1. Introduction
Islands, having discrete boundaries, offer a unique opportunity as living laboratories to gain understanding of management options within sensitive socioecological systems. This paper focuses on Fraser Island in Australia, a World-Heritage-listed landscape with immense cultural and environmental significance. The First Nations People, the Butchulla, have had a long and continuous connection to the land and surrounding waters. Known to the Butchulla People as “K’gari” (henceforth, Fraser Island will be referred to as K’gari), the island has been a site of conflicting views of custodianship, ownership, and management which continue today, providing conditions in which ecological sustainability and cultural equity can be explored through efforts in long-term planning for management [
1].
The purpose of this paper is to draw on existing knowledge of the natural and cultural environment of K’gari as a foundation for future management and provide a model for the management of a unique ecosystem [
2]. We achieve this through a synthesis of existing research, historical documents, government documents, and Butchulla history, knowledge, and understanding of K’gari.
K’gari lies in the subtropics off the eastern coast of Australia between 24 and 26° S and is one of 8411 offshore Australian islands [
3]. It is the largest and northernmost of a chain of prominent barrier islands off the southeast coast of Queensland, formed by South and North Stradbroke Island (Curragee and Minjerribah) and Moreton Island (Moorgumpin). K’gari is frequently cited as the “largest” sand island globally [
4], being ca. 130 km long, 25 km wide, encompassing 1660 km
2, and having a maximum elevation of 277 m a.s.l. [
5,
6].
The outstanding natural value of K’gari was recognized by the United Nations Educational, Scientific, and Cultural Organization (UNESCO) and inscribed on the World Heritage List in 1992 [
7], primarily on account of its wilderness character and the diversity of ecosystems. Amongst features emphasized in the World Heritage listing are the long expanses of ocean-exposed beaches and high-energy surf zones; the combination of shifting sand dunes and tropical rainforests; the presence of half of the world’s perched freshwater dune lakes; and the world’s largest unconfined aquifer on a sand island [
8]. Significantly, K’gari also lies within a biosphere (the Great Sandy Biosphere) and is unique in that it adjoins another biosphere (the Noosa Biosphere).
An estimated 400,000 tourists visit K’gari annually. (Comprehensive monitoring data has not been collated.) Visitor numbers have increased from the 1930s when commercial tours began [
9]. Replacing the historically significant activities of logging (1863–1991) and sand mining (1950–1977), environmental harm resulting from visitation (e.g., damage to habitats and biota caused by off-road vehicles, campfires, and bacterial contamination of freshwater lakes and groundwater from dune campers) is a considered a major threat. Environmental impacts are further exacerbated by unnatural fire regimes, invasive and feral species, and climate change.
Similar to many other small island settings, significant challenges for K’gari include management of tourism, attaining ecological sustainability, and achieving the First Nations heritage goals [
10]. Typically, economic opportunities on islands are limited (other than tourism) and resources can be fragile and difficult to restore. The contrast of preserving the natural values that attract visitors whilst deriving economic benefits from tourism requires a workable and efficient system of management [
10]. Calado et al. [
11] argue that small islands represent some of the most fragile and vulnerable regions in the world. They are also some of the most beautiful regions, sought after by tourists and with unique biodiversity. Small island systems, unfortunately, are over-represented in terms of the need to balance ecological integrity, economic development, and cultural integrity [
11]. The World Heritage list (UNESCO) contains 1154 properties, 218 of which are listed for their natural value. Only 17 properties are composed entirely of islands or archipelagos. Four of these properties are in Australia.
K’gari has a contested social, cultural, and environmental history [
2]. Disparate views exist regarding custodianship, uses, and the management strategies needed to achieve multiple objectives in an environmentally sustainable way whilst creating cultural equity. Management of the recent (2020) wildfire which burnt around 85,000 hectares (over half the island) has demonstrated the variety of views and expectations [
12]. The challenge of managing a fragile landscape of global significance, amidst contested perspectives, provides the stimulus to explore the dimensions and complexities of governance to build a consensus on collaborative management of such a landscape.
In this paper we carry out the following:
Provide a précis of the key historical events that form the current cultural, social, and environmental narrative;
Review key attributes of the major natural features of the island, many of which underpin the value of the island for tourism;
Identify the main shortcomings of our current understanding of the natural assets, processes, and sociocultural issues on the island;
Analyse principal management and conservation challenges and discuss whether the requirements for ecological integrity can be met in the future, given tourism-related impacts, biosecurity threats, current vegetation management practices, and climate change;
Identify commonalities and general principles that may apply globally to other island systems and World Heritage sites listed on the basis of their ecological and cultural integrity. (Noting that K’gari is listed on the basis of its ecological integrity, however, the issue of also listing on the basis of cultural integrity has been raised within the Queensland’s government Fraser Island World Heritage unit).
We base our analysis on papers in the peer-reviewed scientific literature, original reports, letters, and other manuscripts, some of which were previously believed lost, now housed in the K’gari Fraser Island Research Archive [
13] and digitally available. This archive charts the history of the contested area from pre-European colonization to World Heritage status. Through the 1970s, K’gari occupied a critical place in Queensland’s and Australia’s political landscapes. The conflicting interests of scientists, environmentalists, sand miners, and the logging industry impacted all levels of Australian government and resulted, ultimately, in K’gari being included on the UNESCO World Heritage List. The transcripts and exhibits submitted to the 1975 Fraser Island Inquiry, initiated by the Australian Commonwealth Government, form part of the research archive. The archive includes more than 600 photographs which provide a visual narrative of K’gari’s recent history. Historical content includes news items from 19th century newspapers, military records from World War II, parliamentary papers, shipping records, timber industry publications, and interviews and recollections of long-time residents. Documents about forestry include historic, economic, and scientific concerns. Many of the papers dating from the 1970s to the 1990s are associated with political campaigns to end sand mining and logging on the island. Other issues addressed are tourism impacts, recreational and commercial fishing, island geology, and ecology. The Butchulla, the First Nations Peoples, have some presence throughout the documents and their displacement from K’gari is mentioned in many of the papers.
2. History: A Contested Legacy
While K’gari was connected to the mainland during glacial periods throughout the Pleistocene, it also functioned as an island during various interglacial cycles. Most recently (~7000 years ago), K’gari separated from the east Queensland coast to become an island as a result of the early-Holocene sea-level highstand with the sea level at its highest about 6000+ years ago [
14]. Rising sea levels forced the Butchulla People onto higher ground with some persisting on the island and others living on the mainland. The Butchulla People call the island “
K’gari” or “paradise” after a creation spirit who was unable to leave the beauty of the area and instead slept in the waters, transmogrified into landmass through ecstatic belonging [
15]. Archaeological, social, and spiritual significance is evidenced from large numbers of midden heaps, campsites, fish traps, traditional bora rings, and stone tools, suggesting a long cultural occupation. The dates of human occupation pre-colonization have received seminal (and conflicting) ethnohistorical and archaeological study [
16,
17] but are estimated to lie between 1500 and 5000 years before the present time. Based on archaeological findings, ref. [
18] claimed that occupation may extend back to at least 30,000 years ago.
Since K’gari most recently became an island, the productive lands and adjacent waters were home to between 400 and 600 people generally, and up to 2000–3000 people during the winter months, when there was an abundance of sea mullet available for sustenance [
19]. Butchulla oral history gives different accounts of the Traditional Owner groups on the island. However, the modern understanding of the traditional ownership of the island, according to the Butchulla Aboriginal Corporation (BAC), the Butchulla Native Title Aboriginal Corporation (BNTAC), and the Commonwealth government, is that the Butchulla are the sole Traditional Owner group for the island and surrounding waters. The first Native Title claim was granted to the BAC on 24 October 2014, covering approximately 1640 sq km of K’gari and administered by the BAC [
20]. The second claim was determined on the 13 December 2019, and covers the waters surrounding K’gari, alongside parts of the mainland [
20]. This claim was administered by BNTAC.
The Butchulla ancestral message of land stewardship states: What is good for the land comes first; Do not touch or take what does not belong to you; If you have plenty you must share [
21]. However, post-European colonization commercial activities such as logging and sand mining were in sharp contrast to this ethic, and tourism facilitated an increase in recreation-based activities that have, together, visibly contributed to the re-shaping of the island’s ecosystems.
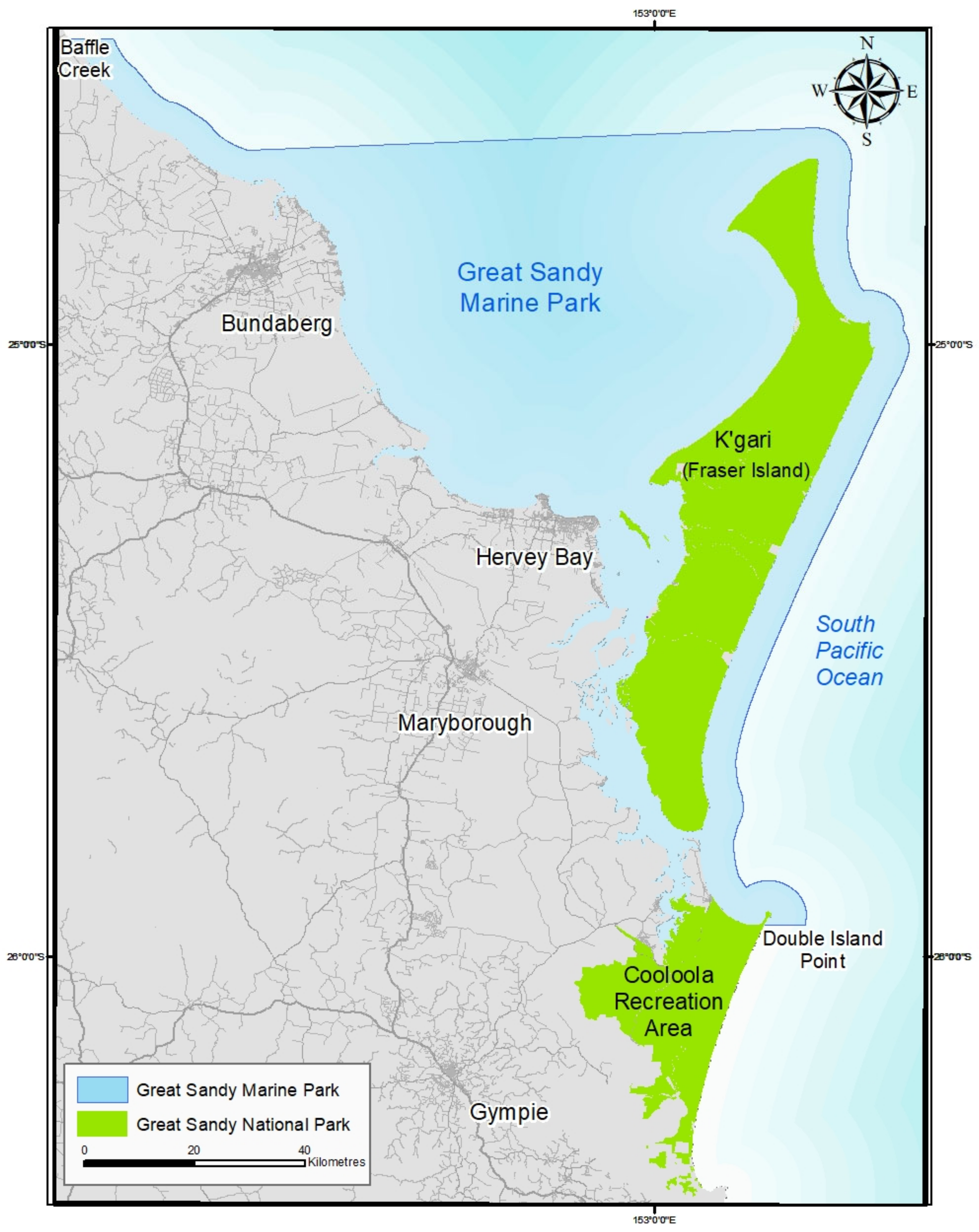
European colonization had a devastating impact on the Butchulla People living on K’gari as it did in other parts of Australia and, indeed, other parts of the world. The first recorded European to have contact with the island was Captain James Cook, who, in 1770, mapped the east coast of Australia and named significant sites including “Indian Head” (Tuckee Wooro is the original Butchulla Name for the headland) (
Figure 1) after seeing Butchulla People on the headland. Other European explorers/invaders followed (Matthew Flinders in 1799 and William Edwardson in 1822).
In 1836, the brig
Stirling Castle, travelling from Sydney to Singapore, was shipwrecked off K’gari. As a result, the traditional culture is loaded in notoriety and colonial mythic significance. The name Fraser is a reminder of Eliza Fraser’s short stay with the Butchulla People. Captain Fraser’s spearing by Butchulla People after the wrecking of his ship and his wife’s (and other survivors’) treatment by the same inhabitants became infamous in colonial foundational narratives [
22,
23]. Much data was suppressed in the national archives of the nineteenth century while myths propagated [
22], a product of Eliza’s own falsification of her experiences and their replication in subsequent texts (for example, Curtis [
24]). Unsubstantiated claims of cannibalism and fire torture were made despite their certain falsity and the far more credible records of the activities of the Butchulla People who assisted the survivors of the wreck [
21,
22,
23].
Although a few descendants continue to live on the island and many live nearby on the mainland, their cultural heritage and ability to maintain their traditional practices had diminished prior to Native Title determination. Native Title has meant that the Butchulla cultural rights and responsibilities for K’gari were restored. The Butchulla People have increasingly taken management responsibilities for the island as they move towards co-management arrangements. They are currently managing the development of a large cultural interpretation centre in conjunction with the Queensland Parks and Wildlife Service (QPWS).
K’gari has a long history of logging operations and sand mining that impacted the Butchulla People, influencing even current island management, due to a transport network established from the mid-1800s to near the end of last century. In 1842, Andrew Petrie’s report on the discovery of kauri pine (
Agathis robusta) and cypress pine (
Callitris columellaris) caught the interest of the entrepreneur William Pettigrew. In 1862, he visited K’gari and the surrounding coast, and finding the area to be well-timbered, opened the Dundathu (the Butchulla name for Kauri pine) timber mill in 1863 [
25]. Other timber mills followed as the capacity of K’gari and the mainland to supply timber burgeoned and the era of intensive logging began. The forests yielded a high-quality timber resource as evidenced by the fact that logging operations continued despite the relative isolation from markets and the need to ship logs to sawmills on the mainland at Maryborough (
Figure 1).
The legacy of logging was a network of roads and old railway sites and the loss of many large trees. By 1971, the timber industry was still removing 22,000 m3 of timber per year. The primary species extracted from the island were kauri pine, blackbutt (Eucalyptus pilularis), tallow-wood (Eucalyptus microcorys), hoop pine (Araucaria cunninghamii), and the satinay or Fraser Island Turpentine (Syncarpia hillii). The wood of the satinay, which is resistant to marine borers, was used in the construction of the Suez Canal (1859–1869) and London’s Falmouth Dock; evidence of the global connections shaping Queensland’s economy from the outset of the colony.
From 1882 the Queensland Government began to exercise some controls over the unrestricted logging of the island [
26]. In 1905, a tramline was built by Wilson, Hart and Co., to move timber more efficiently to the more sheltered west coast (previously moved by bullock teams) and in 1908 a forestry reserve was declared under the control of Forest Services [
27]. This tramline was later moved and overcoming decades a sequence of tramlines and roads criss-crossed the island. Timber-getting practices were so extensive that, by 1920, three mills were in operation and there was a sizeable enough population to support a small school.
From the mid-twentieth century, there was a growing awareness of the mineral wealth hidden in the sands [
28]. The first sand mining leases were granted in 1946 and from 1972 mining companies began intensive strip mining for the sought-after minerals rutile, ilmenite, zircon, and monazite [
26]. Sand mining for minerals, which first stripped an area of vegetation, had a severe impact on part of the island’s coastal environment, but was short-lived, as a strong environmental movement led by John Sinclair’s Fraser Island Defenders Organisation (FIDO) was instrumental in challenging the Commonwealth government. The challenge was strengthened by the Commonwealth’s Environmental Protection (Impact of Proposals) Act (1974–1975), which led to the Commonwealth government’s banning of all mineral exports from the island from December 31, 1976 [
29].
An inspection by ecologists in 2016, forty years after mining operations ceased in 1976, found that it would take hundreds of years, if ever, to re-establish the original plant communities that existed before mining occurred on K’gari [
30]. Even though revegetated, the mined areas do not represent the complex ecosystem that existed prior to sand mining. The mining process on K’gari had reduced an advanced dune system with well-developed soil profiles, which can take hundreds or even thousands of years to develop, to the equivalent of a very young dune system, basically consisting purely of unconsolidated silica sands [
30]. The areas disturbed by mining are also subject to weed infestations, especially the exotic Lantana (
Lantana camara) [
30], an extremely adaptable and rapid-spreading species capable of inhabiting a wide variety of ecosystems, which has been internationally highlighted as a threat to island biodiversity [
31].
The Queensland Forestry Department had almost exclusive management of K’gari up until 1974 [
27]. From the mid-1970s to the 1990s, the general management of K’gari transferred from the Queensland Forestry Department to a fragmented range of diverse stakeholders [
27,
32]. Management was shared among the Queensland Mines, Lands, Forestry and Tourism Departments, the Qld Beach Protection Authority, the Queensland National Park and Wildlife Service, Maryborough City (in the south to protect the forestry industry supporting the City of Maryborough), and Hervey Bay Council (in the north) [
27] (
Figure 1). The emergence of this new diversity of stakeholders in the 1970s resulted in a stifling of effective planning and management and was heavily criticised by conservation groups [
32].
The Commission of Inquiry into the Conservation, Management and Use of Fraser Island and the Great Sandy Region (“Fraser Island Inquiry”) initiated by the Queensland government in 1990, recommended cessation of logging, nomination of the region for World Heritage listing, preparation of a comprehensive management plan, and development of legislation to coordinate management of the region [
33]. Logging finally ceased in 1991 after decades of conservation efforts, legal disputes, and political changes [
34]. K’gari was inscribed on the World Heritage List in 1992 on the basis of three environmental criteria [
4], as follows:
Criterion (vii): Fraser Island is the largest sand island in the world, containing a diverse range of features that are of exceptional natural beauty.
Criterion (viii): The property represents an outstanding example of significant ongoing geological processes including longshore drift.
Criterion (ix): The property represents an outstanding example of significant ongoing biological processes.
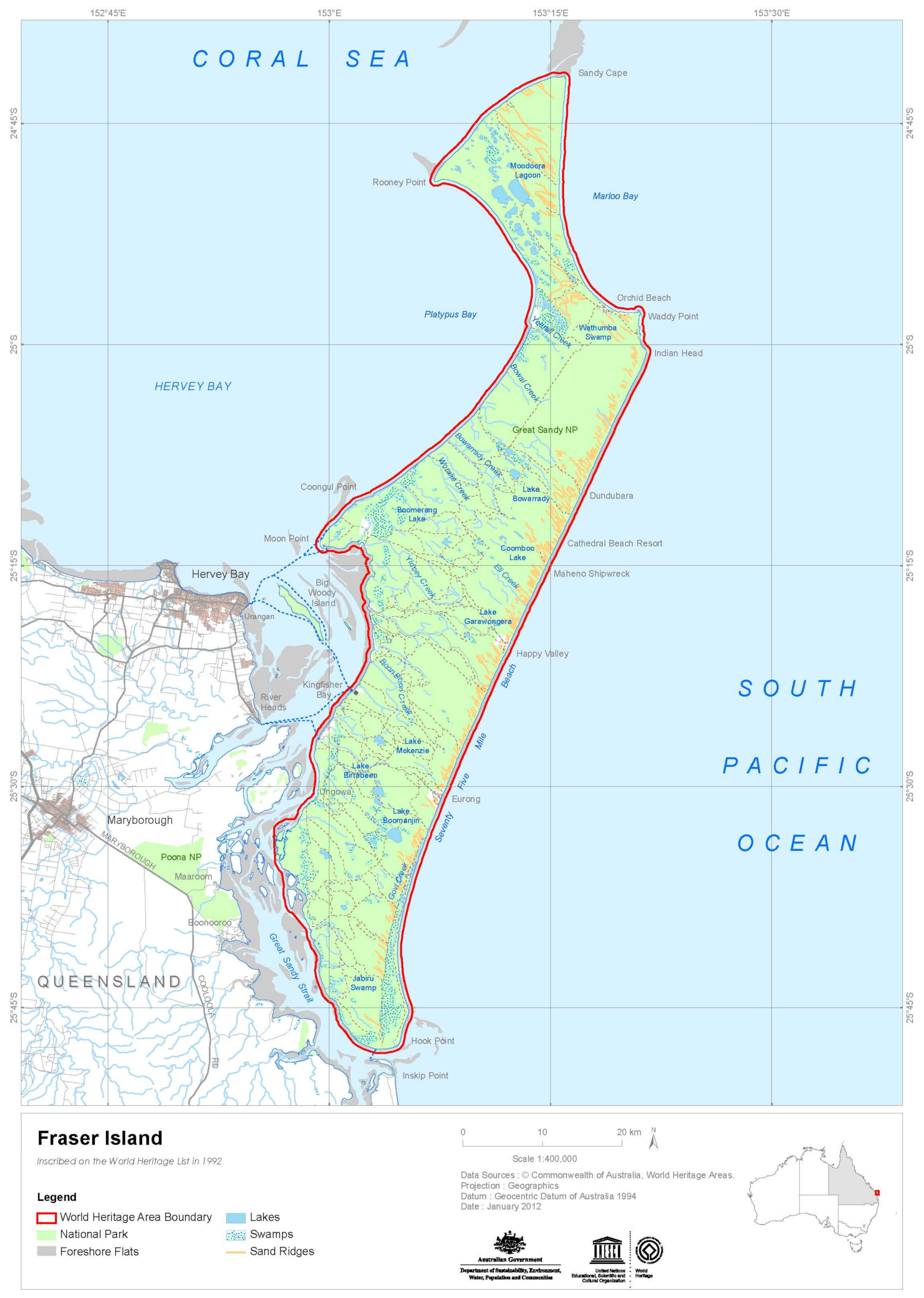
The Fraser Island World Heritage Area incorporates the whole of the island to a distance of 500 m buffer seaward extending from the high-water mark (
Figure 2). Additionally, included are a number of small islands off the west coast: Stewart Island, Dream Island, and small unnamed islands between Dream Island and the mouth of Yankee Jack Creek, including Boonlye Point [
35].
When listed in 1992, the conditions of ecological integrity were met as there was no perceptible human threat to longshore drift and the other ongoing processes that make the property outstanding. The island was considered sufficiently large, diverse, and free from disturbance to contain all ecosystem components required for viable populations of all species and for continued maintenance of all natural phenomena [
4]. It needs to be noted that, at the time of listing, there were no considerations of cultural integrity, nor the human threat to cultural integrity.
Today, invasive species and human activities such as development and tourism are the leading causes of the loss of biodiversity worldwide [
36]. In addition, actions external to the island, such as extraction from interconnected groundwater systems, may affect the ecological integrity of K’gari over time. Likewise, other external but global threats—climate change and climate change adaptation—make the management of World Heritage sites more challenging [
37]. Sustaining the requirements for integrity will become more difficult as current management strategies may not be adequate to maintain the attributes that make K’gari qualify as World Heritage.
It is imperative to understand the natural ecology of K’gari in order to manage the challenges of sustaining the requirements of integrity amidst disparate views on custodianship, uses, threats and management strategies. Therefore, the next four sections focus on the natural ecosystems: dunes and vegetation; freshwater systems; terrestrial fauna and habitats; and ocean beaches and bay.
3. Coastal Dunes and Associated Ecosystems
In granting World Heritage status, UNESCO noted the following:
The property represents an outstanding example of significant ongoing geological processes, including longshore drift. The immense sand dunes are part of the longest and most complete age sequence of coastal dune systems in the world and are still evolving. The superimposition of active parabolic dunes on remnants of older dunes deposited during periods of low sea level, which are stabilized by towering rainforests at elevations of up to 240 m, is considered unique. (UNESCO World Heritage Commission).
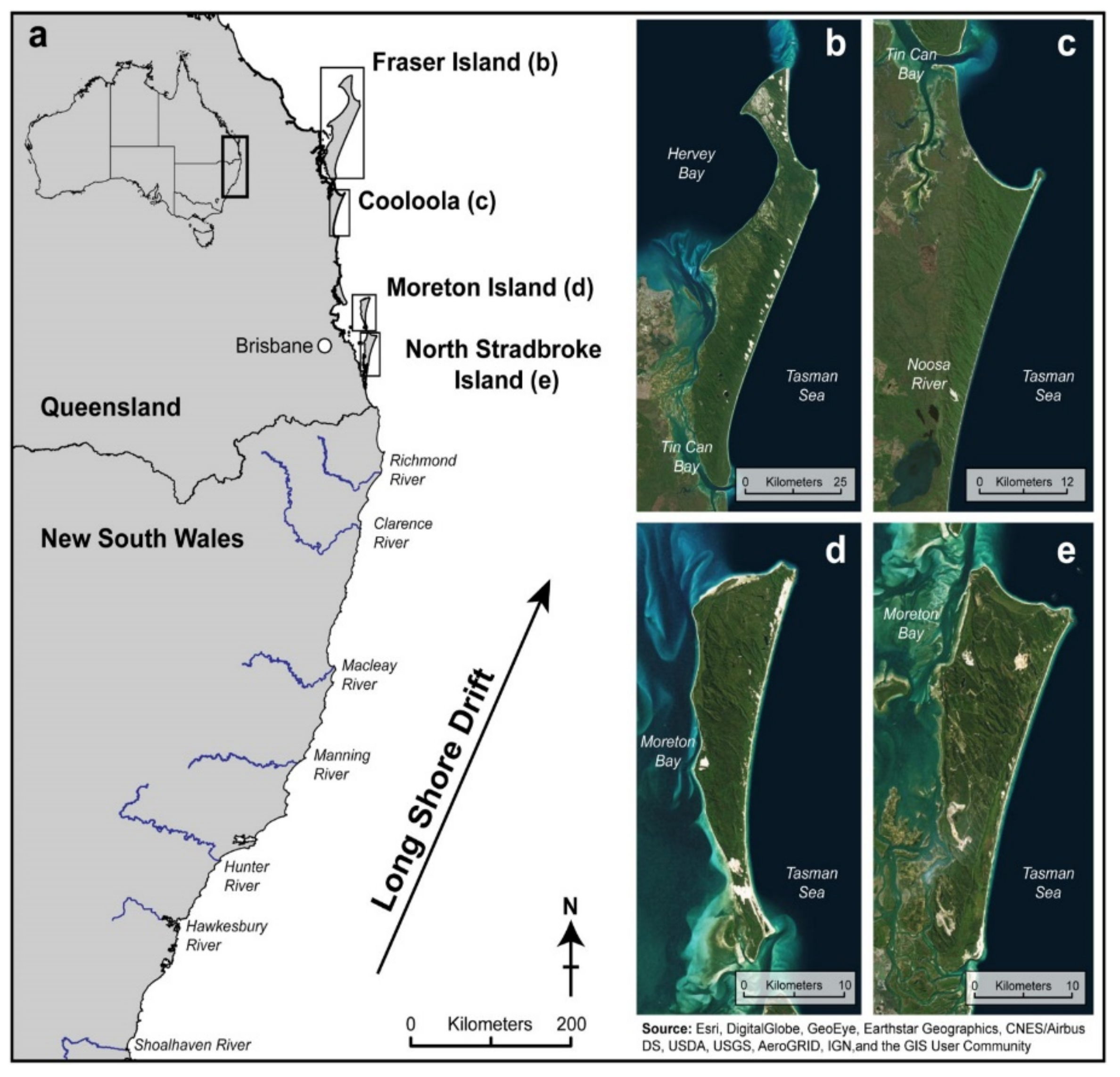
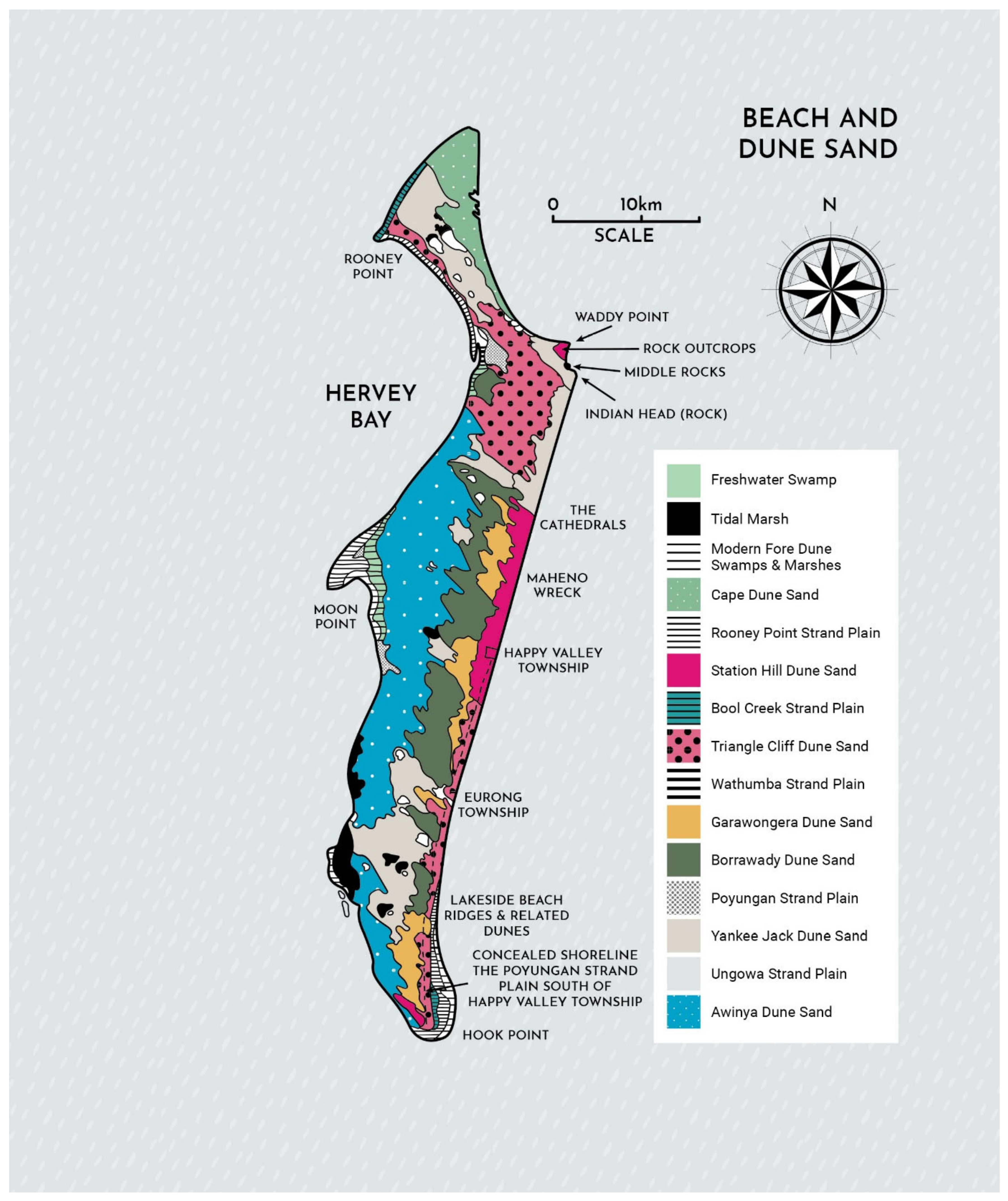
Figure 3 illustrates the effect of the long shore drift in building four large sand islands in southeast Queensland. Panel (a) shows the location of these dune fields. Panel (a) showPanels (b–e) are satellite images of K’gari (b), Cooloola Sand Mass (c), Moreton Island (d), and North Stradbroke Island (e) [
38].
The sands cover older Mesozoic sediments with some of these older sediments visible on the island. These sand masses are mostly of Aeolian origin and were deposited during several periods of dune building in the latter Quaternary period [
39,
40,
41]. The distribution of sand dunes on K’gari is shown in
Figure 4 (based on Figure 1 in Ward [
42]).
Most of the quartz sands probably originated from the rivers of northern New South Wales and were carried north in ocean currents when sea levels were lower. Repeated transportation and exposure to subaerial weathering has removed those differences which occurred in the original source rocks. The size of the sand grains in all the various sand masses is 100–400 μg and sand grains of this size can be readily mobilised and transported by wind. The sands were brought to the beach and blown inland by winds, where protective coastal vegetation trapped and immobilised the sand grains and protected them from further Aeolian activity. The dominant onshore south easterly winds led to the formation of high and often large, U- or V-shaped parabolic dunes aligned southeast–northwest. Local relief on these varies from 40 to 240 m. These dunes have moved inland with advancing younger dunes sometimes burying older dunes. Studies based on aerial photographs suggest the frequency and intensity of passing tropical cyclones have decreased in recent decades meaning fewer recent blow-outs and greater dune stability, although some small mobile blowouts still exist [
43].
Although dating is difficult, there is evidence that deposition has occurred through processes operating over the last 540,000–750,000 years making K’gari an area with one of the longest periods of dune-field evolution in the world [
44,
45]. Dune building has been episodic and mostly synchronous with nine distinct dune sequences of different ages. Miot da Silva and Shulmeister [
45] argue that both climatic cycles and associated changes in sea level were probably involved in the different dune-building episodes.
Vegetation covers much of the island’s dunes, providing stabilisation and protection from wind erosion [
46], although some wind-blows have continued near the coast. Water erosion of sand dunes is common from surface wash and raindrop splash as well as rills and deep gullying on older dunes. Over time, the effect of this erosion has been to make dune crests broader and slopes gentler, thereby converting parabolic dunes to elongated sand ridges, broader convex sandhills, or “whaleback” shapes with more subdued relief.
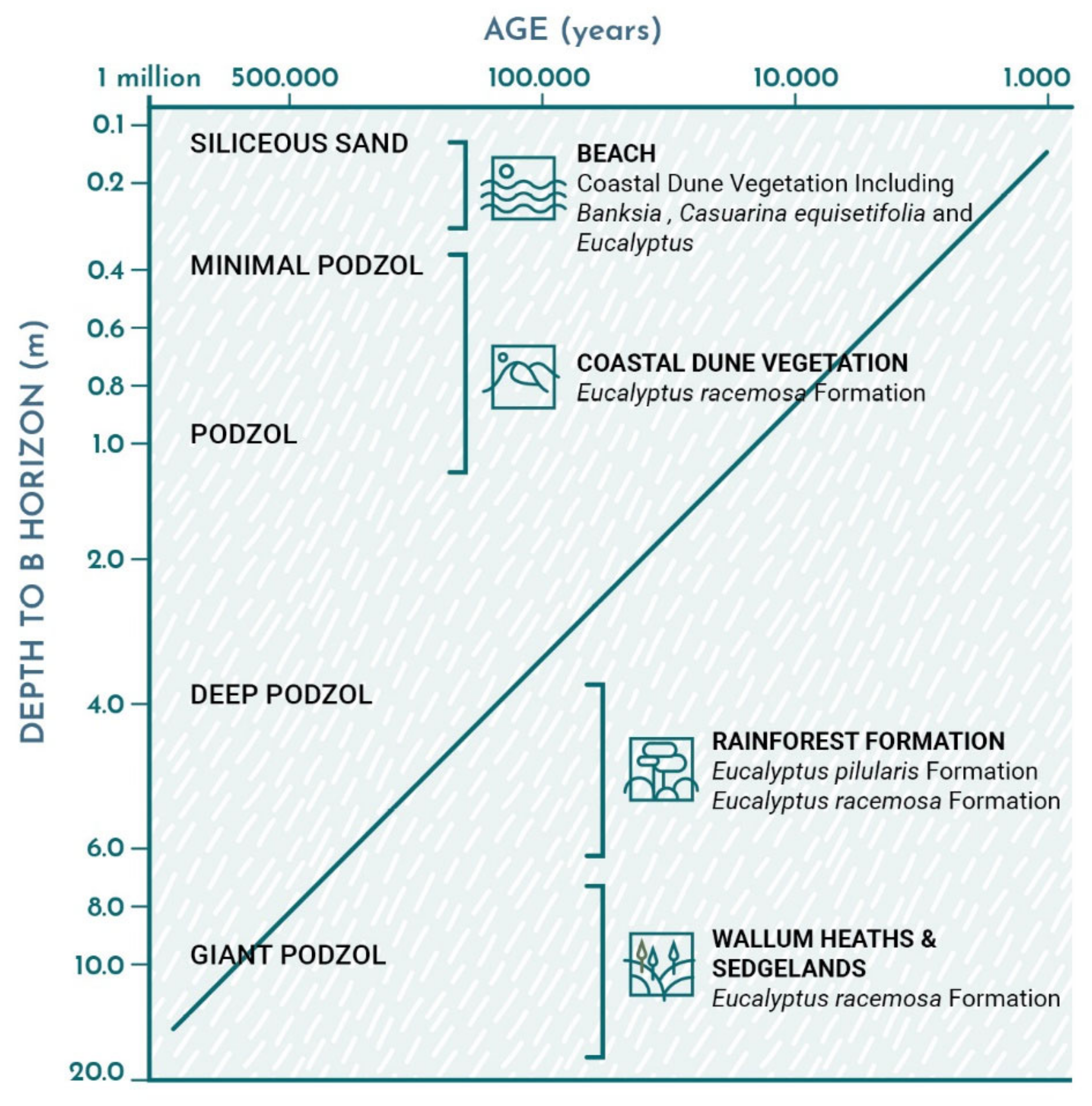
The process of soil formation is also unique. As a result of the successive overlaying of dune systems, a chrono sequence of podzol development from the younger dune systems on the east to the oldest systems on the west change from being less than 0.5 m-thick to being more than 25 m-thick, which far exceeds known depths of podzols anywhere else in the world and has a direct influence on plant succession [
4].
Despite a common sandy parent material, a variety of soils have developed on these dunes. These differences have strongly affected the types of vegetation communities that have subsequently arisen. The soils all have a dark-surface A1 horizon enriched with organic matter and the older soils have an eluviated A2 horizon of “bleached” white sand beneath which lies a yellow, red, brown, or black B horizon with accumulated organic bound iron and aluminium oxides. This layer overlies a C horizon of unweathered sand. A change occurs in the process of B horizon development when it reaches a water table. There can be a loss of iron and a precipitation of organic/aluminium compounds between the sand grains to form the cemented B horizon of humus podzols (
Figure 5) [
47,
48].
The relative depths of these horizons vary. The youngest dunes with the least-weathered soils simply have some darkening and enrichment of the topsoil, but older dunes have increasingly deep A2 horizons. In the older dunes, the A1 horizons are shallower than in dune systems of moderate age, but the A2 horizons can exceed 20 m depth in the older dunes. Some soils develop consolidated layers formed by iron oxides and clay-sized particles and produce hard pans. These hard pans can give rise to perched lakes [
49]. Maximum profile development occurs within dune floors near the apex since this area is subjected to less erosion than soils developing on dune arms. Sandy soils such as these are commonly viewed as being infertile, but many parts of the island support tall forests having a substantial biomass, including rainforests in lower-lying areas. By contrast, other areas are occupied by short heath vegetation with a much lower biomass. Much of this difference is due to differences in soil fertility caused by differences in soil age. All the newly deposited sands are composed of quartz with less than 2% of other minerals such as ilmenite, rutile, zircon, monazite, and K-feldspar. As such, a feature of the sand grains forming beach sands and the youngest dunes is the paucity of bioavailable nutrient sources. However, these modest initial nutrient reserves, combined with the dynamic interplay between various other sources and processes, create a dynamic environment that sustains the development of the wide range of ecosystems. Together, with nutrient addition from litterfall, rainfall (e.g., Mg, K, Ca and Na) [
50], and nitrogen fixation by species of
Acacia,
Allocasuarina,
Casuarina, and
Macrozamia, ongoing edaphic processes (including podsolization) also contribute to soil fertility. Additionally, the mobilisation of phosphorus is facilitated by mycorrhiza including both endo- and ecto-mycorrhizal forms [
51]. These are found even in the youngest dunes and are associated with various colonising plants. The extensive hyphal growth helps aggregate sand grains around the roots of these plants and transfer phosphorus from sand grains into plant biomass and the biogeochemical cycle. Mycorrhiza are found on other plants across the other dunes, and Kurtböke et al. [
52] found species of
Glomus, Gigaspora, and
Acaulospora, common in the plants they studied.
While the types of vegetation communities are heavily influenced by soil changes, the distribution of different communities is also influenced by topography since water (and nutrients) tends to move away from dune crests and accumulate in dune floors or basins. This means that dune crests are commonly occupied by sclerophyll vegetation, but dune floors can be occupied by rainforests.
The youngest dunes are colonised by a variety of woody plants including trees and shrubs. As litter is shed and more nutrients enter the biogeochemical cycle, progressive changes occur in plant biomass and species composition. For example, forests on young dunes are generally <20 m-tall, but in older dunes forests, tree heights exceeding 45 m can be found.
Striking differences in the composition and structure of vegetation communities sometimes occur over very small distances largely due to differences in the ages of the sand dunes from which these soils are derived.
4. Vegetation
Criterion ix of the World heritage listing states that:
Vegetation associations and succession represented on Fraser Island display an unusual level of complexity, with major changes in floristic and structural composition occurring over very short distances [
4].
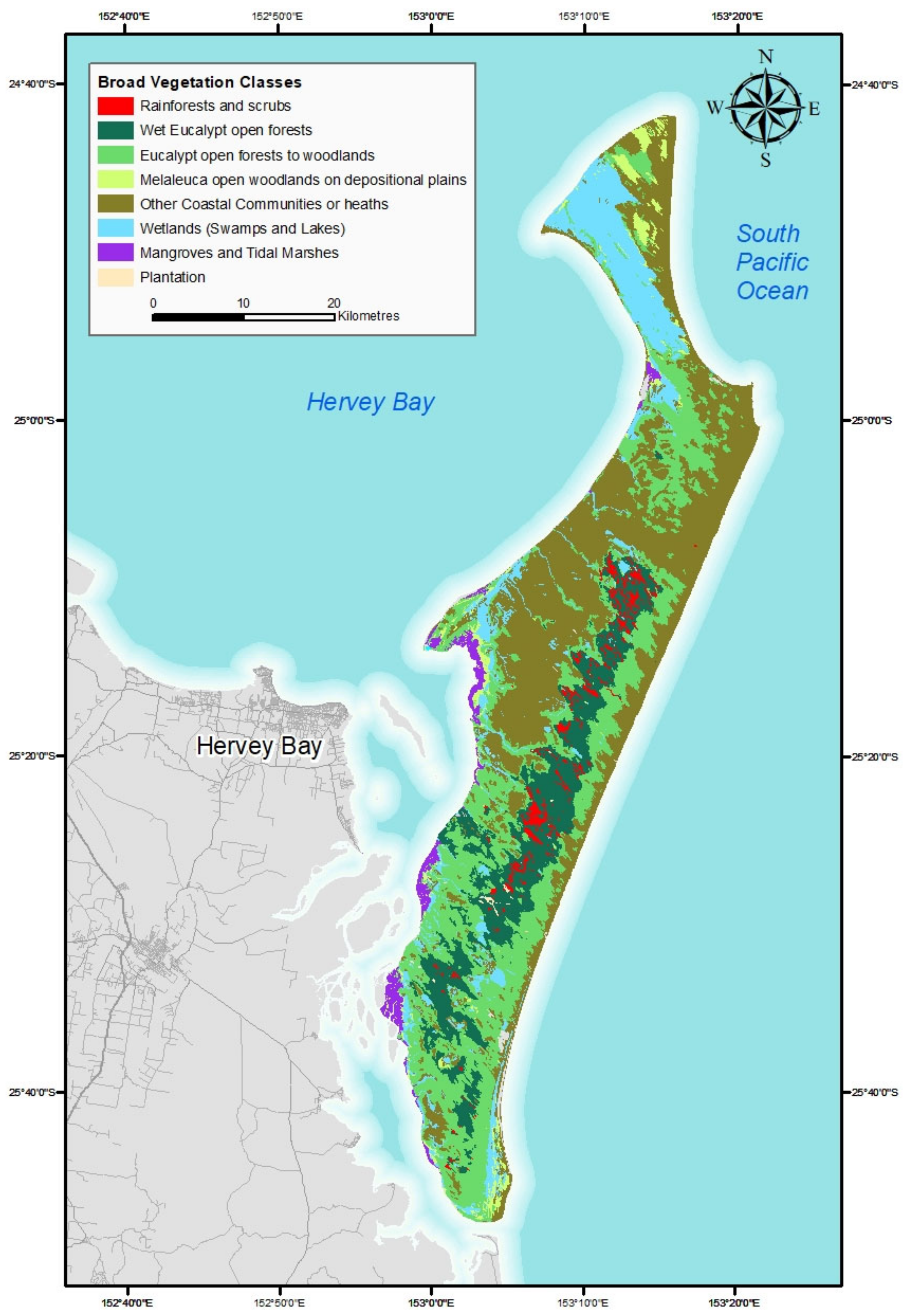
The known vegetation comprises over 830 vascular plants and 57 pteridophytes covering 407 genera in 173 families, which are found in 15 broad structural vegetation types on 5 physiographic units [
53] and 16 broad vegetation groups [
54]. The Proteaceae and Myrtaceae are the dominant trees and shrubs and are represented by 47 species. The structure and floristic composition on a large portion of this sand mass is relatively diverse and broadly similar at roughly equal distances from the sea on either side of the island in a north–south direction. The diversity of vegetation is significant as it serves as a repository for biological endemism, and as a refuge and breeding ground for a diverse range of fauna. Alongside the wide variety of ecosystems, the presence of stands of the vulnerable Goodwood Gum (
Eucalyptus hallii [
55]) and the endangered Pineapple Zamia (
Macrozamia pauli-guilielmi [
55]) highlight the significance of the island’s floral biota. Other endangered taxa such as the Common Swamp Orchid (
Phaius australis [
55]) and Christmas Bells (
Blandfordia grandiflora [
56]) contribute to superlative seasonal wildflower displays, with the latter only flowering immediately following fire and hence under increasing threat from inappropriate fire regimes.
Although dune age and soil fertility affect vegetation development, it is useful to consider vegetation patterns in relation to five main physiographic units; strand, fore dunes, hind dunes, high dunes, and littoral flats [
53]. While a range of vegetation patterns exist over short distances, often with defined narrow transitional boundaries, a broad pattern of vegetation types based on structural attributes from the east coast to the west coast follows these physiographic units [
6,
53].
The vegetation forms broad patterns that are largely influenced by the physiography, the depth to the water table, soil age (depth to, and extent of B Horizon,
Figure 6), topography, proximity to the ocean, underlying geology, geomorphology, and fire regimes. Damage to the dunes and associated ecosystems has occurred through tourism, logging, and sand mining, and has also occurred as a result of the intensity or frequency of events such as cyclones and fires (both anthropogenic and natural). The combination of these factors contributes to the type and extent of the resultant vegetation either directly or indirectly. Contemporary management practices reflect an understanding of these factors in terms of the use of fire or management of fire, forest harvesting, and sand mining rehabilitation.
Vegetation in the eastern areas facing the Pacific Ocean is defined as a strand community, which is characterised by open grassland with sedges and herbs and comprises colonising species such as
Carex spp.,
Spinifex sericeus, and
Centella asiatica. As the elevation increases to 20 m a.s.l., the vegetation changes to scrubland, low woodlands, or low open forest on the fore dunes, with a range of species including
Casuarina equisetifolia var.
incana, Banksia integrifolia, and
Corymbia tessellaris. The fore dunes give way to the hind dunes further inland, which are covered in woodland and open forest formations comprising sclerophyll dominant trees such as
Eucalyptus racemosa,
Corymbia intermedia, and
Callitris columellaris. In the lower stratum members of the Epacridaceae and Proteaceae families persist along with herbs and grasses. Further inland, the high dunes form the backbone of the island rising to a maximum height of 277 m a.s.l. (
Figure 6 and
Figure 7). The dominant vegetation consists of tall open forests on the ridges and slopes with
Eucalyptus pilularis on the more exposed dunes covering 10% of the island. These
E. pilularis dominated forests accumulate biomass at a high rate (nett primary production) in their sapling growth stage, less in the pole stage, and even less in the mature-/over-mature-stage forest with a total biomass (above and below ground expressed as oven dry weight) of 413 t/ha, 639 t/ha, and 1996 t/ha, respectively [
53]. The biomass data from the mature forest after 140 years of logging provide some indication of the potential productivity of these large Myrtaceae-dominated forests growing on siliceous sands. Myrtaceae generally require soil disturbance to regenerate from seed, which they have in huge quantities. Extensive stands of young eucalypts, for example,
E. pilularis and
E. microcorys, have followed logging and burning in the past, thus promoting regeneration. Historically, fire and disturbance from logging have been used to promote regeneration and growth of eucalypts on K’gari.
Today, many remaining forests dominated by
Lophostemon confertus and
Syncarpia hillii are in gullies and in moister parts of the high dunes where fire has not been able to penetrate. They contain little regeneration and exhibit rainforest elements in the understory [
57]. There are also open heaths and woodlands on the flatter areas and closed forests in the protected gullies. In lakes containing permanent freshwater, aquatic species are found on the surrounding terrestrial edges and below the lake surface. There are also vegetation associations that form on flat lands and low rises known as “wallum country”. Wallum was the name given to some species of
Banksia by the First Nations Peoples of southeastern Queensland and the term wallum country is commonly used for coastal plains where these species exist [
5]. The wallum formations vary from (closed) sedgeland (structural classification of vegetation types follows Specht (1970)) (dominated by the Restionaceae) to (closed) open heath (mixtures of families represented) on the lowest lying ground, to open scrub or low open forest of
Banksia spp. and/or
Eucalyptus spp. on the low rises.
The littoral flats on the west coast are associated with the oldest dunes and consist of formations of herblands, sedgelands, woodland, and open forest. There are eight species of mangroves in the intertidal zone, with Casuarina glauca dominating the transition zone between the salt marshes and the dunes.
The peat swamps on K’gari are classed as fens which are normally found in much cooler climates. UNESCO noted:
Since listing, patterned fens have been discovered on the property, which along with those at Cooloola, are the only known examples of subtropical patterned fens in the world. These fens support an unusual number of rare and threatened invertebrates and vertebrates [
4].
The patterned fens developed over 6000 years ago and are amongst the oldest in the world [
58]. They occur close to sea level and merge on the western side of K’gari with mangrove forests. The fens are peat-forming wetlands that obtain nutrients through drainage from upland mineral soils and from groundwater movement from the dunes above. They are able to support diverse animal and plant communities such as grasses, sedges, rushes, and wildflowers [
59]. The patterned fens form an intricate maze of pools (and strings) which are peat ridges of different thicknesses, rather than patterns within the vegetation community with which they are associated. They form at the base of high dunes where there is a constant and high rate of flow of surface freshwater [
47]. Interestingly, the K’gari fens are not cited in the World Heritage listing because, at the time, the fens had not been identified.
The complex and dynamic biota present on the island are a result of a delicate balance between dune-building events, edaphic processes (podsolization), and other biological, geochemical, geomorphic, and hydrological influences over a relatively long time period. However, the persistence of these geological/geomorphic phenomena and associated ecosystems have been compromised by historical disturbance (logging, sand mining). Some of these ecosystems are currently at threat from inappropriate fire regimes and pressures from human activity such as increased erosion and soil compaction (linked to significant vehicle activity and overuse of some sites including sand blows), alongside the incursion of weed species, the altered hydrology from water extraction for human use, and the contamination of ground water from human activity. Ongoing threats to the biota associated with these unique dune systems and soil profiles include Myrtle Rust (which has the capacity to form a significant threat to many of the island’s ecosystems dominated by Myrtaceous taxa) as well as more fundamental threats to geomorphic processes, such as the influence of anthropogenic climate change.
5. Impacts of Logging and Fires
The Myrtaceae-dominated forests of the high dunes support extensive stands of large and tall individuals of
Eucalyptus pilularis, Eucalyptus microcorys (few locations),
Syncarpia hillii, and
Lophostemon confertus. Based on results of macro-charcoal work by [
60], these forests could have regenerated as a result of fire hundreds, if not thousands, of years ago. Ref. [
61] found that fire regimes have fluctuated over the past 24,000 years but are characterised as having low frequency and low return intervals. Longmore [
62] found that, between 600 and 350 years ago, the vegetation on many parts of K’gari was predominantly a closed forest with members of the
Araucariaceae family as emergents and
Podocarpus elatus, and far less Myrtaceous vegetation than that seen today. Thus, the vegetation has undergone successional change influenced by changing climate and fire regimes either as natural events or from anthropogenic causes, including cultural burning practices [
63].
In the recent past (100 years ago), some of the higher dunes were dominated by
Eucalyptus microcorys, the preferred hardwood species by the early saw-millers over
Eucalyptus pilularis. Over time,
E. microcorys has been reduced in number in the older forests and replaced by
E. pilularis and other Myrtaceae members. These species have responded more positively to the possible increase in fire frequency between 800–500 years ago [
61] and the harvesting of
E. microcorys in recent times. Given that
E. pilularis depends on soil disturbance or fire to regenerate, in the absence of such events, the
E. pilularis-dominated forests, which cover 17,000 ha, are likely to change in species composition [
64].
Fire is another contested area of management. Wildfires and intentional fires have shaped the Australian landscape and ecosystem composition for thousands of years. As a result, the existing habitat on K’gari has not only been affected by selective logging of mature, seed-bearing trees, which has opened the canopy for recruiting species, but also by fire. Prescribed low-intensity burns have long been used in mainland Australia to manage fuel load to reduce the likelihood of the damaging, hot wildfires that pose higher risk to property and habitat. Such burns are also used to encourage vegetation regeneration. While some native species are fire-sensitive, others need fire to regenerate, and others are stimulated by the nutrients released by fire (grass trees) or their hard cases release seeds after fire (Banksia spp. and Hakea spp.), but in many cases these are not the only methods of regeneration. The intention has often been that, since fire cannot be completely eradicated, it has to be managed effectively.
Tran and Wild [
65] point out that there has been little scientifically rigorous examination of fire and its effects in southeast Queensland and the majority of work relates to recovery of vegetation species post-fire, rather than a range of indicator species representative of ecosystem health, such as marsupials, avifauna, herpetofauna, and invertebrates. Most of the available work has concentrated on a single fire rather than the effects of return fires, particularly on species diversity. In addition, fire regimes for vegetation types are based on minimum fire intervals to maintain biodiversity, rather than maximum desirable fire regimes to produce mature forests. For example, melaleuca forest should burn no more frequently than 15-year intervals, subtropical rainforest should not burn at all, and wet sclerophyll closed forest should not burn at more than 200-year intervals, in some cases. They note that these are the
minimum fire frequencies to maintain biodiversity, not the suggested fire frequencies, as the frequent misinterpretation [
65].
On K’gari, while fires occur in the
E. pilularis forests, they seldom penetrate into the
Syncarpia hillii- and
Lophostemon confertus-dominated forests. This phenomenon is evidenced by the sharp fire transition boundary and changes in the understory vegetation and ground fuel. These areas are becoming dominated by rainforest elements such as
Syzygium spp. [
57] which regenerate in the absence of fire. Therefore, without fire, a more diverse rainforest community may dominate. Other species that have been logged and might be encouraged to return are
Araucaria cunninghamii—hoop pine. However, studies have shown that forest refugia and postfire regeneration of Araucaria are vulnerable to recurrent fire and fire severity [
66] and vegetation recovery in burned areas have exhibited reduced species richness and diversity [
67]. Thus, management plans need to determine the types of ecosystems which are desired for the future and manage fire accordingly.
Australia’s First Nations Peoples (including the Butchulla) recommend that active fire management should integrate patch–mosaic-style, highly controlled burns in order to preserve dynamic biodiversity and ecosystem complexity over time [
68,
69].
While factors affecting fire behaviour are fairly well understood and prescribed burns are usually well-planned, weather can be unpredictable and prescribed burns may become uncontrollable with unintended effects. The balance of protecting infrastructure and human safety, as well as the hotter more unpredictable climate in the future, may make prescribed burning more precarious, which means that the implementation of fire regimes that aim to conserve and promote biodiversity at a landscape scale are a major challenge. Previous research has highlighted that appropriate fire regimes aimed at balancing biodiversity conservation management will need to incorporate previously underutilised methods of fuel estimation such as LiDAR, alongside further integration of spatial datasets to facilitate efficient utilisation of resources related to fire management [
70].
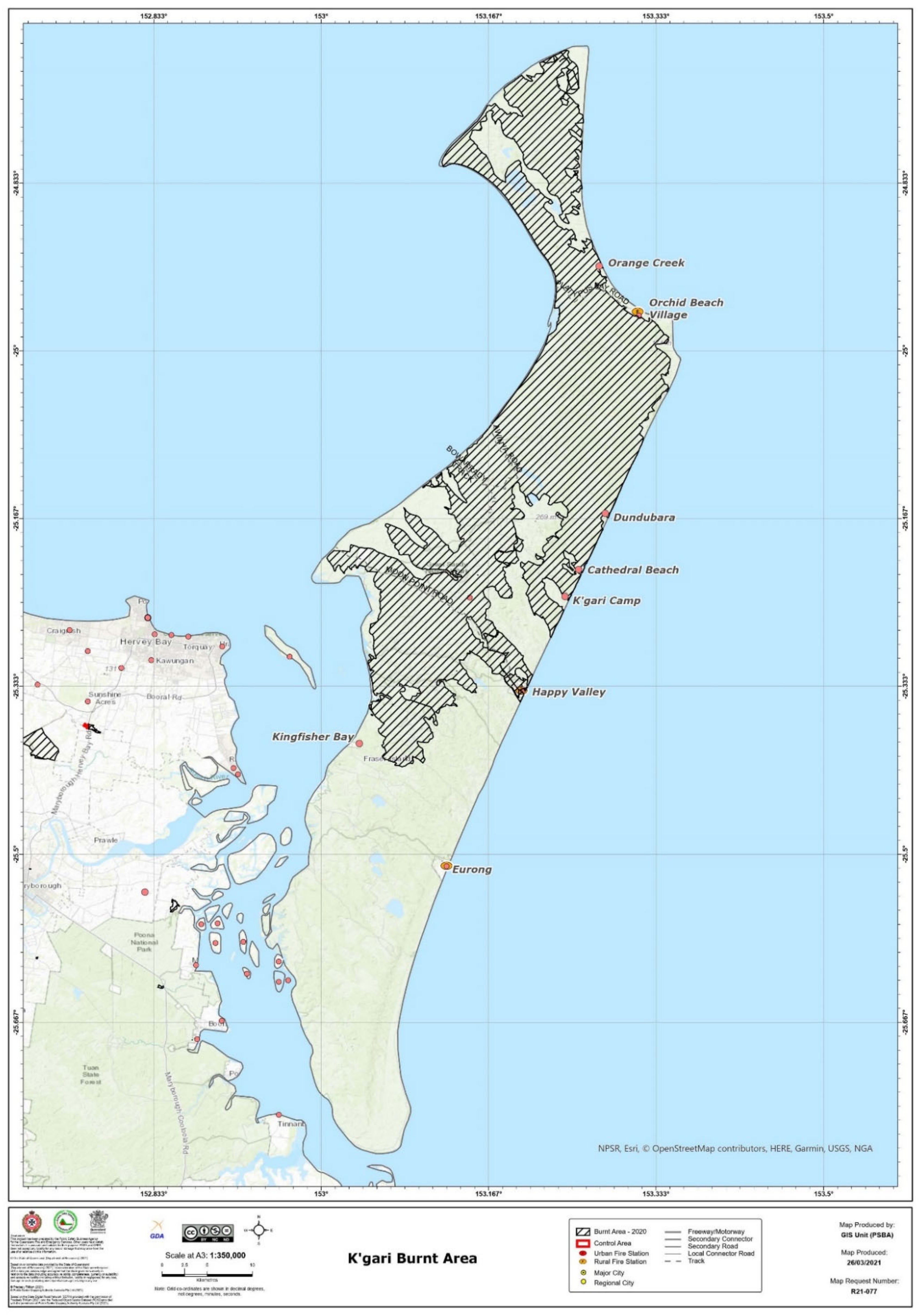
At the end of 2020, a major wildfire burnt for over seven weeks on K’gari, impacting more than half the island (approximately 80,000 ha) (
Figure 8). This fire followed on from a 2019 fire that burnt in the southern part of the island. The result of the 2020 fire was that only half of the island provided habitat and food sources for the entire island’s wildlife, and the remaining habitat did not necessarily duplicate the impacted ecosystems. At the time of writing, comprehensive research has not been undertaken; however, we speculate that certain species will flourish, and others will struggle in the post-fire environment and, due to the fire’s widespread nature, it is possible that the composition of the vegetation will change. This is not the first fire that the island has experienced (nor will it be the last) and past fires have influenced the vegetation which we see today—every fire (based on intensity and speed and fuel loads) is likely to influence the vegetation in addition to faunal recolonisation dynamics. Weeds, for example, may flourish on some of the more barren areas. Certain native species can also regenerate prolifically and dominate in disturbed areas, as evidenced by the dominance of immature
Acacia and
Casuarina species in post-mining areas. Concerns over the use of water extracted from creeks and rivers on the mainland sources for firefighting could, potentially, result in biosecurity issues. Saltwater dumping on burning coastal vegetation, similarly, may also impact these ecosystems and determine rates of recovery (although, the heavy rain which extinguished the fires would have led to some leeching of the water from the soil and washed off the leaves, thus reducing “salt burn”). It is important to note that water from the mainland was taken from town water sources and the buckets used to transport the water were sterilised. Saltwater was mixed with town water and saltwater only was mainly used in salt-exposed coastal areas. Concerns have also been raised about the impact of such an extensive fire on culturally sensitive sites and cultural artefacts.
The K’gari bushfire review conducted by the Office of the Inspector-General Emergency Management, Queensland, assessed the following: (1) the effectiveness of preparedness activities; and (2) the response to the bushfire event by entities responsible for the management of the island and bushfire and disaster management in Queensland. The review was conducted in the context of the cultural and environmental significance of K’gari, as reflected in relevant management plans, and its UNESCO World Heritage listing. The findings of the review and the Queensland government’s response have now been published [
71]. The review makes a number of recommendations for improvements in the management of K’gari, including improved collaboration between the Butchulla People, the Queensland Fire and Emergency Services (QFES), QPWS, and other relevant stakeholders.
6. Freshwater Ecosystems and Threats
Freshwater on the island is integral to the long history of occupation by the Butchulla People and later European colonizers. In granting World Heritage status, UNESCO noted:
Fraser Island also has a variety of freshwater dune lakes which are exceptional in terms of number, diversity, and age. The dynamic interrelationship between the coastal dune sand mass, aquifer hydrology, and freshwater dune lakes provides a sequence of lake formation both spatially and temporally [
4].
There are more than 40 named perched dune lakes on K’gari [
72] which are mostly found in the older dunes along with evidence of many former lakes. Apart from one “barrage” lake, there are two main lake types, “window” lakes (formed as a depression into the regional water table) and “perched” lakes (situated above the regional water table with water percolation retarded by organic-rich indurated sands (humicrete—known locally as “coffee rock”) that play a key role in regulating hydrology. Perched lakes that are not connected to the regional aquifer are vulnerable to the vagaries of local rainfall patterns and their water levels can vary widely over time. Other freshwater systems originate from long, slow filtration via regional aquifers, and are relatively resistant to short-term hydrologic variability. Coastal streams, palustrine wetlands, and Ramsar-listed patterned fens add to the diversity of freshwater ecosystems [
47].
The water of dune lakes is generally characterised by low pH (<6), very low salinity, and extremely low levels of dissolved solids, suspended solids, and nutrients [
73]. Dune lakes can be clear and colourless, tea-coloured, or even opaque, depending on the concentration of terrestrially derived dissolved organic matter and tannic acids. These acidic environs can be loosely classified as “stained” or “clear”, depending on the filtration potential characteristics of the dominant surrounding sedimentary substrate. Younger (Holocene) sands (in terms of time of deposition) are more effective at filtering tannic compounds and other acids sourced from dominant vegetation types on the island, due to their still-intact sesquioxide coatings; hence, they yield clear-water systems. In contrast, “older” (Pleistocene) sands which have had their sesquioxide coatings stripped, and exist as base silica quartz sands, are less effective at filtering these tannic compounds, resulting in “stained” systems.
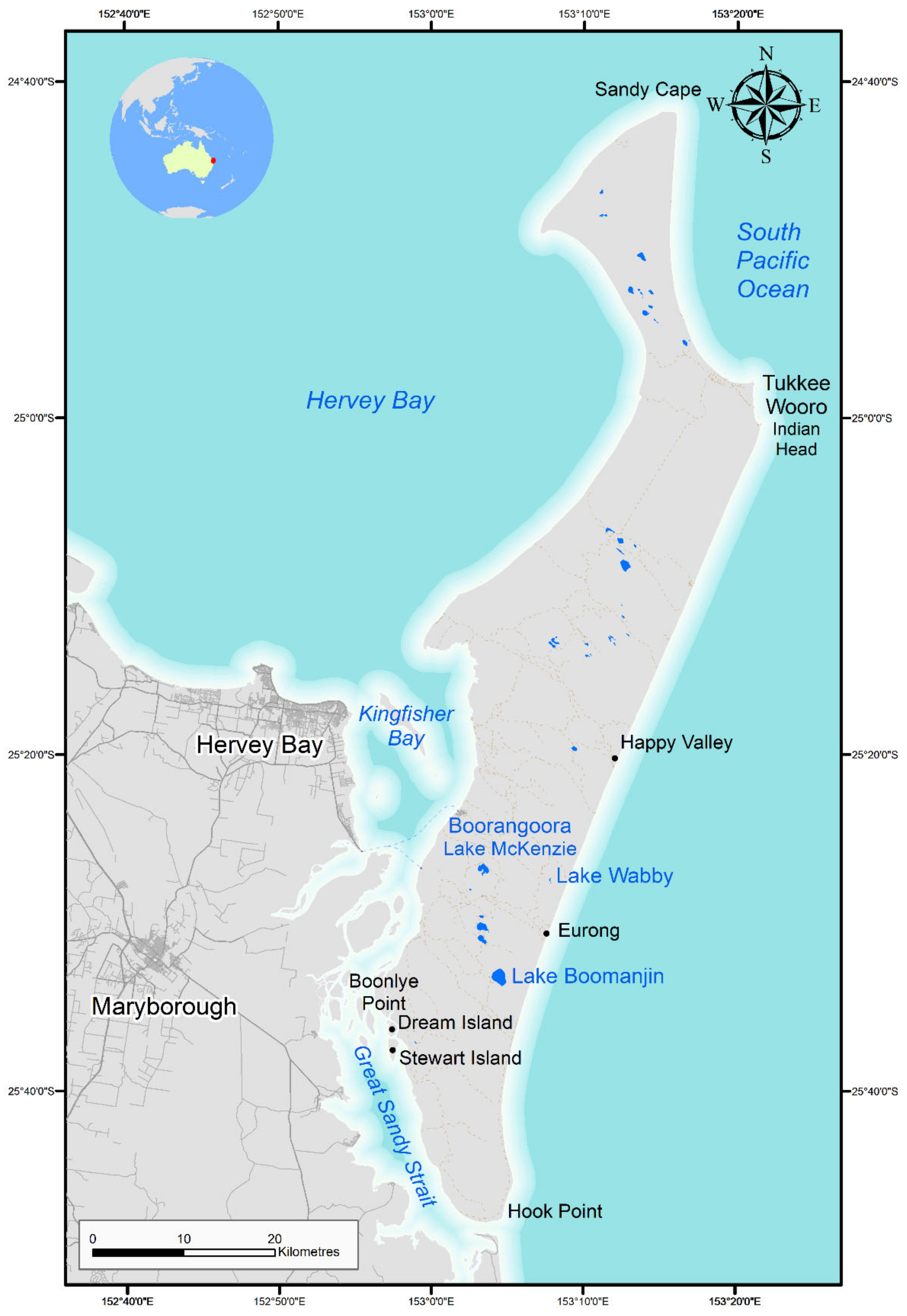
Other systems, such as Lake Wabby (see
Figure 1) and Little Wabby, a barrage lake system that is gradually being infilled via natural sedimentary processes, provide a further layer of habitat complexity. A survey of benthic invertebrates (and vertebrate fish species) in the lake systems indicated far greater diversity in Lake Wabby when compared with the “closed systems” of the island’s perched dune lakes [
74].
Dune lakes are considered to be biologically unproductive. They have low concentrations of essential plant nutrients (nitrogen and phosphorus), are high in dissolved oxygen, and support relatively low floral and faunal biomass [
75]. The island’s lakes (and streams) contain highly specialised, endemic Cyanobacteria—blue-green algae [
76]. The animal plankton of dune lakes is dominated by the copepod
Calamoecia tasmanica, accompanied by small numbers of other copepods, Cladocera, dipteran larvae, and a water mite [
74]. Early studies recorded 84 littoral invertebrate taxa from 29 lakes in the central third of the island between Lake Boomanjin (
Figure 1) in the south and the lesser-known White Lake in the north [
73,
74]. The discovery of specialised Cyanobacteria, a primitive aquatic annelid, three new chironomid species, two new species of Odonata (dragonflies and damselflies), and five new species of Trichoptera (caddisflies) from the dune lakes of southeastern Queensland attests to their unique features [
73,
74,
77,
78].
Pressures from human activities, particularly those associated with tourism, have already left their imprint on lake water quality, biota, and ecosystem processes. Visitors can, potentially, influence the ecology of K’gari’s lake ecosystems either through direct physical disturbance to sediments and vegetation or via the addition of nutrients and other chemicals such as sunscreen into the water column [
79,
80]. Given their high-water residence times and low nutrient concentrations, the perched dune lakes are particularly susceptible to visitor impacts [
81]. Evidence from observational studies suggests that many of the lakes have higher algal biomass than they did in the early 1990s and the rapid growth in visitation since World Heritage status was granted has undoubtedly increased the pressure on these environments [
79]. Some lakes have been closed to swimming for this reason. Experimental investigations have further demonstrated how human inputs of nutrients can stimulate algal growth [
75] and drive the food webs of perched lakes away from a detritus-based system towards one that is dominated by the algal pathway [
82] The long-term implications of this shift are largely unknown, but there are growing concerns surrounding the increased algal biomass in high-use sites as well as the effects on the local ecosystems.
Beach camping is another growing environmental management concern affecting the water quality and ecological balance of dune and beach systems. The issue here is the retention of nutrients with implications for nitrogen-loving weeds and phosphorous-sensitive native taxa [
1], as well as effects on human health.
Climate change represents a threat to the ecosystems of the island. Perched dune lakes are not connected to the regional aquifer and water levels are known to fluctuate widely with local rainfall patterns. Indeed, K’gari is considered to be one of the few regions in Australia where annual precipitation exceeds annual evaporation by about 20% [
72] and this pattern is critical to the existence and longevity of the perched dune lakes.
About 80% of the annual rainfall in Queensland occurs during November–April primarily by low-pressure systems associated with the monsoonal trough and the southeasterly trade winds. Total annual mean rainfall for the island is 1267 mm [
83]. Year-to-year rainfall is highly variable and linked closely with changes in equatorial Pacific Ocean climate and the El Niño Southern Oscillation, compounded by decadal variability.
Current projections for rainfall suggest wetter summers and significantly drier autumns, winters, and springs [
72]. These changes in rainfall patterns, particularly the extended dry spells, are likely to threaten the persistence of many of the shallow perched dune lakes. Furthermore, reduced rainfall means that perched dune lakes may also experience synergistic interactions between changing hydrology, rising water temperature, rising human-generated nutrient levels, and the carbon cycle. Hadwen and Arthington [
72] explored these interacting processes, highlighting how the sequential lowering and raising of lake water levels, as expected with the projected changes in rainfall patterns in the region, will drive pulses of lake productivity stimulated by anthropogenic eutrophication. These pulses of nutrients, which represent simultaneous additions of both current and legacy nutrient inputs from visitors, will likely lead to excessive algal production in lake littoral zones. This excessive algal biomass may have negative consequences both for the lake food web and for the visitors, who may avoid sites with high algal biomass [
72].
7. Terrestrial and Aquatic Fauna: The Importance of Habitat Diversity
K’gari’s habitats range from permanent free-water areas (lakes, streams, wetlands, and soakage areas) to poorly drained flat areas, to beach and dune sands where the water-table is permanently below the ground surface, and to mangroves, which occur in intertidal areas on the west coast and often extend upstream for some distance. In lakes of permanent free water, aquatic species are found in the surrounding terrestrial edge, the shallow littoral zone, the open water, and the substrates of lake basins.
The freshwater systems of K’gari support a number of native freshwater fish species, frogs, and freshwater turtles. However, some lakes do not support fish; instead, a predatory planktonic midge (
Chaoborus, Diptera) and a large aquatic bug (
Anisops, Hemiptera) assume the role of apex predators in the food webs of fishless lakes [
74]. Fish species of conservation concern are the vulnerable honey blue eye (
Pseudomugil mellis), the endangered Oxleyan pygmy perch (
Nannopercaoxleyana) and the restricted ornate rainbowfish (
Rhadinocentrus ornatus) [
84]. The introduced mosquito fish (
Gambusia holbrooki) is a threat to these native fishes [
85]. Four species of “acid frogs” adapted to the unusual water quality of dune lakes and wetlands, in particular their low pH [
86]; these include the wallum froglet (
Crinia tinnula), the Cooloola tree frog (
Litoria cooloolensis), the wallum Freycinet’s frog (
Litoria freycineti), and Olongburra frog (
Litoria olongburensis).
The high degree of terrestrial habitat diversity, in turn, supports a rich faunal diversity which is of significance given that siliciclastic substrates typically yield “low-energy” systems, incapable of supporting highly biodiverse ecosystems. Mammals are well represented with 46 species recorded on the Queensland Government’s WildNet Database [
87], including 13 bat species. Smaller bodied marsupials are also relatively common with 22 taxa recorded [
87] including four glider species and sightings of the vulnerable long-nosed potoroo (
Potorous tridactylus tridactylus) [
55]. The majority of terrestrial ecosystems depend on the occurrence of fire for regeneration, giving rise to niche habitat specialists such as the eastern chestnut mouse (
Pseudomys gracilicaudatus), populations of which exhibit rapid growth in early post-fire successional conditions in wallum heathlands [
88]. Although the swamp wallaby (
Wallabia bicolor) is relatively common, other large macropods are scarce, with a low abundance of eastern grey kangaroos (
Macropus giganteus). Koalas (
Phascolarctos cinereus) are notable in their absence despite anecdotal evidence suggesting their presence prior to 1900, and despite their presence on geographically proximate, sand barrier islands such as Stradbroke Island [
89].
The infamous K’gari wild dingo population (called wongari by the Butchulla People) (
Figure 9) presents a fascinating fauna management case study, partly due to the fact that the island is one of the few places where they are legislatively protected (as a native species within a National Park) and relatively free from hybridisation with domestic dogs. The Australian dingo is a declared pest species under many jurisdictions on mainland Australia and listed as vulnerable (IUCN Red List) due to culling activities and the loss of genetic integrity caused by hybridisation with domestic dogs. The evolutionary history and taxonomy of the dingo remains under debate. Contemporary taxonomic arguments exist for both
Canis dingo and
C. familiaris and although considerable debate also remains regarding the timing of their arrival to Australia, we adopt
C. dingo in this paper due to the unique evolutionary history of the dingo [
90,
91,
92,
93]. The fact that this island-bound population of a highly politicised and contested taxon is relatively small (~70–200 individuals) [
94,
95], is low in genetic diversity, and has become genetically distinct from their mainland counterparts, represents a conservation concern and also adds to their intrigue [
95].
The island offers a rare opportunity for people to observe and encounter wildlife, including dingoes. Although most dingo encounters are benign, occasionally negative and high-risk encounters of aggression towards humans cause injury and, on one occasion, death [
97]. Dingoes identified as posing unacceptable risk are euthanised. Managing the complex issues surrounding human interactions with dingoes on the island is the responsibility of the State Government through the Fraser Island Dingo Conservation and Risk Management Strategy [
98]. Measures have been implemented to mitigate risk, including fencing of residential townships and inland camping areas, interpretation and education, and strict regulations against feeding [
99,
100,
101,
102]. Dingo management is highly controversial and well-publicised, with often-opposing views on the causes of high-risk interactions between dingoes and humans. Clearly, the aim is to mitigate risk to both humans and the dingo [
99,
102]; although, the topic is contentious given the differing expectations and knowledge of wildlife behaviour.
Dingoes are the island’s apex terrestrial predator able to live up to thirteen years [
103]. Whilst they are extremely opportunistic omnivores, that have evolved to hunt prey independently, the most common prey item detected in scats are “high-turnover” medium-sized marsupials with short gestation times, such as the northern brown bandicoot (
Isoodon macrourus) [
104]. Marine strandings are a substantial food source for the dingo, which is particularly sensitive to changes in food availability [
105], as has been reported elsewhere for small island populations of other canids, such as the grey wolf [
106,
107]. Marine strandings are common particularly as the island is located along the seasonal migration routes of humpback whales (
Megaptera novaeangliae) and marine turtles (
Cheloniidae). Many become stranded along the eastern beach and western estuaries becoming an important food source for the dingo population [
105]. Dietary studies suggest that marine subsidisation can directly influence terrestrial carnivore diet, behaviour, and abundance, which may produce indirect effects such as higher rates of depredation and suppression of other native fauna, if (as expected) strandings become more common [
105]. Adaptive hunting techniques for innovative prey handling by dingos have also been observed: a single dingo used the waves on the eastern beach of K’gari to entrap, tire, and deliberately drown an adult swamp wallaby, in one case, as well as an adult short-beaked echidna
Tachyglossus aculeatus in another [
108,
109].
In terms of terrestrial vertebrate richness, the avifauna forms the largest proportional contribution, with 384 recorded species [
110]. This equates to more than half of Australia’s 750 known bird species [
111] and includes taxa such as eastern ground parrots (
Pezoporus wallicus), glossy black-cockatoos (
Calyptorhynchus lathami lathami), and black-breasted button-quails (
Turnix melanogaster). Conversely, some taxa that are ubiquitous, even within highly modified habitats in mainland regions, such as the Australian brush-turkey (
Alectura lathami), are relatively infrequent on the island. The southwestern side of the island adjoins a Ramsar-listed wetland, incorporating the Great Sandy Strait (Korrawinga) (
Figure 2). This region is a hotspot for trans-equatorial migratory wading birds with upwards of 36,000 individuals of species such as Eastern curlew (
Numenius madagascariensis), grey-tailed tattler (
Tringa brevipes), and western Alaskan bar-tailed godwit (
Limosa lapponica baueri) utilising the regions. Given the global decline in these migratory wader species [
112], the closure of most of the islands’ western shores to vehicular traffic is a proactive and practical conservation management measure that should continue.
Over 60 terrestrial and freshwater reptile taxa have been recorded on the island including, the endemic Satinay sand skink (
Coggeria naufragus) and the endemic Fraser Island short-necked turtle,
Emydura krefftii nigra [
113], which displays genetic sub-structuring between different dune lakes [
114] and is also morphologically distinct from mainland populations [
115]. Other freshwater turtles include the broad-shelled turtle (
Chelodina expansa) and the eastern snake-necked turtle (
Chelodina longicollis).
Given the historical disturbances, such as logging and sand mining, it is surprising (pre-wildfire) to find that a relatively intact, unfragmented landscape remains and provides protected habitat for vulnerable common death adders (Acanthophis antarcticus) and red-bellied black snakes (Pseudechis porphyriacus) which are suffering from the negative consequences of habitat destruction on mainland Australia.
Despite the discovery of invertebrate oddities such as the Cooloola monster (
Cooloola propator, Orthoptera) (
Figure 10), which is endemic to the Cooloola Sand Mass (including K’gari), very little work on invertebrate species has occurred, except in studies of freshwater systems. Preliminary surveys indicate that the island’s invertebrate biota is likely to be extremely diverse, with potentially high levels of endemism. We highlight this lack of data as a substantive research gap.
Feral species, such as the black rat (
Rattus rattus), pig (
Sus scrofa), red fox (
Vulpes vulpes), horse (
Equus caballus), and coastal brown ant (
Pheidole megacephala) are present [
117]. However, arguably, the biggest ecological threat posed by feral fauna is from the exotic cane toad (
Ranidella marina) which outcompete native amphibians and are poisonous to native predators; alongside the feral cat (
Felis catus), whose impact on smaller sized marsupials, birds, and reptiles, is likely significant, as elsewhere in Australia. Biosecurity is also a concern, particularly with respect to the risk of fungal pathogens, such as
Phytophthora, chytrid fungus (
Batrachochytrium dendrobatidis), and Myrtle Rust. The latter has the capacity to devastate large swathes of habitat due to its impacts on myrtaceous vegetation whose representatives, including but not limited to,
Eucalyptus,
Melaleuca, and
Leptospermum species, are ubiquitous and often dominant species in many habitat types on the island.
8. Ocean Beaches and the Bay
The ocean beaches are of global significance with over 250 km of clear sandy beaches with long, uninterrupted sweeps of ocean beach [
4]. The beaches are not only ecologically significant but also highly attractive for tourism. They form one of the longest, and least-modified, continuous interfaces between the land and sea [
118].
Beaches are functionally linked to coastal dunes and surf zones by the tides and by fluxes of matter (sediments and nutrients) and organisms (animals and plants) which connect food webs and species pools across the land–ocean ecotone [
119]. These connections shape the distribution and diversity of animals on beaches, resulting in assemblages that are characterized by a mix of terrestrial and marine species [
120,
121]. The beaches support a diversity of marine invertebrates, many of which are harvested for use as bait for recreational fishing (e.g., beach worms,
Australonuphis teres; surf clams (Eugarie),
Donax deltoides) [
122]. As explained earlier, beaches are used as feeding habitats by dingoes and iconic coastal raptors (e.g., white-bellied sea eagle,
Haliaeetus leucogaster), which scavenge marine carrion that is stranded by waves and tides [
104,
123]. Coastal dunes are significant roosting areas for threatened migratory seabirds (e.g., little tern,
Sternula albifrons), and are the nesting sites of vulnerable green (
Chelonia mydas) and endangered loggerhead (
Caretta caretta) turtles [
124,
125,
126]. The surf zones of ocean beaches also provide important feeding and spawning habitat for a diversity of fishes, some of which are prized by recreational anglers (e.g., tailor,
Pomatomus saltatrix) [
127,
128].
The East Australian Current intensifies in the waters offshore from K’gari and collides with the continental shelf, creating a cyclonic eddy (the Fraser Gyre) and an important upwelling zone (the Southeast Fraser Island Upwelling System) [
129,
130]. These hydrological forces combine to produce high marine biodiversity and coastal productivity, support significant fisheries, and promote ecological connectivity between Hervey Bay and the open ocean [
131,
132,
133]. The hydrology and bathymetry of this area is reasonably well known and has been described in detail elsewhere (see [
134,
135]), but the biological assemblages of this area have not been widely studied. Deep-water algal reefs which are dominated by coralline algae from the genera
Phymatolithon,
Lithothamnion, and
Sporolithon grow between depths of 50 and 120 m and can cover 40–50% of the sea floor [
136,
137]. There is, however, no other available data that can be used to describe the biology or ecology of marine species in the waters offshore of K’gari; data that are also critical to understand the exposure of marine species to climate extremes such as marine heat waves [
138].
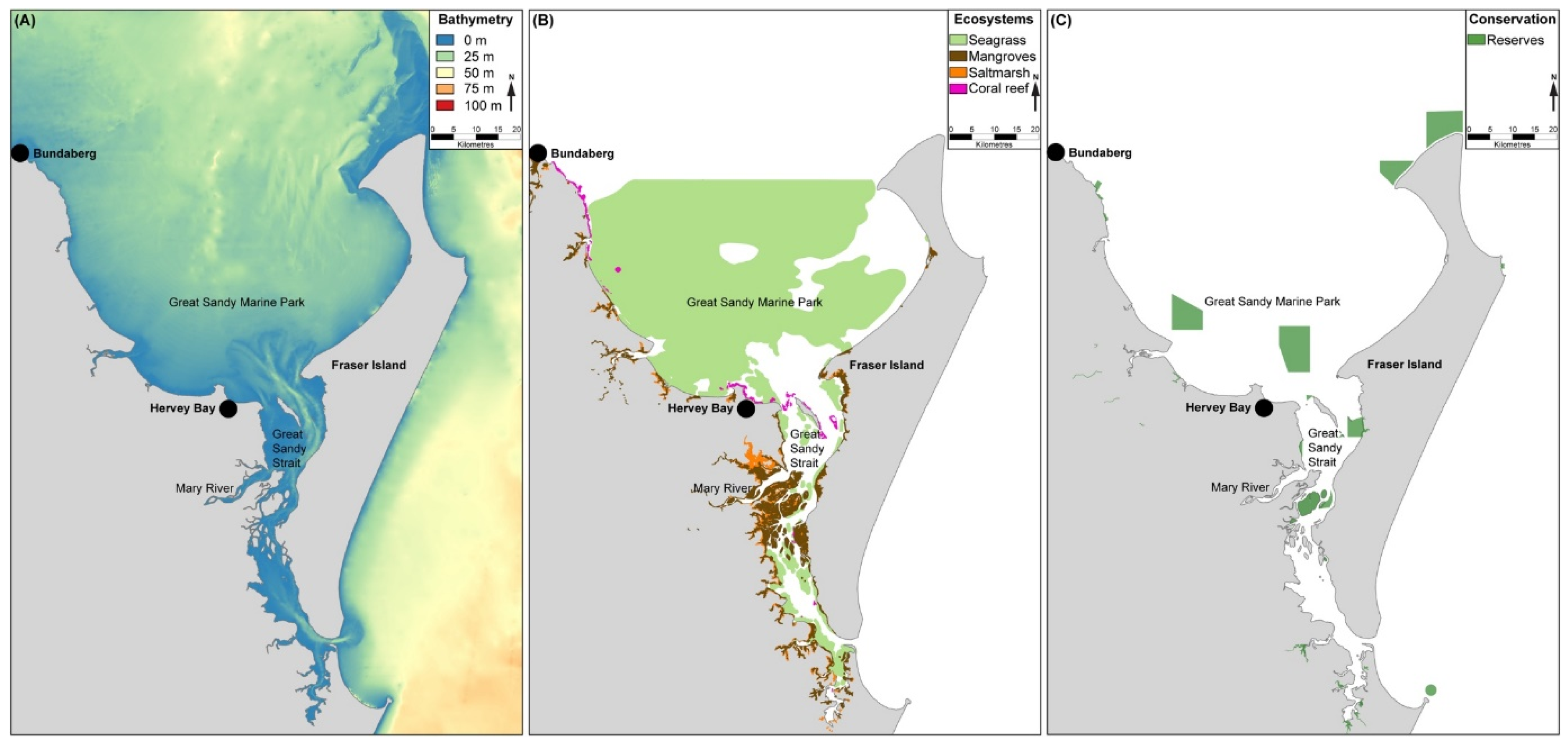
The marine environments of Hervey Bay and the Great Sandy Strait (
Figure 11 and
Figure 12) support a heterogeneous mosaic of ecosystems including saltmarshes, mangrove forests, intertidal flats, seagrass meadows, coral, siliquariid mollusc and rocky reefs, sponge gardens, subtidal soft sediments, and sandy beaches [
54,
134,
139,
140] (
Figure 11B). Marine ecosystems are functionally linked across this coastal seascape by the movement of water, matter, and organisms [
119,
135,
141]. These connections are modified by hydrology, bathymetry, and the spatial arrangement of habitats (
Figure 11A,B), and shape the distribution of organisms, functioning of ecosystems, fisheries’ catches, and the outcomes of conservation [
142,
143].
This region provides critical feeding, breeding and resting habitats for numerous iconic animals and many species that are of international significance for conservation. Hervey Bay is a prominent sanctuary for humpback whales and their major resting area on southern migrations between winter breeding areas in the Great Barrier Reef lagoon and summer feeding areas in Antarctica [
144,
145,
146]. The Great Sandy Strait is a Ramsar wetland of international importance and a significant feeding and roosting site for many endangered shorebird species [
147]. It provides habitat for migratory shorebirds each year and supports, for example, a globally significant population of endangered eastern curlew [
148]. The marine ecosystems of Hervey Bay and the Great Sandy Strait also provide feeding and breeding habitats for vulnerable dugong (
Dugong dugon), vulnerable green and endangered loggerhead turtles, and critically endangered grey nurse shark (
Carcharias taurus) [
125,
149,
150,
151].
The coastal waters of Hervey Bay are widely known as prime sites for visitors who engage in various activities including fishing, and the Great Sandy Strait supports significant fisheries for crabs and finfish [
152,
153]. Despite the social and economic importance of marine ecosystems in this region, few studies have evaluated the impacts of fishing, tourism, or coastal development. Fishing has changed the abundance and diversity of fish assemblages globally [
154], but there are no data that can be used to test for potential impacts from fishing in Hervey Bay [
142]. Interactions with boats alter the behaviour of whales in Hervey Bay [
155] and collisions with boats are a common cause of mortality for sea turtles [
149,
156]. Pollutants, nutrients, and sediments are delivered to Hervey Bay via the Mary River which discharges into the Great Sandy Strait opposite K’gari (
Figure 2 and
Figure 11B). High concentrations of pesticides and herbicides have been reported from seagrasses and sediments in the Great Sandy Strait [
157]. Hervey Bay also receives large nutrient and sediment loads during flood events which have impacted seagrass meadows and coral reefs across the region [
158,
159]. Major floods in 1992 and 1999 significantly reduced the area of seagrass in Hervey Bay leading to a further decline in dugong numbers [
158,
160,
161]. Similarly, extreme and repeated flooding from both the Mary and Burnett Rivers in 2011 and 2013 caused heavy coral mortality (up to 89% at some reefs) and resulted in the cumulative loss of ~56% of coral across the region [
159,
162].
The coastal waters fringing K’gari are within the Great Sandy Marine Park (GSMP) (
Figure 12), managed by Queensland government, and which contains zones with varying degrees of restrictions on fishing. The GSMP contains four no-take “Marine National Park (green) Zones” (
Figure 11C) adjacent to K’gari. In all, green zones conserve approximately 3.9% of the total GSMP area [
163].
Marine reserves are considered an effective conservation tool globally and are known to promote the density, body size, and biomass of harvested species [
143,
164]. Despite the widespread success of marine reserves, only two studies have evaluated their performance in the GSMP [
128,
142]. These studies report higher fish abundance and diversity inside Marine National Park (green) Zones and show that reserve effectiveness is shaped by the spatial properties of both sheltered (i.e., inshore coral reefs) and exposed (i.e., surf zones of ocean beaches) seascapes.
Figure 12.
Great Sandy Marine Park and National Park [
163].
Figure 12.
Great Sandy Marine Park and National Park [
163].
The northeast–east coast of K’gari is prominently exposed to the open ocean and the East Australian current, which transports warm Coral Sea water southward [
165]. Given that the island is also bordered by the shallow waters of Hervey Bay in the west, oceanic conditions such as sea surface temperature could potentially influence local climate and weather conditions [
135]. Current annual mean minimum and maximum temperatures are 18.9 and 25.9 °C, respectively. Climate changes projected for the broader region, underpinned by current and historical trends, suggest a temperature rise of about +1.1–+1.8 °C, a decline in rainfall of about −4–−5%, and an increase in evaporation of about +4–+7% by 2050 [
166]. Furthermore, a future increase in sea level and storm surges is anticipated, leading to more frequent coastal inundation events. An increased risk from tropical cyclone impacts is projected. This risk is most likely due to the southward shift in the cyclone generation area [
166] and a continuation of the annual historical ocean warming trend of about +0.02 °C per year since 1990 [
138].
Many tropical species are moving towards the poles with rising sea temperatures and their arrival in cooler waters is altering the structure and functioning of subtropical and temperate ecosystems [
167,
168]. The coastal waters of Hervey Bay are in a hotspot for species range shifts [
169] and are recognised as a potential refuge for tropical species that are migrating south with climate change [
142]. Future conservation planning will require empirical data to test whether, and how, immigrating tropical taxa alter species interactions, habitat selection, and conservation performance in this region. Given the highly unique environment, visitor impact needs to be carefully managed.
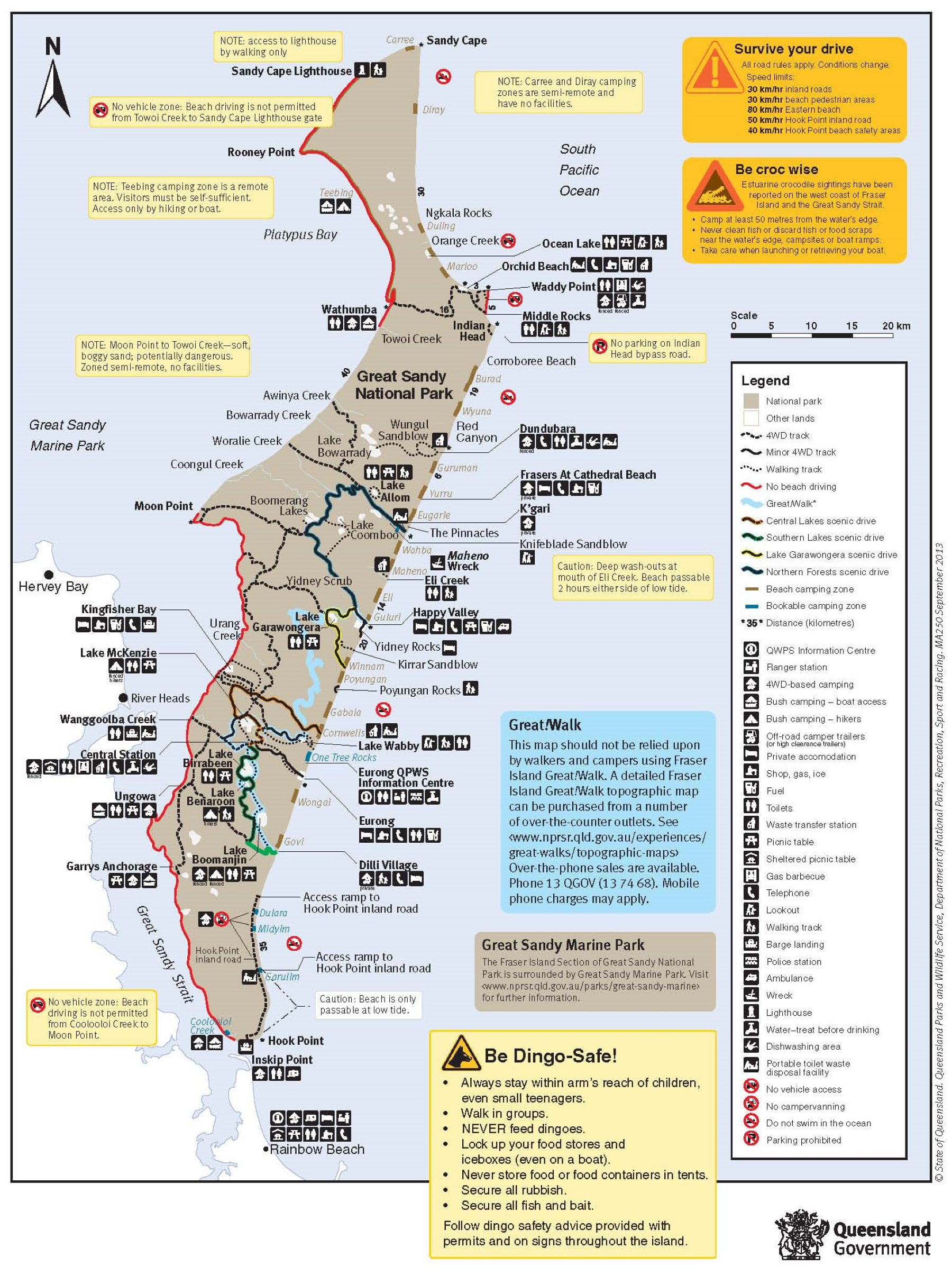
9. Impact of Beach Roads and Island Tracks
Most visitors coming to the island travel in 4 × 4 vehicles to camp in coastal dunes that fringe the ocean beaches, or to fish in the abutting surf zone [
118,
170,
171]. While the majority of the west coast of the island is closed to vehicular access, most of the east coast beaches, exposed to the open ocean, are accessible and used extensively by vehicles ranging from conventional 4 × 4 vehicles to large (64-seat) commercial 4 × 4 buses (
Figure 13). These activities are of immense social significance to visitors, but they also impact upon many physical and biological properties of the surf–beach–dune interface. For example, vehicle-access tracks change the physical structure of foredunes and alter the distribution and diversity of dune plants and animals (e.g., ghost crabs,
Ocypode spp.) [
118]. The use of 4 × 4 vehicles on beaches has a direct impact on the abundance, diversity, and behaviour of animals and indirect effects on the fitness of many species [
172,
173]. On the beaches of K’gari, these types of impacts are particularly well documented for beach birds (e.g., pied oystercatchers,
Haematopus longirostris; crested terns,
Thalasseus bergii) and invertebrates (e.g., surf clams) [
122,
174,
175,
176,
177]. Recreational anglers also catch large numbers of fish from the beaches each year which modifies the abundance and diversity of fish in the surf zone [
128,
170,
178] and alters the foraging behaviour of dingoes [
179]. Vehicle-based recreation, camping, and fishing on the beaches needs tighter management to meet the conservation objectives for the region [
118,
125].
K’gari has about 210 km of trafficable but tide-dependent beach, providing the main north–south transport route between Hook Point and Sandy Cape (
Figure 12). Whilst the vast network of over 800 kms of inland roads and tracks provides access to many areas, much of the island is still inaccessible.
The inland roads are currently managed primarily by QPWS which is responsible for their maintenance and redevelopment within an allocated budget and in concordance with the Great Sandy Region Management Plan (GSRMP) [
180]. Furthermore, the QPWS has been developing a road management strategy which aims to: categorise K’gari’s roads, provide operational guidelines, ensure quality pre-visit information about roads and driving, and encourage tour operators and organisations to gradually convert to more sustainable vehicles [
180].
Given the history of these roads, they often have a poor engineering layout and alignment [
181]. Only within the small settlements such as Eurong and Kingfisher Bay (
Figure 13) are roads paved. The vast majority of roads are composed primarily of sand and are vulnerable to rapid deterioration from erosion damage from surface runoff [
181] and the “downcutting” of roads due to vehicle movements displacing and churning sand [
171]. Detrimental environmental impacts occur due to erosive processes on roads depositing sand in the surrounding area [
181].
The rate of sand movement is influenced by rainfall volume and intensity, surface organic debris, the degree of canopy cover, and the degree of repellence within the surface sand layer. Due to the high variability of sand regions, different road surfaces present a challenge to the sustainable management of the inland roads, because some road areas are more vulnerable to erosion from weather and vehicle impacts than others. Tailoring engineering solutions to specific road surfaces based on their specific conditions is an issue for sustainable management of inland roads.
According to the GSRMP [
110], one-way access roads are provided to minimise environmental impacts, protect scenic amenities, and minimise the risk of vehicle collisions. Extensive track hardening with pallets (timber and plastic) has also been used in places to minimise erosion.
FIDO has been advocating for revitalising the rail network and establishing a light rail people mover since 1974 [
180]. Advantages would be to reduce use of internal roads and consequent environmental impacts, reduce the need for additional “hardening” of surfaces, provide a unique visitor experience with a higher standard of interpretation and information, and facilitate access for people with disabilities. Currently, there is no public transport, and the existing modes of transport impact the environment. The proposal, though, is controversial because of concerns that building a light rail system would have an extensive environmental footprint, exacerbated during construction and during the ongoing operational phase.
Beach driving on K’gari is a popular and valued visitor activity; however, it is not without incident. Between 2002 and 2015, there were over 160 reported crashes, including 4 fatalities involving 4 × 4 vehicles [
182]. Since 2015, there has been another fatality and a further 15 crashes requiring medical intervention. The beach roadway environment presents unique challenges for drivers. It is one of rapid change due to tidal flux, unstable surfaces, pedestrian priority, and distraction [
182,
183].
The beach roadway “75 Mile Beach road”, is an unfamiliar driving experience for many of the drivers, particularly overseas visitors and backpackers. A standard recommendation is that the beach roads should only be used for vehicular travel two hours either side of low tide, yet many users push these limits, resulting in regular bogging and rescues from incoming tides, shifting sands, and drainage lines. This time limit essentially condenses the period for travel, resulting in congestion on an otherwise pristine beach. Given that 80% of camping is along the beach and access is typically only along the beach, drivers need to be constantly alert to campers, picnickers, young children, and anglers accessing the water’s edge. The beach road near Eurong village is also shared as an airstrip for commercial air-chartered tourist flights.
While standard road safety interventions are applied, particularly when visitor numbers are high, Stevens and Salmon [
183] argue that the unique context of beach driving renders conventional approaches inappropriate. Stevens and Salmon used a work domain analysis to demonstrate the disparity between the purposes of the use of a “beach as a road”. The first purpose is as a “safe vehicle access to (coastal) destinations” while the second is to “limit the need for roadway infrastructure”. What is immediately recognisable is that these two purposes are likely in conflict. It is questionable whether it is possible to prioritise safety whilst at the same time limiting roadway infrastructure [
182]. Analyses provide a picture of a complex, high-risk driving environment in which multiple factors and stakeholders influence driving and safety.
The GSRMP [
110] acknowledged the need to balance “the practicality, comfort and convenience” of beach driving, with the protection of beaches and their wildlife; and use of beaches for other recreational purposes.
One of the challenges is to minimise human impact. While many of the overnight visitors stay in built accommodation, camping is very popular (
Figure 13). All camping areas have capacity limits and tent distance restrictions, to maintain visitor experience. Issues concerning damage caused by vehicles and human waste have already been discussed. A requirement to bring portable chemical toilets into beach camp zones was introduced from 2009. In spite of this intervention, elevated nutrient levels were still found in the water table in camping zones with faecal coliforms in beach flows [
1]. The risk implications for human health are significant, while the extent of the ecological threat is still speculative. An experimental adaptive management approach which includes resting or temporary closures of camping zones and ongoing monitoring would assist in gaining information to inform management decisions [
1].
Increasing visitation poses significant threats to the natural environment and to the visitor experience [
184], while potentially generating fiscal challenges in the case of visitor numbers declining. Conversely, higher camping and access fees could be charged to generate resources for managing environmental impacts. These fees could, arguably, include some form of income for the Butchulla People, providing the capital to re-invest in Indigenous tourism, interpretation, and management.
10. Governance and Management Planning: Opportunities for Collaboration
World Heritage Listing: UNESCO’s Role.
UNESCO aims to protect and preserve cultural and natural heritage around the world considered to be of outstanding universal value. This aim is embodied in the international treaty, ‘Convention Concerning the Protection of the World Cultural and Natural Heritage’ adopted by UNESCO in 1972
There are 213 World Heritage Area (WHA) sites designated globally for their outstanding natural attributes and, of these properties, only 17 are composed entirely of islands or archipelagos [
4]. These island-based sites are located in Australia, Madagascar, Cuba, Fiji, Thailand, Japan, Kiribati, New Zealand, Palau, and Papua New Guinea [
4].
Australia’s offshore islands contribute 40% of Australia’s coastline and, as such, a significant portion of Australia’s coastal landscape [
3]. Four of Australia’s islands are World-Heritage-listed islands—K’gari, Heard-McDonald, Lord Howe, and Macquarie Islands—but only K’gari has a long history of human occupation [
185] and is close to the coast. The generalised threats to the natural value of these islands are similar to those faced by most Australian islands and include climate change, biosecurity, feral species, and human usage, including tourism [
185].
Tourism is typically the current driver for economic growth, development, and the conservation and preservation of island environments [
186]. Islands are repositories for a great deal of biodiversity and are hotspots of biodiversity extinction [
10]. They are unique in that they are enclosed areas where threatening processes such as feral animals, weeds, and diseases that impact biodiversity may be managed, eliminated, or prevented from entering. However, these ecosystems are vulnerable to perturbation from extrinsic sources. Invasive species and human activities are the leading causes of the loss of biodiversity on islands [
36].
The modern tourist is increasingly searching for unique and meaningful travel experiences to satisfy their specific needs and desires [
187]. Islands have long been a magnetic draw for people seeking escape or adventure in paradise. While other arguments are considered in this paper, economic arguments for conservation are particularly powerful in political contexts [
188].
However, sound data are essential. Accessing data on island tourism is problematic as statistics are inconsistently collected, inherently difficult to collect, and factors such as contribution to local economy are often absorbed and aggregated at the broader regional, state, or country level [
189]. This applies in the case of K’gari, where there is no coordinated effort to collect visitor data.
In addition to economic benefits, wildlife tourism is important in connecting people and nature [
190]. Pyle [
191] claims that a strong individual sense of connection to nature and natural processes is significant for people’s good health. Tourism in natural environments can facilitate meaning and potential transformative experiences. Consequently, these tourism experiences in nature can stimulate pro-environmental intentions and behaviours [
192]. Destinations such as K’gari, when well-managed to achieve the intended outcomes, offer significant opportunities to learn about, connect with, and benefit from environmental and cultural immersion.
The designation of World Heritage listing offers brand marketing and awareness [
193] that serves to stimulate tourism. Tourism is critical to the economy of many islands and conservation of natural resources is essential to sustain the industry. However, not all tourism operators actively support the conservation of the natural assets that underpin their business [
194]. A World Heritage listing is not given in perpetuity and is reliant on conserving that which was deemed special at the time of listing, including the natural environment. In remote destinations such as islands, it is the uniqueness and the beauty of the natural environment which is essential to sustaining the tourism industry. World Heritage listing of Australia’s Great Barrier Reef, for example, supported new alliances between the tourism sector and national and international organisations, based on shared understanding of conservation values [
195].
Conservation management of islands is critical for the protection of biodiversity, geodiversity, and cultural and historic heritage values [
185]. Aboriginal and Torres Strait Islanders have had a long connection to most of Australia’s islands and many islands are being returned to First Nations Peoples [
196]. Co-management and joint management agreements are already in place, or proposed, for some of Australia’s protected estate, including islands that are gazetted in part or in whole as National Parks.
We argue that the management of tourism on K’gari remains a challenge, requiring robust and long-term planning and a commitment to long-term funding for implementation. Islands have not received commensurate management attention. Poor or absent biosecurity arrangements, pollution by waste and human use (e.g., sunscreen), impacts on coastlines by vehicles, inappropriate fire control and management and, increasingly, climate change, all have an effect. Yet, islands are logical places to safeguard biodiversity. We propose a management model involving a holistic co-management approach with the Butchulla People of K’gari which is necessary to ensure that actions taken to address certain needs do not have unintended consequences.
11. Governance Structures: Multiple Players and Instruments
Whilst geographically separate, islands are also part of a larger system: the biodiversity and associated ecosystem services are often connected to proximate terrestrial, freshwater, and marine systems. Likewise, they are often part of broader governance arrangements. K’gari’s model of governance involves local, state, and federal government jurisdictions, and the Native Title Determination Area and requires that they work collaboratively to achieve sustainable outcomes. Set in a regional context, at least twelve different formal spatial governance arrangements have effects on K’gari. This model challenges decision makers to react in a timely fashion to new challenges and opportunities to maintain and enhance the attributes that contributed to gaining the World Heritage listing.
K’gari has a particularly complex institutional arrangement. In 1971, the northern half of the island was declared a Queensland State National Park, which was extended in 1992 when a World Heritage listing was granted to include almost all of the island as a section of the Great Sandy National Park. It is administered by QPWS within the Department of Environment and Science.
In addition to World Heritage status, two other international commitments apply. One is the Great Sandy Biosphere Reserve 2009 (including the island as well as a sizeable area of the mainland). The Burnett Mary Regional Group (BMRG) is a community-based, not-for-profit organization that delivers targeted natural resource management outcomes for the region (Burnett Mary Regional Group). It played a major role in the nomination of the Great Sandy Biosphere Reserve and continues to play a coordination and supportive role in many community initiatives and collaborations. There is no management plan for the Biosphere Reserve, but the overarching aim of the UNESCO Man and Biosphere (MAB) program is to advance sustainable use, conserve biodiversity and advance climate change education within reserve boundaries [
197]. The UNESCO MAB vision emphasises a social learning platform to support adaptive management based on different social groups and strategies for public engagement [
198]. Objectives are to assist in ‘maintaining local livelihoods, including local people in decisions, and maintaining respect for the rights and responsibilities of local and Indigenous peoples’ (UNESCO 2000, 2002 as cited in [
198]). Without an updated management plan that covers the Great Sandy National Park and other reserves and conservation areas on K’gari, developed through established collaborative processes with stakeholders, it is difficult to achieve such objectives.
The second international commitment is the Great Sandy Strait Ramsar Site, designated in 1999 [
148]. The Ramsar listing includes the Mary River estuary and sand passage receiving tidal flows from Hervey Bay in the north and Wide Bay in the south, situated partially within and between the Fraser Island World Heritage Area and the mainland coast (
Figure 2). Furthermore, the Great Sandy Strait Conservation Park, 1999, includes the adjacent Ramsar-listed wetlands but not K’gari. The island is also within the jurisdiction of the Fraser Coast Regional Council, formed from amalgamated local governments in 2008. Thus, the council has administrative responsibility for the small freehold settlements on the island. Although non-exclusive Native Title rights were granted to the Butchulla People in 2014 (BAC), and a second claim was made in 2019 (BNTAC), the implications for the shared governance model are yet to be determined.
Many islands such as K’gari are protected through tiers of governance arrangements involving multiple agencies. Currently, a number of stakeholders have interests in the management of K’gari, namely: Queensland State Government departments, including QPWS; the Department of Agriculture and Fisheries; Queensland Police Service; Queensland Ambulance Service; Department of Transport and Main Roads; Fraser Coast Regional Council; BAC; BNTAC; community-based organisations; tourism operators; visitors; residents; research teams; conservation groups. While this layered protective framework aims to be inclusive of interests and responsibilities, good coordination among key stakeholders representing these diverse governance arrangements is essential for avoiding multiple barriers to effective conservation management and preventing ongoing environmental degradation.
Under the auspices of World Heritage, Scientific and Community Advisory Committees were formed in the late 1990s to support K’gari management, followed by an Indigenous Advisory Committee in 2005 (now absorbed in the community and scientific committees at the request of the BAC). These advisory committees have been effective in implementing community involvement in the monitoring and protection of K’gari and the associated additional funding required to maintain World Heritage value [
199]. The advisory committees have encouraged employment of the Butchulla People as land and sea rangers and investigated potential extensions to the current World Heritage boundary [
199]. The advisory committees also support the BAC’s claim for World Heritage status to include a cultural criterion.
Currently, the advisory committees are being restructured; however, it is assumed that membership will include the BAC and BNTAC, private sector, relevant Queensland and local government agencies, Fraser Coast Council, community interest groups, and experts in the following: coastal evolution; biogeography; geomorphology and water management; climate change; fisheries and coastal management; recreation and park management; social science; Indigenous archaeology.
FIDO has played a significant role in past campaigning to protect K’gari from sandmining and logging. Its aim is “to ensure the wisest use of Fraser Island’s natural resources” [
30] and for K’gari to be a well-managed National Park with managed tourism and other compatible uses. FIDO continues to contribute to both policy and management as well as direct operational tasks such as volunteer weed-control programs.
Given the range of stakeholders, it became clear that a coordination mechanism was needed. The Fraser Island Natural Integrity Alliance (FINIA) was established in 2005. It is a community-based partnership dedicated to the protection and restoration of the island’s “natural integrity, ecological assets and unique beauty through collaborative management, community education and targeted rehabilitation works” [
200]. Members include representatives from the BAC, BNTAC, conservation groups, local council, community groups, university researchers, and others, with Queensland government departments, QPWS, and Queensland Department of Environment and Science attending as required. It is a means to “bring stakeholders together to implement common goals with respect to often differing organizational objectives, in a proactive and respectful manner” [
200].
Regional-scale governance arrangements and coordination mechanisms have advantages that include the capacity to integrate across social, environmental, and economic issues; improve investment efficiency; establish appropriate power-sharing and partnership arrangements; and capability building [
201]. However, governance is complicated. Lockwood et al.’s [
201] study refers to the need for supportive rather than absentee or controlling central governments, and community and stakeholder collaboration based on trust. While K’gari’s coordination and partnership arrangements are well-established, new challenges emerge as the Butchulla People increasingly aim to play a major management role, which is important and appropriate. Given the long and continual connection of the Butchulla People with the island, they will play a key role in achieving new forms of management that tangibly values their unique and irreplaceable roles as custodians of the island.
Ball et al. [
194] refer to Kania and Kramer’s [
202] 4Cs approach to management planning, comprising conservation, community, cultures, and commerce. They have added another C, that is, collective—the commitment of a group of important actors from different sectors to a common agenda for solving a specific problem [
194]. For collaboration to be successful, they suggest the need for a common agenda and shared vision; shared measurement; mutually reinforcing activities; and continuous communication, appreciation, and recognition.
The outcome will mean sharing and/or devolving power to the Butchulla People and community-based groups to commensurate with their roles, enabling a distinct identity, and sufficient, but not excessive, accountability measures [
201]. To be successful in its mission, the BAC and BNTAC and their partners can build on the existing formal links between government bodies and community groups to strengthen efficiencies, complementary skills, and legitimacy. Good multilevel governance requires effective multilateral engagement as well as mutual respect and transparency in decision making.
12. World Heritage and Great Sandy Region Management
K’gari’s gazetted management plan [
110] was originally approved in 1994 and has changed little since. The plan proposed seven purposes of management including the protection of the physical and cultural values of the region, involvement of the Butchulla People, World Heritage obligations, and the provision of recreational opportunities in an economically, socially, and culturally sustainable manner [
110].
Queensland Parks and Wildlife Service and Partnerships (QPWS&P) provide park management through its values-based management framework (VBMF), an adaptive management approach based on international guidelines for effective and efficient protected area management. Current management of K’gari is supported by the VBMF. Monitoring the condition of the values and identification of emerging threats is key to effective management. An advanced draft management plan for the Great Sandy Area—including the Great Sandy National Park, Fraser Island World Heritage Area, Fraser Island Recreation Area, Cooloola Recreation Area, Cooloola (Noosa River) Resources Reserve, Great Sandy Conservation Park, Great Sandy Resources Reserve, Sandy Cape Conservation Park, Double Island Point Conservation Park, and Womalah Resources Reserve (
Figure 2)—is currently being developed in collaboration with the Butchulla and Kabi Kabi Peoples.
Since Native Title rights and interests were granted to the Butchulla People, K’gari’s management now must be in accordance with the Butchulla People’s Native Title rights as set out in an Indigenous Land Use Agreement (currently being reviewed). A cooperative management agreement between the department and the Butchulla People sets out the agreed approach for cooperative management of the protected areas.
The current in-force GSRMP mid-term review (2005) noted changes in visitor numbers, amount of area protected, coordinating committees, legislation, Native Title claims, and growth in residents and visitor numbers. While the review aimed to “ensure the Plan remained relevant to current conditions”, it did not alter the directions and outcomes of the 1994 plan [
110].
In spite of management plans being required for protected areas under the Queensland Nature Conservation Act, 1992 [
56], and recreation areas under the Recreation Areas Management Act, 1988 [
203], as well as obligations under the World Heritage Convention, Fraser Island National Park has no current approved management plan and is managed in accordance with the GSRMP. (The GSRMP states: “The Great Sandy Regional Management Plan, in conjunction with management strategies and guidelines in relation to the Fraser Island World Heritage property, will be used to guide management there until a property-specific management plan is prepared” ([
110] p. 8). It is also managed in accordance with the revised approved management plan 1994, the draft management plan for the Great Sandy Area, and the Butchulla People’s rights and interests. There are, however, management plans for areas adjacent to the Fraser Island Park, the Great Sandy Marine Park, and the Great Sandy Conservation Park covering islands and headlands in the Great Sandy Strait. The GSRMP, which gives guidance for management of the World Heritage Area, is now 24 years old. Finalising the draft management plan for the Great Sandy Area should be a major priority.
Many of the planning provisions in the GSRMP are focussed on constraining expansion of township reserves and ensuring appropriate services in those areas [
110]. Curiously, the GSRMP states that future planning schemes will be required to comply with the GSRMP, even though there is no legal basis in legislation. The plan specifically states: “This management plan is not a statutory management plan under the Nature Conservation Act 1992, Recreation Areas Management Act 2006 or the Marine Parks Act 2004” [
110]. It is considered a statement for the purpose of providing management direction for the region. As of 2011 (and more recently with the value-based management planning process currently being undertaken), “This plan is currently being reviewed” [
110] and prepared for public consultation but has not been completed. The complexity of management for K’gari continues to present challenges in completing a planning process; however, considering the international obligations, the changes in governance arrangements and visitor impacts since 1994, and the clear need for a holistic approach to island management, completing a statutory management instrument for K’gari remains a high priority for Queensland Parks and Wildlife Services and Partnerships (QPWS&P). Management planning will continue to progress in partnership and agreement with the Butchulla People. The World Heritage Unit (Queensland Department of Environment and Science) also plans to develop a strategic plan for the World Heritage Area.
13. Co-Management: Issues and Opportunities
Co-management has long been regarded as a process of power sharing in management decision making related to natural resources including protected areas [
204]. The substantive nature of co-management arrangements must be seen in light of the broader context of First Nations Peoples within the jurisdiction. The scope of arrangements varies depending on whether co-management derives from a treaty settlement (e.g., New Zealand), land rights claims (e.g., Northern Canada), or native title (e.g., Australia).
In New Zealand, only one example of joint management of a national park exists—Te Urewera—which became its own living entity as part of the Tūhoe Treaty Settlement process and the subsequent Te Urewera Act 2014. The act provides for a shift from equal to two-thirds Indigenous-majority board with powers including, but not limited to, making by-laws, developing management and operational plans, and monitoring of management. It also includes guidelines for decision-making processes, clearly preferencing unanimous agreement, followed by consensus and mediation. This example has arisen from a history during which Tūhoe saw themselves as independent from government [
205] and with the views held being in tension with those of the government [
206].
In the Canadian Arctic, experiences follow those in Ethiopia and Norway, where a mixture of agreements operate at specific scales, affecting the shape of both formal and informal co-management arrangements, e.g., Nunavut Land Claims Agreement between the Canadian Government and Inuit Territorial Authority. These agreements include associated arrangements, such as a benefits agreement, a joint park management committee, a planning team, First Nations knowledge working groups, and a separate set of arrangements for wildlife management [
207]. In Norway, a similar process exists except that administration is fully devolved to Sami administration, with reports of increasing Sami support at higher rates than non-Sami support [
208]. In the Phillipines, it is argued that higher levels of government support enable co-managing parties to be held to account where preexisting juristictional laws and traditional owner territories are in tension or contested [
209].
In Australia, co-management of protected areas exists at two levels (Federal and State), largely facilitated through responses to the Commonwealth Native Title Act 1993. Federal models are generously resourced, with Traditional Owner lease of land to the federal government as National Park for a fee (perpetual or limited term) and other concessions, among which the requirement for plans of management that are endorsed by majority Traditional Owner Boards is key. For federally managed parks in Australia, management plans must be passed through Federal Parliament, which can limit First Nations’ agency in decision making (for example, delaying prohibition of climbing of Uluru (Ayers Rock) until 2019).
At the state government level in Australia, multiple examples of co-management exist in Indigenous Land Use agreements, Memorandums of Understanding, and other mechanisms (see Bauman et al. [
210] for a comprehensive review). These agreements include: with or without title; leaseback with or without fee; absence, presence, and/or majority of Traditional Owners on management boards; with or without the right to live in, use, and gain preferential employment. In Queensland, formal co-management of parks occurs whether lands are First-Nations-owned (or with title) or not, and leased to government in perpetuity with or without fees, but with no guarantee of a First Nations majority on management boards. Unsurprisingly, this arrangement has been viewed as “not meeting aspirations” and ineffective [
210]. A more recent form of co-management specifically negotiated for parks in Queensland’s Cape York provides for First Nations’ ownership, including, in some cases, the ability to limit public access to nature reserves.
Co-management can also be viewed as an evolving problem-solving arrangement between First Nations Peoples and the state, that can involve a suite of formal and informal arrangements. A well-known example is that of Girringun Aboriginal Corporation, near Mission Beach, which includes an Indigenous Protected Area, an Indigenous Ranger Unit, and a Traditional Use of Marine Resources Agreement [
211,
212,
213]. Such arrangements are now becoming more common (see [
214,
215]), as there is recognition of the tensions between park boundaries which do not necessarily align with those of First Nations Countries.
The variety of co-management arrangements makes building a suite of determinants for the success of co-management difficult. As Hill [
216] acknowledges, “research has yet to illuminate under what conditions and at what cultural consequences Indigenous people (sic) attain equity in protected areas”. She further argues that effective planning (a common element across examples) must acknowledge a foundational platform of recognising rights, responsibilities, and legacy issues, enabling effective organisations that support the roles of key actors and providing effective mechanisms for working together (place, people, and engagement) so as to shape equitable intercultural places for ongoing negotation of co-management.
In the case of K’gari, Native Title determination provides an opportunity for Butchulla and all levels of government to reflect on existing protected area co-management models, and to consider multi-layered arrangements which acknowledge cultural and spiritual connections, laws, activities, and responsibilities. There is no single model to success, and the process continues to be negotiated between the BAC, BNTAC, QPWS, and numerous stakeholder groups.
14. World Heritage Sites and the Role of Research: Mapping Research
An issue raised in this paper is the lack, or patchiness, of research for many World Heritage sites, including K’gari. In preparing this paper, a Web of Science and Scopus search was conducted with the aim of determining whether, and how, research topics related to K’gari have changed over time, who is involved in research, and in which fields. The aim was to identify and understand the major research gaps and opportunities.
The string, “Fraser Island” was searched in the fields “Topic” and “Article title, abstract and keyword” in the databases, respectively. The search in Web of Science resulted in 144 documents including articles, proceedings, papers, editorial material, book reviews, and literature reviews. The Scopus search resulted in 186 documents including articles, conference papers, book chapters, reviews, and conference reviews. The VOS viewer and R software packages were used for data processing and analysis, mostly with the use of the bibliometrix package [
217]. Duplicates were automatically excluded in the process of merging the records from the two sources. A further manual search excluded irrelevant documents, resulting in a total of 206 articles retrieved for the analyses.
Publications searched dated from 1930 to 2017. The growth rate for the period between 1930 and 2017 was 5.78% p.a., and between 2017 and July 2021 it was 9.4% p.a. There were 70 new publications between 18 November 2017 and 30 June 2021. This rate exceeded the annual growth rate (2.96%) for total publications in Web of Science between 1980 and 2012 [
218], and was within the range of growth rates found for 12 databases for the period 1997–2006 (2.70–13.5%) [
219]. Given the island’s World Heritage status, it was not surprising that there was a relative growth in publications.
A total of 88 organisations from 21 countries were involved in research related to K’gari. Unsurprisingly, Australia was predominant in the list of authors’ affiliations in all but twelve documents. The USA was the second most frequent country listed, followed by Canada, Spain, the United Kingdom, and Japan. Considering documents from all years, the organisation with the largest contribution to the literature was the University of Queensland (UQ). When considering only the publications from 2015 to 2017, the University of the Sunshine Coast (USC) was the leading organisation. Given USC’s proximity to K’gari, along with the fact that USC has a research and teaching facility on the island (since 2000), these results were expected.
The most frequent topic in the compiled publications was wildlife, with 56 publications, 22 of which focus on dingoes. Dingoes are also a topic of discussion in tourism and entertainment publications. Geomorphology was the second most frequent subject addressed, often focusing on dunes. Terms related to geomorphology were present through time in the publications’ keywords (
Figure 14).
Variation was observed in the thematic focus (a) of all studies (n = 68), the number of papers for each type of animal examined (b), and the effect of humans on marine systems (c). The number of studies conducted is shown for each research theme animal type and human influence, illustrated as a proportion of all studies.
Given the significance of the waterways adjacent to K’gari, a further search of Scopus and Web of Knowledge was conducted. The following keywords were searched: Fraser Island, Hervey Bay, Great Sandy Strait, Great Sandy Marine Park, and marine. This search yielded 68 studies (
Figure 15), most focussed on describing the ecology of marine animals, particularly mammals, birds, fish, reptiles, and corals. A smaller proportion of research also addressed questions about hydrology (e.g., circulation, residence times, freshwater balance), water (e.g., chemistry, nutrients, toxins), plants (e.g., seagrass, algal reefs), geomorphology (e.g., sand transport, beach profiles), and sediments (e.g., grain size, nutrients, toxins). Most research was conducted in coastal waters, on sandy beaches, or in seagrass meadows. Few studies examined the effects of floods or measured the impacts of human activities (e.g., 4 × 4 vehicles, boats, pollution, beach camping) or the performance of marine reserves in the coastal waters that fringe the island.
In the 78 publications of the initial study, no specific research location was identified. Instead, the island was discussed as a whole, particularly in terms of land use and management, history, human occupation, and culture. Lake McKenzie is the most popular research location. Indeed, lakes were the most frequently studied environment, followed by dunes and creeks. Lakes were the focus of research in relation to freshwater ecology, tourism, recreation, wildlife, and geomorphology. Eight publications focused on the oceans on the eastern side, five of which focused on geomorphology. Frequently, publications focused on a broader geographic scale. Nine studies related to K’gari also focused on North Stradbroke Island, and five focused on the Southern Great Barrier Reef. A few studies also had an international focus. For instance, Povilanskas et al. [
220] compared factors affecting sustainability on K’gari to those on another World Heritage island, Curonian Spit, Lithuania.
It should be noted that research on the island is often related to the more easily accessed sites and visitation hotspots, such as Lake McKenzie and the ocean beaches, which reflects access restrictions or challenges for many parts of the island.
The temporal evolution of keywords suggests a trend to a broader perspective in research on K’gari. In the early 2000s, the focus was on the biophysical aspects of the island. Between 2005 and 2010, human-related terms, such as ecotourism, began gaining importance in the literature. Frequent keywords in the later years, such as climate change and world heritage, demonstrated that there is a current focus on topics with impacts beyond the local level. Interestingly, K’gari has been a frequent term in recent years, demonstrating that researchers are adapting to acknowledge the Butchulla People. Nevertheless, other terms related to Butchulla occupation and culture on the island and region are missing. Indigenous Knowledge is glaringly absent from this research summary.
The most frequent sources of Fraser Island research among the 206 publications found are listed in
Table 1. Despite the number of countries involved in research on the island, the most frequent sources were from Australian publications with relatively low impact factors. It could be speculated that “uniqueness” and “local interest” may be part of the challenge of publishing research in higher-impact journals. Analysing organisational studies, Mone and McKinley [
221] pointed out four negative consequences of “uniqueness” in the research arena, as follows: few standard concepts to describe organisations; disciplinary fragmentation; information overload; reduced probability of replication. In the case of research related to K’gari, reduced comparability may be an effect of reduced probability of replication due to its uniqueness.
There are known constraints for the development of research in unique and relatively isolated areas. Beyond logistical issues, unsupportive local management and limited funding can also be barriers. Since 2014 the Australian government has committed over AUD two billion for protection of the Great Barrier Reef (GBR) World Heritage Area, involving numerous stakeholders, such as industries, governmental and non-governmental organisations, academia, First Nations communities, and the public [
222]. Data on the funding invested in research on K’gari are not available in public sources, with the implication that funding levels are not significant enough to be reported.
One possibility for the probable low investment in K’gari research compared with the GBR is the economic value of the sites. GBR economic activities are estimated at AUD 1.2 billion per annum, including tourism, commercial fishing, and recreational fishing. Tourism alone accounts for AUD 700 million [
223]. Tourism in the Fraser Coast Region, of which K’gari is a part, contributed AUD 430 million [
224]. Another possible explanation for the difference between research funding on K’gari and the GBR is that World Heritage protection on the GBR was initiated by the Australian Federal Government which offered joint funding as an incentive to gain Queensland support. Nomination of the Fraser Island World Heritage Area, however, was initiated by the state government.
15. Discussion—Challenges, Limitations, and Future Research
This synthesis of existing research about K’gari has highlighted three key areas for further consideration:
Gaps in data about the ecological and cultural resources, as well as the limited evaluation of the effects of visitor use on K’gari’s values;
Limitations of current governance approaches, with co-governance proposed for negotiated decision making about research and management;
Implications for the resilience of a fragile island environment that continues to meet World Heritage and Biosphere Reserve values in a time of changing climate.
15.1. Data Gaps
This work identified a lack of basic data about certain ecosystems including wildlife; minimal documentation of cultural heritage; and a lack of thorough evaluation of visitor use and management effectiveness including carrying capacity. Yet, research is crucial to both understanding and managing the natural integrity of K’gari. Universities are well placed to be partners in research and contribute to strategic and management planning and ongoing monitoring. With a dearth of research in key areas, we suggest that research funding is not commensurate with other similar unique natural environments and World Heritage Areas. Research and monitoring urgently needs to be supported to provide evidence for the retention of the World Heritage status based on K’gari’s natural values, expanding the World Heritage designation based on cultural values, and commitment to the Biosphere Reserve as a living lab and educational resource.
Indigenous Knowledge has not historically played any role in the Westernised approach to research on the island. The scientific community has either dismissed Indigenous Knowledge as being a quasi-science, or taken the view of romantic primitivism, where the actions of a First Nations group are frozen in time [
225]. Protocols are currently being negotiated between the QPWS and the BAC. Similarly, research protocols are being negotiated between tertiary institutions—the BAC and BNTAC. Prior to these negotiations, few scientific research permits were endorsed since the Native Title was declared in 2014. The protocols should assist in facilitating a rebalance of documented knowledge about the island which has thus far been principally from a postcolonial world view. The history of colonization and the ongoing dominance of Western governance regimes, characterized by controversy and conflict, needs to be acknowledged and then redressed in contemporary management, and cannot be divorced from the historical context of the Butchulla experience and the collective memory of colonization. The need to actively incorporate Indigenous Knowledge systems in conservation management planning is a rising priority globally, but practical applications and successes vary locally, and transparent approaches to complimentary knowledges can be enhanced [
226]. In the K’gari instance, such complimentary epistemological synthesis in conservation practice needs to be concurrent with strong recognition of the relationship between colonial power, resistance, and ecology in this region as part of the emergent truth-telling reconciliation imperatives. There is contemporary progress in the process acknowledging Butchulla deep knowledge of and connection with K’gari. The formal reclaiming of the Butchulla name for the island on 19 September 2021 was a result of ‘a decades-long campaign by Butchulla Elders and community members… endorsed by the Queensland government and adopted by the World Heritage Committee’ [
227].
An appropriate model of collaboration and a protocol for the development of research exists between the Rainforest Aboriginal people of Far North Queensland, the Wet Tropics Management Authority, and the State of Queensland, incorporating both Indigenous and scientific knowledge systems. Like K’gari, the Wet Tropics is World Heritage listed for its outstanding natural value but is not currently recognised by UNESCO for its cultural value. This is in spite of a widely held opinion that the Rainforest Aboriginal Peoples’ cultural landscape within the Wet Tropics is of World Heritage significance [
228].
Cullen-Unsworth et al. [
228] draw on the work of Rössler [
229] and Walker and Salt [
230] arguing that biological diversity is declining through the loss of species and habitats, while Rainforest Aboriginal cultures are being simultaneously eroded. They explain that Indigenous Ecological Knowledge has been accumulated and used over a very long time and is contained within First Nations Peoples’ language and stories. The need to integrate Indigenous Knowledge and science into the management of vulnerable natural environments is widely recognized but there are few successful examples. They used a cooperative research or co-research model conducted with multiple stakeholders to provide joint learning. Such an integrated knowledge system is a model for co-management frameworks and is directly related to governance, as discussed in the next section.
15.2. Governance and Co-Management
QPWS have a predominant role in conserving and managing the protected areas which comprise most of the island. They are required to achieve this task through research, education, and cooperative involvement of the community, landholders, First Nations Peoples, and Torres Strait Islanders [
56]. In practice, the management of K’gari now draws on a dynamic form of negotiation and consensus building, principally between QPWS and the Butchulla Peoples. Other relevant stakeholders are involved, with varying and shifting degrees of engagement and divergent agendas, facilitated through interagency and cross-sectoral advisory groups, such as FINIA. While there may be overall agreement on the need to maintain the World Heritage values, the question of how to manage such unique areas for sustainable outcomes, given visitor use, is a global challenge.
Although contemporary management of the island recognises and respects Butchulla responsibilities, interests, and aspirations, meaningful involvement in public land management, including the development and management of tourism, is still evolving. To effectively achieve understanding, informed management, and appropriate use, there is increased recognition of the need for inclusive involvement of the Butchulla People and local communities at all stages in the World Heritage process [
231]. Governments and First Nations Peoples are interested in tourism for political and economic reasons, since tourism is a central dimension of nation-building politics [
232,
233].
We argue here that a culturally responsive management plan is needed for K’gari, and that it should be developed in partnership with the BAC and BNTAC, and it should account for Indigenous Knowledge. Any discussion of issues related to K’gari, such as climate change and visitor use, as well as future research and monitoring, need to involve partnership with the Butchulla People to ensure that the natural and cultural values of the island are maintained. We propose a management model that includes Butchulla People in basic ecological and cultural assessments, ongoing monitoring, research, management, and restoration. Integral to this goal is their inclusion in governance. This paper establishes a foundation on which to build such a model.
As part of co-governance, a model of Indigenous and scientific knowledge integration through co-research should be aimed at creating new knowledge to support natural resources management [
228]. The following five principles are relevant: (1) equity for co-researchers; (2) resource provision for co-researchers; (3) research strengthens Indigenous Ecological Knowledge; (4) Indigenous Ecological Knowledge is valued alongside scientific knowledge; and (5) the approach supports the redefinition of research, as described in an agreed protocol [
228]. Seven determinants were deemed critical for the successful implementation of the five principles, as follows: (1) Indigenous governance; (2) problem framing and conceptualisation; (3) relationship building; (4) data collection and management; (5) considerations of scale; (6) dissemination of results; and (7) evaluation [
228].
In the Cullen-Unsworth et al. [
228] study, Rainforest Aboriginal Peoples were uncomfortable with linking science-derived biophysical indicators with their cultural indicators because of concerns of cultural appropriation and vies of scientists as knowledge-takers. Instead, First Nations Peoples’ cultural indicators were linked with biophysical reality, as perceived by Rainforest Aboriginal Peoples. They developed a series of linked biophysical and cultural indicators which were appropriate for monitoring and evaluating the condition of the cultural landscape for current and potential future reporting processes [
234]. The questions posed were on issues that had practical and local relevance, the focus being research “
with”—rather than research “
for”—Rainforest Aboriginal Peoples [
235].
The Western research perspective is that scientific validity comes from the process of generating, documenting, and analysing information, grounded by theoretical concepts in the scientific literature. First Nations Peoples’ validity comes from the use and oral transmission of information and the associated transgenerational transfer of this information [
228]. The concept of Indigenous Knowledge as information that is contextualised is challenging to the scientific community, in the way that scientific method should allow for reproducibility [
225]. The issue here is that the scientific framework that is used to judge the voracity of any knowledge is not seen as a framework in itself, which can be judged. In the scientific community, it is difficult to produce evidence to challenge or change the framework and, unfortunately, there is significant vested interest in the extant framework to maintain hegemony [
225].
The co-research method presented by Cullen-Unsworth [
228] provides a way of integrating scientific knowledge and Indigenous Ecological Knowledge, and an approach for the two groups to work together to develop solutions to real-world problems by co-producing knowledge. Importantly, the researchers noted that time spent together “on country”, developing cross-cultural understanding, was critical for research to go ahead and for successful research outcomes.
15.3. Resilience of a Fragile Island Environment in a Changing Climate
Similar to other small-island settings, K’gari has unique biodiversity and natural features that are vulnerable to human interference, yet attractive to visitors. Ocean warming and acidification and extreme weather regimes are expected to increasingly affect the terrestrial, freshwater, groundwater, coastal, and marine biodiversity and ecosystem services of islands [
236]. Pressures from pests, diseases, and fire, as well as from weather, effect tourism and facilities; such pressures will challenge island management. Meanwhile, management is more costly due to accessibility constraints.
On the other hand, islands do offer an opportunity to trial management within a somewhat bounded system. The Great Barrier Reef World Heritage Area’s cross-governmental and Aboriginal and Torres Strait Island ranger co-management approach to islands may provide lessons and insights, including insights into a co-funding arrangement.
16. Conclusions
The purpose of this paper was to draw on existing knowledge of the natural and cultural environment of the unique K’gari landscape and to provide a foundation on which to build a management model that includes First Nations Peoples in research, monitoring, and governance.
Based on the documented knowledge, we argue the following points:
Research is crucial to both understanding and managing the natural and cultural integrity of K’gari, yet key gaps exist in ecological and cultural knowledge about the island.
A new model of co-research that integrates Indigenous Ecological Knowledge is needed to redress the postcolonial and Western scientific information systems.
A culturally responsive management plan is needed for K’gari. This should be developed in partnership with the BAC and BNTAC. Butchulla People need to be included in basic ecological and cultural assessments, ongoing monitoring, research, management, restoration, and governance.
The development of protocols that improve the engagement and involvement of First Nations Peoples in scientific research has the capacity to expand knowledge, well beyond the frameworks which are embedded within a singular world view.
Natural World Heritage sites are social–ecological systems. As such, the natural integrity of these sites is vulnerable to political change, economic fluctuations, and ecological variance [
37].
K’gari is a contested space between vast numbers of annual visitors, assisted by large commercial tour operations, which directly or indirectly cause unavoidable environmental impacts. The island has a rich history of the Butchulla Peoples, unique ecological diversity, and breath-taking aesthetic beauty. This juxtaposition has prompted scholars to attempt to redefine our perception of K’gari in terms of culture, ecology, and the value of these elements in the modern day [
29,
111,
237,
238].
Before colonization, K’gari was home to the Butchulla People and was managed in accordance with their cultural traditions. Post-colonization, the Butchulla People were displaced, and K’gari was managed for its valued raw natural resources—its timber and mineral sand. K’gari is now managed as a National Park and World Heritage site and is valued for tourism opportunities and income derived from its isolation, “wilderness” setting, and its unique and outstanding beauty. The Butchulla People value their long cultural history and, as such, there is growing evidence to suggest that a case could be put to the World Heritage Committee that K’gari’s World Heritage status should be expanded beyond its unique natural values and be recognised for its cultural values.
Climate change is a pressing problem around the world, with particular impact on islands. Any strategy devised to protect the natural and cultural value of K’gari needs to be based on accurate data. Yet, there are a dearth of data and knowledge about the ramifications of climate change on K’gari. The island’s freshwater ecosystems are highly susceptible to activities associated with tourism and recreation, groundwater extraction, water pollution, and feral animals and plants. Climate change is likely to exacerbate these impacts, particularly in perched aquatic systems which are dependent on rainfall and surface hydrology. Research is warranted on biodiversity patterns in unexplored ecosystem types (e.g., patterned fens), modelling of ecosystem processes, monitoring to track responses to management intervention (e.g., restrictions on beach camping; fire management), and documenting/forecasting the effects of climate change.
Although the island harbours relatively unfragmented swathes of key habitat for a diverse array of flora and fauna, the effects of climate change, alongside high ongoing human visitation rates to some environments (e.g., lakes and streams) also mean that ecological disturbance will continue into the future. Ineffective management of tourism has the propensity to destroy not only the environment but the very experience which is being sought by visitors. Given that many parts of the island are inaccessible by road, visitor numbers, visitor behaviour, effective fire management, effective biosecurity measures, and developing an awareness of ecological management under a changing climate are all significant challenges. It is crucial that conservation management is underpinned by effective visitor management, including the continued restriction of vehicular traffic to large tracts of the island; ongoing research that reviews both baseline fire ecology and climate data; and continuing efforts to catalogue the unique biota and ecology.
A co-management plan that acknowledges the contributions of community-driven conservation programs, provides cultural partnerships, and addresses visitor management is essential. A co-research process, within the island’s living laboratory, that cooperatively creates new information, is required to gain an understanding of management effects. The aim is not only for the conservation of biological and cultural diversity, but one which establishes practices that build resilience for K’gari in the face of exogenous pressures, driven by a rapidly changing environment.