Meiofauna from Almirante Câmara Canyon and Its Adjacent Open Slope, Southwest Atlantic Ocean
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Sample Processing
2.3. Data Analysis
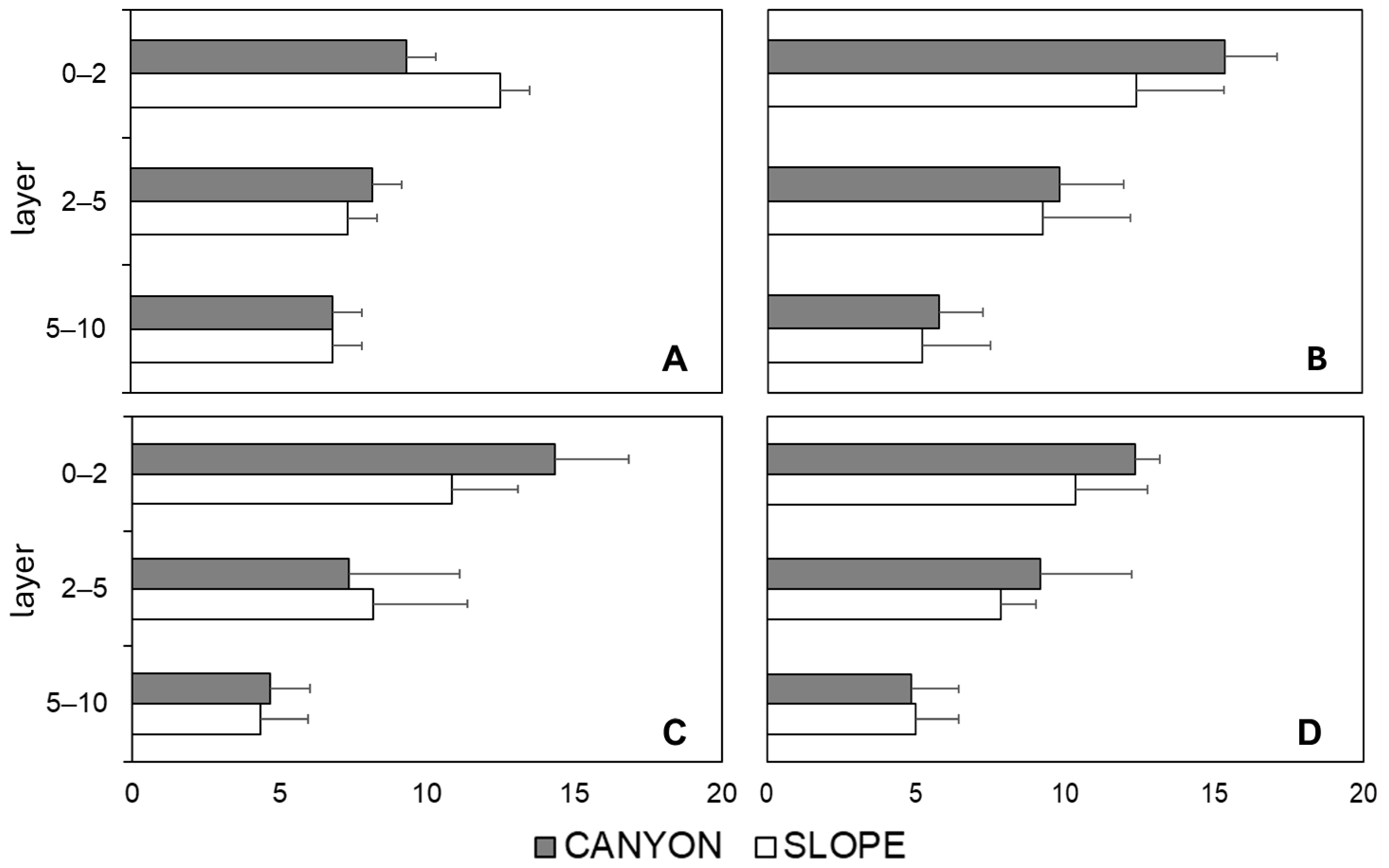
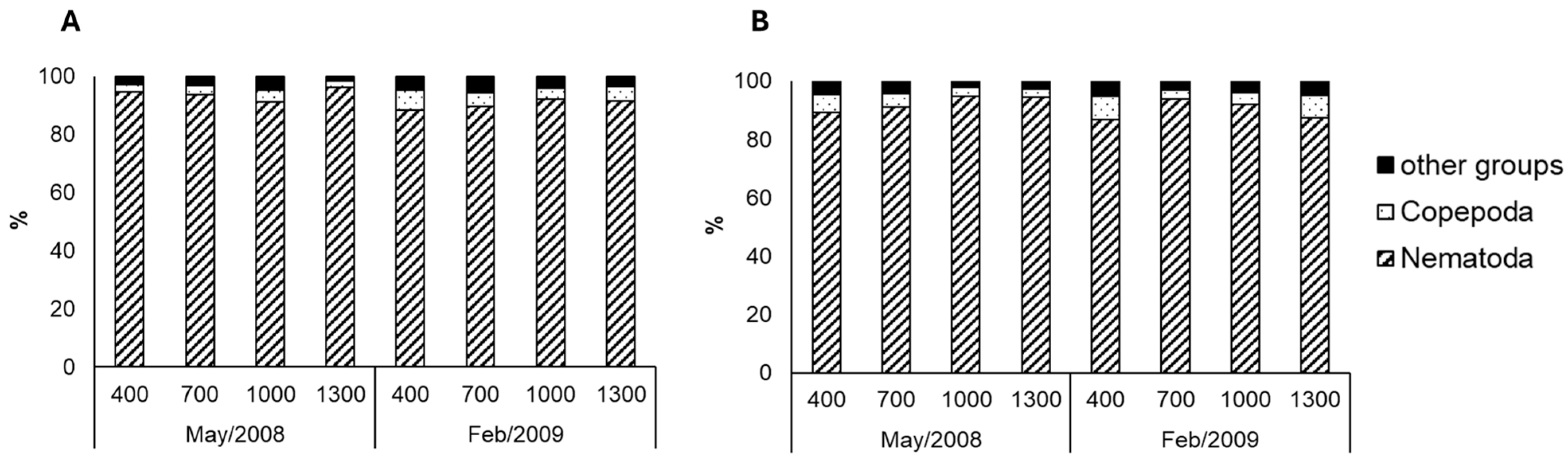
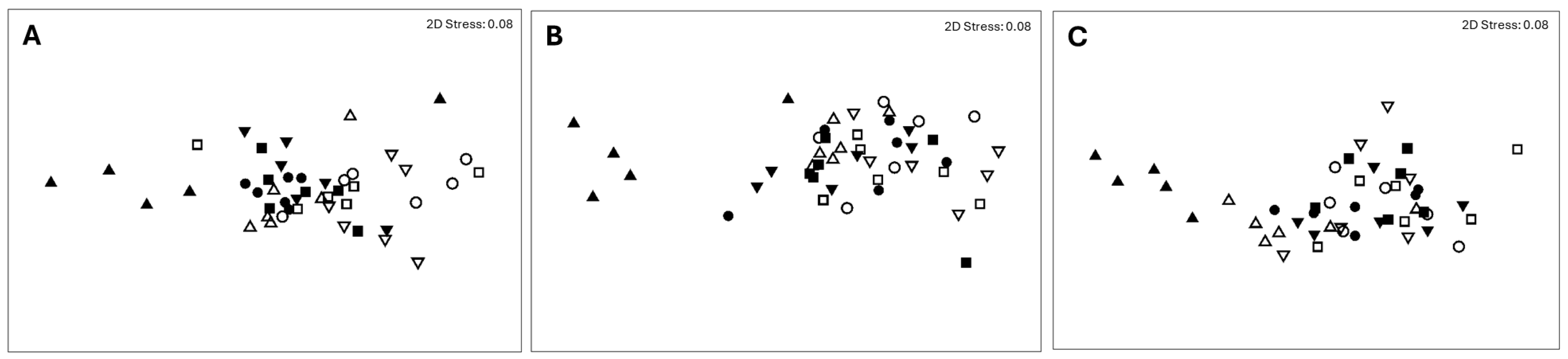
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| RICHNESS | 400 m | 700 m | 1000 m | 1300 m |
| 0–2 cm | 0.347 | 0.090 | 0.039 | 0.076 |
| 2–5 cm | 0.532 | 0.736 | 0.648 | 0.245 |
| 5–10 cm | 0.149 | 0.681 | 0.733 | 0.891 |
| RARE TAXA | 400 | 700 | 1000 | 1300 |
| 0–2 cm | 0.066 | 0.209 | 0.085 | 0.336 |
| 2–5 cm | 0.007 | 0.717 | 0.002 | 0.070 |
| 5–10 cm | 0.002 | 0.517 | 0.821 | 0.337 |
| MEIO ASSEMBLAGE | 400 | 700 | 1000 | 1300 |
| 0–2 cm | 0.139 | 0.320 | 0.002 | 0.009 |
| 2–5 cm | 0.020 | 0.642 | 0.118 | 0.026 |
| 5–10 cm | 0.038 | 0.604 | 0.406 | 0.843 |
References
- Trincardi, F.; Foglini, F.; Verdicchio, G.; Asioli, A.; Correggiari, A.; Minisini, D.; Piva, A.; Remia, A.; Ridente, D.; Taviani, M. The impact of cascading currents on the Bari Canyon System, SW-Adriatic Margin (Central Mediterranean). Mar. Geol. 2007, 246, 208–230. [Google Scholar] [CrossRef]
- Skliris, N.; Djenidi, S. Plankton dynamics controlled by hydrodynamic processes near a submarine canyon off NW corsican coast: A numerical modelling study. Cont. Shelf Res. 2006, 26, 1336–1358. [Google Scholar] [CrossRef]
- Machado, L.; Kowsmann, R.; Almeida, W., Jr.; Murakami, C.; Schreiner, S.; Miller, D.; Piauilino, P. Geometry of the proximal part of the modern turbidite depositional system of the Carapebus Formation, Campos Basin: A model for reservoir heterogeneities. Bolm. Geoc. Petrob. 2004, 12, 287–315. Available online: https://bgp.petrobras.com.br/bgp/article/view/182 (accessed on 5 February 2025).
- Bianchelli, S.; Gambi, C.; Zeppilli, D.; Danovaro, R. Metazoan meiofauna in deep-sea canyons and adjacent open slopes: A large-scale comparison with focus on the rare taxa. Deep Sea Res. Part I 2010, 5, 420–433. [Google Scholar] [CrossRef]
- Sousa, S.H.M.; Yamashita, C.; Vicente, T.M.; Mendes, R.N.M.; Licari, L.; Martins, M.V.A.; Kim, B.S.M.; Millo, C.; Carreira, R.; Montoya-Montes, I.; et al. Living benthic foraminifera from Almirante Câmara and Grussai canyons and adjacent slope areas (Campos Basin, Southwest Atlantic): Response to trophic and hydrodynamic conditions. Deep Sea Res. I 2024, 204, 104231. [Google Scholar] [CrossRef]
- Ciraolo, A.C.; Snelgrove, P.V.R. Contrasting benthic ecological functions in deep-sea canyon and non-canyon habitats: Macrofaunal diversity and nutrient cycling. Deep Sea Res. I 2023, 197, 104073. [Google Scholar] [CrossRef]
- Giere, O. Meiobenthology: The Microscopic Fauna in Aquatic Sediments, 2nd ed.; Springer: Berlin, Germany, 2009; 527p. [Google Scholar]
- Vanreusel, A.; Fonseca, G.; Danovaro, R.; da Silva, M.C.; Esteves, A.M.; Ferrero, T.J.; Gunnar, G.; Galtsova, V.; Gambi, C.; Genevois, V.F.; et al. The contribution of deep-sea macrohabitat heterogeneity to meiofaunal biodiversity in the deep Mediterranean Sea. Mar. Ecol. 2010, 31, 6–20. [Google Scholar] [CrossRef]
- Schratzberger, M.; Ingels, J. Meiofauna matters: The roles of meiofauna in benthic ecosystems. J. Exp. Mar. Biol. Ecol. 2017, 502, 12–25. [Google Scholar] [CrossRef]
- Duineveld, G.; Lavaleye, M.; Berghuis, E.; de Wilde, P. Activity and composition of the benthic fauna in the Whittard Canyon and the adjacent continental slope (NE Atlantic). Oceanol. Acta 2001, 24, 69–83. [Google Scholar] [CrossRef]
- Viana, A.; Faugéres, J.; Kowsmann, R.; Lima, J.; Caddah, L.; Rizzo, J. Hydrology, morphology and sedimentology of the Campos continental margin, offshore Brazil. Sed. Geol. 1998, 115, 133–157. [Google Scholar] [CrossRef]
- Almada, G.V.M.B.; Bernardino, A.F. Conservation of deep-sea ecosystems within offshore oil fields on the Brazilian margin, SW Atlantic. Biol. Conserv. 2017, 206, 92–101. [Google Scholar] [CrossRef]
- Ribeiro-Ferreira, V.; Curbelo-Fernandez, M.; Filgueiras, V.; Mello, R.; Falcão, A.; Disaró, S.; Mello e Sousa, S.; Lavrado, H.; Veloso, V.; Esteves, A.; et al. Métodos empregados na avaliação do compartimento bentônico da Bacia de Campos. In Ambiente Bentônico: Caracterização Ambiental Regional da Bacia de Campos, Atlântico Sudoeste, 1st ed.; Falcão, A.P.C., Lavrado, H.P., Eds.; Elsevier: Rio de Janeiro, Brazil, 2017; Volume 3, pp. 15–39. [Google Scholar] [CrossRef]
- Somerfield, P.J.; Warwick, R.M.; Moens, T. Meiofauna techniques. In Methods for the Study of Marine Benthos, 3rd ed.; Eleftheriou, A., McIntyre, A., Eds.; Blackwell: Oxford, UK, 2005; pp. 229–272. [Google Scholar] [CrossRef]
- Anderson, M.; Robinson, J. Generalized discriminant analysis based on distances. Aust. N. Z. J. Stat. 2003, 45, 301–318. [Google Scholar] [CrossRef]
- Clark, K.; Warwick, R. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; Plymouth Marine Laboratory, Natural Environment Research Council of UK: Plymouth, UK, 2001; 144p. [Google Scholar]
- Anderson, M.; Gorley, R.; Clarke, K. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008; 274p. [Google Scholar]
- Danovaro, R.; Tselepides, A.; Otegui, A.; Della Croce, N. Dynamics of meiofaunal assemblages on the continental shelf and deep-sea sediments of the Cretan Sea (NE Mediterranean): Relationships with seasonal changes in food supply. Progr. Oceanogr. 2000, 46, 367–400. [Google Scholar] [CrossRef]
- Ramalho, S.F.; Adão, H.; Kiriakoulakis, K.; Wolff, G.A.; Vanreusel, A.; Ingels, I. Temporal and spatial variation in the Nazaré Canyon (Western Iberian margin): Inter-annual and canyon heterogeneity effects on meiofauna biomass and diversity. Deep Sea Res. Part I 2014, 83, 102–114. [Google Scholar] [CrossRef]
- Danovaro, R.; Della Croce, N.; Eleftheriou, A.; Fabiano, M.; Papadopoulou, N.; Smith, C.; Tselepides, A. Meiofauna of the deep Eastern Mediterranean Sea: Distribution and abundance in relation to bacterial biomass, organic matter composition and other environmental factors. Progr. Oceanogr. 1995, 36, 329–341. [Google Scholar] [CrossRef]
- Baguley, J.G.; Montagna, P.A.; Hyde, L.J.; Kalke, R.D.; Rowe, G.T. Metazoan meiofauna abundance in relation to environmental variables in the northern Gulf of Mexico deep sea. Deep Sea Res. Part I 2006, 53, 1344–1362. [Google Scholar] [CrossRef]
- Netto, S.; Gallucci, F.; Fonseca, G. Deep-sea meiofauna response to synthetic-based drilling mud discharge off SE Brazil. Deep Sea Res. Part II 2009, 56, 41–49. [Google Scholar] [CrossRef]
- Van Gaever, S.; Raes, M.; Pasotti, F.; Vanreusel, A. Spatial scale and habitat-dependent diversity patterns in nematode communities in three seepage related sites along the Norwegian Sea margin. Mar. Ecol. 2010, 31, 66–77. [Google Scholar] [CrossRef]
- Soetaert, K.; Heip, C.; Vincx, M. The meiobenthos along a Mediterranean deep-transect off Calvi (Corsica) and in an adjacent canyon. Mar. Ecol. 1991, 12, 227–242. [Google Scholar] [CrossRef]
- Garcia, R.; Koho, K.A.; De Stigter, H.C.; Epping, E.; Koning, E.; Thomsen, L. Distribution of meiobenthos in the Nazaré canyon and adjacent slope (western Iberian Margin) in relation to sedimentary composition. Mar. Ecol. Prog. Ser. 2007, 340, 207–220. [Google Scholar] [CrossRef]
- Lavrado, H.; Omena, E.; Bernardino, A. Macrofauna bentônica do talude continental e cânions da Bacia de Campos. In Ambiente Bentônico: Caracterização Ambiental Regional da Bacia de Campos, Atlântico Sudoeste; Lavrado, H.P., Brasil, A.C.S., Eds.; Elsevier: Rio de Janeiro, Brazil, 2010. [Google Scholar] [CrossRef]
- Soetaert, K.; Muthumbi, A.; Heip, C. Size and shape of ocean margin nematodes: Morphological diversity and depth-related patterns. Mar. Ecol. Prog. Ser. 2002, 242, 179–1793. [Google Scholar] [CrossRef]
- Danovaro, R.; Dinet, A.; Duineveld, G.; Tselepides, A. Benthic response to particulate fluxes in different trophic environments: A comparison between the Gulf of Lions–Catalan Sea (western-Mediterranean) and the Cretan Sea (eastern-Mediterranean). Progr. Oceanogr. 1999, 44, 287–312. [Google Scholar] [CrossRef]
- Pusceddu, A.; Gambi, C.; Zeppilli, D.; Bianchelli, S.; Danovaro, R. Organic matter composition, metazoan meiofauna, and nematode biodiversity in sediments of the deep Mediterranean Sea. Deep Sea Res. II 2009, 56, 755–762. [Google Scholar] [CrossRef]
- Ingels, J.; Kiriakoulakis, K.; Wolff, G.A.; Vanreusel, A. Nematode diversity and its relation to the quantity and quality of sedimentary organic matter in the deep Nazare’ Canyon, Western Iberian Margin. Deep Sea Res. I 2009, 56, 1521–1539. [Google Scholar] [CrossRef]
- Miramontes, E.; Garreau, P.; Caillaud, M.; Jouet, G.; Pellen, R.; Hernández-Molina, F.J.; Clare, M.A.; Cattaneo, A. Contourite distribution and bottom currents in the NW Mediterranean Sea: Coupling seafloor geomorphology and hydrodynamic modelling. Geomorphology 2019, 333, 43–60. [Google Scholar] [CrossRef]
- Schmiedl, G.; deBove’e, F.; Buscail, R.; Charriere, B.; Hemleben, C.; Medernach, L.; Picon, P. Trophic control of benthic foraminiferal abundance and microhabitat in the bathyal Gulf of Lions, western Mediterranean Sea. Mar. Micropaleontol. 2000, 40, 167–188. [Google Scholar] [CrossRef]
- Polymenakou, P.N.; Lampadariou, N.; Tselepides, A. Exo-enzymatic activities and organic matter properties in deep-sea canyon and slope systems off the southern Cretan margin. Deep Sea Res. I 2008, 55, 1318–1329. [Google Scholar] [CrossRef]
- Nardelli, M.P.; Jorissen, F.J.; Pusceddu, A.; Morigi, C.; Dell’Anno, A.; Danovaro, R.; De Stigter, H.C.; Negri, A. Living benthic foraminiferal assemblages along a latitudinal transect at 1000m depth off the Portuguese margin. Micropaleontology 2010, 56, 323–344. Available online: https://www.researchgate.net/publication/233414762_Living_benthic_foraminiferal_assemblages_along_a_latitudinal_transect_at_1000m_depth_off_the_Portuguese_margin (accessed on 3 February 2025). [CrossRef]
- Vicente, T.M.; Yamashita, C.; Sousa, S.H.M.; Ciotti, A.M. Evaluation of the relationship between biomass of living (stained) benthic foraminifera and particulate organic matter vertical flux in an oligotrophic region, Campos Basin, southeastern Brazilian continental margin. J. Sea Res. 2021, 176, 102110. [Google Scholar] [CrossRef]

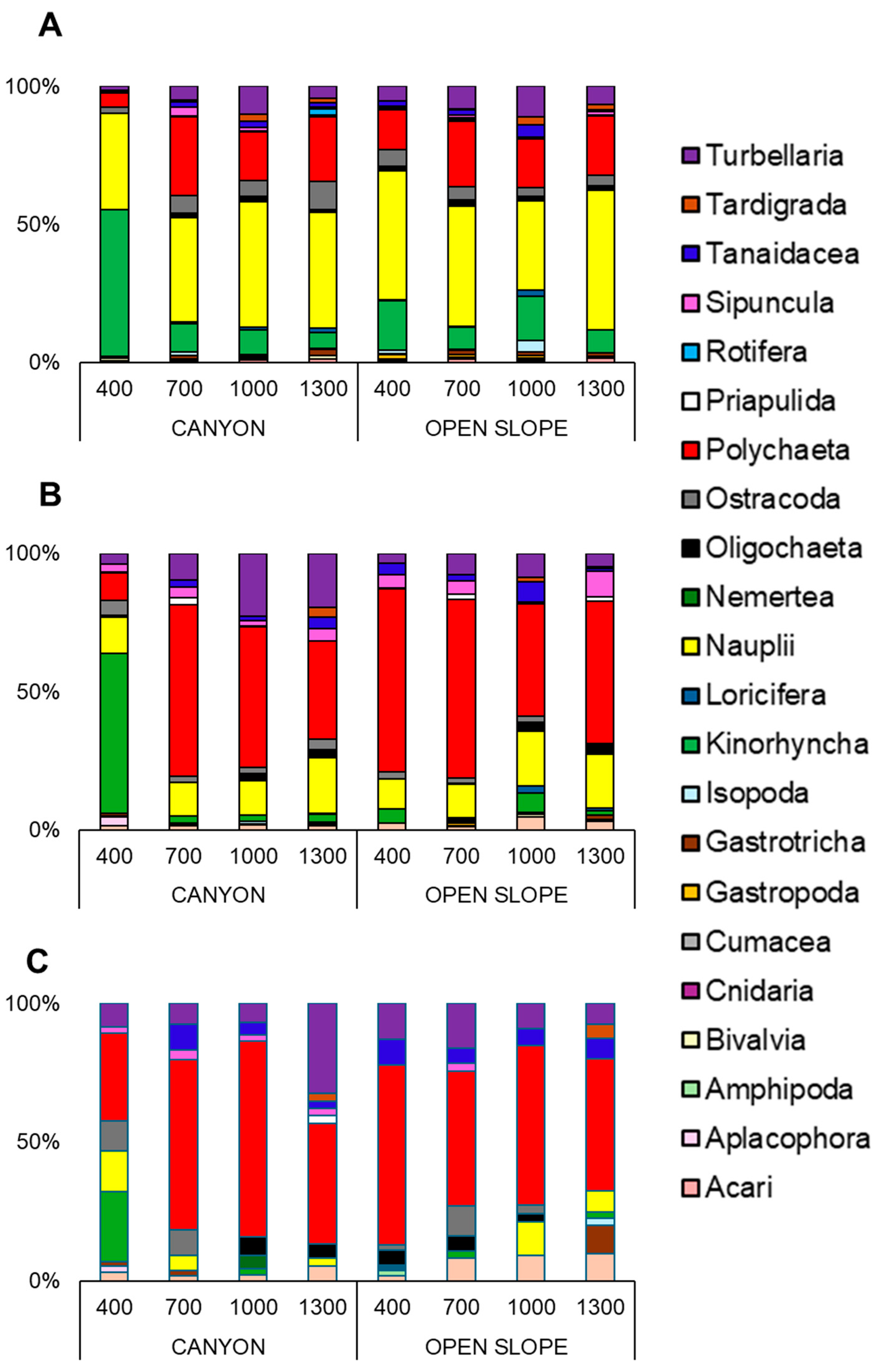

| Factors/Variables | Richness | Density | Assemblage | Rare Taxa | |
|---|---|---|---|---|---|
| HA (1, 143) | MS | 23.3 | <0.001 | 4090.2 | 4401.8 |
| pseudo-F | 3.82 | 23.46 | 8.05 | 3.09 | |
| p (perm) | 0.0543 | 0.0001 | 0.0001 | 0.0011 | |
| ISO (3, 143) | MS | 1.9 | <0.001 | 2969.3 | 4087.4 |
| pseudo-F | 0.31 | 16.22 | 5.84 | 2.87 | |
| p (perm) | 0.8168 | 0.0001 | 0.0001 | 0.0001 | |
| LAY (2, 143) | MS | 558.2 | <0.001 | 40735.0 | 43243.0 |
| pseudo-F | 91.32 | 36.39 | 80.18 | 30.33 | |
| p (perm) | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |
| PD (1, 143) | MS | 3.4 | <0.001 | 794.3 | 2217.0 |
| pseudo-F | 0.55 | 4.76 | 1.56 | 1.56 | |
| p (perm) | 0.460 | 0.0277 | 0.1617 | 0.1248 | |
| HA × ISO (3, 143) | MS | 3.0 | <0.001 | 1151.4 | 3525.2 |
| pseudo-F | 0.49 | 10.70 | 2.27 | 2.40 | |
| p (perm) | 0.6894 | 0.0001 | 0.0077 | 0.0002 | |
| HA × PD (1, 143) | MS | 28.4 | <0.001 | 1287.2 | 2296.6 |
| pseudo-F | 4.64 | 10.77 | 2.53 | 1.61 | |
| p (perm) | 0.0326 | 0.0005 | 0.0382 | 0.1108 | |
| HA × LAY (2, 143) | MS | 2.0 | <0.001 | 891.2 | 2349.4 |
| pseudo-F | 0.32 | 6.60 | 1.75 | 1.65 | |
| p (perm) | 0.7337 | 0.0007 | 0.0747 | 0.0458 | |
| ISO × PD (3, 143) | MS | 7.2 | <0.001 | 816.3 | 1502.5 |
| pseudo-F | 1.18 | 4.94 | 1.61 | 1.05 | |
| p (perm) | 0.3144 | 0.0014 | 0.0851 | 0.3905 | |
| ISO × LAY (6, 143) | MS | 6.1 | <0.001 | 1636.3 | 2742.8 |
| pseudo-F | 1.00 | 3.38 | 3.22 | 1.92 | |
| p (perm) | 0.4301 | 0.0019 | 0.0001 | 0.0002 | |
| PD × LAY (2, 143) | MS | 5.7 | <0.001 | 416.2 | 2021.4 |
| pseudo-F | 0.93 | 2.74 | 0.8192 | 1.42 | |
| p (perm) | 0.3969 | 0.0584 | 0.5964 | 0.1166 | |
| HA × ISO × PD (3, 143) | MS | 13.1 | <0.001 | 602.5 | 1300.5 |
| pseudo-F | 2.14 | 2.61 | 1.18 | 0.91 | |
| p (perm) | 0.0973 | 0.0500 | 0.2782 | 0.5908 | |
| HA × ISO × LAY (6, 143) | MS | 14.9 | <0.001 | 979.3 | 2584.8 |
| pseudo-F | 2.43 | 2.67 | 1.93 | 1.81 | |
| p (perm) | 0.0285 | 0.0104 | 0.0051 | 0.0005 | |
| HA × PD × LAY (2, 143) | MS | 6.0 | <0.001 | 547.9 | 1870.1 |
| pseudo-F | 0.99 | 7.23 | 1.08 | 1.31 | |
| p (perm) | 0.3666 | 0.0004 | 0.3613 | 0.1763 | |
| IS × PD × LAY (6, 143) | MS | 3.8 | <0.001 | 196.0 | 892.0 |
| pseudo-F | 0.61 | 3.00 | 0.39 | 0.62 | |
| p (perm) | 0.7281 | 0.0027 | 0.9993 | 0.9833 | |
| HA × IS × PD × LAY (6, 143) | MS | 4.2 | <0.001 | 302.1 | 1533.1 |
| pseudo-F | 0.68 | 1.97 | 0.59 | 1.07 | |
| p (perm) | 0.6678 | 0.0622 | 0.9537 | 0.3323 |
| Mediterranean Sea [24] | Nazaré Canyon [25] Iberian Margin North Atlantic | Almirante Câmara Canyon Southeast Atlantic | ||||||
|---|---|---|---|---|---|---|---|---|
| Box Corer | Multiple Corer | Box Corer | ||||||
| Depths (m) | Mean ±SE | Depth (m) | Mean ±SE | Depth (m) | Mean ±SE | |||
| Canyon | Adjacent | Canyon | Adjacent | Canyon | Adjacent | |||
| 165 | 1399 ** | 743 ** | 300 | 60 ±25 | 150 ±30 | 400 | 2843 ±603 | 580 ±105 |
| 370 | 1856 ** | 629 ** | 1100 | 250 100 | 110 ±20 | 700 | 527 ±149 | 308 ±60 |
| 990 | * | 611 ** | 2850 | 10 ±2 | * | 1000 | 662 ±269 | 224 ±40 |
| 1220 | * | 383 ** | 4800 | 20 ±5 | 30 ±5 | 1300 | 782 ±183 | 229 ±46 |
| Layer | 2008 | 2009 |
|---|---|---|
| 0–2 cm | 0.0006 | 0.7135 |
| 2–5 cm | 0.0150 | 0.1024 |
| 5–10 cm | 0.0044 | 0.3157 |
| Area | Environmental Variables | Total Meiofauna | Copepoda | Nematoda |
|---|---|---|---|---|
| Canyon | Mean grain size (Phi) | −0.01 | −0.29 | 0.01 |
| Carbonates (%) | 0.60 * | 0.71 * | 0.57 * | |
| TOC (%) | 0.22 | 0.33 | 0.21 | |
| Chlorophyll a (μg) | 0.69 * | 0.69 * | 0.67 * | |
| Pheophytin a (μg) | 0.62 * | 0.62 * | 0.60 | |
| Adjacent open slope | Mean grain size (Phi) | −0.38 | −0.89 * | −0.30 |
| Carbonates (%) | 0.40 | 0.87 * | 0.32 | |
| TOC (%) | 0.00 | −0.37 | 0.03 | |
| Chlorophyll a (μg) | 0.28 | 0.47 | 0.24 | |
| Pheophytin a (μg) | 0.39 | 0.47 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteves, A.M.; Oliveira, V.S.; Santos, P.J.P.d.; Maria, T.F.; Wandeness, A.P. Meiofauna from Almirante Câmara Canyon and Its Adjacent Open Slope, Southwest Atlantic Ocean. Coasts 2025, 5, 14. https://doi.org/10.3390/coasts5020014
Esteves AM, Oliveira VS, Santos PJPd, Maria TF, Wandeness AP. Meiofauna from Almirante Câmara Canyon and Its Adjacent Open Slope, Southwest Atlantic Ocean. Coasts. 2025; 5(2):14. https://doi.org/10.3390/coasts5020014
Chicago/Turabian StyleEsteves, André M., Verônica S. Oliveira, Paulo J. P. dos Santos, Tatiana F. Maria, and Adriane P. Wandeness. 2025. "Meiofauna from Almirante Câmara Canyon and Its Adjacent Open Slope, Southwest Atlantic Ocean" Coasts 5, no. 2: 14. https://doi.org/10.3390/coasts5020014
APA StyleEsteves, A. M., Oliveira, V. S., Santos, P. J. P. d., Maria, T. F., & Wandeness, A. P. (2025). Meiofauna from Almirante Câmara Canyon and Its Adjacent Open Slope, Southwest Atlantic Ocean. Coasts, 5(2), 14. https://doi.org/10.3390/coasts5020014






