Carotenoids Extraction from Orange Peels Using a Thymol-Based Hydrophobic Eutectic Solvent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Orange Sample Preparation
2.3. HDES Synthesis
2.4. Extraction Process
2.5. Total Carotenoid Content (TCC)
2.6. Antiradical Activity (DPPH Assay)
2.7. Ferric Reducing Antioxidant Power (FRAP) Assay
2.8. Color Analysis
2.8.1. Colorimeter Method
2.8.2. Absorbance Method
2.9. Statistical Analysis
3. Results and Discussion
3.1. Choice of Solvent
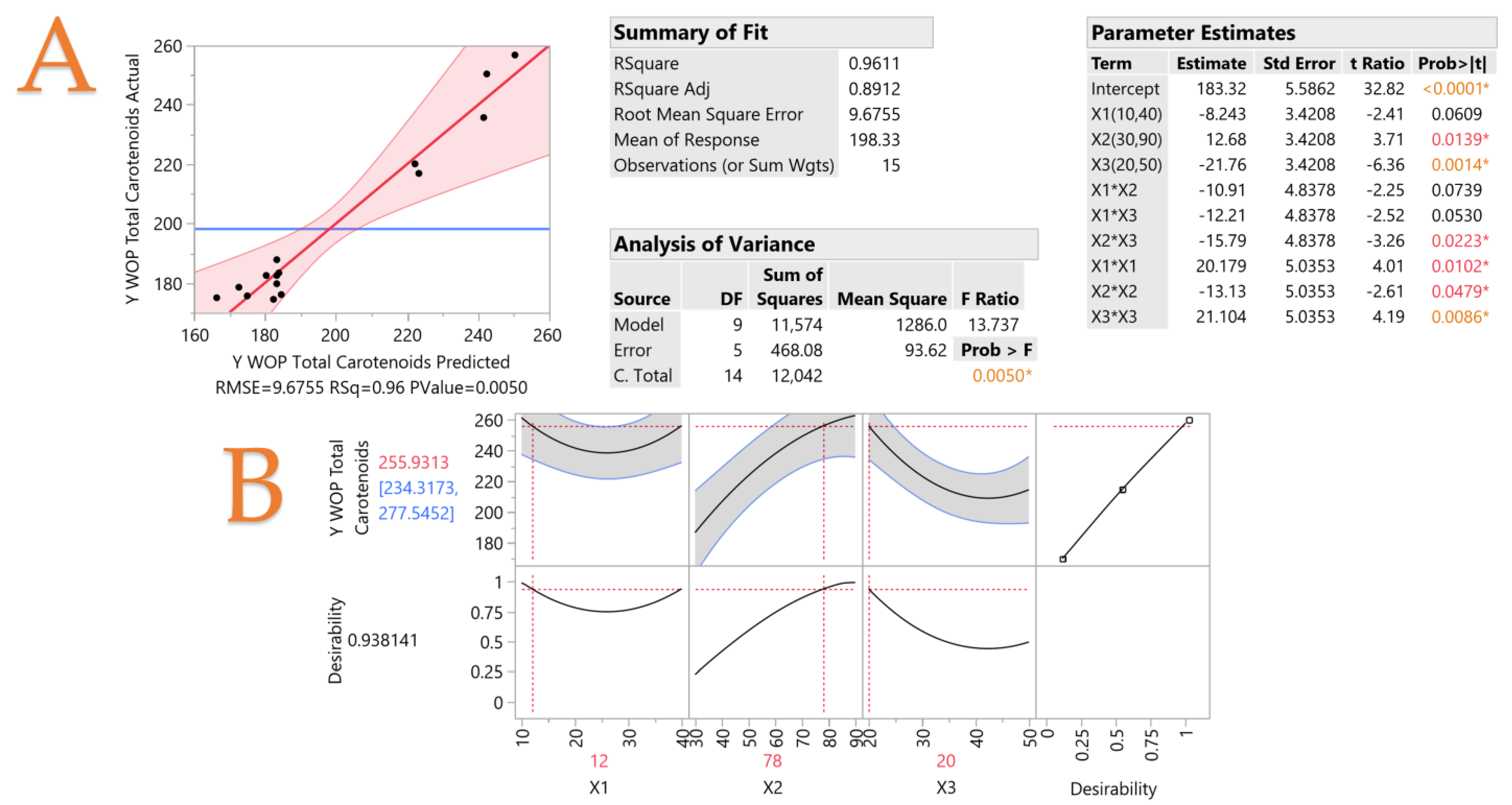
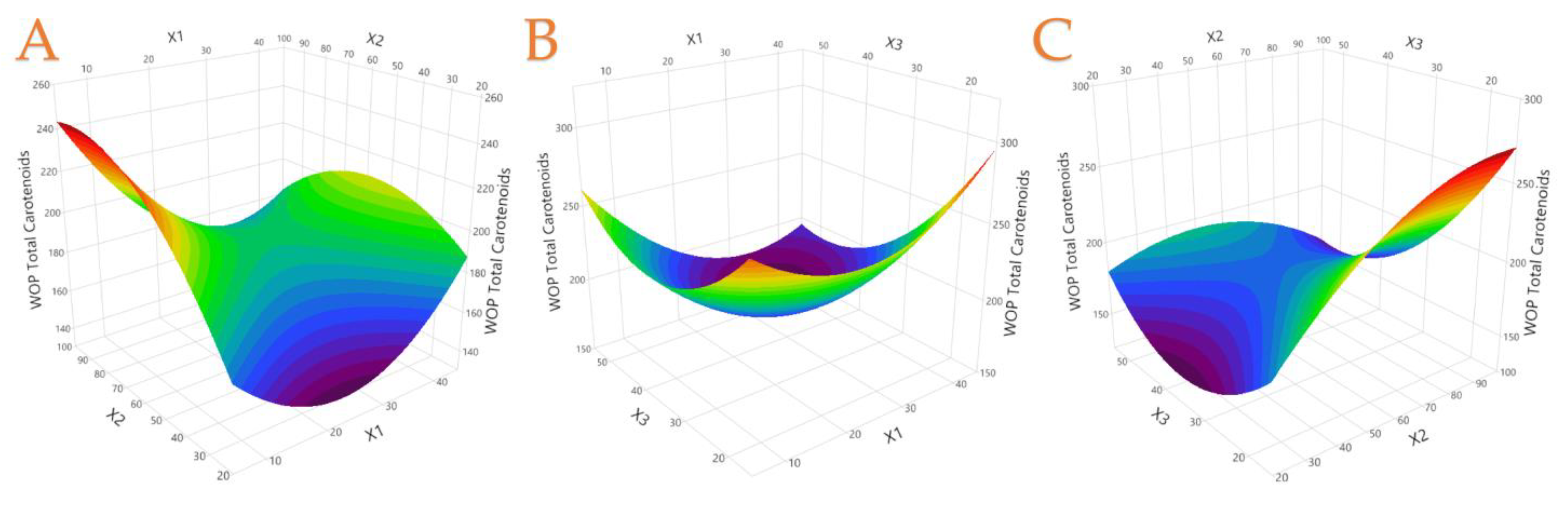
3.2. Extraction Optimization
3.3. TCC of the Extracts
3.4. Antioxidant Properties of the Extracts
3.5. Color Analysis of the Extracts
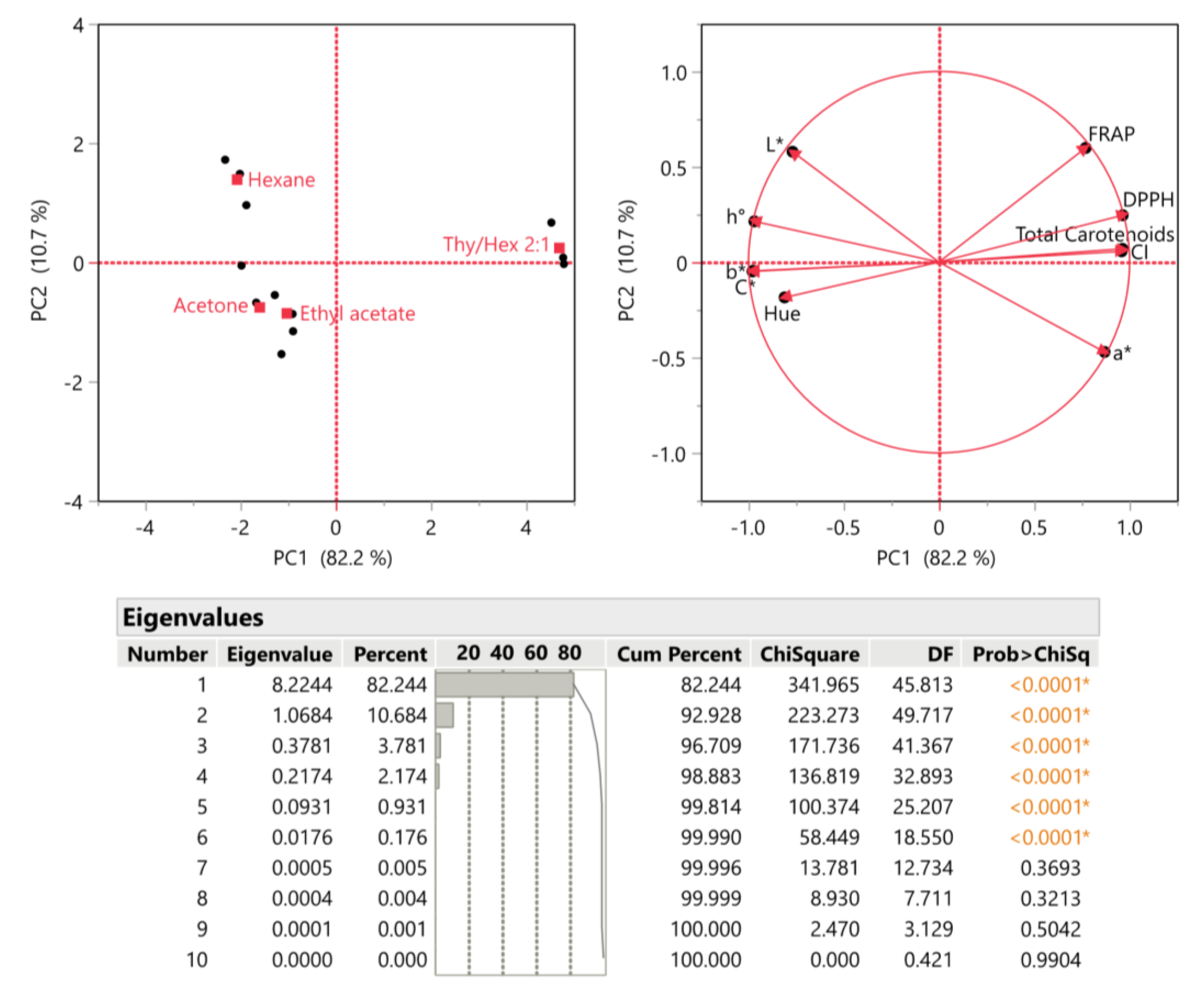
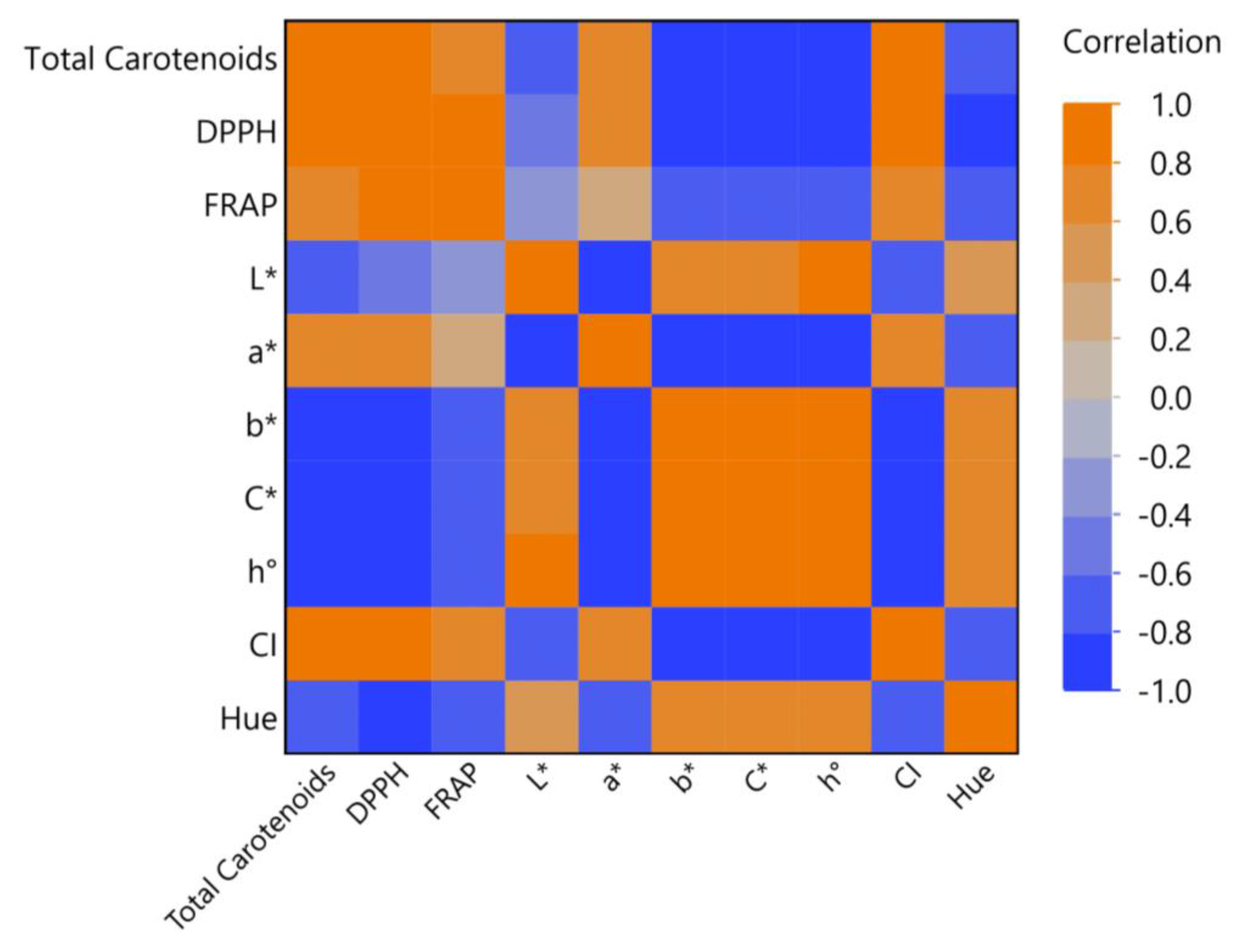
3.6. Principal Component Analysis (PCA) and Multivariate Correlation Analysis (MCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garzón, C.G.; Hours, R.A. Citrus Waste: An Alternative Substrate for Pectinase Production in Solid-State Culture. Bioresour. Technol. 1992, 39, 93–95. [Google Scholar] [CrossRef]
- Mamma, D.; Christakopoulos, P. Biotransformation of Citrus By-Products into Value Added Products. Waste Biomass Valorization 2014, 5, 529–549. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K. Citrus Processing Wastes: Environmental Impacts, Recent Advances, and Future Perspectives in Total Valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Chuang, Y.-C.; Hsu, H.-W. The Flavonoid, Carotenoid and Pectin Content in Peels of Citrus Cultivated in Taiwan. Food Chem. 2008, 106, 277–284. [Google Scholar] [CrossRef]
- Martins, N.; Ferreira, I.C.F.R. Wastes and By-Products: Upcoming Sources of Carotenoids for Biotechnological Purposes and Health-Related Applications. Trends Food Sci. Technol. 2017, 62, 33–48. [Google Scholar] [CrossRef]
- Murador, D.C.; Braga, A.R.C.; Martins, P.L.G.; Mercadante, A.Z.; De Rosso, V.V. Ionic Liquid Associated with Ultrasonic-Assisted Extraction: A New Approach to Obtain Carotenoids from Orange Peel. Food Res. Int. 2019, 126, 108653. [Google Scholar] [CrossRef]
- Viñas-Ospino, A.; Panić, M.; Bagović, M.; Radošević, K.; Esteve, M.J.; Radojčić Redovniković, I. Green Approach to Extract Bioactive Compounds from Orange Peel Employing Hydrophilic and Hydrophobic Deep Eutectic Solvents. Sustain. Chem. Pharm. 2023, 31, 100942. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Rutkowska, M.; Owczarek, K.; Tobiszewski, M.; Namieśnik, J. Extraction with Environmentally Friendly Solvents. TrAC Trends Anal. Chem. 2017, 91, 12–25. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Chloride/Urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Frigerio, J.; Giustra, C.M.; Labra, M.; Campone, L. Natural Deep Eutectic Solvents (NADESs) Combined with Sustainable Extraction Techniques: A Review of the Green Chemistry Approach in Food Analysis. Foods 2022, 12, 56. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and Properties of Deep Eutectic Solvents: A Review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Ping-Kou; Jiang, Y.-W.; Wang, L.-T.; Niu, L.-J.; Liu, Z.-M.; Fu, Y.-J. Natural Deep Eutectic Solvents Couple with Integrative Extraction Technique as an Effective Approach for Mulberry Anthocyanin Extraction. Food Chem. 2019, 296, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Urios, C.; Viñas-Ospino, A.; Puchades-Colera, P.; López-Malo, D.; Frígola, A.; Esteve, M.J.; Blesa, J. Sustainable Development and Storage Stability of Orange By-Products Extract Using Natural Deep Eutectic Solvents. Foods 2022, 11, 2457. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring Properties of Natural Deep Eutectic Solvents with Water to Facilitate Their Applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Van Osch, D.J.G.P.; Zubeir, L.F.; Van Den Bruinhorst, A.; Rocha, M.A.A.; Kroon, M.C. Hydrophobic Deep Eutectic Solvents as Water-Immiscible Extractants. Green Chem. 2015, 17, 4518–4521. [Google Scholar] [CrossRef]
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Development of Hydrophobic Deep Eutectic Solvents for Extraction of Pesticides from Aqueous Environments. Fluid Phase Equilibria 2017, 448, 135–142. [Google Scholar] [CrossRef]
- Elgharbawy, A.; Syed Putra, S.; Khan, H.; Azmi, N.; Sani, M.; Ab Llah, N.; Hayyan, A.; Jewaratnam, J.; Basirun, W. Menthol and Fatty Acid-Based Hydrophobic Deep Eutectic Solvents as Media for Enzyme Activation. Processes 2023, 11, 547. [Google Scholar] [CrossRef]
- Boldrini, C.L.; Manfredi, N.; Perna, F.M.; Capriati, V.; Abbotto, A. Designing Eco-Sustainable Dye-Sensitized Solar Cells by the Use of a Menthol-Based Hydrophobic Eutectic Solvent as an Effective Electrolyte Medium. Chem. Eur. J. 2018, 24, 17656–17659. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of Polyphenolic Antioxidants from Orange Peel Waste Using Deep Eutectic Solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Development of a Method Based on Natural Deep Eutectic Solvents for Extraction of Flavonoids from Food Samples. Food Anal. Methods 2018, 11, 1330–1344. [Google Scholar] [CrossRef]
- Gómez-Urios, C.; Viñas-Ospino, A.; Puchades-Colera, P.; Blesa, J.; López-Malo, D.; Frígola, A.; Esteve, M.J. Choline Chloride-Based Natural Deep Eutectic Solvents for the Extraction and Stability of Phenolic Compounds, Ascorbic Acid, and Antioxidant Capacity from Citrus Sinensis Peel. LWT—Food Sci. Technol. 2023, 177, 114595. [Google Scholar] [CrossRef]
- Tejero, A.; Martín, M.E.; López-Malo, D.; Esteve, M.J.; Frigola, A.; Blesa, J. Assessment of the Use of a Selection of Natural Deep Eutectic Solvents in the Extraction of Polar Bioactive Compounds from Orange Peel. Biol. Life Sci. Forum 2021, 6, 14. [Google Scholar] [CrossRef]
- Viñas-Ospino, A.; Panić, M.; Radojčić- Redovniković, I.; Blesa, J.; Esteve, M.J. Using Novel Hydrophobic Deep Eutectic Solvents to Improve a Sustainable Carotenoid Extraction from Orange Peels. Food Biosci. 2023, 53, 102570. [Google Scholar] [CrossRef]
- Stupar, A.; Šeregelj, V.; Ribeiro, B.D.; Pezo, L.; Cvetanović, A.; Mišan, A.; Marrucho, I. Recovery of β-Carotene from Pumpkin Using Switchable Natural Deep Eutectic Solvents. Ultrason. Sonochem. 2021, 76, 105638. [Google Scholar] [CrossRef]
- Khuri, A.I.; Mukhopadhyay, S. Response Surface Methodology. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 128–149. [Google Scholar] [CrossRef]
- Dean, A.; Voss, D.; Draguljić, D. Design and Analysis of Experiments; Springer Texts in Statistics; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001; ISBN 978-1-57881-072-7. [Google Scholar]
- Li, H.; Zhao, C.; Tian, H.; Yang, Y.; Li, W. Liquid–Liquid Microextraction Based on Acid–Base-Induced Deep Eutectic Solvents for Determination of β-Carotene and Lycopene in Fruit Juices. Food Anal. Methods 2019, 12, 2777–2784. [Google Scholar] [CrossRef]
- Biswas, A.K.; Sahoo, J.; Chatli, M.K. A Simple UV-Vis Spectrophotometric Method for Determination of β-Carotene Content in Raw Carrot, Sweet Potato and Supplemented Chicken Meat Nuggets. LWT—Food Sci. Technol. 2011, 44, 1809–1813. [Google Scholar] [CrossRef]
- Karvela, E.; Makris, D.P.; Karathanos, V.T. Implementation of Response Surface Methodology to Assess the Antiradical Behaviour in Mixtures of Ascorbic Acid and α-Tocopherol with Grape (Vitis Vinifera) Stem Extracts. Food Chem. 2012, 132, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Paleologou, I.; Vasiliou, A.; Grigorakis, S.; Makris, D.P. Optimisation of a Green Ultrasound-Assisted Extraction Process for Potato Peel (Solanum Tuberosum) Polyphenols Using Bio-Solvents and Response Surface Methodology. Biomass Convers. Biorefinery 2016, 6, 289–299. [Google Scholar] [CrossRef]
- Robertson, A.R. The CIE 1976 Color-Difference Formulae. Color Res. Appl. 1977, 2, 7–11. [Google Scholar] [CrossRef]
- Martínez-Pérez, M.P.; Bautista-Ortín, A.B.; Pérez-Porras, P.; Jurado, R.; Gómez-Plaza, E. A New Approach to the Reduction of Alcohol Content in Red Wines: The Use of High-Power Ultrasounds. Foods 2020, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.D.; Florindo, C.; Iff, L.C.; Coelho, M.A.Z.; Marrucho, I.M. Menthol-Based Eutectic Mixtures: Hydrophobic Low Viscosity Solvents. ACS Sustain. Chem. Eng. 2015, 3, 2469–2477. [Google Scholar] [CrossRef]
- Florindo, C.; Romero, L.; Rintoul, I.; Branco, L.C.; Marrucho, I.M. From Phase Change Materials to Green Solvents: Hydrophobic Low Viscous Fatty Acid–Based Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2018, 6, 3888–3895. [Google Scholar] [CrossRef]
- Verma, R.; Banerjee, T. Liquid–Liquid Extraction of Lower Alcohols Using Menthol-Based Hydrophobic Deep Eutectic Solvent: Experiments and COSMO-SAC Predictions. Ind. Eng. Chem. Res. 2018, 57, 3371–3381. [Google Scholar] [CrossRef]
- Rodrigo, M.-J.; Marcos, J.F.; Zacarías, L. Biochemical and Molecular Analysis of Carotenoid Biosynthesis in Flavedo of Orange (Citrus sinensis L.) during Fruit Development and Maturation. J. Agric. Food Chem. 2004, 52, 6724–6731. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Carotenoid Extraction Methods: A Review of Recent Developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Crespo, E.A.; Pontes, P.V.A.; Silva, L.P.; Bülow, M.; Maximo, G.J.; Batista, E.A.C.; Held, C.; Pinho, S.P.; Coutinho, J.A.P. Tunable Hydrophobic Eutectic Solvents Based on Terpenes and Monocarboxylic Acids. ACS Sustain. Chem. Eng. 2018, 6, 8836–8846. [Google Scholar] [CrossRef]
- Li, K.; Jin, Y.; Jung, D.; Park, K.; Kim, H.; Lee, J. In Situ Formation of Thymol-Based Hydrophobic Deep Eutectic Solvents: Application to Antibiotics Analysis in Surface Water Based on Liquid-Liquid Microextraction Followed by Liquid Chromatography. J. Chromatogr. A 2020, 1614, 460730. [Google Scholar] [CrossRef] [PubMed]
- Yara-Varón, E.; Fabiano-Tixier, A.S.; Balcells, M.; Canela-Garayoa, R.; Bily, A.; Chemat, F. Is It Possible to Substitute Hexane with Green Solvents for Extraction of Carotenoids? A Theoretical versus Experimental Solubility Study. RSC Adv. 2016, 6, 27750–27759. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Tsiouras, A.; Mourtzinos, I. Extraction of Lycopene from Tomato Using Hydrophobic Natural Deep Eutectic Solvents Based on Terpenes and Fatty Acids. Foods 2022, 11, 2645. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Liu, Y.; Shan, Y.; Cao, X. A Priori Design of New Natural Deep Eutectic Solvent for Lutein Recovery from Microalgae. Food Chem. 2022, 376, 131930. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.A.; Ferreira, T.A.P.C.; Jiao, G.; Brooks, M.S. Sustainable Approach for Lycopene Extraction from Tomato Processing By-Product Using Hydrophobic Eutectic Solvents. J. Food Sci. Technol. 2019, 56, 1649–1654. [Google Scholar] [CrossRef]
- Lee, S.Y.; Liang, Y.N.; Stuckey, D.C.; Hu, X. Single-Step Extraction of Bioactive Compounds from Cruciferous Vegetable (Kale) Waste Using Natural Deep Eutectic Solvents. Sep. Purif. Technol. 2023, 317, 123677. [Google Scholar] [CrossRef]
- Yaqoob, S.; Riaz, M.; Shabbir, A.; Zia-Ul-Haq, M.; Alwakeel, S.S.; Bin-Jumah, M. Commercialization and Marketing Potential of Carotenoids. In Carotenoids: Structure and Function in the Human Body; Zia-Ul-Haq, M., Dewanjee, S., Riaz, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 799–826. [Google Scholar] [CrossRef]
- Grewal, J.; Woła̧cewicz, M.; Pyter, W.; Joshi, N.; Drewniak, L.; Pranaw, K. Colorful Treasure From Agro-Industrial Wastes: A Sustainable Chassis for Microbial Pigment Production. Front. Microbiol. 2022, 13, 832918. [Google Scholar] [CrossRef]
- Alsaud, N.; Shahbaz, K.; Farid, M. Antioxidant and Antibacterial Evaluation of Manuka Leaves (Leptospermum scoparium) Extracted by Hydrophobic Deep Eutectic Solvent. Chem. Eng. Res. Des. 2021, 174, 96–106. [Google Scholar] [CrossRef]
- Durand, E.; Lecomte, J.; Upasani, R.; Chabi, B.; Bayrasy, C.; Baréa, B.; Jublanc, E.; Clarke, M.J.; Moore, D.J.; Crowther, J.; et al. Evaluation of the ROS Inhibiting Activity and Mitochondrial Targeting of Phenolic Compounds in Fibroblast Cells Model System and Enhancement of Efficiency by Natural Deep Eutectic Solvent (NADES) Formulation. Pharm. Res. 2017, 34, 1134–1146. [Google Scholar] [CrossRef]
- Li, Y.; Hu, K.; Huang, C.; Hu, Y.; Ji, H.; Liu, S.; Gao, J. Improvement of Solubility, Stability and Antioxidant Activity of Carotenoids Using Deep Eutectic Solvent-Based Microemulsions. Colloids Surf. B Biointerfaces 2022, 217, 112591. [Google Scholar] [CrossRef]
- Lee, S.-J.; Umano, K.; Shibamoto, T.; Lee, K.-G. Identification of Volatile Components in Basil (Ocimum basilicum L.) and Thyme Leaves (Thymus vulgaris L.) and Their Antioxidant Properties. Food Chem. 2005, 91, 131–137. [Google Scholar] [CrossRef]
- Youdim, K.A.; Deans, S.G.; Finlayson, H.J. The Antioxidant Properties of Thyme (Thymus zygis L.) Essential Oil: An Inhibitor of Lipid Peroxidation and a Free Radical Scavenger. J. Essent. Oil Res. 2002, 14, 210–215. [Google Scholar] [CrossRef]
- Aeschbach, R.; Löliger, J.; Scott, B.C.; Murcia, A.; Butler, J.; Halliwell, B.; Aruoma, O.I. Antioxidant Actions of Thymol, Carvacrol, 6-Gingerol, Zingerone and Hydroxytyrosol. Food Chem. Toxicol. 1994, 32, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Lagouri, V.; Boskou, D. Screening for Antioxidant Activity of Essential Oils Obtained from Spices. In Developments in Food Science; Elsevier: Amsterdam, The Netherlands, 1995; Volume 37, pp. 869–879. [Google Scholar] [CrossRef]
- Kishimoto, S.; Sumitomo, K.; Yagi, M.; Nakayama, M.; Ohmiya, A. Three Routes to Orange Petal Color via Carotenoid Components in 9 Compositae Species. J. Jpn. Soc. Hortic. Sci. 2007, 76, 250–257. [Google Scholar] [CrossRef]
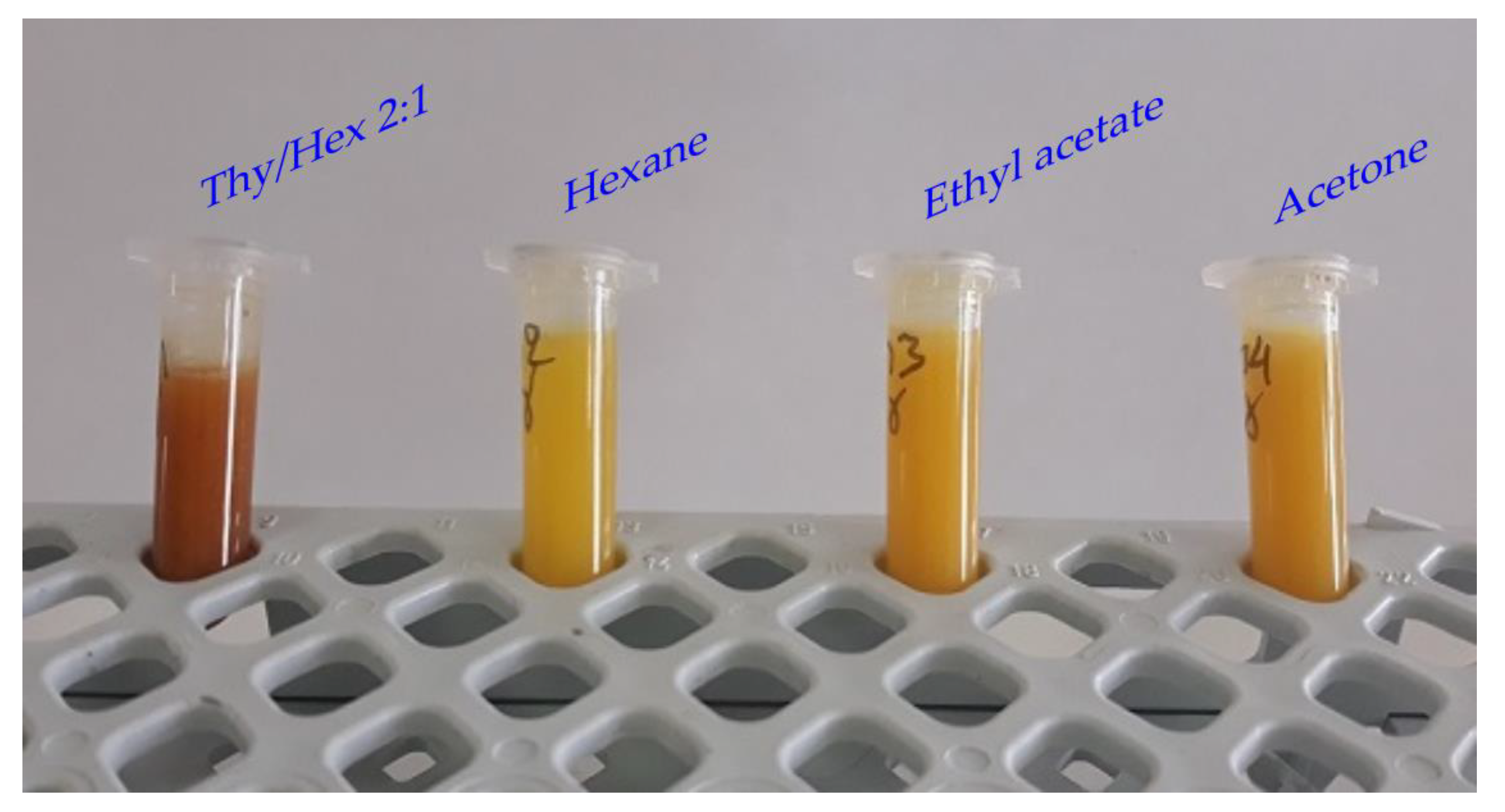
| HBA | HBD | Molar Ratio | Abbreviation | Density (g/mL) |
|---|---|---|---|---|
| Thymol | Hexanoic acid | 2:1 | Thy/Hex 2:1 | 0.838 |
| 1:1 | Thy/Hex 1:1 | 0.862 | ||
| 1:2 | Thy/Hex 1:2 | 0.855 | ||
| Thymol | Octanoic acid | 2:1 | Thy/Oct 2:1 | 0.870 |
| 1:1 | Thy/Oct 1:1 | 0.830 | ||
| 1:2 | Thy/Oct 1:2 | 0.863 | ||
| Hexanoic acid | Octanoic acid | 2:1 | Hex/Oct 2:1 | 0.869 |
| 1:1 | Hex/Oct 1:1 | 0.858 | ||
| 1:2 | Hex/Oct 1:2 | 0.848 |
| Independent Variables | Coded Units | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Solvent-to-solid ratio (mL/g) | X1 | 10 | 25 | 40 |
| t (min) | X2 | 30 | 60 | 90 |
| T (°C) | X3 | 20 | 35 | 50 |
| Solvent | YTCn (µg CtE/g dm) |
|---|---|
| Thy/Hex 2:1 | 184.94 ± 4.69 a |
| Thy/Hex 1:1 | 166.97 ± 4.11 b |
| Thy/Hex 1:2 | 165.39 ± 3.82 b |
| Thy/Oct 2:1 | 180.42 ± 9.02 a,b |
| Thy/Oct 1:1 | 134.98 ± 5.71 c |
| Thy/Oct 1:2 | 164.12 ± 7.7 b |
| Hex/Oct 2:1 | 179.52 ± 5.8 a,b |
| Hex/Oct 1:1 | 171.15 ± 2.76 a,b |
| Hex/Oct 1:2 | 175.17 ± 5.79 a,b |
| Hexane | 141.58 ± 3.49 c |
| Ethyl Acetate | 171.2 ± 8.16 a,b |
| Acetone | 128.15 ± 6.9 c |
| Design Point | Independent Variables | YTCn (µg CtE/g dm) | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | Measured | Predicted | |
| 1 | 10 | 30 | 35 | 175.6 | 175.0 |
| 2 | 10 | 90 | 35 | 220.1 | 222.2 |
| 3 | 40 | 30 | 35 | 182.5 | 180.4 |
| 4 | 40 | 90 | 35 | 183.3 | 183.9 |
| 5 | 25 | 30 | 20 | 176.0 | 184.6 |
| 6 | 25 | 30 | 50 | 178.5 | 172.7 |
| 7 | 25 | 90 | 20 | 235.7 | 241.5 |
| 8 | 25 | 90 | 50 | 175.0 | 166.4 |
| 9 | 10 | 60 | 20 | 250.4 | 242.4 |
| 10 | 40 | 60 | 20 | 256.8 | 250.3 |
| 11 | 10 | 60 | 50 | 216.9 | 223.3 |
| 12 | 40 | 60 | 50 | 174.4 | 182.4 |
| 13 | 25 | 60 | 35 | 182.5 | 183.3 |
| 14 | 25 | 60 | 35 | 187.8 | 183.3 |
| 15 | 25 | 60 | 35 | 179.7 | 183.3 |
| Solvent | Total Carotenoids Content (YTCn) (μg CtE/g dm) | Antiradical Activity (AAR) (μmol AAE/g dm) | Reducing Power (PR) (μmol AAE/g dm) |
|---|---|---|---|
| Thy/Hex 2:1 | 259.45 ± 3.46 a | 72.32 ± 0.45 a | 29.48 ± 0.15 a |
| Hexane | 163 ± 4.78 c | 24.24 ± 1 b | 21.27 ± 1.87 b |
| Ethyl acetate | 188.27 ± 4.54 b | 16.61 ± 0.4 c | 11.66 ± 0.25 c |
| Acetone | 147.38 ± 6.15 d | 18.16 ± 0.41 c | 10.75 ± 0.12 c |
| Solvent | L* | a* | b* | C* | h° |
|---|---|---|---|---|---|
| Thy/Hex 2:1 | 64.6 ± 0.8 b | 11.4 ± 1.4 a | 32.8 ± 1.6 b | 34.7 ± 2 b | 70.9 ± 1.4 c |
| Hexane | 69.3 ± 1 a | −4 ± 1.2 c | 67.3 ± 3 a | 67.4 ± 2.9 a | 93.5 ± 1.2 a |
| Ethyl acetate | 66.3 ± 0.8 a,b | 3.5 ± 0.8 b | 65.5 ± 2.8 a | 65.6 ± 2.8 a | 86.9 ± 0.7 b |
| Acetone | 67.3 ± 2 a,b | 3.8 ± 1.6 b | 62.9 ± 2.5 a | 63 ± 2.4 a | 86.5 ± 1.6 b |
| Solvent | CI | Hue | % Yellow | % Red | % Blue |
|---|---|---|---|---|---|
| Thy/Hex 2:1 | 6.5 ± 0.02 a | 8.16 ± 0.2 b | 87.91 | 10.78 | 1.31 |
| Hexane | 4.1 ± 0.07 c | 11.07 ± 1.84 a | 91.21 | 8.24 | 0.55 |
| Ethyl acetate | 4.71 ± 0.02 b | 11.16 ± 0.48 a | 91.29 | 8.18 | 0.53 |
| Acetone | 3.74 ± 0.05 d | 11.1 ± 0.86 a | 91.18 | 8.22 | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terlidis, K.; Athanasiadis, V.; Chatzimitakos, T.; Bozinou, E.; Lalas, S.I. Carotenoids Extraction from Orange Peels Using a Thymol-Based Hydrophobic Eutectic Solvent. AppliedChem 2023, 3, 437-451. https://doi.org/10.3390/appliedchem3040028
Terlidis K, Athanasiadis V, Chatzimitakos T, Bozinou E, Lalas SI. Carotenoids Extraction from Orange Peels Using a Thymol-Based Hydrophobic Eutectic Solvent. AppliedChem. 2023; 3(4):437-451. https://doi.org/10.3390/appliedchem3040028
Chicago/Turabian StyleTerlidis, Konstantinos, Vassilis Athanasiadis, Theodoros Chatzimitakos, Eleni Bozinou, and Stavros I. Lalas. 2023. "Carotenoids Extraction from Orange Peels Using a Thymol-Based Hydrophobic Eutectic Solvent" AppliedChem 3, no. 4: 437-451. https://doi.org/10.3390/appliedchem3040028
APA StyleTerlidis, K., Athanasiadis, V., Chatzimitakos, T., Bozinou, E., & Lalas, S. I. (2023). Carotenoids Extraction from Orange Peels Using a Thymol-Based Hydrophobic Eutectic Solvent. AppliedChem, 3(4), 437-451. https://doi.org/10.3390/appliedchem3040028










