Abstract
Dietary nucleotides and nucleosides, primarily inosine monophosphate (IMP) and the adenine nucleotide pool (ANP), are widely considered as essential nutrients responsible for multiple biological functions. Food prepared from meat and fish is the main source of these substances in the human diet, and it is extremely important to implement storage and processing techniques ensuring their maximum preservation and even accumulation during maturation or conditioning. In experiments with freshly refrigerated grass carp and defrosted Alaska pollock fillets it was discovered, initially using Fast Protein and Metabolites Liquid Chromatography and the ATP-bioluminescence test, and afterwards validated by NMR spectroscopy, that heat treatment identical to conventional culinary processing in aqueous or wet media at temperatures above 62 °C leads to nucleotide salvage (recovery) in aged fish. A significant increase in the concentration of IMP, and even an emergence of ANP substances, were reliably demonstrated in fish samples which had already partially or fully lost these components during prolonged storage due to the ATP breakdown metabolic reactions. Owing to this recovery, the nutritive value of ready-to-eat food can be higher than was initially evaluated in raw products before heat treatment: an effect that should certainly be considered in practical nutrition. Moreover, it is necessary to reconsider the widely acknowledged system of indices of freshness based on nucleotides and nucleosides elaborated a long time ago for raw meat and fish products.
1. Introduction
There is a growing tendency to consider dietary nucleotides and nucleosides as essential nutrients responsible for numerous biological functions including immunity boosting and the regulation of intestinal flora; accordingly, it has been proposed that the nutritive index of nucleotides and nucleosides be added to the database []. Food prepared from meat and fish products are among the main sources of exogeneous nucleotides and nucleosides in the human diet, and it is extremely important to adopt and implement storage and processing techniques (both in the industry and at home) ensuring maximum preservation and even accumulation of these nutrients during maturation or conditioning, i.e., the conception of food umamification [].
Umamification is associated with an increase of the role of inosine monophosphate (IMP) C10H13N4O8P, adenosine monophosphate (AMP) C10H14N5O7P [], and, possibly, adenosine triphosphate C10H16N5O13P3 (ATP) and adenosine diphosphate C10H15N5O10P2 (ADP) in nutrition. It was noticed decades ago that a period of postmortem aging of meat improves its flavor and taste (see, e.g., [] and references therein). Nowadays IMP is often even added to food products artificially as a highly expensive flavor enhancer from the E630 series, but for multiple reasons it is preferable to preserve and increase endogenous IMP content in meat and fish.
A common phenomenon observed in the preparation of meat and fish dishes by heat treatment is a decrease in the content of nucleotides, primarily in IMP which is the most valuable nucleotide in mammal meat [,,,,,,] and fish []. It was shown that the simplest way to preserve IMP is to save the IMP-rich juice that leaked from the meat when it was heated [,] and use it as a seasoning []. However, the achievement of the best results can be hampered by poor knowledge of the processes occurring during heat treatment, even although the use of fire and heat goes back in history for many millennia.
There is one more aspect concerning the quality of nutrition, i.e., the question of whether animal meat must be consumed as fresh as possible, or whether its storage (maturation or conditioning) can be beneficial not only in terms of creating palatability and exquisite taste, but also in terms of nutritional value. The storage of meat and fish induces numerous changes in the structure and composition of the muscle tissue, and we will focus our attention on the evolution of nucleotides and nucleosides, particularly IMP and the adenine nucleotide pool (ANP) ATP + ADP + AMP, during refrigerated storage as an essential element in healthy nutrition.
The complete conversion of ATP into IMP occurs during the first 24–48 h after the fish’s death according to the following pathway: . Afterwards, during chilled storage, IMP degrades to Ino by the action of a 5′-nucleotidase or some phosphatases. Ino, in turn, is further transformed into Hx with purine nucleoside phosphorylase or inosine nucleosidase [,]. Alternatively, IMP degradation is closely associated with specific spoilage organisms (SSOs), which are both endogenous, from fish microbiota, and introduced during fish processing, possibly via the production of enzymes that catalyze IMP degradation by bacteria [,,]. The chemical processes related to ATP breakdown occurring in mammal meat are very similar to those occurring in fish but slightly slower.
It is important to notice that the effects of storage are intertwined with the effects of heat treatment. This phenomenon is manifested as nucleotide salvage or recovery in aged meat or fish. Along with the well-known facts regarding IMP loss upon heating, exceptions such as an increase in the content of IMP and other nucleotides were observed. The most comprehensive data of this kind was obtained by the method of preparing broth by boiling minced beef [,] or shrimp []. In beef broth heated from room temperature up to >100 °C, the concentration of IMP increased at 85–95 °C by a factor of 2.6 in comparison to the initial level in the raw extract [], and by factor of 8 at 95 °C []. Even more pronounced was the effect of the emergence of AMP, ADP, and ATP in the same broth at temperatures ≥65 °C, while only traces of these nucleotides were initially recorded in raw products.
A significant increase of 1.8 up to 3.4 times in AMP content in heated beef, pork, and lamb was observed in [], while an enormous 18-fold increase in goat meat and a 16-fold increase for pork meat were reported in [] and [], respectively. T. Nishimura et al. recorded the changes in the content of AMP and ADP metabolites in soup from beef, pork and chicken depending on the storage time at 4 °C []. It was found that the AMP content in the first and third cases increased after 12 and 2 days of meat storage, respectively. In the pork soup, after 6 days of storage the AMP content remained almost unchanged, but ADP emerged. The concentration of IMP in all soups decreased as a result of meat storage. The emergence in heated products of nucleotides which had already disappeared and the dependance of this phenomenon on the meat storage time is probably the most intriguing observation and was documented a considerable time ago [,,,].
The ATP, ADP, AMP, and IMP nucleotides can be divided into two groups according to their characteristic behavior in experiments with heating. Adenylates are typically present at very low concentrations in commercial meat and fish which are usually available a relatively long time after slaughter or catch. Therefore, all experiments with them which aim to detect adenylates can be normally carried out according to the most convincing scheme “No–Yes” (“There was no signal—There is a signal”). In particular, this applies to ATP, the most important representative of adenylates. In studies [,] with beef and shrimp, it was noted that at temperatures <75 °C, HPLC signals from adenylates were mainly at noise level and, therefore, the concentrations were characterized as “traces”. Because of this, the low concentration of adenylates produced by heating could be considered as a quantitative fact, despite the fact that in all cases the measurement deviations were significant: STDEV/Average ≈ 20% [].
The situation with IMP is much more complex from a methodological point of view. Most often, in meat and fish suitable for consumption, IMP is present in significant amounts (several µmol/g), but it is subject to large losses during heating (see Table 1). These losses inside pieces of meat in samples are, at least partly, associated with outward leaching of IMP. For example, in [] it is shown that at the end of a 180 min heating circle of pork, the concentration of IMP in the leaked juice was almost twice as high as in a piece of meat. The experiments, which allow accurate IMP determination, require special techniques, for example, sous vide heating with the obligatory collection of juice and homogenization of the solid material for the measurement of residual IMP.

Table 1.
Content of residual Res[IMP] in animal meats and fish after heating.
Perhaps these cumbersome procedures did prevent the authors from detecting the appearance of small additional quantities of IMP generated during heating. In any case, we did not find any note of these in the core publications in the area. Therefore, we chose an alternative approach and used fish (Pollock) without any presence of IMP before heating. In our pilot investigation, [] five fish species were inspected, and manifestations of heat-induced emergence of IMP and, possibly, other nucleotides from the ANP, were initially revealed by Fast Protein and Metabolite Liquid Chromatography (FPMLC) and were afterwards validated by NMR spectroscopy for Alaska pollock treated in water steam.
The results presented in [] should be considered as only preliminary because the conditions of heat treatment necessary for the manifestation of nucleotide recovery and the origin of this effect remained mostly unclear and need further investigation, which is the main objective of this research. Additionally, it is extremely important to understand to what extent simplified rapid methods for on-site testing, i.e., FPLMC and ATP-bioluminescence assay, can provide reliable information about nucleotide recovery compared to more sophisticated laboratory techniques such as Nuclear Magnetic Resonance (NMR) spectroscopy.
To verify the discovered effects of heat treatment on nucleotide salvage in fish, which were found at the very beginning using the FPMLC technique, we have undertaken a complex investigation using Alaska pollock stored in a frozen condition for a long time. In our previous work [], we found that pollock had two properties that made it an attractive subject for further research. Firstly, the NMR spectra did not contain any signals from IMP, which made it possible to work in the most heuristic mode of the appearance of the signal “No” → “Yes”. Secondly, in the NMR spectrum in the region relevant for the registration of nucleotides 6–9 ppm, there are few extrinsic lines and the most relevant for us, the ATP, ADP, AMP, and IMP signals in the range of 8.5–8.7 ppm, are clearly separated from each other. Pollock is a very convenient model subject for work with nucleotides.
The FPMLC, NMR spectroscopy, and ATP-bioluminescence assay have been combined for the first time for the highly selective detecting and quantitative determination of specific nucleotides which emerged for the first time, or which accumulated, after the heat treatment of aged fish. Extensive experiments were conducted to investigate the impact of treatment temperature (from room temperatures to the water boiling point), medium (wet or dry heating), and heat treatment options (steam cooking, microwave oven) on nucleotides’ recovery, which is a key to the better understanding of the background of this effect.
2. Materials and Methods
2.1. Sample Preparation
The experiments using the FPMLC technique were performed with two fish species, i.e., defrosted Alaska pollock (Theragra calcogramma) and freshly refrigerated White Amur or grass carp (Ctenopharyngodon idella), according to the measurement protocol described in detail in []. The choice of fish species was based on commercial availability and dominant positions in aquaculture (White Amur) or fisheries (Alaska pollock): White Amur (grass carp) is the most important freshwater fish species cultured in many countries, including Russia [] and China []. Alaska pollock is the world’s second most important fish species, after the Peruvian anchoveta, in terms of total catch []. An in-depth analysis of dietary nucleotides content before and after heat treatment was performed for the pollock samples with an additional and rather intense use of the ATP-bioluminescence test and Nuclear Magnetic Resonance (NMR) spectroscopy.
Fish samples were purchased from local supermarkets: frozen Alaska pollock fillets in Tartu (Estonia) and fresh farmed Amur (from an aquarium) in St. Petersburg (Russia). The Alaska pollock was frozen on 10 January 2021 and the best before day (BBD) is 2 August 2023. It was defrosted at room temperature before the experiments. The first measurements using FPMLC were performed for the Amur within one hour after capture. Afterwards, the fish was kept refrigerated in a closed plastic container at 2–4 °C for 10 days; it was sampled and analyzed every other day during this period.
Ethical aspects of the research were handled as described in the Singapore Statement on Research Integrity [] and the Montreal Statement on Research Integrity []. As the fish were purchased from commercial providers post-mortem and no laboratory animals were involved, approval from ethical commissions is not required.
Muscle tissue sampled from the back of a whole fish (for fresh Amur) or a large piece of flesh from defrosted fillets (for Alaska pollock) were manually cut into small pieces of 2 × 2 × (2–5) mm3. A set of identical samples was prepared for both the Amur and the Alaska pollock; some samples were left intact (raw), while the others afterwards underwent heat treatment (heated). It should be noted that the investigations into the Alaska Pollock and the Amur were undertaken separately.
Several samples of the Alaska pollock were heated in four different modes: in water steam, in a broth-like liquid, in a microwave oven, and in open air on the surface of food grade aluminum foil. Wet treatment was carried out in Eppendorf tubes at different temperatures between 22 and 100 °C for the intervals of 15 to 60 min in the dry block thermostat Biosan TDB-120 (Biosan, Riga, Latvia). The Amur was heated in water steam only in a conventional steam cooker at 100 °C for 45 min. The choice of heating conditions in this research was based on the common heating temperature for fish processing: 100 °C for cooking in boiling water and lower temperatures 22–100 °C for smoking or sous vide preparation. Processing at higher temperatures typical for frying in oil or canning was not investigated.
Liquid extracts were prepared from all the samples taken and were processed at a previous stage (both raw and heated in various conditions) according to []. The samples were large enough to prepare separate extracts from each sample to provide for all analytical methods used in this research (we need only 2 g of fish flesh to make an extract).
The extracts and broths from the Alaska pollock were centrifuged at 13,000 rpm and filtered with the syringe 0.45 µm filters Whatman® GF/B from Merck KGaA (Darmstadt, Germany). The same protocol was applied to the Amur, except the centrifuging. The extracts from the Amur and the Pollock (both raw and heated in various conditions) were rapidly tested by FPMLC (immediately after preparation). The extracts from pollock were also analyzed by NMR spectroscopy and the ATP test (they were frozen after preparation and stored at −17 °C before measurements). We did not perform NMR spectroscopy for the Amur because the interpretation of NMR spectra of fresh fish is far more complicated and needs more effort because, in this case, a considerable amount of the adenylate nucleotides could be detected in raw samples.
2.2. Methods
NMR spectroscopy was the main analytical technique used in this study. Since NMR is quite expensive and requires a lot of time for registration, it is reasonable to preselect fairly promising samples which show the most pronounced effects of nucleotide salvage by a simple rapid method. We used for the FPMLC instrument and technique designed and manufactured by Ldiamon AS (Tartu, Estonia) [] to meet this aim. The FPMLC sensor is a compact affordable analytical device with photometric detection at a wavelength of 260 nm; the basic operation principle of the sensor is the separation of molecules by size by gel chromatography with PD-10 protein desalting columns from GE Healthcare® Bio-Sciences AB (Uppsala, Sweden) [].
The FPMLC sensor was calibrated with model solutions of bovine serum albumin (BSA, heat shock fraction, pH 7, ≥98%, Product No. A7906) and the main nutritional nucleotides and nucleosides, i.e., ATP (disodium salt hydrate, Grade II, ≥97% HPLC, from microbial, Product No. A3377), ADP (sodium salt; >95% HPLC, bacterial, Product No. A2754), AMP (sodium salt, from yeast, ≥99%, Product No. A1752), IMP (disodium salt hydrate, ≥99.0% HPLC, Product No. 57510), inosine (Ino, ≥99% HPLC, Product No. I4125), and hypoxanthine (Hx, ≥99.0%, Product No. H9377) in TRIS buffer (pH 8.0) []; the BSA, the nucleotides, and the nucleoside standards were purchased from Sigma-Aldrich, Burlington, MA, USA. The FPMLC chromatograms of ATP, ADP, AMP, IMP, Ino, and Hx are presented in Figure 1; BSA solution was added to all samples to produce a highly distinctive reference protein peak. The timescale of the chromatograms was adjusted in such a way that the BSA peak is at point zero. The elution times of the nucleotides and nucleosides are presented in Table 2 the values were determined relative to the BSA peak. The higher elution times are characteristic of the lower molecular weight substances (Ino and Hx) which emerge at the later stages of the ATP breakdown chain [].
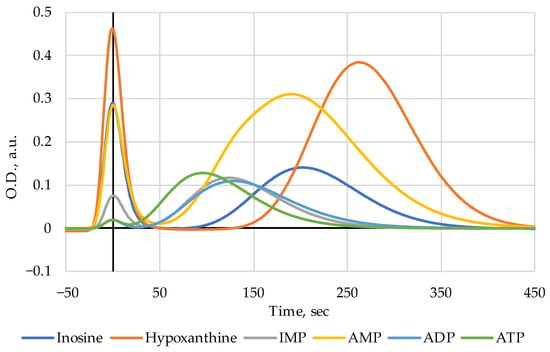
Figure 1.
The FPMLC chromatograms of ATP, ADP, AMP, IMP, Inosine, and Hypoxanthine.

Table 2.
Elution times of the main nutritional nucleotides and nucleosides.
This method enables us, in the short runs of 15 min duration (per sample), to see the shift of the characteristic index Time (the time interval between the protein peak and the combined peak of nucleotides and nucleosides in the chromatograms of liquid meat extracts) to smaller values as a result of heat treatment (Figure 2c). Such shift could be interpreted as evidence of heat-induced nucleotide salvage (see, also []).
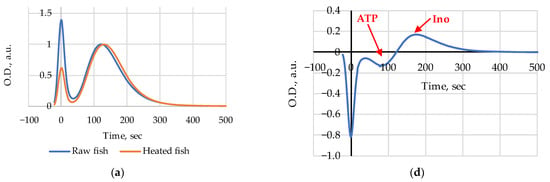
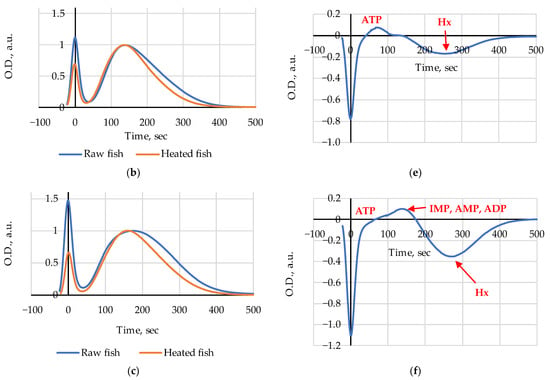
Figure 2.
Chromatograms of heated (steamed) and raw Amur: (a) at Day 1, (b) at Day 5, (c) at Day 8; (d–f) difference chromatograms (Heated–Raw). Chromatograms (a–c) are normalized at maxima of broad elution bands.
NMR measurements were performed using the Bruker Avance III 700 NMR spectrometer equipped with a 5 mm Broadband Observe (BBO) probe. The 1H Larmor frequency was 700.08 MHz. The ¹H NMR spectra were measured at 298 K with solvent suppression using the noesypr1d pulse sequence. The acquisition time was 3.67 s; the recycle delay was set to 4.00 s. NMR solutions were prepared by adding approximately 20% of deuterium oxide D2O (purity: 99.92 atom% D) containing 0.05% (w/v) sodium 2,2,3,3-tetradeuterio-3-trimethylsilylpropanoate TSP-d4 (acquired from Sigma-Aldrich, product number: 450510-25ML, batch number: MKBS0535V) to the aqueous samples. All spectra were referenced to TSP-d4 (0 ppm). Corrections to phase and baseline were performed with the Bruker Topspin 4.1.4 (Bruker, Rheinstetten, Germany). The free induction decays (FIDs) were multiplied by a line-broadening function of 0.1 Hz prior to Fourier transformation.
As an additional express method for the qualitative estimation of ATP content in fish muscle tissue, the UltraSnap Surface ATP test and a luminometer from Hygiena, Camarillo, CA, USA [] were applied for the first time. The UltraSnap Surface swabs’ performance was verified with the model solutions of single nucleotides ATP, ADP, and AMP in the TRIS buffer. The concentrations of all solutions were 1 µM. The recorded signal intensities were approximately as follows ATP:ADP:AMP = 550:50:1. It was concluded that the UltraSnap Surface swabs are suitable for use in selective determination of ATP presence in biological fluids such as the extracts used in this study.
3. Results
The FPMLC chromatograms of liquid extracts prepared from raw and heated Amur samples on days 1, 5 and 8 of the 10 days storage period are presented in Figure 2a–c; the differences of the two chromatograms (Heated–Raw) as the change of optical density are presented in Figure 2d–f.
The index Time evolution for the raw and heated Amur during the 10 days’ storage period is presented in Figure 3. The index Time values for the heated (steamed) Amur were slightly higher than those for the raw samples on days 1 and 3 (Figure 2a and Figure 3). After day 5 (Figure 2b and Figure 3), the initial index Time value before heat treatment became higher than after steam cooking (Figure 2c and Figure 3).
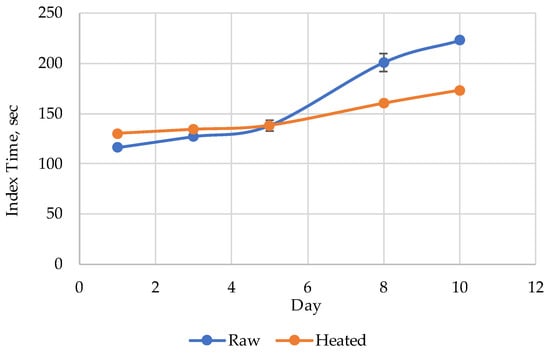
Figure 3.
The Time index evolution for raw and heated Amur during the 10 days’ storage period.
In the difference chromatograms (Heated–Raw), special attention should be given to the interval of elution times below 300 s which are characteristic for all nucleotides and nucleosides relevant for the current research []. The amplitude and the sign of peaks indicate heat-induced emergence or disappearance and changes in the relative concentrations of substances in fish extracts. Following this assumption, it is reasonable to conclude that a decrease in ATP and possibly ADP content (a negative peak at 80–100 s) and an increase in Ino (a positive peak at 180–200 s) are clearly discernible on day 1 (Figure 2d); on day 5 (Figure 2e) we see a slight increase in ATP and possibly ADP content (a positive peak at 80–100 s) and a relative decrease in the Hx level (a negative peak at 250–270 s); on day 8 (Figure 2f) an increase in ATP (a small positive peak at 90–100 s), IMP, ADP, and AMP (a wide positive peak at 120–170 s) and also a relative decrease in the Hx level (a negative peak at 250–270 s) are visible as the results of heating in water steam are noticeable.
The decrease in the index Time after heat treatment of Amur on day 10 (50 s) is the largest observed by us so far, indirectly indicating strong effects of nucleotide salvage. Nevertheless, to continue the experiments, we worked with pollock. Its rather unique characteristic was the complete absence of nucleotides in the initial raw state, which made it easy to detect even the slightest changes induced by heat. The difference of the two chromatograms (Boiled–Raw) for Alaska pollock samples heated in broth at various temperatures are presented in Figure 4.
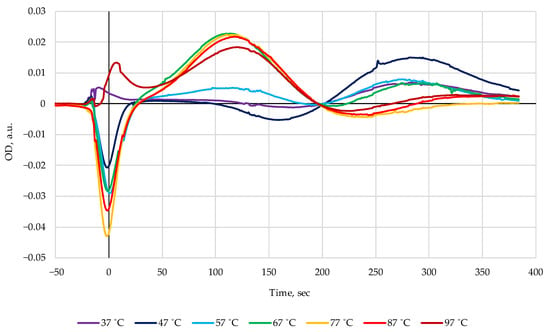
Figure 4.
The difference chromatograms (Boiled–Raw) of Alaska pollack heated in broth at different temperatures.
A clearly distinctive peak at 120 s with almost constant amplitude appeared consistently after heat treatment of pollock in the temperature interval 67–97 °C, while at lower temperatures of 37–57 °C the changes in the chromatograms were rather non-monotonous with a sharp threshold-like rise in the peak amplitude as a reaction to a small increase in the treatment temperature from 57 to 67 °C.
Further experiments were undertaken to clarify the identity of the maximum centered at 120 s and to explain its nonmonotonic behavior in the range 37–57 °C by the ATP bioluminescence assay. The ATP results in luminescence units (LU) together with the change of optical density values in Figure 4 are represented in Figure 5. One can see a highly correlated development (r = 0.999) of both characteristics in the range from 57 to 97 °C, pointing to the highly probable connection of the band 120 s with the ATP nucleotide. On the other hand, it can be seen from the data in [] and in Table 2 that the elution time of ATP in FPMLC is only 95 s. This means that other substances contribute to the absorption band at 120 s in Figure 4. The answer to this was obtained in the course of the NMR measurements.
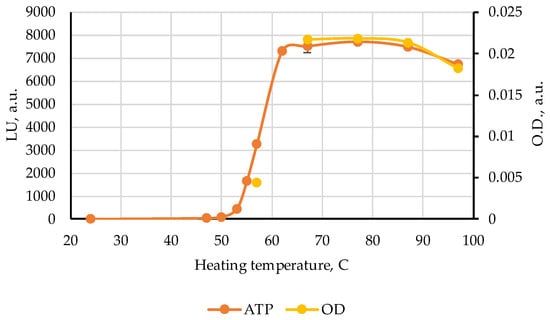
Figure 5.
The results of the ATP test (LU) and the amplitude of the characteristic peak at 120 s in the difference chromatograms (O.D.) of Alaska pollack samples heated in broth at different temperatures.
NMR spectra were recorded for the raw sample at room temperature and for the samples heated at various temperatures in the interval from 37 to 97 °C. In the samples heated at 67, 77, 87, and 97 °C the characteristic lines for the whole ANP triad (ATP, ADP, and AMP) were observed (see as an example Figure 6c), while in the samples heated at 37, 47, and 57 °C these lines were not present. Surprisingly, the lines of IMP were very weak in this measurement when in the spectra; when belonging to the samples heated in water steam or in a microwave oven, the line of IMP was relatively strong (Figure 6b).
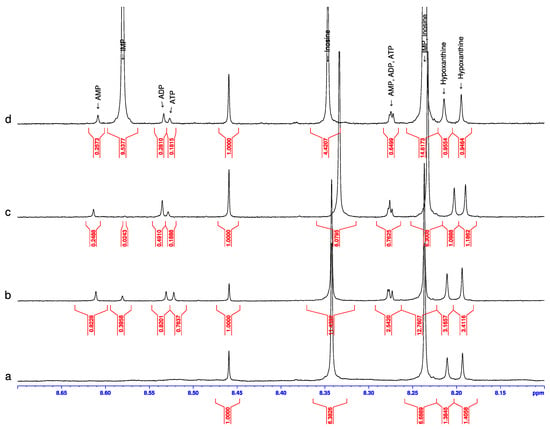
Figure 6.
1H NMR spectra for pollock samples: (a)—raw at 24 °C; (b)—after heating in microwave oven at 400 W for 1 min; (c)—after boiling in broth at 77 °C (there is a weak IMP signal at 8.58 ppm); (d)—the same as (c) with indication and concentration estimation as follows: AMP—0.036, ADP—0.039, ATP—0.025, inosine—0.614 and hypoxanthine—0.131 µmol/mL. The strong IMP signal in (d) is due to spiking with IMP, whereas the original weak signal in (c) corresponds to a concentration of 0.004 µmol/mL.
The NMR spectra of the analyzed thawed pollock did not show any lines of IMP, ATP, ADP, or AMP in the initial measurements at room temperatures (Figure 6a). The absence of ANP representatives serves as a relatively certain indication that this pollock was not frozen according to rapid shock technology when at least some representatives of ANP can survive. The absence of IMP probably points to a too prolonged pause between the catching and preparation of the fillets. Thus, the quality of these fillets was not high but, on the other hand, it was very convenient for us to observe the new emergence (renaissance) of nucleotides without interference from the nucleotides present before heating.
Three of the four modes used for pollock heat treatment (water steam, boiling in broth, and microwave irradiation) induced new NMR lines for all four nucleotides, IMP, AMP, ADP, and ATP. The dry heating in open air produced no effect (this peculiarity will be touched upon later on). Positive results usually gave NMR lines of different relative intensities with a trend towards a lower intensity of the ATP signal as can be seen in Figure 6c,d. Nevertheless, in the NMR spectrum of the pollock sample heated in a microwave oven at 400 W for 1 min, all three lines of the ANP are practically of equal intensity, pointing to the almost equal concentration of generated nucleotides. The equal intensities of lines in the family of AMDT nucleotides given by the relatively intense ATP signal are detected for the first time if we compare with [,,,].
The relatively stable presence of AMDT nucleotides in the heated extracts allows us to easily explain the properties of the band 120 s in the difference chromatograms in Figure 4. Evidently, this band is the evolution of the elution times of the three nucleotides AMP, ADP, and ATP, with some dominance of ADP (see Figure 6c) which has an elution time of 127 s.
The absolute concentrations of the newly appeared nucleotides have been estimated by the addition of AMP, ADP, and ATP solutions (spiking) to the broths heated at 77 °C (Table 3). This allowed us to compare the obtained values with those given in numerical or diagram form in [,,].

Table 3.
Content of AMDT nucleotides in animal meats and fish after heating in broth.
One can see that the values of concentrations in Table 3 are in a reasonable correlation, which is natural since the heat treatments applied were similar, i.e., boiling in broth. The concentrations of IMP cannot, however, be compared because of its initial presence in quite a high concentration in [,,].
4. Discussion
The heat-induced nucleotide salvage in aged fish revealed in this work, together with the results obtained earlier for beef and shrimp [,], make it possible to raise the question of introducing significant amendments to the existing approaches for quantitative assessment of meat and fish freshness (quality), based on the nucleotide–nucleoside indices K, Ki, etc. [,].
For example, the most widely used freshness index K is calculated as the ratio of ATP metabolites concentrations (usually presented in µmol/L) []:
The simplified index Ki was specifically designed to assess freshness after more than 24 h post-mortem, when almost all ATP, ADP and AMP are already catabolized:
As far as we are familiar with the situation in this area, it is automatically assumed that the value of very fresh meat or fish remains at its highest level for ready-to-eat food after preparation by means of heat treatment. No account is taken of the fact that as a result of heating there is a strong decrease in the IMP nucleotide content which is widely recognized as a valuable component in food.
As a matter of fact, in studies [,,,,], where very fresh animal meats or fish were used, a dramatic change in the IMP content after heating was observed (Table 1). Thus, it appears to be reasonably certain that an average of one third of the IMP is lost during heat treatment of both fresh animal and fish meat: these losses are large enough to be noteworthy. Moreover, the potential possibility of giant losses of up to two thirds of the IMP should also be taken into account [].
Such a large loss of IMP as the most valuable dietary nucleotide requires considerably more attention to be paid to the process of thermal action. Nevertheless, to the best of our knowledge there is only one paper [] reporting comprehensive research into IMP enzymatic decomposition during cooking at different temperatures. In this research, the authors came to the conclusion that IMP decomposition enzymes are inactive at temperatures above 64.1 °C. If this estimation is correct, the search for IMP salvage should be only effective at temperatures above 64 °C and should be ineffective at lower heating temperatures.
Nucleotide leakage can be a major contributor to IMP losses. It was shown in [,] that upon stepwise heating of pork samples the concentration of IMP in the inside of the sample decreased, while it increased on the surface [] and in the leaked juice []. In [] a very high anti-correlation between the values of [IMP] “in” and “out” with r = −0.99132 was observed.
The leaching process can also fluctuate, especially in the cases large samples. For instance, for the whole shrimps used in research [], the increase in heating time at 85 °C from 30 to 45 min induced an increase in IMP in broth ≈400% and by the next step 45→60 min the decrease of 41% occurred. High deviations were also observed for adenylates with the outliers of ATP and ADP close to zero by the averaged values of these adenylates of tens µmol/100 mL [].
Something like this evidently took place in our case with the IMP in the broth (Figure 6c). The study of the leaching details was beyond the scope of the present work. This investigation was organized in a binary mode “No” → “Yes” which usually gives the most convincing results.
We were able to obtain the lines of all the nucleotides ATP, ADP, AMP, and IMP in the NMR spectra of some pollock samples heated at 62 °C, but only with a very large number of scans. Therefore, for samples with heating temperature lower than 62 °C we did not perform the NMR measurements. On the other hand, the ultrasensitive ATP tests showed that meaningful content ATP can be generated in pollock heated to 55 °C (Figure 5). Additional studies are required to clarify more exactly the temperature interval for nucleotides to survive the enzyme activity in pollock and in other types of meat and fish as well.
It is possible to give this discussion one more quantitative dimension. In Table 4, we give the freshness index values before (Ki) and their change after heat treatment (ΔKi = Ki − K) for pollock (our data), minced beef [] and whole shrimp []. For pollock, the largest decrease in the Ki value was obtained by water steam heating for 40 min (−ΔKi = 0.189), but the most efficient method in the sense of energy consumption could be heating in a microwave oven (400 watts, 1 min). One can see that the changes in the freshness indices are rather high and can be essential in nutrition praxis.

Table 4.
The changes in values of indices Ki as a result of heating in pollock fish, beef and shrimp.
The effects of the emergence or strong increase in the concentration of nucleotides unveiled in [,,,,,] and in this research create three new circumstances.
Firstly, it is very reasonable to continue to study the dependence of the of nucleotide salvage phenomena on the storage time for various types of animal and fish meat, with particular attention centered on the period of a shelf-life edge. Because the concentrations of the resulting nucleotides are not very high in a “primitive” heating mode, it is necessary to look for the ways to additionally stimulate the generation of nucleotides to obtain higher concentrations.
Secondly, it is necessary to investigate the effect of all nucleotides on the taste and nutritional qualities of ready-to-eat foods. There is a firm acknowledgement that AMP has a synergetic role in enhancing umami flavor together with IMP [,,], but the role of ATP and ADP is usually not mentioned. Relatively rich data in [,,] show that the concentrations of emerged ATP and ADP in broth were much lower than that of AMP, so the effect on palatability was studied taking only IMP and AMP into consideration.
On the other hand, our NMR spectra of the pollock heated in a microwave oven demonstrate (Figure 6b) that the intensities of the lines in the triplet of ATP, ADP and AMP are close, pointing to equal concentrations of these nucleotides. Therefore, it is of particular importance not to ignore a priori the role of ATP and ADP in studies of the nutritional quality of processed foods.
Thirdly, the indices of freshness for ready-to-eat foods, if introduced by analogy to the existing indices for raw meats and fish, should be examined thoroughly in the pilot investigations. This idea is actually very close to the proposal of Chinese specialists to introduce into practice a nutritive nucleotide–nucleosides index []. Both suggestions correlate very well with the proposed umamification of food which facilitates the green transition []. The balanced and careful prolongation of the shelf life of meats/fish could be a direct measure to facilitate the green transition.
The analysis and establishment of the biochemical mechanisms of the nucleotide renaissance in animal meat [,,], shrimp [] and fish (this work) was beyond the scope of the research tasks. Nevertheless, the fact that we have found that dry heating is ineffective indicates that it is reasonable to analyze, first of all, the effect of autolytic hydrolysis on the cells of biological tissues. The solid experimental facts obtained by us on the manifestation of exothermic activation of nucleotide production and on the blocking of this production under dry conditions qualitatively coincide with characteristics of hydrolysis processes []. Hydrolysis of nucleic acids with the cleaving of the chains and “cutting out” of free nucleotides is very likely. However, it is necessary to show how nucleotides leach into the extracellular space and to establish whether the energy threshold at ≈60 °C is associated only with hydrolysis or if it is also modulated by changing of enzyme activity in this temperature region.
5. Conclusions and Perspectives
As a final conclusion, one can say that the generally accepted opinion that food nucleotides irretrievably disappear during the prolonged storage of meat and fish should be corrected. Nucleotide renaissance as the result of the inevitable heat treatment of main feedstocks such as animal meat, fish, and shrimps is a phenomenon which is typically present. Thanks to this recovery, the nutritive value of ready-to-eat food can be higher than it was evaluated as being before heating.
The matter has additional dimensions and three more concrete results have been obtained.
- The discovery of the renaissance of adenylate group nucleotides in fish in this work, together with the data on beef [] and shrimp [], allows one to conclude with great deal of certainty that this phenomenon is typical and exists in the main food sources: animal meat, fish and, maybe, all shellfish which have undergone conventional heat treatment.
- The fact that we used samples in their macroform for investigations, with no homogenization or protein removal having been carried out, brings this research work as close as possible to reality and enables us to project the results on practice at a 1:1 proportion.
- It is important for further investigations and practice that a triad of techniques has been successfully tested: the classic NMR, the relatively new and low-cost FPMLC as well as the widely known ATP test used in a new context. Such a combination of techniques makes it easy to verify and apply our results both in scientific laboratories equipped with heavy apparatus (NMR) and in industrial or veterinary laboratories and in the field (FPMLC, ATP test).
It is important to reveal the mechanisms of the new emergence of free nucleotides. This aspect is especially interesting in relation to the adenylate pool (ATP + ADP + AMP). It has been noted in the scientific literature that there was even a strong doubt and theological reasoning regarding the appearance of extracellular ATP formulated as “the cells should guard their intracellular ATP at all costs” []. Perhaps this idea and the difficulties encountered in observing ATP [] prevented researchers from appreciating the fact that the adenylate group can still be present in ready-to-eat food in micromolar concentrations, and therefore it is necessary to reconsider the widely-acknowledged system of indices of freshness based on nucleotides and nucleosides elaborated for raw food products a long time ago (see, e.g., [,]). Even micromolar concentrations, which are typical for ATP and ADP, may influence many biological processes in living organisms []. In a recent paper, the pair [ATP]/[ADP] × [Pi] is classified as a member of the “great” controlling nucleotide coenzymes []; thanks to the research reported in [,,,], these coenzymes could be appreciated even more.
Nowadays, the list of drugs exclusively based on ATP includes ten items [], something which indicates the recognition of the pharmaceutical applications of this nucleotide. In parallel, difficult but steady development is being made towards the use of ATP as a functional bioactive supplement. After inconclusive results with enteric-coated oral ATP [], progress has been achieved in oral uncoated ATP disodium supplementation [,]. The intake of an ATP dose of 400 mg/day was positive for increasing the abilities of athletes []. An interesting question arises as to how much food one has to eat to get a sufficient quantity of ATP.
If one ate meat or fish broth as an ATP-containing food (see Table 3), one would need to drink 32 L of liquid to get the required dose of ATP. Obviously, it would be necessary to concentrate the ATP content, which is quite a costly affair with an unclear result in terms of the taste of such a drink. Of course, one can use absolutely fresh meat or fish with a starting concentration of ATP of several µmol/g, but this is another specific situation.
In short, this small detail illustrates that there is a lot of interesting work ahead to find optimal solutions in increasing free adenylate content in ready-to eat-food. The concentration of adenylates in living cells is at a millimolar level []. Thus, the source of adenylates seems to be limitless, the task will be to utilize this opportunity.
Author Contributions
Conceptualization, A.K., G.K., P.R. and T.P.; methodology, A.K., A.S., G.K. and M.R.; validation, L.T., M.R. and D.L.; formal analysis, O.S.S.; investigation, A.S., L.T., O.V.S. and D.L.; resources, M.R. and A.F.; writing—original draft preparation, A.K.; writing—review and editing, G.K. and O.S.S.; visualization, O.V.S. and L.T.; supervision, A.K. and G.K.; project administration, A.F., M.R., P.R. and T.P.; funding acquisition, M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially financially supported by the Estonian Research Council grant (PRG 1441) of the Estonian University of Life Sciences (A.S., P.R., T.P., M.R.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request due to Ldiamon AS commercial secret.
Acknowledgments
The authors express their gratitude to Roman and Vadim Korsakov for valuable technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ding, T.; Song, G.; Liu, X.; Xu, M.; Li, Y. Nucleotides as optimal candidates for essential nutrients in living organisms: A review. J. Funct. Foods 2021, 82, 104498. [Google Scholar] [CrossRef]
- Mouritsen, O.G. Umamification of food facilitates the green transition. Soil Ecol. Lett. 2022, 5, 9. [Google Scholar] [CrossRef]
- Nishimura, T.; Ra Rhue, M.; Okitani, A.; Kato, H. Components Contributing to the Improvement of Meat Taste during Storage. Agric. Biol. Chem. 1988, 52, 2323–2330. [Google Scholar] [CrossRef]
- Macy, R.L.; Naumann, H.D.; Bailey, M.E. Water-Soluble Flavor and Odor Precursors of Meat. 3. Changes in Nucleotides, Total Nusleosides and Bases of Beef, Pork and Lamb During Heating. J. Food Sci. 1970, 35, 78–80. [Google Scholar] [CrossRef]
- Macy, R.L.; Naumann, H.D.; Bailey, M.E. Water-Soluble Flavor and Odor Precursors of Meat. 4. Influence of Cooking on Nucleosides and Bases of Beef Steaks and Roasts and Their Relationship to Flavor, Aroma and Juiciness. J. Food Sci. 1970, 35, 81–83. [Google Scholar] [CrossRef]
- Arya, S.S.; Parihar, D.B.; Vijayaraghavan, P.K. Changes in free nucleotides, nucleosides and bases during preparation of pre-cooked dehdyrated minced meats. Nahrung 1979, 23, 495–499. [Google Scholar] [CrossRef]
- Tikk, M.; Tikk, K.; Tørngren, M.A.; Meinert, L.; Aaslyng, M.D.; Karlsson, A.H.; Andersen, H.J. Development of Inosine Monophosphate and Its Degradation Products during Aging of Pork of Different Qualities in Relation to Basic Taste and Retronasal Flavor Perception of the Meat. J. Agric. Food Chem. 2006, 54, 7769–7777. [Google Scholar] [CrossRef]
- Madruga, M.S.; Elmore, J.S.; Oruna-Concha, M.J.; Balagiannis, D.; Mottram, D.S. Determination of some water-soluble aroma precursors in goat meat and their enrolment on flavour profile of goat meat. Food Chem. 2010, 123, 513–520. [Google Scholar] [CrossRef]
- Piskarev, A.; Dibrasulaev, M.; Korzhenko, V. Changes in the level of free amino acids and nucleotides in fresh-killed and aged meat sterilization. Myas. Ind. SSSR 1972, 2, 34–37. (In Russian) [Google Scholar]
- Rotola-Pukkila, M.K.; Pihlajaviita, S.T.; Kaimainen, M.T.; Hopia, A.I. Concentration of Umami Compounds in Pork Meat and Cooking Juice with Different Cooking Times and Temperatures. J. Food Sci. 2015, 80, C2711–C2716. [Google Scholar] [CrossRef]
- Hattula, T.; Kiesvaara, M. Breakdown Products of Adenosine Triphosphate in Heated Fishery Products as an Indicator of Raw Material Freshness and of Storage Quality. LWT-Food Sci. Technol. 1996, 29, 135–139. [Google Scholar] [CrossRef]
- Sasaki, K.; Motoyama, M.; Mitsumoto, M. Changes in the amounts of water-soluble umami-related substances in porcine longissimus and biceps femoris muscles during moist heat cooking. Meat Sci. 2007, 77, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Howgate, P. A review of the kinetics of degradation of inosine monophosphate in some species of fish during chilled storage. Int. J. Food Sci. Technol. 2006, 41, 341–353. [Google Scholar] [CrossRef]
- Vilas, C.; Alonso, A.A.; Herrera, J.R.; García-Blanco, A.; García, M.R. A model for the biochemical degradation of inosine monophosphate in hake (Merluccius merluccius). J. Food Eng. 2017, 200, 95–101. [Google Scholar] [CrossRef]
- Li, D.; Zhuang, S.; Peng, Y.; Tan, Y.; Hong, H.; Luo, Y. Mechanism of Inosine Monophosphate Degradation by Specific Spoilage Organism from Grass Carp in Fish Juice System. Foods 2022, 11, 2672. [Google Scholar] [CrossRef]
- Boziaris, I.S.; Parlapani, F.F. Specific Spoilage Organisms (SSOs) in Fish. In The Microbiological Quality of Food, 1st ed.; Bevilacqua, A., Corbo, M., Sinigaglia, M., Eds.; Elsevier: Cambridge, UK, 2017; pp. 61–98. ISBN 9780081005026. [Google Scholar]
- Li, J.; Zhou, G.; Xue, P.; Dong, X.; Xia, Y.; Regenstein, J.; Du, M.; Sun, L. Spoilage microbes’ effect on freshness and IMP degradation in sturgeon fillets during chilled storage. Food Biosci. 2021, 41, 101008. [Google Scholar] [CrossRef]
- Cambero, M.I.; Pereira-Lima, C.I.; Ordoez, J.A.; Garca de Fernando, G.D. Beef broth flavour: Relation of components with the flavour developed at different cooking temperatures. J. Sci. Food Agric. 2000, 80, 1519–1528. [Google Scholar] [CrossRef]
- Cambero, M.I.; Seuss, I.; Honikel, K.O. Flavor Compounds of Beef Broth as Affected by Cooking Temperature. J. Food Sci. 1992, 57, 1285–1290. [Google Scholar] [CrossRef]
- Cambero, M.I.; Jaramillo, C.J.; Ordoñez, J.A.; Cobos, A.; Pereira-Lima, C.I.; García de Fernando, G.D. Effect of cooking conditions on the flavour compounds and composition of shrimp (Parapenaeus longirostris) broth. Z. Lebensm. Forsch. A 1998, 206, 311–322. [Google Scholar] [CrossRef]
- Kuznetsov, A.; Frorip, A.; Sünter, A.; Kasvand, N.; Korsakov, V.; Konoplev, G.; Stepanova, O.; Rusalepp, L.; Anton, D.; Püssa, T.; et al. Fast Protein and Metabolites (Nucleotides and Nucleosides) Liquid Chromatography Technique and Chemical Sensor for the Assessment of Fish and Meat Freshness. Chemosensors 2023, 11, 69. [Google Scholar] [CrossRef]
- Servetnik, G.E. White Amur—Perspective Object for Reservoirs of Agricultural Purpose. Vestn. Rus. Agric. Sci. 2016, 2, 59–61. (In Russian) [Google Scholar]
- Gui, J.-F.; Tang, Q.; Li, Z.; Liu, J.; de Silva, S.S. Aquaculture in China: Success Stories and Modern Trends, 1st ed.; John Wiley & Sons Ltd.: Chichester, UK, 2018; 677p, ISBN 978-1-119-12076-6. [Google Scholar]
- Food and Agriculture Organization of the United Nations. State of World Fisheries and Aquaculture. 2010. Available online: https://www.fao.org/3/i1820e/i1820e.pdf (accessed on 18 April 2023).
- Singapore Statement on Research Integrity. Available online: https://www.wcrif.org/downloads/main-website/singapore-statements/223-singpore-statement-a4size (accessed on 18 April 2023).
- Montreal Statement on Research Integrity in Cross-Boundary Research Collaborations. Available online: https://www.wcrif.org/downloads/main-website/montreal-statement/123-montreal-statement-english (accessed on 18 April 2023).
- GE Healthcare. Instructions 52-1308-00 BB. Available online: http://wwwuser.gwdg.de/~jgrossh/protocols/protein-purification/PD10.pdf (accessed on 18 April 2023).
- Hong, H.; Regenstein, J.M.; Luo, Y. The importance of ATP-related compounds for the freshness and flavor of post-mortem fish and shellfish muscle: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1787–1798. [Google Scholar] [CrossRef]
- UltraSnap Surface ATP Test—Hygiena. Available online: https://www.hygiena.com/aiovg_videos/ultrasnap-surface-atp-test (accessed on 10 April 2023).
- Zhang, Z.; Sun, Y.; Sang, S.; Jia, L.; Ou, C. Emerging Approach for Fish Freshness Evaluation: Principle, Application and Challenges. Foods 2022, 11, 1897. [Google Scholar] [CrossRef] [PubMed]
- Ishiwatari, N.; Fukuoka, M.; Hamada-Sato, N.; Sakai, N. Decomposition kinetics of umami component during meat cooking. J. Food Eng. 2013, 119, 324–331. [Google Scholar] [CrossRef]
- Greiner, J.V.; Glonek, T. Intracellular ATP Concentration and Implication for Cellular Evolution. Biology 2021, 10, 1166. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Ismail, I.; Joo, S.-T. Identification of Umami Taste in Sous-Vide Beef by Chemical Analyses, Equivalent Umami Concentration, and Electronic Tongue System. Foods 2020, 9, 251. [Google Scholar] [CrossRef]
- Sarower, M.G.; Hasanuzzaman, A.F.M.D.; Biswas, B.; Abe, H. Taste producing components in fish and fisheries products: A review. J. Food. Ferment. Technol. 2012, 2, 113–121. [Google Scholar]
- Hou, C.; Xiao, G.; Amakye, W.K.; Sun, J.; Xu, Z.; Ren, J. Guidelines for purine extraction and determination in foods. Food Front. 2021, 2, 557–573. [Google Scholar] [CrossRef]
- Gordon, J.L. Extracellular ATP: Effects, sources and fate. Biochem. J. 1986, 233, 309–319. [Google Scholar] [CrossRef]
- Veech, R.L.; Todd King, M.; Pawlosky, R.; Kashiwaya, Y.; Bradshaw, P.C.; Curtis, W. The “great” controlling nucleotide coenzymes. IUBMB Life 2019, 71, 565–579. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 238, Triphosadenine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Triphosadenine#section=Synonyms (accessed on 18 April 2023).
- Arts, I.C.; Coolen, E.J.; Bours, M.J.; Huyghebaert, N.; Stuart, M.A.C.; Bast, A.; Dagnelie, P.C. Adenosine 5′-triphosphate (ATP) supplements are not orally bioavailable: A randomized, placebo-controlled cross-over trial in healthy humans. J. Int. Soc. Sport. Nutr. 2012, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Joy, J.M.; Lowery, R.P.; Roberts, M.D.; Lockwood, C.M.; Manninen, A.H.; Fuller, J.C.; De Souza, E.O.; Baier, S.M.; Wilson, S.M.; et al. Effects of oral adenosine-5′-triphosphate supplementation on athletic performance, skeletal muscle hypertrophy and recovery in resistance-trained men. Nutr. Metab. 2013, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Purpura, M.; Rathmacher, J.A.; Fuller, J.C.; Pitchford, L.M.; Rossi, F.E.; Kerksick, C.M. Health and ergogenic potential of oral adenosine-5′-triphosphate (ATP) supplementation. J. Funct. Foods 2021, 78, 104357. [Google Scholar] [CrossRef]
- Purpura, M.; Rathmacher, J.A.; Sharp, M.H.; Lowery, R.P.; Shields, K.A.; Partl, J.M.; Wilson, J.M.; Jäger, R. Oral Adenosine-5′-triphosphate (ATP) Administration Increases Postexercise ATP Levels, Muscle Excitability, and Athletic Performance Following a Repeated Sprint Bout. J. Am. Coll. Nutr. 2017, 36, 177–183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).