Aroma Compounds of Carrier Oils
Abstract
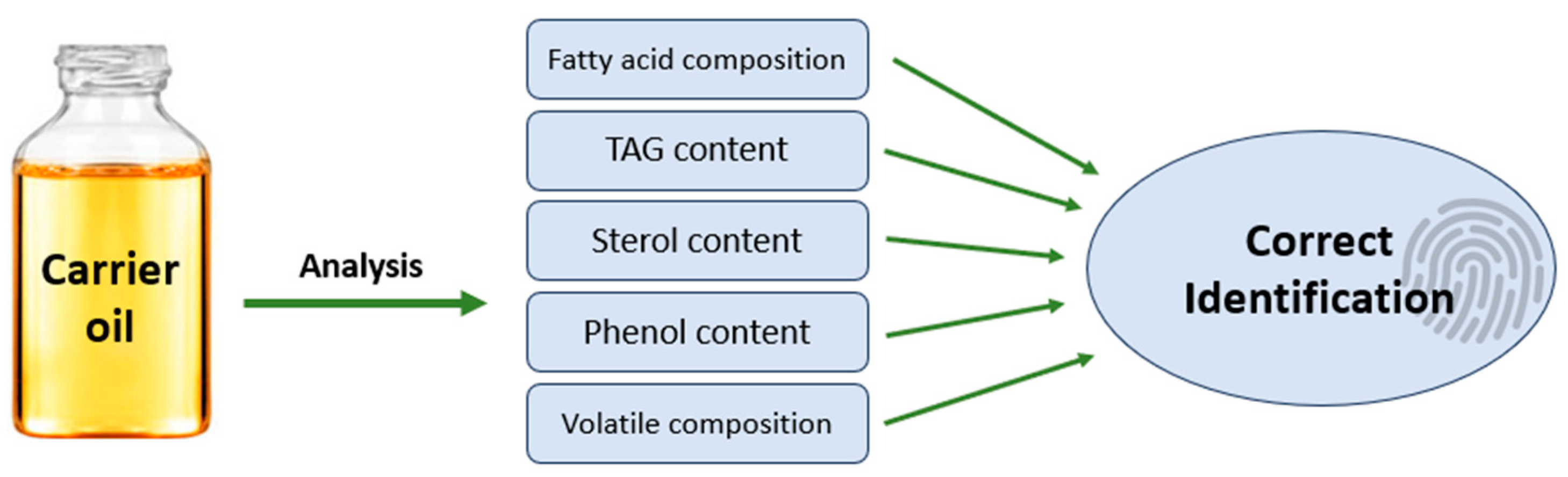
1. Introduction
2. Methodology
3. Almond Oil
4. Apricot Seed Oil
5. Argan Oil
6. Avocado Oil
7. Babassu Oil
8. Baobab Oil
9. Black Cumin Oil
10. Brazil Nut Oil
11. Camelina Oil
12. Castor Oil
13. Chia Seed Oil
14. Coconut Oil
15. Cranberry Seed Oil
16. Grapeseed Oil
17. Hazelnut Oil
18. Hemp Seed Oil
19. Jojoba Oil
20. Kukui Nut Oil
21. Macadamia Oil
22. Marula Oil
23. Moringa Oil
24. Neem Seed Oil
25. Olive Oil
26. Palm Kernel Oil
27. Passionfruit Seed Oil
28. Pomegranate Seed Oil
29. Evening Primrose Oil
30. Pumpkin Oil
31. Raspberry Seed Oil
32. Rosehip Oil
33. Sacha Inchi Oil
34. Safflower Oil
35. Sea Buckthorn Oil
36. Sesame Seed Oil
37. Shea Butter
38. Soybean Oil
39. Sunflower Oil
40. Tamanu Oil
41. Volatile Composition
42. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alhasso, B.; Ghori, M.U.; Conway, B.R. Systematic review on the effectiveness of essential and carrier oils as skin penetration enhancers in pharmaceutical formulations. Sci. Pharm. 2022, 90, 14. [Google Scholar] [CrossRef]
- Oji, A.; Vivian, I.C. Extraction and characterization of selected carrier oils. Chem. Sci. Int. J. 2020, 29, 48–54. [Google Scholar] [CrossRef]
- Michalak, M. The use of carrier oils in aromatherapy massage and their effect on skin. Arch. Physiother. Global Res. 2018, 22, 23–31. [Google Scholar]
- Orchard, A.; Kamatou, G.; Viljoen, A.M.; Patel, N.; Mawela, P.; van Vuuren, S.F. The influence of carrier oils on the antimicrobial activity and cytotoxicity of essential oils. Evid. Based Complement. Altern. Med. 2019, 2019, 6981305. [Google Scholar] [CrossRef] [PubMed]
- Akhone, M.A.; Bains, A.; Tosif, M.M.; Chawla, P.; Fogarasi, M.; Fogarasi, S. Apricot kernel: Bioactivity, characterization, applications, and health attributes. Foods 2022, 11, 2184. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.; Sekhon, J.J.K.; Rosentrater, K.A.; Wang, T.; Jung, S.; Johnson, L.A. Environmental impact assessment of soybean oil production: Extruding-expelling process, hexane extraction and aqueous extraction. Food Bioprod. Process. 2018, 108, 58–68. [Google Scholar] [CrossRef]
- Naksuk, A.; Sabatini, D.A.; Tongcumpou, C. Microemulsion-based palm kernel oil extraction using mixed surfactant solutions. Ind. Crop. Prod. 2009, 30, 194–198. [Google Scholar] [CrossRef]
- Arturo-Perdomo, D.; Mora, J.P.J.; Ibáñez, E.; Cifuentes, A.; Hurtado-Benavides, A.; Montero, L. Extraction and characterization of the polar lipid fraction of blackberry and passion fruit seeds oils using supercritical fluid extraction. Food Anal. Methods 2021, 14, 2026–2037. [Google Scholar] [CrossRef]
- Martínez-Gómez, P.; Sánchez-Pérez, R.; Dicenta, F.; Howad, W.; Arús, P.; Gradziel, T.M. Almond. In Fruits and Nuts; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 229–242. [Google Scholar]
- Kodad, O.; Estopañán, G.; Juan, T.; Rafel Socias i Company. Protein content and oil composition of almond from Moroccan seedlings: Genetic diversity, oil quality and geographical origin. J. Am. Oil Chem. Soc. 2013, 90, 243–252. [Google Scholar] [CrossRef]
- Martínez, M.L.; Bordón, M.G.; Bodoira, R.M.; Penci, M.C.; Ribotta, P.D.; Maestri, D.M. Walnut and almond oil screw-press extraction at industrial scale: Effects of process parameters on oil yield and quality. Grasas Aceites 2017, 68, 10–2017. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R. Almond Production in California. Fertilizer Research and Education Program; California Department of Food and Agriculture: Sacramento, CA, USA, 2014. [Google Scholar]
- Čolić, S.; Zec, G.; Natić, M.; Fotirić-Akšić, M. Almond (Prunus dulcis) oil. In Fruit Oils: Chemistry and Functionality; Ramadan, M.F., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 149–180. [Google Scholar]
- Roncero, J.M.; Álvarez-Ortí, M.; Pardo-Giménez, A.; Gómez, R.; Rabadán, A.; Pardo, J.E. Virgin almond oil: Extraction methods and composition. Grasas Aceites 2016, 67, e143. [Google Scholar] [CrossRef]
- Zhou, Q.; Jia, X.; Liu, Y.; Wan, C.; Zheng, C.; Li, S.; Huang, F. The effect of the subcritical fluid extraction on the quality of almond oils: Compared to conventional mechanical pressing method. Food Sci. Nutr. 2019, 7, 2231–2241. [Google Scholar] [CrossRef]
- Roncero, J.M.; Álvarez-Ortí, M.; Pardo-Giménez, A.; Rabadán, A.; Pardo, J.E. Influence of pressure extraction systems on the performance, quality and composition of virgin almond oil and defatted flours. Foods 2021, 10, 1049. [Google Scholar] [CrossRef] [PubMed]
- Givianrad, M.H.; Saber-Tehrani, M.; Jafari Mohammadi, S.A. Chemical composition of oils from wild almond (Prunus scoparia) and wild pistachio (Pistacia atlantica). Grasas Aceites 2013, 64, 77–84. [Google Scholar] [CrossRef]
- Kesen, S.; Amanpour, A.; Selli, S. Comparative evaluation of the fatty acids and aroma compounds in selected Iranian nut oils. Eur. J. Lipid Sci. Technol. 2018, 120, 1800152. [Google Scholar] [CrossRef]
- Zhebentyayeva, T.; Ledbetter, C.; Burgos, L.; Llácer, G. Apricot. In Fruit Breeding; Badenes, M., Byrne, D., Eds.; Springer: Boston, MA, USA, 2012; pp. 415–458. [Google Scholar]
- Groppi, A.; Liu, S.; Cornille, A.; Decroocq, S.; Bui, Q.T.; Tricon, D.; Cruaud, C.; Arribat, S.; Belser, C.; Marande, W.; et al. Population genomics of apricots unravels domestication history and adaptive events. Nat. Commun. 2021, 12, 3956. [Google Scholar] [CrossRef] [PubMed]
- Kiralan, M.; Özkan, G.; Kucukoner, E.; Ozcelik, M.M. Apricot (Prunus armeniaca L.) oil. In Fruit Oils: Chemistry and Functionality; Ramadan, M.F., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 505–519. [Google Scholar]
- Lee, H.; Ahn, J.-H.; Kwon, A.-R.; Lee, E.S.; Kwak, J.-H.; Min, Y.-H. Chemical composition and antimicrobial activity of the essential oil of apricot seed. Phytother. Res. 2014, 28, 1867–1872. [Google Scholar] [CrossRef]
- Manzoor, M.; Anwar, F.; Ashraf, M.; Alkharfy, K.M. Physico-chemical characteristics of seed oils extracted from different apricot (Prunus armeniaca L.) varieties from Pakistan. Grasas Aceites 2012, 63, 193–201. [Google Scholar] [CrossRef]
- Uluata, S. Effect of extraction method on biochemical properties and oxidative stability of apricot seed oil. Akad. Gıda 2016, 14, 333–340. [Google Scholar]
- Orhan, I.; Koca, U.; Aslan, S.; Kartal, M.; Küsmenoǧlu, Ş. Fatty acid analysis of some Turkish apricot seed oils by GC and GC-MS techniques. Turk. J. Pharm. Sci. 2008, 5, 29–34. [Google Scholar]
- Kiralan, M.; Ketenoglu, O. Apricot (Prunus armeniaca L.) kernel: A valuable by-product. In Mediterranean Fruits Bio-Wastes; Ramadan, M.F., Farag, M.A., Eds.; Springer International: Cham, Switzerland, 2022; pp. 547–558. [Google Scholar]
- Kaya, C.; Kola, O.; Ozer, M.S.; Altan, A. Some characteristics and fatty acids composition of wild apricot (Prunus pseudoarmeniaca L.) kernel oil. Asian J. Chem. 2008, 20, 2597–2602. [Google Scholar]
- Matthaus, B.; Özcan, M.M.; Al Juhaimi, F. Fatty acid composition and tocopherol content of the kernel oil from apricot varieties (Hasanbey, Hacihaliloglu, Kabaasi and Soganci) collected at different harvest times. Eur. Food Res. Technol. 2016, 242, 221–226. [Google Scholar] [CrossRef]
- Rudzińska, M.; Górnaś, P.; Raczyk, M.; Soliven, A. Sterols and squalene in apricot (Prunus armeniaca L.) kernel oils: The variety as a key factor. Nat. Prod. Res. 2017, 31, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Makrygiannis, I.; Athanasiadis, V.; Chatzimitakos, T.; Bozinou, E.; Mantzourani, C.; Chatzilazarou, A.; Makris, D.P.; Lalas, S.I. Exploring the chemical composition and antioxidant properties of apricot kernel oil. Separations 2023, 10, 332. [Google Scholar] [CrossRef]
- Lybbert, T.J.; Aboudrare, A.; Chaloud, D.; Magnan, N.; Nash, M. Booming markets for Moroccan argan oil appear to benefit some rural households while threatening the endemic argan forest. Proc. Natl. Acad. Sci. USA 2011, 108, 13963–13968. [Google Scholar] [CrossRef] [PubMed]
- Charrouf, Z.; Guillaume, D. Argan oil: Occurrence, composition and impact on human health. Eur. J. Lipid Sci. Technol. 2008, 110, 632–636. [Google Scholar] [CrossRef]
- Belarbi-Benmahdi, M.; Khaldi, D.; Beghdad, C.; Gouzi, H.; Bendimerad, N.; Hammouti, B. Physicochemical and nutritional study of argan oil (Argania spinosa L.) in south-western Algeria. Pigment. Resin Technol. 2009, 38, 96–99. [Google Scholar] [CrossRef]
- Gharby, S.; Charrouf, Z. Argan oil: Chemical composition, extraction process, and quality control. Front. Nutr. 2022, 8, 804587. [Google Scholar] [CrossRef]
- Kouidri, M.; Saadi, A.K.; Noui, A.; Medjahed, F. The chemical composition of argan oil. Int. J. Adv. Stud. Comput. Sci. Eng. 2015, 4, 24–28. [Google Scholar]
- Sevindik, O.; Amanpour, A.; Tsouli Sarhir, S.; Kelebek, H.; Selli, S. Characterization of key odorants in Moroccan argan oil by aroma extract dilution analysis. Eur. J. Lipid Sci. Technol. 2019, 121, 1800437. [Google Scholar] [CrossRef]
- Khallouki, F.; Younos, C.; Soulimani, R.; Oster, T.; Charrouf, Z.; Spiegelhalder, B.; Bartsch, H.; Owen, R.W. Consumption of argan oil (Morocco) with its unique profile of fatty acids, tocopherols, squalene, sterols, and phenolic compounds should confer valuable cancer chemopreventive effects. Eur. J. Cancer Prev. 2003, 12, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Costagli, G.; Betti, M. Avocado oil extraction processes: Method for cold-pressed high-quality edible oil production versus traditional production. J. Agric. Eng. 2015, 46, 115–122. [Google Scholar] [CrossRef]
- Berasategi, I.; Barriuso, B.; Ansorena, D.; Astiasarán, I. Stability of avocado oil during heating: Comparative study to olive oil. Food Chem. 2012, 132, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Woolf, A.; Wong, M.; Eyres, L.; McGhie, T.; Lund, C.; Olsson, S.; Wang, Y.; Bulley, C.; Wang, M.; Friel, E.; et al. Avocado Oil. In Gourmet and Health-Promoting Specialty Oils; Moreau, R.A., Kamal-Eldin, A., Eds.; AOCS Press: Urbana, IL, USA, 2009; pp. 73–125. [Google Scholar]
- Tan, C.X. Virgin avocado oil: An emerging source of functional fruit oil. J. Funct. Foods 2019, 54, 381–392. [Google Scholar] [CrossRef]
- Cervantes-Paz, B.; Yahia, E.M. Avocado oil: Production and market demand, bioactive components, implications in health, and tendencies and potential uses. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4120–4158. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhong, J. A review of extraction techniques for avocado oil. J. Oleo Sci. 2016, 65, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Yanty, N.A.M.; Marikkar, J.M.N.; Long, K. Effect of varietal differences on composition and thermal characteristics of avocado oil. J. Am. Oil Chem. Soc. 2011, 88, 1997–2003. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R.; Rosado, A.; Fuentes, V. Volatile components of avocado (Persea americana Mill.) cv. Moro grown in Cuba. J. Essent. Oil Res. 2004, 16, 139–140. [Google Scholar] [CrossRef]
- Pessoa, R.; França, E.; Ribeiro, E.; Lanes, P.; Chaud, N.; Moraes, L.; Honorio-França, A. Microemulsion of babassu oil as a natural product to improve human immune system function. Drug Des. Dev. Ther. 2014, 9, 21–31. [Google Scholar] [CrossRef]
- Anderson, A.B.; Balick, M.J. Taxonomy of the babassu complex (Orbignya spp.: Palmae). Syst. Bot. 1988, 13, 32–50. [Google Scholar] [CrossRef]
- Cavallari, M.M.; Toledo, M.M. What is the name of the babassu? A note on the confusing use of scientific names for this important palm tree. Rodriguésia 2016, 67, 533–538. [Google Scholar] [CrossRef]
- Melo, E.; Michels, F.; Arakaki, D.; Lima, N.; Gonçalves, D.; Cavalheiro, L.; Oliveira, L.; Caires, A.; Hiane, P.; Nascimento, V. First study on the oxidative stability and elemental analysis of babassu (Attalea speciosa) edible oil produced in Brazil using a domestic extraction machine. Molecules 2019, 24, 4235. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.H.S.L.; Monteiro, C.A.; Figueredo, P.M.S.; Nascimento, F.R.F.; Guerra, R.N.M. Ethnopharmacological use of babassu (Orbignya phalerata Mart) in communities of babassu nut breakers in Maranhão, Brazil. J. Ethnopharmacol. 2011, 133, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Silva Ferreira, B.; Pereira Faza, L.; Le Hyaric, M. A Comparison of the physicochemical properties and fatty acid composition of indaiá (Attalea dubia) and babassu (Orbignya phalerata) oils. Sci. World J. 2012, 2012, 532374. [Google Scholar] [CrossRef]
- Rahul, J.; Jain, M.K.; Singh, S.P.; Kamal, R.K.; Naz, A.; Gupta, A.K.; Mrityunjay, S.K. Adansonia digitata L. (baobab): A review of traditional information and taxonomic description. Asian Pac. J. Trop. Biomed. 2015, 5, 79–84. [Google Scholar] [CrossRef]
- Pock Tsy, J.M.L.; Lumaret, R.; Mayne, D.; Vall, A.O.M.; Abutaba, Y.I.M.; Sagna, M.; Raoseta, S.O.R.; Danthu, P. Chloroplast DNA phylogeography suggests a West African centre of origin for the baobab, Adansonia digitata L. (Bombacoideae, Malvaceae). Mol. Ecol. 2009, 18, 1707–1715. [Google Scholar] [CrossRef]
- Msalilwa, U.L.; Makule, E.E.; Munishi, L.K.; Ndakidemi, P.A. Physicochemical properties, fatty acid composition, and the effect of heating on the reduction of cyclopropenoid fatty acids on baobab (Adansonia digitata L.) crude seed oil. J. Lipids 2020, 2020, 6691298. [Google Scholar] [CrossRef]
- Cissé, M.; Sow, A.; Poucheret, P.; Margout, D.; Ayessou, N.C.; Faye, P.G.; Sakho, M.; Diop, C.M.G. Impact of extraction method on physicochemical characteristics and antioxidant potential of Adansonia digitata oil. Food Nutr. Sci. 2018, 9, 937–955. [Google Scholar] [CrossRef][Green Version]
- Bazongo, P.; Bassolé, I.; Nielsen, S.; Hilou, A.; Dicko, M.; Shukla, V. Characteristics, composition and oxidative stability of Lannea microcarpa seed and seed oil. Molecules 2014, 19, 2684–2693. [Google Scholar] [CrossRef]
- Cravotto, G.; Binello, A.; Orio, L. Green extraction techniques for high-quality natural products. Agro Food Ind. Hi Tech 2011, 22, 57–59. [Google Scholar]
- Abeer, A.I.; Azhari, H.N.; Mahmoud, M.A.; Ibrahim, Y.E.; Omer, A.O.I. Physicochemical properties and fatty acids composition of Sudanese baobab (Adansonia digitata L.) seed oil. Int. J. Pharma Bio Sci. 2020, 11, 34–42. [Google Scholar] [CrossRef]
- Ramadan, M.F. Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa L.): An overview. Int. J. Food Sci. Technol. 2007, 42, 1208–1218. [Google Scholar] [CrossRef]
- Datta, A.K.; Saha, A.; Bhattacharya, A.; Mandal, A.; Paul, R.; Sengupta, S. Black cumin (Nigella sativa L.)—A review. J. Plant Develop Sci. 2012, 4, 1–43. [Google Scholar]
- Ahmad, M.F.; Ahmad, F.A.; Ashraf, S.A.; Saad, H.H.; Wahab, S.; Khan, M.I.; Ali, M.; Mohan, S.; Hakeem, K.R.; Athar, M.T. An updated knowledge of black seed (Nigella sativa Linn.): Review of phytochemical constituents and pharmacological properties. J. Herb. Med. 2021, 25, 100404. [Google Scholar] [CrossRef] [PubMed]
- Kiralan, M.; Özkan, G.; Bayrak, A.; Ramadan, M.F. Physicochemical properties and stability of black cumin (Nigella sativa) seed oil as affected by different extraction methods. Ind. Crop. Prod. 2014, 57, 52–58. [Google Scholar] [CrossRef]
- Rahim, M.A.; Shoukat, A.; Khalid, W.; Ejaz, A.; Itrat, N.; Majeed, I.; Koraqi, H.; Imran, M.; Nisa, M.U.; Nazir, A.; et al. A narrative review on various oil extraction methods, encapsulation processes, fatty acid profiles, oxidative stability, and medicinal properties of black seed (Nigella sativa). Foods 2022, 11, 2826. [Google Scholar] [CrossRef] [PubMed]
- Dinagaran, S.; Sridhar, S.; Eganathan, P. Chemical composition and antioxidant activities of black seed oil (Nigella sativa L.). Int. J. Pharm. Sci. Res. 2016, 7, 4473–4479. [Google Scholar] [CrossRef]
- Soleimanifar, M.; Niazmand, R.; Jafari, S.M. Evaluation of oxidative stability, fatty acid profile, and antioxidant properties of black cumin seed oil and extract. J. Food Meas. Character 2019, 13, 383–389. [Google Scholar] [CrossRef]
- Albakry, Z.; Karrar, E.; Ahmed, I.A.M.; Oz, E.; Proestos, C.; El Sheikha, A.F.; Oz, F.; Wu, G.; Wang, X. Nutritional composition and volatile compounds of black cumin (Nigella sativa L.) seed, fatty acid composition and tocopherols, polyphenols, and antioxidant activity of its essential oil. Horticulturae 2022, 8, 575. [Google Scholar] [CrossRef]
- Shepard, G.H.; Ramirez, H. “Made in Brazil”: Human dispersal of the Brazil nut (Bertholletia excelsa, Lecythidaceae) in ancient Amazonia. Econ. Bot. 2011, 65, 44–65. [Google Scholar] [CrossRef]
- Ariane, M.K.; Maristela, M.; Silmara, M.M.; Renata, H.S.; Karine, S.N.; Helyde, A.M.; Augusto, K.J. Properties of Brazil nuts: A review. Afr. J. Biotechnol. 2015, 14, 642–648. [Google Scholar] [CrossRef]
- Silva, P.C.; Resende, O.; Ferreira Junior, W.N.; Silva, L.C.d.M.; Quequeto, W.D.; Silva, F.A. Drying kinetics of Brazil nuts. Food Sci. Technol. 2022, 42, e64620. [Google Scholar] [CrossRef]
- Sousa de Oliveira, T.; Freitas-Silva, O.; Mendonça Kluczkovski, A.; Henrique Campelo, P. Potential use of vegetable proteins to reduce Brazil nut oil oxidation in microparticle systems. Food Res. Int. 2020, 137, 109526. [Google Scholar] [CrossRef] [PubMed]
- Santos, O.V.; Corrêa, N.C.F.; Soares, F.A.S.M.; Gioielli, L.A.; Costa, C.E.F.; Lannes, S.C.S. Chemical evaluation and thermal behavior of Brazil nut oil obtained by different extraction processes. Food Res. Int. 2012, 47, 253–258. [Google Scholar] [CrossRef]
- Yang, J. Brazil nuts and associated health benefits: A review. LWT—Food Sci. Technol. 2009, 42, 1573–1580. [Google Scholar] [CrossRef]
- Özcan, M.M.; Al Juhaimi, F.; Uslu, N. The effect of heat treatment on phenolic compounds and fatty acid composition of Brazilian nut and hazelnut. J. Food Sci. Technol. 2018, 55, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Chunhieng, T.; Hafidi, A.; Pioch, D.; Brochier, J.; Didier, M. Detailed study of Brazil nut (Bertholletia excelsa) oil micro-compounds: Phospholipids, tocopherols, and sterols. J. Braz. Chem. Soc. 2008, 19, 1374–1380. [Google Scholar] [CrossRef]
- Cadwallader, K.R.; Puangpraphant, S. Flavor and volatile compounds in tree nuts. In Tree Nuts: Composition, Phytochemicals, and Health Effects; Alasavar, C., Shahidi, F., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 109–126. [Google Scholar]
- Brock, J.R.; Dönmez, A.A.; Beilstein, M.A.; Olsen, K.M. Phylogenetics of Camelina Crantz. (Brassicaceae) and insights on the origin of gold-of-pleasure (Camelina sativa). Mol. Phylogenetics Evol. 2018, 127, 834–842. [Google Scholar] [CrossRef]
- Ghamkhar, K.; Croser, J.; Aryamanesh, N.; Campbell, M.; Kon’kova, N.; Francis, C. Camelina (Camelina sativa (L.) Crantz) as an alternative oilseed: Molecular and ecogeographic analyses. Genome 2010, 53, 558–567. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmed, Z.; Ahmad, R.; Ashraf, M.Y.; Naeem, M.S.; Rengel, Z. ‘Camelina sativa’, a climate proof crop, has high nutritive value and multiple-uses: A review. Aust. J. Crop Sci. 2013, 7, 1551–1559. [Google Scholar]
- Sampath, A. Chemical Characterization of Camelina Seed Oil. Master’s Thesis, Rutgers University, New Brunswick, NJ, USA, 2009. [Google Scholar]
- Popa, A.-L.; Jurcoane, Ș.; Dumitriu, B. Camelina sativa oil-A review. Sci. Bull. F Biotechnol. 2017, 21, 233–238. [Google Scholar]
- Abramovič, H.; Abram, V. Physico-chemical properties, composition and oxidative stability of Camelina sativa oil. Food Technol. Biotechnol. 2005, 43, 63–70. [Google Scholar]
- Ratusz, K.; Symoniuk, E.; Wroniak, M.; Rudzińska, M. Bioactive compounds, nutritional quality and oxidative stability of cold-pressed camelina (Camelina sativa L.) Oils. Appl. Sci. 2018, 8, 2606. [Google Scholar] [CrossRef]
- Patel, V.R.; Dumancas, G.G.; Viswanath, L.C.K.; Maples, R.; Subong, B.J.J. Castor oil: Properties, uses, and optimization of processing parameters in commercial production. Lipid Insights 2016, 9, LPI-S40233. [Google Scholar] [CrossRef]
- Yeboah, A.; Ying, S.; Lu, J.; Xie, Y.; Amoanimaa-Dede, H.; Boateng, K.G.A.; Chen, M.; Yin, X. Castor oil (Ricinus communis): A review on the chemical composition and physicochemical properties. Food Sci. Technol. 2021, 41, 399–413. [Google Scholar] [CrossRef]
- Said, G.; Daniel, P.; Badr, K.; Mohamed, I.; Zoubida, C. Chemical characterization and oxidative stability of castor oil grown in Morocco. Moroc. J. Chem. 2016, 4, 279–284. [Google Scholar]
- Anjani, K. Castor genetic resources: A primary gene pool for exploitation. Ind. Crop. Prod. 2012, 35, 1–14. [Google Scholar] [CrossRef]
- Bhaskar, T.; Pandey, A.; Rene, E.R.; Tsang, D.C.W. Waste Biorefinery; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Akpan, U.G.; Jimoh, A.; Mohammed, A.D. Extraction, characterization and modification of castor seed oil. Leonardo J. Sci. 2006, 5, 43–52. [Google Scholar]
- Jamshidi, A.M.; Amato, M.; Ahmadi, A.; Bochicchio, R.; Rossi, R. Chia (Salvia hispanica L.) as a novel forage and feed source: A review. Ital. J. Agron. 2019, 14, 1297. [Google Scholar] [CrossRef]
- Kulczyński, B.; Kobus-Cisowska, J.; Taczanowski, M.; Kmiecik, D.; Gramza-Michałowska, A. The chemical composition and nutritional value of chia seeds—Current state of knowledge. Nutrients 2019, 11, 1242. [Google Scholar] [CrossRef]
- Motyka, S.; Koc, K.; Ekiert, H.; Blicharska, E.; Czarnek, K.; Szopa, A. The current state of knowledge on Salvia hispanica and Salviae hispanicae semen (chia seeds). Molecules 2022, 27, 1207. [Google Scholar] [CrossRef]
- Ullah, R.; Nadeem, M.; Khalique, A.; Imran, M.; Mehmood, S.; Javid, A.; Hussain, J. Nutritional and therapeutic perspectives of chia (Salvia hispanica L.): A review. J. Food Sci. Technol. 2016, 53, 1750–1758. [Google Scholar] [CrossRef]
- Mohd Ali, N.; Yeap, S.K.; Ho, W.Y.; Beh, B.K.; Tan, S.W.; Tan, S.G. The promising future of chia, Salvia hispanica L. J. Biomed. Biotechnol. 2012, 2012, 171956. [Google Scholar] [CrossRef]
- Ixtaina, V.Y.; Martínez, M.L.; Spotorno, V.; Mateo, C.M.; Maestri, D.M.; Diehl, B.W.K.; Nolasco, S.M.; Tomás, M.C. Characterization of chia seed oils obtained by pressing and solvent extraction. J. Food Compos. Anal. 2011, 24, 166–174. [Google Scholar] [CrossRef]
- Ixtaina, V.Y.; Vega, A.; Nolasco, S.M.; Tomás, M.C.; Gimeno, M.; Bárzana, E.; Tecante, A. Supercritical carbon dioxide extraction of oil from Mexican chia seed (Salvia hispanica L.): Characterization and process optimization. J. Supercritic Fluids 2010, 55, 192–199. [Google Scholar] [CrossRef]
- Martínez, M.L.; Marín, M.A.; Salgado Faller, C.M.; Revol, J.; Penci, M.C.; Ribotta, P.D. Chia (Salvia hispanica L.) oil extraction: Study of processing parameters. LWT—Food Sci. Technol. 2012, 47, 78–82. [Google Scholar] [CrossRef]
- Chan, E.; Elevitch, C.R. Cocos Nucifera (Coconut): Species Profiles for Pacific Island Agroforestry; Permanent Agriculture Resources (PAR): Hōlualoa, HI, USA, 2006. [Google Scholar]
- DebMandal, M.; Mandal, S. Coconut (Cocos nucifera L.: Arecaceae): In health promotion and disease prevention. Asian Pac. J. Trop. Med. 2011, 4, 241–247. [Google Scholar] [CrossRef]
- Shankar, P.; Ahuja, S.; Tracchio, A. Coconut oil: A review. Agro Food Ind. Hi Tech 2013, 24, 62–64. [Google Scholar]
- Marina, A.M.; Che Man, Y.B.; Nazimah, S.A.H.; Amin, I. Chemical properties of virgin coconut oil. J. Am. Oil Chem. Soc. 2009, 86, 301–307. [Google Scholar] [CrossRef]
- Srivastava, Y.; Semwal, A.D.; Majumdar, A. Quantitative and qualitative analysis of bioactive components present in virgin coconut oil. Cogent Food Agric. 2016, 2, 1164929. [Google Scholar] [CrossRef]
- Enig, M.G. Coconut: In support of good health in the 21st century. In Proceedings of the 36th Meeting of Asian Pacific Coconut Community, Federated States of Micronesia, Pohnpei; 1999. [Google Scholar]
- Ng, Y.J.; Tham, P.E.; Khoo, K.S.; Cheng, C.K.; Chew, K.W.; Show, P.L. A comprehensive review on the techniques for coconut oil extraction and its application. Bioprocess. Biosyst. Eng. 2021, 44, 1807–1818. [Google Scholar] [CrossRef]
- Santos, J.E.R.; Villarino, B.J.; Zosa, A.R.; Dayrit, F.M. Analysis of volatile organic compounds in virgin coconut oil and their sensory attibutes. Philipp. J. Sci. 2011, 140, 161–171. [Google Scholar]
- Schlautman, B.; Diaz-Garcia, L.; Covarrubias-Pazaran, G.; Schlautman, N.; Vorsa, N.; Polashock, J.; Ogden, E.L.; Brown, A.; Lin, Y.C.; Bassil, N.; et al. Comparative genetic mapping reveals synteny and collinearity between the American cranberry and diploid blueberry genomes. Mol. Breed. 2018, 38, 9. [Google Scholar] [CrossRef]
- Jurikova, T.; Skrovankova, S.; Mlcek, J.; Balla, S.; Snopek, L. Bioactive compounds, antioxidant activity, and biological effects of European cranberry (Vaccinium oxycoccos). Molecules 2018, 24, 24. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. Cranberries (Vaccinium macrocarpon) and cardiovascular disease risk factors. Nutr. Rev. 2008, 65, 490–502. [Google Scholar] [CrossRef]
- Thyagarajan, P. Evaluation and Optimization of Cranberry Seed Oil Extraction Methods. Master’s Thesis, McGill University, Montreal, QC, Canada, 2012. [Google Scholar]
- Bederska-łojewska, D.; Pieszka, M.; Marzec, A.; Rudzińska, M.; Grygier, A.; Siger, A.; Cieślik-Boczula, K.; Orczewska-Dudek, S.; Migdał, W. Physicochemical properties, fatty acid composition, volatile compounds of blueberries, cranberries, raspberries, and cuckooflower seeds obtained using sonication method. Molecules 2021, 26, 7446. [Google Scholar] [CrossRef]
- Khan, N.; Fahad, S.; Naushad, M.; Faisal, S. Grape production critical review in the world. SSRN Electron. J. 2020, 3595842. [Google Scholar] [CrossRef]
- Imazio, S.; Labra, M.; Grassi, F.; Scienza, A.; Failla, O. Chloroplast microsatellites to investigate the origin of grapevine. Genet. Resour. Crop Evol. 2006, 53, 1003–1101. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape seed oil compounds: Biological and chemical actions for health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef]
- Rombaut, N.; Savoire, R.; Thomasset, B.; Bélliard, T.; Castello, J.; Van Hecke, É.; Lanoisellé, J.L. Grape seed oil extraction: Interest of supercritical fluid extraction and gas-assisted mechanical extraction for enhancing polyphenol co-extraction in oil. Comptes Rendus Chim. 2014, 17, 284–292. [Google Scholar] [CrossRef]
- Fiori, L. Grape seed oil supercritical extraction kinetic and solubility data: Critical approach and modeling. J. Supercrit. Fluids 2007, 43, 43–54. [Google Scholar] [CrossRef]
- Baydar, N.G.; Akkurt, M. Oil content and oil quality properties of some grape seeds. Turk. J. Agric. For. 2001, 25, 163–168. [Google Scholar]
- Yalcin, H.; Kavuncuoglu, H.; Ekici, L.; Sagdic, O. Determination of fatty acid composition, volatile components, physico-chemical and bioactive properties of grape (Vitis vinifera) seed and seed oil. J. Food Process. Preserv. 2017, 41, e12854. [Google Scholar] [CrossRef]
- Erfatpour, M.; Hamidogli, Y.; Kaviani, B.; Fatahi, R.; Falahati, M.; Javadi, D.; Hashemabadi, D. Assessment of genetic diversity among some Iranian hazelnut genotypes using SSR markers. Aust. J. Crop Sci. 2011, 5, 1286–1291. [Google Scholar]
- Król, K.; Gantner, M. Morphological traits and chemical composition of hazelnut from different geographical origins: A review. Agriculture 2020, 10, 375. [Google Scholar] [CrossRef]
- Santamaría, R.I.; Soto, C.; Zúñiga, M.E.; Chamy, R.; López-Munguía, A. Enzymatic extraction of oil from Gevuina avellana, the Chilean hazelnut. J. Am. Oil Chem. Soc. 2003, 80, 33–36. [Google Scholar] [CrossRef]
- Sun, J.; Feng, X.; Lyu, C.; Zhou, S.; Liu, Z. Effects of different processing methods on the lipid composition of hazelnut oil: A lipidomics analysis. Food Sci. Hum. Wellness 2022, 11, 427–435. [Google Scholar] [CrossRef]
- Karabulut, I.; Topcu, A.; Yorulmaz, A.; Tekin, A.; Ozay, D.S. Effects of the industrial refining process on some properties of hazelnut oil. Eur. J. Lipid Sci. Technol. 2005, 107, 476–480. [Google Scholar] [CrossRef]
- Topkafa, M.; Ayyildiz, H.F.; Kara, H. Hazelnut (Corylus avellana) oil. In Fruit Oils: Chemistry and Functionality; Ramadan, M.F., Ed.; Springer: Cham, Switzerland, 2019; pp. 223–241. [Google Scholar]
- Benitez-Sánchez, P.L.; León-Camacho, M.; Aparicio, R. A comprehensive study of hazelnut oil composition with comparisons to other vegetable oils, particularly olive oil. Eur. Food Res. Technol. 2003, 218, 13–19. [Google Scholar] [CrossRef]
- Liu, F.H.; Hu, H.R.; Du, G.H.; Deng, G.; Yang, Y. Ethnobotanical research on origin, cultivation, distribution and utilization of hemp (Cannabis sativa L.) in China. Indian J. Tradit. Knowl. 2017, 16, 235–242. [Google Scholar]
- Burton, R.A.; Andres, M.; Cole, M.; Cowley, J.M.; Augustin, M.A. Industrial hemp seed: From the field to value-added food ingredients. J. Cannabis Res. 2022, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Busson, M.; Godfrey, D.V.; Drover, J.C.G. Characteristics of hemp (Cannabis sativa L.) seed oil. Food Chem. 2002, 76, 33–43. [Google Scholar] [CrossRef]
- Aladić, K.; Jarni, K.; Barbir, T.; Vidović, S.; Vladić, J.; Bilić, M.; Jokić, S. Supercritical CO2 extraction of hemp (Cannabis sativa L.) seed oil. Ind. Crop. Prod. 2015, 76, 472–478. [Google Scholar] [CrossRef]
- Kumeroa, F.; Komahan, S.; Sofkova-Bobcheva, S.; Clavijo McCormick, A. Characterization of the volatile profiles of six industrial hemp (Cannabis sativa L.) cultivars. Agronomy 2022, 12, 2651. [Google Scholar] [CrossRef]
- Daconta, L.V.; Minger, T.; Nedelkova, V.; Zikopoulos, J.N. Organic chemistry and the native plants of the Sonoran Desert: Conversion of jojoba oil to biodiesel. J. Chem. Educ. 2015, 92, 1741–1744. [Google Scholar] [CrossRef]
- Abu-Arabi, M.K.; Allawzi, M.A.; Al-Zoubi, H.S.; Tamimi, A. Extraction of jojoba oil by pressing and leaching. Chem. Eng. J. 2000, 76, 61–65. [Google Scholar] [CrossRef]
- Salgin, U.; Çlimli, A.; Uysal, B.Z. Supercritical fluid extraction of jojoba oil. J. Am. Oil Chem. Soc. 2004, 81, 293–296. [Google Scholar] [CrossRef]
- Hegel, P.; Mabe, G.; Brignole, E.A.; Pereda, S. Phase equilibrium engineering of jojoba oil extraction with mixed-CO2 + propane solvent. J. Supercrit. Fluids 2013, 79, 114–122. [Google Scholar] [CrossRef]
- Wisniak, J. Potential uses of jojoba oil and meal—A review. Ind. Crop. Prod. 1994, 3, 43–68. [Google Scholar] [CrossRef]
- Gad, H.A.; Roberts, A.; Hamzi, S.H.; Gad, H.A.; Touiss, I.; Altyar, A.E.; Kensara, O.A.; Ashour, M.L. Jojoba oil: An updated comprehensive review on chemistry, pharmaceutical uses, and toxicity. Polymers 2021, 13, 1711. [Google Scholar] [CrossRef]
- El-Mallah, M.H.; El-Shami, S.M. Investigation of liquid wax components of Egyptian jojoba seeds. J. Oleo Sci. 2009, 58, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Ako, H.; Kong, N.; Brown, A. Fatty acid profiles of kukui nut oils over time and from different sources. Ind. Crop. Prod. 2005, 22, 169–174. [Google Scholar] [CrossRef]
- Riebsomer, J.L.; Foote, N. The chemical composition of the fatty oil From kukui nuts. Proc. Indiana Acad. Sci. 1935, 45, 116–119. [Google Scholar]
- Pimentel, L.D. The culture of macadamia nut. Rev. Bras. Frutic. 2007, 29, 414–415. [Google Scholar]
- McHargue, L.T. Macadamia production in Southern California. In Progress in New Crops; Janick, J., Ed.; ASHS Press: Arlington, VA, USA, 1996; pp. 458–462. [Google Scholar]
- Rodrigues, C.E.C.; Silva, F.A.; Marsaioli, A.; Meirelles, A.J.A. Deacidification of Brazil nut and macadamia nut oils by solvent extraction: Liquid-liquid equilibrium data at 298.2 K. J. Chem. Eng. Data 2005, 50, 517–523. [Google Scholar] [CrossRef]
- Kaseke, T.; Fawole, O.A.; Opara, U.L. Chemistry and functionality of cold-pressed macadamia nut oil. Processes 2021, 10, 56. [Google Scholar] [CrossRef]
- Pino, J.A.; Cuevas-Glory, L.; Marbot, R.; Fuentes, V. Volatile compounds of the nut of Macadamia integrifolia Maiden et Betche. J. Essent. Oil Res. 2009, 21, 159–161. [Google Scholar] [CrossRef]
- Gutman, F.; Nerd, A.; Mizrahi, Y.; Bar-Zvi, D.; Raveh, D. Application of random amplified polymorphic DNA markers for identification of marula genotypes. Hortscience 1999, 34, 1256–1258. [Google Scholar] [CrossRef]
- Mokgolodi, N.C.; Ding, Y.F.; Setshogo, M.P.; Ma, C.; Liu, Y.J. The importance of an indigenous tree to southern African communities with specific relevance to its domestication and commercialization: A case of the marula tree. For. Stud. China 2011, 13, 36–44. [Google Scholar] [CrossRef]
- Gandure, J.; Ketlogetswe, C. Chemical extraction and property analyses of marula nut oil for biodiesel production. Adv. Chem. Eng. Sci. 2011, 1, 96–101. [Google Scholar] [CrossRef]
- Nwabuebo, A.T. The Effect of Extraction Methods on the Oxidatives Stability of Marula and Moringa Seed Oil. Master’s Thesis, University of KwaZulu-Natal, Westville, South Africa, 2017. [Google Scholar]
- Mariod, A.; Matthäus, B.; Eichner, K. Fatty acid, tocopherol and sterol composition as well as oxidative stability of three unusual Sudanese oils. J. Food Lipids 2004, 11, 179–189. [Google Scholar] [CrossRef]
- Viljoen, A.M.; Kamatou, G.P.P.; Başer, K.H.C. Head-space volatiles of marula (Sclerocarya birrea subsp. caffra). S. Afr. J. Bot. 2008, 74, 325–326. [Google Scholar] [CrossRef]
- Boukandoul, S.; Casal, S.; Zaidi, F. The potential of some moringa species for seed oil production. Agriculture 2018, 8, 150. [Google Scholar] [CrossRef]
- Gharsallah, K.; Rezig, L.; Msaada, K.; Chalh, A.; Soltani, T. Chemical composition and profile characterization of Moringa oleifera seed oil. S. Afr. J. Bot. 2021, 137, 475–482. [Google Scholar] [CrossRef]
- Koul, O. Neem: A global perspective. In Neem: Today and in the New Millennium; Koul, O., Wahab, S., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 1–19. [Google Scholar]
- Awolu, O. Optimization of solvent extraction of oil from neem (Azadirachta indica) and its characterizations. J. Sci. Res. Rep. 2013, 2, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, B.; Tefera, T. Extraction of essential oil from neem seed using Soxhlet extraction methods. Int. Adv. Eng. Manag. Sci. 2017, 3, 239870. [Google Scholar] [CrossRef][Green Version]
- Usman, J.G.; Okonkwo, P.C.; Shehu, M.S. Investigation into the usage of solvent for extracting neem oil from neem seed for industrial application. Acad. J. Interdis Stud. 2014, 3, 39–46. [Google Scholar]
- Djibril, D.; Mamadou, F.; Gérard, V.; Geuye, M.D.C.; Oumar, S.; Luc, R. Physical characteristics, chemical composition and distribution of constituents of the neem seeds (Azadirachta indica A. Juss) collected in Senegal. Res. J. Chem. Sci. 2015, 3, 606–612. [Google Scholar]
- Swapna Sonale, R.; Ramalakshmi, K.; Udaya Sankar, K. Characterization of neem (Azadirachta indica A. Juss) seed volatile compounds obtained by supercritical carbon dioxide process. J. Food Sci. Technol. 2018, 55, 1444–1454. [Google Scholar] [CrossRef]
- Breton, C.; Terral, J.F.; Pinatel, C.; Médail, F.; Bonhomme, F.; Bervillé, A. The origins of the domestication of the olive tree. Comptes Rendus Biol. 2009, 332, 1059–1064. [Google Scholar] [CrossRef]
- Bombarely, A.; Doulis, A.G.; Lambrou, K.K.; Zioutis, C.; Margaritis, E.; Koubouris, G. Elucidation of the origin of the monumental olive tree of Vouves in Crete, Greece. Plants 2021, 10, 2374. [Google Scholar] [CrossRef] [PubMed]
- Boskou, D. Characteristics of the olive tree and olive fruit. In Olive Oil: Chemistry and Technology, Second Edition; Boskou, D., Ed.; AOCS Press: Champaign, IL, USA, 2006; pp. 13–19. [Google Scholar]
- Yüksel Aydar, A. Emerging extraction technologies in olive oil production. In Technological Innovation in the Olive Oil Production Chain; Muzzalupo, I., Ed.; InTech Open: London, UK, 2019. [Google Scholar]
- Aparicio, R.; Harwood, J. Handbook of Olive Oil: Analysis and Properties; Springer: New York, NY, USA, 2013. [Google Scholar]
- Ramírez-Tortosa, M.C.; Granados, S.; Quiles, J.L. Chemical composition, types and characteristics of olive oil. In Olive Oil and Health; Quiles, J.L., Ramírez-Tortosa, M.C., Yaqoob, P., Eds.; CABI Publishing: Wallingford, UK, 2006; pp. 45–62. [Google Scholar] [CrossRef]
- Fernandes-Silva, A.A.; Falco, V.; Correia, C.M.; Villalobos, F.J. Sensory analysis and volatile compounds of olive oil (cv. Cobrançosa) from different irrigation regimes. Grasas Aceites 2013, 64, 59–67. [Google Scholar] [CrossRef]
- Zeven, A.C. On the origin of the oil palm (Elaeis guineensis Jacq.). Grana 1964, 5, 121–123. [Google Scholar] [CrossRef]
- Zaidul, I.S.M.; Nik Norulaini, N.A.; Mohd Omar, A.K.; Smith, R.L. Supercritical carbon dioxide (SC-CO2) extraction of palm kernel oil from palm kernel. J. Food Eng. 2007, 79, 1007–1014. [Google Scholar] [CrossRef]
- Atasie, V.N.; Akinhanmi, T.F. Extraction, compositional studies and physico-chemical characteristics of palm kernel oil. Pak. J. Nutr. 2009, 8, 800–803. [Google Scholar] [CrossRef]
- Thokchom, R.; Mandal, G. Production preference and importance of passion fruit (Passiflora edulis): A review. J. Agric. Eng. Food Technol. 2017, 4, 27–30. [Google Scholar]
- Biswas, S.; Mishra, R.; Bist, A.S. Passion to profession: A review of passion fruit processing. Aptisi Trans. Technopreneurship (ATT) 2021, 3, 48–57. [Google Scholar] [CrossRef]
- Malacrida, C.R.; Jorge, N. Yellow passion fruit seed oil (Passiflora edulis f. flavicarpa): Physical and chemical characteristics. Braz. Arch. Biol. Technol. 2012, 55, 127–134. [Google Scholar] [CrossRef]
- Piombo, G.; Barouh, N.; Barea, B.; Boulanger, R.; Brat, P.; Pina, M.; Villeneuve, P. Characterization of the seed oils from kiwi (Actinidia chinensis), passion fruit (Passiflora edulis) and guava (Psidium Guajava). Oilseeds Crop. Fats Lipids 2006, 13, 195–199. [Google Scholar] [CrossRef]
- De Paula, R.C.M.; Soares, A.G.; Freitas, S.P. Volatile compounds in passion fruit seed oil (Passiflora setacea BRS Pérola Do Cerrado and Passiflora alata BRS Doce Mel). Chem. Eng. Trans. 2015, 44, 103–108. [Google Scholar] [CrossRef]
- Chandra, R.; Babu, K.D.; Jadhav, V.T.; Jaime, A.; Silva, T.D. Origin, history, and domestication of pomegranate. Fruit. Veg. Cereal Sci. Biotechnol. 2010, 2, 1–6. [Google Scholar]
- Paul, A.; Radhakrishnan, M. Pomegranate seed oil in the food industry: Extraction, characterization, and applications. Trends Food Sci. Technol. 2020, 105, 273–283. [Google Scholar] [CrossRef]
- Abbasi, H.; Rezaei, K.; Rashidi, L. Extraction of essential oils from the seeds of pomegranate using organic solvents and supercritical CO2. J. Am. Oil Chem. Soc. 2008, 85, 83–89. [Google Scholar] [CrossRef]
- Loukhmas, S.; Kerak, E.; Elgadi, S.; Ettalibi, F.; El Antari, A.; Harrak, H. Oil content, fatty acid composition, physicochemical properties, and antioxidant activity of seed oils of ten Moroccan pomegranate cultivars. J. Food Qual. 2021, 2021, 6617863. [Google Scholar] [CrossRef]
- de Melo, I.L.P.; de Carvalho, E.B.T.; de Silva, A.M.d.O.; Yoshime, L.T.; Sattler, J.A.G.; Pavan, R.T.; Mancini-Filho, J. Characterization of constituents, quality and stability of pomegranate seed oil (Punica granatum L.). Food Sci. Technol. 2016, 36, 132–139. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, L.; Liu, J.; Zhang, X.; Lu, Y. Analysis of the volatile flavor compounds of pomegranate seeds at different processing temperatures by GC-IMS. Molecules 2023, 28, 2717. [Google Scholar] [CrossRef]
- Deng, Y.-C.; Hua, H.-M.; Li, J.; Lapinskas, P. Studies on the cultivation and uses of evening primrose (Oenothera spp.) in China. Econ. Bot. 2001, 55, 83–92. [Google Scholar] [CrossRef]
- Steckel, L.E.; Sosnoskie, L.M.; Steckel, S.J. Common evening-primrose (Oenothera biennis L.). Weed Technol. 2019, 33, 757–760. [Google Scholar] [CrossRef]
- Christie, W.W. The analysis of evening primrose oil. Ind. Crop. Prod. 1999, 10, 73–83. [Google Scholar] [CrossRef]
- Timoszuk, M.; Bielawska, K.; Skrzydlewska, E. Evening primrose (Oenothera biennis) biological activity dependent on chemical composition. Antioxidants 2018, 7, 108. [Google Scholar] [CrossRef]
- Zhao, B.; Gong, H.; Li, H.; Zhang, Y.; Deng, J.; Chen, Z. Fatty acid, triacylglycerol and unsaponifiable matters profiles and physicochemical properties of Chinese evening primrose oil. J. Oleo Sci. 2019, 68, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.G.; Chen, X.M.; Pang, Y.; Yang, E.Q.; Wang, S.Y.; Wang, Y.F.; Liu, B.Q. Characterization of volatile compounds in evening primrose oil after γ-irradiation. Flavour. Fragr. J. 2022, 37, 181–191. [Google Scholar] [CrossRef]
- Shaban, A.; Sahu, R.P. Pumpkin seed oil: An alternative medicine. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar, V. Phyto-chemical and bioactive compounds of pumpkin seed oil as affected by different extraction methods. Food Chem. Adv. 2023, 2, 100211. [Google Scholar] [CrossRef]
- Procida, G.; Stancher, B.; Cateni, F.; Zacchigna, M. Chemical composition and functional characterisation of commercial pumpkin seed oil. J. Sci. Food Agric. 2013, 93, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Neđeral, S.; Škevin, D.; Kraljić, K.; Obranović, M.; Papeša, S.; Bataljaku, A. Chemical composition and oxidative stability of roasted and cold pressed pumpkin seed oils. J. Am. Oil Chem. Soc. 2012, 89, 1763–1770. [Google Scholar] [CrossRef]
- Rezig, L.; Chouaibi, M.; Msaada, K.; Hamdi, S. Chemical composition and profile characterization of pumpkin (Cucurbita maxima) seed oil. Ind. Crop. Prod. 2012, 37, 82–87. [Google Scholar] [CrossRef]
- Hagos, M.; Yaya, E.E.; Chandravanshi, B.S.; Redi-Abshiro, M. Analysis of volatile compounds in flesh, peel and seed parts of pumpkin (Cucurbita maxima) cultivated in Ethiopia using gas chromatography-mass spectrometry (GC-MS). Int. J. Food Prop. 2022, 25, 1498–1512. [Google Scholar] [CrossRef]
- Ispiryan, A.; Viškelis, J.; Viškelis, P. Red raspberry (Rubus idaeus L.) seed oil: A review. Plants 2021, 10, 944. [Google Scholar] [CrossRef]
- Oomah, B.D.; Ladet, S.; Godfrey, D.V.; Liang, J.; Girard, B. Characteristics of raspberry (Rubus idaeus L.) seed oil. Food Chem. 2000, 69, 187–193. [Google Scholar] [CrossRef]
- Bilgin, N.A.; Mısırlı, A.; Şen, F.; Türk, B.; Yağmur, B. Fruit pomological, phytochemical characteristic and mineral content of rosehip genotypes. ETP Int. J. Food Eng. 2020, 6, 18–23. [Google Scholar] [CrossRef]
- Ercişli, S.; Eşitken, A. Fruit characteristics of native rose hip (Rosa spp.) selections from the Erzurum Province of Turkey. N. Z. J. Crop Hortic. Sci. 2004, 32, 51–53. [Google Scholar] [CrossRef]
- Georgieva, S.; Angelov, G.; Boyadzhieva, S. Concentration of vitamin C and antioxidant activity of rosehip extracts. J. Chem. Technol. Metall. 2014, 49, 451–454. [Google Scholar]
- del Valle, J.M.; Bello, S.; Thiel, J.; Allen, A.; Chordia, L. Comparison of conventional and supercritical CO2-extracted rosehip oil. Braz. J. Chem. Eng. 2000, 17, 335–348. [Google Scholar] [CrossRef]
- Machmudah, S.; Kawahito, Y.; Sasaki, M.; Goto, M. Supercritical CO2 extraction of rosehip seed oil: Fatty acids composition and process optimization. J. Supercrit. Fluids 2007, 41, 421–428. [Google Scholar] [CrossRef]
- Ilyasoğlu, H. Characterization of rosehip (Rosa canina L.) seed and seed oil. Int. J. Food Prop. 2014, 17, 1591–1598. [Google Scholar] [CrossRef]
- Murathan, Z.T.; Zarifikhosroshahi, M.; Kafkas, N.E. Determination of fatty acids and volatile compounds in fruits of rosehip (Rosa spp.) species by HS-SPME/GC-MS and IM-SPME/GC-MS techniques. Turk. J. Agric. For. 2016, 40, 269–279. [Google Scholar] [CrossRef]
- Cárdenas, D.M.; Rave, L.J.G.; Soto, J.A. Biological activity of sacha inchi (Plukenetia volubilis Linneo) and potential uses in human health: A review. Food Technol. Biotechnol. 2021, 59, 253–266. [Google Scholar] [CrossRef]
- Nusselder, H.; Cloesen, P. Noble seeds: Sacha inchi from Amazonia to the Caribbean Basin? In A Stroll along Local Lines. Contributions to Public Policy in the Rural Sector of Central America, the Caribbean and the Andean Region; Centro de Estudios para el Desarrollo Rural: San José, Costa Rica, 2014; Volume 1980, pp. 5–14. [Google Scholar]
- Gutiérrez, L.F.; Rosada, L.M.; Jiméneza, Á. Chemical composition of sacha inchi (Plukenetia volubilis L.) seeds and characteristics of their lipid fraction. Grasas Aceites 2011, 62, 76–83. [Google Scholar] [CrossRef]
- Fanali, C.; Dugo, L.; Cacciola, F.; Beccaria, M.; Grasso, S.; Dachà, M.; Dugo, P.; Mondello, L. Chemical characterization of sacha inchi (Plukenetia volubilis L.) oil. J. Agric. Food Chem. 2011, 59, 13043–13049. [Google Scholar] [CrossRef]
- Chapman, M.A.; Burke, J.M. DNA sequence diversity and the origin of cultivated safflower (Carthamus tinctorius L.; Asteraceae). BMC Plant Biol. 2007, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Cheng, L.; Zhang, R.; Bi, J. Extraction of safflower seed oil by supercritical CO2. J. Food Eng. 2009, 92, 370–376. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Uslu, N.; Babiker, E.E.; Ghafoor, K.; Mohamed Ahmed, I.A.; Özcan, M.M. The effect of different solvent types and extraction methods on oil yields and fatty acid composition of safflower seed. J. Oleo Sci. 2019, 68, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Khan, R.S.; Hussain, M.I.; Farooq, M.; Ahmad, A.; Ahmed, I. A comprehensive characterization of safflower oil for its potential applications as a bioactive food ingredient: A review. Trends Food Sci. Technol. 2017, 66, 176–186. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Z.; Han, B.; Wu, W.; Zhao, Q.; Wei, C.; Liu, W. Comprehensive analysis of volatile compounds in cold-pressed safflower seed oil from Xinjiang, China. Food Sci. Nutr. 2020, 8, 903–914. [Google Scholar] [CrossRef]
- Zeb, A. Important therapeutic uses of sea buckthorn (Hippophae): A review. J. Biol. Sci. 2004, 4, 687–693. [Google Scholar]
- Zielińska, A.; Nowak, I. Abundance of active ingredients in sea-buckthorn oil. Lipids Health Dis. 2017, 16, 95. [Google Scholar] [CrossRef]
- Li, T.S.C.; Beveridge, T.H.J.; Drover, J.C.G. Phytosterol content of sea buckthorn (Hippophae rhamnoides L.) seed oil: Extraction and identification. Food Chem. 2007, 101, 1633–1639. [Google Scholar] [CrossRef]
- Tudor, C.; Bohn, T.; Iddir, M.; Dulf, F.V.; Focşan, M.; Rugină, D.O.; Pintea, A. Sea buckthorn oil as a valuable source of bioaccessible xanthophylls. Nutrients 2020, 12, 76. [Google Scholar] [CrossRef]
- Kumar, R.; Phani Kumar, G.; Chaurasia, O.P.; Singh, S.B. Phytochemical and pharmacological profile of sea buckthorn oil: A review. Res. J. Med. Plant 2011, 5, 491–499. [Google Scholar] [CrossRef]
- Pham, T.D.; Nguyen, T.D.T.; Carlsson, A.S.; Bui, T.M. Morphological evaluation of sesame (Sesamum indicum L.) varieties from different origins. Aust. J. Crop Sci. 2010, 4, 498–504. [Google Scholar]
- Nayar, N.M.; Mehra, K.L. Sesame: Its uses, botany, cytogenetics, and origin. Econ. Bot. 1970, 24, 20–31. [Google Scholar] [CrossRef]
- Warra, A. Sesame (Sesamum indicum L.) seed oil methods of extraction and its prospects in cosmetic industry: A review. Bayero J. Pure Appl. Sci. 2012, 4, 164–168. [Google Scholar] [CrossRef]
- Kheirati Rounizi, S.; Akrami Mohajeri, F.; Moshtaghi Broujeni, H.; Pourramezani, F.; Jambarsang, S.; Kiani, H.; Khalili Sadrabad, E. The chemical composition and heavy metal content of sesame oil produced by different methods: A risk assessment study. Food Sci. Nutr. 2021, 9, 2886–2893. [Google Scholar] [CrossRef]
- Ivanova-Petropulos, V.; Mitrev, S.; Stafilov, T.; Markova, N.; Leitner, E.; Lankmayr, E.; Siegmund, B. Characterization of traditional Macedonian edible oils by their fatty acid composition and their volatile compounds. Food Res. Int. 2015, 77, 506–514. [Google Scholar] [CrossRef]
- Allal, F.; Piombo, G.; Kelly, B.A.; Okullo, J.B.L.; Thiam, M.; Diallo, O.B.; Nyarko, G.; Davrieux, F.; Lovett, P.N.; Bouvet, J.M. Fatty acid and tocopherol patterns of variation within the natural range of the shea tree (Vitellaria paradoxa). Agrofor. Syst. 2013, 87, 1065–1082. [Google Scholar] [CrossRef]
- Alonge, A.F.; Olaniyan, A.M. Problems of shea butter processing in Africa. In Proceedings of the American Society of Agricultural and Biological Engineers—International Conference on Crop Harvesting and Processing, Louisville, KY, USA, 11–14 February 2007; p. 701. [Google Scholar] [CrossRef]
- Bariwere Samuel, C. Physicochemical properties and fatty acid profile of shea butter and fluted pumpkin seed oil, a suitable blend in bakery fat production. Int. J. Nutr. Food Sci. 2017, 6, 122–128. [Google Scholar] [CrossRef]
- Abdel-Razek, A.G.; Abo-Elwafa, G.A.; Al-Amrousi, E.F.; Badr, A.N.; Hassanein, M.M.M.; Qian, Y.; Siger, A.; Grygier, A.; Radziejewska-Kubzdela, E.; Rudzińska, M. Effect of refining and fractionation processes on minor components, fatty acids, antioxidant and antimicrobial activities of shea butter. Foods 2023, 12, 1626. [Google Scholar] [CrossRef]
- Sedivy, E.J.; Wu, F.; Hanzawa, Y. Soybean domestication: The origin, genetic architecture and molecular bases. New Phytol. 2017, 214, 539–553. [Google Scholar] [CrossRef]
- Moses, R. Performance evaluation of continuous screw press for extraction soybean oil. Am. J. Sci. Technol. 2014, 1, 238–242. [Google Scholar]
- Wills, D.M.; Burke, J.M. Chloroplast DNA variation confirms a single origin of domesticated sunflower (Helianthus annuus L.). J. Hered. 2006, 97, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Amalia Kartika, I.; Pontalier, P.Y.; Rigal, L. Extraction of sunflower oil by twin screw extruder: Screw configuration and operating condition effects. Bioresour. Technol. 2006, 97, 2302–2310. [Google Scholar] [CrossRef] [PubMed]
- Gotor, A.A.; Rhazi, L. Effects of refining process on sunflower oil minor components: A review. Oilseeds Fats Crop. Lipids 2016, 23, D207. [Google Scholar] [CrossRef]
- Ginigini, J.; Lecellier, G.J.; Nicolas, M.; Nour, M.; Hnawia, E.; Lebouvier, N.; Herbette, G.; Lockhart, P.; Raharivelomanana, P. Chemo-diversity of Calophyllum inophyllum L. oil bioactive components related to their specific geographical distribution in the South Pacific Region. PeerJ 2019, 2019, e6896. [Google Scholar] [CrossRef]
- Dweck, A.C.; Meadows, T. Tamanu (Calophyllum inophyllum)—The African, Asian, Polynesian and Pacific panacea. Int. J. Cosmet. Sci. 2002, 24, 341–348. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Tran, T.T.M. Chemical composition analysis and antibacterial-anti-inflammatory activity tests of tamanu seed oil extracted by supercritical fluid technology. Sci. Technol. Dev. J. 2016, 19, 146–154. [Google Scholar] [CrossRef]
- Levent, O. A detailed comparative study on some physicochemical properties, volatile composition, fatty acid, and mineral profile of different almond (Prunus dulcis L.) varieties. Horticulturae 2022, 8, 488. [Google Scholar] [CrossRef]
- Doğan, A.; Karaat, F.E.; Levent, O.; Asma, B.M. Characterization of new late-spring-frost-tolerant apricot hybrids: Physical and biochemical fruit quality attributes, volatile aroma compounds. Cienc. Rural 2023, 53, e20220144. [Google Scholar] [CrossRef]
- Kilic-Buyukkurt, O. Characterization of aroma compounds of cold-pressed avocado oil using solid-phase microextraction techniques with gas chromatography–mass spectrometry. J. Raw Mat. Process. Foods 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Kiralan, M.; Kiralan, S.S. Changes in volatile compounds of black cumin oil and hazelnut oil by microwave heating process. J. Am. Oil Chem. Soc. 2015, 92, 1445–1450. [Google Scholar] [CrossRef]
- Sartori, A.G.d.O.; Sampaio, G.R.; Bastos, D.H.M.; Regitano d’Arce, M.A.B.; Skibsted, L.H. Volatiles and tendency of radical formation of cold-pressed Brazil nut oil during ambient storage. J. Am. Oil Chem. Soc. 2018, 95, 721–730. [Google Scholar] [CrossRef]
- Jung, H.; Kim, I.; Jung, S.; Lee, J. Oxidative stability of chia seed oil and flax seed oil and impact of rosemary (Rosmarinus officinalis L.) and garlic (Allium cepa L.) extracts on the prevention of lipid oxidation. Appl. Biol. Chem. 2021, 64, 6. [Google Scholar] [CrossRef]
- Sevindik, O.; Guclu, G.; Bombai, G.; Rombolá, A.D.; Kelebek, H.; Selli, S. Volatile compounds of cvs Magliocco Canino and Dimrit grape seed oils. J. Raw Mater. Process. Foods 2020, 1, 47–54. [Google Scholar]
- Da Porto, C.; Decorti, D.; Natolino, A. Separation of aroma compounds from industrial hemp inflorescences (Cannabis sativa L.) by supercritical CO2 extraction and on-line fractionation. Ind. Crop. Prod. 2014, 58, 99–103. [Google Scholar] [CrossRef]
- Daniel, M.; John, N.-A.; Zhang, X.; Arthur, G.; Eric, K.; Godelieve, M. Comparison of volatile profile of Moringa oleifera leaves from Rwanda and China using HS-SPME. Pak. J. Nutr. 2011, 10, 602–608. [Google Scholar] [CrossRef]
- Cavalli, J.-F.; Fernandez, X.; Lizzani-Cuvelier, L.; Loiseau, A.-M. Characterization of volatile compounds of French and Spanish virgin olive oils by HS-SPME: Identification of quality-freshness markers. Food Chem. 2004, 88, 151–157. [Google Scholar] [CrossRef]
- Mamede, A.M.G.N.; Soares, A.G.; Oliveira, E.J.; Farah, A. Volatile composition of sweet passion fruit (Passiflora alata Curtis). J. Chem. 2017, 2017, 3497216. [Google Scholar] [CrossRef]
- Costa, A.M.M.; Silva, L.O.; Torres, A.G. Chemical composition of commercial cold-pressed pomegranate (Punica granatum) seed oil from Turkey and Israel, and the use of bioactive compounds for samples’ origin preliminary discrimination. J. Food Compos. Anal. 2019, 75, 8–16. [Google Scholar] [CrossRef]
- Karabagias, V.K.; Karabagias, I.K.; Gatzias, I.; Badeka, A.V. Prickly pear seed oil by shelf-grown cactus fruits: Waste or maste? Processes 2020, 8, 132. [Google Scholar] [CrossRef]
- Kiralan, M.; Yildirim, G. Rosehip (Rosa canina L.) oil. In Fruit Oils: Chemistry and Functionality; Springer International Publishing: Cham, Switzerland, 2019; pp. 803–814. [Google Scholar]
- Aydeniz, B.; Güneşer, O.; Yılmaz, E. Physico-chemical, sensory and aromatic properties of cold press produced safflower oil. J. Am. Oil Chem. Soc. 2014, 91, 99–110. [Google Scholar] [CrossRef]
- Jia, X.; Zhou, Q.; Wang, J.; Liu, C.; Huang, F.; Huang, Y. Identification of key aroma-active compounds in sesame oil from microwaved seeds using E-nose and HS-SPME-GC×GC-TOF/MS. J. Food Biochem. 2019, 43, e12786. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Nishimura, O.; Masuda, H.; Mihara, S. Identification of volatile flavor components of the oil from roasted sesame seeds. Agric. Biol. Chem. 1989, 53, 1891–1899. [Google Scholar] [CrossRef]
- Oussou, K.F.; Guclu, G.; Sevindik, O.; Starowicz, M.; Kelebek, H.; Selli, S. Comparative elucidation of aroma, key odorants, and fatty acid profiles of Ivorian shea butter prepared by three different extraction methods. Separations 2022, 9, 245. [Google Scholar] [CrossRef]
- Xiao, L.; Li, C.; Chai, D.; Chen, Y.; Wang, Z.; Xu, X.; Wang, Y.; Geng, Y.; Dong, L. Volatile compound profiling from soybean oil in the heating process. Food Sci. Nutr. 2020, 8, 1139–1149. [Google Scholar] [CrossRef]
| Carrier Oil | VOC Extraction Method | Origin | Volatile Organic Compounds | Ref. |
|---|---|---|---|---|
| Bitter Almond Oil (Cold pressed) | Purge and Trap Extraction | Iran | hexanal, nonanal, benzaldehyde, (E)-decenal, (E,E)-2,4-nonadienal, 2-phenyl-2-propanol, benzyl alcohol, octadecanol, hexanoic acid, heptanoic acid, octanoic acid, benzoic acid, 2-pentylfuran, α-pinene, β-pinene, sabinene, δ-3-carene, limonene, γ-terpinene, p-cymene, 4-terpineol | [18] |
| Sweet Almond Oil (Cold pressed) | Purge and Trap Extraction | Iran | hexanal, octanal, nonanal, furfural, benzaldehyde, (E,E)-nonadienal, 2-undecenal, (E,E)-2,4-decadienal, 3-pentel-2-ol, octanol, 2-phenyl-2-propanol, benzyl alcohol, hexanoic acid, octanoic acid, 2-octanone, 4-nonanone, methylpyrazine, 2,5-dimethylpyrazine, 2,6-dimethylpyrazine, methylethylpyrazine, 2-ethylpyrazine, δ-3-carene, limonene, p-cymene | [18] |
| Ferragnes Almonds (Soxhlet Solvent Extraction Using n-Hexane) | Solid Phase Microextraction | France | butanal, toluene, butyl acetate, 2-methyl-1-propanol, 4-methyl-6-hepten-3-one, ethylbenzene, 2,5-dimethyl-3-hexanone, butanol, 3-heptanone, 3-methylbutanol, 2-hexanol, 4-octanone, 3-octanone, butyl valerate, pinacol, 1-hexanol, 2-nonanone, 2-methylpentanal, isobutyric acid, valeric acid, 2-phenyl-2-propanol, γ-decalactone, γ-undecalactone, diethyl phthalate | [230] |
| Ferraduel Almonds (Soxhlet Solvent Extraction Using n-Hexane) | Solid Phase Microextraction | France | butanal, 3-methyl-2-pentanone, toluene, butyl acetate, 2-methyl-1-propanol, isoamyl acetate, ethylbenzene, 2.5-dimethyl-3-hexanone, butyl isobutyrate, butanol, 3-heptanone, butyl butyrate, 4-octanone, 3-octanone, m-cymene, butyl valerate, 2-nonanone, 2-methylpentanal, isobutyric acid, valeric acid, 2-phenyl-2-propanol, γ-undecalactone, diethyl phthalate | [230] |
| Marta Almonds (Soxhlet Solvent Extraction Using n-Hexane) | Solid Phase Microextraction | France | 3-methyl-2-pentanone, toluene, butyl acetate, hexanal, isoamyl acetate, ethylbenzene, butanol, limonene, 2-hexanol, 4-octanone, 2-heptenal, pinacol, 2-nonanone, 2-methylpentanal, benzaldehyde, γ-butyrolactone, 2-decenal, phenylacetaldehyde, 2-phenyl-2-propanol, γ-decalactone, diethyl phthalate | [230] |
| Lauranne Almonds (Soxhlet Solvent Extraction Using n-Hexane) | Solid Phase Microextraction | France | butanal, toluene, butyl acetate, 2-methyl-1-propanol, ethylbenzene, butyl isobutyrate, butanol, 3-heptanone, 3-methyl-butanol, butyl butyrate, butyl butyrate, 4-octanone, m-cymene, pinacol, 2-nonanone, 2-methylpentanal, benzaldehyde, isobutyric acid, phenylacetaldehyde, 2-phenyl-2-propanol, γ-undecalactone | [230] |
| Texas Almonds (Soxhlet Solvent Extraction Using n-Hexane) | Solid Phase Microextraction | United States | 2-methylbutanal, ethanol, α-pinene, toluene, butyl acetate, hexanal, 2-methyl-1-propanol, 4-methyl-6-hepten-3-one, 2.5-dimethyl-3-hexanone, butanol, 2-heptanone, 3-methyl-butanol, 2-hexanol, 4-octanone, 3-octanone, m-cymene, pinacol, 1-hexanol, 2-nonanone, 2-methylpentanal, benzaldehyde, 2-decenal, valeric acid, 2-phenyl-2-propanol, γ-undecalactone | [230] |
| Nonpareil Almonds (Soxhlet Solvent Extraction Using n-Hexane) | Solid Phase Microextraction | United States | 2-methylbutanal, 3-methylbutanal, ethanol, α-pinene, toluene, butyl acetate, hexanal, 2,5-dimethyl-3-hexanone, butanol, limonene, 4-octanone, 2-heptenal, pinacol, 2-nonanone, 2-methylpentanal, benzaldehyde, 2-decenal, phenylacetaldehyde, 2-phenyl-2-propanol, diethyl phthalate | [230] |
| Guara Almonds (Soxhlet Solvent Extraction Using n-Hexane) | Solid Phase Microextraction | Spain | butanal, α-pinene, toluene, butyl acetate, hexanal, ethylbenzene, butanol, 3-heptanone, limonene, 2-hexanol, 4-octanone, 3-octanone, 2-heptenal, pinacol, 2-nonanone, 2-methylpentanal, benzaldehyde, 2-decenal, phenylacetaldehyde, 2-phenyl-2-propanol, γ-decalactone | [230] |
| Yaltinskii Almonds (Soxhlet Solvent Extraction Using n-Hexane) | Solid Phase Microextraction | Russia | butanal, 3-methyl-2-pentanone, α-pinene, toluene, butyl acetate, 4-methyl-6-hepten-3-one, ethylbenzene, butanol, 2-heptanone, 3-methyl-butanol, 2-hexanol, 4-octanone, m-cymene, butyl valerate, pinacol, 1-hexanol, 2-methylpentanal, benzaldehyde, 2-decenal, valeric acid, 2-phenyl-2-propanol, γ-undecalactone | [230] |
| Nurlu Almonds (Soxhlet Solvent Extraction Using n-Hexane) | Solid Phase Microextraction | Turkey | butanal, 3-methylbutanal, ethanol, α-pinene, toluene, butyl acetate, 2-methyl-1-propanol, ethylbenzene, 2.5-dimethyl-3-hexanone, butyl isobutyrate, butanol, 2-heptanone, limonene, 2-hexanol, 4-octanone, 2-heptenal, pinacol, 2-nonanone, 2-methylpentanal, γ-butyrolactone, valeric acid, 2-phenyl-2-propanol, γ-decalactone, diethyl phthalate | [230] |
| Acıbadem Almonds (Soxhlet Solvent Extraction Using n-Hexane) | Solid Phase Microextraction | Turkey | butanal, 2-methylbutanal, 3-methylbutanal, toluene, butyl acetate, hexanal, 4-methyl-6-hepten-3-one, ethylbenzene, 2,5-dimethyl-3-hexanone, butanol, 3-heptanone, 2-hexanol, 4-octanone, 2-heptenal, pinacol, 2-nonanone, 2-methylpentanal, benzaldehyde, 2-decenal, valeric acid, 2-phenyl-2-propanol, γ-decalactone, diethyl phthalate | [230] |
| Apricot Fruit | Solid Phase Microextraction | Turkey | 2-methylbutanal, 3-methylbutanal, hexanal, 4-pentenal, heptanal, (E)-2-hexenal, nonanal, 2-octenal, benzaldehyde, 2,3-butandione, 3-hydroxy-2-butanone, 6-methyl-5-hepten-2-one, β-ionone, γ-decalactone, ethyl acetate, methyl propaonate, methyl butanoate, ethyl butanoate, butyl acetate, butyl butanoate, hexyl acetate, (Z)-3-hexenylacetate, (E)-2-hexenylacetate, ethanol, 1-butanol, 1-pentanol, 1-hexanol, 3-hexanol, 2-hexen-1-ol, linalool, α-pinene, sabinene, β-myrcene, limonene, β-phellandrene, 2-methylpropanoic acid, 2-methylbutanoic acid, decane, toluene, 2-methyltetrahydrofuran, tert-butyl-benzene | [231] |
| Apricot Kernel Oil | Unknown | Korea | benzaldehyde, mandelonitrile, benzoic acid | [26] |
| Apricot Kernel Oil (Hydrodistillation) | Head Space Solid Phase Microextraction | Greece | toluene, 2,3-butanediol, ethylbenzene, 2-methyl-propanal, 1,3-dimethyl-benzene, nonane, benzaldehyde, 1,2,4-trimethyl-benzene, 1,2,3-trimethyl-benzene, decane, benzyl alcohol, butyl-cyclohexane, 1,2-diethylbenzene, 1-methyl-3-propylbenzene, 1-methyl-2-propylbenzene, 1-ethyl-3,5-dimethylbenzene, 2-ethyl-1,3-dimethylbenzene, o-cymene, 2-ethyl-1,4-dimethylbenzene, decahydro-2-methylnaphthalene, 1,2,3,5-tetramethylbenzene, 1,2,4,5-tetramethylbenzene, undecane, 2,3-dihydro-4-methyl-1H-indene, 1-phenyl-1-butene, 1,2,3,4-tetramethyl-5-methylene-1,3-cyclopentadiene, 1-phenyl-1,2-propanedione, benzyl acetate, azulene, ethyl benzoate, 2,4-diethyl-1-methyl-benzene, 1-methyl-4-(1-methylpropyl)-benzene, 6-methylundecane, 2-methylundecane, benzoin, dodecane, 2,6-dimethyl-undecane, tridecane | [30] |
| Argan Oil (cold pressed) | Purge and Trap Extraction | Morocco | 2-methyl-2-butanol, 3-penten-2-ol, isoamyl alcohol, pentanol, 2-hexanol, 2,3-butanediol, 1,3-butanediol, 1,2-propanediol, benzyl alcohol, phenylethyl alcohol, methyl pyrazine, 2,5-dimethylpyrazine, 2,6-dimethylpyrazine, ethylpyrazine, 2-ethyl-6-methylpyrazine, 2-ethyl-5-methylpyrazine, 2,3,5-trimethylpyrazine, 2,5-dimethyl-3-ethylpyrazine, 2,3-diethyl-5-methylpyrazine, acetic acid, propanoic acid, isobutanoic acid, butanoic acid, 2-methylbutanoic acid, hexanoic acid, furfuryl alcohol, 5-methylfurfuryl alcohol, 1-methyl-1H-pyrrole, 2-formylpyrrole, γ-valerolactone, γ-butyrolactone, phenol, p-cresol, 2-octanone, nonanal | [36] |
| Avocado Fruit | Likens-Nickerson Extraction | Cuba | acetaldehyde, ethanol, acetic acid, diacetyl, 3-penten-2-one, pyridine, pentanol, toluene, hexanal, 2-furfural, (Z)-3-hexenol, hexanol, methional, α-pinene, (E)-2-heptenal, benzaldehyde, β-pinene, 2-pentylfuran, p-cymene, limonene, (Z)-β-ocimene, cyclohexyl acetate, (E)-β-ocimene, γ-terpinene, (E)-2-octenal, p-methylbenzaldehyde, perillene, (E)-2-nonenal, (Z)-4-decenal, (Z)-7-decenol, decanal, benzothiazole, (E)-anethole, (E,Z)-2,4-decadienal, 10-undecenal, 4-vinylguaiacol, (E,E)-2,4-decadienal, cyclosativene, α-copaene, β-elemene, (Z)-jasmone, β-caryophyllene, trans-α-bergamotene, α-humulene, allo-aromadendrene, 9-epi-β-caryophyllene, germacrene D, bicyclogermacrene, (E,E)-α-farnesene, δ-cadinene, β-sesquiphellandrene, (Z)-nerolidol, caryophyllene oxide, guaiol, tetradecanal, α-cadinol, bulnesol, α-bisabolol, mintsulfide, tetradecanoic acid, (E)-β-santalol, (E,E)-farnesyl acetate, methyl hexadecanoate, hexadecanoic acid | [45] |
| Avocado Seed Oil (cold pressed) | Solid Phase Microextraction | Turkey | d-limonene, α-cubebene, β-caryophyllene, pulegone, β-curcumene, hexanal, heptanal, octanal, nonanal, (Z)-5-tridecene, 2-heptanone, 2-octanone, 2-decanone, durene, isodurene, biphenyl, undecane, dodecane, tridecane | [232] |
| Black Cumin Seed | DBWAX Column GC-MS | China | β-thujene, β-pinene, α-terpinolene, d-limonene, γ-terpinene, o-cymene, cis-4-methoxy thujane, p-mentha-1,5,8-triene, p-cymenene, acetic acid, (E)-longipinene, ylangene, trans-2-caren-4-ol, 1,3,4-trimethyl-3-cyclohexene-1-carboxaldehyde, verbenone, longifolene, butanoic acid, estragole, carvone, thymoquinone, anethole, p-cymen-8-ol, isolongifolyl acetate, (Z)-18-octadec-9-enolide, 9(E),11(E)-conjugated linoleic acid, nonanoic acid, phenol, 2-methyl-5-(1-methylethyl)phenol, 6-methyl-5-(1-methylethyl)-5-hepten-3-yn-2-ol, p-cymene-2,5-diol, (Z,Z,Z)-9,12,15-octadecatrienoic acid, 3,6-dimethylbenzo[b]thiophene | [66] |
| Black Cumin Seed Extract | Purge and Trap Extraction | Turkey | acetic acid, propanoic acid, isobutanoic acid, butanoic acid, propenoic acid, pentanoic acid, hexanoic acid, (E)-3-hexenoic acid, heptanoic acid, octanoic acid, nonanoic acid, hexadecanoic acid, octadecanoic acid, 2-methyl-3-butanol, 3-penten-2-ol, (Z)-2-methyl-2-buten-1-ol, furfuryl alcohol, 2-(2-ethoxyethoxy)ethanol, benzyl alcohol, phenethyl alcohol, guaiacol, phenol, eugenol, limonene, benzyl acetate, ethyl 4-ethoxybenzoate, acetoin, hydroxyacetone, furfural, butyrolactone, m-xylene, styrene | [18] |
| Black Cumin Oil (cold pressed) | Solid Phase Microextraction | Turkey | hexanal, α-thujene, α-pinene, sabinene, β-pinene, 2-heptenal, α-terpinene, limonene, p-cymene, γ-terpinene, (E)-2-octenal, nonanal, 4-terpineol, thymoquinone, (E,E)-2,4-decadienal, α-longipinene, isolongifolene | [233] |
| Brazil Nut | GCO with both Carbowax 20M and SE30 Columns | Brazil | hexanal, heptanal, 2,4-nonadienal, 2,4-decadienal, ethanol, 1-butanol, 1-pentanol, 2-methyl-2-butanol, 1-hexanol, 1-octanol, 2-nonanol, phenol, cresol, 2-heptanone, 2-nonanone, 2-decanone, 2-undecanone, 2-dodecanone, benzaldehyde, n-nonane, n-decane, n-undecane, n-dodecane, n-tridecane, toluene, ethylbenzene, styrene, n-butylbenzene, 1,2,3-trimethylbenzene, 1,2,4-trimethylbenzenene, 1,3,5- trimethylbenzenene, naphthalene, 2-methylnaphthalene, limonene, ethyl acetate, benzofuran, methylbenzofuran, cyanobenzene, chloroform | [75] |
| Brazil Nut Oil (cold pressed) | Head Space Solid Phase Microextraction | Brazil | hexanal, (E)-2-heptenal, (E)-2-octenal, nonanal, 3-octen-2-one, 2-nonanone, 1-heptanol, 1-octen-3-ol | [234] |
| Chia Seed Oil | Head Space Solid Phase Microextraction | The United States | hexanal, 2-pentenal, (2Z)-heptanal, nonanal, 2-octanal, (2E,4E)-heptadienal, 3-octen-2-one, (3E,5E)- octadien-2-one, 1-penten-3-ol, 2-methyl-3-pentanol, 1-hexanol, 1-octen-3-ol, propanoic acid, butanoic acid, hexanoic acid, octanoic acid, nonanoic acid, 2-ethylfuran, 2-pentylfuran, α-Pinene, sabinene, β-pinene, o-xylene, β-myrcene, limonene, p-cymene, phenol | [235] |
| Coconut Oil (expeller) | Head Space Solid Phase Microextraction | Philippines | acetic acid, hexanal, 2-heptanone, limonene, nonanal, δ-octalactone, δ-decalactone, dodecanoic acid | [104] |
| Coconut Oil (centrifuge) | Head Space Solid Phase Microextraction | Philippines | hexanal, 2-heptanone, octanoic acid, δ-octalactone, dodecanoic acid | [104] |
| Coconut Oil (fermentation without heat) | Head Space Solid Phase Microextraction | Philippines | ethyl acetate, acetic acid, hexanal, 2-heptanone, limonene, nonanal, octanoic acid, ethyl octanoate, δ-octalactone, δ-decalactone, dodecanoic acid | [104] |
| Coconut Oil (fermentation without heat) | Head Space Solid Phase Microextraction | Philippines | acetic acid, 2-pentanone, hexanal, nonanal, octanoic acid, δ-octalactone, dodecanoic acid | [104] |
| Cranberry Seeds (sonication) | Head Space Solid Phase Microextraction | Poland | 1,2-dimethyl cyclopropane, 1,3,5,7-cyclooctatetraene, 1-penten-3-one, (E,E)-2,4-heptadienal, (E)-2-butenal, (Z)-2-heptenal, 2-hexenal, (E)-2-Penten-1-ol, 2-pentenal, (E)-2-pentene, (Z)-4-heptenal, acetic acid, ethyl acetate, 2-ethylfuran, furfural, hexanal, hexane, (S)-methyloxirane, pentanal, trimethylene oxide | [109] |
| Grape Seeds | Turkey | ethyl octanoate, ethyl decanoate, ethyl dodecanoate, and hexyl hexadecanoate | [116] | |
| Cabernet Grapeseeds | Turkey | isoamyl acetate, isoamyl alcohol, ethyl heptanoate, hexyl acetate, octanal, nonanal, ethyl octanoate, 1-octen-3-ol, heptyl alcohol, n-decanal, nonanol, ethyl laurate, isovaleric acid, valeric acid, phenylethyl acetate, hexanoic acid, phenylethyl alcohol, heptanoic acid, octanoic acid, nonanoic acid | [116] | |
| Gamay Grapeseeds | Turkey | isoamyl acetate, isoamyl alcohol, ethyl heptanoate, hexyl acetate, octanal, 2-nonanone, nonanal, ethyl octanoate, 1-octen-3-ol, heptyl alcohol, n-decanal, 2,3-butanediol diacetate, benzaldehyde, nonanol, ethyl laurate, isovaleric acid, valeric acid, phenylethyl acetate, hexanoic acid, phenylethyl alcohol, heptanoic acid, octanoic acid, nonanoic acid, β-caryophyllene, myrcene, valencene, benzyl acetate | [116] | |
| Kalecik Karasi Grapeseeds | Turkey | isoamyl acetate, ethyl heptanoate, hexyl acetate, octanal, nonanal, ethyl octanoate, 1-octen-3-ol, heptyl alcohol, 2,3-butanediol diacetate, benzaldehyde, nonanol, ethyl laurate, isovaleric acid, benzyl acetate, phenylethyl acetate, hexanoic acid, phenylethyl alcohol, heptanoic acid, octanoic acid | [116] | |
| Okuzgozu Grapeseeds | Turkey | isoamyl acetate, ethyl heptanoate, hexyl acetate, octanal, nonanal, ethyl octanoate, 1-octen-3-ol, heptyl alcohol, benzaldehyde, nonanol, ethyl laurate, phenylethyl alcohol, octanoic acid | [116] | |
| Senso Grapeseeds | Turkey | isoamyl acetate, ethyl heptanoate, hexyl acetate, octanal, nonanal, ethyl octanoate, 1-octen-3-ol, heptyl alcohol, n-decanal, benzaldehyde, nonanol, ethyl laurate, isovaleric acid, phenyl-ethyl acetate, hexanoic acid, phenyl-ethyl alcohol, octanoic acid | [116] | |
| Magliocco Canino Grape Seed Oil (solvent-assissted extraction) | Purge and Trap Extraction | Italy | 3-penten-2-ol, 3-hexanol, 2-hexanol, 1-methyl cyclopentanol, 3-methyl-cyclopentanol, 1-hexanol, 1-octanol, 2-phenyl-2-propanol, phenylethyl alcohol, isoamyl acetate, ethyl octanoate, phenylethyl acetate, ethyl dodecanoate, hexanal, nonanal, 2-nonanone, 4-ethoxybenzoic acid, 2-methyl-2-buten-1-ol, 2-butoxyethanol, 1-octen-3-ol, 2,3-butanediol, benzyl alcohol, 2-phenoxyethanol, ethyl decanoate, (E,E)-2,4 heptadienal, acetophenone, 2-dodecanone, β-cubebene, germacrene, phenol, hexanoic acid, octanoic acid, nonanoic acid, γ-butyrolactone | [236] |
| Dimrit Grape Seed Oil (solvent-assissted extraction) | Purge and Trap Extraction | Turkey | 3-hexanol, 2-hexanol, 1-hexanol, 1-octen-3-ol, 2-phenyl-2-propanol, benzyl alcohol, phenylethyl alcohol, 2-phenoxyethanol, isoamyl acetate, ethyl octanoate, ethyl dodecanoate, hexanal, (E)-2-heptanal, nonanal, (E)-2-nonenal, benzene, acetaldehyde, acetophenone, isocumene, phenol, 3,5-xylenol, 2,4-dimethylphenol, carvacrol, 3,4-dimethylphenol, 2,4-di-tert-butyl phenol, hexanoic acid, 2-ethylhexanoic acid, octanoic acid, 2-pentylfuran, γ-butyrolactone, dihydroxymaltol, 2-butoxyethanol | [236] |
| Hazelnut Oil (cold pressed) | Solid Phase Microextraction | Turkey | 2-heptenal, nonanal, (E,E) 2,4-decadienal, (E)-2-decenal, (E,Z)-2,4-decadienal, (E)-2-tridecenal, heptanal, hexanal | [233] |
| Hemp Flowers and Leaves | Dynamic Head Space Collection | Canada, France, Finland | (Z)-3-hexen1-yl acetate, α-pinene, β-pinene, β-myrcene, limonene, (Z)-β-ocimene, (Z)-β-caryophyllene, α-humulene | [128] |
| Hemp (Hydrodistillation) | Head Space Solid Phase Microextraction | Italy | α-pinene, camphene, β-pinene, myrcene, limonene, 1,8-cineol, (Z)-β-ocimene, (E)-β-ocimene, γ-terpinene, terpinolene, linalool, β-caryophyllene, (E)-β-farnesene, α-humulene, caryophyllene oxide, β-eudesmol, β-bisabolol, α-bisabolol | [237] |
| Hemp (Supercritical Fluid CO2) Extract | Head Space Solid Phase Microextraction | Italy | α-pinene, camphene, β-pinene, myrcene, limonene, 1,8-cineol, (Z)-β-ocimene, (E)-β-ocimene, γ-terpinene, terpinolene, linalool, β-caryophyllene, (E)-β-farnesene, α-humulene, caryophyllene oxide, β-eudesmol, β-bisabolol, α-bisabolol | [237] |
| Macadamia Nut | Likens-Nickerson Extraction | Cuba | hexanal, ethyl butyrate, butyl acetate, 2-furfural, ethylbenzene, p-xylene, hexanol, isoamyl acetate, o-xylene, 2-heptanone, heptanal, cumene, benzaldehyde, propylbenzene, β-pinene, 6-methy-5-hepten-2-ol, ethyl hexanoate, octanal, p-cymene, limonene, cyclohexyl acetate, 2-phenylacetaldehyde, salicylaldehyde, (E)-β-ocimene, γ-terpinene, acetophenone, trans-linalool oxide (furanoid), p-tolualdehyde, terpinoiene, p-cymenene, 2-nonanone, methyl benzoate, ethyl heptanoate, nonanal, p-mentha-1,3,8-triene, isophorone, 1-phenyl-2-propanone, cis-limonene oxide, trans-limonene oxide, camphor, (Z)-3-hexenyl isobutyrate, citronellal, ethyl benzoate, ethyl octanoate, α-terpineol, methylthymol, neral, cuminaldehyde, carvone, ethyl 2-phenylacetate, edulan I, (E)-2-decenal, geranial, ethyl salicylate, perillaldehyde, (E)-anethole, isobornyl acetate, safrole, edulan II, benzyl butyrate, α-terpinyl acetate, citronellyl acetate, eugenol, γ-nonalactone, neryl acetate, benzyl isothiocyanate, α-ylangene, biphenyl, geranyl acetate, hexyl hexanoate, β-cubebene, β-elemene, ethyl decanoate, methyl eugenol, α-cedrene, cis-α-bergamotene, β-caryophyllene, (E)-α-ionone, trans-α-bergamotene, 2-phenylethyl butyrate, benzyl valerate, α-humulene, geranyl acetone, geranyl propionate, ar-curcumene, valencene, (E,E)-α-farnesene, γ-cadinene, δ-cadinene, α-calacorene, (E)-nerolidol, γ-undecalactone, dodecanoic acid, ethyl dodecanoate, hexadecane, methyl tridecanoate, dill apiol, 7-dodecalactone, heptadecane, 8-dodecalactone, methyl tetradecanoate, tetradecanoic acid, ethyl tetradecanoate, pentadecanoic acid, hexadecanoic acid, isopropyl hexadecanoate, methyl octadecanoate, oleic acid, octadecanoic acid, ethyl octadecanoate | [142] |
| Marula Intact Fruits | Head Space Solid Phase Microextraction | South Africa | ethyl isovalerate, ethyl hexanoate, ethyl octanoate, isoamyl hexanoate, ethyl (E)-4-octenoate, pentadecane, cyclopentadecane, β-caryophyllene, hexadecene, isoamyl octanoate, α-humulene, ethyl (E)-4-decenoate, heptadecane, (Z)-3-decenyl acetate, heptadecene, benzyl acetate, (Z)-3-decen-1-ol, 1-octen-3-yl butyrate, benzyl butyrate, nonadecane, 6-dodecen-1-ol, cyclodecene, benzyl methacrylate, benzyl 4-methylpentanoate, benzyl tiglate, hexadecanal, 11-hexadecenal, ethyl 9-hexadecenoate, benzyl octanoate, (Z)-13-octadecenal | [148] |
| Marula Fruit Pulp | Head Space Solid Phase Microextraction | South Africa | germacrene D, α-humulene, β-caryophyllene | [148] |
| Moringa Leaves | Head Space Solid Phase Microextraction | Rwanda | (E)-2-pentenal, 2-hexenal, (Z)-2-heptenal, nonanal, (E,E)-2,4-hexadienal, (E,E)-2,4-heptadienal, benzaldehyde, (E)-2-nonenal, 2-phenylacetaldehyde, γ-nonalactone, 1-pentanol, (Z)-2-pentenol, hexanol, (Z)-3-octenol, octanol, 3,3-dimethylcyclohexanol, (E)-2,6-dimethyl-3,5,7-octatriene-2-ol, 1-pentadecanol, 1-nonanol, 1-undecanol, benzyl alcohol, phenethyl alcohol, methylheptenone, (E,E)-3,5-octadien-2-one, methyl heptadienone, 2-hexen-4-olide, 2-acetylpyrrole, 3-ethyl-4-methyl-1H-pyrrole-2,5-dione, dihydroactinidolide, tridecane, pentadecane, 1-tridecyne, heptadecane, methyl hexanoate, dibutyl phthalate, α-himachalene, (E)-geranyl acetone, (E)-β-ionone, β-ionone epoxide, hexahydrofarnesyl acetone, (E,E)-farnesyl acetone, acetic acid, dimethylpropanedioic acid, pentanoic acid, 3-methylbutanoic acid, pentanoic acid, hexanoic acid, 4-hexenoic acid, 2-hexenoic acid, octanoic acid, nonanoic acid, decanoic acid, dodecanoic acid, octadecanoic acid, tetradecanoic acid, (E)-9-octadecenoic acid, hexadecanoic acid, phenylacetonitrile | [238] |
| Moringa Leaves | Head Space Solid Phase Microextraction | China | (E)-2-butenal, hexanal, 2,4-pentadienal, (E)-2-pentenal, 2-hexenal, (Z)-2-heptenal, (E,E)-2,4-hexadienal, 2-methylfuran, 2-ethylcyclobutanol, (Z)-2-pentenol, (E)-2-ethyl-2-hexen-1-ol, 2,4-dimethylcyclohexanol, 3,3-dimethylcyclohexanol, benzyl alcohol, 1-dodecanol, methylheptenone, 1-(furan-2-yl)ethanone, (E,E)-3,5-octadien-2-one, γ-butyrolactone, 4-isopropyl-2-cyclohexenone, 2-hexen-4-olide, 2-acetylpyrrole, 7-octen-2-one, 3-ethyl-4-methyl-1H-pyrrole-2,5-dione, dihydroactinidolide, tridecane, 2-methyltridecane, pentadecane, 2-methyltetradecane, 2-methyl-1-tetradecene, 3-methylpentadecane, [R,R-(E)]-4,5-dimethyl-2-undecene, (Z)-hexyl oleate, methyl acetate, hexyl 3-methylbutanoate, (Z)-3-hexen-1-yl valerate, propyl 3-methylbutanoate, octyl 2-methyl butyrate, diethyl phthalate, dibutyl phthalate, o-cymene, α-himachalene, longifolene, carvone, citronellyl valerate, (E)-geranyl acetone, (E)-β-ionone, β-ionone epoxide, acetic acid, propionic acid, butyric acid, pentanoic acid, hexanoic acid, octadecanoic acid, (E)-9-octadecenoic acid, hexadecanoic acid, toluene, 2,4-lutidine, dimethylsulfoxide | [238] |
| Neem Seed (hydrodistillation) | GC-MS with PDMS coated column | India | 1,3-propanedithiol, allyl mercaptan, 1-dodecene, 4,6-dimethyl-[1,2,3]trithiane, 1,3-propanedithiol, 3,5-diethyl-1,2,4-trithiolane, 1-hexadecanol, 2-epi-α-funebrene, cis-α-bergamotene, γ-muurolene, β-acoradiene, 5,6-dihydro-2,4,6-triethyl-4H-1,3,5-dithiazine, cycloisolongifolene, valencene, α-bisabolene, di-tert-butylphenol, (E)-nerolidol, 1-hexadecanol, juniper camphor, (E)-15-heptadecenal, oxacycloheptadec-8-en-2-one, (8Z)-cycloeicosane, 4,6-diethyl-1,2,3,5-tetrathiolane, 1-Tetracosanol, 9-hexacosene | [156] |
| Olive Oil, Cailletier Variety | Head Space Solid Phase Microextraction | France | ethanol, propan-2-one, pent-2-ene, acetic acid, pentan-2-one, pentan-3-one, heptane, 3-methylbutanol, pent-2-enal, (Z)-pent-2-enol, toluene, hex-3-enal, hexanal, octane, (E)-hex-2-enal, (Z)-hex-3-enol, (E)-hex-2-enol, hexanol, p-xylene, hexa-2,4-dienal, o-xylene, 3,4-diethylhexa-1,5-diene, benzaldehyde, α-pinene, 3-ethylocta-1,5-diene, octanal, (Z)-hex-3-enyl acetate, deca-3,7-diene, δ-3-carene, α-terpinene, limonene, β-Ocimene, γ-terpinene, nonanal, (Z)-4,8-dimethylnona-1,3,7-triene, farnesene | [239] |
| Olive Oil, Blanquettier Variety | Head Space Solid Phase Microextraction | France | ethanol, pent-2-ene, acetic acid, pentan-2-one, pentan-3-one, 3-methylbutanol, toluene, hexanal, octane, (E)-hex-2-enal, (Z)-hex-3-enol, (E)-hex-2-enol, hexanol, p-xylene, hexa-2,4-dienal, benzaldehyde, α-pinene, 3-ethylocta-1,5-diene, (Z)-hex-3-enyl acetate, deca-3,7-diene, β-ocimene, nonanal, (Z)-4,8-dimethylnona-1,3,7-triene, farnesene | [239] |
| Olive Oil, Arbequines Variety | Head Space Solid Phase Microextraction | Spain | ethanol, pent-2-ene, qcetic acid, pentan-2-one, pentan-3-one, 3-methylbutanol, pent-2-enal, toluene, hexanal, octane, (E)-hex-2-enal, (Z)-hex-3-enol, (E)-hex-2-enol, hexanol, p-xylene, α-pinene, 3-ethylocta-1,5-diene, (Z)-hex-3-enyl acetate, hexyl acetate, β-ocimene, nonanal, (Z)-4,8-dimethylnona-1,3,7-triene, farnesene | [239] |
| Passionfruit Fruit Pulp | Head Space Solid Phase Microextraction | Brazil | methyl acetate, ethyl acetate, methyl butanoate, methyl (E)-2-butenoate, ethy butanoate, ethyl (E)-2-butenoate, methyl-2-pentenoate, methyl 3-hydroxybutanoate, propyl butanoate, methyl hexanoate, methyl 2-hexenoate, butyl butanoate, ethyl hexanoate, ethyl-2-hexenoate, methyl benzoate, hexyl butanoate, octyl acetate, hexyl (2E) butenoate, ethyl (E)-2-octenoate, methyl geranate, benzyl butanoate, methyl (E)-cinnamate, hexyl hexanoate, octyl butanoate, hexyl octanoate, methyl dihydrojasmonate, δ-3-carene, p-cymene, limonene, (Z)-β-ocimene, (E)-β-ocimene, γ-terpinene, α-terpinolene, 1,3,8-p-menthatriene, allo-ocimene, neo-allo-ocimene, bornylene, β-cyclocitral, β-ionone, 1-hexanol, 6,10-dimethyl-2-undecanone | [240] |
| Passionfruit Seed Oil (expeller extraction) | Head Space Solid Phase Microextraction | Brazil | ethyl 2-butenoate, methyl 2-butenoate, ethyl acetate, ethyl 3-hydroxybutanoate, methyl 3-butenoate, methyl butyrate, 2-methylbutanal, 3-methylbutanal, acetaldehyde, phenylacetaldehyde, hexanal, isobutanal, 3-methyl-1-butanol, 2-methyl-1-butanol, phenylethyl alcohol, 2-(3H)dihydrofuranone, (E)-β-ocimene, dodecane | [171] |
| Pomegranate Seed Oil (cold pressed) | Head Space Solid Phase Microextraction | Turkey | 3-methyl-1-butanol, 2,3-butanediol, phenylethyl alcohol, ethyl butanoate, ethyl 2-hydroxy-propanoate, pentyl acetate, 1-butanyl 2-methylacetate, ethyl hexanoate, hexyl acetate, diethyl butanedioate | [241] |
| Pomegranate Seed Oil (cold pressed) | Head Space Solid Phase Microextraction | Israel | 2,3-butanediol, phenylethyl alcohol, 1-pentanol, 2,4-nonadienal, 2-hexenal, (Z)-2-heptenal, hexanal, 5-decanone, pentanoic acid, hexanoic acid, limonene | [241] |
| Prickly Pear Seed Oil (hexane extract) | Head Space Solid Phase Microextraction | Greece | (2Z)-heptenal, hexanal, (2E)-octenal, 2-pentylfuran, (2E,4E)-decadienal, nonanal | [242] |
| Evening Primrose Oil | Head Space Solid Phase Microextraction | China | (E)-2-octen-ol, 2-ethyl-1-hexanol, 2-propanol, 1-octen-3-ol, 3-methyl-1-pentanol, 1-butanol, (E)-2-heptenal, nonanal, hexanal, (E)-2-nonenal, (E,E)-2,4-dodecadienal, (E,E)-2,4-decadienal, (E)-2-octenal, octanal, (Z)-9,17-octadecadienal, dodecanal, pentanal, heptanal, (E,E)-2,4-nonadienal, (E)-2-decenal, (E,Z)-2,4-decadienal, 2-tridecanone, 3-octen-2-one, 1-nonen-3-one, 2-hexenoic acid, 2-octynoic acid, acetic acid, heptanoic acid, hexanoic acid, octadecanoic acid, docosyl docosanoate, methyl (Z,Z)-9,12-octadecadienoate, methyl hexadecanoate, (Z)-9-octadecenyl octadecanoate, 4,6-dimethyldodecane, 2,6,10-trimethyltridecane, (E)-3-octadecene, 3,7-dimethyldecane, dodecane, heptadecane, octadecane, pentane, 5-methyl-tetradecane, tridecane, undecane | [183] |
| Pumpkin Seeds | Hydro-distillation | Ethiopia | 2-phenylbut-2-enal, 2-phenylacetaldehyde, nonanal, decanal, (2E,4E)-deca-2,4-dienal, hexadecanal, 1-nonanol, 2,4-di-tert-butylphenol, 2-ethyl-1-hexanol, eicosane, hexacosane, (E)-undec-3-ene, hexadecane, 9-methylnonadecane, heptadecane, heneicosane, 5-butylhexadecane, [2,2,4-trimethyl-3-(2-methylpropanoyloxy)pentyl] 2-methylpropanoate, (3-hydroxy-2,4,4-trimethylpentyl) 2-methylpropanoate, methyl hexadecanoate, methyl 9-octadecenoate, Methyl octadecanoate, 1,8-cineole, δ-3-carene, (E)-geranyl acetone, 5,6-dihydro 2,4,6-trimethyl-4H-1,3,5-dithiazine, 1,3-benzothiazole, 2-hexylthiophene | [189] |
| Pumpkin Seed Oil | Purge and Trap Extraction | Italy, Slovenia | methanol, scetaldehyde, methanethiol, methylformate, ethanol, acetone, pentane, ethyl formate, dimethylsulfide, methylacetate, carbon disulfide, dimethylsulfone, isobutanal, 2-butenal (crotonal), 2,3-butanedione, butanal, 2-butanone, hexane, ethyl acetate, isobutanol, 3-methylbutanal, 2-pentanone, butyl formate, 2-methylbutanal, 1-penten-3-ol, pentanal, heptane, 2-methyl-2-butenal, dimethyldisulfide, 1-pentanol, 2-hexanone, hexanal, 4-octene, 2-methyltetrahydrofuran-3-one, 2-methylpyrazine, furfural, 4-hydroxy-4-methyl-2-pentanone, 2-hexenal, 2-heptanone, heptanal, 2,6-dimethylpyrazine, α-pinene, 2-heptenal, 6-methyl-5-hepten-2-one, 2-octanone, dimethyltrisulfide, 2-pentylfuran, octanal, 5-methyl-2-ethylpyrazine, limonene, nonanal, fenchone | [186]. |
| Raspberry Seeds (sonication) | Head Space Solid Phase Microextraction | Poland | (E,E)-3,5-heptatriene, 1,6-heptadien-4-ol, 3-methyl-1 butanol, 3-methyl-1-butanyl acetate, 2-methyl-1-propanol, (E)-2-hexenal, 3-methyl-3-buten-1-ol, (Z)-3-hexen-1-yl acetate, methyl acetate, benzene, ethanol, ethyl acetate, hexanal, 2,2-dimethylpropanal, propylene oxide, p-xylene | [109]. |
| Rosehip R. dumalis fruit | Head Space Solid Phase Microextraction | Turkey | acetic acid, butanoic acid, 3-eethylpentanoic acid, oxalic acid, 2(3H)-furanone, 2-heptanone, 1-hydroxy-2-propanone, hexanal, 2-hexenal, acetaldehyde, nonanal, furfural, decanal, benzaldehyde, dodecanal, isoamyl isovalerate, butyl hexanoate, hexyl hexanoate, 1,2-propanediol, 1-penten-3-ol, 2-methyl-1-propanol, 3-methyl-1-butanol, 1-pentanol, 2-nonen-1-ol, 4-hexen-1-ol, 2-furanmethanol, 3,7-dimethyl-1,6-octadien-3-ol, 2-ethyl-1-hexanol, dodecanol, α-terpineol, 1-butanol, 2,4-bis(1,1-dimethylethyl)phenol, phenol, m-xylene | [198] |
| Rosehip R. canina fruit | Head Space Solid Phase Microextraction | Turkey | formic acid, acetic acid, ionone, 3-methylbutanoic acid, butanoic acid, 2-methyl-2-propenoic acid, hexanoic acid, heptanoic acid, octanoic acid, nonanoic acid, decanoic acid, 2(3H)-furanone, 6-methyl-5-hepten-2-one, hexanal, acetaldehyde, benzaldehyde, ethyl acetate, methyl acetate, hexyl hexanoate, 1,2-propanediol, 2-methyl-1-propanol, 3-methyl-1-butanol, 1-pentanol, 4-methyl-1-heptanol, 2-furanmethanol, 3,7-dimethyl-1,6-octadien-3-ol, α-terpineol, phenylethyl alcohol, 1-hexadecanol, α-humulene, naphthalene, 2,4-di-tert-butylphenol, phenol, m-xylene | [198] |
| Rosehip R. pimpinellifolia fruit | Head Space Solid Phase Microextraction | Turkey | 2,4-dimethoxycinnamic acid, sinapic acid, formic acid, acetic acid, ionone, 3-methylbutanoic acid, hexanoic acid, octanoic acid, nonanoic acid, decanoic acid, benzoic acid, dodecanoic acid, 1,2-benzenedicarboxylic acid, Aacetone, 6-methyl-5-hepten-2-one, 1-hydroxy-2-propanone, 2H-pyran-2,6(3H)-dione, furfural, 3-caren-10-al, 2-nonen-1-ol, 2-furanmethanol, naphthalene, 2,4-di-tert-butylphenol, phenol | [198] |
| R. villosa | Head Space Solid Phase Microextraction | Turkey | formic acid, acetic acid, 3-methylbutanoic acid, butanoic acid, hexanoic acid, octanoic acid, benzoic acid, 1,2-benzenedicarboxylic acid, pentadecanoic acid, 2(3H)-furanone, 6-methyl-5-hepten-2-one, 1-hydroxy-2-propanone, furfural, benzaldehyde, ethyl acetate, ethanol, 3-methyl-1-butanol, 1-pentanol, 2-furanmethanol, 3,7-dimethyl-1,6-octadien-3-ol, α-terpineol, α-humulene, naphthalene, 2,4-di-tert-butylphenol, phenol | [198] |
| Rosehip Oil | Superheated Water Extraction | NA | benzaldehyde, benzyl alcohol, phenyl ethyl alcohol, 2,6,11-trimethyl dodecane and eicosane | [243] |
| Dincer Safflower Seed Oil (cold pressed) | Head Space Solid Phase Microextraction | Turkey | acetoin, toluene, pentanal, 2,3-butanediol, hexanal, 2-hexenal, ethylbenzene, p-xylene, heptanal, γ-butyrolactone, α-pinene, p-cymene, limonene, benzyl alcohol, 1,5-octadien-3-ol, benzene acetaldehyde, 2-octenal, heptadecanoic acid, thiazolidine, 2-hepten-1-ol, nonanal, phenylethyl alcohol, 2-nonenal, 2,4-nonadienal, octanoic acid, 4-ethylbenzaldehyde, naphthalene, decanal, (E,E),-2,4-nonadienal, γ-octalactone, 3-dodecen-1-al, 6-dodecanone, thymol, 2,4-decadienal, undecanal, (E,E)2,4-decadienal, 5-pentyl-2(5H)-furanone, 2-dodecenal, 2-cyclohexen-1-one, 4-heptenal, 5-tetradecene, 1-tetradecene, 5,5-dimethyl-4(3-oxo-butyl)-2(3H)-furanone, methyl eugenol, dodecanal, β-caryophyllene, (Z)-geranyl acetone, tetradecanal, 2,4-dodecadienal, nonylbenzene, 17-octadecenal, isopropyl myristate, γ-dodecalactone, methyl 9-octadecenoate | [244] |
| Microwaved Dincer Safflower Seed Oil (cold pressed) | Head Space Solid Phase Microextraction | Turkey | 2-methyl-1-butanol, pentanal, acetoin, pyrazine, 1-pentanal, 2,3-butanediol, hexanal, methylpyrazine, furfural, ethylbenzene, p-xylene, heptanone, heptanal, 2,5-dimethylpyrazine, methyl hexanoate, 2-ethylpyrazine, α-pinene, p-cymene, limonene, 1,5-octadien-3-ol, benzene acetaldehyde, 1-ethyl-2-formylpyrrol, 2-octenal, thiazolidine, 3,5-dimethyl-2-ethylpyrazine, 1-propylpentyl butyrate, nonanal, phenylethyl alcohol, 2-acetyl-6-methylpyrazine, (E)-3-nonene-2-one, 2-nonenal, 2,4-nonadienal, octanoic acid, naphthalene, 2-methyl-5H-6,7-dihydrocyclopentapyrazine, decanal, (E,E)-2,4-nonadienal, γ-octalactone, 3-dodecen-1-al, γ-nonalactone, 2,4-decadienal, undecanal, (E,E)-2,4-decadienal, 5-pentyl-2(5H)-furanone, 2-dodecenal, 2-cyclohexen-1-one, 4-heptenal, 5-tetradecene, 1-tetradecene, 5,5-dimethyl-4(3-oxo-butyl)-2(3H)-furanone, methyl eugenol, dodecanal, 2,4-undecadienal, β-caryophyllene, (Z)-geranyl acetone, tetradecanal, 2,4-dodecadienal, lauric acid, nonylbenzene, myristic acid, 17-octadecenal, isopropyl myristate, γ-dodecalactone, methyl 9-octadecanoate | [244] |
| Roasted Dincer Safflower Seed Oil (cold pressed) | Head Space Solid Phase Microextraction | Turkey | isoamyl alcohol, toluene, 2,3-butanediol, hexanal, 2-hexenal, ethylbenzene, p-xylene, heptanal, γ-butyrolactone, α-pinene, α-phellandrene, p-cymene, limonene, 1,5-octadien-3-ol, 2-octenal, isophytol, nonanal, phenylethyl alcohol, 2-nonenal, 2,4-nonadienal, octanoic acid, naphthalene, (E,E)-2,4-nonadienal, 3-dodecen-1-al, γ-nonalactone, 6-dodecanone, 2,4-decadienal, 2-n-heptylfuran, undecanal, (E,E)-2,4-decadienal, 2-dodecenal, 2-cyclohexen-1-one, 5-tetradecene, 1-tetradecene, 5,5-dimethyl-4(3-oxo-butyl)-2(3H)-furanone, methyl eugenol, 2,4-undecadienal, β-caryophyllene, tetradecanal, 2,4-dodecadienal, lauric acid, nonylbenzene, 17-octadecenal, γ-dodecalactone, methyl 9-octadecanoate | [244] |
| Sesame Seed Oil (Screw-Press Extraction) | Head Space Solid Phase Microextraction | China | pentanal, hexanal, heptanal, benzaldehyde, octanal, benzeneacetaldehyde, (E)−2-octenal, nonanal, decanal, 2-heptanone, 6-methyl−5-hepten−2-one, 3-octanone, 3-methyl−1-butanol, 1-pentanol, 1-heptanol, 1-octanol, acetic acid, pyridine, ethyl-pyrazine, trimethyl-pyrazine, methyl-pyrazine, 3-ethyl−2,5-dimethyl-pyrazine, 2-methoxy−3-(2-methylpropyl)-pyrazine, dimethyl sulfide, dimethyl disulfide, dimethylsulfoxide, 2,4-dimethylthiazole, butyrolactone | [245] |
| Roasted Sesame Seed Oil | GC-FID with Carbowax coated column | Korea | 1-octen-3-ol, 3-methyl-2-butanone, furfuryl alcohol, guaiacol, 2-methylpyrazine, 2,5-dimethylpyrazine, 2,6-dimethylpyrazine, 2,3,5-trimethylpyrazine, pyrazine, 2-ethylpyrazine, 2-acety1pyrazine, 2-acetyl-5-methylpyrazine, 2-ethyl-6-methylpyrazine, 2,3-dimethylpyrazine, pyrrole, 2-acetylpyrrole, 2-pyrrolecarbaldehyde | [246] |
| Shea Butter (cold pressed) | Purge and Trap Extraction | Ivory Coast | 2-methylbutanal, 3-methylbutanal, (Z)-2-hexenal, (E)-2-hexenal, (E)-2-heptenal, (Z)-6-nonenal, benzaldehyde, (E,E)-2,4-nonadienal, (E,E)-2,4-decadienal, 4-methoxybenzaldehyde, 1-penten-3-ol, 3-hexanol, 1-hexanol, 2-methyl-2-butenol, 2-heptanol, (E)-2-hexenol, 3-octanol, 2,3-butanediol, benzyl alcohol, phenylethyl alcohol, 3-methoxy-2-butanol, 4-methyl-3-penten-2-one, 3-hydroxy-2-butanone, acetophenone, ethyl acetate, butyl acetate, acetic acid, propanoic acid, butyric acid, valeric acid, hexanoic acid, heptanoic acid, nonanoic acid, 2-methylfuran, gurfural, 2-scetylfuran, 2-hydroxymethylfuran, α-pinene, sabinene, α-terpinene, limonene, p-cymene, β-myrcene, phenol, p-cresol, o-xylene, styrene, γ-butyrolactone, 2-acetylpyrrole, 2-ethylpyrazine, 2-acetyl-3-methylpyrazine | [247] |
| Shea Butter (Solvent extraction) | Purge and Trap Extraction | Ivory Coast | 2-methylbutanal, 3-methylbutanal, (Z)-2-hexenal, (E)-2-hexenal, (E)-2-heptenal, (Z)-6-nonenal, benzaldehyde, (E,E)-2,4-nonadienal, (E,E)-2,4-decadienal, 4-methoxybenzaldehyde, 1-penten-3-ol, 3-hexanol, 1-hexanol, 2-methyl-2-butenol, 2-heptanol, (E)-2-hexenol, 3-octanol, 2,3-butanediol, benzyl alcohol, phenylethyl alcohol, 3-methoxy-2-butanol, 4-methyl-3-penten-2-one, 3-hydroxy-2-butanone, acetophenone, pheny lacetate, acetic acid, propanoic acid, butyric acid, valeric acid, hexanoic acid, heptanoic acid, nonanoic acid, furfural, α-pinene, sabinene, α-terpinene, limonene, p-cymene, β-myrcene, phenol, o-xylene, styrene, γ-valerolactone, γ-butyrolactone, 2-acetylpyrrole, 2-ethylpyrazine, 2-acetyl-3-methylpyrazine | [247] |
| Shea Butter | Purge and Trap Extraction | Ivory Coast | 2-methylbutanal, 3-methylbutanal, (E)-2-heptenal, benzaldehyde, (E,E)-2,4-nonadienal, 1-penten-3-ol, 3-hexanol, 1-hexanol, 2-methyl-2-butenol, 2-heptanol, 3-cctanol, 2,3-butanediol, benzyl alcohol, phenylethyl alcohol, ethyl acetate, butyl acetate, phenyl acetate, acetic acid, propanoic acid, butyric acid, valeric acid, hexanoic acid, heptanoic acid, nonanoic acid, 2-methylfuran, furfural, 2-acetylfuran, 2-hydroxymethylfuran, α-pinene, sabinene, α-terpinene, limonene, p-cymene, guaiacol, phenol, p-cresol, o-xylene, styrene, γ-valerolactone, γ-butyrolactone, 2-acetylpyrrole, 2-ethylpyrazine, 2-acetyl-3-methylpyrazine | [247] |
| Soy Bean Oil (expeller extraction) | Vacuum-Assisted Head Space Solid Phase Microextraction | China | hexanal, (Z)-2-heptenal, nonanal, decanal, (E)-2-hepten-1-ol, (E,E)-3,5-octadien-2-one, toluene, ethylbenzene, p-xylene, styrene, butyl butanoate | [248] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marshall, T.; Dosoky, N.S.; Satyal, P.; Setzer, W.N. Aroma Compounds of Carrier Oils. AppliedChem 2023, 3, 546-580. https://doi.org/10.3390/appliedchem3040034
Marshall T, Dosoky NS, Satyal P, Setzer WN. Aroma Compounds of Carrier Oils. AppliedChem. 2023; 3(4):546-580. https://doi.org/10.3390/appliedchem3040034
Chicago/Turabian StyleMarshall, Tyler, Noura S. Dosoky, Prabodh Satyal, and William N. Setzer. 2023. "Aroma Compounds of Carrier Oils" AppliedChem 3, no. 4: 546-580. https://doi.org/10.3390/appliedchem3040034
APA StyleMarshall, T., Dosoky, N. S., Satyal, P., & Setzer, W. N. (2023). Aroma Compounds of Carrier Oils. AppliedChem, 3(4), 546-580. https://doi.org/10.3390/appliedchem3040034







