strategies for Augmenting Organism Recruitment on Coastal Defense Structures. J. Mar. Sci. Eng. 2025, 13, 95. [Google Scholar] [CrossRef]
- Masria, A.; Iskander, M.; Negm, A. Coastal protection measures, case study (Mediterranean zone, Egypt). J. Coast. Conserv. 2015, 19, 281–294. [Google Scholar] [CrossRef]
- Perkol-Finkel, S.; Hadary, T.; Rella, A.; Shirazi, R.; Sella, I. Seascape architecture–incorporating ecological considerations in design of coastal and marine infrastructure. Ecol. Eng. 2018, 120, 645–654. [Google Scholar] [CrossRef]
- Evans, A.J.; Firth, L.B.; Hawkins, S.J.; Morris, E.S.; Goudge, H.; Moore, P.J. Drill-cored rock pools: An effective method of ecological enhancement on artificial structures. Mar. Freshw. Res. 2015, 67, 123–130. [Google Scholar] [CrossRef]
- Santin, S.; Willis, T.J. Direct versus indirect effects of wave exposure as a structuring force on temperate cryptobenthic Wsh assemblages. Mar. Biol. 2007, 151, 1683–1694. [Google Scholar] [CrossRef]
- Reidenbach, M.A.; Stocking, J.B.; Szczyrba, L.; Wendelken, C. Hydrodynamic interactions with coral topography and its impact on larval settlement. Coral Reefs 2021, 40, 505–519. [Google Scholar] [CrossRef]
- Dollar, S.J. Wave stress and coral community structure in Hawaii. Coral Reefs 1982, 1, 71–81. [Google Scholar] [CrossRef]
- Page, C.E.; Leggat, W.; Heron, S.F.; Fordyce, A.J.; Ainsworth, T.D. High flow conditions mediate damaging impacts of sub-lethal thermal stress on corals’ endosymbiotic algae. Conserv. Physiol. 2021, 9, coab046. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 30 November 2023).
- Horoszowski-Fridman, Y.B.; Brêthes, J.C.; Rahmani, N.; Rinkevich, B. Marine silviculture: Incorporating ecosystem engineering properties into reef restoration acts. Ecol. Eng. 2015, 82, 201–213. [Google Scholar] [CrossRef]
- Goel, M.K.; Khanna, P.; Kishore, J. Understanding survival analysis: Kaplan-Meier estimate. Int. J. Ayurveda Res. 2010, 1, 274. [Google Scholar] [PubMed]
- Doty, M.S. Measurement of water movement in reference to benthic algal growth. Bot. Mar. 1971, XIV, 32–35. [Google Scholar] [CrossRef]
- Shaked, Y.; Fine, M. Annual Report of the Israel National Monitoring Program in the Northern Gulf of Aqaba Eilat. 2023. Available online: http://iui-eilat.ac.il/Research/NMPMeteoData.aspx (accessed on 3 July 2024).
- Salinas-de-León, P.; Costales-Carrera, A.; Zeljkovic, S.; Smith, D.J.; Bell, J.J. Scleractinian settlement patterns to natural cleared reef substrata and artificial settlement panels on an Indonesian coral reef. Estuar. Coast. Shelf Sci. 2011, 93, 80–85. [Google Scholar] [CrossRef]
- Carr, M.H.; Hixon, M.A. Artificial reefs: The importance of comparisons with natural reefs. Fisheries 1997, 22, 28–33. [Google Scholar] [CrossRef]
- Viyakarn, V.; Chavanich, S.; Raksasab, C.; Loyjiw, T. New coral community on a breakwater in Thailand. Coral Reefs 2009, 28, 427. [Google Scholar] [CrossRef][Green Version]
- Manasrah, R.; Zibdah, M.; Al-Ougaily, F.; Yusuf, N.; Al-Najjar, T. Seasonal changes of water properties and current in the northernmost Gulf of Aqaba, Red Sea. Ocean Sci. J. 2007, 42, 103–116. [Google Scholar] [CrossRef]
- Guerrini, G.; Yerushalmy, M.; Shefy, D.; Shashar, N.; Rinkevich, B. Apparent recruitment failure for the vast majority of coral species at Eilat, Red Sea. Coral Reefs 2020, 39, 1715–1726. [Google Scholar] [CrossRef]
- Tynyakov, J.; Rousseau, M.; Chen, M.; Figus, O.; Belhassen, Y.; Shashar, N. Artificial reefs as a means of spreading diving pressure in a coral reef environment. Ocean Coast. Manag. 2017, 149, 159–164. [Google Scholar] [CrossRef]
- Zakai, D.; Chadwick-Furman, N.E. Impacts of intensive recreational diving on reef corals at Eilat, northern Red Sea. Biol. Conserv. 2002, 105, 179–187. [Google Scholar] [CrossRef]
- Sella, I.; Hadary, T.; Rella, A.J.; Riegl, B.; Swack, D.; Perkol-Finkel, S. Design, production, and validation of the biological and structural performance of an ecologically engineered concrete block mattress: A nature-inclusive design for shoreline and offshore construction. Integr. Environ. Assess. Manag. 2022, 18, 148–162. [Google Scholar] [CrossRef]
- Reguero, B.G.; Beck, M.W.; Agostini, V.N.; Kramer, P.; Hancock, B. Coral reefs for coastal protection: A new methodological approach and engineering case study in Grenada. J. Environ. Manag. 2018, 210, 146–161. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Natan, A.; Chernihovsky, N.; Shashar, N. Augmenting Coral Growth on Breakwaters: A Shelter-Based Approach. Coasts 2025, 5, 18. https://doi.org/10.3390/coasts5020018
Ben Natan A, Chernihovsky N, Shashar N. Augmenting Coral Growth on Breakwaters: A Shelter-Based Approach. Coasts. 2025; 5(2):18. https://doi.org/10.3390/coasts5020018
Chicago/Turabian StyleBen Natan, Almog, Natalie Chernihovsky, and Nadav Shashar. 2025. "Augmenting Coral Growth on Breakwaters: A Shelter-Based Approach" Coasts 5, no. 2: 18. https://doi.org/10.3390/coasts5020018
APA StyleBen Natan, A., Chernihovsky, N., & Shashar, N. (2025). Augmenting Coral Growth on Breakwaters: A Shelter-Based Approach. Coasts, 5(2), 18. https://doi.org/10.3390/coasts5020018






