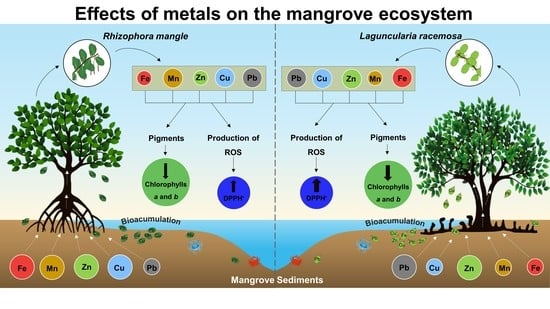
Impact of Metal Accumulation on Photosynthetic Pigments, Carbon Assimilation, and Oxidative Metabolism in Mangroves Affected by the Fundāo Dam Tailings Plume
Abstract
1. Introduction
2. Materials and Methods
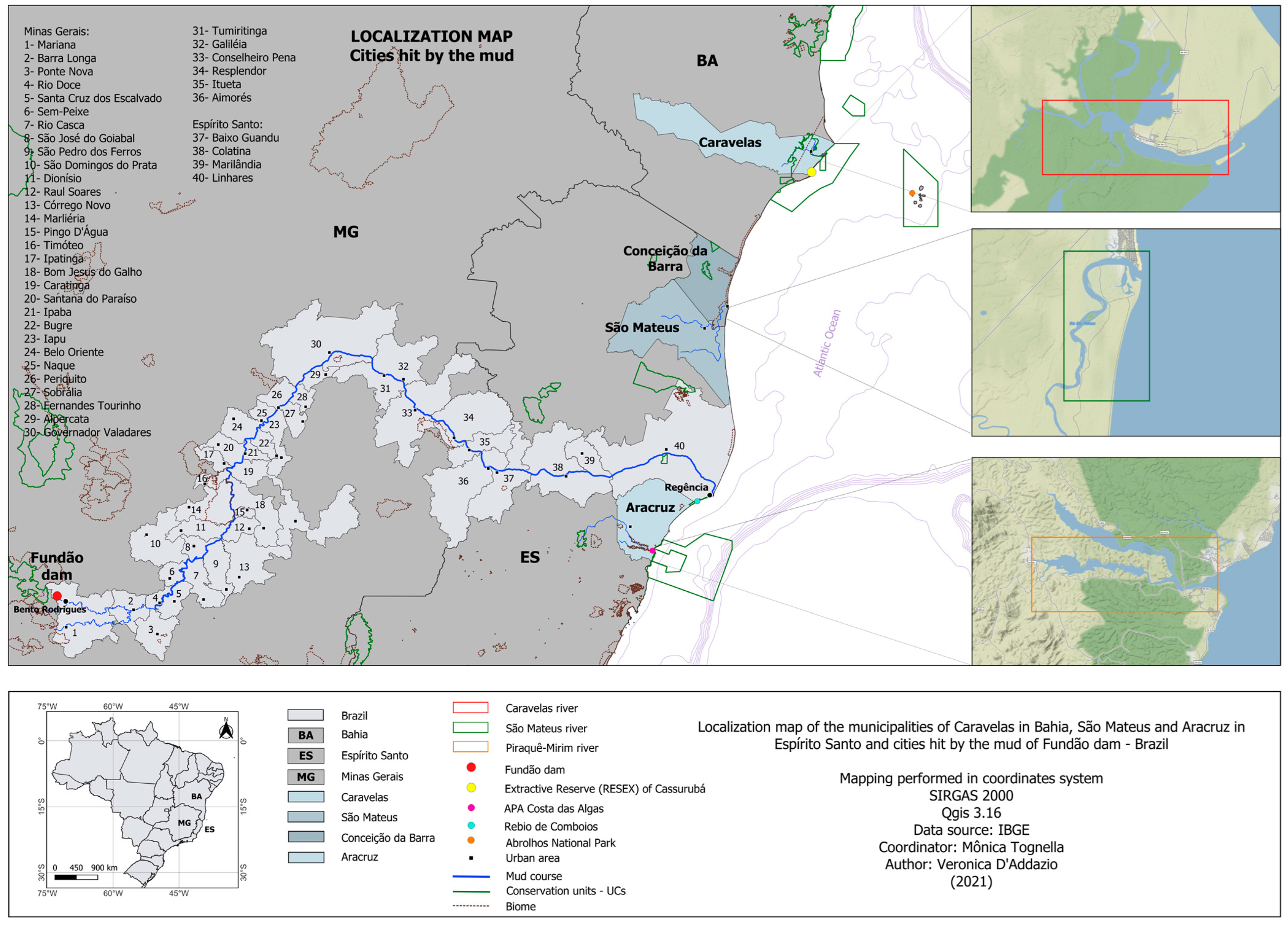
2.1. Study Area
2.2. Sediments
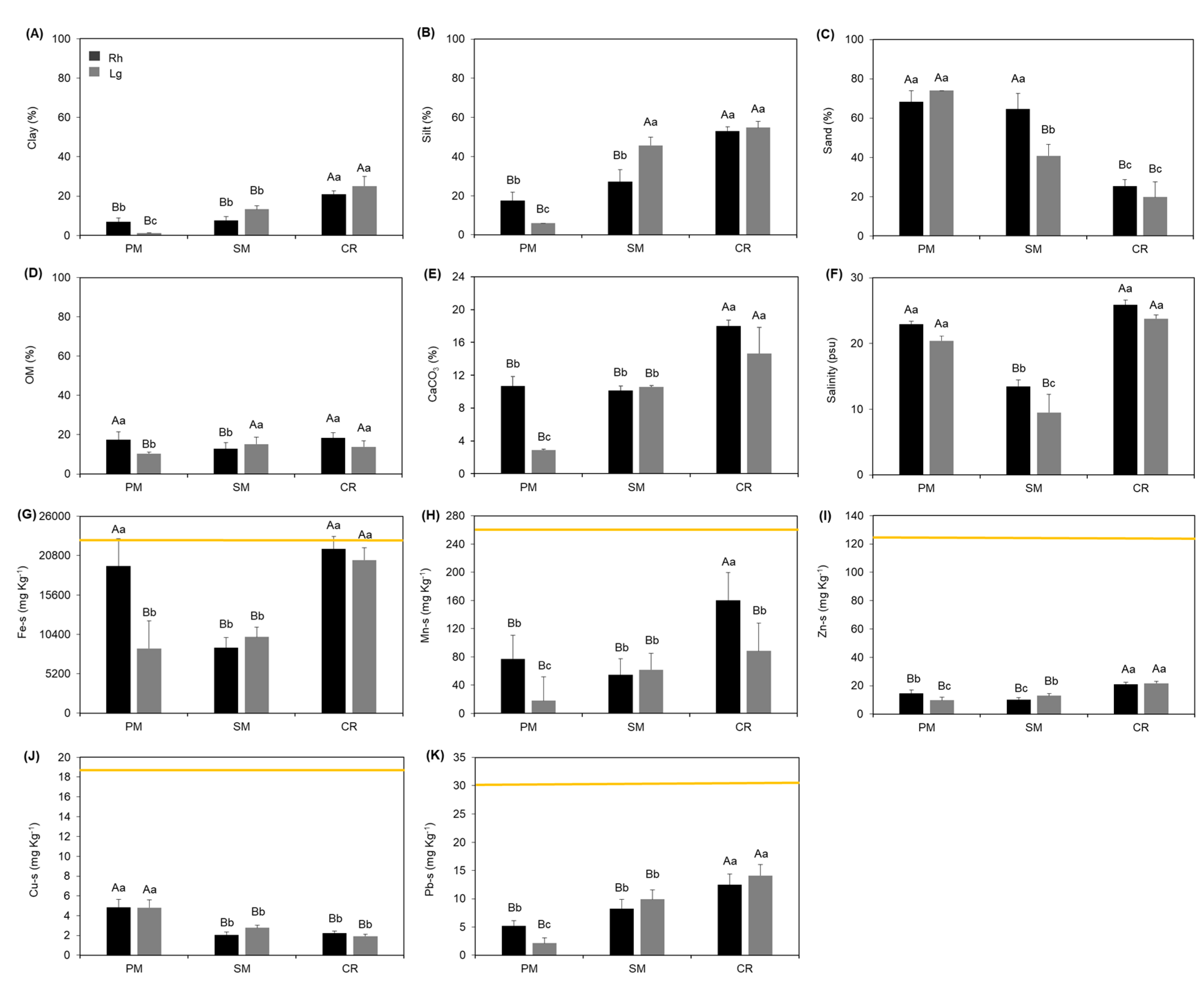
2.3. Granulometry, Organic Matter, and Calcium Carbonate
2.4. Metals in Sediments
2.5. Salinity
2.6. Leaf Samples
2.7. Leaf Analysis of Macro and Micronutrients
2.8. Carbon Assimilation
2.9. DPPH• (2,2-Diphenyl-1-picrylhydrazyl)
2.10. Photosynthetic Pigments
2.11. Bioconcentration Factor
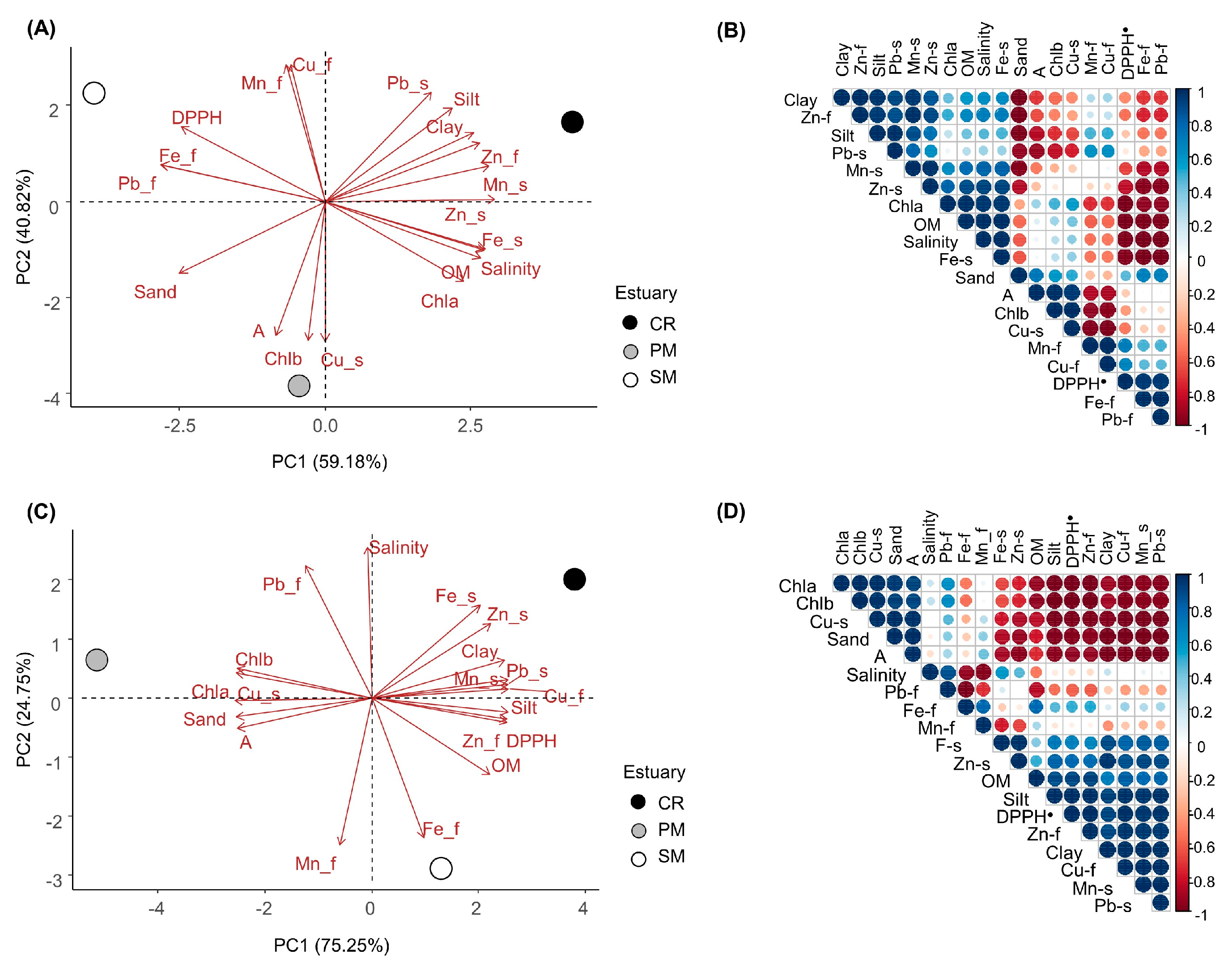
2.12. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chai, M.; Shen, X.; Li, R.; Qiu, G. The risk assessment of heavy metals in Futian mangrove forest sediment in Shenzhen Bay (South China) based on SEM–AVS analysis. Mar. Pollut. Bull. 2015, 97, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Tam, N.F.Y.; Wong, Y.S. Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Environ. Pollut. 2000, 110, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, S.; Borges, A.V.; Castañeda-Moya, E.; Diele, K.; Dittmar, T.; Duke, N.C.; Twilley, R.R. Mangrove production and carbon sinks: A revision of global budget estimates. Glob. Biogeochem. Cycles 2008, 22, 1–12. [Google Scholar] [CrossRef]
- Sasmito, S.D.; Sillanpaa, M.; Hayes, M.A.; Bachri, S.; Saragi-Sasmito, M.F.; Sidik, F.; Hanggara, B.B.; Mofu, W.Y.; Rumbiak, V.I.; Hendri, T.S.; et al. Mangrove blue carbon stocks and dynamics are controlled by hydrogeomorphic settings and land-use change. Glob. Chang. Biol. 2020, 26, 3028–3039. [Google Scholar] [CrossRef] [PubMed]
- CONAMA (Conselho Nacional de Meio Ambiente). Resolução Conama N. 457 de 01 de Novembro de 2012. 2012. Available online: http://www.mma.gov.br (accessed on 10 January 2019).
- Marta-Almeida, M.; Mendes, R.; Amorim, F.N.; Cirano, M.; Dias, J.M. Fundão Dam collapse: Oceanic dispersion of River Doce after the greatest Brazilian environmental accident. Mar. Pollut. Bull. 2016, 112, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Segura, F.R.; Nunes, E.A.; Paniz, F.P.; Paulelli, A.C.C.; Rodrigues, G.B.; Braga, W.; Filho, W.R.P.; Barbosa Júnior, F.; Cerchiaro, G.; Silva, F.F.; et al. Potential risks of the residue from Samarco’s mine dam burst (Bento Rodrigues, Brazil). Environ. Pollut. 2016, 218, 813–825. [Google Scholar] [CrossRef]
- Bianchini, A.; Da Silva, C.C.; Lauer, M.M.; Jorge, M.B.; Costa, P.G.; Marques, J.A.; Marangoni, L.F.B.; Jesulich, A.C.; Taylor, A.J.; Luz, D.C.; et al. Avaliação do Impacto da Lama/Pluma Samarco Sobre os Ambientes Costeiros e Marinhos (ES e BA) Com ênfase Nas Unidades de Conservação. 1a Expedição do Navio de Pesquisa Soloncy Moura do CEPSUL/ICMBio; Ministério do Meio Ambiente. Instituto Chico Mendes de Conservação da Biodiversidade—ICMBio; Diretoria de Pesquisa, Avaliação e Monitoramento da Biodiversidade: Brasília, DF, Brazil, 2016; 62p.
- Queiroz, H.M.; Nóbrega, G.N.; Ferreira, T.O.; Almeida, L.S.; Romero, T.B.; Santaella, S.T.; Bernardino, A.F.; Otero, X.L. The Samarco mine tailing disaster: A possible time-bomb for heavy metals contamination? Sci. Total Environ. 2018, 637, 498–506. [Google Scholar] [CrossRef]
- Tognella, M.M.P.; Falqueto, A.R.; Espinoza, H.D.C.F.; Gontijo, I.; Gontijo, A.B.P.L.; Fernandes, A.A.; Schmildt, E.R.; Soares, M.L.G.; Chaves, F.O.; Schmidt, A.J.; et al. Mangroves as traps for environmental damage to metals: The case study of the Fundão Dam. Sci. Total Environ. 2022, 806, 150452. [Google Scholar] [CrossRef]
- Conrad, S.R.; Santos, I.R.; Brown, D.R.; Sanders, L.M.; van Santen, M.L.; Sanders, C.J. Mangrove sediments reveal records of development during the previous century (Coffs Creek estuary, Australia). Mar. Pollut. Bull. 2017, 122, 441–445. [Google Scholar] [CrossRef]
- Machado, A.A.S.; Wood, C.M.; Bianchini, A.; Gillis, P.A. Responses of biomarkers in wild freshwater mussels chronically exposed to complex contaminant mixtures. Ecotoxicology 2014, 23, 1345–1358. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, J.; Fu, J.; Shi, J.; Jiang, G. Biomonitoring: An appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal. Chim. Acta 2008, 606, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Lasat, M.M. Phytoextraction of toxic metals. J. Environ. Qual. 2002, 31, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef] [PubMed]
- Onofre, D.E.; Regina, C.; José, J.; Nano, W.; Maria, R.; Queiroz, D.S. Biodisponibilidade de metais traços nos sedimentos de manguezais da porção norte da Baía de Todos os Santos, Bahia, Brasil. Rev. Biol. E Ciências Terra 2007, 7, 65–82. Available online: https://www.redalyc.org/articulo.oa?id=50007208 (accessed on 13 September 2020).
- Mehana, E.S.E.; Khafaga, A.F.; Elbehi, S.S.; El-Hack, M.E.A.; Naiel, M.A.E.; Bin-Jumah, M.; Othaman, S.I.; Allam, A.A. Biomonitoring of heavy metal pollution using acanthocephalans parasite in ecosystem: An updated overview. Animals 2020, 10, 811. [Google Scholar] [CrossRef]
- MacFarlane, G.R.; Koller, C.E.; Blomberg, S.P. Accumulation and partitioning of heavy metals in mangroves: A synthesis of field-based studies. Chemosphere 2007, 69, 1454–1464. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, X.; Xu, Y.; Zhang, Q.; Li, X. Accumulation and Tolerance of Mangroves to Heavy Metals: A Review. Curr. Pollut. Rep. 2017, 3, 302–317. [Google Scholar] [CrossRef]
- Agoramoorthy, G.; Chenfa, H.M.J. Threat of heavy metal pollution in halophytic and mangrove plants of Tamil Nadu, India. Environ. Pollut. 2008, 155, 320–326. [Google Scholar] [CrossRef]
- Vangronsveld, J.; Clijsters, H. Toxic effects of metals. In Plants and the Chemical Elements: Biochemistry, Uptake, Tolerance and Toxicity; Farago, M.E., Ed.; VCH Press: Weinheim, Germany, 1994; pp. 149–177. [Google Scholar] [CrossRef]
- Bhaduri, A.; Fulekar, M.H. Antioxidant enzyme response of plants to heavy metal stress. Rev. Environ. Sci. Biotechnol. 2012, 11, 55–69. [Google Scholar] [CrossRef]
- Balk, J.; Pilon, M. Ancient and essential: The assembly of iron–sulfur clusters in plants. Trends Plant Sci. 2011, 16, 218–226. [Google Scholar] [CrossRef]
- Ure, A.M.; Davidson, C.M. Chemical Speciation in the Environment; Blackwell Science: Glasgow, UK, 2001. [Google Scholar] [CrossRef]
- Du Laing, G.; Bogaert, N.; Tack, F.M.G.; Verloo, M.G.; Hendrickx, F. Heavy metal contents (Cd, Cu, Zn) in spiders (Pirata piraticus) living in intertidal sediments of the river Scheldt estuary (Belgium) as affected by substrate characteristics. Sci. Total Environ. 2002, 289, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Marchand, C.; Lallier-Vergès, E.; Baltzer, F.; Albéric, P.; Cossa, D.; Baillif, P. Heavy metals distribution in mangrove sediments along the mobile coastline of French Guiana. Mar. Chem. 2006, 98, 1–17. [Google Scholar] [CrossRef]
- Ahmed, K.; Mehedi, Y.; Haque, R.; Mondol, P. Heavy metal concentrations in some macrobenthic fauna of the Sundarbans mangrove forest, southwest coast of Bangladesh. Environ. Monit. Assess. 2011, 177, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Ibama. Laudo Técnico Preliminar: Impactos Ambientais Decorrentes do Desastre Envolvendo o Rompimento da Barragem de Fundão, em Mariana, Minas Gerais. Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis. 2015. Available online: http://www.ibama.gov.br/phocadownload/barragemdefundao/laudos/laudo_tecnico_preliminar_Ibama.pdf (accessed on 13 February 2019).
- Hadlich, H.L.; Venturini, N.; Martins, C.C.; Hatje, V.; Tinelli, P.; Gomes, L.E.O.; Bernardino, A.F. Multiple biogeochemical indicators of environmental quality in tropical estuaries reveal contrasting conservation opportunities. Ecol. Indic. 2018, 95, 21–31. [Google Scholar] [CrossRef]
- Tognella, M.M.P.; Leopoldo, R.V.S.; Oliveira, C.P.; Pascoalini, S.S.; Silva, E.D. Diversidade estrutural das florestas de mangue da costa central e norte do Espírito Santo: Contribuições para entendimento de funções ecossistêmicas. Enciclopédia Biosf. 2020, 17, 178–193. [Google Scholar] [CrossRef]
- Dias, J.A. Análise Sedimentar e o Conhecimento dos Sistemas Marinhos—Uma Introdução à Oceanografia Geológica; Faro, Universidade do Algarve: Algarve, Portugal, 2004; Available online: https://www.researchgate.net/publication/236551412_A_ANALISE_SEDIMENTAR_E_O_CONHECIMENTOS_DOS_SISTEMAS_MARINHOS_Uma_Introducao_a_Oceanografia_Geologica (accessed on 25 March 2019).
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. J. Geol. 1922, 30, 377–392. Available online: http://www.jstor.org/stable/30063207 (accessed on 13 January 2019). [CrossRef]
- Suguio, K. Geologia Sedimentar; Blucher: São Paulo, Brazil, 2003; ISBN 9788521203179. [Google Scholar]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.Á.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Filho, J.A.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos; Embrapa Solos: Brasília, Brazil, 2018; ISBN 9788570358004. [Google Scholar]
- United States Environmental Protection Agency (USEPA) Electronic Code of Federal Regulations, Title 40-Protection of Environment, Part 423—Steam Electric Power Generating Point Source Category, Appendix A to Part 423–126, Priority Pollutants. 2013. Available online: https://www.ecfr.gov/cgi-bin/text-idx?node=pt40.31.423&rgn=div5 (accessed on 16 July 2020).
- Buchman, M.F. NOAA Screening Quick Reference Tables, NOAA OR&R Report 08-1, Seattle WA, Office of Response and Restoration Division, National Oceanic and Atmospheric Administration. 2008. Available online: https://repository.library.noaa.gov/view/noaa/9327 (accessed on 10 January 2019).
- Blanchar, R.W.; Rehm, G.; Caldwell, A.C. Sulfur in plant material digestion with nitric and perchloric acids. Soil Sci. Soc. Am. Proc. 1965, 29, 71–72. [Google Scholar] [CrossRef]
- Malavolta, E.; Vitti, C.C.; Oliveira, S.A. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações; Esalq-USP: Piracicaba, Brazil, 1997. [Google Scholar]
- Cuzzuol, G.R.F.; Campos, A. Aspectos nutricionais na vegetação de manguezal do estuário do rio Mucuri, Bahia, Brasil. Rev. Bras. Bot. 2001, 24, 227–234. [Google Scholar] [CrossRef]
- Machado, W.; Silva-Filho, E.V.; Oliveira, R.R.; Lacerda, L.D. Trace metal retention in mangrove ecosystems in Guanabra Bay, SE Brazil. Mar. Pollut. Bull. 2002, 44, 1277–1280. [Google Scholar] [CrossRef]
- Souza, I.C.; Morozesk, M.; Duarte, I.D.; Bonomo, M.M.; Rocha, L.D.; Furlan, L.M.; Arrivabene, H.P.; Monferran, M.V.; Matsumoto, S.T.; Milanez, C.R.D.; et al. Matching pollution with adaptive changes in mangrove plants by multivariate statistics. A case study, Rhizophora mangle from four neotropical mangroves in Brazil. Chemosphere 2014, 108, 115–124. [Google Scholar] [CrossRef]
- Dal Prá, V.; Dolwitsch, C.B.; Da Silveira, G.D.; Porte, L.; Frizzo, C.; Tres, M.V.; Da Rosa, M.B. Supercritical CO2 extraction, chemical characterization and antioxidant potential of Brassica oleracea var capitata against HO·, O·−2 and ROO·. Food Chem. 2013, 141, 3954–3959. [Google Scholar] [CrossRef] [PubMed]
- Arar, E.J. Determination of Chlorophylls a and b and Identification of Other Pigments of Interest in Marine and Freshwater Algae Using High-Performance Liquid Chromatography with Visible Wavelength Detection. EPA Method 447.0 447, 1–20. 1997. Available online: https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NERL&dirEntryId=309414 (accessed on 18 December 2018).
- Cruz, C.D. Programa Genes—Ampliado e integrado aos aplicativos R, Matlab e Selegen. Acta Sci. Agron. 2016, 38, 547–552. [Google Scholar] [CrossRef]
- R Core Team. The R Project for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 25 November 2021).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses, R Package Version 1.0.5; 2017. Available online: https://CRAN.Rproject.org/package=factoextra (accessed on 25 November 2021).
- Thuleau, S.; Husson, F. FactoInvestigate: Automatic Description of Factorial Analysis, R Package Version 1.7. 2020. Available online: https://CRAN.R-project.org/package=FactoInvestigate (accessed on 25 November 2021).
- Bartoli, G.; Papa, S.; Sagnella, E.; Fioretto, A. Heavy metal content in sediments along the Calore River: Relationships with physical e chemical characteristics. J. Environ. Manag. 2012, 95, S9–S14. [Google Scholar] [CrossRef] [PubMed]
- Miola, B.; Morais, J.O.D.; Pinheiro, L.S. Trace metal concentrations in tropical mangrove sediments, NE Brazil. Mar. Pollut. Bull. 2016, 102, 206–209. [Google Scholar] [CrossRef]
- Marchand, C.; Fernandez, J.M.; Moreton, B. Trace metal geochemistry in mangrove sediments and their transfer to mangrove plants (New Caledonia). Sci. Total Environ. 2016, 562, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Zhu, Y.G.; Hu, Y.; Williams, P.H.; Gault, A.G.; Meharg, A.A.; Charnock, J.M.; Smith, F.A. Arsenic sequestration in iron plaque, its accumulation and speciation in mature rice plants (Oryza sativa L.). Environ. Sci. Technol. 2006, 40, 5730–5736. [Google Scholar] [CrossRef]
- Tam, N.F.Y.; Wong, Y.S. Mangrove soils in removing pollutants from municipal wastewater of different salinities. J. Environ. Qual. 1999, 28, 556–564. [Google Scholar] [CrossRef]
- Ye, H.; Zang, S.; Xiao, H.; Zhang, L. Speciation and ecological risk of heavy metals and metalloid in the sediments of Zhalong Wetland in China. Int. J. Environ. Sci. Technol. 2015, 12, 115–124. [Google Scholar] [CrossRef]
- Analuddin, K.; Sharma, S.; Jamili; Septiana, A.; Sahidind, I.; Riansee, U.; Nadaoka, K. Heavy metal bioaccumulation in mangrove ecosystem at the coral triangle ecoregion, Southeast Sulawesi, Indonesia. Mar. Pollut. Bull. 2017, 125, 472–480. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FA, USA, 2010. [Google Scholar] [CrossRef]
- Araújo Júnior, C.J.M.; Ferreira, T.O.; Suarez-Abelenda, M.; Nóbrega, G.N.; Albuquerque, A.G.B.M.; de Ca Bezerra, A.; Otero, X.L. The role of bioturbation by Ucides cordatus crab in the fractionation and bioavailability of trace metals in tropical semiarid mangroves. Mar. Pollut. Bull. 2016, 111, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 2004, 49, 219–286. [Google Scholar] [CrossRef] [PubMed]
- RRDM. Relatório Anual 2019 do PMBA/Fest-RRDM—Anexo 5-Manguezal-RT-21 RRDM/NOV 19; Fundação Espírito-Santense de Teconologia (Fest)/Rede Rio Doce Mar (RRDM): Vitória, ES, Brazil, 2019; 600p. [Google Scholar]
- RRDM. Relatório Anual 2020 do PMBA/Fest-RRDM-Evolução Espaço-Temporal na Qualidade Ambiental e na Biodiversidade no Ambiente Costeiro-RT-36C RRDM/DEZ 20; Fundação Espírito-Santense de Teconologia (Fest)/Rede Rio Doce Mar (RRDM): Vitória, ES, Brazil, 2020; 422p. [Google Scholar]
- Chakraborty, P.; Ramteke, D.; Chakraborty, S. Geochemical partitioning of Cu and Ni in mangrove sediments: Relationships with their bioavailability. Mar. Pollut. Bull. 2015, 93, 194–201. [Google Scholar] [CrossRef]
- Chakraborty, P.; Chakraborty, S.; Ramteke, D.; Chennuri, K. Kinetic speciation and bioavailability of copper and nickel in mangrove sediments. Mar. Pollut. Bull. 2014, 88, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.M.M.; Souza, R.C.; Anunciação, D.S.; Moreira, I.T.A.; Santos, V.L.C.S.; Viana, Z.C.V. Evaluation of chemical elements content in mangroves island of Itaparica, Bahia, Brazil. Acta Bras. 2018, 2, 15–20. [Google Scholar] [CrossRef]
- Sipos, P.; Nemeth, T.; Mohai, I. Distribution and possible immobilization of lead in a forest soil (Luvisol) profile. Environ. Geochem. Health 2005, 27, 1–10. [Google Scholar] [CrossRef]
- Bromenschenkel, V.C.S.; Tognella, M.M.P. Population estimate and extractive potential of uçá crab in the post-closed season: Subsidies for management in a Conservation Unit of sustainable use. Res. Soc. Dev. 2020, 9, e25791210992. [Google Scholar] [CrossRef]
- ICMBio. Plano de Manejo da Reserva Extrativista de Cassurubá—Diagnóstico—Volume I. Instituto Chico Mendes de Conservação da Biodiversidade. 2018. Available online: https://www.gov.br/icmbio/pt-br/assuntos/biodiversidade/unidade-de-conservacao/unidades-de-biomas/marinho/lista-de-ucs/resex-de-cassuruba/arquivos/plano_de_manejo_resex_de_cassuruba_diagnostico_vol1.pdf (accessed on 19 April 2022).
- Lutts, S.; Lefèvre, I. How can we take advantage of halophyte properties to cope with heavy metal toxicity in salt-affected areas? Ann. Bot. 2015, 115, 1–20. [Google Scholar] [CrossRef]
- Machado, W.; Gueiros, B.B.; Lisboa-Filho, S.D.; Lacerda, L.D. Trace metals in mangrove seedlings: Role of iron plaque formation. Wetl. Ecol. Manag. 2005, 13, 199–206. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Rezende, C.E.; José, D.V.; Francisco, M.C. Metallic composition of leaves from the Southeastern Brazilian coast. Rev. Bras. Biol. 1986, 46, 395–399. [Google Scholar]
- Medina, E.; Giarizzo, T.; Menezes, M.; Carvalholira, M.; Carvalho, E.A.; Peres, A.; Silva, B.; Vilhena, R.; Reise, A.; Braga, F.C. Mangal communities of the Salgado Paraense: Ecological heterogeneity along the Bragança peninsula assessed through soil and leaf analysis. Amazoniana 2001, 16, 397–416. Available online: http://hdl.handle.net/21.11116/0000-0004-9629-5 (accessed on 11 April 2023).
- Arrivabene, H.P.; Souza, I.C.; Có, W.L.O.; Conti, M.M.; Wunderlin, D.A.; Milanez, C.R.D. Effect of pollution by particulate iron on the morphoanatomy, histochemistry, and bioaccumulation of three mangrove plant species in Brazil. Chemosphere 2015, 127, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Bernini, E.; Silva, M.A.B.; Carmo, T.M.; Cuzzuol, G.R.F. Composição química do sedimento e de folhas das espécies do manguezal do estuário do Rio São Mateus, Espírito Santo, Brasil. Rev. Bras. Bot. 2006, 29, 689–699. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Martinelli, L.A.; Rezende, C.E.; Mozeto, A.A.; Ovalle, A.R.C.; Victoria, R.L.; Silva, C.A.R.; Nogueira, F.B. The fate of trace metals in suspended matter in a mangrove creek during a tidal cycle. Sci. Total Environ. 1988, 75, 169–180. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Rezende, C.E.; José, D.V.; Wasserman, J.C.; Francisco, M.C. Mineral concentrations in leaves of mangrove trees. Biotropica 1985, 17, 21–27. [Google Scholar] [CrossRef]
- Dechen, A.R.; Nachtigall, G. Micronutrientes. In Nutrição Mineral de Plantas; Fernandes, M.S., Ed.; SBCS: Viçosa, Brazil, 2006; pp. 327–354. ISBN 8586504025. [Google Scholar]
- Khan, M.; Neeha, N.; Ifthekhar, A.; Muhammad, A.; Muhammad, R.; Parvaiz, A.; Shafaqat, A. Regulation of Photosynthesis Under Metal Stress. In Photosynthesis, Productivity and Environmental Stress; Ahmad, P., Ahanger, M.A., Alyemeni, M.N., Alam, P., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 95–105. [Google Scholar]
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Gupta, M.; Cuypers, A.; Vangronsveld, J.; Clijsters, H. Copper affects the enzymes of the ascorbate-glutathione cycle and its related metabolites in the roots of Phaseolus vulgaris. Physiol. Plant. 1999, 106, 262–267. [Google Scholar] [CrossRef]
- Pätsikkä, E.; Kairavuo, M.; Sersen, F.; Aro, E.M.; Tyystjärvi, E. Excess copper predisposes photosystem II to photoinhibition in vivo by outcompeting iron and causing decrease in leaf chlorophyll. Plant Physiol. 2002, 129, 1359–1367. [Google Scholar] [CrossRef]
- Millaleo, R.; Reyes-Diaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 476–494. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef]
- Fernando, D.R.; Marshall, A.L.T.; Forster, P.I.; Hoebee, S.E.; Siegele, R.A. Multiple metal accumulation within, a manganese-specific genus. Am. J. Bot. 2013, 100, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Singh, H.P.; Khan, M.I.R.; Masood, A.; Per, T.S.; Negi, A.; Batish, D.R.; Khan, N.A.; Duarte, A.C.; Pereira, E.; et al. Too much is bad—An appraisal of phytotoxicity of elevated plant-beneficial heavy metal ions. Environ. Sci. Pollut. Res. 2015, 22, 3361–3382. [Google Scholar] [CrossRef]
- Marschner, P. Marchner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Wang, B.; Zhang, C.; Shi, X.; Zhu, C. Level and fate of heavy metals in the Changjiang estuary and its adjacent waters. Oceanology 2009, 49, 64–72. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.; Zheng, X.; Qian, P.; Wu, Y. Influence of Spartina alterniflora on the mobility of heavy metals in saltmarsh sediments of the Yangtze River estuary, China. Environ. Sci. Pollut Res. 2013, 20, 1675–1685. [Google Scholar] [CrossRef]
- Küpper, H.; Šetlík, I.; Spiller, M.; Küpper, F.C.; Prášil, O. Heavy metal-induced inhibition of photosynthesis: Targets of in vivo heavy metal chlorophyll formation. J. Phycol. 2002, 38, 429–441. [Google Scholar] [CrossRef]
- Cheng, H.; Tam, N.F.Y.; Wang, Y.S.; Li, S.Y.; Chen, G.Z.; Ye, Z.H. Effect of copper on growth, radial oxygen loss and root permeability of seedlings of mangroves Bruguiera gymnorrhiza and Rhizophora stylosa. Plant Soil 2012, 359, 255–266. [Google Scholar] [CrossRef]
- Naidoo, G.; Hiralal, T.; Naidoo, Y. Ecophysiological response of the mangrove Avicennia marina to trace metal contamination. Flora 2014, 209, 63–72. [Google Scholar] [CrossRef]
- MacFarlane, G.R.; Pulkownik, A.; Burchett, M.D. Accumulation and distribution of heavy metals in the grey mangrove Avicennia marina (Forsk.) Vierh.: Biological indication potential. Environ. Pollut. 2003, 123, 139–151. [Google Scholar] [CrossRef]
- Elefteriou, E.P.; Karataglis, S. Ultrastructural and morphological characteristics of cultivated wheat growing on copper-polluted fields. Bot. Acta 1989, 102, 134–140. [Google Scholar] [CrossRef]
- Kocheva, K.; Lambrev, P.; Georgiev, G.; Goltsev, V.; Karabaliev, M. Evaluation of chlorophyll fluorescence and membrane injury in the leaves of barley cultivars under osmotic stress. Bioelectrochemistry 2004, 63, 121–124. [Google Scholar] [CrossRef]
- Chettri, M.K.; Cook, C.M.; Vardaka, E.; Sawidis, T.; Lanaras, T. The effect of Cu, Zn and Pb on the chlorophyll content of the lichens Cladonia convoluta and Cladonia rangiformis. Environ. Exp. Bot. 1998, 39, 1–10. [Google Scholar] [CrossRef]
- Ebbs, S.; Uchil, S. Cadmium and zinc induced chlorosis in Indian mustard [Brassica juncea (L.) Czern] involves preferential loss of chlorophyll b. Photosynthetica 2008, 46, 49–55. [Google Scholar] [CrossRef]
- Bakshi, M.; Ghosh, S.; Chakraborty, D.; Hazra, S.; Chaudhuri, P. Assessment of potentially toxic metal (PTM) pollution in mangrove habitats using biochemical markers: A case study on Avicennia officinalis L. in and around Sundarban, India. Mar. Pollut. Bull. 2018, 133, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Petranich, E.; Acquavita, A.; Covelli, S.; Emili, A. Potential bioaccumulation of trace metals in halophytes from salt marshes of a northern Adriatic coastal lagoon. J. Soils Sediments 2017, 17, 1986–1998. [Google Scholar] [CrossRef]
- Ong Che, R.G. Concentration of 7 heavy metals in sediments and mangrove root samples from Mai Po, Hong Kong. Mar. Pollut. Bull. 1999, 39, 269–279. [Google Scholar] [CrossRef]


| Percentage (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sediment 1 | Leaf 2 | ||||||||
| PM | SM | CR | PM | SM | CR | ||||
| Rh | Lg | Rh | Lg | Rh | Lg | ||||
| Fe | 36 | 7 | 46 | 76 | 0 | 93 | 60 | 78 | 0 |
| Mn | 9 | 0 | 21 | 0 | 20 | 0 | 53 | 0 | 0 |
| Zn | 0 | 0 | 0 | 37 | 0 | 23 | 60 | 58 | 50 |
| Cu | 2 | 0 | 0 | 52 | 20 | 70 | 100 | 56 | 40 |
| Pb | 0 | 5 | 14 | 100 | 100 | 100 | 100 | 100 | 100 |
| BCF | |||||
|---|---|---|---|---|---|
| Fe | Mn | Zn | Cu | Pb | |
| Rhizophora mangle | |||||
| Piraquê-Mirim | 0.01 | 2.36 | 0.32 | 0.43 | 1.50 |
| São Mateus | 0.01 | 5.97 | 0.46 | 1.37 | 1.15 |
| Caravelas | 0.04 | 1.76 | 0.25 | 1.27 | 0.56 |
| Laguncularia racemosa | |||||
| Piraquê-Mirim | 0.01 | 3.31 | 0.64 | 0.34 | 3.56 |
| São Mateus | 0.08 | 1.47 | 0.94 | 1.47 | 0.70 |
| Caravelas | 0.01 | 0.36 | 0.61 | 3.21 | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Addazio, V.; Tognella, M.M.P.; Fernandes, A.A.; Falqueto, A.R.; da Rosa, M.B.; Gontijo, I.; de Oliveira, M.A. Impact of Metal Accumulation on Photosynthetic Pigments, Carbon Assimilation, and Oxidative Metabolism in Mangroves Affected by the Fundāo Dam Tailings Plume. Coasts 2023, 3, 125-144. https://doi.org/10.3390/coasts3020008
D’Addazio V, Tognella MMP, Fernandes AA, Falqueto AR, da Rosa MB, Gontijo I, de Oliveira MA. Impact of Metal Accumulation on Photosynthetic Pigments, Carbon Assimilation, and Oxidative Metabolism in Mangroves Affected by the Fundāo Dam Tailings Plume. Coasts. 2023; 3(2):125-144. https://doi.org/10.3390/coasts3020008
Chicago/Turabian StyleD’Addazio, Veronica, Monica Maria Pereira Tognella, Adriano Alves Fernandes, Antelmo Ralph Falqueto, Marcelo Barcellos da Rosa, Ivoney Gontijo, and Marcelo Antônio de Oliveira. 2023. "Impact of Metal Accumulation on Photosynthetic Pigments, Carbon Assimilation, and Oxidative Metabolism in Mangroves Affected by the Fundāo Dam Tailings Plume" Coasts 3, no. 2: 125-144. https://doi.org/10.3390/coasts3020008
APA StyleD’Addazio, V., Tognella, M. M. P., Fernandes, A. A., Falqueto, A. R., da Rosa, M. B., Gontijo, I., & de Oliveira, M. A. (2023). Impact of Metal Accumulation on Photosynthetic Pigments, Carbon Assimilation, and Oxidative Metabolism in Mangroves Affected by the Fundāo Dam Tailings Plume. Coasts, 3(2), 125-144. https://doi.org/10.3390/coasts3020008