Abstract
Ecosystem engineers are organisms that cause changes in the physical state of biotic and abiotic structures that modulate the availability of resources to other species, thus affecting biochemical cycles. Molluscs, especially bivalves such as mussels, are widespread in coastal environments and they are excellent ecosystem engineers because of the durability of their shells, which add complexity and heterogeneity to benthic environments. The presence of mussel farms favours the accumulation of shells in benthic environments and may influence surrounding bare sediments, with potential legacy effects on benthic communities. We studied the effects of the accumulation of mussel shells at finfish farms and mussel farms by experimentally comparing bare sediment and sediment with fragmented shells in terms of the abundance of the most relevant faunal groups, specifically polychaete families as well as physical–chemical variables in sediment water samples, specifically organic matter (OM), redox potential, and acid-volatile sulphides (AVS) NH4+ and PO43−. The experiment was replicated under two environmental conditions over a period of 35 days: eutrophic muddy sediments and oligotrophic sandy sediments. The OM and AVS values were significantly higher in the eutrophic sediment with mussel shells. Only NH4+ was positively affected by the mussel shells in the oligotrophic conditions. Differences between the two environments were observed, and the effect of the mussel shells on the polychaete assemblages was more significant in the oligotrophic conditions. Mussel shell accumulations affected the structure of benthic assemblages by modifying their heterogeneity and complexity, which suggests that the presence of mussel farms above bare sediment may affect ecosystem functioning. Aquaculture has potentially negative or positive effects that must be addressed on a large scale, considering the increased input of organic matter and also the simultaneous presence of mussel shell waste, both of which alter the surrounding environment. This is particularly important in oligotrophic sandy sediment.
1. Introduction
Among marine molluscs, mussels are known to act as ecosystem engineers in coastal systems, and their ecological effects have been well studied [1,2,3]. Mussel beds occur naturally on intertidal sand bars and rocky platforms, usually in inshore waters and estuaries and in open coastal areas. Mussel beds can have indirect effects on the surrounding ecosystem because the massive accumulation of dead whole (empty) or crushed shells (i.e., shell hash) on bare sediment can potentially impact the structure and functioning of soft substrates [4].
Finfish aquaculture, which is widespread across the world [5], generates artificial habitats that can potentially be colonized by mussels (amongst other species) transported to the sites by sea currents [6,7]. Thus, mussel shells often accumulate around these artificial habitats, generally in locations far away from their natural habitats on the coast [8]. Mussel aquaculture, which can cause fouling [9], is also a source of mussel shell waste [10,11]. Therefore, throughout the lifespan of fish farms and mussel rafts, which is usually tens of years, mussel shells accumulate below the facilities and have potentially important environmental effects, especially in regions with high concentrations of aquaculture installations.
Around aquaculture installations, the accumulation of shell hash may have important effects on benthic habitats, in addition to the effects of increased amounts of organic matter in the surrounding area [12,13]. Thus, the physical structure provided by the mussels and associated organisms that become detached from suspended bivalves together provide a habitat for species generally associated with hard-bottom communities, thus, often increasing local benthic diversity and related parameters [11]. Furthermore, the accreted shell hash acts as a boundary between the sediment and the water column, affecting the surface microlayer sediment and modifying biological processes such as recruitment, food supply and predation [14,15]. The physical presence of mussel shells and the associated microbiota can also alter hydrological flows, increasing the biochemical heterogeneity [16]. The shell hash can also have indirect effects, mitigating the effects of organic matter loads [17] and increasing bioturbation [18]. In addition to the physical effect of the mussel shells that occurs over time, the effects that biodeposits from live mussels have on the benthic community, especially scavengers, should also be considered.
The effects of organic matter enrichment in sediments due to aquaculture have been widely studied; however, other inputs such as shell waste from mussels growing on floating structures have been scarcely studied. These ecological effects may be modulated by the natural characteristics of seabed sediments, such as the particle size [19] and the natural organic matter load [20]. Therefore, the objectives of the present study were as follows: (a) to evaluate the changes in physicochemical variables due to the presence of mussel shell hash; (b) to test the biogenic effects by comparing the community structure of macrofauna in bare sediment and sediment with fragmented shells after a short period of colonization; (c) to correlate the changes in physicochemical variables with the changes in macrofaunal assemblages, especially of polychaetes; and (d) to compare the effects in two environmental conditions, i.e., eutrophic muddy sediments and oligotrophic sandy sediments.
2. Materials and Methods
2.1. Study Area
The study was conducted simultaneously on the Atlantic and Mediterranean coasts (Figure 1). On the Atlantic coast, the rías that occur along the coast of Galicia (NW Spain) are among the most productive areas in the world [21]. The high primary productivity is largely supported by inputs of nutrients from the upwelling of the sub-superficial nutrient-rich North Atlantic Central Waters linked to the Canary Current System [22]. The high productivity of the rías enables successful production of Mytilus galloprovincialis Lamarck, 1819 on vertical ropes suspended from floating wooden rafts or platforms (of an area of about 100 m2) [11]. Galicia is one of the top mussel producing regions worldwide, and, in 2019, mussel production on 3386 rafts amounted to 255,514 tons [23]. In the Mediterranean region, a high concentration of fish-fattening cages, mainly used for sea bass, sea bream and meagre, can be found in some areas in SE Spain [24]; the total production, from 22 fish farms, reached 35,475 tons in 2019. The marine environment in this region is generally oligotrophic due to the oceanographic conditions of the Mediterranean Sea [25]. In both areas, mussel shells are accumulating on the bare sediment due to different processes, and the associated ecological effects are poorly known.
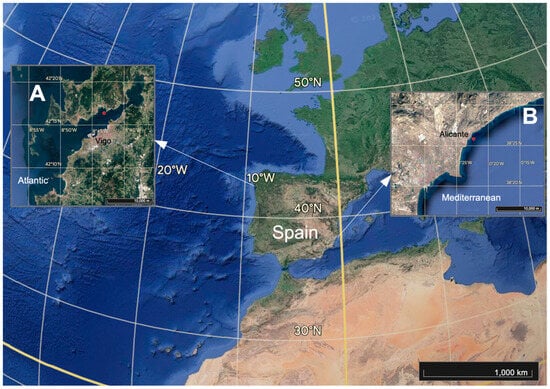
Figure 1.
Spatial localization of experiments in the Atlantic location (Ría de Vigo) (A) around mussel farms and the Mediterranean location (Alicante Bay) (B) around a fish farm.
2.2. Experimental Set-Up
The experimental units consisted of plastic containers of volume 0.036 m3 (24 × 15 × 6 cm; Figure S1), fitted with airtight lids. Each container was filled with sediment from which all faunas had been removed by sieving through a 0.5 mm mesh to remove macrofauna. The sediment used in the Mediterranean location was sandy sediment (79.56% sand, 16.13% clay and 4.3% silt) with a low organic matter (OM) content (0.83%), measured by a loss on the ignition method. The sediment was collected from a beach (Gola, Santa Pola, SE Spain: 38°11,018′81″ N; 0°35,034′55″ W) close to a fish farming region. The sediment used in the Atlantic location was silty sediment (11.94% sand, 11.21% clay and 76.83% silt) with a high OM content (3.64%). The sediment was collected in the Ría de Vigo, NW Spain (42°16.544′ N; 008°43.494′ W), with a Van-Veen grab with a maximum penetration depth of 10 cm. In both cases, the grain size and the OM content of the sediment were very similar to those in the sediment underneath the aquaculture facilities. To test the effect of mussel shells on sediment, 2 Kg of mussel shell hash (without flesh) per m2 of sediment were added to half of the experimental units. Empty shells, which were broken to produce fragments sized between 1.5 and 4 cm, were mixed in the first cm of the sediment to simulate gravity-fed deposition from the surface. This amount corresponds to a realistic input to the sediment under aquaculture facilities [26,27].
In the summer of 2016, the experimental units were placed in both locations, on the Mediterranean and Atlantic coastlines, at a distance of around 200 m from the aquaculture facilities. At this distance, the environmental conditions are very similar to those around the aquaculture installations, but distant enough to prevent any direct impact from the facilities [27,28]. In the Mediterranean location, the aquaculture facility was a fish farm (El Campello, Alicante, SE Spain: 38°25′34.7″ N 0°22′29.3″ W) producing gilthead sea bream (Sparus aurata Linnaeus, 1758), which were fed daily with pellets. The farm, which comprises 12 rounded cages and produces ~600 t per year of fish, is located offshore (2.8 km from the coast at a depth of 34 m) where oligotrophic conditions prevail. In the Atlantic location, the aquaculture facility was a mussel (M. galloprovincialis) farm (Ría de Vigo, NO Spain 42°16′35.04″ N 008°43′23.477″ W), located one km from the coast, at 12 m depth.
Twenty-four experimental units were placed in each location: twelve with mussel shell hash on the sediment surface and twelve consisting of bare sediment. Three sites were randomly selected for each treatment in each location. Four experimental units were placed in each of the sites by scuba divers. The experimental units were buried in the natural sediment to prevent an edge effect, which could prevent colonization by macrofauna. The experimental system has been already tested as a tool for monitoring the impact of aquaculture on benthic habitats [29].
After 35 days, each experimental unit was covered with a lid and transported to the boat by scuba divers. One month of colonisation was enough time to identify changes among treatments, following the indications of Martinez et al., 2019 [29]. A portion of the surface sediment in each experimental unit (160 cm3) was removed with a syringe for determination of the OM content, the redox potential, accumulation of acid volatile sulphides (AVS) and the NH4+ and PO43− porewater concentrations. These physicochemical parameters are commonly used for monitoring aquaculture activities [30] and to help us understand the relationship between fauna and sediment [17,19]. The OM content (%) was measured by a loss in the ignition method, at 450 °C for four hours. The redox potential (mV) was measured with an electrochemical sensor (Hamilton Liq-Glass ORP) inserted in the upper 3 cm of the sediment. AVS accumulation (mg Kg−1) was quantified in 5 mL of wet sediment by the distillation method proposed by Allen et al. (1993) [31]. Porewater was extracted from 20 mL of wet sediment with a vacuum pump. The concentrations of NH4+ and the PO43− (µM) were determined in the porewater. The porewater was filtered (Syringe Filter-13 mm Ø 0.22 µm) and transferred to 15 mL plastic vials, which were transported while being refrigerated at 5 °C, in darkness (for no more than 4 h) and frozen (−20 °C) until analysis. The NH4+ and the PO43− concentrations were determined in an Automated Wet Chemistry Analyzer–Continuous Flow Analyzer (Skalar Analytical B.V., Breda, The Netherlands). The remaining sediment (around 2000 cm3) in each experimental unit was sieved through a 500 µm mesh, to ensure retention of the macrofauna, which was preserved in a 70% ethanol seawater solution. In the laboratory, the individual specimens were separated into Crustacea, Mollusca and Polychaeta, and the samples stored in a 70% alcohol solution for later identification. Polychaetes were identified to family level. The macrofaunal abundance was reported as individuals m−2.
2.3. Data Analysis
The physicochemical parameters of the sediment (OM content, redox, AVS accumulation, NH4+ and PO43− porewater concentration) were compared for the different locations (Atlantic and Mediterranean) and treatments (with and without mussel shell hash). The abundance of the main taxa (crustaceans, molluscs, polychaetes) and total macrofauna abundance were compared in the same way. For this purpose, we used a three-way analysis of the variance (ANOVA) including the factors Location (fixed and with two treatments), Mussel shells (fixed, orthogonal, and with two treatments) and Site (random and nested in the interaction between Location and Mussel shells), n = 4 (Figure S2). The abundance of the main families of polychaetes was analysed independently for each location, due to the different composition in terms of families. In this case, the abundance of the Capitellidae, Paraonidae, Spionidae, Cirratulidae, Nereididae, Syllidae, Dorvelleidae and Lumbrineridae families sampled in the Atlantic location and the Syllidae, Capitellidae, Chaetopteridae, Poecilochaetidae, Paraonidae, Nereididae, Lumbrineridae and Dorvilleidae families sampled in the Mediterranean location were analysed by a two-way ANOVA including the factors Mussel shells (fixed, orthogonal, and with two treatments) and Site (random and nested in Mussel shells), n = 4.
Before ANOVA, the normality of variance was checked using Cochran’s test, and the data were logarithmically transformed when necessary [32]. The redox potential values, which were all negative, were log(X × (−1)) transformed. If the Cochran’s C test with transformed data was significant, a lower level of significance was applied in the ANOVA. When differences of any factors or their interaction were significant, the post hoc Student-Newman–Keuls (SNK) test for multiple comparisons was applied. Analyses were conducted using the GAD package [33] in R (4.0.0 version; http://www.R-project.org; accessed on 20 February 2018). Differences between the polychaete assemblages, between treatments and between sites for each location, were determined using a permutational multivariate analysis of variance (PERMANOVA) [34]. PERMANOVA was calculated on the basis of the Bray–Curtis similarity matrix, with square root data transformation. The contribution of each family to the average sample dissimilarity was determined with the SIMPER routine. The SIMPER functions perform pairwise comparisons of groups of sampling units and find the average contributions of each species to the average overall Bray–Curtis dissimilarity. Samples with no individuals were excluded from the analysis. All statistical tests were conducted by applying a significance level of α = 0.05. To extract and summarize the variation in polychaete assemblages which could be explained by the physicochemical variables, non-metric redundancy analysis (RDA) was applied using a Bray–Curtis matrix. Collinearity of explanatory variables was explored by pairwise correlations (Spearmen coefficient; Table S1). Since the level of collinearity was not high, and when OM was excluded from the analysis no changes on the sign of estimated parameters occurred, we used the five variables. Families present in a very low abundance, less than 10% of samples, were excluded from the RDA. ANOVA permutation test for RDA was applied to assess the significance of the explanatory model and axes. BIOENV calculated which set of environmental variables was best correlated with the structure of polychaete assemblages. For each possible combination of environmental factors, a dissimilarity matrix based on normalized Euclidean distances was calculated. The agreement between the biotic matrix and matrices of environmental factors was expressed as the Spearman rank correlation coefficient. All the multivariate analyses were conducted using the VEGAN 2.5-6 [35] package in R. Data and were plotted using the GGPLOT2 package in R [36].
3. Results
3.1. Physicochemical Variables
Overall, the presence of mussel shells generated an increase in OM, which was greater in the experiment conducted in the Atlantic location. The values of the other variables, especially AVS and NH4+, were also higher in the treatment with shells (Table S2). As we expected, the OM content was higher in the sediment in the experimental units from the Atlantic location (4.14 ± 0.56%) than in those from the Mediterranean location (0.93 ± 0.16%) (Figure 2). The ANOVA revealed statistically significant differences in the Location x Mussel interaction (Table 1). The SNK tests indicated statistically significant differences between mussel shell treatments in the Atlantic location, with the OM content increasing by 1% in the treatment with mussel shells. The same pattern was observed in the Mediterranean location, i.e., a higher OM content when mussel shells were present, although the difference was not statistically significant (Figure 2). The opposite trend was observed for the redox potential, with higher values in the Mediterranean location. Thus, in the experimental unit in the Atlantic location the mean value was −405 ± 18 mV, and in the Mediterranean location the mean value was −40 ± 39.3 (Table 1). Despite the high variability at the site level, the ANOVA revealed a marginal significant interaction between the factors Location and Mussel shells (Table 1; p = 0.056). SNK tests of this interaction showed significant differences in the Mussel shells factor in the Mediterranean location, with higher values in the treatment without mussel shells (−20.2 ± 23.2 mV versus −61.4 ± 443.1 mV; Figure 2). AVS concentration was much higher in the Atlantic (286 ± 155 mg Kg−1) than in the Mediterranean location (17.6 ± 15.9 mg Kg−1; Figure 2). This variable also differed significantly between the mussel shell treatments, but depending on the location, i.e., significant interaction of Location x Mussel shells (Table 1). In the Atlantic location, there were statistically significant differences between mussel shell treatments, increasing from 188 ± 81.4 mg Kg−1 to 384 ± 151 mg Kg−1 due to the presence of mussel shells (Table S2). The NH4+ concentrations were fairly similar in the Atlantic and Mediterranean locations (89 ± 2.3 and 105 ± 41.9 µM, respectively; Table S2; Figure 2), but in the Mediterranean location the concentration was significantly higher in the treatment with mussel shells (129 ± 15.2 µM) than in the bare sediment (81.9 ± 47.2 µM) (Figure 2), as revealed by the statistically significant differences in the Location x Mussel shells interaction (Table 1). The concentration of PO43− was significantly higher in the Atlantic location: 37.8 ± 1.72 µM, compared to 10.9 ± 3.16 µM in the Mediterranean location (Table S2; Figure 2). Marginally significant differences were detected between the mussel shell treatments (Table 1), with higher concentration in the treatment with shells.
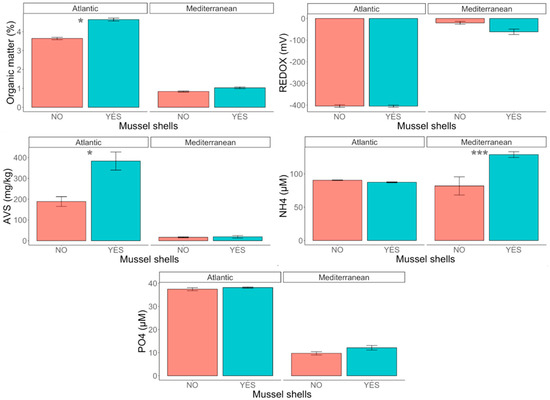
Figure 2.
Percentage of organic matter (OM %), redox potential (mV), AVS (mg Kg−1), NH4+ (µM) and PO43− concentration (µM) in the two locations (Atlantic and Mediterranean), and for treatments with and without mussel shells. * p < 0.05, *** p < 0.0001.

Table 1.
Analysis of variance for physicochemical variables (OM, Redox Potential, AVS, NH4+ and PO43−) L = location, M = mussel shells, S = site). MS = mean square, p = p value of F test. Cochran = Cochran’s C test value and significance level (p).
3.2. Macrofaunal Assemblages
Molluscs were present in relatively low mean abundances in both the Atlantic (106 ± 92.2 ind m−2) and Mediterranean locations (75.2 ± 216 ind m−2; Table 2), followed by crustaceans (Atlantic: 496 ± 514; Mediterranean: 325 ± 282 ind m−2). Polychaetes were the most abundant group, with 19,106 ± 1440 ind m−2 in the Atlantic location and 11,306 ± 1290 ind m−2 in the Mediterranean location (Figure 3). Regarding the comparison between experimental treatments, the density of molluscs and crustaceans did not differ in relation to the presence of mussel shells (Figure 3; Table 3), but there were marginally significant differences in the numbers of molluscs between locations, and the number of crustaceans varied widely between sites. Polychaetes were significantly more abundant in the Atlantic than in the Mediterranean location (Figure 3; Table 3), and the small difference between mussel shells treatments was statistically significant (shells: 1170 ± 886 ind m−2; no shells: 1100 ± 1620 ind m−2). As the total macrofauna abundance was dominated by polychaetes, the pattern was similar, with a significantly greater abundance in the Atlantic location; however, the differences due to the mussel shells were significant only in the Mediterranean location (SNK test, p < 0.01) (Figure 3; Table 3).

Table 2.
Mean, standard deviation, minimum and maximum values of high taxa of fauna in the two locations by mussel shells treatment.
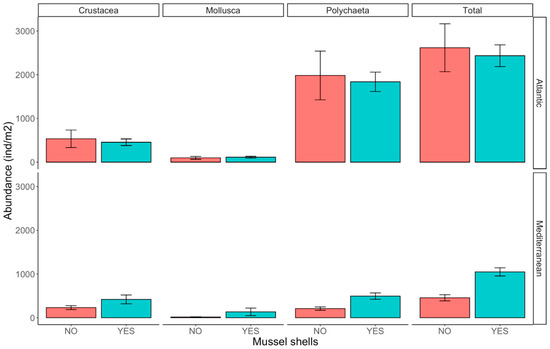
Figure 3.
Abundance (ind m−2) of fauna in higher taxonomic groups in the two locations, and for treatments with and without mussel shells.

Table 3.
Analysis of variance for higher taxonomic groups Crustacea, Mollusca, Polychaeta and total abundance (data were transformed log10 (ind m−2 + 1). L = location, M = mussel shells, S = sites. Cochran = Cochran’s C test value and significance level (p).
The polychaete assemblage in the Atlantic location was composed of 23 families (Table S3), with abundances of between 1550 ± 1410 ind m−2 for Capitellidae and 1.16 ± 5.67 ind m−2 for Nephtyidae. The most abundant families were Capitellidae, Paraonidae, Spionidae, Cirratulidae, Nereididae, Syllidae, Dorvilleidae and Lumbrineridae, but there were no significant differences between the treatments (Figure 4). Several families were present at very low abundances (Figure 4), with some individuals present in treatments with mussel shells (Aphroditidae, Eunicidae, Onuphidae, Polynoidae and Scalibragmatidae) and without mussel shells (Cossuridae, Maldanidae, Nephtyidae, Orbiniidae, Poecilochaetidae and Sigalionidae). In the Mediterranean location, 26 polychaete families were identified (Table S4), with abundance ranging between 1.16 ± 5.57 ind m−2 for Ampharetidae and 88 ± 77.1 ind m−2 for Syllidae. The most abundant families were Syllidae, Capitellidae, Chaetopteridae, Poecilochaetidae, Paraonidae, Nereididae, Lumbrineridae and Dorvilleidae (Figure 5). The least abundant families, only present in the treatment with mussel shells, were Ampharetidae, Magelonidae, Maldanidae, Onuphidae, Opheliidae, Oweniidae and Sigalionidae (Figure 5). By contrast, members of the Pectinariidae and Sabellidae families were only present in the treatment without shells. Capitellidae was the only family for which statistically significant differences were observed (Factor Mussel shells, p = 0.0148; Cochran’s C test p = 0.4343), with Capitellidae being more abundant in the treatment with mussel shells.
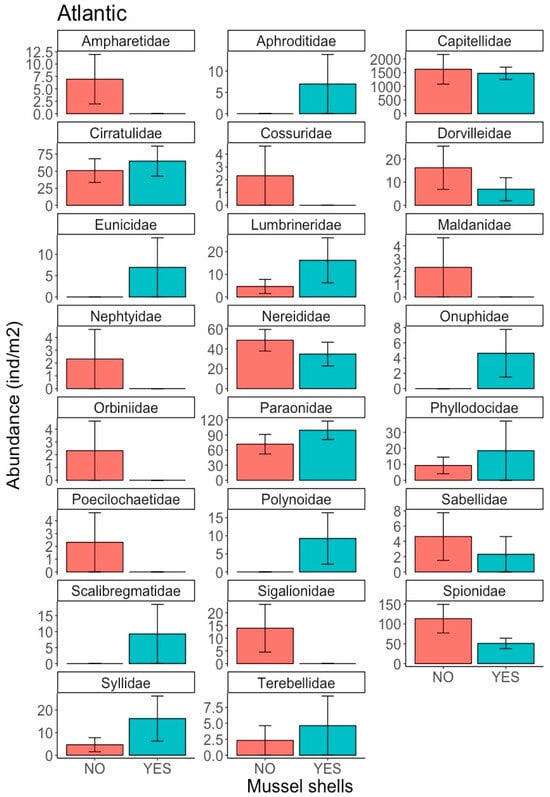
Figure 4.
Abundance (ind m−2) of polychaete families in the Atlantic region for treatments with and without mussel shells.
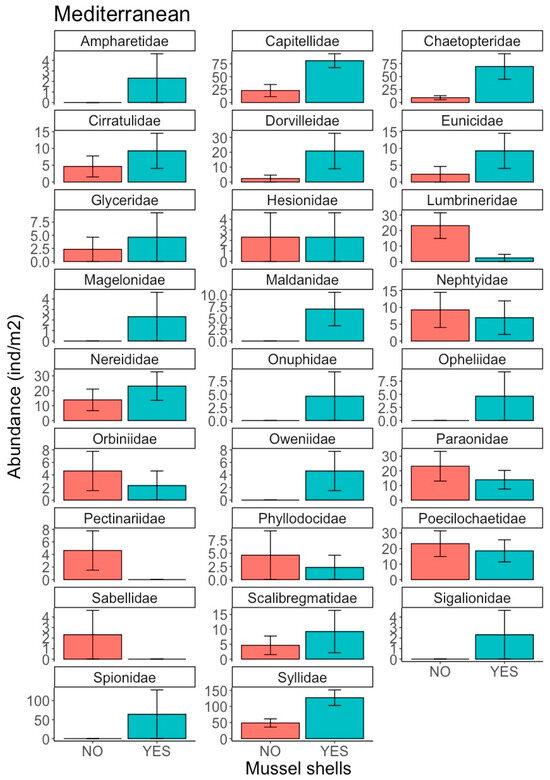
Figure 5.
Abundance (ind m−2) of polychaete families in the Mediterranean location for treatments with and without mussel shells.
3.3. Relationship with Environmental Variables
Polychaete assemblages in the Atlantic location were quite similar in both treatments, and there were no statistically significant differences (PERMANOVA; p = 0.440). The one-way SIMPER test showed that the families Cirratulidae, Paraonidae, Nereididae, Spionidae, Dorvilleidae, Phyllodocidae, Lumbrineridae and Syllidae were the major contributors to the dissimilarity between treatments (Table 4), but the differences were not statistically significant. In the Mediterranean location, PERMANOVA revealed significant differences in the structure of polychaete assemblage between treatments for the Mussel shell factor (PERMANOVA, p = 0.03). In this case, SIMPER indicated that the families Capitellidae, Chaetopteridae, Syllidae, Poecilochaetidae Nereididae, Lumbrineridae, Paraonidae and Dorvilleidae were the most important in relation to dissimilarities between treatments (Table 5). Some families showed a significant influence in the dissimilarity between treatments, such as Capitellidae, Chaetopteridae, Syllidae and Dorvilleidae.

Table 4.
SIMPER analysis of polychaete assemblages in the Atlantic region for both treatments: with shells and no shells. p = permutation p-value; cumsum = ordered cumulative contribution; av = average abundances per group; YES = with shells; NO = without shells; ratio = average to sd ratio; sd = standard deviation of contribution; average = average contribution to overall dissimilarity.

Table 5.
SIMPER analysis of polychaete assemblages in the Mediterranean region for both treatments: with shells and no shells. p = permutation p-value; cumsum = ordered cumulative contribution; av = average abundances per group; YES = with shells; NO = without shells; ratio = average to sd ratio; sd = standard deviation of contribution; average = average contribution to overall dissimilarity.
The results of the RDA indicated that the relationships between the changes in physicochemical variables due to mussel shells and the polychaete assemblages were not strong in either the Atlantic or Mediterranean locations (Figure 6). In the Atlantic location, the first two axes explained 42.1 and 24.3% of the total variance, but there were no significant correlations (RD1 p = 0.287; RD2 p = 0.894). The full model of the physicochemical variables was not significant (ANOVA; p = 0.419). BIOENV analysis indicated that AVS and PO43− were the physicochemical variables that best explained the polychaete assemblages, with a correlation of only 0.123. In the Mediterranean location, the first two axes explained 52.6 and 20.46% of the total accumulated variance. In this case, the RDA1 was significantly correlated (ANOVA, p = 0.009) with NH4+, but RDA2 was not significantly correlated with this variable (ANOVA, p = 0.894). However, the full model of the physicochemical variables was not significant (ANOVA; p = 0.373). After BIOENV, as expected, the patterns in the structure of the polychaete assemblages were best explained by NH4+, with a weak correlation of 0.125.
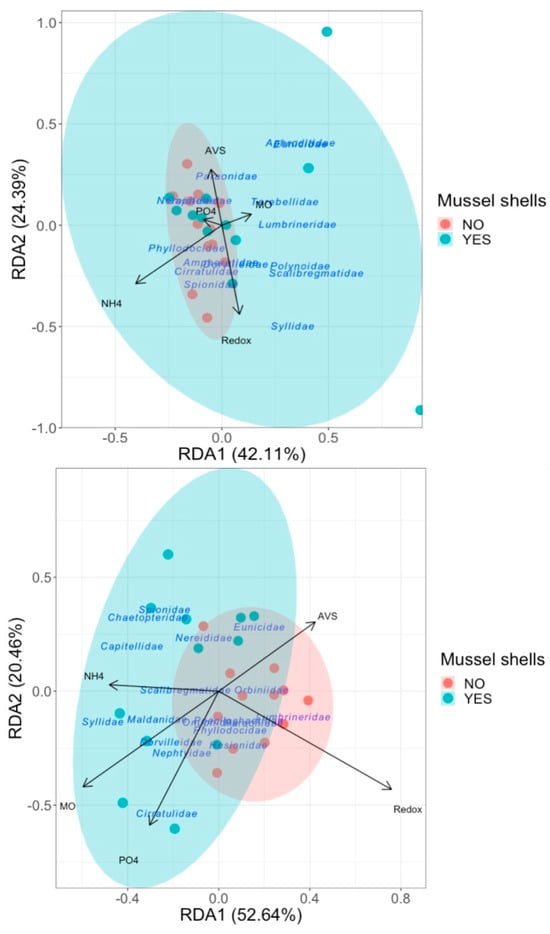
Figure 6.
RDA plot of polychaete assemblages in relation to the physicochemical variables in the Atlantic (upper graph) and Mediterranean (lower graph) locations for both treatments with shells and without shells. The most relevant families of polychaetes in the distribution of the samples are indicated.
4. Discussion
Marine farming activities potentially affect the benthic marine habitat via sedimentation of biodeposits, although accumulation of mussel shells may also be an important factor. These effects are modulated by the fact that local conditions, in terms of eutrophication levels, sediment characteristics, etc., are more important in oligotrophic habitats, where the presence of mussel shells produces significant changes in polychaete assemblages. Nevertheless, the accumulation of mussel shells has not been taken into account in previous studies about the effects of aquaculture in the Mediterranean Sea [37]. However, in the present study, despite the significant changes in organic matter in eutrophic muddy sediment due to mussel shells with an influence on AVS accumulation, we did not observe any effect on polychaete assemblages. This study was carried out with fragmented mussel shells (1.5–4 cm length); the size and structure of the mussel shell are important in relation to understanding the ecological effects [3]. Therefore, the conclusions of the present study are restricted to the effects of mussel shell hash on the benthic assemblages because massive accumulation of mussel shells on eutrophic environments under mussel production systems has important effects on benthic fauna [26]. The differences between regions can be explained in terms of the natural concentration of OM in different types of sediments, as indicated by the measurement of redox potential [19].
The effects on the sediment may be caused by the modifications produced by the shells in the suspended-sediment flux. Suspended-sediment flux is affected by bed micromorphology; benthic biological processes act in complex interactions with physical processes to control bed micromorphology [38], and the presence of mussel shells on the sediment will affect the bottom boundary-layer fluid and particulate transport across the sediment. In the present study, the increased roughness due to the presence of mussel shells may have reduced particle transport because of the change from laminar to turbulent flow at the microscale of the sediment surface. This change may favour the retention of particulate OM in the shell interstices, which become buried in the sediment. In the future, it would be interesting to study the effect of changes in roughness on sedimentation and resuspension in the presence and absence of mussel shells.
The increase in AVS related to the increase in OM suggested that mineralization of the organic matter mainly occurs via sulphate reduction. This indicates that the presence of mussel shells can promote anoxic conditions by reducing the oxygen interchange across the sediment surface, thus increasing the importance of anaerobic metabolic pathways. However, this process would only occur in the presence of relatively high levels of organic matter in the sediment, and not in oligotrophic sediments, as in the Mediterranean location. Previous laboratory experiments investigating the effect of mussel shells on sediment with organic matter contents of less than 1% showed a significant effect on AVS [17]. The physical effects of mussel shells without flesh may involve two mechanisms: first, mussel shells favour the settlement of low-density OM particles, and second, OM input can often be disproportionately higher than the potential of the sediment for decomposition, increasing AVS. However, mesocosm experiments indicated that mussel shell debris, linked to bioturbation, lowers the AVS accumulation under organic pollution conditions [17]. However, this effect is probably related to the OM input and to the carrying capacity of the sediment. In general, studies on the impact of mollusc aquaculture on OM inputs and the effects on AVS concentration do not consider the influence of piles of shells on the seabed (e.g., [39]). Therefore, the presence of mussel shells must be also considered in relation to sulphide formation among other factors such as OM reactivity and quantity or sediment grain size [19], and future studies concerning the effects of aquaculture activities on biochemical cycles in sediment must also consider this aspect [40].
In addition to OM, some changes in nutrient accumulation in the sediment were detected. Benthic ammonium and phosphate fluxes are typically higher within mussel culture sites than in control zones [11]. Similar patterns occur in relation to fish farms [13]. Increased ammonium and phosphate releases measured at the benthic interface in culture zones probably partly occur due to the degradation of mussel or fish biodeposits rich in nitrogen and phosphorus [19]. However, the influence of mussel shells on biogeochemical fluxes around fish farms is not well understood. The accumulation of nutrients, such as NH4+ and PO43−, on sediments is controlled by processes occurring within the sediment column and at the sediment–water interface. Fragmented shells may be important in controlling nutrient fluxes because they can often be observed in dense, tightly packed patches that may act as a barrier between the sediment interstitial water and the water column [3]. Although we did not study nutrient fluxes, we found that the presence of mussel shells contributed to increasing the concentration of NH4+ in the sandy sediment. Nitrification involves the biological oxidation of ammonia to nitrite followed by the oxidation of the nitrite to nitrate [11], and the biofilm growing on the shell surface may favour this process [41]. In the Atlantic location, with muddy sediment, the predominance of anaerobic conditions may increase the potential for denitrification (i.e., microbial reduction of nitrate to gaseous nitrogen, either as molecular nitrogen or as a nitrogen oxide) because this process is carried out by anaerobic microorganisms [16]. Therefore, nitrification and denitrification can be modulated by the presence of mollusc shells and the oxygen content of the interstitial water in the sediment, which can affect the biogeochemical cycling of N [42]. Increased sedimentation rates observed around mussel farms on the west coast of Sweden were found to be accompanied by increased NH4+ efflux from sediment under mussel lines [43]. The relatively large effect on NH4+ release and porewater concentration suggests stimulated benthic metabolism, in which ammonium is released as the end product of OM decomposition. By contrast, the mussel shells did not appear to affect the PO43− concentration, and the values were highest in the Atlantic sediment. This could be explained by the intense upwelling, high pelagic primary production and subsequent sedimentation. Upwelling of nutrient-rich water pulses is accompanied by large decreases in oxygen and pH and increases in phosphate (>0.5 µmol Kg−1; [44]).
Regarding the effect of mussel shells on benthic fauna, it has been demonstrated that soft-bottom Mytilus edulis Linnaeus, 1798 beds can alter the abundance of macrofauna relative to adjacent bare sediment because of the accumulation of shells [45]. In areas affected by mussel aquaculture, the benthic community structure is strongly influenced by mussels growing on longlines, which affect habitat heterogeneity [46]. In addition to the physical effect of the mussel shell, the effect of the mussel flesh on benthic fauna may also be important. An increase in abundance of benthic fauna around mussel farms in Canada has been reported [47], probably reflecting the attraction of mobile fauna due to better food supply and possibly to the creation of a more heterogeneous habitat. Similarly, on the west coast of Scotland, the presence of mussel farms was found to cause an increase in the amount of shell hash present, which frequently dominated the sediment in close proximity to the mussel lines and influenced the macrobenthic assemblage structure [27]. In the Mediterranean location (oligotrophic conditions), we detected changes in the community assemblage, with an increase in total macrofauna abundance, experimentally demonstrating that the existence of mussel shells, independently of an additional organic matter input, affects the colonization of soft sediments, especially by polychaetes.
Polychaetes were very abundant in the Atlantic location, with up to 2000 ind m−2 present after only 35 days from the start of the experiment. The structure of the assemblage was dominated by families tolerant of organic matter, such as Capitellidae, Spionidae, Nereididae, Oweniidae and Dorvilleidae, and the mussel shells did not have any clear influence on this. The families Capitellidae, Spionidae and Dorvelleidae are known to be tolerant to organic loads [48,49]. The presence of Nereididae and Oweniidae may be an early indicator of organic enrichment [48,50,51]. In highly productive regions, such as the Galician rias, sedimentation of particulate OM may mask the effects of fragments of mussel shells on the sediment, stimulating the colonization of organic matter tolerant species. The findings of a study on the effect of mussel bed and mussel farming in Denmark and Spain (Vigo) indicated that the impacts of ecosystem engineers are strongest in extreme physical environments where they ameliorate physical stress [10]. The authors of the study argued that in less stressful conditions the effect of ecosystem engineers may be reduced to the provision of competitors or predator-free spaces. However, we found the opposite was true for the mussel shells.
Mussel shells act like ecosystem engineers by modifying benthic habitats and thus disturbing the natural environment, especially in oligotrophic sandy sediments, enhancing the abundance of some species due to ecological process, which are difficult to establish (e.g., increased settlement and availability of trophic resources availability and reduced predation rates). The families Capitellidae, Chaetopteridae and Syllidae were more abundant in experimental units with mussel shells in the Mediterranean location. The habitat was probably optimal for recruitment of polychaetes, or was attractive to adults in terms of trophic resources [29]. The populations of Capitellidae may have increased due to a concomitant enhancement in the organic matter content (see references above), caused by the presence of mussel shells and a reduction in mortality due to predation [52]. This family includes species that are generally motile and surface deposit feeders, tolerant to organic matter enrichment and rather non-selective feeders [53]. Similarly, Chaetopterids are tubicolous polychaetes common in shallow waters, and are considered sessile filter feeders that pump water through their mouths and trap particles with mucus. The gut contents of this species include planktonic skeleta and detritus, indicating a pelagic origin of the food [53]. Increasing OM due to the presence of mussel shells at the water–sediment interface may benefit this family, in addition to the increase in turbulence at the sediment surface microscale, which may favour the catchability of pelagic trophic resources. Syllids are most frequent in shallow water associated with hard substrata [54] and the mussel shells probably represent an optimal hard substratum for these organisms. In a study using the same type of experimental units as ours, greater abundance of syllids was observed on sandy sediments than on muddy sediments [19]. The study’s authors suggested that the presence of mussel shells also favoured colonization of sandy substrates by syllids relative to colonization of muddy sediments.
We suggest that the presence of aquaculture installations on oligotrophic, sandy bare sediment in coastal environments has a detectable effect on the ecosystem functioning due to the increased input of biodeposits and also to the simultaneous presence of mussel shells acting like ecosystem engineers. Ecosystem engineers can affect biogeochemical processing by altering the availability of resources for microbes or by altering abiotic conditions affecting microbial process rates, and thus create biogeochemical heterogeneity in sediments [16]. The biogenic changes observed can persist beyond the presence of the ecosystem engineer with the modifications known as legacy effects [55]. On bare oligotrophic sediments, accretion of mussel shell hash affects the complexity of benthic habitats by modifying some biological processes at the boundary layer of the sediment, e.g., larvae settlement [56], thus influencing macroinvertebrate assemblage structure. Mussel shells probably also change the availability of resources to macroinvertebrates as a direct consequence of the habitat structure created by mussel shells (e.g., increase in OM) or indirectly through the modulation of biotic forces, such as predation rates, due to changes in complexity. The potential mitigating effect of the presence of mussel shells on the sediment [17] is enhanced by the presence of polychaetes [40] in sediments impacted by biodeposits from aquaculture. An increase in the abundance of burrowing animals can affect nutrient cycling in different ways, altering decomposition, respiration and remineralization. At the seascape spatial level, the mussel shell accumulations build a heterogeneous mosaic of habitats that will affect biodiversity patterns at the regional scale. Appropriate decision-making practices require detailed knowledge of aquaculture–sediment system interactions. For example, there is an ongoing debate as to whether the best strategy is to remove the mussel shells that accumulate under aquaculture facilities or to leave them. Further studies are essential to further our understanding of how mussel shells alter ecosystems toward optimal ecosystem-based management in regions with clustering of aquaculture facilities and different natural environmental conditions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/coasts3040020/s1, Figure S1: Experimental units, with different type of sediment depending on the location (region) and two treatments, with mussel shells and without mussel shells, were placed on the sea bottom and collected after 35 days; Figure S2: Experimental design for comparison of physicochemical variables and faunal abundance. Polychaete families were analysed separately by location, due to the different assemblage structure; Table S1: Pairwise correlations between physicochemical variables (Spearman coefficient) for each location; Table S2: Mean, standard deviation, minimum and maximum values of physicochemical variables in the two locations for the mussel shells treatment, the treatment without mussel shells, = NO, and with mussel shells = YES. Table S3: Mean ± standard deviation (SD), median and minimum (Min) and maximum (Max) values of polychaete family abundances (ind m−2) in the Atlantic location: treatments without mussel shells = NO, and with mussel shells = YES; Table S4: Mean ± standard deviation (SD), median and minimum (Min) and maximum (Max) values of polychaete family abundances (ind m−2) in the Mediterranean location: treatments without mussel shells = NO, and with mussel shells = YES.
Author Contributions
Conceptualization, P.S.-J., C.S.-L. and N.C.-C.; methodology P.S.-J., C.S.-L. and C.V.-P.; formal analysis, P.S.-J. and C.O.; investigation, C.O., N.C.-C. and C.V.-P.; data curation, P.S.-J. and C.O.; writing—original draft preparation, P.S.-J., J.S.T., C.S.-L. and N.C.-C.; writing—review and editing, P.S.-J., C.S.-L., C.O., N.C.-C. and C.M.S.; supervision, P.S.-J. and J.S.T.; funding acquisition, P.S.-J. and C.S.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project CGL2015-70136-R from the Spanish National Agency for Research (MINECO/FEDER).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to Elena Martinez-Garcia for her assistance with the polychaete identification and with the collection of experimental units. We would like to express our gratitude to ECIMAT for the assistance in setting up and dismantling the experiment in Vigo and to CUDOMAR fish farm Company for its readiness to let us perform the experiment on its lease.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Svane, I.; Setyobudiandi, I. Diversily of associated fauna in beds of the blue mussel Mytilus edulis L.: Effects of location, patch size, and position within a patch. Ophelia 1996, 45, 39–53. [Google Scholar] [CrossRef]
- Buschbaum, C.; Dittmann, S.; Hong, J.H.; Hwang, I.S.; Strasser, M.; Thiel, M.; Valdiva, N.; Yoon, S.P.; Reise, K. Mytilid mussels: Global habitat engineers in coastal sediments. Helgol. Mar. Res. 2009, 63, 47–58. [Google Scholar] [CrossRef]
- Commito, J.A.; Como, S.; Grupe, B.M.; Dow, W.E. Species diversity in the soft-bottom intertidal zone: Biogenic structure, sediment, and macrofauna across mussel bed spatial scales. J. Exp. Mar. Biol. Ecol. 2008, 366, 70–81. [Google Scholar] [CrossRef]
- Commito, J.A.; Jones, B.R.; Jones, M.A.; Winders, S.E.; Como, S. What happens after mussels die? Biogenic legacy effects on community structure and ecosystem processes. J. Exp. Mar. Biol. Ecol. 2018, 506, 30–41. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture—2020. Available online: http://www.fao.org/publications/sofia/en/ (accessed on 20 February 2023).
- Dürr, S.; Watson, D.I. Biofouling and antifouling in aquaculture. In Biofouling; Dürr, S., Thomason, J.C., Eds.; Wiley-Blackwell: Chichester, UK, 2010; pp. 267–287. [Google Scholar]
- Fernandez-Gonzalez, V.; Sanchez-Jerez, P. Fouling assemblages associated with off-coast aquaculture facilities: An overall assessment of the Mediterranean Sea. Mediterr. Mar. Sci. 2017, 18, 87–96. [Google Scholar] [CrossRef]
- Sanchez-Jerez, P.; Krüger, L.; Casado-Coy, N.; Valle, C.; Sanz-Lazaro, C. Mollusk Shell Debris Accumulation in the Seabed Derived from Coastal Fish Farming. J. Mar. Sci. Eng. 2019, 7, 335. [Google Scholar] [CrossRef]
- Fitridge, I.; Dempster, T.; Guenther, J.; de Nys, R. The impact and control of biofouling in marine aquaculture: A review. Biofouling 2012, 28, 649–669. [Google Scholar] [CrossRef]
- Ysebaert, T.; Hart, M.; Herman, P.M.J. Impacts of bottom and suspended cultures of mussels Mytilus spp. on the surrounding sedimentary environment and macrobenthic biodiversity. Helgol. Mar. Res. 2009, 63, 59–74. [Google Scholar] [CrossRef]
- McKindsey, C.W.; Archambault, P.; Callier, M.D.; Olivier, B. Influence of suspended and off-bottom mussel culture on the sea bottom and benthic habitats: A review. Can. J. Zool. 2011, 89, 622–646. [Google Scholar] [CrossRef]
- Holmer, M.; Barry, W. Organic enrichment from marine finfish aquaculture and effects on sediment biogeochemical processes. In Handbook of Environmental Chemistry; Hargrave, B.T., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 181–206. [Google Scholar] [CrossRef]
- Sanz-Lazaro, C.; Marín, A. Assessment of Finfish Aquaculture Impact on the Benthic Communities in the Mediterranean Sea. Dyn. Biochem. Process Biotechnol. Mol. Biol. 2008, 2, 21–32. [Google Scholar]
- Gutierrez, J.L.; Jones, C.G.; Strayer, D.L.; Iribarne, O.O. Mollusks as ecosystem engineers: The role of shell production in aquatic habitats. Oikos 2003, 101, 79–90. [Google Scholar] [CrossRef]
- Gutierrez, J.L.; Jones, C.; Byers, J.; Arkema, K.; Berkenbusch, K.; Commito, J.; Duarte, C.; Hacker, S.; Lambrinos, J.; Hendriks, I.; et al. Physical Ecosystem Engineers and the Functioning of Estuaries and Coasts. In Treatise on Estuarine and Coastal Science; Wolanski, E., McLusky, D.S., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Waltham, MA, USA, 2011; Volume 7, pp. 53–81. [Google Scholar] [CrossRef]
- Gutierrez, J.L.; Jones, C.G. Physical ecosystem engineers as agents of biogeochemical heterogeneity. BioScience 2006, 56, 227–236. [Google Scholar] [CrossRef]
- Casado-Coy, N.; Martinez-Garcia, E.; Sanchez-Jerez, P.; Sanz-Lazaro, C. Mollusc-shell debris can mitigate the deleterious effects of organic pollution on marine sediments. J. Appl. Ecol. 2017, 54, 547–556. [Google Scholar] [CrossRef]
- Casado-Coy, N.; Sanchez-Jerez, P.; Troncoso, J.S.; Sanz-Lazaro, C. Mollusc-shell debris derived from aquaculture can promote macrofaunal communities with a high bioturbation capacity. Aquaculture 2022, 548, 737642. [Google Scholar] [CrossRef]
- Martinez-Garcia, E.; Carlsson, M.S.; Sanchez-Jerez, P.; Sánchez-Lizaso, J.L.; Sanz-Lazaro, C.; Holmer, M. Effect of sediment grain size and bioturbation on decomposition of organic matter from aquaculture. Biogeochemistry 2015, 125, 133–148. [Google Scholar] [CrossRef]
- Sweetman, A.K.; Norling, K.; Gunderstad, C.; Haugland, B.T.; Dale, T. Benthic ecosystem functioning beneath fish farms in different hydrodynamic environments. Limnol Ocean. 2014, 59, 1139–1151. [Google Scholar] [CrossRef]
- Tenore, K.R.; Corral, J.; Gonzalez, N. Effects of intense mussel culture on food chain patterns and production in coastal Galicia, NW Spain. In Proceedings of the International Symposium on Utilization of Coastal Ecosystems: Planning, Pollution and Productivity, Rio Grande, Brazil, 21–27 November 1982. [Google Scholar]
- Blanton, J.O.; Tenore, K.R.; Castillejo, F.; Atkinson, L.P.; Schwing, F.B.; Lavin, A. The relationship of upwelling to mussel production in the rias on the western coast of Spain. J. Mar. Res. 1987, 45, 497–511. [Google Scholar] [CrossRef]
- Instituto Geológico de Estadística. Available online: www.ige.eu (accessed on 20 February 2023).
- APROMAR. 2019. La Acuicultura en España. Available online: www.apromar.com (accessed on 20 February 2023).
- D’Ortenzio, F.; Marullo, S.; Ragni, M.; Ribera d’Alcalà, M.; Santoleri, R. Validation of empirical SeaWiFS algorithms for chlorophyll-a retrieval in the Mediterranean Sea: A case study for oligotrophic seas. Remote Sens. Environ. 1987, 82, 79–94. [Google Scholar] [CrossRef]
- Sanchez-Jerez, P.; Couret Huertas, M.; Pérez-Benavente, G.; Uglem, I.; Casado Coy, N.; Fernández González, V.; Toledo Guedes, K.; Sanz-Lázaro, C. Ecological effects of mussel shells from marine aquaculture on benthic macrofauna. Foro Rec. Mar. Ac. Rías Gal. 2019, 21, 355–362. [Google Scholar]
- Wilding, T.A.; Nickell, T.D. Changes in Benthos Associated with Mussel (Mytilus edulis L.) Farms on the West-Coast of Scotland. PLoS ONE 2013, 8, e68313. [Google Scholar] [CrossRef]
- Sanz-Lazaro, C.; Belando, M.D.; Marín-Guirao, L.; Navarrete-Mier, F.; Marín, A. Relationship between sedimentation rates and benthic impact on Maërl beds derived from fish farming in the Mediterranean. Mar. Environ. Res. 2011, 71, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, E.; Capaccioni-Azzati, R.; Sánchez-Lizaso, J.L.; Sanchez-Jerez, P. Application of a new protocol to evaluate the benthic impacts of aquaculture: Colonization of experimental units for monitoring by polychaeta. Ecol. Indic. 2019, 101, 50–61. [Google Scholar] [CrossRef]
- Martinez-Garcia, E.; Fernandez-Gonzalez, V.; Aguado-Giménez, F.; Sánchez-Lizaso, J.L.; Sanchez-Jerez, P. From paper to practice: An initial approach to implementation of the environmental monitoring plan for fish farming proposed by JACUMAR. Sci. Mar. 2018, 82, 27–34. [Google Scholar] [CrossRef]
- Allen, H.E.; Fu, G.; Deng, B. Analysis of acid-volatile sulfide (AVS) and simultaneously extracted metals (SEM) for the estimation of potential toxicity in aquatic sediments. Environ. Toxicol. Chem. 1993, 12, 1441–1453. [Google Scholar] [CrossRef]
- Underwood, A.J. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance; Cambridge University Press: Cambridge, UK, 1997; p. 522. [Google Scholar]
- Sandrini-Neto, L.; Camargo, M.G. GAD: An R Package for ANOVA Designs from General Principles. 2018. Available online: https://cran.r-project.org/package=gad (accessed on 10 February 2018).
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.B.; Simpson, G.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version. 2.5-6. 2019. Available online: https://cran.r-project.org/web/packages/vegan (accessed on 9 March 2017).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 9783319242774. Available online: https://ggplot2.tidyverse.org (accessed on 20 June 2017).
- Tičina, V.; Katavić, I.; Grubišić, L. Marine Aquaculture Impacts on Marine Biota in Oligotrophic Environments of the Mediterranean Sea–A Review. Front. Mar. Sci. 2020, 7, 217. [Google Scholar] [CrossRef]
- Wright, L.; Friedrichs, C.; Hepworth, D. Effects of benthic biology on bottom boundary layer processes, Dry Tortugas Bank, Florida Keys. Geo. Mar. Lett. 1997, 17, 291–298. [Google Scholar] [CrossRef]
- Otero, X.L.; Calvo de Anta, R.M.; Macías, F. Sulphur partitioning in sediments and biodeposits below mussel rafts in the Ria de Arousa (Galicia, NW Spain). Mar. Environ. Res. 2006, 61, 305–325. [Google Scholar] [CrossRef]
- Bergström, P.; Durland, Y.; Lindegarth, M. Deposition of shells modify nutrient fluxes in marine sediments: Implications for effects of nutrient enrichment and mitigation by bioturbation below mussel farms. Aquac. Environ. Interact. 2020, 12, 315–325. [Google Scholar] [CrossRef]
- Svenningsen, N.B.; Heisterkamp, I.M.; Sigby-Clausen, M.; Larsen, L.H.; Nielsen, L.P.; Stief, P.; Schramm, A. Shell Biofilm Nitrification and Gut Denitrification Contribute to Emission of Nitrous Oxide by the Invasive Freshwater Mussel Dreissena polymorpha (Zebra Mussel). Appl. Environ. Microbiol. 2012, 78, 4505–4509. [Google Scholar] [CrossRef]
- Caffrey, J.M.; Hollibaugh, J.T.; Mortazavi, B. Living oysters and their shells as sites of nitrification and denitrification. Mar. Pollut. Bull. 2016, 112, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, M.S.; Engström, P.; Lindahl, O.; Ljungqvist, L.; Petersen, J.K.; Svanberg, L.; Holmer, M. Effects of mussel farms on the benthic nitrogen cycle on the Swedish west coast. Aquac. Environ. Interact. 2012, 12, 177–191. [Google Scholar] [CrossRef]
- Rosón, G.; Pérez, F.F.; Álvarez-Salgado, X.A.; Figueiras, F.G. Variation of Both Thermohaline and Chemical Properties in an Estuarine Upwelling Ecosystem: Ria de Arousa; I. Time Evolution. Estuar. Coast. Shelf Sci. 1995, 41, 195–213. [Google Scholar] [CrossRef]
- Commito, J.A.; Dankers, N. Dynamics of spatial and temporal complexity in European and North American soft- bottom mussel beds. In Ecological Comparisons of Sedimentary Shores; Reise, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 39–59. [Google Scholar]
- Grant, J.; Hatcher, A.; Scott, D.B.; Pocklington, P.; Schafer, C.T.; Winters, G.V. A multidisciplinary approach to evaluating impacts of shellfish aquaculture on benthic communities. Estuaries 1995, 18, 124–144. [Google Scholar] [CrossRef]
- D’Amours, O.; Archambault, P.; McKindsey, C.W.; Johnson, L.E. Local enhancement of epibenthic macrofauna by aquaculture activities. Mar. Ecol. Prog. Ser. 2008, 371, 73–84. [Google Scholar] [CrossRef]
- Lee, H.W.; Bailey-Brock, J.H.; McGurr, M.M. Temporal changes in the polychaetes infaunal community surrounding a Hawaiian mariculture operation. Mar. Ecol. Prog. Ser. 2008, 307, 175–185. [Google Scholar] [CrossRef]
- Dean, H.K. The use of polychaetes (Annelida) as indicator species of marine pollution: A review. J. Trop. Biol. Conserv. 2008, 56, 11–38. Available online: https://www.redalyc.org/pdf/449/44919934004.pdf (accessed on 19 September 2018).
- Pagliosa, P.R. Another diet of worms: The applicability of polychaetes feeding guilds as a useful conceptual framework and biological variable. Mar. Ecol. 2005, 26, 246–254. [Google Scholar] [CrossRef]
- Méndez, N.; Flos, J.; Romero, J. Littoral soft-bottom polychaetes communities in a pollution gradient in front of Barcelona (Western Mediterranean, Spain). Bull. Mar. Sci. 1998, 63, 167–178. [Google Scholar]
- Nelson, W.G.; Capone, M.A. Experimental studies of predation on polychaetes associated with seagrass beds. Estuaries 1990, 13, 51–58. [Google Scholar] [CrossRef]
- Fauchald, K.; Jumars, P.A. The diet of worms: A study of polychaete feeding guilds. Ocean. Mar. Biol. Annu. Rev. 1979, 17, 193–284. [Google Scholar]
- Kohn, A.J.; Lloyd, M.C. Polychaetes of Truncated Reef Limestone Substrates on Eastern Indian Ocean Coral Reefs: Diversity, Abundance, and Taxonomy. Int. Rev. Ges. Hydrobiol. Hydrogr. 1973, 58, 369–400. [Google Scholar] [CrossRef]
- Albertson, L.K.; Sklar, L.S.; Tumolo, B.B.; Cross, W.F.; Collins, S.F.; Woods, H.A. The ghosts of ecosystem engineers: Legacy effects of biogenic modifications. Funct. Ecol. 2022. [Google Scholar] [CrossRef]
- Commito, J.A.; Celano, E.A.; Celico, H.J.; Como, S.; Johnson, C.P. Mussels matter: Post-larval dispersal dynamics altered by a spatially complex ecosystem engineer. J. Exp. Mar. Biol. Ecol. 2005, 316, 133–147. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).