The Influence of Functional Materials on the Size of the Lipid Vesicles in Beverages
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Lipid Vesicles
2.3. Microscopic Observation of Lipid Vesicls
2.4. Statistical Analysis
3. Results and Discussion
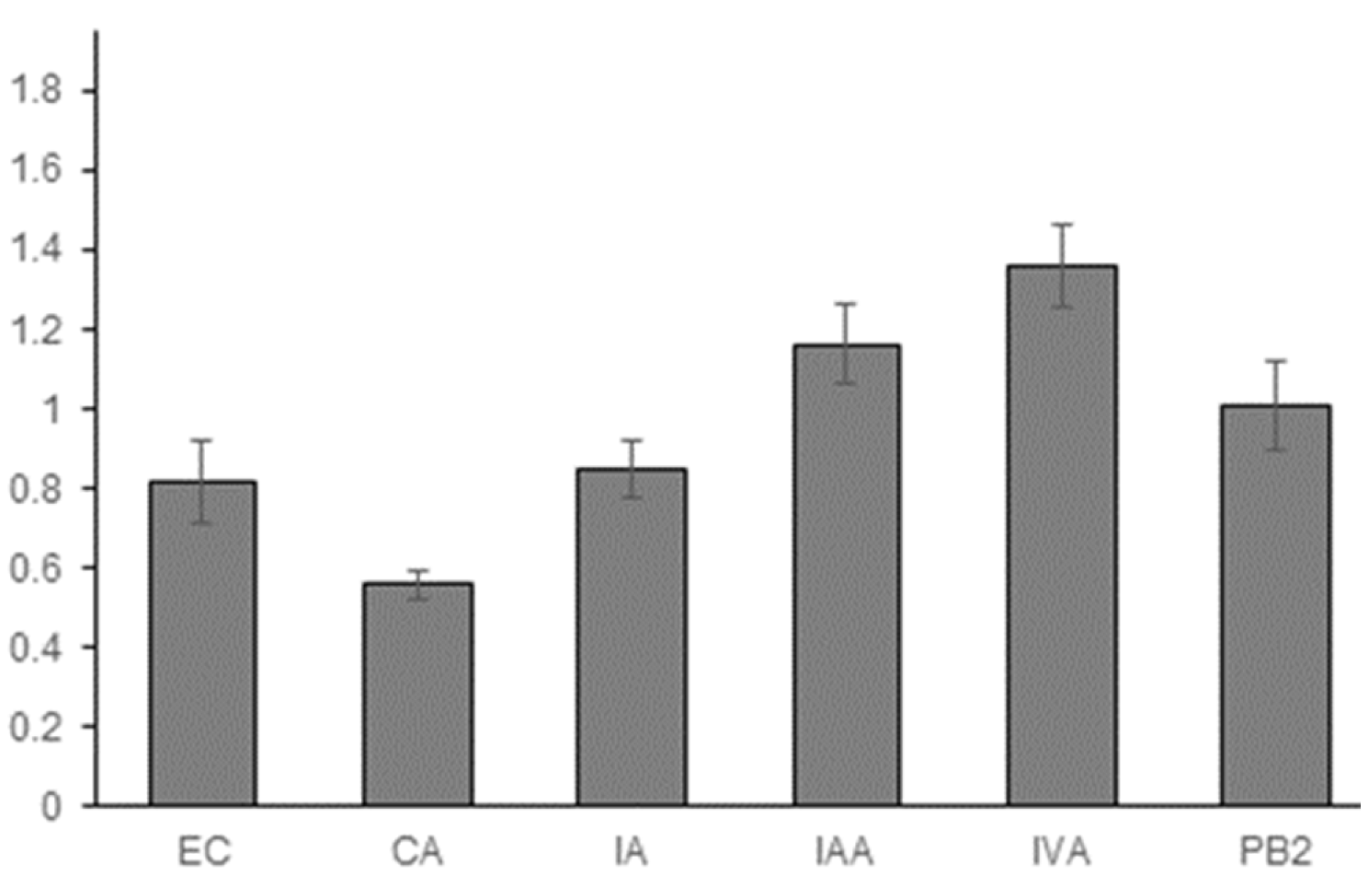
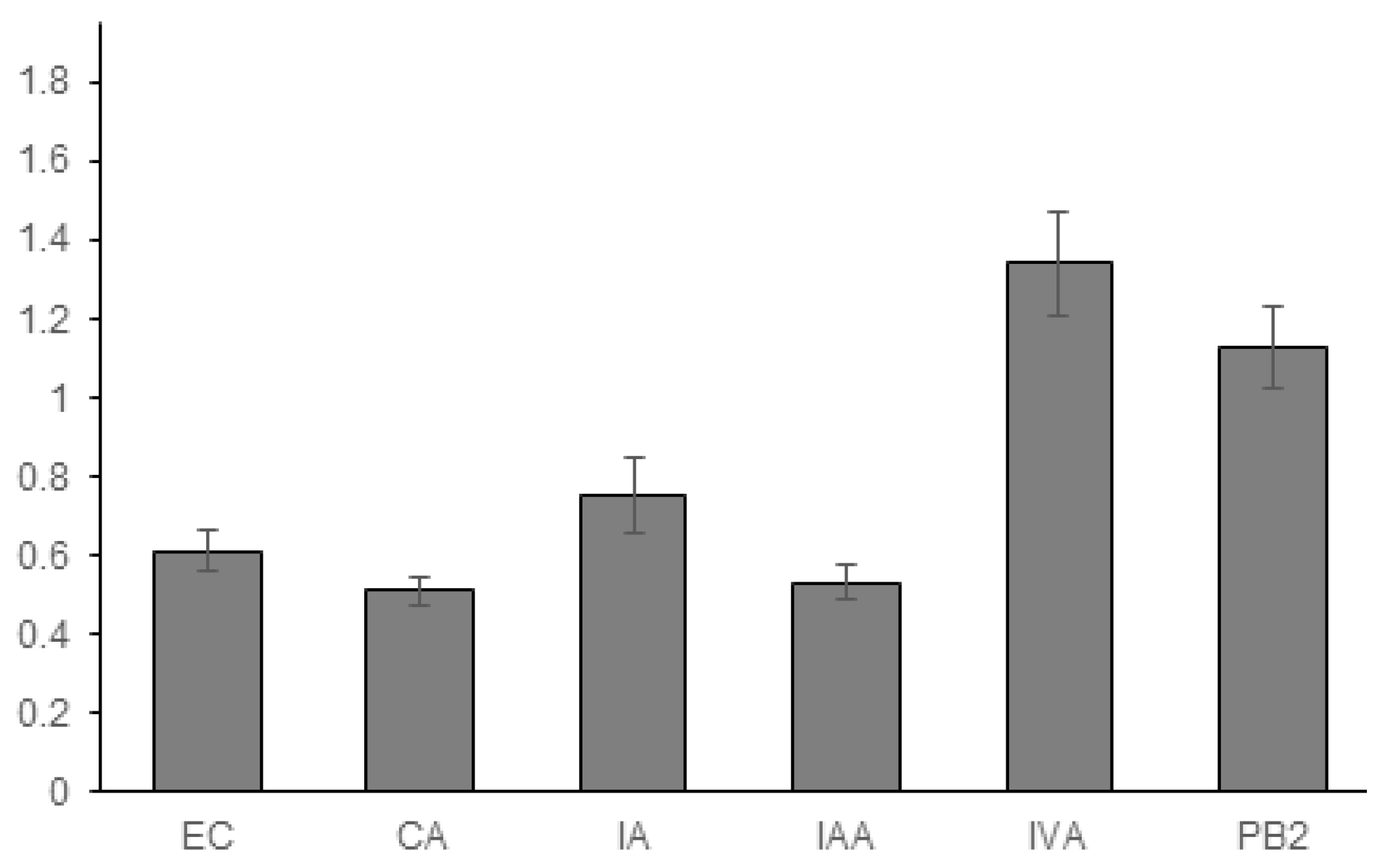
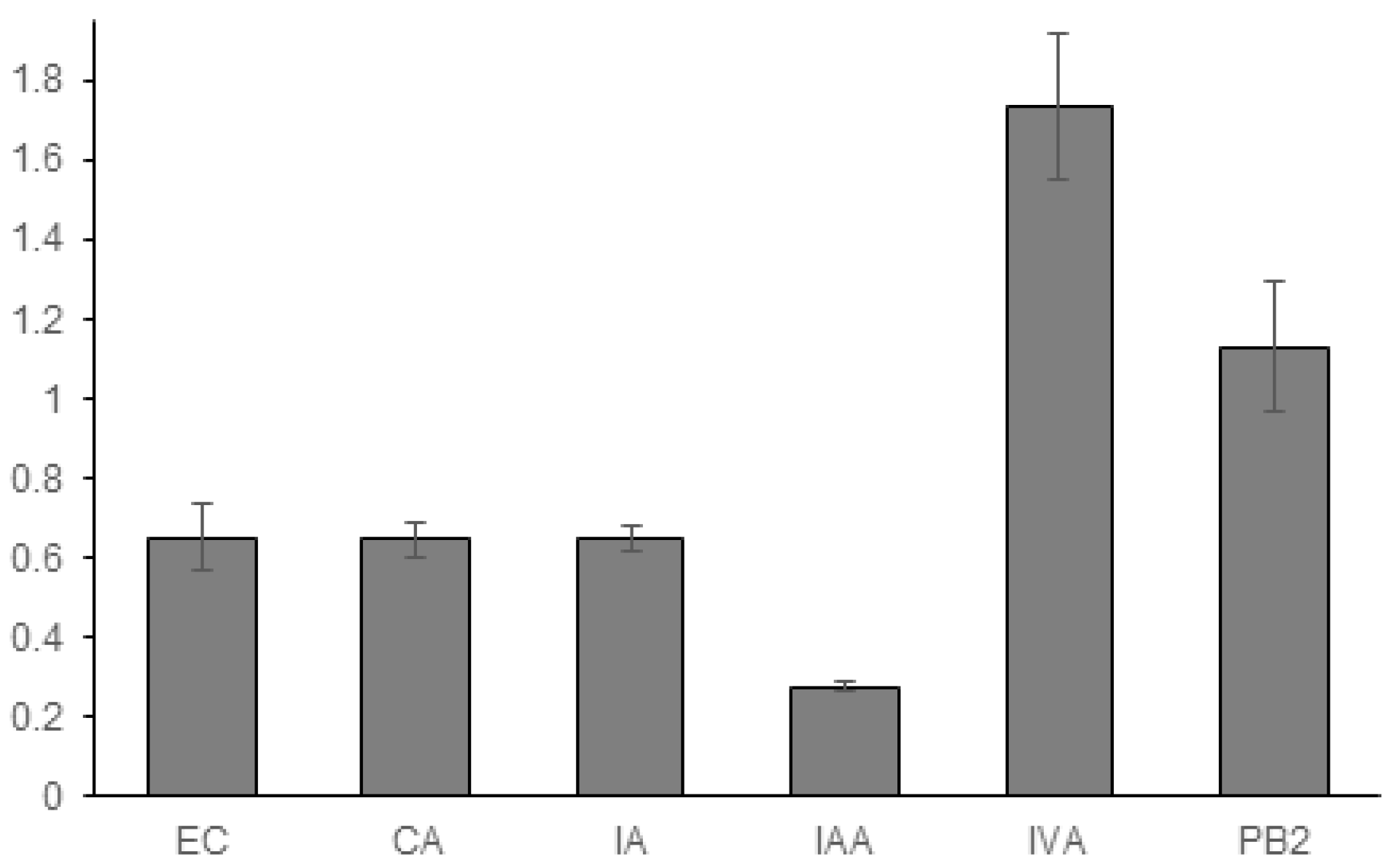
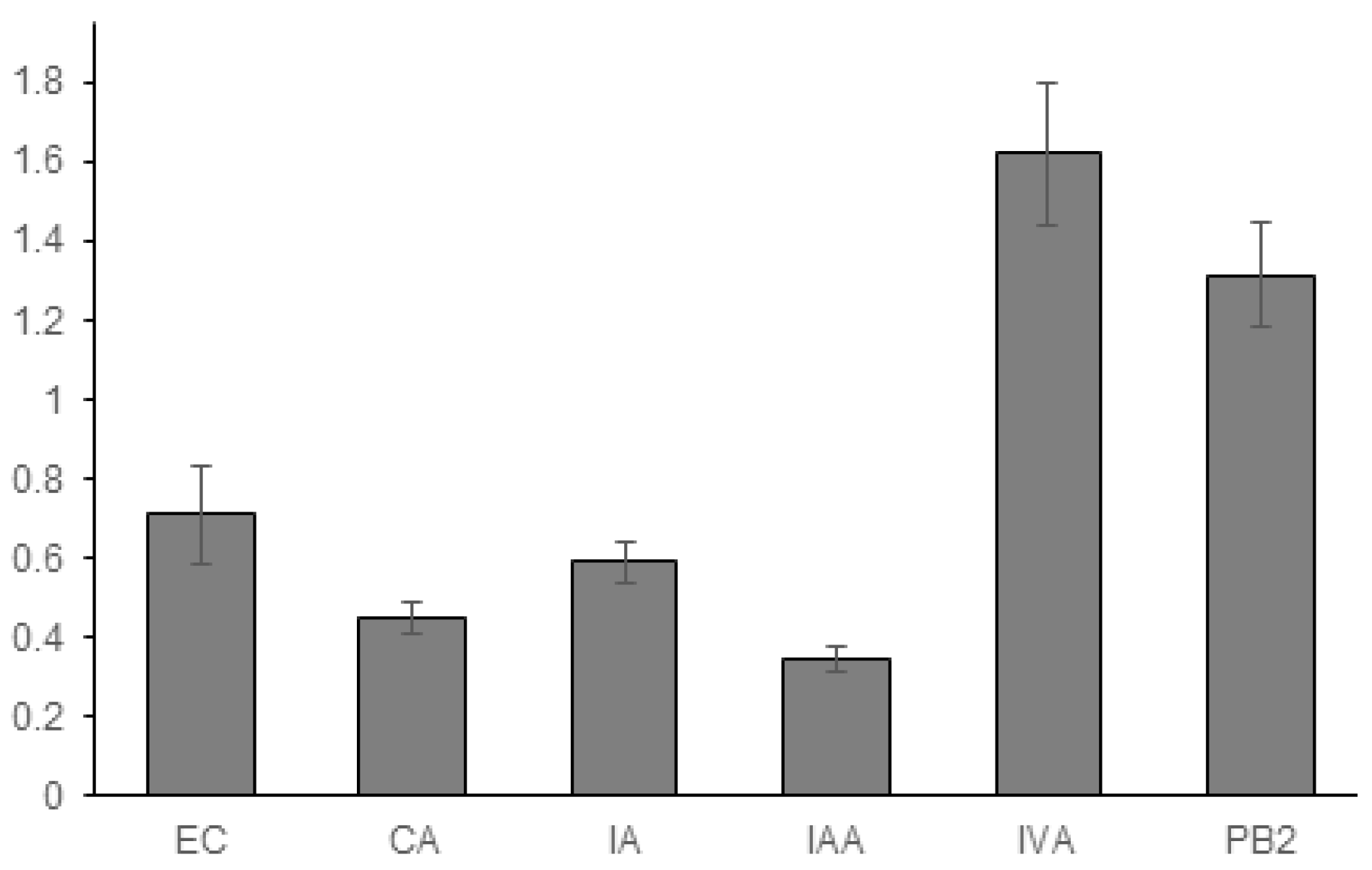
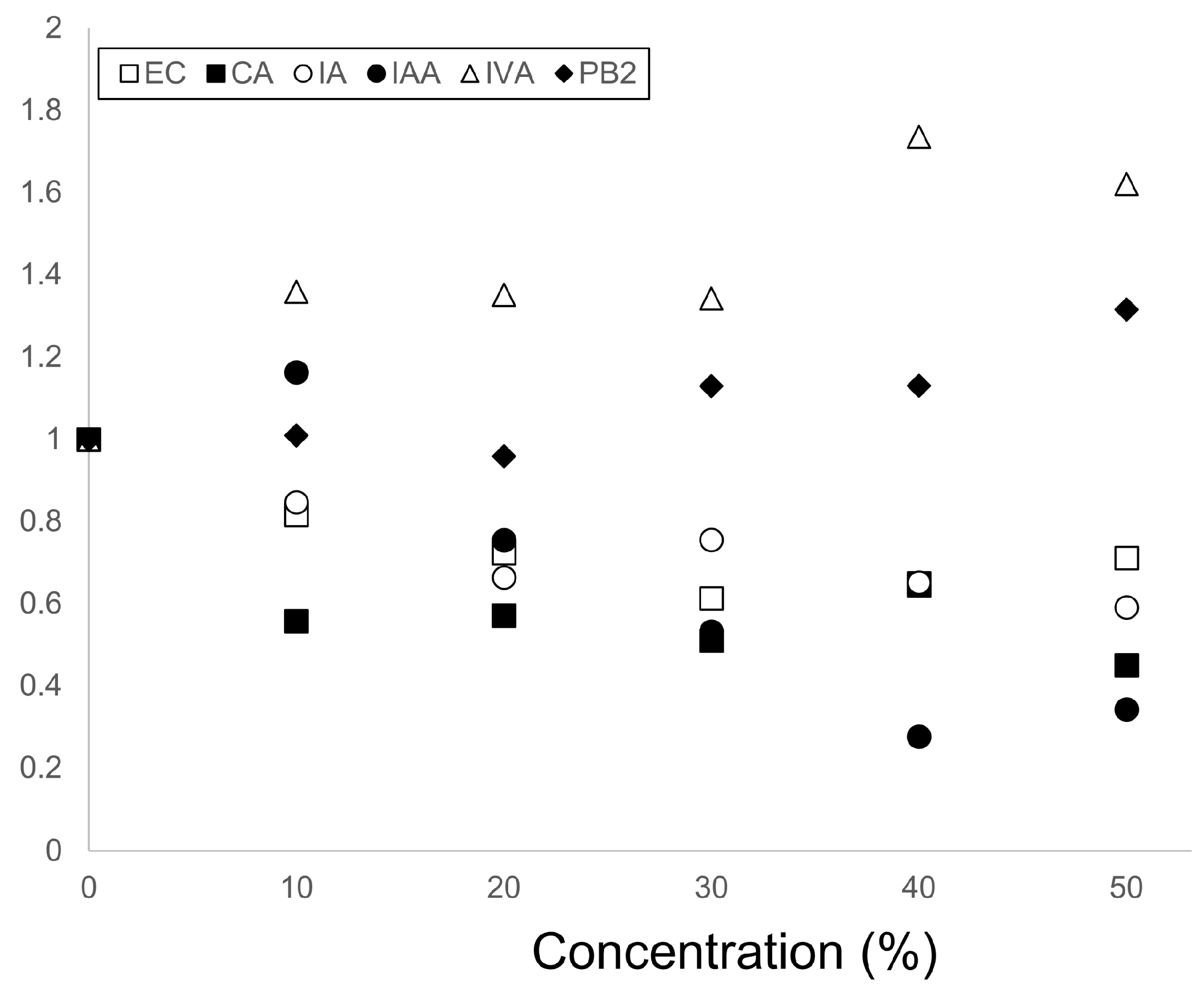
3.1. Experimental Results
3.2. Discussion of Molecular Properties
3.3. Influence of Membrane Fluidity
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, S.J.; Nicolson, G.L. The Fluid Mosaic Model of the Structure of Cell Membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Yoshikawa, K. Cell-Sized Liposomes and Droplets: Real-World Modeling of Living Cells. Materials 2012, 5, 2292–2305. [Google Scholar] [CrossRef]
- Dimova, R.; Stano, P.; Marques, C.M.; Walde, P. Preparation methods for giant unilamellar vesicles. In The Giant Vesicle Book; Dimova, R., Marques, C.M., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 3–20. [Google Scholar]
- Vestergaard, M.C.; Yoda, T.; Hamada, T.; Akazawa (Ogawa), Y.; Yoshida, Y.; Takagi, M. The effect of oxycholesterols on thermo-induced membrane dynamics. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2245–2251. [Google Scholar] [CrossRef] [PubMed]
- Yoda, T.; Vestergaard, M.C.; Hamada, T.; Le, P.T.M.; Takagi, M. Thermo-induced Vesicular Dynamics of Membranes Containing Cholesterol Derivatives. Lipids 2012, 47, 813–820. [Google Scholar] [CrossRef]
- Vestergaard, M.C.; Yoda, T.; Hamada, T.; Akazawa, Y.; Yoshida, Y.; Takagi, M. Thermo-responsiveness of auto-oxidized cholesterol-containing lipid membranes, observed in real-time. In Proceedings of the 2011 International Symposium on Micro-NanoMechatronics and Human Science, Nagoya, Japan, 6–9 November 2011; pp. 451–455. [Google Scholar]
- Phan, H.T.T.; Hata, T.; Morita, M.; Yoda, T.; Hamada, T.; Vestergaard, M.C.; Takagi, M. The effect of oxysterols on the interaction of Alzheimer’s amyloid beta with model membranes. Biochim. Biophys. Acta Biomembr. 2013, 1828, 2487–2495. [Google Scholar] [CrossRef]
- Yoda, T.; Phan, H.T.T.; Vestergaard, M.C.; Hamada, T.; Takagi, M. Thermo-induced Dynamics of Membranes and Liquid Crystals Containing Cholesterol Derivatives. In Proceedings of the 2012 International Symposium on Micro-NanoMechatronics and Human Science (MHS), Nagoya, Japan, 4–7 November 2012; pp. 87–92. [Google Scholar]
- Sharma, N.; Phan, H.T.T.; Yoda, T.; Shimokawa, N.; Vestergaard, M.C.; Takagi, M. Effects of Capsaicin on Biomimetic Membranes. Biomimetics 2019, 4, 17. [Google Scholar] [CrossRef]
- Chahal, B.; Vestergaard, M.C.; Yoda, T.; Morita, M.; Takagi, M. Structure-Dependent Membrane Interaction and bioactivity of Flavonoids with Lipid Bilayers. In Proceedings of the 2012 International Symposium on Micro-NanoMechatronics and Human Science (MHS), Nagoya, Japan, 4–7 November 2012; pp. 106–110. [Google Scholar]
- Phan, H.T.T.; Yoda, T.; Chahal, B.; Morita, M.; Takagi, M.; Vestergaard, M.C. Structure-dependent interactions of polyphenols with a biomimetic membrane system. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2670–2677. [Google Scholar] [CrossRef]
- Ichikawa, E.; Hosokawa, N.; Hata, Y.; Abe, Y.; Suginami, K.; Imayasu, S. Breeding of a sake yeast with improved ethyl hexanoate productivity. Agric. Biol. Chem. 1991, 55, 2153–2154. [Google Scholar]
- Ichikawa, E. Sake yeast with improved ethyl caproate productivity. Nihon Jozo gakkaishi 1993, 88, 101–105. (In Japanese) [Google Scholar]
- Akita, O. Breeding of sake yeast producing a large quantity of aroma. Nihon Jozo gakkaishi 1992, 87, 621–625. (In Japanese) [Google Scholar]
- Verstrepen, K.J.; Derdelinckx, G.; Dufour, J.P.; Winderickx, J.; Thevelein, J.M.; Pretorius, I.S.; Delvaux, F.R. Flavor-active esters: Adding fruitiness to beer. J. Biosci. Bioeng. 2003, 96, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, H. Seishu kōbo no kōki seisei no kenkyū (Study on aroma production of sake yeast). Seibutsu-Koggakaishi 2011, 12, 717–719. (In Japanese) [Google Scholar]
- Tsutsumi, S.; Mochizuki, M.; Sakai, K.; Ieda, A.; Ohara, R.; Mitsui, S.; Ito, A.; Hirano, T.; Shimizu, M.; Kato, M. Ability of Saccharomyces cerevisiae MC87-46 to assimilate isomaltose and its effects on sake taste. Sci. Rep. 2019, 9, 13908. [Google Scholar] [CrossRef] [PubMed]
- Aritomi, K.; Hirosawa, I.; Hoshida, H.; Shigi, M.; Nishizawa, Y.; Kashiwagi, S.; Akada, R. Self-cloning yeast strains containing novel FAS2 mutations produce a higher amount of ethyl caproate in Japanese sake. Biosci. Biotechnol. Biochem. 2004, 68, 206–214. [Google Scholar] [CrossRef]
- Goshima, T.; Nakamura, R.; Kume, K.; Okada, H.; Ichikawa, E.; Tamura, H.; Hasuda, H.; Inahashi, M.; Okazaki, N.; Akao, T.; et al. Identification of a mutation causing a defective spindle assembly checkpoint in high ethyl caproate- producing sake yeast strain K1801. Biosci. Biotechnol. Biochem. 2016, 80, 1657–1662. [Google Scholar] [CrossRef]
- Ashida, S.; Ichdcawa, E.; Suginami, K.; Imayasu, S. Isolation and application of mutants producing sufficient isoamyl acetate a sake flavor component. Agric. Biol. Chem. 1987, 51, 2061–2065. [Google Scholar]
- Mason, A.B.; Dufour, J. Alcohol acetyltransferases and the significance of ester synthesis in yeast. Yeast 2000, 16, 1287–1298. [Google Scholar] [CrossRef]
- Blanco, C.A.; Andrés-Iglesias, C.; Montero, O. Low-alcohol beers: Flavor compounds, defects, and improvement strategies. Crit. Rev. Food Sci. 2016, 56, 1379–1388. [Google Scholar] [CrossRef]
- Tian, H.Y.; Zhang, J.; Sun, B.G.; Huang, M.Q.; Li, J.R.; Han., J.X.X. Preparation of natural isovaleraldehyde by the Maillard reaction. Chin. Chem. Lett. 2007, 18, 1049–1052. [Google Scholar] [CrossRef]
- Priegue, J.M.; Crisan, D.N.; Martínez-Costas, J.; Granja, J.R.; Fernandez-Trillo, F.; Montenegro, J. In situ functionalized polymers for siRNA delivery. Angew. Chem. 2016, 128, 7618–7621. [Google Scholar] [CrossRef]
- Kanamoto, Y.; Yamashita, Y.; Nanba, F.; Yoshida, T.; Tsuda, T.; Fukuda, I.; Nakamura-Tsuruta, S.; Ashida, H. A Black Soybean Seed Coat Extract Prevents Obesity and Glucose Intolerance by Up-regulating Uncoupling Proteins and Down-regulating Inflammatory Cytokines in High-Fat Diet-Fed Mice. J. Agric. Food Chem. 2011, 59, 8985–8993. [Google Scholar] [CrossRef] [PubMed]
- Yoda, T.; Miyaki, H.; Saito, T. Effect of container shape on freeze concentration of apple juice. PLoS ONE 2021, 16, e0245606. [Google Scholar] [CrossRef] [PubMed]
- Yoda, T.; Miyaki, H.; Saito, T. Freeze concentrated apple juice maintains its flavor. Sci. Rep. 2021, 11, 12679. [Google Scholar] [CrossRef] [PubMed]
- Yoda, T.; Ogura, A.; Saito, T. Influence of Ethyl Caproate on the Size of Lipid Vesicles and Yeast Cells. Biomimetics 2020, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Yoda, T.; Saito, T. Size of Cells and Physicochemical Properties of Membranes are Related to Flavor Production during Sake Brewing in the Yeast Saccharomyces cerevisiae. Membranes 2020, 10, 440. [Google Scholar] [CrossRef]
- Yoda, T.; Yamada, Y.; Chounan, Y. Effects of isovaleraldehyde on cell-sized lipid bilayer vesicles. Biophys. Chem. 2021, 279, 106698. [Google Scholar] [CrossRef]
- Yoda, T. Direct Observation of Cell-sized Liposomes Containing a Functional Polyphenol Procyanidin B2 from Apple. ChemistrySelect 2022, 7, e01808. [Google Scholar] [CrossRef]
- Image, J. A Soft-Ware for Image Analysis, It Was Download from Official Site. Available online: https://imagej.nih.gov/ij/download.html (accessed on 12 July 2023).
- Graphical Abstract of Ref. 30. Available online: https://ars.els-cdn.com/content/image/1-s2.0-S0301462221001812-ga1_lrg.jpg (accessed on 12 July 2023).
- Arisawa, K.; Mitsudome, H.; Yoshida, K.; Sugimoto, S.; Ishikawa, T.; Fujiwara, Y.; Ichi, I. Saturated fatty acid in the phospholipid monolayer contributes to the formation of large lipid droplets. Biochem. Biophys. Res. Commun. 2016, 480, 641–647. [Google Scholar] [CrossRef]
- Hamada, T.; Miura, Y.; Komatsu, K.; Kishimoto, Y.; Vestergaard, M.; Takag, M. Construction of asymmetric cell-sized lipid vesicles from lipid-coated water-in-oil micro-droplets. J. Phys. Chem. B 2008, 112, 14678–14681. [Google Scholar] [CrossRef]
- Morita, M.; Onoe, H.; Yanagisawa, M.; Ito, H.; Ichikawa, M.; Fujiwara, K.; Saito, H.; Takinoue, M. Droplet-Shooting and Size-Filtration (DSSF) Method for Synthesis of Cell-Sized Liposomes with Controlled Lipid Compositions. ChemBioChem 2015, 16, 2029–2035. [Google Scholar] [CrossRef]
- Ohno, M.; Hamada, T.; Takiguchi, K.; Homma, M. Dynamic behavior of giant liposomes at desired osmotic pressures. Langmuir 2009, 25, 11680–11685. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Fujimoto, R.; Shimobayashi, S.F.; Ichikawa, M.; Takagi, M. Molecular behavior of DNA in a cell-sized compartment coated by lipids. Phys. Rev. E 2015, 91, 062717. [Google Scholar] [CrossRef] [PubMed]
- Chadani, T.; Ohnuki, S.; Isogai, A.; Goshima, T.; Kashima, M.; Ghanegolmohammadi, F.; Nishi, T.; Hirata, D.; Watanabe, D.; Kitamoto, K.; et al. Genome editing to generate sake yeast strains with eight mutations that confer excellent brewing characteristics. Cells 2021, 10, 1299. [Google Scholar] [CrossRef]
- Wang, R.; Dang, M.; Zhu, W.; Li, C. Galloyl Group in B-type proanthocyanidin dimers was responsible for its differential inhibitory activity on 3T3-L1 preadipocytes due to the strong lipid raft-perturbing potency. J. Agric. Food Chem. 2021, 69, 5216–5225. [Google Scholar] [CrossRef] [PubMed]
- Yoda, T. The Flavonoid Molecule Procyanidin Reduces Phase Separation in Model Membranes. Membranes 2022, 12, 943. [Google Scholar] [CrossRef] [PubMed]
- Yoda, T. Phase-Separated structures of sake flavors-containing cell model membranes. Chem. Biodiver. 2023, 20, e202200750. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoda, T. The Influence of Functional Materials on the Size of the Lipid Vesicles in Beverages. AppliedChem 2023, 3, 366-377. https://doi.org/10.3390/appliedchem3030023
Yoda T. The Influence of Functional Materials on the Size of the Lipid Vesicles in Beverages. AppliedChem. 2023; 3(3):366-377. https://doi.org/10.3390/appliedchem3030023
Chicago/Turabian StyleYoda, Tsuyoshi. 2023. "The Influence of Functional Materials on the Size of the Lipid Vesicles in Beverages" AppliedChem 3, no. 3: 366-377. https://doi.org/10.3390/appliedchem3030023
APA StyleYoda, T. (2023). The Influence of Functional Materials on the Size of the Lipid Vesicles in Beverages. AppliedChem, 3(3), 366-377. https://doi.org/10.3390/appliedchem3030023





