Australian Native Lemongrass (Cymbopogon ambiguus A. Camus): An Underestimated Herbal Plant
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Extract Preparation
2.2. Chemicals, Reagents and Tested Microorganisms
2.3. Nutritional Composition Analysis
2.4. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.5. Determination of Ferric-Reducing Antioxidant Power of Plasma (FRAP Assay)
2.6. Determination of Citral Content
2.7. Determination of Antimicrobial Assay
2.8. Determination of Antidiabetic Capacity
2.9. Statistical Analyses
3. Results
3.1. Nutritional Composition
3.2. Phenolic Content and Antioxidant Properties
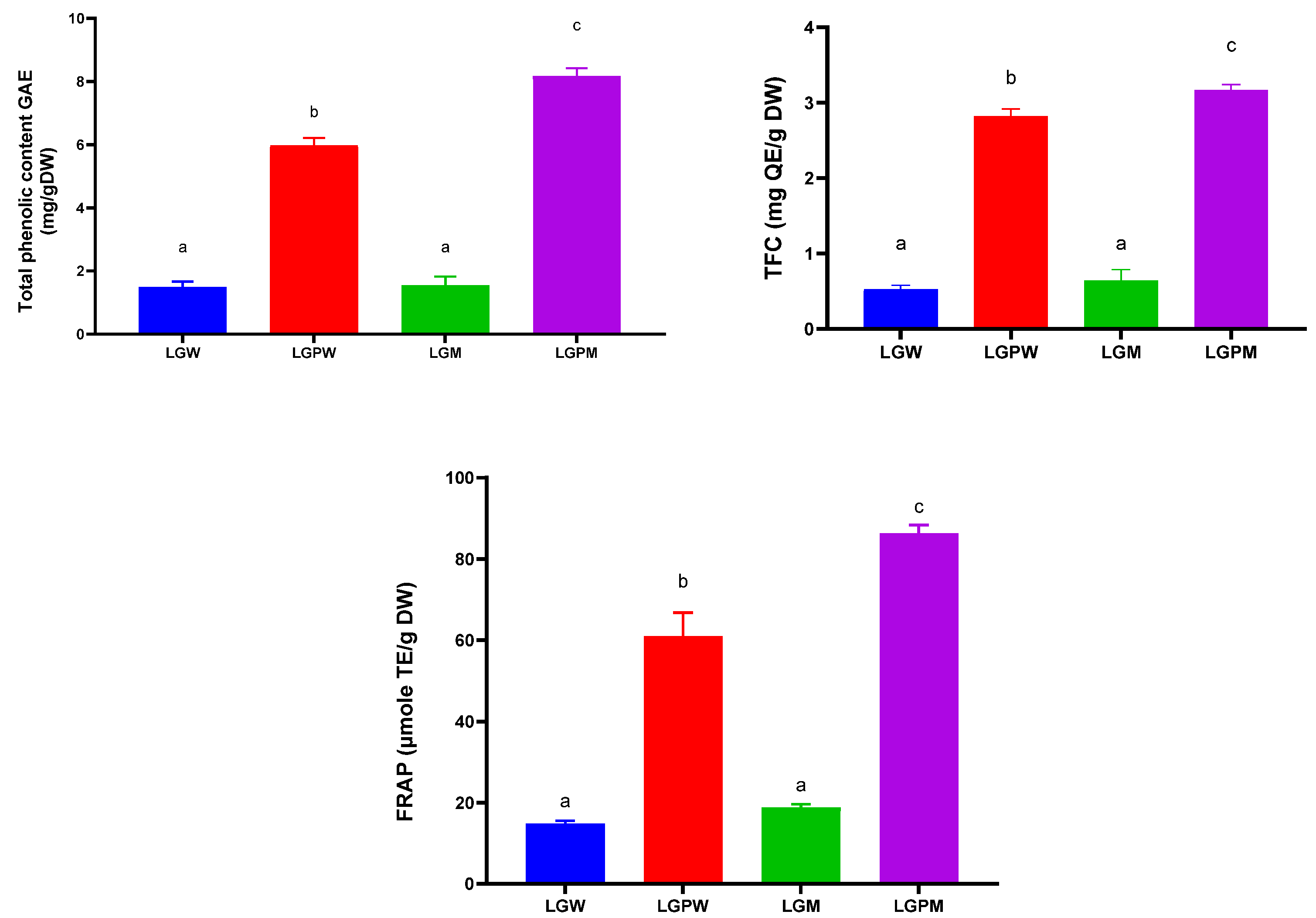
3.2.1. Total Phenolic Content
3.2.2. Total Flavonoid Content (TFC)
3.2.3. Antioxidant Properties—Ferric-Reducing Antioxidant Power of Plasma (FRAP)
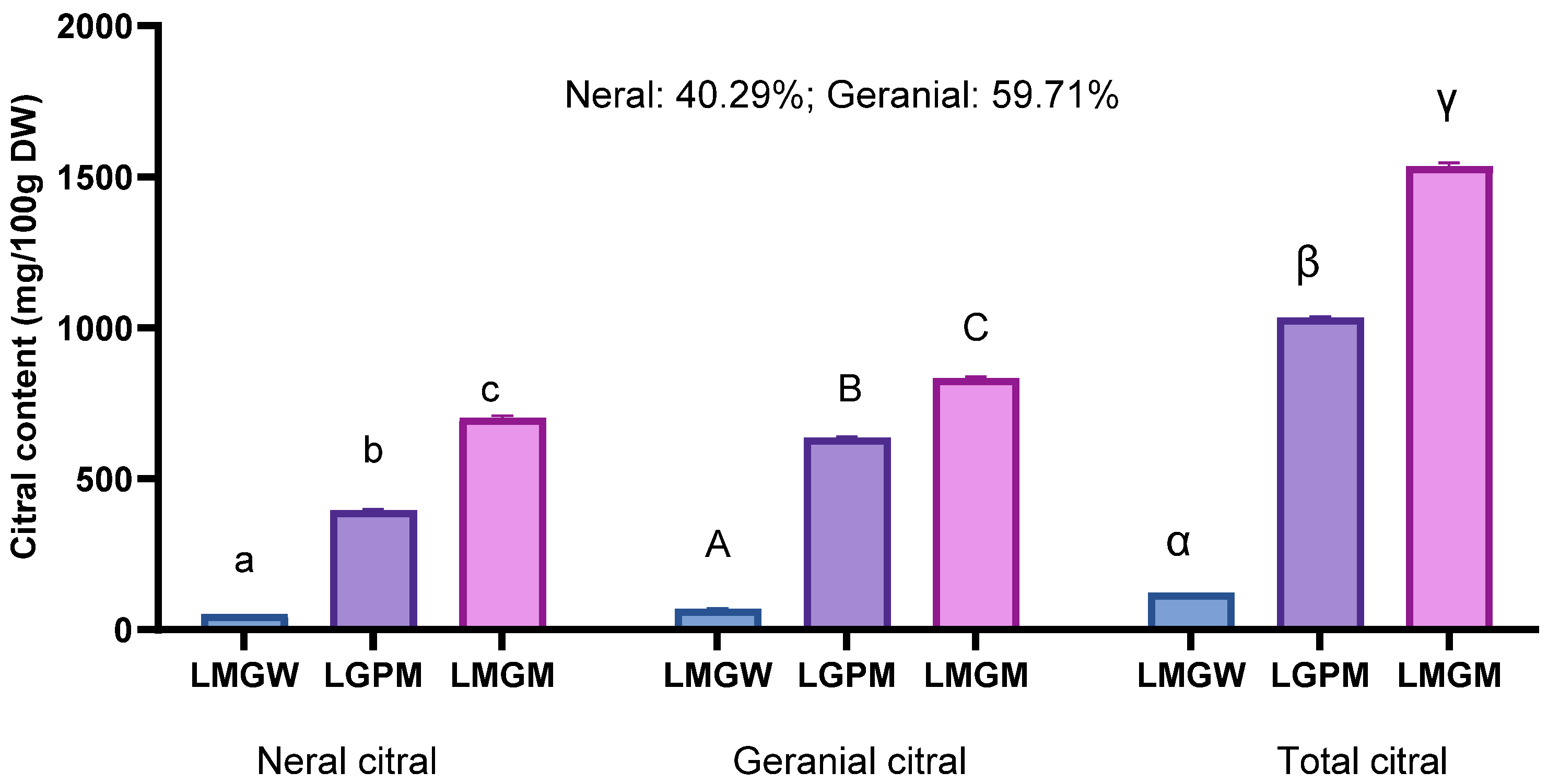
3.3. Citral Content
3.4. Antimicrobial Assay
3.5. Antidiabetic Assay
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grice, I.D.; Rogers, K.L.; Griffiths, L.R. Isolation of Bioactive Compounds That Relate to the Anti-Platelet Activity of Cymbopogon ambiguus. In Evidence-Based Complementary and Alternative Medicine; Hindawi Publishing Corporation: London, UK, 2011. [Google Scholar]
- Simpson, M.G. (Ed.) 7—Diversity and Classification of Flowering Plants: Amborellales, Nymphaeales, Austrobaileyales, Magnoliids, Ceratophyllales, and Monocots. In Plant Systematics, 2nd ed.; Academic Press: San Diego, CA, USA, 2010; pp. 181–274. [Google Scholar]
- Toungos, M.D. Lemongrass (Cymbopogon, L. Spreng) valuable grass but underutilized in Northern Nigeria. Int. J. Innov. Food Nutr. Sustain. Agric. 2019, 7, 6–14. [Google Scholar]
- Majewska, E.; Kozlowska, M.; Gruszczynska-Sekowska, E.; Kowalska, D.; Tarnowska, K. Lemongrass (Cymbopogon citratus) essential oil: Extraction, composition, bioactivity and uses for food preservation—A review. Pol. J. Food Nutr. Sci. 2019, 69, 327–341. [Google Scholar] [CrossRef]
- Tibenda, J.J.; Yi, Q.; Wang, X.; Zhao, Q. Review of phytomedicine, phytochemistry, ethnopharmacology, toxicology, and pharmacological activities of Cymbopogon genus. Front. Pharmacol. 2022, 13, 997918. [Google Scholar] [CrossRef] [PubMed]
- Gaba, J.; Bhardwaj, G.; Sharma, A. Lemongrass. In Antioxidants in Vegetables and Nuts-Properties and Health Benefits; Springer: Berlin/Heidelberg, Germany, 2020; pp. 75–103. [Google Scholar]
- Irfan, S.; Ranjha, M.M.A.N.; Nadeem, M.; Safdar, M.N.; Jabbar, S.; Mahmood, S.; Murtaza, M.A.; Ameer, K.; Ibrahim, S.A. Antioxidant Activity and Phenolic Content of Sonication- and Maceration-Assisted Ethanol and Acetone Extracts of Cymbopogon citratus Leaves. Separations 2022, 9, 244. [Google Scholar] [CrossRef]
- Le, Q.U.; Lay, H.L.; Wu, M.C. The isolation, structural characterization, and anticancer activity from the aerial parts of Cymbopogon flexuosus. J. Food Biochem. 2019, 43, e12718. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.; Shanbhoge, R.; Rao, B.; Adiga, S.K.; Upadhya, D.; Aithal, B.; Kumar, M. Preventive efficacy of hydroalcoholic extract of Cymbopogon citratus against radiation-induced DNA damage on V79 cells and free radical scavenging ability against radicals generated in vitro. Hum. Exp. Toxicol. 2009, 28, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Halabi, M.F.; Sheikh, B.Y. Anti-proliferative effect and phytochemical analysis of Cymbopogon citratus extract. BioMed Res. Int. 2014, 2014, 906239. [Google Scholar] [CrossRef]
- Bhatnagar, A. Chemical composition and antioxidant activity of essential oil of Cymbopogon flexuosus. J. Appl. Nat. Sci. 2020, 12, 25. [Google Scholar] [CrossRef]
- Silva, C.d.B.d.; Guterres, S.S.; Weisheimer, V.; Schapoval, E.E. Antifungal activity of the lemongrass oil and citral against Candida spp. Braz. J. Infect. Dis. 2008, 12, 63–66. [Google Scholar] [CrossRef]
- Leimann, F.V.; Gonçalves, O.H.; Machado, R.A.; Bolzan, A. Antimicrobial activity of microencapsulated lemongrass essential oil and the effect of experimental parameters on microcapsules size and morphology. Mater. Sci. Eng. C 2009, 29, 430–436. [Google Scholar] [CrossRef]
- Singh, B.R.; Singh, V.; Singh, R.K.; Ebibeni, N. Antimicrobial activity of lemongrass (Cymbopogon citratus) oil against microbes of environmental, clinical and food origin. Int. Res. J. Pharm. Pharmacol. 2011, 1, 228–236. [Google Scholar]
- Hajizadeh, M.R.; Maleki, H.; Barani, M.; Fahmidehkar, M.A.; Mahmoodi, M.; Torkzadeh-Mahani, M. In vitro cytotoxicity assay of D-limonene niosomes: An efficient nano-carrier for enhancing solubility of plant-extracted agents. Res. Pharm. Sci. 2019, 14, 448. [Google Scholar] [PubMed]
- Idrees, M.; Hakkim, F.L.; Naikoo, G.A.; Ul Hassan, I. Recent Advances in Extraction, Characterization, and Potential Use of Citral. In Natural Bio-Active Compounds: Volume 3: Biotechnology, Bioengineering, and Molecular Approaches; Springer: Berlin/Heidelberg, Germany, 2019; pp. 225–236. [Google Scholar]
- Bailly, C. Targets and pathways involved in the antitumor activity of citral and its stereo-isomers. Eur. J. Pharmacol. 2020, 871, 172945. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, J.; Pachaiappan, P.; Thangarasu, R. Citronellol, an acyclic monoterpene induces mitochondrial-mediated apoptosis through activation of proapoptotic factors in MCF-7 and MDA-MB-231 human mammary tumor cells. Nutr. Cancer 2021, 73, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Machado, L.; Bica, C.G.; Machado, A.K.; Steffani, J.A.; Cadoná, F.C. In vitro evaluation of antioxidant and anticancer activity of lemongrass (Cymbopogon citratus (DC) Stapf). Nutr. Cancer 2022, 74, 1474–1488. [Google Scholar] [CrossRef]
- Quinto, E.J.; Caro, I.; Villalobos-Delgado, L.H.; Mateo, J.; De-Mateo-Silleras, B.; Redondo-Del-Río, M.P. Food safety through natural antimicrobials. Antibiotics 2019, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, G.; Yew, X.Y.; Sivasamugham, L.A. Antibacterial activity of Cymbopogon citratus against clinically important bacteria. S. Afr. J. Chem. Eng. 2020, 34, 26–30. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Eller, G.; Ngassoum, M.B.; Maponmetsem, P.M. Composition and antimicrobial activity of Cymbopogon giganteus (Hochst.) chiov. essential flower, leaf and stem oils from Cameroon. J. Essent. Oil Res. 2007, 19, 485–489. [Google Scholar] [CrossRef]
- Gupta, A.K.; Muhury, R.; Ganjewala, D. A study on antimicrobial activities of essential oils of different cultivars of lemongrass (Cymbopogon flexuosus). Pharm. Sci. 2016, 22, 164–169. [Google Scholar] [CrossRef]
- Murbach Teles Andrade, B.F.; Nunes Barbosa, L.; Bérgamo Alves, F.C.; Albano, M.; Mores Rall, V.L.; Sforcin, J.M.; Fernandes, A.A.H.; Fernandes Júnior, A. antibacterial effects of Melaleuca alternifolia, Pelargonium graveolens and Cymbopogon martinii essential oils and major compounds on liquid and vapor phase. J. Essent. Oil Res. 2016, 28, 227–233. [Google Scholar] [CrossRef]
- Manosroi, J.; Dhumtanom, P.; Manosroi, A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006, 235, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Whelan, R.J.; Pattnaik, B.R.; Ludwig, K.; Subudhi, E.; Rowland, H.; Claussen, N.; Zucker, N.; Uppal, S.; Kushner, D.M. Terpenoids from Zingiber officinale (Ginger) induce apoptosis in endometrial cancer cells through the activation of p53. PLoS ONE 2012, 7, e53178. [Google Scholar] [CrossRef] [PubMed]
- Najar, B.; Shortrede, J.E.; Pistelli, L.; Buhagiar, J. Chemical composition and in vitro cytotoxic screening of sixteen commercial essential oils on five cancer cell lines. Chem. Biodivers. 2020, 17, e1900478. [Google Scholar] [CrossRef] [PubMed]
- AOAC Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000; Methods 934.01, 942.05.
- AOAC Official Methods of Analysis, 16th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1997; Method 990.03.
- AOAC Official Methods of Analysis, 16th ed.; 5th revision; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1999; Method 991.36.
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1975; Volume 222. [Google Scholar]
- Muala, W.C.B.; Desobgo, Z.S.C.; Jong, N.E. Optimization of extraction conditions of phenolic compounds from Cymbopogon citratus and evaluation of phenolics and aroma profiles of extract. Heliyon 2021, 7, e06744. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Phan, A.D.T.; Akter, S.; Bobasa, E.M.; Seididamyeh, M.; Sivakumar, D.; Sultanbawa, Y. Bioactive Properties of Kakadu Plum-Blended Products. Molecules 2023, 28, 2828. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Zhang, H.; Wang, X.; Fan, J.; Zhang, X. Phenolic compounds and bioactivity evaluation of aqueous and methanol extracts of Allium mongolicum Regel. Food Sci. Nutr. 2019, 7, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P. Antioxidant capacity and hydrophilic phytochemicals in commercially grown native Australian fruits. Food Chem. 2010, 123, 1048–1054. [Google Scholar] [CrossRef]
- Gaonkar, R.; Yallappa, S.; Dhananjaya, B.; Hegde, G. Development and validation of reverse phase high performance liquid chromatography for citral analysis from essential oils. J. Chromatogr. B 2016, 1036, 50–56. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Chaliha, M.; Sultanbawa, Y.; Netzel, M.E. Nutritional characteristics and antimicrobial activity of Australian grown feijoa (Acca sellowiana). Foods 2019, 8, 376. [Google Scholar] [CrossRef]
- Djenane, D.; Yangüela, J.; Montañés, L.; Djerbal, M.; Roncalés, P. Antimicrobial activity of Pistacia lentiscus and Satureja montana essential oils against Listeria monocytogenes CECT 935 using laboratory media: Efficacy and synergistic potential in minced beef. Food Control 2011, 22, 1046–1053. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, S.; Yin, P.; Yan, L.; Han, J.; Shi, L.; Zhou, X.; Liu, Y.; Ma, C. α-Glucosidase inhibitory activity of polyphenols from the burs of castanea mollissima blume. Molecules 2014, 19, 8373–8386. [Google Scholar] [CrossRef] [PubMed]
- Uraku, A.J.; Onuoha, S.C.; Edwin, N.; Ezeani, N.; Ogbanshi, M.E.; Ezeali, C.; Nwali, B.U.; Ominyi, M.C. Nutritional and anti-nutritional quantification assessment of Cymbopopgon citratus leaf. Pharmacol. Pharm. 2015, 6, 401. [Google Scholar] [CrossRef]
- Unuigbe, C.; Enahoro, J.; Erharuyi, O.; Okeri, H. Phytochemical analysis and antioxidant evaluation of lemon grass (Cymbopogon citratus DC.) Stapf leaves. J. Appl. Sci. Environ. Manag. 2019, 23, 223–228. [Google Scholar] [CrossRef]
- Abdulmajid, A. Evaluation of Chemical Compositions and Antioxidant Properties of Mentha Spicata and Cymbopogon Citratus leaves. 2012. Available online: http://138.197.184.166/chemistry/evaluation-of-chemical-compositions-and-antioxidant-properties-of-mentha-spicata-and-cymbopogon-citratus-leaves/index.html (accessed on 12 October 2023).
- Guleria, K.; Sehgal, A. Appraisal of antioxidant effect of fresh and dried leaves of lemongrass (Cymbopogon citratus). Plant Arch. 2020, 20, 2554–2557. [Google Scholar]
- Tran, T.T.; Nguyen, H.V. Effects of spray-drying temperatures and carriers on physical and antioxidant properties of lemongrass leaf extract powder. Beverages 2018, 4, 84. [Google Scholar] [CrossRef]
- Naseem, Z.; Zahid, M.; Hanif, M.A.; Shahid, M. Green extraction of ethnomedicinal compounds from Cymbopogon citratus Stapf using hydrogen-bonded supramolecular network. Sep. Sci. Technol. 2021, 56, 1520–1533. [Google Scholar] [CrossRef]
- Gazwi, H.S.S. Preventive effect of lemongrass (Cymbopogon citratus) against oxidation in soybean oil. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 151–159. [Google Scholar] [CrossRef]
- Ng, Z.X.; Yong, P.H.; Lim, S.Y. Customized drying treatments increased the extraction of phytochemicals and antioxidant activity from economically viable medicinal plants. Ind. Crops Prod. 2020, 155, 112815. [Google Scholar] [CrossRef]
- Hagvall, L.; Bruze, M.; Engfeldt, M.; Isaksson, M.; Lindberg, M.; Ryberg, K.; Stenberg, B.; Svedman, C.; Karlberg, A.T.; Bråred Christensson, J. Contact allergy to citral and its constituents geranial and neral, coupled with reactions to the prehapten and prohapten geraniol. Contact Dermat. 2020, 82, 31–38. [Google Scholar] [CrossRef]
- Oliveira, J.; Reygaert, W.C. Gram Negative Bacteria; StatPearls: Boston, MA, USA, 2019. [Google Scholar]
- Hassan, A.; Madu, A.; Ozojiofor, U.; Galadanci, A.; Mato, I.; Jafaru, R. Antimicrobial Activities of Cymbopogon citratus and Ximenia Americana Leaf Extracts Against Some Selected Bacterial and Yeast Clinical Isolates. Asian J. Biochem. Genet. Mol. Biol. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Bassolé, I.; Lamien-Meda, A.; Bayala, B.; Obame, L.; Ilboudo, A.; Franz, C.; Novak, J.; Nebié, R.; Dicko, M. Chemical composition and antimicrobial activity of Cymbopogon citratus and Cymbopogon giganteus essential oils alone and in combination. Phytomedicine 2011, 18, 1070–1074. [Google Scholar] [CrossRef]
- Kausar, R.; Kausar, S.; Chiragh, S. In vitro antifungal activity of Aloe vera and Cymbopogon citratus against Candida albicans. Biomedica 2017, 33, 1. [Google Scholar]
- Alzobaay, A.H.; Khadim, B. Phytochemical Screening, Chemical Composition and Antibacterial Activity of Lemongrass (Cymbopogon citratus) Leaves Extracts. Int. J. Nat. Sci. 2018, 9, 15306–15315. [Google Scholar]
- AG, H.B.B. Pharmacology of α-glucosidase inhibition. Eur. J. Clin. Investig. 1994, 24, 3–10. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, G.; Dong, J. Inhibitory properties of aqueous ethanol extracts of propolis on alpha-glucosidase. Evid. Based Complement. Altern. Med. 2015, 2015, 587383. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, R.; Zhang, K.; Chen, X.; Zhang, Y. Antioxidant, Hypoglycemic and Molecular Docking Studies of Methanolic Extract, Fractions and Isolated Compounds from Aerial Parts of Cymbopogon citratus (DC.) Stapf. Molecules 2022, 27, 2858. [Google Scholar] [CrossRef]
- Proença, C.; Ribeiro, D.; Freitas, M.; Fernandes, E. Flavonoids as potential agents in the management of type 2 diabetes through the modulation of α-amylase and α-glucosidase activity: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 3137–3207. [Google Scholar] [CrossRef]
- Nassar, M.I. Chapter 15—Bee Venom: Antitumor Activity and Its Therapeutic Applications. In Bee Products and Their Applications in the Food and Pharmaceutical Industries; Boyacioglu, D., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 369–390. [Google Scholar]

| Proximate Composition | Australian Native Lemongrass C. ambiguus |
|---|---|
| Moisture (%) | 4.74 ± 0.10 |
| Ash (%) | 4.32 ± 0.22 |
| Fat (%) | 6.79 ± 0.01 |
| Protein (%) | 3.30 ± 0.07 |
| Fibre (%) | 67.55 ± 0.10 |
| Microorganism | Zone of Inhibition (mm) | ||||
|---|---|---|---|---|---|
| LGW | LGPW | LGM | LGPM | Streptomycin | |
| Staphylococcus aureus | - | - | 2.97 ± 0.33 a | 2.02 ± 0.08 b | 11.58 ± 0.33 c |
| Escherichia coli | - | - | 8.64 ± 0.57 a | 4.70 ± 1.14 b | 16.09 ± 0.06 c |
| Candida albicans | - | - | - | - | - |
| Samples | IC50 α-glucosidase (mg/mL) |
|---|---|
| Acarbose (standard) | 0.38 ± 0.02 a |
| LGW | 0.18 ± 0.05 ab |
| LGPW | 0.27 ± 0.14 ab |
| LGM | 0.17 ± 0.04 ab |
| LGPM | 0.16 ± 0.09 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Akter, S.; Phan, A.D.T.; Bobasa, E.M.; Seididamyeh, M.; Sivakumar, D.; Sultanbawa, Y. Australian Native Lemongrass (Cymbopogon ambiguus A. Camus): An Underestimated Herbal Plant. AppliedChem 2024, 4, 212-223. https://doi.org/10.3390/appliedchem4020014
Zhou Y, Akter S, Phan ADT, Bobasa EM, Seididamyeh M, Sivakumar D, Sultanbawa Y. Australian Native Lemongrass (Cymbopogon ambiguus A. Camus): An Underestimated Herbal Plant. AppliedChem. 2024; 4(2):212-223. https://doi.org/10.3390/appliedchem4020014
Chicago/Turabian StyleZhou, Yuntao, Saleha Akter, Anh Dao Thi Phan, Eshetu Mulisa Bobasa, Maral Seididamyeh, Dharini Sivakumar, and Yasmina Sultanbawa. 2024. "Australian Native Lemongrass (Cymbopogon ambiguus A. Camus): An Underestimated Herbal Plant" AppliedChem 4, no. 2: 212-223. https://doi.org/10.3390/appliedchem4020014
APA StyleZhou, Y., Akter, S., Phan, A. D. T., Bobasa, E. M., Seididamyeh, M., Sivakumar, D., & Sultanbawa, Y. (2024). Australian Native Lemongrass (Cymbopogon ambiguus A. Camus): An Underestimated Herbal Plant. AppliedChem, 4(2), 212-223. https://doi.org/10.3390/appliedchem4020014










