A Comparative Review on Biodegradation of Poly(Lactic Acid) in Soil, Compost, Water, and Wastewater Environments: Incorporating Mathematical Modeling Perspectives
Abstract
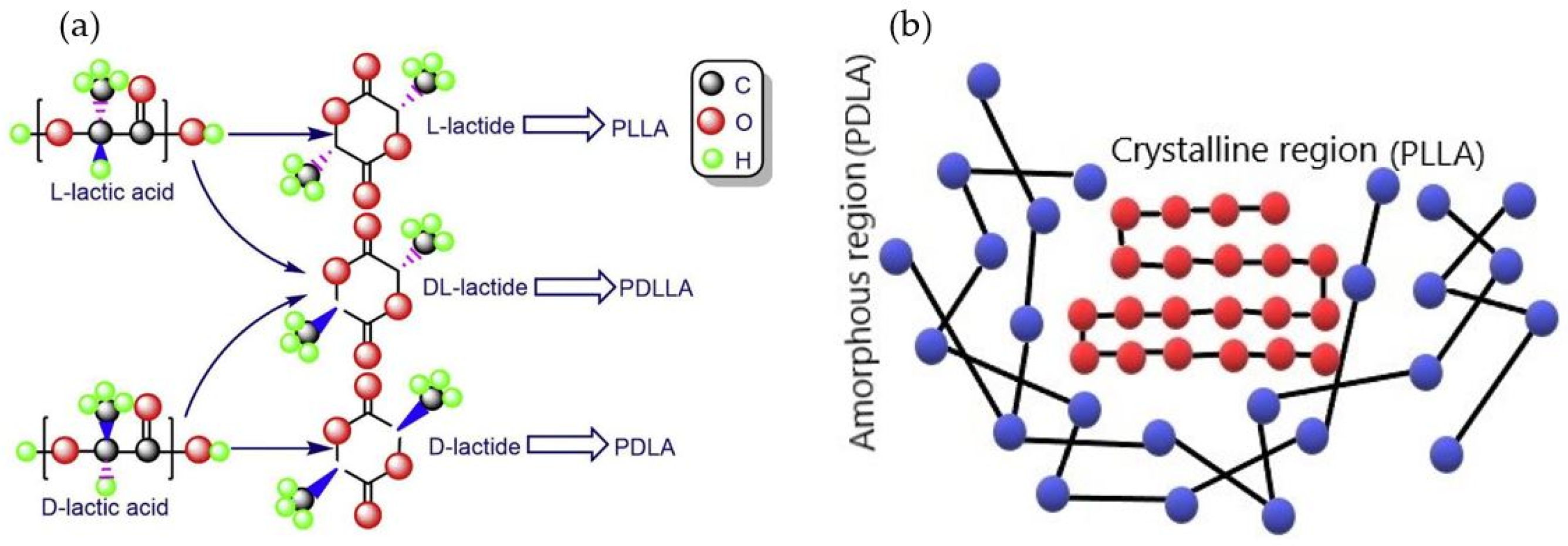
1. Introduction
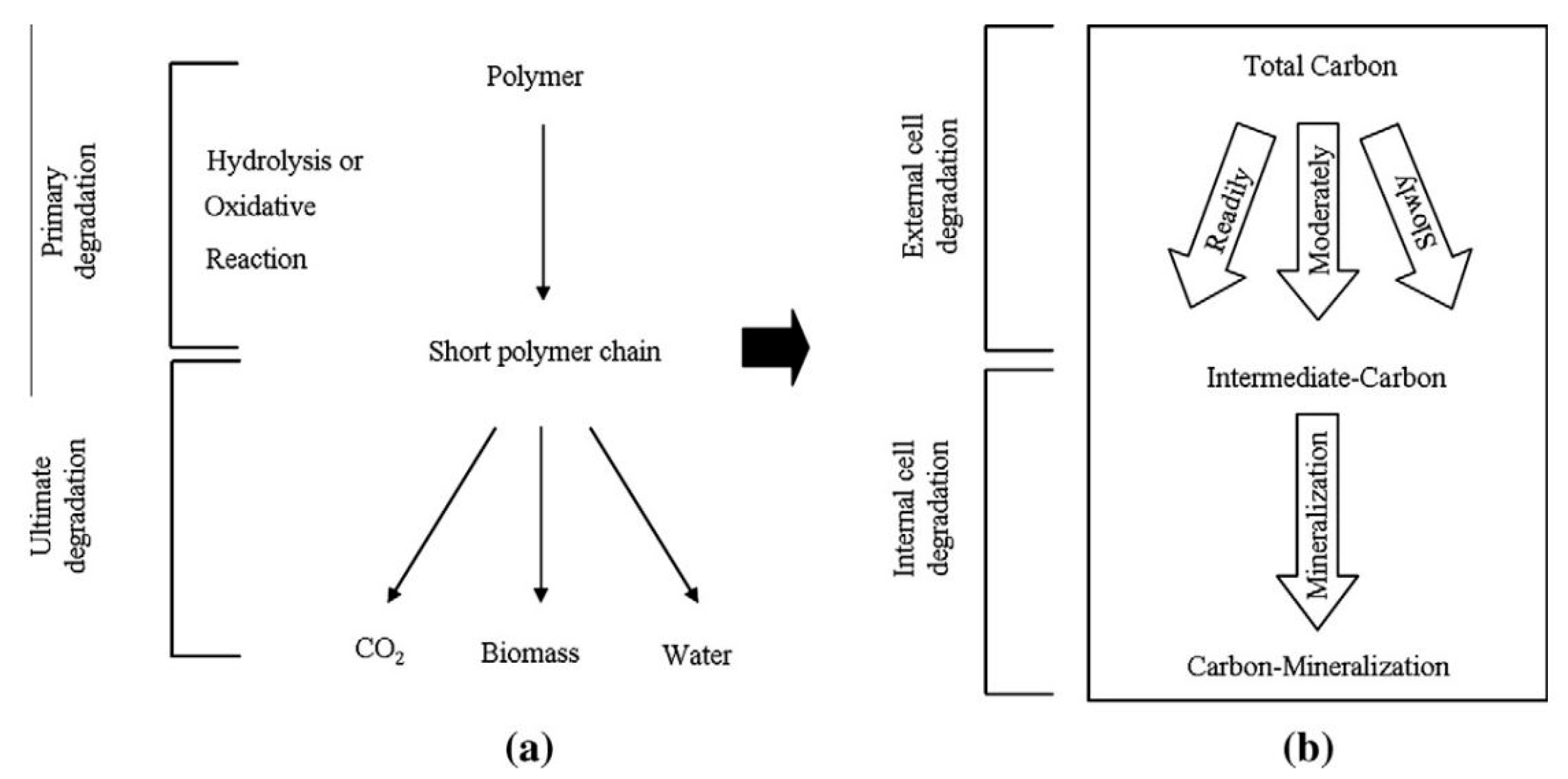
2. Biodegradation of PLA
2.1. Biodegradation in Soil and Compost by Enzymatic Mechanism
| Microorganism | Substrate | Mechanism | Secreted Enzyme | T (°C) | pH | Biodegradation Criteria | Ref. |
|---|---|---|---|---|---|---|---|
| In soil and compost | |||||||
| Actinomycetes | |||||||
| Amycolatopsis sp. 1 strain HT32 | PLLA | Enzymatic | Protease | 30 | 7 | %TOC 2 = −72.5 (20 days) | [68] |
| Bacillus stearothermophilus | PDLA | Lipase | 58 | 7 | %TOC~−62.5 (20 days) | [69] | |
| Amycolatopsis sp. strain 3118 | PLLA | Enzymatic | Protease | 30 | 7 | %WL 3 = 9.2 (11 days) | [70] |
| Pseudonocardia alni AS4.1531(T) | PLLA | Enzymatic | Protease | 30 | 8 | %WL = 71.6 (8 days) | [84] |
| Pseudonocardia sp. RM423 | PLLA | Enzymatic | Protease | 30 | 7 | %WL = 0.4 ± 0.2 (7 days) | [85] |
| Amycolatopsis sp. strain KT-s-9 | PLLA | Enzymatic | Protease | 30 | 7 | - | [67] |
| Amycolatopsis sp. strain 41 | PLLA | Enzymatic | Protease, Lipase | 30 | 6 | - | [67] |
| Amycolatopsis sp. strain K104-1 | PLLA | Enzymatic | Protease | 37 | 9.5 | %RA 4~200 (7 days) | [86] |
| Amycolatopsis thailandensis PLA07 | PLLA | Enzymatic | Protease | 25–37 | 6–10 | - | [74] |
| Amycolatopsis strain SCM_MK2-4 | PLLA | Enzymatic | Protease | 30 | 7 | EA 5~ 0.05 U/mL 6 (7 days) | [75] |
| Actinomadura strain T16-1 | PLLA | Enzymatic | Protease | 70 | 10 | EA = 46 ± 2 U/mL (6 days) | [76] |
| Laceyella sacchari LP175 | PLLA | Enzymatic | Protease | 50 | 9 | EA = 5.07 ± 0.25 U/mL (4 days) | [77] |
| Bacillus brevis | PLLA | Enzymatic | Protease | 58 | 6.9 | - | [78] |
| Other Bacteria | |||||||
| Geobacillus thermocatenulatus | PLLA | Enzymatic | Esterase | 60 | 7 | - | [67] |
| Pseudomonas geniculata WS3 | PLA | Enzymatic | Protease | 30 | 7 | %WL~85 (30 days) | [19] |
| Serratia plymuthica | PLA | Biofilm | Lipase | 24 | 7.5 | %WL < 10 (6 months) | [79] |
| Arthrobacter sulfonivorans | PLA | Biofilm | Amylase, Lipase | 24 | 7.5 | %WL < 10 (6 months) | [79] |
| Fungus | |||||||
| Clitocybe sp (Clit) | PLA | Enzymatic | Cellulase | 12.5 | 7 | %WL < 10 (6 months) | [79] |
| Laccaria laccata (Lac) | PLA | Enzymatic | Cellulase | 12.5 | 7 | %WL < 10 (6 months) | [79] |
| Aspergillus oryzae RIB40 | PDLLA | Enzymatic | Cutinase | 20–80 | 8 | - | [71] |
| Trichoderma viride | PLLA | Enzymatic | Cutinase | - | - | - | [72] |
| In liquid culture medium | |||||||
| Actinomycetes | |||||||
| Amycolatopsis orientalis IFO12362 | PLLA | Enzymatic | Protease | 30, 40 | 7 | 600 mg/L water-soluble TOC | [80] |
| Kibdelosporangium aridum | PLLA | Enzymatic | Protease | 30 | 6–7 | >97% degradation in 14 days | [81] |
| Saccharothrix waywayandensis | PLLA | Enzymatic | Protease | 30 | 7–8 | >95% degradation in 4 days | [87] |
| Paenibacillus amylolyticus strain TB-13 | PDLA, PLLA | Enzymatic | Lipase | 45–55 | 10 | - | [88] |
| Fungus | |||||||
| Tritirachium album ATCC 22563 | PLLA | Enzymatic | Protease, Lipase | 30 | 7 | 76% degradation in 14 days | [89] |
| Cryptococcus sp. strain S-2 | PLLA | Waste water | Cutinase | 37 | 7 | - | [73] |
2.2. Biodegradation in Liquid Media
2.2.1. Hydrolytic Degradation in Freshwater
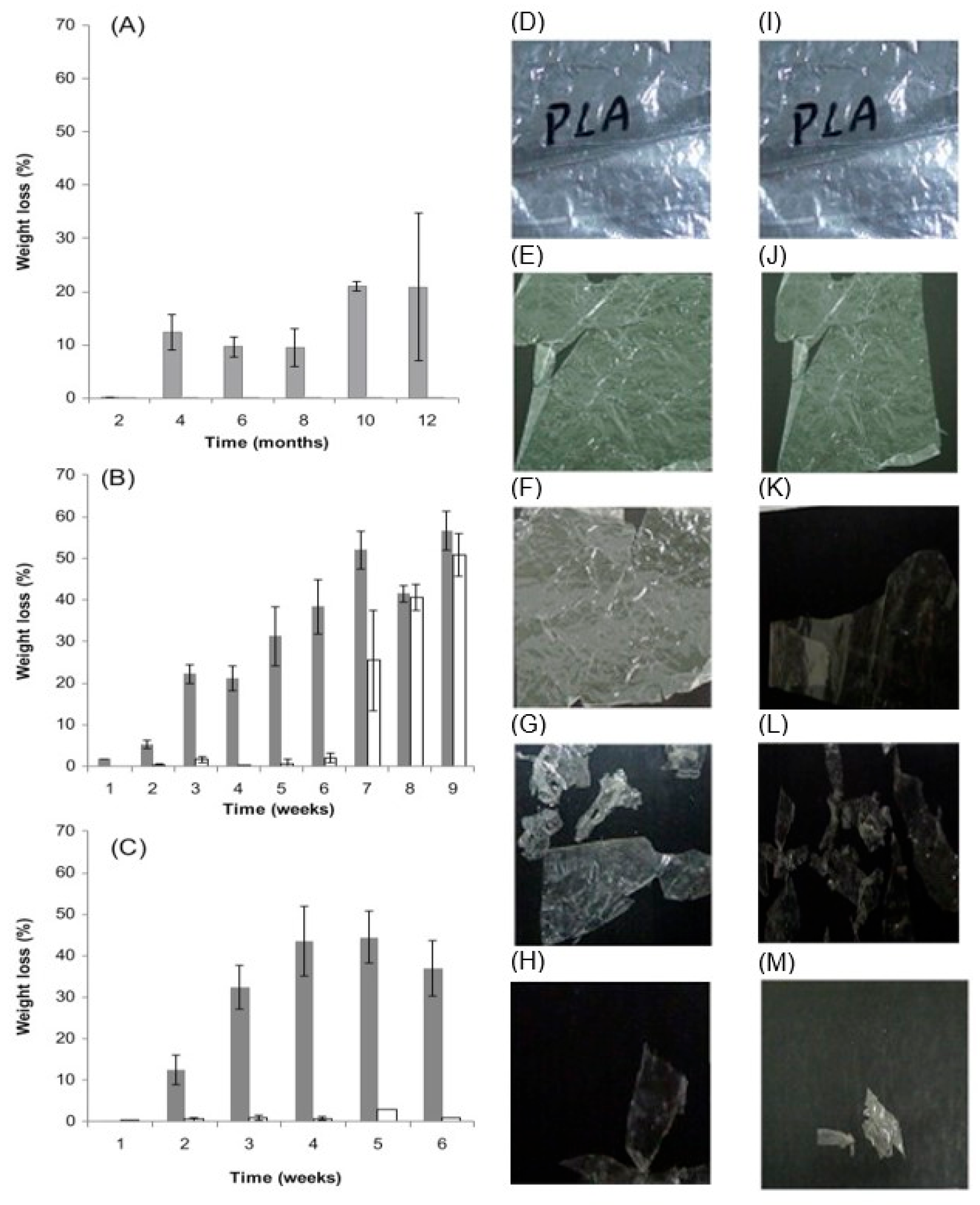
2.2.2. Biodegradation in Wastewater and Landfills
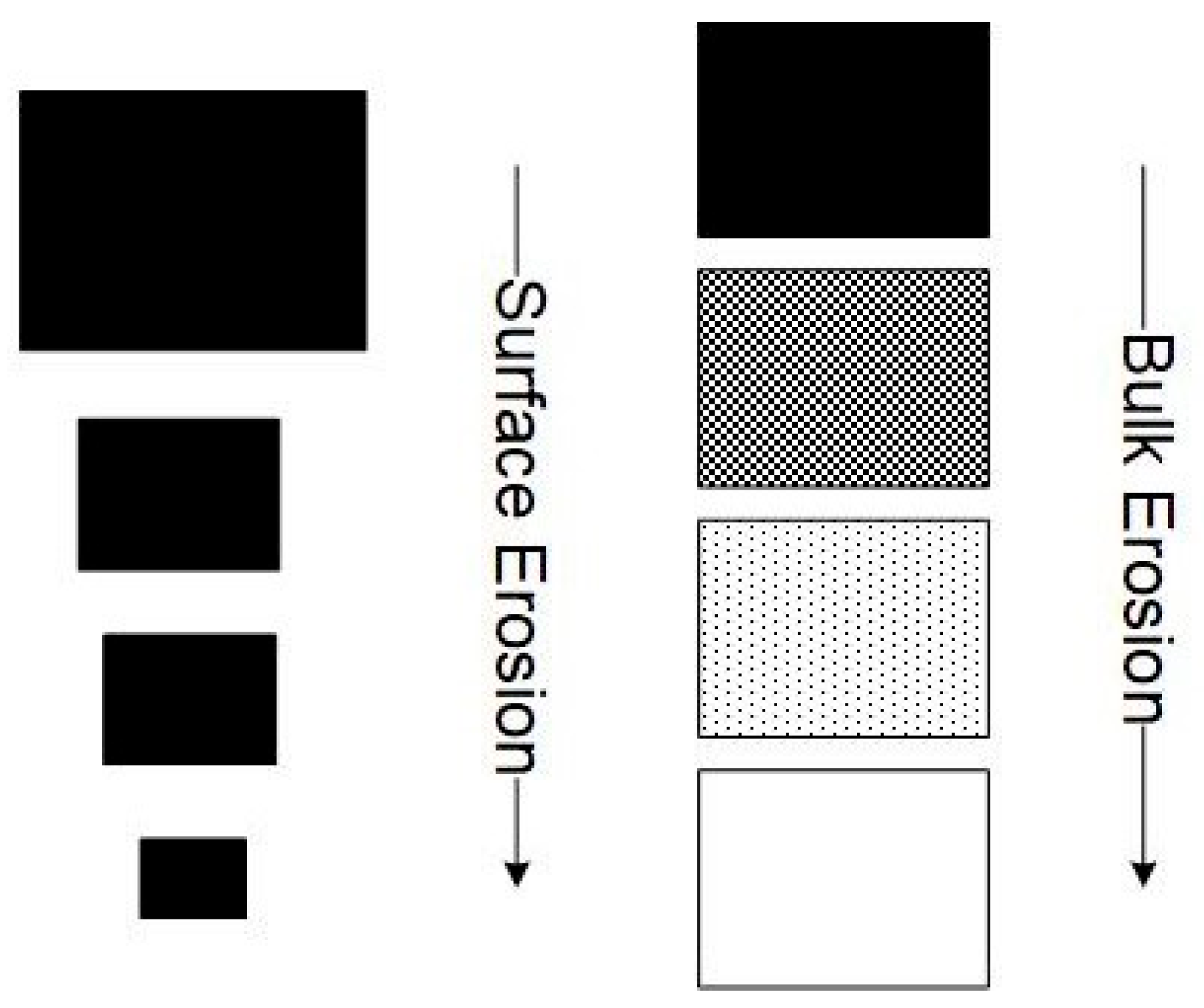
3. Mathematical Modeling of PLA Biodegradation Linked to the Experiments
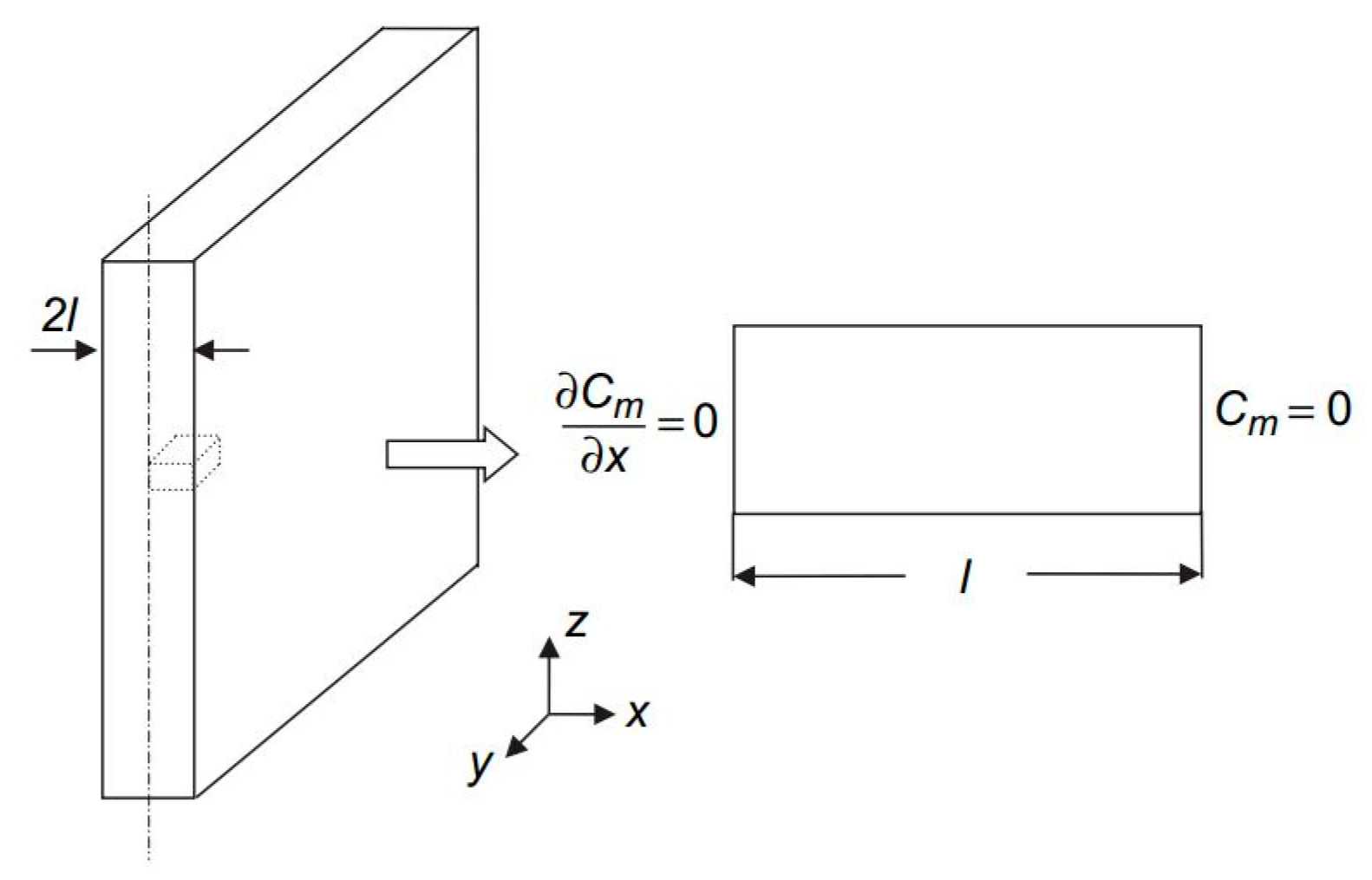
3.1. Hydrolytical Degradation Model
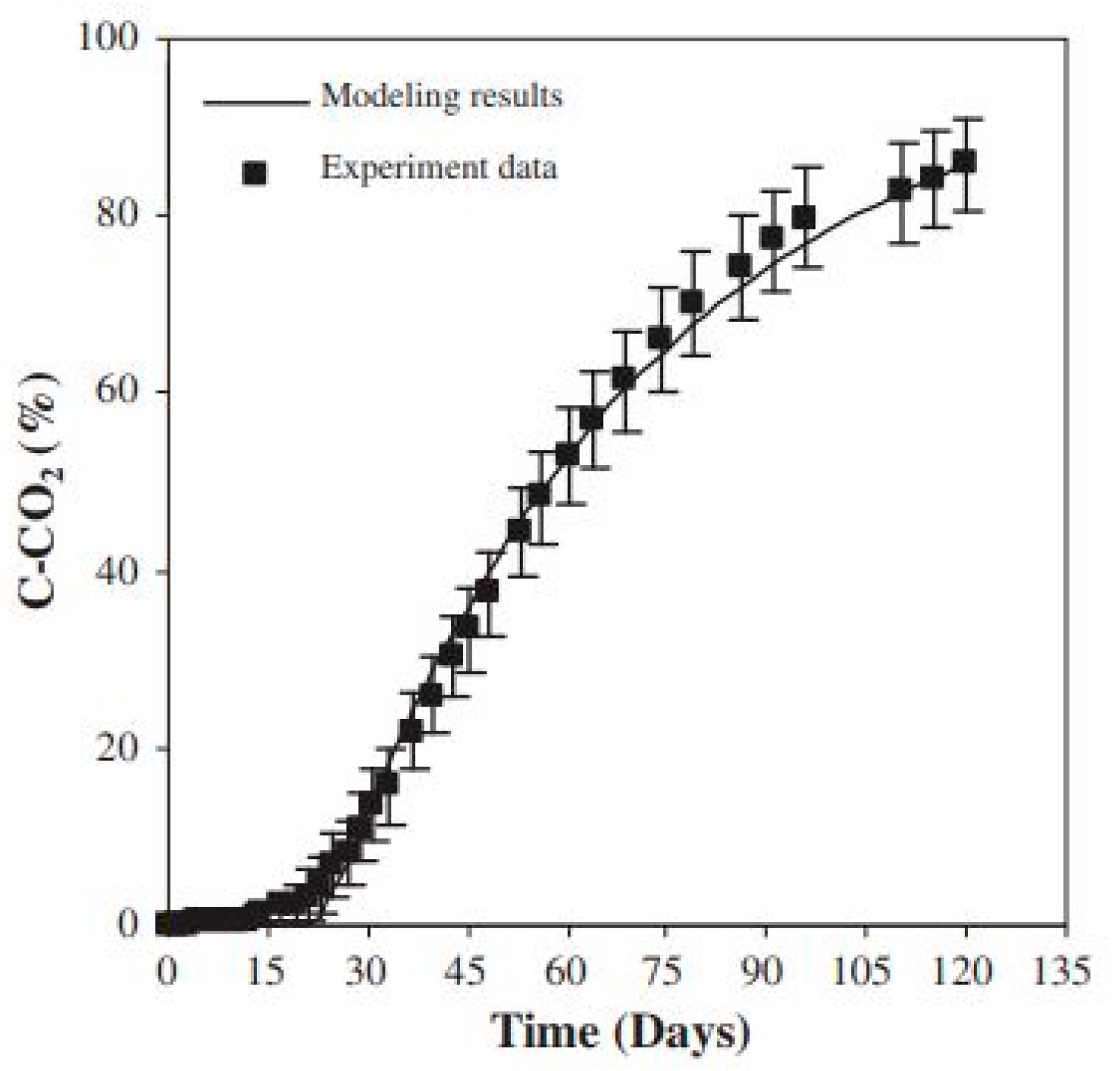
3.2. Biodegradation Model
4. Micro/Nano-Plastics (MPs and NPs) of PLA
5. Perspectives
6. Conclusions
- From a biodegradation viewpoint, PLA demonstrates biodegradability in compost, wastewater, soil, under accelerated landfill conditions, and in water in descending order.
- The primary mechanism driving PLA degradation involves the hydrolysis of ester bonds. This process occurs via autocatalytic hydrolysis in water, facilitated by carboxylic acid end groups of PLA.
- PLA undergoes enzymatic degradation in compost and soil, catalyzed by different enzymes secreted by microorganisms.
- While temperature significantly influences PLA biodegradation under both aerobic (compost) and anaerobic (digested sludge) conditions, degradation occurs at a slower rate in anaerobic environments.
- It should be emphasized that neat PLA cannot be classified as a completely biodegradable polymer, as it generates microplastics (MPs) during biodegradation.
- The generation of PLA MPs is inevitable; however, the utilization of synthetic enzymes, like metal oxides, can significantly reduce MP production.
- To streamline the assessment of PLA degradability, alternative methods such as diffusion–reaction and zero-order kinetic models can be employed, bypassing the time-consuming conventional approaches.
Funding
Conflicts of Interest
References
- Lunetta, E.; Cacciotti, I. Chapter 1—An Overview of the Packaging Industry: State of the Art, Opportunities, Challenges, Criticisms, and Solutions. In Nanostructured Materials for Food Packaging Applications; Jacob, J., Cacciotti, I., Thomas, S., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–30. ISBN 978-0-323-99525-2. [Google Scholar]
- EUBIO_Admin Market. Eur. Bioplastics EV. Available online: https://www.european-bioplastics.org/market (accessed on 15 October 2024).
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Chandra, R.; Rustgi, R. Biodegradable polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Lin, Z.; Jin, T.; Zou, T.; Xu, L.; Xi, B.; Xu, D.; He, J.; Xiong, L.; Tang, C.; Peng, J.; et al. Current Progress on Plastic/Microplastic Degradation: Fact Influences and Mechanism. Environ. Pollut. 2022, 304, 119159. [Google Scholar] [CrossRef] [PubMed]
- Zaaba, N.F.; Jaafar, M. A Review on Degradation Mechanisms of Polylactic Acid: Hydrolytic, Photodegradative, Microbial, and Enzymatic Degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Qi, X.; Ren, Y.; Wang, X. New Advances in the Biodegradation of Poly(Lactic) Acid. Int. Biodeterior. Biodegrad. 2017, 117, 215–223. [Google Scholar] [CrossRef]
- Kim, K.W.; Woo, S.I. Synthesis of High-Molecular-Weight Poly(L-Lactic Acid) by Direct Polycondensation. Macromol. Chem. Phys. 2002, 203, 2245–2250. [Google Scholar] [CrossRef]
- Kimura, N.; Kabe, T.; Kimura, S.; Kasuya, K.; Iwata, T. Enzymatic Degradation Mechanism of the Lamellar Stacking Structures of Poly([R]-3-Hydroxybutyrate-Co-[R]-3-Hydroxyvalerate) Fibers. Polym. Degrad. Stab. 2024, 219, 110607. [Google Scholar] [CrossRef]
- Huang, Q.; Kimura, S.; Iwata, T. Thermal Embedding of Humicola Insolens Cutinase: A Strategy for Improving Polyester Biodegradation in Seawater. Biomacromolecules 2023, 24, 5836–5846. [Google Scholar] [CrossRef]
- Pantani, R.; Sorrentino, A. Influence of Crystallinity on the Biodegradation Rate of Injection-Moulded Poly(Lactic Acid) Samples in Controlled Composting Conditions. Polym. Degrad. Stab. 2013, 98, 1089–1096. [Google Scholar] [CrossRef]
- Ke, T.; Sun, X. Thermal and mechanical properties of poly(lactic acid) and starch blends with various lasticizers. Trans. ASAE 2001, 44, 945. [Google Scholar] [CrossRef]
- Cai, H.; Dave, V.; Gross, R.A.; McCarthy, S.P. Effects of Physical Aging, Crystallinity, and Orientation on the Enzymatic Degradation of Poly(Lactic Acid). J. Polym. Sci. Part B Polym. Phys. 1996, 34, 2701–2708. [Google Scholar] [CrossRef]
- Li, Y.; Xin, S.; Bian, Y.; Dong, Q.; Han, C.; Xu, K.; Dong, L. Stereocomplex Crystallite Network in Poly(D,L-Lactide): Formation, Structure and the Effect on Shape Memory Behaviors and Enzymatic Hydrolysis of Poly(D,L-Lactide). RSC Adv. 2015, 5, 24352–24362. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R. Effect of PLA Grades and Morphologies on Hydrolytic Degradation at Composting Temperature: Assessment of Structural Modification and Kinetic Parameters. Polym. Degrad. Stab. 2013, 98, 1006–1014. [Google Scholar] [CrossRef]
- Limsukon, W.; Rubino, M.; Rabnawaz, M.; Lim, L.-T.; Auras, R. Hydrolytic Degradation of Poly(Lactic Acid): Unraveling Correlations between Temperature and the Three Phase Structures. Polym. Degrad. Stab. 2023, 217, 110537. [Google Scholar] [CrossRef]
- Omura, T.; Tsujimoto, S.; Kimura, S.; Maehara, A.; Kabe, T.; Iwata, T. Marine Biodegradation of Poly[(R)-3-Hydroxybutyrate-Co-4-Hydroxybutyrate] Elastic Fibers in Seawater: Dependence of Decomposition Rate on Highly Ordered Structure. Front. Bioeng. Biotechnol. 2023, 11, 1303830. [Google Scholar] [CrossRef]
- Omura, T.; Komiyama, K.; Maehara, A.; Kabe, T.; Iwata, T. Real-Time Structural Changes of Poly[(R)-3-Hydroxybutyrate-Co-4-Hydroxybutyrate] Biodegradable Elastic Fibers by Using Synchrotron Radiation. In Sustainable Green Chemistry in Polymer Research. Volume 1. Biocatalysis and Biobased Materials; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2023; Volume 1450, pp. 217–236. [Google Scholar]
- Boonluksiri, Y.; Prapagdee, B.; Sombatsompop, N. Promotion of Polylactic Acid Biodegradation by a Combined Addition of PLA-Degrading Bacterium and Nitrogen Source under Submerged and Soil Burial Conditions. Polym. Degrad. Stab. 2021, 188, 109562. [Google Scholar] [CrossRef]
- Smit, K.D.; Marien, Y.W.; Steenberge, P.H.M.V.; D’hooge, D.R.; Edeleva, M. Playing with Process Conditions to Increase the Industrial Sustainability of Poly(Lactic Acid)-Based Materials. React. Chem. Eng. 2023, 8, 1598–1612. [Google Scholar] [CrossRef]
- Vayshbeyn, L.I.; Mastalygina, E.E.; Olkhov, A.A.; Podzorova, M.V. Poly(Lactic Acid)-Based Blends: A Comprehensive Review. Appl. Sci. 2023, 13, 5148. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and Mechanical Properties of PLA, and Their Functions in Widespread Applications—A Comprehensive Review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Siakeng, R.; Jawaid, M.; Ariffin, H.; Sapuan, S.M.; Asim, M.; Saba, N. Natural Fiber Reinforced Polylactic Acid Composites: A Review. Polym. Compos. 2019, 40, 446–463. [Google Scholar] [CrossRef]
- Pan, J.; Chen, X. 2—Modelling Degradation of Amorphous Biodegradable Polyesters: Basic Model. In Modelling Degradation of Bioresorbable Polymeric Medical Devices; Pan, J., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2015; pp. 15–31. ISBN 978-1-78242-016-3. [Google Scholar]
- Leejarkpai, T.; Suwanmanee, U.; Rudeekit, Y.; Mungcharoen, T. Biodegradable Kinetics of Plastics under Controlled Composting Conditions. Waste Manag. 2011, 31, 1153–1161. [Google Scholar] [CrossRef]
- Sevim, K.; Pan, J. A Model for Hydrolytic Degradation and Erosion of Biodegradable Polymers. Acta Biomater. 2018, 66, 192–199. [Google Scholar] [CrossRef]
- Kolstad, J.J.; Vink, E.T.H.; De Wilde, B.; Debeer, L. Assessment of Anaerobic Degradation of IngeoTM Polylactides under Accelerated Landfill Conditions. Polym. Degrad. Stab. 2012, 97, 1131–1141. [Google Scholar] [CrossRef]
- Chomnutcha Boonmee, C.K. Degradation of Poly(Lactic Acid) under Simulated Landfill Conditions. Environ. Nat. Resour. J. 2016, 14, 2. [Google Scholar] [CrossRef]
- Krause, M.J.; Townsend, T.G. Life-Cycle Assumptions of Landfilled Polylactic Acid Underpredict Methane Generation. Environ. Sci. Technol. Lett. 2016, 3, 166–169. [Google Scholar] [CrossRef]
- Chuensangjun, C.; Pechyen, C.; Sirisansaneeyakul, S. Degradation Behaviors of Different Blends of Polylactic Acid Buried in Soil. Energy Procedia 2013, 34, 73–82. [Google Scholar] [CrossRef]
- Liu, L.; Xu, M.; Ye, Y.; Zhang, B. On the Degradation of (Micro)Plastics: Degradation Methods, Influencing Factors, Environmental Impacts. Sci. Total Environ. 2022, 806, 151312. [Google Scholar] [CrossRef] [PubMed]
- Kunduru, K.R.; Basu, A.; Haim Zada, M.; Domb, A.J. Castor Oil-Based Biodegradable Polyesters. Biomacromolecules 2015, 16, 2572–2587. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kawazoe, N.; Tateishi, T.; Chen, G. In Vitro Evaluation of Biodegradation of Poly(Lactic-Co-Glycolic Acid) Sponges. Biomaterials 2008, 29, 3438–3443. [Google Scholar] [CrossRef]
- Koitabashi, M.; Noguchi, M.T.; Sameshima-Yamashita, Y.; Hiradate, S.; Suzuki, K.; Yoshida, S.; Watanabe, T.; Shinozaki, Y.; Tsushima, S.; Kitamoto, H.K. Degradation of Biodegradable Plastic Mulch Films in Soil Environment by Phylloplane Fungi Isolated from Gramineous Plants. AMB Express 2012, 2, 40. [Google Scholar] [CrossRef] [PubMed]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Laforsch, C.; Greiner, A.; Agarwal, S. Fate of So-Called Biodegradable Polymers in Seawater and Freshwater. Glob. Chall. 2017, 1, 1700048. [Google Scholar] [CrossRef]
- Ndazi, B. Characterization of Hydrolytic Degradation of Polylactic Acid/Rice Hulls Composites in Water at Different Temperatures. Express Polym. Lett. 2011, 5, 119–131. [Google Scholar] [CrossRef]
- Huang, D.; Hu, Z.-D.; Liu, T.-Y.; Lu, B.; Zhen, Z.-C.; Wang, G.-X.; Ji, J.-H. Seawater Degradation of PLA Accelerated by Water-Soluble PVA. e-Polymers 2020, 20, 759–772. [Google Scholar] [CrossRef]
- Marín, A.; Feijoo, P.; de Llanos, R.; Carbonetto, B.; González-Torres, P.; Tena-Medialdea, J.; García-March, J.R.; Gámez-Pérez, J.; Cabedo, L. Microbiological Characterization of the Biofilms Colonizing Bioplastics in Natural Marine Conditions: A Comparison between PHBV and PLA. Microorganisms 2023, 11, 1461. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, L.; Xiao, P.; Huang, Y.; Chen, P.; Wang, X.; Gu, J.; Zhang, J.; Chen, T. Biodegradable PLA Nonwoven Fabric with Controllable Wettability for Efficient Water Purification and Photocatalysis Degradation. ACS Sustain. Chem. Eng. 2018, 6, 2445–2452. [Google Scholar] [CrossRef]
- Deroiné, M.; Le Duigou, A.; Corre, Y.-M.; Le Gac, P.-Y.; Davies, P.; César, G.; Bruzaud, S. Accelerated Ageing of Polylactide in Aqueous Environments: Comparative Study between Distilled Water and Seawater. Polym. Degrad. Stab. 2014, 108, 319–329. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An Overview of the Recent Developments in Polylactide (PLA) Research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef]
- Odobel, C.; Dussud, C.; Philip, L.; Derippe, G.; Lauters, M.; Eyheraguibel, B.; Burgaud, G.; Ter Halle, A.; Meistertzheim, A.-L.; Bruzaud, S.; et al. Bacterial Abundance, Diversity and Activity During Long-Term Colonization of Non-Biodegradable and Biodegradable Plastics in Seawater. Front. Microbiol. 2021, 12, 734782. [Google Scholar] [CrossRef]
- Jacquin, J.; Callac, N.; Cheng, J.; Giraud, C.; Gorand, Y.; Denoual, C.; Pujo-Pay, M.; Conan, P.; Meistertzheim, A.-L.; Barbe, V.; et al. Microbial Diversity and Activity During the Biodegradation in Seawater of Various Substitutes to Conventional Plastic Cotton Swab Sticks. Front. Microbiol. 2021, 12, 604395. [Google Scholar] [CrossRef]
- Kawai, F.; Kawabata, T.; Oda, M. Current Knowledge on Enzymatic PET Degradation and Its Possible Application to Waste Stream Management and Other Fields. Appl. Microbiol. Biotechnol. 2019, 103, 4253–4268. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Hecht, K.; Buller, R. Enzymatic PET Degradation. Chimia 2019, 73, 743. [Google Scholar] [CrossRef] [PubMed]
- Sang, T.; Wallis, C.J.; Hill, G.; Britovsek, G.J.P. Polyethylene Terephthalate Degradation under Natural and Accelerated Weathering Conditions. Eur. Polym. J. 2020, 136, 109873. [Google Scholar] [CrossRef]
- Ahmaditabatabaei, S.; Kyazze, G.; Iqbal, H.M.N.; Keshavarz, T. Fungal Enzymes as Catalytic Tools for Polyethylene Terephthalate (PET) Degradation. J. Fungi 2021, 7, 931. [Google Scholar] [CrossRef]
- Yao, J.; Liu, Y.; Gu, Z.; Zhang, L.; Guo, Z. Deconstructing PET: Advances in Enzyme Engineering for Sustainable Plastic Degradation. Chem. Eng. J. 2024, 497, 154183. [Google Scholar] [CrossRef]
- Fotopoulou, K.N.; Karapanagioti, H.K. Degradation of Various Plastics in the Environment. In Hazardous Chemicals Associated with Plastics in the Marine Environment; Takada, H., Karapanagioti, H.K., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 71–92. ISBN 978-3-319-95568-1. [Google Scholar]
- Khairul Anuar, N.F.S.; Huyop, F.; Ur-Rehman, G.; Abdullah, F.; Normi, Y.M.; Sabullah, M.K.; Abdul Wahab, R. An Overview into Polyethylene Terephthalate (PET) Hydrolases and Efforts in Tailoring Enzymes for Improved Plastic Degradation. Int. J. Mol. Sci. 2022, 23, 12644. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, Y.; Zhang, L.; Wu, J.; Zeng, X.; Shi, X.; Liu, L.; Chen, J. A Comprehensive Review on Enzymatic Biodegradation of Polyethylene Terephthalate. Environ. Res. 2024, 240, 117427. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Pan, H.; Kai, L.; Han, K.; Lian, J. Microbial Degradation and Valorization of Poly(Ethylene Terephthalate) (PET) Monomers. World J. Microbiol. Biotechnol. 2022, 38, 89. [Google Scholar] [CrossRef]
- Webb, H.K.; Arnott, J.; Crawford, R.J.; Ivanova, E.P. Plastic Degradation and Its Environmental Implications with Special Reference to Poly(Ethylene Terephthalate). Polymers 2013, 5, 1–18. [Google Scholar] [CrossRef]
- Taib, N.-A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Julaihi, M.R.M.B.; Khan, A. A Review on Poly Lactic Acid (PLA) as a Biodegradable Polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic Acid (PLA) Synthesis and Modifications: A Review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA Composites: From Production to Properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-Based Nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Bajpai, P.K.; Singh, I.; Madaan, J. Development and Characterization of PLA-Based Green Composites: A Review. J. Thermoplast. Compos. Mater. 2014, 27, 52–81. [Google Scholar] [CrossRef]
- Inkinen, S.; Hakkarainen, M.; Albertsson, A.-C.; Södergård, A. From Lactic Acid to Poly(Lactic Acid) (PLA): Characterization and Analysis of PLA and Its Precursors. Biomacromolecules 2011, 12, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, T.; Kao, N. PLA Based Biopolymer Reinforced with Natural Fibre: A Review. J. Polym. Environ. 2011, 19, 714–725. [Google Scholar] [CrossRef]
- Trivedi, A.K.; Gupta, M.K.; Singh, H. PLA Based Biocomposites for Sustainable Products: A Review. Adv. Ind. Eng. Polym. Res. 2023, 6, 382–395. [Google Scholar] [CrossRef]
- Bergström, J.S.; Hayman, D. An Overview of Mechanical Properties and Material Modeling of Polylactide (PLA) for Medical Applications. Ann. Biomed. Eng. 2016, 44, 330–340. [Google Scholar] [CrossRef]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and Safety of PLA and Its Copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Amass, W.; Amass, A.; Tighe, B. A Review of Biodegradable Polymers: Uses, Current Developments in the Synthesis and Characterization of Biodegradable Polyesters, Blends of Biodegradable Polymers and Recent Advances in Biodegradation Studies. Polym. Int. 1998, 47, 89–144. [Google Scholar] [CrossRef]
- Kawai, F. Polylactic Acid (PLA)-Degrading Microorganisms and PLA Depolymerases. In ACS Symposium Series; Cheng, H.N., Gross, R.A., Eds.; American Chemical Society: Washington, DC, USA, 2010; Voluem 1043, pp. 405–414. ISBN 978-0-8412-2581-7. [Google Scholar]
- Pranamuda, H.; Tokiwa, Y.; Tanaka, H. Polylactide Degradation by an Amycolatopsis sp. Appl. Environ. Microbiol. 1997, 63, 1637–1640. [Google Scholar] [CrossRef]
- Tomita, K.; Tsuji, H.; Nakajima, T.; Kikuchi, Y.; Ikarashi, K.; Ikeda, N. Degradation of Poly(d-Lactic Acid) by a Thermophile. Polym. Degrad. Stab. 2003, 81, 167–171. [Google Scholar] [CrossRef]
- Ikura, Y.; Kudo, T. Isolation of a Microorganism Capable of Degrading Poly-(L-Lactide). J. Gen. Appl. Microbiol. 1999, 45, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Yamagata, Y.; Abe, K.; Hasegawa, F.; Machida, M.; Ishioka, R.; Gomi, K.; Nakajima, T. Purification and Characterization of a Biodegradable Plastic-Degrading Enzyme from Aspergillus Oryzae. Appl. Microbiol. Biotechnol. 2005, 67, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Lipsa, R.; Tudorachi, N.; Darie-Nita, R.N.; Oprică, L.; Vasile, C.; Chiriac, A. Biodegradation of Poly(Lactic Acid) and Some of Its Based Systems with Trichoderma viride. Int. J. Biol. Macromol. 2016, 88, 515–526. [Google Scholar] [CrossRef]
- Masaki, K.; Kamini, N.R.; Ikeda, H.; Iefuji, H. Cutinase-Like Enzyme from the Yeast Cryptococcus Sp. Strain S-2 Hydrolyzes Polylactic Acid and Other Biodegradable Plastics. Appl. Environ. Microbiol. 2005, 71, 7548–7550. [Google Scholar] [CrossRef]
- Chomchoei, A.; Pathom-aree, W.; Yokota, A.; Kanongnuch, C.; Lumyong, S. Amycolatopsis Thailandensis sp. nov., a Poly(l-Lactic Acid)-Degrading Actinomycete, Isolated from Soil. Int. J. Syst. Evol. Microbiol. 2011, 61, 839–843. [Google Scholar] [CrossRef]
- Penkhrue, W.; Khanongnuch, C.; Masaki, K.; Pathom-aree, W.; Punyodom, W.; Lumyong, S. Isolation and Screening of Biopolymer-Degrading Microorganisms from Northern Thailand. World J. Microbiol. Biotechnol. 2015, 31, 1431–1442. [Google Scholar] [CrossRef]
- Sukkhum, S.; Tokuyama, S.; Tamura, T.; Kitpreechavanich, V. A Novel Poly (L-Lactide) Degrading Actinomycetes Isolated from Thai Forest Soil, Phylogenic Relationship and the Enzyme Characterization. J. Gen. Appl. Microbiol. 2009, 55, 459–467. [Google Scholar] [CrossRef]
- Hanphakphoom, S.; Maneewong, N.; Sukkhum, S.; Tokuyama, S.; Kitpreechavanich, V. Characterization of Poly(L-Lactide)-Degrading Enzyme Produced by Thermophilic Filamentous Bacteria Laceyella Sacchari LP175. J. Gen. Appl. Microbiol. 2014, 60, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Kuroki, Y.; Nagai, K. Isolation of Thermophiles Degrading Poly(l-Lactic Acid). J. Biosci. Bioeng. 1999, 87, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Janczak, K.; Hrynkiewicz, K.; Znajewska, Z.; Dąbrowska, G. Use of Rhizosphere Microorganisms in the Biodegradation of PLA and PET Polymers in Compost Soil. Int. Biodeterior. Biodegrad. 2018, 130, 65–75. [Google Scholar] [CrossRef]
- Jarerat, A.; Tokiwa, Y.; Tanaka, H. Production of Poly(l-Lactide)-Degrading Enzyme by Amycolatopsis Orientalis for Biological Recycling of Poly(l-Lactide). Appl. Microbiol. Biotechnol. 2006, 72, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Jarerat, A.; Tokiwa, Y.; Tanaka, H. Poly(l-Lactide) Degradation by Kibdelosporangium aridum. Biotechnol. Lett. 2003, 25, 2035–2038. [Google Scholar] [CrossRef]
- Weng, Y.-X.; Wang, L.; Zhang, M.; Wang, X.-L.; Wang, Y.-Z. Biodegradation Behavior of P(3HB,4HB)/PLA Blends in Real Soil Environments. Polym. Test. 2013, 32, 60–70. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and Biodegradation of Poly(Lactide). Appl. Microbiol. Biotechnol. 2006, 72, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Konkit, M.; Lumyong, S. Poly(Lactide) Degradation By Pseudonocardia Alni. Chiang Mai J. Sci. 2012, 39, 128–132. [Google Scholar]
- Apinya, T.; Sombatsompop, N.; Prapagdee, B. Selection of a Pseudonocardia Sp. RM423 That Accelerates the Biodegradation of Poly(Lactic) Acid in Submerged Cultures and in Soil Microcosms. Int. Biodeterior. Biodegrad. 2015, 99, 23–30. [Google Scholar] [CrossRef]
- Nakamura, K.; Tomita, T.; Abe, N.; Kamio, Y. Purification and Characterization of an Extracellular Poly(l-Lactic Acid) Depolymerase from a Soil Isolate, Amycolatopsis Sp. Strain K104-1. Appl. Environ. Microbiol. 2001, 67, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Jarerat, A.; Tokiwa, Y. Poly(L-Lactide) Degradation by Saccharothrix Waywayandensis. Biotechnol. Lett. 2003, 25, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Akutsu-Shigeno, Y.; Teeraphatpornchai, T.; Teamtisong, K.; Nomura, N.; Uchiyama, H.; Nakahara, T.; Nakajima-Kambe, T. Cloning and Sequencing of a Poly(Dl-Lactic Acid) Depolymerase Gene from Paenibacillus Amylolyticus Strain TB-13 and Its Functional Expression in Escherichia Coli. Appl. Environ. Microbiol. 2003, 69, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Jarerat, A.; Tokiwa, Y. Degradation of Poly(L-Lactide) by a Fungus. Macromol. Biosci. 2001, 1, 136–140. [Google Scholar] [CrossRef]
- Sukhumaporn, S.; Shinji, T.; Prachumporn, K.; Tomohiko, T.; Yuumi, I.; Vichien, K. A Novel Poly (L-Lactide) Degrading Thermophilic Actinomycetes, Actinomadura Keratinilytica Strain T16-1 and Pla Sequencing. Afr. J. Microbiol. Res. 2011, 5, 2575–2582. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and Biotic Environmental Degradation of the Bioplastic Polymer Poly(Lactic Acid): A Review. Polym. Degrad. Stab. 2017, 137, 122–130. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Râpă, M.; Darie-Niţă, R.N.; Mitelut, A.C.; Popa, E.E.; Popescu, P.A.; Draghici, M.C.; Popa, M.E. Study of the Soil Burial Degradation of Some PLA/CS Biocomposites. Compos. Part B Eng. 2018, 142, 251–262. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Robson, G.D. The Influence of Biotic and Abiotic Factors on the Rate of Degradation of Poly(Lactic) Acid (PLA) Coupons Buried in Compost and Soil. Polym. Degrad. Stab. 2013, 98, 2063–2071. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Kim, K.-H.; Park, J.-W.; Deep, A. Hydrolytic Degradation of Polylactic Acid (PLA) and Its Composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Brzeska, J.; Heimowska, A.; Sikorska, W.; Jasińska-Walc, L.; Kowalczuk, M.; Rutkowska, M. Chemical and Enzymatic Hydrolysis of Polyurethane/Polylactide Blends. Int. J. Polym. Sci. 2015, 2015, e795985. [Google Scholar] [CrossRef]
- Krasowska, K.; Heimowska, A. Degradability of Polylactide in Natural Aqueous Environments. Water 2023, 15, 198. [Google Scholar] [CrossRef]
- Piemonte, V.; Gironi, F. Kinetics of Hydrolytic Degradation of PLA. J. Polym. Environ. 2013, 21, 313–318. [Google Scholar] [CrossRef]
- Iñiguez-Franco, F.; Auras, R.; Burgess, G.; Holmes, D.; Fang, X.; Rubino, M.; Soto-Valdez, H. Concurrent Solvent Induced Crystallization and Hydrolytic Degradation of PLA by Water-Ethanol Solutions. Polymer 2016, 99, 315–323. [Google Scholar] [CrossRef]
- Moreno Nieto, D.; Alonso-García, M.; Pardo-Vicente, M.-A.; Rodríguez-Parada, L. Product Design by Additive Manufacturing for Water Environments: Study of Degradation and Absorption Behavior of PLA and PETG. Polymers 2021, 13, 1036. [Google Scholar] [CrossRef] [PubMed]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(Lactic Acid)—Mass Production, Processing, Industrial Applications, and End of Life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Rom, M.; Fabia, J.; Grübel, K.; Sarna, E.; Graczyk, T.; Janicki, J. Study of the Biodegradability of Polylactide Fibers in Wastewater Treatment Processes. Polimery 2017, 62, 834–840. [Google Scholar] [CrossRef]
- Pattanasuttichonlakul, W.; Sombatsompop, N.; Prapagdee, B. Accelerating Biodegradation of PLA Using Microbial Consortium from Dairy Wastewater Sludge Combined with PLA-Degrading Bacterium. Int. Biodeterior. Biodegrad. 2018, 132, 74–83. [Google Scholar] [CrossRef]
- Shi, B.; Palfery, D. Temperature-Dependent Polylactic Acid (PLA) Anaerobic Biodegradability. Int. J. Environ. Waste Manag. 2012, 10, 297–306. [Google Scholar] [CrossRef]
- Walczak, M.; Swiontek Brzezinska, M.; Sionkowska, A.; Michalska, M.; Jankiewicz, U.; Deja-Sikora, E. Biofilm Formation on the Surface of Polylactide during Its Biodegradation in Different Environments. Colloids Surf. B Biointerfaces 2015, 136, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Swiontek Brzezinska, M.; Walczak, M.; Kalwasińska, A.; Richert, A.; Świątczak, J.; Deja-Sikora, E.; Burkowska-But, A. Biofilm Formation during Biodegradation of Polylactide, Poly (3,4 Hydroxybutyrate) and Poly(ε-Caprolactone) in Activated Sludge. Int. J. Biol. Macromol. 2020, 159, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Morohoshi, T.; Oi, T.; Aiso, H.; Suzuki, T.; Okura, T.; Sato, S. Biofilm Formation and Degradation of Commercially Available Biodegradable Plastic Films by Bacterial Consortiums in Freshwater Environments. Microbes Environ. 2018, 33, 332–335. [Google Scholar] [CrossRef]
- Delacuvellerie, A.; Benali, S.; Cyriaque, V.; Moins, S.; Raquez, J.-M.; Gobert, S.; Wattiez, R. Microbial Biofilm Composition and Polymer Degradation of Compostable and Non-Compostable Plastics Immersed in the Marine Environment. J. Hazard. Mater. 2021, 419, 126526. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Kim, C.; Moon, J.; Heo, J.; Jung, S.P.; Kim, J.R. Polymer Film-Based Screening and Isolation of Polylactic Acid (PLA)-Degrading Microorganisms. J. Microbiol. Biotechnol. 2017, 27, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Ribba, L.; Lopretti, M.; Oca-Vásquez, G.M.d.; Batista, D.; Goyanes, S.; Vega-Baudrit, J.R. Biodegradable Plastics in Aquatic Ecosystems: Latest Findings, Research Gaps, and Recommendations. Environ. Res. Lett. 2022, 17, 033003. [Google Scholar] [CrossRef]
- Flemming, H.-C. Relevance of Biofilms for the Biodeterioration of Surfaces of Polymeric Materials*. Polym. Degrad. Stab. 1998, 59, 309–315. [Google Scholar] [CrossRef]
- Tsuji, H.; Suzuyoshi, K. Environmental Degradation of Biodegradable Polyesters 1. Poly(ε-Caprolactone), Poly[(R)-3-Hydroxybutyrate], and Poly(L-Lactide) Films in Controlled Static Seawater. Polym. Degrad. Stab. 2002, 75, 347–355. [Google Scholar] [CrossRef]
- Tsuji, H.; Suzuyoshi, K. Environmental Degradation of Biodegradable Polyesters 2. Poly(ε-Caprolactone), Poly[(R)-3-Hydroxybutyrate], and Poly(L-Lactide) Films in Natural Dynamic Seawater. Polym. Degrad. Stab. 2002, 75, 357–365. [Google Scholar] [CrossRef]
- Żenkiewicz, M.; Malinowski, R.; Rytlewski, P.; Richert, A.; Sikorska, W.; Krasowska, K. Some Composting and Biodegradation Effects of Physically or Chemically Crosslinked Poly(Lactic Acid). Polym. Test. 2012, 31, 83–92. [Google Scholar] [CrossRef]
- Martin, R.T.; Camargo, L.P.; Miller, S.A. Marine-Degradable Polylactic Acid. Green Chem. 2014, 16, 1768–1773. [Google Scholar] [CrossRef]
- ISO 14855-2:2018; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials Under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide—Part 2: Gravimetric Measurement of Carbon Dioxide Evolved in a Laboratory-Scale Test. International Organization for Standardization: Geneva, Switzerland, 2018.
- Gautieri, A.; Mezzanzanica, A.; Motta, A.; Redealli, A.; Vesentini, S. Atomistic Modeling of Water Diffusion in Hydrolytic Biomaterials. J. Mol. Model. 2012, 18, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhou, S.; Gao, X.; Yang, Z.; Sun, L.; Zhang, D. A Multi-Scale Method for Modeling Degradation of Bioresorbable Polyesters. Acta Biomater. 2017, 50, 462–475. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, J.; Han, X.; Sinka, C.; Ding, L. A Phenomenological Model for the Degradation of Biodegradable Polymers. Biomaterials 2008, 29, 3393–3401. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Pan, J.; Buchanan, F.; Weir, N.; Farrar, D. Analysis of Degradation Data of Poly(l-Lactide–Co-l,d-Lactide) and Poly(l-Lactide) Obtained at Elevated and Physiological Temperatures Using Mathematical Models. Acta Biomater. 2010, 6, 3882–3889. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Pan, J. A Model for Simultaneous Crystallisation and Biodegradation of Biodegradable Polymers. Biomaterials 2009, 30, 423–430. [Google Scholar] [CrossRef]
- Gleadall, A.; Pan, J.; Atkinson, H. A Simplified Theory of Crystallisation Induced by Polymer Chain Scissions for Biodegradable Polyesters. Polym. Degrad. Stab. 2012, 97, 1616–1620. [Google Scholar] [CrossRef]
- Arosio, P.; Busini, V.; Perale, G.; Moscatelli, D.; Masi, M. A New Model of Resorbable Device Degradation and Drug Release—Part I: Zero Order Model. Polym. Int. 2008, 57, 912–920. [Google Scholar] [CrossRef]
- Perale, G.; Arosio, P.; Moscatelli, D.; Barri, V.; Müller, M.; Maccagnan, S.; Masi, M. A New Model of Resorbable Device Degradation and Drug Release: Transient 1-Dimension Diffusional Model. J. Control. Release 2009, 136, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Righetti, M.C.; Gazzano, M.; Di Lorenzo, M.L.; Androsch, R. Enthalpy of Melting of A′- and α-Crystals of Poly(l-Lactic Acid). Eur. Polym. J. 2015, 70, 215–220. [Google Scholar] [CrossRef]
- ISO 11734:1995; Water Quality—Evaluation of the “Ultimate” Anaerobic Biodegradability of Organic Compounds in Digested Sludge—Method by Measurement of the Biogas Production. International Organization for Standardization: Geneva, Switzerland, 1995.
- Molina, J.a.E.; Clapp, C.E.; Larson, W.E. Potentially Mineralizable Nitrogen in Soil: The Simple Exponential Model Does Not Apply for the First 12 Weeks of Incubation. Soil Sci. Soc. Am. J. 1980, 44, 442–443. [Google Scholar] [CrossRef]
- Jones, C.A. Estimation of an Active Fraction of Soil Nitrogen. Commun. Soil Sci. Plant Anal. 1984, 15, 23–32. [Google Scholar] [CrossRef]
- Bonde, T.A.; Rosswall, T. Seasonal Variation of Potentially Mineralizable Nitrogen in Four Cropping Systems. Soil Sci. Soc. Am. J. 1987, 51, 1508–1514. [Google Scholar] [CrossRef]
- Murwira, H.K.; Kirchmann, H.; Swift, M.J. The Effect of Moisture on the Decomposition Rate of Cattle Manure. Plant Soil 1990, 122, 197–199. [Google Scholar] [CrossRef]
- Komilis, D.P. A Kinetic Analysis of Solid Waste Composting at Optimal Conditions. Waste Manag. 2006, 26, 82–91. [Google Scholar] [CrossRef]
- Gan, H.; Okada, T.; Kimura, S.; Kasuya, K.; Iwata, T. Manufacture, Physical Properties, and Degradation of Biodegradable Polyester Microbeads. Polym. Degrad. Stab. 2023, 208, 110239. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S.; Shetti, N.P.; Nadagouda, M.N.; Aminabhavi, T.M. Microplastics in the Environment: Occurrence, Perils, and Eradication. Chem. Eng. J. 2021, 408, 127317. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Liang, B.; Peng, Z.; Lin, C. Generation of Microplastic Particles during Degradation of Polycarbonate Films in Various Aqueous Media and Their Characterization. J. Hazard. Mater. 2021, 415, 125640. [Google Scholar] [CrossRef]
- Othman, A.R.; Hasan, H.A.; Muhamad, M.H.; Ismail, N.I.; Abdullah, S.R.S. Microbial Degradation of Microplastics by Enzymatic Processes: A Review. Environ. Chem. Lett. 2021, 19, 3057–3073. [Google Scholar] [CrossRef]
- Bacha, A.-U.-R.; Nabi, I.; Zhang, L. Mechanisms and the Engineering Approaches for the Degradation of Microplastics. ACS EST Eng. 2021, 1, 1481–1501. [Google Scholar] [CrossRef]
- Ahmed, Q.; Ali, Q.M.; Bat, L.; Öztekin, A.; Memon, S.; Baloch, A. Preliminary Study on Abundance of Microplastic in Sediments and Water Samples Along the Coast of Pakistan (Sindh and Balochistan)-Northern Arabian Sea. Turk. J. Fish. Aquat. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of Microplastics in Raw and Treated Drinking Water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Ainali, N.M.; Kalaronis, D.; Evgenidou, E.; Kyzas, G.Z.; Bobori, D.C.; Kaloyianni, M.; Yang, X.; Bikiaris, D.N.; Lambropoulou, D.A. Do Poly(Lactic Acid) Microplastics Instigate a Threat? A Perception for Their Dynamic towards Environmental Pollution and Toxicity. Sci. Total Environ. 2022, 832, 155014. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Sherrell, P.C.; Chen, J.; Wang, H.; Zhang, W.; Yang, J. How to Build a Microplastics-Free Environment: Strategies for Microplastics Degradation and Plastics Recycling. Adv. Sci. 2022, 9, 2103764. [Google Scholar] [CrossRef] [PubMed]
- Munno, K.; Helm, P.A.; Jackson, D.A.; Rochman, C.; Sims, A. Impacts of Temperature and Selected Chemical Digestion Methods on Microplastic Particles. Environ. Toxicol. Chem. 2018, 37, 91–98. [Google Scholar] [CrossRef]
- Grbic, J.; Nguyen, B.; Guo, E.; You, J.B.; Sinton, D.; Rochman, C.M. Magnetic Extraction of Microplastics from Environmental Samples. Environ. Sci. Technol. Lett. 2019, 6, 68–72. [Google Scholar] [CrossRef]
- Kedzierski, M.; Le Tilly, V.; Bourseau, P.; Bellegou, H.; César, G.; Sire, O.; Bruzaud, S. Microplastics Elutriation from Sandy Sediments: A Granulometric Approach. Mar. Pollut. Bull. 2016, 107, 315–323. [Google Scholar] [CrossRef]
- Fuller, S.; Gautam, A. A Procedure for Measuring Microplastics Using Pressurized Fluid Extraction. Environ. Sci. Technol. 2016, 50, 5774–5780. [Google Scholar] [CrossRef] [PubMed]
- Radford, F.; Zapata-Restrepo, L.M.; Horton, A.A.; Hudson, M.D.; Shaw, P.J.; Williams, I.D. Developing a Systematic Method for Extraction of Microplastics in Soils. Anal. Methods 2021, 13, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Felsing, S.; Kochleus, C.; Buchinger, S.; Brennholt, N.; Stock, F.; Reifferscheid, G. A New Approach in Separating Microplastics from Environmental Samples Based on Their Electrostatic Behavior. Environ. Pollut. 2018, 234, 20–28. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Zhang, X.; Hu, J. Methods for Separating Microplastics from Complex Solid Matrices: Comparative Analysis. J. Hazard. Mater. 2021, 409, 124640. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.; Wei, M.; Zheng, Z.; Vu, D.D.; Bui, T.T.X.; Huang, X. Preparation, Characterization, and Properties of Graphene Oxide/Urushiol-Formaldehyde Polymer Composite Coating. J. Coat. Technol. Res. 2018, 15, 1343–1356. [Google Scholar] [CrossRef]
- Crichton, E.M.; Noël, M.; Gies, E.A.; Ross, P.S. A Novel, Density-Independent and FTIR-Compatible Approach for the Rapid Extraction of Microplastics from Aquatic Sediments. Anal. Methods 2017, 9, 1419–1428. [Google Scholar] [CrossRef]
- Qin, Q.; Yang, Y.; Yang, C.; Zhang, L.; Yin, H.; Yu, F.; Ma, J. Degradation and Adsorption Behavior of Biodegradable Plastic PLA under Conventional Weathering Conditions. Sci. Total Environ. 2022, 842, 156775. [Google Scholar] [CrossRef]
- Fan, X.; Gan, R.; Liu, J.; Xie, Y.; Xu, D.; Xiang, Y.; Su, J.; Teng, Z.; Hou, J. Adsorption and Desorption Behaviors of Antibiotics by Tire Wear Particles and Polyethylene Microplastics with or without Aging Processes. Sci. Total Environ. 2021, 771, 145451. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Yu, X.; Liu, J.; Xia, T.; Wang, T.; Jia, H.; Guo, X. Fenton Aging Significantly Affects the Heavy Metal Adsorption Capacity of Polystyrene Microplastics. Sci. Total Environ. 2020, 722, 137762. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hu, X.; Qin, L.; Yin, D. Evaluating the Effect of Different Modified Microplastics on the Availability of Polycyclic Aromatic Hydrocarbons. Water Res. 2020, 170, 115290. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lu, K.; Li, J.; Wu, X.; Qian, L.; Wang, M.; Gao, S. Effect of Aging on Adsorption Behavior of Polystyrene Microplastics for Pharmaceuticals: Adsorption Mechanism and Role of Aging Intermediates. J. Hazard. Mater. 2020, 384, 121193. [Google Scholar] [CrossRef] [PubMed]
- Barlucchi, L.; Biale, G.; La Nasa, J.; Mattonai, M.; Pezzini, S.; Corti, A.; Castelvetro, V.; Modugno, F. Abiotic Degradation and Accelerated Ageing of Microplastics from Biodegradable and Recycled Materials in Artificial Seawater. Sci. Total Environ. 2024, 954, 176832. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, H.; Ołdak, D.; Malanowski, P.; Chaberska, H. Effect of Short Wavelength UV-Irradiation on Ageing of Polypropylene/Cellulose Compositions. Polym. Degrad. Stab. 2005, 88, 189–198. [Google Scholar] [CrossRef]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and Its Blends with Poly(Butylene Succinate) (PBS): A Brief Review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef]
- Navarro-Baena, I.; Sessini, V.; Dominici, F.; Torre, L.; Kenny, J.M.; Peponi, L. Design of Biodegradable Blends Based on PLA and PCL: From Morphological, Thermal and Mechanical Studies to Shape Memory Behavior. Polym. Degrad. Stab. 2016, 132, 97–108. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Gu, J.; Tan, H. Biodegradation Behavior and Modelling of Soil Burial Effect on Degradation Rate of PLA Blended with Starch and Wood Flour. Colloids Surf. B Biointerfaces 2017, 159, 800–808. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, X.; Cai, T. The Study of Surface Properties of ZrO2. Appl. Surf. Sci. 2004, 225, 7–13. [Google Scholar] [CrossRef]
- Ghanbariha, M.; Farvizi, M.; Ebadzadeh, T.; Alizadeh Samiyan, A. Effect of ZrO2 Particles on the Nanomechanical Properties and Wear Behavior of AlCoCrFeNi–ZrO2 High Entropy Alloy Composites. Wear 2021, 484–485, 204032. [Google Scholar] [CrossRef]
- Kuang, Q.; Wang, X.; Jiang, Z.; Xie, Z.; Zheng, L. High-Energy-Surface Engineered Metal Oxide Micro- and Nanocrystallites and Their Applications. Acc. Chem. Res. 2014, 47, 308–318. [Google Scholar] [CrossRef]
- D’Souza, L.; Suchopar, A.; Zhu, K.; Balyozova, D.; Devadas, M.; Richards, R.M. Preparation of Thermally Stable High Surface Area Mesoporous Tetragonal ZrO2 and Pt/ZrO2: An Active Hydrogenation Catalyst. Microporous Mesoporous Mater. 2006, 88, 22–30. [Google Scholar] [CrossRef]
- Hajilou, N.; Javaheri, M.; Ebadzadeh, T.; Farvizi, M. Investigation of the Electrochemical Behavior of AlCoCrFeNi–ZrO2 High Entropy Alloy Composites Prepared with Mechanical Alloying and Spark Plasma Sintering. J. Appl. Electrochem. 2024, 54, 457–466. [Google Scholar] [CrossRef]
- Luo, Y.-B.; Wang, X.-L.; Wang, Y.-Z. Effect of TiO2 Nanoparticles on the Long-Term Hydrolytic Degradation Behavior of PLA. Polym. Degrad. Stab. 2012, 97, 721–728. [Google Scholar] [CrossRef]
- Zhou, Q.; Xanthos, M. Nanoclay and Crystallinity Effects on the Hydrolytic Degradation of Polylactides. Polym. Degrad. Stab. 2008, 93, 1450–1459. [Google Scholar] [CrossRef]
- Pan, H.; Qiu, Z. Biodegradable Poly(l-Lactide)/Polyhedral Oligomeric Silsesquioxanes Nanocomposites: Enhanced Crystallization, Mechanical Properties, and Hydrolytic Degradation. Macromolecules 2010, 43, 1499–1506. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Kim, H.-I. Synthesis, Characterization, and Hydrolytic Degradation of Polylactide/Poly(Ethylene Glycol)/Nano-Silica Composite Films. J. Macromol. Sci. Part A 2012, 49, 348–354. [Google Scholar] [CrossRef]
- Qu, M.; Tu, H.; Amarante, M.; Song, Y.-Q.; Zhu, S.S. Zinc Oxide Nanoparticles Catalyze Rapid Hydrolysis of Poly(Lactic Acid) at Low Temperatures. J. Appl. Polym. Sci. 2014, 131, 40287. [Google Scholar] [CrossRef]
- Huang, Q.; Kimura, S.; Iwata, T. Development of Self-Degradable Aliphatic Polyesters by Embedding Lipases via Melt Extrusion. Polym. Degrad. Stab. 2021, 190, 109647. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Lammel, G.; Samara, C.; Ernst, M.; Wenk, J.; Torretta, V.; Voutsa, D.; Vollertsen, J.; Bucheli, T.D.; Godbersen, L.; et al. Innovative aspects of environmental chemistry and technology regarding air, water, and soil pollution. Environ. Sci. Pollut. Res. 2021, 28, 58958–58968. [Google Scholar] [CrossRef]
- Almeshal, I.; Tayeh, B.A.; Alyousef, R.; Alabduljabbar, H.; Mustafa Mohamed, A.; Alaskar, A. Use of Recycled Plastic as Fine Aggregate in Cementitious Composites: A Review. Constr. Build. Mater. 2020, 253, 119146. [Google Scholar] [CrossRef]
- McKeown, P.; Jones, M.D. The Chemical Recycling of PLA: A Review. Sustain. Chem. 2020, 1, 1–22. [Google Scholar] [CrossRef]
- Faraj, R.H.; Hama Ali, H.F.; Sherwani, A.F.H.; Hassan, B.R.; Karim, H. Use of Recycled Plastic in Self-Compacting Concrete: A Comprehensive Review on Fresh and Mechanical Properties. J. Build. Eng. 2020, 30, 101283. [Google Scholar] [CrossRef]
- Beltrán, F.R.; Infante, C.; de la Orden, M.U.; Martínez Urreaga, J. Mechanical Recycling of Poly(Lactic Acid): Evaluation of a Chain Extender and a Peroxide as Additives for Upgrading the Recycled Plastic. J. Clean. Prod. 2019, 219, 46–56. [Google Scholar] [CrossRef]
- Atakok, G.; Kam, M.; Koc, H.B. A Review of Mechanical and Thermal Properties of Products Printed with Recycled Filaments for Use in 3d Printers. Surf. Rev. Lett. 2022, 29, 2230002. [Google Scholar] [CrossRef]
- Beltrán, F.R.; Arrieta, M.P.; Moreno, E.; Gaspar, G.; Muneta, L.M.; Carrasco-Gallego, R.; Yáñez, S.; Hidalgo-Carvajal, D.; de la Orden, M.U.; Martínez Urreaga, J. Evaluation of the Technical Viability of Distributed Mechanical Recycling of PLA 3D Printing Wastes. Polymers 2021, 13, 1247. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Beltrán, F.R.; Arrieta, M.P.; Gaspar, G.; Muneta, L.M.; Carrasco-Gallego, R.; Yáñez, S.; Hidalgo-Carvajal, D.; Orden, M.U.d.l.; Urreaga, J.M. Technical Evaluation of Mechanical Recycling of PLA 3D Printing Wastes. Proceedings 2020, 69, 19. [Google Scholar] [CrossRef]
- Hong, J.-H.; Yu, T.; Park, S.-J.; Kim, Y.-H. Repetitive Recycling of 3D Printing PLA Filament as Renewable Resources on Mechanical and Thermal Loads. Int. J. Mod. Phys. B 2020, 34, 2040147. [Google Scholar] [CrossRef]
- Pinho, A.C.; Amaro, A.M.; Piedade, A.P. 3D Printing Goes Greener: Study of the Properties of Post-Consumer Recycled Polymers for the Manufacturing of Engineering Components. Waste Manag. 2020, 118, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Koca, N.; Aversa, C.; Barletta, M. Recycling of Poly(Lactic Acid)/Poly(Butylene Succinate) (PLA/PBS) Blends with High Amounts of Secondary Raw Material. J. Appl. Polym. Sci. 2023, 140, e54659. [Google Scholar] [CrossRef]
- Delgado-Aguilar, M.; Puig, R.; Sazdovski, I.; Fullana-i-Palmer, P. Polylactic Acid/Polycaprolactone Blends: On the Path to Circular Economy, Substituting Single-Use Commodity Plastic Products. Materials 2020, 13, 2655. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.N.; Redhwi, H.H.; Al-Arfaj, A.A.; Achilias, D.S. Chemical Recycling of PET in the Presence of the Bio-Based Polymers, PLA, PHB and PEF: A Review. Sustainability 2021, 13, 10528. [Google Scholar] [CrossRef]
- Lagazzo, A.; Moliner, C.; Bosio, B.; Botter, R.; Arato, E. Evaluation of the Mechanical and Thermal Properties Decay of PHBV/Sisal and PLA/Sisal Biocomposites at Different Recycle Steps. Polymers 2019, 11, 1477. [Google Scholar] [CrossRef] [PubMed]




| Materials and Condition | Mechanisms | Key Findings | Ref. |
|---|---|---|---|
| Materials: PLA Environment: Water |
|
| [6,94] |
| Materials: Crystalline, semi-crystalline, and amorphous PLA. Environment: HPLC 1-grade water Temperatures: 45, 65, 75, and 85 °C under an unbuffered condition |
|
| [16] |
| Materials: PLA Environment: Water Temperatures: 140–180 °C Condition: water/PLA ratio up to 50% of PLA by weight |
|
| [97] |
| Materials: PLA Environments: Water, water/ethanol Temperature: 40 °C |
|
| [98] |
| Materials: PLA Environments: Water, and saturated solutions of water with maritime salt and sugar together Temperature: 20 °C |
|
| [99] |
| Materials and Condition | Mechanisms | Key Findings | Ref. |
|---|---|---|---|
| Materials: PLA Environment: wastewater Temperatures: 36, 56 °C |
|
| [101] |
| Materials: PLA Environment: wastewater sludges from dairy, rice vermicelli and coconut milk factories in soil Temperatures: 37 °C |
|
| [102] |
| Materials: PLA Environment: digested sludge Temperatures: 50, 65 °C |
|
| [103] |
| Materials: PLA Environments: activated sludge Temperature: 20 °C |
|
| [105] |
| Materials: PLA Environments: mixture of landfill soil and sludge Temperature: 61 °C |
|
| [28] |
| Materials: semi-crystalline PLA Environments: accelerated landfill condition Temperature: 21, 35 °C |
|
| [27] |
| Materials: semi-crystalline PLA Environments: landfill Temperature: 35, 55 °C |
|
| [29] |
| Aspect | Hydrolytic Degradation Model | Biodegradation Model |
|---|---|---|
| Purpose | Predict degradation rate of PLA and its mechanism in water media. | Facilitate study of biodegradable devices and degradation under composting conditions. |
| Key Mechanisms | Hydrolysis involving non-catalytic and autocatalytic reactions influenced by molecular concentration. | Two stages: primary degradation (cleaving long chains to oligomers) and ultimate degradation (microbial assimilation). |
| Main Equations | Ester bonds’ scission rate (first-order equation) Equation (6). | Hydrolysable carbon Equation (26). |
| Modeling Approach | Incorporates diffusion and crystallinity, utilizes finite element method for model solving. | Utilizes first-order kinetics; focuses on mineralization of carbon dioxide production. |
| Predictions/Outcomes | Controls degradation by reaction and diffusion interplay; predicts normalized molecular weight ) changes over time. | Cumulative CO2 production correlates well with experimental data under composting conditions. |
| Strengths | Comprehensive model by including temperature and crystallinity as influencing factors. | Straightforward experimental validation by either gas analyzer or titration method. |
| Drawbacks | Indirect experimental validation, which increases error probability using Equation (21). | Models are based on first-order kinetics, potentially oversimplifying complex degradation processes. |
| Aspect | Description/Findings | Ref. |
|---|---|---|
| Definition of Microplastics (MPs) | Small plastic pieces less than 5 mm resulting from the breakdown of larger plastics or intentionally manufactured. | [133,134,135,136] |
| Types of Microplastics |
| [131] |
| Challenges in Detection | Lack of standardized methods hinders assessment of MPs’ impact on soil environments; systematic approaches exist for isolation. | [133,134,135] |
| Degradation Techniques |
| [140,141,142,143,144,145,147,148,149,150,151,152,153,170] |
| Blend Composition Effects | Blending PLA with PBS, PCL, and PHAs can accelerate degradation but may still produce MPs. | [82,156,157] |
| Additive Effects on Degradation | Additives like metals and metal oxides such as TiO2 nanofillers and ZnO nanoparticles enhance hydrolysis and degradation rates. | [164,168] |
| Innovative Methods | Embedding enzymes during the melt extrusion or casting process to create self-degradable polymers. | [169] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajilou, N.; Mostafayi, S.S.; Yarin, A.L.; Shokuhfar, T. A Comparative Review on Biodegradation of Poly(Lactic Acid) in Soil, Compost, Water, and Wastewater Environments: Incorporating Mathematical Modeling Perspectives. AppliedChem 2025, 5, 1. https://doi.org/10.3390/appliedchem5010001
Hajilou N, Mostafayi SS, Yarin AL, Shokuhfar T. A Comparative Review on Biodegradation of Poly(Lactic Acid) in Soil, Compost, Water, and Wastewater Environments: Incorporating Mathematical Modeling Perspectives. AppliedChem. 2025; 5(1):1. https://doi.org/10.3390/appliedchem5010001
Chicago/Turabian StyleHajilou, Narjess, Seyed Sepehr Mostafayi, Alexander L. Yarin, and Tolou Shokuhfar. 2025. "A Comparative Review on Biodegradation of Poly(Lactic Acid) in Soil, Compost, Water, and Wastewater Environments: Incorporating Mathematical Modeling Perspectives" AppliedChem 5, no. 1: 1. https://doi.org/10.3390/appliedchem5010001
APA StyleHajilou, N., Mostafayi, S. S., Yarin, A. L., & Shokuhfar, T. (2025). A Comparative Review on Biodegradation of Poly(Lactic Acid) in Soil, Compost, Water, and Wastewater Environments: Incorporating Mathematical Modeling Perspectives. AppliedChem, 5(1), 1. https://doi.org/10.3390/appliedchem5010001






