Abstract
To deal with climate emergency and reduce environmental impact, agro-industrial wastes are gradually gaining interest and are being used for new products and applications. The large production of watermelons represents an opportunity because of the many byproducts that can be transformed into innovative and valuable foodstuffs. In this study, we examined the lycopene-rich whole dietary fiber (WDF) obtained from the watermelon pomace of a peculiar cultivar, Gavina® (Oristano, Italy) a seedless fruit from Sardinia (Italy). The volatile chemical composition of the WDF was investigated using Solid-Phase Microextraction-Gas Chromatography/Mass Spectrometry (SPME-GC/MS). The aim was to follow the evolution of the Volatile Organic Compounds (VOCs) fraction during storage and verify its stability over time. Since watermelon is an excellent source of carotenoids, their byproducts were the most abundant VOCs of the freshly prepared samples, but their overall abundance decreased significantly during storage. The opposite trend was observed for acids and aldehydes, whose increase over time is related to amino acid degradation. Freshly prepared WDF can be used in the food industry as an antioxidant-rich dietary fiber that imparts a characteristic and pleasant aroma. Over time, its aroma profile and carotenoid content change considerably, reducing its health properties and limiting its potential application as a natural flavor.
1. Introduction
The analysis of the volatile fraction of food is essential for understanding, planning, and improving the sensory characteristics of new products [1]. The aroma of fruits and vegetables strictly depends on their chemical composition, as well as on any possible constitutive variation [2,3]. Furthermore, it is determined by unique combinations of Volatile Organic Compounds (VOCs) with different physicochemical properties and perceptual thresholds [4]. These VOCs confer specific quality characteristics and represent valuable and interesting sensory parameters, particularly for the food industry.
The aroma of a food product is directly related to its acceptability by consumers, nutritional and qualitative characteristics, health, and safety [3]. The measurement and identification of VOCs, in the specific case of fruits, allow to highlight any differences between different cultivars of the same species [5]. These variations are linked to geographical origin, cultivation site, production methods, harvesting technologies, degree of ripeness, and aging [2,3,6].
Monitoring VOCs over time allows the achievement of different goals. For example, it is possible to establish authenticity and traceability of food products. These controls are more effective when targeting specific molecules that act as unique quality markers. Some analytes may develop over time, which reflects the age of the product. In addition, they could result from the manipulations to which the material is subjected (i.e., prolonged thermal stress, sterilization, cooking, drying, etc.). These studies are important because, among the countless VOCs, some are not particularly desirable because they impart unpleasant aromas (the “off-flavors”), compromising the acceptability of the product by the final consumers [7,8,9,10].
The VOC fraction also persists in the dietary fiber (DF) that can be obtained from fruits or vegetables. Consumption of DF in human nutrition plays a functional role in maintaining good health. Therefore, nutritionists have largely encouraged an increase in its consumption [11,12] as the modern diet of Western countries is particularly devoid of it. In addition, animals also benefit from a high-fiber diet [13].
According to the Food and Agriculture Organization of the United Nations (FAO), one of the Sustainable Development Goals is the transformation and conversion of poor and waste materials into value-added resources. A significant amount of food is wasted directly in the field, and approximately one-third of the agri-products for food supply are wasted [14]. The recovery of these materials allows their conversion into new opportunities to obtain functional foods. In fact, agri-food waste is often rich in nutrients such as dietary fibers, polyphenols, vitamins, and minerals [15]. The recovery of this edible waste perfectly follows the circular economy principles, aimed at achieving a “zero-waste society” [16]. In addition, it can help mitigate the low nutrient intake that is typical of modern diets.
Pursuing our previous works regarding the recovery of dietary fiber from agri-food waste [5,17,18], in this study, we obtained and examined the whole dietary fiber (WDF) from watermelon pomace. Pomace is the pulp residue remaining after the fruit has been crushed to extract its juice.
Watermelon (Citrullus Lanatus L.), belonging to the Cucurbitaceae family, is widely consumed and is the second most common fruit crop worldwide [19]. According to statistics from the Food and Agriculture Organization, global watermelon production reached approximately 104 million tons in 2019 [20]. The Italian Institute of Statistics (ISTAT) estimated that 8.5% of the total production was not harvested during the 2020 cultivation cycle, corresponding to approximately 55 Ktons/year [21]. These data are impressive considering the worldwide production of watermelons. Sorting operations carried out during the cultivation cycle generate food loss, such as fruits with defects caused by hail, those with spots or malformations, or those that are over-ripened; these fruits are left on the cultivation field at the end of the growing season. It is necessary to add the amount of fruit discarded or unsold by retailers and the by-products derived from industrial processing to these quantities.
Therefore, we recovered and enhanced the pomace of pre-waste watermelons, obtaining a DF rich in bioactive components such as polyphenols, carotenoids, and vitamins, among other species that exert powerful antioxidant and anti-inflammatory effects [22]. Many studies have shown that the volatile fraction of watermelon originates from a combination of different classes of molecules, including esters, alcohols, organic acids, aldehydes, ketones, heterocyclic compounds, terpenoids, and volatile apocarotenoids [4,6,23]. The great variety of molecules that define watermelon aroma is the result of different biosynthetic or degradative metabolic pathways involving lipids, amino acids, and carotenoids, giving rise to a rich and complex VOCs population [3].
In this study, we analyzed the VOCs of a set of WDF samples by applying HS-SPME-GC-MS technique. The goal was to identify VOCs that could represent quality markers of the fiber and evaluate their stability over time. For this research, we chose the pre-waste watermelons of a particularly valuable cultivar, Gavina®, in Italy covered by the trademark. To our knowledge, there is little evidence in literature regarding this uncommon and extremely peculiar watermelon cultivar. The analysis was performed immediately after obtaining the fiber (WDF_t0), and subsequently, the differences following storage were evaluated. We considered two aging steps: 30 days (WDF_t1) and 24 months (WDF_t2) of storage. The compositional stability and resistance of watermelon fibers to the aging processes can be evaluated both macroscopically, i.e., following the temporal evolution of the color point [24], and microscopically, through the variation of the VOCs fraction. It is well known that when β-carotene and lycopene break down, many different volatiles are released, indicating the loss of these valuable bioactive components [4,25]. Furthermore, the degradation of these carotenoids, which are natural chromophores, involves variations in the color of the food containing them. The loss of these antioxidant nutrients leads to a decrease in the nutritional value of the WDF, on which the possible technological applications depend. The content of lycopene and β-carotene, which are beneficial to human health, can significantly determine their specific use in various production sectors, such as food. Moreover, the aroma of a product is particularly important in defining its possible applications, especially in the food and flavoring sectors. Therefore, this study aimed to obtain preliminary information to evaluate the possible technological applications of the sample according to its storage time.
2. Materials and Methods
2.1. Samples Preparation
Five Gavina® watermelons produced in the Sardinia region (Italy), were purchased from different local markets in Modena. This choice allowed us to achieve a greater representation of the cultivation areas. The fresh fruits were washed with distilled water. Approximately 300 g of the edible fraction of each fruit was separated, weighed, and homogenized using a kitchen mixer. Paper filtration was performed to separate the juice from the crude fiber. A known weight of the wet sample was then vacuum freeze dried using a Christ Alpha freeze-dryer (Christ Alpha 1-2 LDPlus, Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany), to obtain the whole dietary fiber (WDF). Immediately after drying, the resulting fibers were homogenized in a grinding mill equipped with a rotor made of Ti and a sieve of 500 μm, maintaining the equipment at a low temperature with a few drops of liquid N2. This strategy was adopted to preserve the aroma profile of the fibers as much as possible. One set of samples was prepared for each aging step, with each containing three replicates. VOCs analysis was carried out on samples that were freshly prepared (WDF_t0), samples after 30 days of storage (WDF_t1), and samples after 24 months of storage (WDF_t2). For each sample, about 0.5 g of Gavina® WDF was transferred into 10 mL glass vials, which were sealed tightly with Teflon/silicone septa. The samples were stored in the dark at room temperature and in the same closed vials during the aging period. The 1-month and 24-month aging steps were chosen to evaluate the flavor profile variations relating to short- and long-term storage, respectively.
2.2. Volatile Organic Compounds Sampling: HS-SPME
All samples were sonicated for 30 min in a thermostated bath at 40.0 ± 0.1 °C to facilitate the VOCs transfer to the vial headspace (HS). VOCs sampling was performed by exposing a CW/DVB/PDMSO Solid Phase Micro-Extraction (SPME) fiber (Supelco, Bellefonte, PA, USA) to the HS for 15 min. The SPME holder (Supelco Inc., Bellefonte, PA, USA) was used to manually perform the analysis. The SPME fiber was then inserted into the injector of the GC-MS system to desorb the analytes at 250 °C for 15 min. The SPME fiber being composed of three materials with different polarity allows to sample analytes of different chemical properties and molecular size.
To obtain good reproducibility of the experimental procedures, the analyses were performed on at least three replicate samples of the same matrix. Blank tests were performed by analyzing a standard solution of 1-decanol (conc. 150 µg/g ethanolic solution) after five chromatographic runs of real samples.
2.3. GC-MS Analysis
GC-MS analysis was performed using an Agilent 6890N Network gas chromatography system coupled with a 5973N mass spectrometer (Agilent Technologies, CA, USA). A DB-5MS UI column (60 m × 0.25 mm i.d., 1.00 μm film thickness; J&W Scientific, Folsom, CA, USA) was used for chromatographic separation. SPME injections were performed in splitless mode. The carrier gas (He) was fluxed at a constant flow rate of 1 mL/min with a column head pressure of 15 psi. The initial oven temperature was 40 °C (held for 5 min), followed by a heating ramp set at 10 °C/min up to 160 °C, and then at 8 °C/min to reach a final temperature of 270 °C, held for 5 min. The transfer line was heated to 270 °C. The mass spectrometer was operated in the electron impact (EI) ionization mode at 70 eV in the full scan acquisition mode, with a m/z scanning range from 25 to 300.
Chromatograms and mass spectra were analyzed using Enhanced ChemStation software (Agilent Technologies, Santa Clara, CA, USA). The volatiles were tentatively identified by matching the mass spectra with the data system library (NIST14/NIST05/WILEY275/NBS75K) and using Web databases, such as the National Institute for Standards and Technology (NIST database https://webbook.nist.gov, accessed on 15 November 2022) and Mass Bank of North America (https://mona.fiehnlab.ucdavis.edu, accessed on 15 November 2022).
The Linear Retention Index (LRI) was used for an additional comparison between our data and those reported in the literature and in the NIST Standard Reference Database, considering only values referring to analyses performed under the same operating conditions (instrumental specifications and heating ramp). The LRI of the detected compounds were calculated from a standard solution of n-alkanes (C6, C9, C12, C14, and C16) analyzed following the same procedure used for the samples. It proved to be particularly useful for the distinction of E/Z isomers because these species produce mass spectra that are difficult to differentiate.
Finally, some analytes were identified by comparing their mass spectra with those of their respective pure standards (when available) and analyzed using HS-SPME-GC-MS under the same operating conditions used for the samples. The pure standard used are as follows: 2-methyl-1-butanol; 1-pentanol; 1-hexanol; benzyl alcohol; 2-methylbutanal; 3-methylbutanal; 2-methyl-2-butenal; methyl-hexanoate; 2-methylbutanoic acid; 3-methylbutanoic acid; and decanal.
VOCs related to the chromatographic column or to the sorbent fiber, such as silane and siloxane derivatives, were discarded in order to collect only the information related to our samples. The amount of each identified volatile is expressed as the Total Ion Current (TIC) peak area. The data are expressed as the mean of three replicates ± Standard Deviation (SD).
2.4. Chemicals and Reagents
The chemicals used during the study were the following: (i) 2-methyl-1-butanol; 1-pentanol; 1-hexanol; benzyl alcohol; 2-methylbutanal; 3-methylbutanal; 2-methyl-2-butenal; methyl-hexanoate; 2-methylbutanoic acid; and 3-methylbutanoic acid; all were obtained from Sigma–Aldrich, distributed by Merck KGaA, Darmstadt, Germany. (ii) decanal; n-hexane; nonane; dodecane; tetradecane; and hexadecane were obtained from Carlo Erba Reagents, Milano (Italy).
2.5. Statistical Analysis
Experimental data were compared by applying analysis of variance (one-way ANOVA), by running in the Matlab® 2020b environment (The Mathworks Inc., Natick, MA, USA). The level of significance was determined at p < 0.05 to see whether there are statistical differences between the mean values.
3. Results and Discussion
The Gavina® WDF samples were obtained by freeze-drying. Unlike oven drying, this technique allows the nutritional value and the volatile fraction of the matrix to be maintained more unaltered. Therefore, we believe that the WDF_t0 aroma and bioactive molecules are extremely similar to those of fresh Gavina® watermelon. The oven-drying process leads to the loss of volatile VOCs even at low temperatures. Moreover, thermal stress accelerates the degradation of unstable bioactive molecules, thereby reducing the functional properties of the matrix. The low water content (3.5%) after freeze-drying prevents the proliferation of molds and bacteria. In fact, even after two years of storage, Gavina® WDF was free of mold and unpleasant odors, as confirmed using the HS-SPME-GC-MS analysis.
Figure 1a–c shows the chromatograms obtained using HS-SPME-GC MS from a sample of Gavina® watermelon WDF: freshly prepared (t0) (Figure 1a), after 30 days (t1) (Figure 1b), and after 24 months (t2) (Figure 1c).
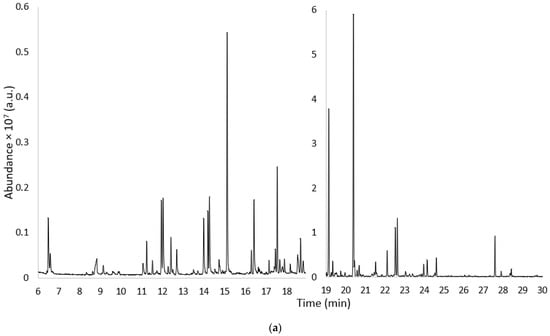
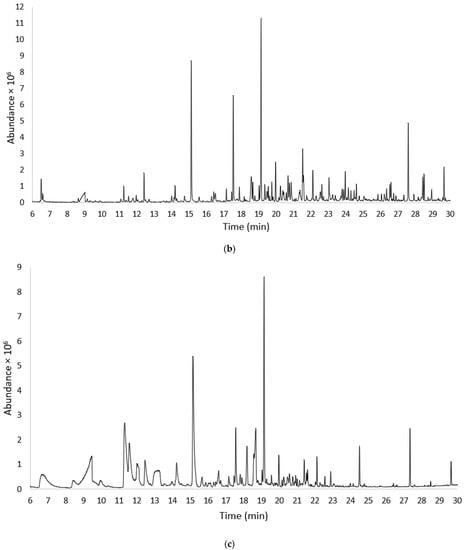
Figure 1.
Chromatograms obtained using HS-SPME-GC-MS from samples of Gavina® watermelon WDF; (a) freshly prepared (t0); (b) after 30 days of storage (t1); and (c) after 24 months of storage (t2).
The chromatogram profile (a) relating to the WDF_t0 sample was divided into two sections with scale expansion to better visualize the less intense peaks. Comparing the TIC scales of the three chromatograms in Figure 1, an overall decrease in analytical signals was observed with increasing sample storage time.
As shown in Figure 1a (WDF_t0), 102 compounds were detected, including 34 aldehydes (ALD), 15 alcohols (ALC), 1 ester (EST), 8 ketones (KET), 2 acids (ACD), 7 hydrocarbons (AHA), 25 terpenes and derivatives (TER), and 10 other compounds (OTH). After 30 days of aging (Figure 1b, WDF_t1), 88 compounds were identified, including 27 aldehydes, 14 alcohols, 2 esters, 10 ketones, 7 acids, 3 hydrocarbons, 16 terpenes and derivatives, and 9 other compounds. Finally, in the samples stored for 24 months (Figure 1c, WDF_t2), only 45 analytes were identified: 9 aldehydes, 7 alcohols, 2 esters, 2 ketones, 7 acids, 1 hydrocarbon, 10 terpenes and derivatives, and 6 other compounds. Figure 2 shows the trend of the eight molecular classes for each sample and provides a better representation of the data.
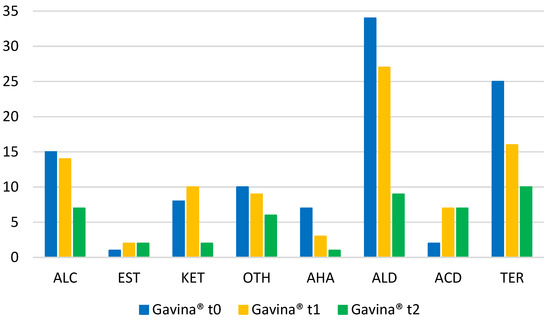
Figure 2.
Representation of the VOC number belonging to each molecular class of the Gavina® WDF samples at different aging times (ti).
The identified analytes are listed in the tables below and divided into their chemical classes.
3.1. Aldehydes
The number of aldehydes was the highest in the WDF_t0 sample, with 34 out of the 102 molecules detected being aldehydes, and was the second class in terms of total absolute abundance. During storage, the number of identified aldehydes decreased (27 and 9 for WDF_t1 and WDF_t2, respectively). The total abundance in terms of the TIC area decreased by 45% after 30 days of storage, from 1109 × 106 to 608.5 × 106, and subsequently increased to a value of 940.4 × 106. This increase is related to the degradation of more complex molecules, such as amino acids, fatty acids, and carotenoids, which release volatile aldehydes as byproducts. In contrast, the TIC area of more unstable aldehydes decreased over time, some of which eventually disappeared. They characterize the WDF_t0 aroma and are typical flavor compounds of the fresh fruit, i.e., Gavina® watermelon. They probably underwent degradation due to oxidative, biological, and photochemical processes, thus giving rise to other species, and leading to a change in the aroma profile of the sample. Table 1 collects the results relating to this molecular class.

Table 1.
Composition of the VOC fraction of Gavina® watermelon pomace WDF identified using HS-SPME-GC-MS analysis: aldehydes class.
C9 aldehydes characterize the flavor and aroma of different species of Cucurbitaceae [3,6,26,33,34,35] and have been previously reported in several studies on similar samples [5,29,30,33]. We identified 4-nonenal, nonanal, (Z,Z)-3,6-nonadienal, (Z)-2-nonenal, (E,E)-2,6-nonadiena€(E)-2-nonenal, and 2,4-nonadienal. They are among the species that mainly define the characteristic aroma of Gavina® watermelon, since they were particularly abundant in WDF_t0. The abundance of these species decreased sharply during storage, and only nonanal was detected after 24 months (WDF_t2). The most unstable and rapidly degrading species (4-nonenal, (Z)-2-nonadienal, 3,6-nonadienal, and (E,E)-2,6-nonadienal) were not detected after 30 days (WDF_t1). At the biological level, C9 aliphatic aldehydes are produced by the enzymatic oxidation of polyunsaturated fatty acids (PUFA), particularly linolenic and linoleic acids, by lipoxygenase [6,34]. Figures S1 and S2 show the degradation mechanisms of these fatty acids and the VOCs formation. Some enzymes such as alcohol dehydrogenase and cis/trans isomerase convert these compounds into their corresponding alcohols and cis/trans isomers, respectively [30].
(Z,Z)-3,6-nonadienal is often cited in the literature, but is not easily identified because it rapidly isomerizes to 2,6-nonadienal and 2-nonenal [31,34,36]. Some authors have defined it “watermelon aldehyde” [30], since it has a low odor threshold and a strong aroma of “freshly-cut watermelon”. Despite its instability, (Z,Z)-3,6-nonadienal was detected in WDF_t0. It is likely that the fresh fruit of this cultivar had a particularly strong aroma that persisted in WDF_t0. 2,6-nonadienal and 2-nonenal are also particularly unstable. They have a “green” and “waxy” aroma and are characteristic of cucumber flavor. A progressive decrease in their abundance occurred over time until complete disappearance after 24 months. Various mechanisms have been proposed to explain this decrease during storage, including hydration followed by condensation [36], oxidation to the corresponding acids, or enzymatic reduction [34].
In contrast, other aldehydes are considered to contribute to a lesser extent [26,30], but they enrich the aroma of WDF samples with pleasant fragrances. Hexanal, 2-hexenal, 4-heptenal, 2,4-hexadienal, 2-heptenal, 2,4-heptadienal, and 2,4-decadienal can be generated by the oxidation of polyunsaturated fatty acids and, to a lesser extent, by autoxidation [1,3,6,37]. These molecules, already detected in some watermelon cultivars, are particularly abundant in foods rich in fatty acids such as avocado, fish oil, and olive oil [5,37].
The C6 aldehydes hexanal and 2-hexenal play very important roles in the food and perfume industries, since they impart “green” and fresh fragrances, which are particularly appreciated and sought after [1]. Some authors [25] have suggested the possible formation of hexanal in small quantities due to lycopene degradation, a carotenoid abundant in watermelon fruit. This aldehyde reached an amount 2.5 times higher after 24 months (WDF_t2).
Similarly, 2-methyl-propanal, pentanal, 2-methyl-butanal, and 3-methyl-butanal levels increased over time. The origin of these last two compounds has been associated with the catabolism of the amino acid leucine [38] in a complex degradation process that involves some sugars, which are certainly present in the analyzed samples. The oxidation of 2-methyl-butanal and 3-methyl-butanal gave rise to volatile compounds present only in aged samples, such as 2-methyl butanoic and 3-methyl butanoic acids, species absent in WDF_t0, and present in increasing quantities after 30 days (WDF_t1) and 24 months (WDF_t2) of storage (Table 1). 2-methyl-propanal was also assumed to have the same origin [38], which could explain the significant increase in its concentration over time. These analytes impart a “green”, “fermented”, and “fresh” aroma.
The results of HS-SPME-GC-MS analysis showed a strong loss of the typical aroma of the fresh fruit, watermelon, during storage. The abundance of C9 aldehydes decreased significantly over time and was practically absent after 24 months. After 30 days, the “green” and “herbaceous” notes prevail with some “fruity” nuances, which disappear completely in WDF_t2, where the pleasant fragrance perceived is given only by the first two aromas.
3.2. Ketones
Ketones constitute only a restricted fraction of the headspace of the samples in terms of both the number of analytes identified and their abundance. Their number strongly decreased after 24 months (2 analytes against the initial 8), but the total abundance increased from WDF_t0 to WDF_t1 (30 days) samples and subsequently remained almost constant in WDF_t2 (24 months). All data related to ketones is shown in Table 2.

Table 2.
Composition of the VOC fraction of Gavina® watermelon pomace WDF identified using HS-SPME-GC-MS analysis: ketones class.
3,5-Octadien-2-one, 1-penten-3-one, and 2,3-pentanedione are involved in the degradation of polyunsaturated fatty acids (PUFAs) [37]. The abundances of 3,5-octadien-2-one and 1-penten-3-one increased after 30 days (WDF_t1) and then reset to zero after 24 months (WDF_t2). Each of them imparts specific notes that contribute to enriching the aroma profiles of WDF_t0 and WDF_t1.
In contrast, acetone was detected in higher quantities in WDF_t2 than in the previous aging steps. This was probably due to the degradation of complex molecules with different natures present in the initial matrix.
Acetoin has a pleasant yogurt smell, with buttery and creamy notes, and is used as a food additive to enhance the aroma of some preparations [40]. It is often detected together with its precursor species on HS-SPME-GC-MS analysis. Among these, 2,3-butanediol was identified in the WDF samples. They are molecules with similar biological and biosynthetic pathways, and they also showed a similar behavior over time, as they were detected only in WDF_t0 and WDF_t1.
3.3. Alcohols
In WDF_t0, alcohols accounted for 15 out of the 102 detected analytes, with an overall TIC area of 369.2 × 106. During storage, a reduction in their number was observed (14 after 30 days and 7 after 24 months), together with their absolute abundance (184.1 × 106 after 30 d, 173.6 × 106 after 24 months). The alcohol class did not define the aroma of Gavina® WDF samples predominantly, and this was true for all the three aging steps examined. The results for the alcohol class are shown in Table 3.

Table 3.
Composition of the VOC fraction of Gavina® watermelon pomace WDF identified using HS-SPME-GC-MS analysis: alcohols class.
C9 alcohols were not the most prevalent species in any of the aging steps. This observation is in contrast to that reported in the literature [2,6,18,23,29,32]. In fact, these species are considered particularly characteristic of the aroma of Cucurbitaceae fruits including watermelon by imparting “green, fresh, waxy, and cucumber or melon notes”. However, none of the cited studies examined the Gavina® variety. The different genotypes, growing areas, and cultivation methods could explain these differences in the flavor profiles. We identified only 2,6-nonadien-1-ol and 6-nonen-1-ol in WDF_t0.
The amount of 1-pentanol significantly increased from WDF_t1 to WDF_t2, and progressively imparted fermented notes. This compound mainly originates from the radical oxidation of linoleic acid and, to a lesser extent, from lycopene degradation [25]. Benzyl alcohol was the only aromatic alcohol detected after 30 days (WDF_t1) and, to a lesser extent, after 24 months (WDF_t2) of storage. It imparts sweet, floral, and fruity notes, and is associated with the degradation of phenylalanine.
3.4. Acids
Organic acids were present in extremely small quantities in WDF_t0, as the overall TIC area was only 33.5 × 106. They increased in both number and abundance in the aged samples, and were among the species that most characterized the aroma profile of WDF_t2. All data relating to the acid class are shown in Table 4.

Table 4.
Composition of the VOC fraction of Gavina® watermelon pomace WDF identified using HS-SPME-GC-MS analysis: acids class.
The amount of acetic acid progressively increased over time, probably because of the combined effects of degradation and oxidation of molecules of different kinds originally present in the initial matrix, such as sugars. The 3-methyl-butanoic and 2-methyl butanoic acids could reasonably originate from the oxidation of the respective aldehydes, 3-methyl-butanal, and 2-methyl-butanal [38]. In general, it can be assumed that all acids formed over time are derived from chemical and biochemical processes similar to those indicated for acetic acid. In conclusion, acids were among the predominant aromas formed during WDF aging, giving the sample a more pungent aroma.
3.5. Esters
Esters were not particularly abundant in the WDF samples, with an overall TIC area of 50.6 × 106 (WDF_t0), 142.0 × 106 (WDF_t1), and 98.3 × 106 at (WDF_t2), as shown in Table 5. Our results are in line with those reported in several studies on some seedless watermelon cultivars [4,6,23]. Unlike aldehydes, alcohols, and terpenes, the aroma of these varieties is not particularly characterized by esters. Ethylene synthesis in seedless watermelons is minimal, and this molecule is required for all enzymatic activities related to the production of volatile esters [23]. Ethylene is synthesized in higher quantities in seeded cultivars and in some melon varieties. This information suggests that genetic factors play a particularly important role in watermelon aroma.

Table 5.
Composition of the VOC fraction of Gavina® watermelon pomace WDF identified using HS-SPME-GC-MS analysis: ester class.
3.6. Hydrocarbons
The number of identified hydrocarbons was extremely small and progressively decreased from WDF_t0 to WDF_t2, as shown in Table 6. Their abundance was quite low, also because of their low vapor pressure due to their high molecular weight. Therefore, only a very small fraction passed into the headspace during the sampling phase.

Table 6.
Composition of the VOC fraction of Gavina® watermelon pomace WDF identified using HS-SPME-GC-MS analysis: hydrocarbon class.
3.7. Terpenes and Derivatives
Terpenes play a fundamental role in defining the aroma and fragrance of fruits, flowers, and spices [1,4,42,43]. They probably constitute one of the largest groups of compounds known in nature, with approximately 30,000 molecules identified to date, isolated from plants, microorganisms, and animals. Lighter species are important constituents of essential oils and plant resins, whereas heavier species include carotenoids. Similar to monoterpenes, carotenoids are biosynthesized starting from geranyl diphosphate (Figure S6), which, through further coupling of isoprene units, each catalyzed by specific enzymes, progressively forms derivatives with a lipophilic chain gradually longer, up to the tetraterpenes (C40). They typically have a 40-carbon atom chain backbone, and are among the most important pigments in fruits and flowers [4,44]. They show different colors, from the yellow of lutein, to the orange of β-carotene, to the red of lycopene, owing to their system of conjugated double bonds, on which their chemical, physical, and biological properties depend. The high delocalization of π electrons allows them to stabilize reactive intermediates, such as carbocations or radicals, by resonance. Therefore, they have efficient antioxidant properties and protect cells from oxidative stress induced by reactive species, such as free radicals. Foods containing carotenoids have a high antioxidant capacity [45,46], and their nutritional intake is strongly recommended, since it is associated with the prevention of several diseases, including cardiovascular diseases and cancers, and with increased immune response [4,44,46]. However, because of their high number of double bonds, carotenoids are particularly sensitive to degradation induced by heat or atmospheric oxygen, biological oxidation processes, and exposure to electromagnetic radiation, leading to the formation of characteristic volatile compounds. For this reason, many carotenoids have considerable commercial value in the food and cosmetic industries, not only as pigments but also as substances capable of imparting pleasant aromas to products [47]. Given the increase in demand for natural and healthy products and the problems related to the synthesis of these analytes, the flavor and fragrance industry is increasingly searching for new methods to obtain natural species to replace their synthetic counterparts. Among the emerging production methods, the use and transformation of matrices containing carotenoids are becoming increasingly invasive, as a natural alternative capable of supplying precious aroma volatiles [48,49].
The monitoring of carotenoid degradation compounds in commercial products can provide information on storage time, product quality and acceptability, nutritional value, vitamin and aroma content [48], color, and visual impact. Based on their detection, it is possible to draw conclusions regarding the presence of a given carotenoid. These observations applied to the watermelon WDF can be exploited to evaluate, in an approximate way, the variation of the carotenoid content during storage and to make considerations both on its nutritional value and on its aroma. All the results relating to the terpenes and derivative class are shown in Table 7.

Table 7.
Composition of the VOC fraction of Gavina® watermelon pomace WDF identified using HS-SPME-GC-MS analysis: terpenes and derivatives class.
Monoterpenes and monoterpenoids were identified in WDF_t0 only. After 30 days, these volatile analytes were not detected probably because they underwent degradation.
HS-SPME-GC-MS analysis highlighted the presence of limonene in an unexpected and positive way. This analyte is one of the characteristic constituents of the citrus family but has also been found in other fruits, including watermelon [6,10,26]. It is a particularly sought-after molecule, appreciated in the food, cosmetic, and soap industries, as it provides a very pleasant citrus and fresh fragrance. It was the most abundant species in WDF_t0, but it was not detected in any of the aged samples, probably because natural limonene is highly unstable and undergoes isomerization and autoxidation [51].
Another monoterpene is linalool (Figure S3), which is typically present in the essential oils of lavender, citrus, jasmine, and rosewood, to which it provides citrus and floral notes. This species undergoes epoxidation, followed by rearrangement, which leads to the formation of trans-linalool oxide. This explains the decrease of linalool and the increase of its oxidized product in the aged samples.
Β-Terpinene, γ-Terpinene, and α-Terpineol (Figure S4) are cyclic monoterpenes only present in WDF_t0, while isoterpinolene was detected only after 24 months of storage. We do not know whether this analyte is formed over time or if it was already present in WDF_t0 and had not been identified because of the high complexity of the chromatogram. Although present in modest quantities, they help enrich and complete the aroma of Gavina® WDF with floral, citrus, and woody notes.
Cymene was identified only in WDF_t0. It is an aromatic volatile compound that is very common in thyme and oregano essential oils but has been found in more than 100 plants and 200 fruits [1,50]. It performs various biological functions, and it also possesses anxiolytic, anticancer, and antimicrobial properties, as well as antiviral and antifungal activities [1].
Borneol (Figure S5) is a bicyclic molecule with a fresh and woody fragrance, which was detected in WDF_t0 and t1 in comparable quantities.
As shown in Figure 3, lycopene degradation gives rise to several VOCs, following various degradation pathways.
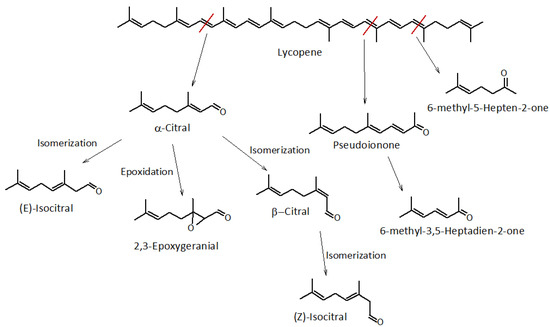
Figure 3.
Fragmentation scheme of lycopene and analytes of successive orders. The red line indicates the bond where the fragmentation of the molecule occurs.
With the term “citral”, or 3,7-Dimethyl-2,6-octadienal, we mean the cis- and trans- mixture of the neral and geranial acyclic monoterpenes, unsaturated aldehydes that impart a “citrus-like fresh and fruity” aroma. In nature, they are found mainly in citrus fruits and are exploited in the food and cosmetic industries because of their characteristic pleasant fragrances. However, these are unstable molecules that are sensitive to oxidation [25].
The highest concentrations of (Z)-3,7-Dimethyl-2,6-octadienal and (E)-3,7-Dimethyl-2,6-octadienal were detected in WDF_t0 (85.4 × 106 and 89.8 × 106 on a TIC basis, respectively). These quantities are reduced to approximately a quarter in WDF_t1 (21.6 × 106 and 24.1 × 106 for the (Z) and (E) isomer, respectively) and reduced to zero after 24 months of storage. In addition to watermelon, they are also detected in some cultivars of apples, tomatoes, and paprika [2,29], and more generally, in species containing high levels of lycopene or its precursors [4]. Their formation is probably due to the interaction and subsequent degradation of lycopene with atmospheric [2,25,29]. We also identified 2,3-epoxy-geranial, the epoxidation product of citral, previously detected in similar matrices by other authors [25]. It is formed by a mechanism that is easily established in contact with atmospheric oxygen. Furthermore, only in WDF_t0, the citral structural isomer is present, isocitral, in a cis-trans mixture.
The most abundant species associated with lycopene degradation was 6-methyl-5-hepten-2-one, which accounted for approximately 17% of the VOCs fraction of WDF_t0, with a TIC abundance of 810 × 106. It imparts a fruity, waxy, green fragrance with citrus notes, and its abundance tends to decrease strongly (approximately −70%) after 30 days and remains almost constant over time up to 24 months. Several authors have correlated its formation with the oxidation or degradation of lycopene, α-farnesene, citral, or conjugated trienols [2,23,29]. The presence of 6-methyl-5-hepten-2-one in non-negligible abundance in the freshly prepared sample highlights the high instability of lycopene, which underwent rapid degradation.
The species shown in Figure 4 are commonly related to β-carotene degradation [29,48] by breaking bonds in the different structural positions of the molecule. Among the degradation products, the most representative ones are:
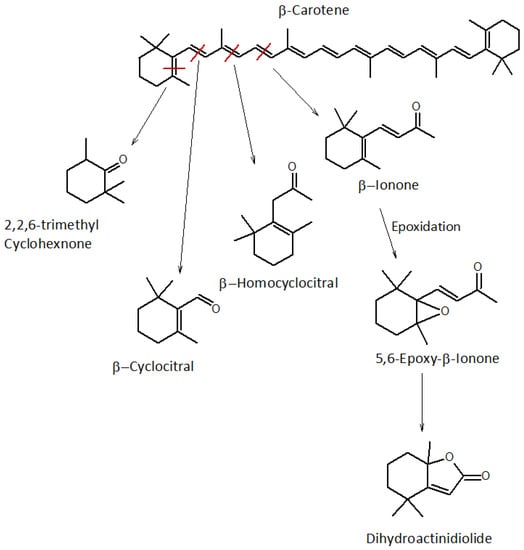
Figure 4.
Fragmentation scheme of β-carotene and analytes of successive orders. The red line indicates the bond where the fragmentation of the molecule occurs.
- β-ionone, produced following the breaking of the C9-C10 bond, is present in comparable quantities in WDF_t0 and WDF_t1 (35.8 × 106 and 31.2 × 106, respectively), while it is much less abundant after 24 months of storage (0.93 × 106). It is a molecule with a floral, slightly fruity, and pleasant aroma [44]; is widely used in all sectors of perfumery; and is used industrially for the synthesis of vitamin A [1]. In our samples, β-ionone was also identified in its epoxidized form (5,6-ionone epoxide), which reached a higher abundance in WDF_t1 (34.4 × 106). In this aging step, the quantities of β-ionone and 5,6-ionone epoxide were comparable. As previously reported for 2,3-epoxy-geranial, the epoxidation of these terpenes occurs quite easily in the presence of atmospheric oxygen. These two species were the most abundant among those involved in β-carotene degradation. Therefore, we can hypothesize that the C9-C10 bond is the most susceptible to breaking.
- Dihydroactinidiolide, whose formation mechanism is not well understood, is hypothesized to form starting from 5,6-ionone epoxide according to a radical oxidation mechanism [44,48] or following the oxidation of the C8-C9 bond. Like 5,6-ionone epoxide, dihydroactinidiolide also reaches its maximum quantity after 30 days of storage, and then halves after two years (44.7 × 106 and 22.0 × 106 for WDF_t1 and WDF_t2 samples, respectively), giving the aged fiber a fruity aroma with woody notes.
Although β-carotene is not the most abundant carotenoid in watermelon pulp, it contributes significantly to its aroma, as its degradation products have a particularly low odor threshold. Therefore, even if present in small quantities, they are easily perceived by humans sense of smell [4,48]. After 24 months of storage, the revealed the presence of some species considered secondary in the degradation process of β-carotene, such as 2,2,6-trimethylcyclohexanone and β-cyclocytral.
ζ-carotene is an acyclic, faint yellow carotenoid [4], a precursor of lycopene and β-carotene, structurally similar to lycopene, but with a lower number of unsaturations (Figure 5). Its presence has been hypothesized in several fruits that contain carotenoids, including watermelons and tomatoes.
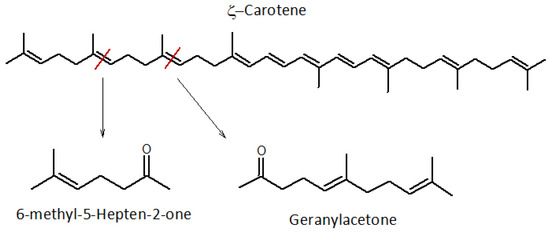
Figure 5.
Fragmentation scheme of the ζ-carotene molecule and higher order analytes. The red line indicates the bond where the fragmentation of the molecule occurs.
We assume that ζ-carotene is present in our samples because its main degradation product, geranylacetone, was detected at each aging step, albeit in progressively lower quantities over time (181 × 106, 104 × 106, and 2.36 × 106 for WDF_t0, WDF_t1, and WDF_t2, respectively). Geranylacetone is a volatile molecule with a floral and fruity aroma and a high odor threshold [39], lacking a distinctive effect on the overall fragrance of our samples. Furthermore, 6-methyl-5-hepten-2-one is derived from ζ-carotene, a ketone already described above in relation to lycopene, and has a high odor threshold [28]. As 6-methyl-5-hepten-2-one can result from the simultaneous degradation of two precursor analytes, the significant amount detected in the WDF samples could be justified.
The results of the HS-SPME-GC-MS analysis showed a strong loss of terpenes during storage. They provide the samples with a characteristic and pleasant aroma, and their decrease contributes to the loss of the typical fragrance of the initial fresh matrix. Carotenoids degradation VOCs were mainly identified in WDF_t0, while in the aged samples, their content is minimal. This leads to a significant change in the WDF aroma profile, considering that these VOCs impart pleasant nuances and have a high odor threshold. Moreover, the carotenoid content probably decreases drastically during storage. It follows that the beneficial properties associated with the carotenoid content of Gavina® WDF are also compromised.
3.8. Other Compounds
Table 8 shows some analytes of different nature, scarcely described in the literature as volatile aromas of Citrullus Lanatus. Among the identified molecules, there are some furans, a phenolic derivative (2,4-dimethyl phenol), and some more complex analytes with different functional groups, such as 3-hydroxy-2,2,4-trimethilpentyl ester of 2-methyl propanoic acid.

Table 8.
Composition of the VOC fraction of Gavina® watermelon pomace WDF identified using HS-SPME-GC-MS analysis: other compounds.
Some authors [1,32] associate 2-pentylfuran with the radical peroxidation of fatty acids, a mechanism already mentioned above. Another compound, 3,4-dimethyl-2,5-furandione, has been described as a bioactive molecule naturally produced by various plants, with efficient insecticidal and biofumigant activity [52]. This analyte was identified in modest quantities in the sample aged for 30 days and decreased by approximately 50% after 24 months of storage.
3.9. Summary Data
Table 9 shows all the results obtained through HS-SPME-GC-MS analysis. The data are expressed as the sum of the average values of the total ion current (TIC) of each analyte, classified according to the chemical class.

Table 9.
Compound classes identified in the HS-SPME-GC-MS analysis of the Gavina® water-melon pomace WDF: freshly prepared (t0), t1 (30 days), and t2 (24 months).
A one-way analysis of variance (ANOVA) was performed to assess whether there was a statistically significant difference between the quantities of each compound class among WDF_t0, WDF_t1, and WDF_t2 samples. The p-value for all the investigated compounds was smaller than the significant level (0.05). Therefore, we can conclude that, globally, the investigated WDF samples are statistically different.
3.10. Potential Applications of Gavina® Watermelon WDF
In addition to the environmental benefits of sustainable development, the enhancement of watermelon WDF is extremely interesting because of its numerous beneficial effects on human health. The lycopene and β-carotene contents, confirmed using HS-SPME-GC-MS analysis (Section 3.8), make the freshly prepared sample (WDF_t0) a source of antioxidants that protect against several health issues [4,44,47], as outlined in the previous sections. Moreover, the food industry is increasingly looking for dietary fiber sources, as they can physically and chemically modify food preparations and enhance specific sought-after properties [53,54]. Dietary fibers also have numerous health benefits, since their consumption is associated with the prevention of several diseases, such as cardiovascular disease, diabetes, and colon cancer [11,12,13,54]. In recent years, the consumer demand for foods enriched with natural supplements capable of conferring health benefits has increased significantly [53,54]. Consumers are more concerned about the consumption of healthy foods with a high content of dietary fibers, polyphenols, vitamins, and minerals, and low-calorie intake. Gavina® watermelon WDF not only fully satisfies this demand, but is also extremely low-cost, since it can be recovered from pre-waste fruits or from industrial processing that considers it a by-product. Therefore, this represents an opportunity for the food industry.
The products in which dietary fibers are mostly included are bakery goods [54,55,56,57], beverages, dairy products, frozen dairy products [58], pasta, meat [59], and soups. For example, several studies used fruit powder to partially replace traditional wheat flour to produce biscuits [55,57,60]. The final products had significantly higher antioxidant activity, mineral and fiber contents, and reduced starch content.
In several studies, the inclusion of fruit pomace powder led to a significant increase in sensory characteristics, i.e., aroma and flavor [58,61,62,63]. In fact, they impart fruity notes and improve consumer acceptability of certain food products, such as cakes, ice cream, beverages, and confectionery.
The high potential of watermelon WDF can also been extended to other sectors, such as animal feeds and nutraceuticals [62,64,65]. The use of fruit pomace for feeding can lead to significant changes in animal growth and health.
However, carotenoid degradation, highlighted using HS-SPME-GC-MS analysis, leads to a decrease in the antioxidant content of Gavina® watermelon WDF. Therefore, some of the beneficial effects were reduced over time, but those associated with dietary fiber remained unchanged. To maximize its benefits, it must be consumed within a short time after preparation. A possible solution may concern changing the storage method. In this study, WDF samples were stored at room temperature in the dark. Under these conditions, a significant loss of aromas related to carotenoids occurred after only 30 days of storage. Probably, refrigeration can increase the conservation of watermelon WDF for a longer time.
4. Conclusions
In this study, a dietary fiber was obtained from Gavina® watermelon pomace. This allows to deal with overproduction in the agri-food sector and contributes to minimize waste production.
The study of WDF_t0 aroma profile allowed to obtain important information regarding its chemical composition. The carotenoids content, especially lycopene and β-carotene, also present in the fresh fruit, has been confirmed by the detection of their volatile byproducts. These VOCs, together with C9 aldehydes, impart a characteristic and pleasant aroma to the fiber. Therefore, this raw material can find application in the food industry as an antioxidant-rich dietary fiber, as it preserves the bioactive molecules typical of watermelon. It can be used as an enriching ingredient in healthy food formulations, such as snacks or smoothies. The antioxidant power can also be exploited in the formulation of natural food supplements, thus finding use also in the pharmaceutical sector.
The study of the aroma profile during storage showed significant changes in the chemical composition of the Gavina® WDF over time. VOCs relating to carotenoids are already absent after 30 days of storage. This may mean that their precursors have completely oxidized. It follows that our sample loses part of its bioactive compounds over time. The aroma profile notes during storage become increasingly grassy, green, and pungent. Consequently, this aged WDF can find application as a simple dietary fiber. It lacks a peculiar aroma and antioxidant carotenoids such as lycopene and β-carotene.
In this study, storage was performed at room temperature and in the dark. It will be interesting to evaluate whether it will be possible to better preserve the bioactive molecules detected in the WDF_t0 by varying the storage conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/appliedchem3010006/s1, Figure S1: Proposed biosynthetic pathway for watermelon VOCs: linolenic acid degradation; Figure S2: Proposed biosynthetic pathway for watermelon VOCs: linoleic acid degradation; Figure S3: Epoxidation of linalool to linalool oxide; Figure S4: Structure of some cyclic monoterpenes identified in the WDF samples; Figure S5: Chemical structure of cymene (left) and borneol (right); Figure S6: Lycopene, a carotenoid biosynthesized starting from geranyl pyrophosphate.
Author Contributions
Conceptualization, L.M. and V.D.; methodology, L.M. and V.D.; software, V.D. and L.M.; validation, V.D., L.M. and L.T.; formal analysis, A.M., F.R. and L.T.; investigation, L.M. and V.D.; resources, A.M. and L.T.; data curation, L.M. and F.R.; writing—original draft preparation, L.T.; writing—review and editing, V.D., F.R. and A.M.; visualization, L.T., L.M. and V.D.; supervision, L.T., F.R. and A.M.; project administration, L.T. and F.R.; funding acquisition, L.T. and L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research program has been funded by MIUR, the Italian Ministry of Education, University and Research. L.M. gratefully acknowledges financial support from POR FSE 2014-20 of Emilia-Romagna region (Italy) for doctoral fellowship.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Berger, R.G. Flavours and Fragances: Chemistry, Bioprocessing and Sustainability; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-49338-9. [Google Scholar]
- Xisto, A.L.R.P.; Boas, E.V.d.B.V.; Nunes, E.E.; Federal, B.M.V.B.; Guerreiro, M.C. Volatile profile and physical, chemical, and biochemical changes in fresh cut watermelon during storage. Food Sci. Technol. 2012, 32, 173–178. [Google Scholar] [CrossRef]
- El Hadi, M.; Zhang, F.-J.; Wu, F.-F.; Zhou, C.-H.; Tao, J. Advances in Fruit Aroma Volatile Research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef] [PubMed]
- Lewinsohn, E.; Sitrit, Y.; Bar, E.; Azulay, Y.; Ibdah, M.; Meir, A.; Yosef, E.; Zamir, D.; Tadmor, Y. Not just colors—Carotenoid degradation as a link between pigmentation and aroma in tomato and watermelon fruit. Trends Food Sci. Technol. 2005, 16, 407–415. [Google Scholar] [CrossRef]
- Maletti, L.; D’Eusanio, V.; Durante, C.; Marchetti, A.; Tassi, L. VOCs Analysis of Three Different Cultivars of Watermelon (Citrullus lanatus L.) Whole Dietary Fiber. Molecules 2022, 27, 8747. [Google Scholar] [CrossRef] [PubMed]
- Dima, G.; Tripodi, G.; Condurso, C.; Verzera, A. Volatile constituents of mini-watermelon fruits. J. Essent. Oil Res. 2014, 26, 323–327. [Google Scholar] [CrossRef]
- Benzo, M.; Gilardoni, G.; Gandini, C.; Caccialanza, G.; Finzi, P.V.; Vidari, G.; Abdo, S.; Layedra, P. Determination of the threshold odor concentration of main odorants in essential oils using gas chromatography–olfactometry incremental dilution technique. J. Chromatogr. A 2007, 1150, 131–135. [Google Scholar] [CrossRef]
- Brattoli, M.; De Gennaro, G.; De Pinto, V.; Demarinis Loiotile, A.; Lovascio, S.; Penza, M. Odour Detection Methods: Olfactometry and Chemical Sensors. Sensors 2011, 11, 5290–5322. [Google Scholar] [CrossRef]
- Chambers, E.; Koppel, K. Associations of Volatile Compounds with Sensory Aroma and Flavor: The Complex Nature of Flavor. Molecules 2013, 18, 4887–4905. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y.; Wang, B.; Song, H.; Zou, T. Screening of the volatile compounds in fresh and thermally treated watermelon juice via headspace-gas chromatography-ion mobility spectrometry and comprehensive two-dimensional gas chromatography-olfactory-mass spectrometry analysis. LWT 2021, 137, 110478. [Google Scholar] [CrossRef]
- Dahl, W.J.; Stewart, M.L. Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber. J. Acad. Nutr. Diet. 2015, 115, 1861–1870. [Google Scholar] [CrossRef]
- Galanakis, C.M. Dietary Fiber: Properties, Recovery, and Applications; Academic Press: London, UK, 2019; ISBN 978-0-12-816495-2. [Google Scholar]
- Li, H.; Yin, J.; Tan, B.; Chen, J.; Zhang, H.; Li, Z.; Ma, X. Physiological function and application of dietary fiber in pig nutrition: A review. Anim. Nutr. 2021, 7, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.A.; Sanguansri, L.; Fox, E.M.; Cobiac, L.; Cole, M.B. Recovery of wasted fruit and vegetables for improving sustainable diets. Trends Food Sci. Technol. 2020, 95, 75–85. [Google Scholar] [CrossRef]
- Khattak, K.F.; Rahman, T.U. Analysis of vegetable’s peels as a natural source of vitamins and minerals. Int. Food Res. J. 2017, 24, 292–297. [Google Scholar]
- Hamam, M.; Chinnici, G.; Di Vita, G.; Pappalardo, G.; Pecorino, B.; Maesano, G.; D’Amico, M. Circular Economy Models in Agro-Food Systems: A Review. Sustainability 2021, 13, 3453. [Google Scholar] [CrossRef]
- Maletti, L.; D’Eusanio, V.; Lancellotti, L.; Marchetti, A.; Pincelli, L.; Strani, L.; Tassi, L. Candying process for enhancing pre-waste watermelon rinds to increase food sustainability. Future Foods 2022, 6, 100182. [Google Scholar] [CrossRef]
- Maletti, L.; D’Eusanio, V.; Durante, C.; Marchetti, A.; Pincelli, L.; Tassi, L. Comparative Analysis of VOCs from Winter Melon Pomace Fibers before and after Bleaching Treatment with H2O2. Molecules 2022, 27, 2336. [Google Scholar] [CrossRef]
- Méndez, D.A.; Fabra, M.J.; Gómez-Mascaraque, L.; López-Rubio, A.; Martinez-Abad, A. Modelling the Extraction of Pectin towards the Valorisation of Watermelon Rind Waste. Foods 2021, 10, 738. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data (accessed on 17 October 2022).
- Istat.it. Available online: https://www.istat.it/ (accessed on 20 November 2022).
- Lum, T.; Connolly, M.; Marx, A.; Beidler, J.; Hooshmand, S.; Kern, M.; Liu, C.; Hong, M. Effects of Fresh Watermelon Consumption on the Acute Satiety Response and Cardiometabolic Risk Factors in Overweight and Obese Adults. Nutrients 2019, 11, 595. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Lea, J.M. Characterization and Semiquantitative Analysis of Volatiles in Seedless Watermelon Varieties Using Solid-Phase Microextraction. J. Agric. Food Chem. 2006, 54, 7789–7793. [Google Scholar] [CrossRef]
- Arocho, Y.D.; Bellmer, D.; Maness, N.; McGlynn, W.; Rayas-Duarte, P. Watermelon Pomace Composition and the Effect of Drying and Storage on Lycopene Content and Color: Watermelon Pomace. J. Food Qual. 2012, 35, 331–340. [Google Scholar] [CrossRef]
- Kobori, C.N.; Wagner, R.; Padula, M.; Rodriguez-Amaya, D.B. Formation of volatile compounds from lycopene by autoxidation in a model system simulating dehydrated foods. Food Res. Int. 2014, 63, 49–54. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Grimm, C.C. Identification of Volatile Compounds in Cantaloupe at Various Developmental Stages Using Solid Phase Microextraction. J. Agric. Food Chem. 2001, 49, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Saftner, R.; Luo, Y.; McEvoy, J.; Abbott, J.A.; Vinyard, B. Quality characteristics of fresh-cut watermelon slices from non-treated and 1-methylcyclopropene- and/or ethylene-treated whole fruit. Postharvest Biol. Technol. 2007, 44, 71–79. [Google Scholar] [CrossRef]
- Cho, M.J.; Buescher, R. Degradation of cucumber flavor aldehydes in juice. Food Res. Int. 2011, 44, 2975–2977. [Google Scholar] [CrossRef]
- Liu, Y.; He, C.; Song, H. Comparison of fresh watermelon juice aroma characteristics of five varieties based on gas chromatography-olfactometry-mass spectrometry. Food Res. Int. 2018, 107, 119–129. [Google Scholar] [CrossRef]
- Genthner, E.R. Identification of Key Odorants in Fresh-Cut Watermelon Aroma and Structure-Odor Relationships of Cis,Cis-3,6-Nonadienal and Ester Analogs with Cis,Cis-3,6-Nonadiene, Cis-3-Nonene and Cis-6-Nonene Backbone Structures; University of Illinois at Urbana-Champaign: Urbana, IL, USA, 2010. [Google Scholar]
- Tripodi, G.; Condurso, C.; Cincotta, F.; Merlino, M.; Verzera, A. Aroma compounds in mini-watermelon fruits from different grafting combinations. J. Sci. Food Agric. 2020, 100, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Yajima, I.; Sakakibara, H.; Ide, J.; Yanai, T.; Kazuo, H. Volatile Flavor Components of Watermelon (Citrullus vulgaris). Agric. Biol. Chem. 1985, 49, 3145–3150. [Google Scholar] [CrossRef]
- Kemp, T.R.; Knavel, D.E.; Stoltz, L.P.; Lundin, R.E. 3,6-Nonadien-1-ol from Citrullus vulgaris and Cucumis melo. Phytochemistry 1974, 13, 1167–1170. [Google Scholar] [CrossRef]
- Hatanaka, A.; Kajiwara, T.; Harada, T. Biosynthetic pathway of cucumber alcohol: Trans-2,cis-6-nonadienol via cis-3,cis-6-nonadienal. Phytochemistry 1975, 14, 2589–2592. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Role of free radicals and catalytic metal ions in human disease: An overview. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1990; Volume 186, pp. 1–85. ISBN 978-0-12-182087-9. [Google Scholar]
- Josephson, D.B.; Lindsay, R.C. Retro-aldol degradations of unsaturated aldehydes: Role in the formation ofc4-heptenal fromt2,c6-nonadienal in fish, oyster and other flavors. J. Am. Oil Chem. Soc. 1987, 64, 132–138. [Google Scholar] [CrossRef]
- Hammer, M.; Schieberle, P. Model Studies on the Key Aroma Compounds Formed by an Oxidative Degradation of ω-3 Fatty Acids Initiated by either Copper (II) Ions or Lipoxygenase. J. Agric. Food Chem. 2013, 61, 10891–10900. [Google Scholar] [CrossRef] [PubMed]
- Smit, B.A.; Engels, W.J.M.; Smit, G. Branched chain aldehydes: Production and breakdown pathways and relevance for flavour in foods. Appl. Microbiol. Biotechnol. 2009, 81, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Fan, L.; Beaudry, R.M. Application of Solid Phase Microextraction and Gas Chromatography/Time-of-Flight Mass Spectrometry for Rapid Analysis of Flavor Volatiles in Tomato and Strawberry Fruits. J. Agric. Food Chem. 1998, 46, 3721–3726. [Google Scholar] [CrossRef]
- Xiao, Z.; Lu, J.R. Generation of Acetoin and Its Derivatives in Foods. J. Agric. Food Chem. 2014, 62, 6487–6497. [Google Scholar] [CrossRef]
- Leffingwell, J.C.; Alford, E.D.; Leffingwell, D. Identification of the Volatile Constituents of Raw Pumpkin (Cucurbita pepo L.) by Dynamic Headspace Analyses. Leffingwell Rep. 2015, 7, 293–301. [Google Scholar]
- Oldfield, E.; Lin, F.-Y. Terpene Biosynthesis: Modularity Rules. Angew. Chem. Int. Ed. 2012, 51, 1124–1137. [Google Scholar] [CrossRef]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural Products (Secondary Metabolites). Biochem. Mol. Biol. Plants 2000, 24, 1250–1319. [Google Scholar]
- Pénicaud, C.; Achir, N.; Dhuique-Mayer, C.; Dornier, M.; Bohuon, P. Degradation of β-carotene during fruit and vegetable processing or storage: Reaction mechanisms and kinetic aspects: A review. Fruits 2011, 66, 417–440. [Google Scholar] [CrossRef]
- Powell, Z.D.; Lakesha, C. Antioxidant capacity of lycopene-containing foods. Int. J. Food Sci. Nutr. 2001, 52, 143–149. [Google Scholar] [CrossRef]
- Gul, K.; Tak, A.; Singh, A.K.; Singh, P.; Yousuf, B.; Wani, A.A. Chemistry, encapsulation, and health benefits of β-carotene—A review. Cogent Food Agric. 2015, 1, 1018696. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Glória, M.B.A.; Grulke, E.A.; Gray, J.I. Effect of type of oxidation on beta-carotene loss and volatile products formation in model systems. Food Chem. 1993, 46, 401–406. [Google Scholar] [CrossRef]
- Bruno, A.; Durante, M.; Marrese, P.P.; Migoni, D.; Laus, M.N.; Pace, E.; Pastore, D.; Mita, G.; Piro, G.; Lenucci, M.S. Shades of red: Comparative study on supercritical CO 2 extraction of lycopene-rich oleoresins from gac, tomato and watermelon fruits and effect of the α-cyclodextrin clathrated extracts on cultured lung adenocarcinoma cells’ viability. J. Food Compos. Anal. 2018, 65, 23–32. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.; Barbieri, R.; Silva, A.; Nabavi, S.; Tsetegho Sokeng, A.; Izadi, M.; Jafari, N.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- Bernhard, R.A.; Marr, A.G. The Oxidation of Terpenes. I. Mechanism and Reaction Products of d-Limonene Autoxidation. J. Food Sci. 1960, 25, 517–530. [Google Scholar] [CrossRef]
- Rajashekar, Y.; Tonsing, N.; Shantibala, T.; Manjunath, J.R. 2, 3-Dimethylmaleic anhydride (3, 4-Dimethyl-2, 5-furandione): A plant derived insecticidal molecule from Colocasia esculenta var. esculenta (L.) Schott. Sci. Rep. 2016, 6, 20546. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Amezquita, L.E.; Tejada-Ortigoza, V.; Serna-Saldivar, S.O.; Welti-Chanes, J. Dietary Fiber Concentrates from Fruit and Vegetable By-products: Processing, Modification, and Application as Functional Ingredients. Food Bioprocess Technol. 2018, 11, 1439–1463. [Google Scholar] [CrossRef]
- Pop, C.; Suharoschi, R.; Pop, O.L. Dietary Fiber and Prebiotic Compounds in Fruits and Vegetables Food Waste. Sustainability 2021, 13, 7219. [Google Scholar] [CrossRef]
- Salehi, F. Recent applications of powdered fruits and vegetables as novel ingredients in biscuits: A review. Nutrire 2020, 45, 1. [Google Scholar] [CrossRef]
- Al-Sayed, H.M.A.; Ahmed, A.R. Utilization of watermelon rinds and sharlyn melon peels as a natural source of dietary fiber and antioxidants in cake. Ann. Agric. Sci. 2013, 58, 83–95. [Google Scholar] [CrossRef]
- Sangeeta, S.; Kalphana, K. Peach Juice and Pomace Powder; Nutritive Value and Use of Pomace Powder in Biscuits. Int. J. Food Sci. Technol. 2016, 6, 5–16. [Google Scholar]
- Shelke, G.; Kad, V.; Yenge, G.; Desai, S.; Kakde, S. Utilization of jamun pomace as functional ingredients to enhance the physico-chemical and sensory characteristics of ice cream. J. Food Process. Preserv. 2020, 44, e14736. [Google Scholar] [CrossRef]
- Ahmad, S.R.; Gokulakrishnan, P.; Giriprasad, R.; Yatoo, M.A. Fruit-based Natural Antioxidants in Meat and Meat Products: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1503–1513. [Google Scholar] [CrossRef]
- Parveen, H.; Bajpai, A.; Bhatia, S.; Singh, S. Analysis of Biscuits Enriched With Fibre by Incorporating Carrot and Beetroot Pomace Powder. IJND 2017, 54, 403. [Google Scholar] [CrossRef]
- Salehi, F.; Aghajanzadeh, S. Effect of dried fruits and vegetables powder on cakes quality: A review. Trends Food Sci. Technol. 2020, 95, 162–172. [Google Scholar] [CrossRef]
- Iqbal, A.; Schulz, P.; Rizvi, S.S.H. Valorization of bioactive compounds in fruit pomace from agro-fruit industries: Present Insights and future challenges. Food Biosci. 2021, 44, 101384. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Oracz, J.; Bilicka, M.; Kulbat-Warycha, K.; Klewicka, E. Influence of Freeze-Dried Phenolic-Rich Plant Powders on the Bioactive Compounds Profile, Antioxidant Activity and Aroma of Different Types of Chocolates. Molecules 2021, 26, 7058. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable valorisation of tomato pomace: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Manju Wadhwa, M.W.; Bakshi, M.P.S.; Makkar, H.P.S. Waste to worth: Fruit wastes and by-products as animal feed. CABI Rev. 2015, 2015, 1–26. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).