Synthesis of Graphene and Related Materials by Microwave-Excited Surface Wave Plasma CVD Methods
Abstract
1. Introduction
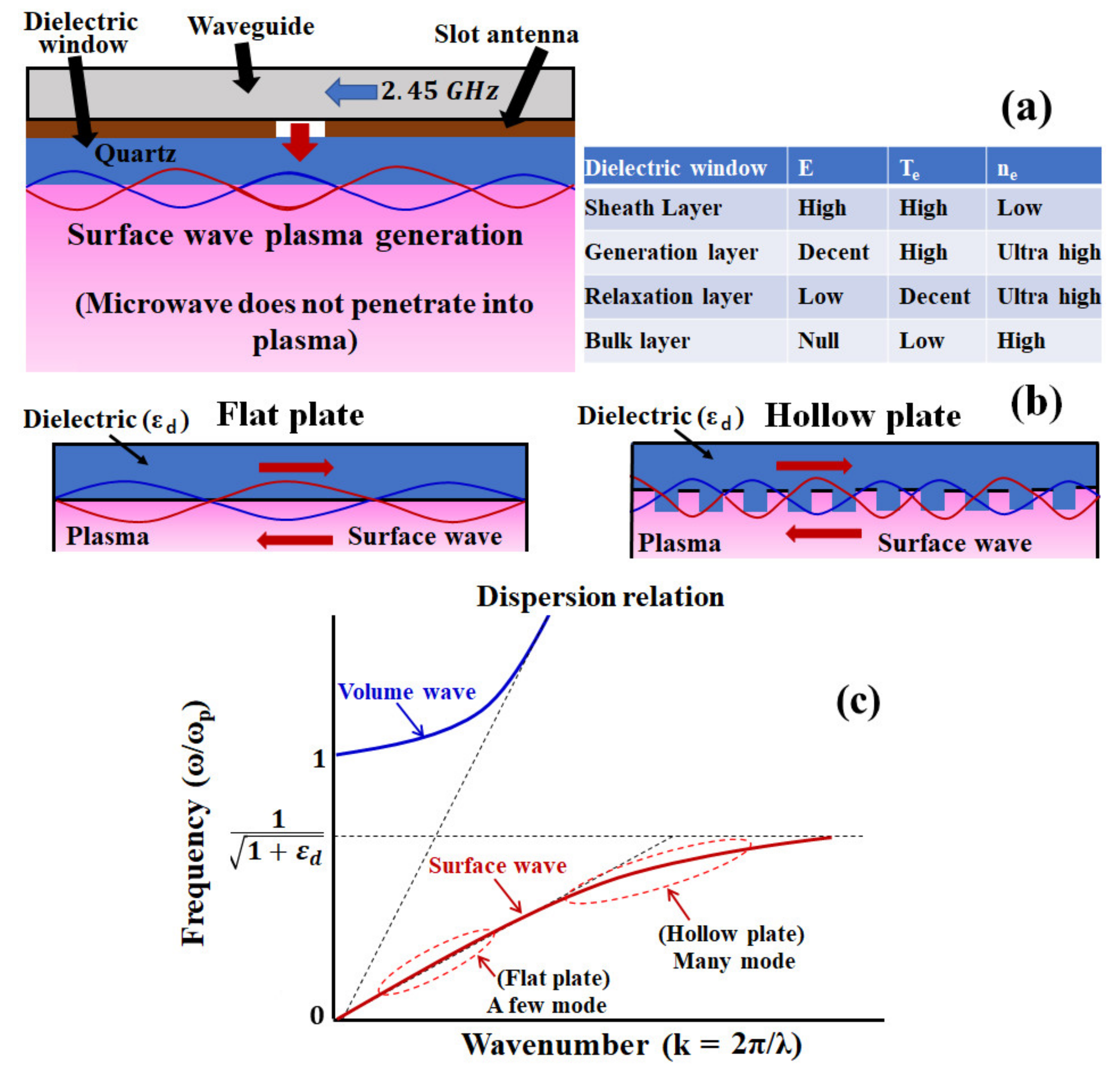
2. Microwave-Excited Surface Wave Plasma CVD (MW-SWP CVD)
3. Growth of Graphene and 2D Materials
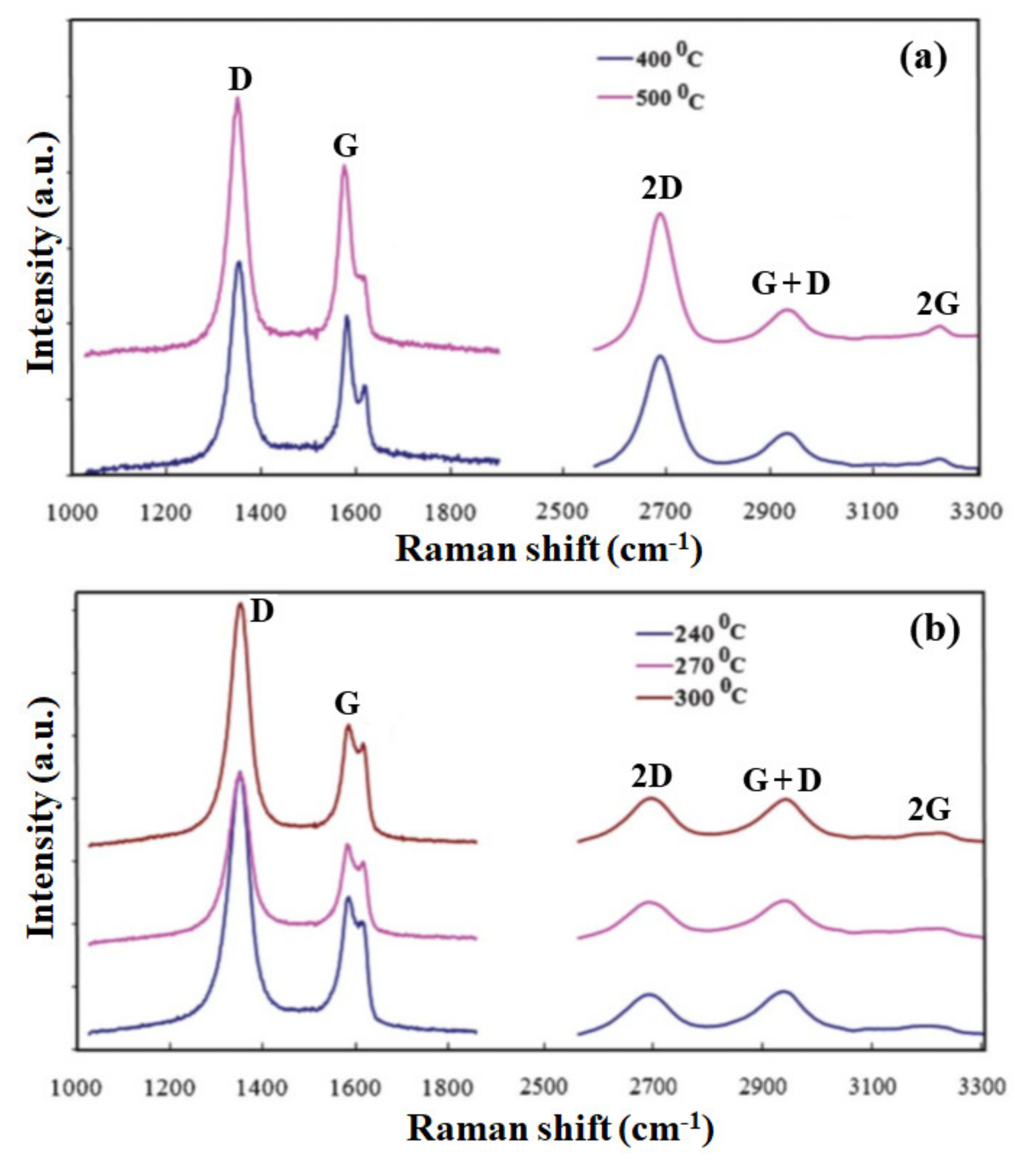
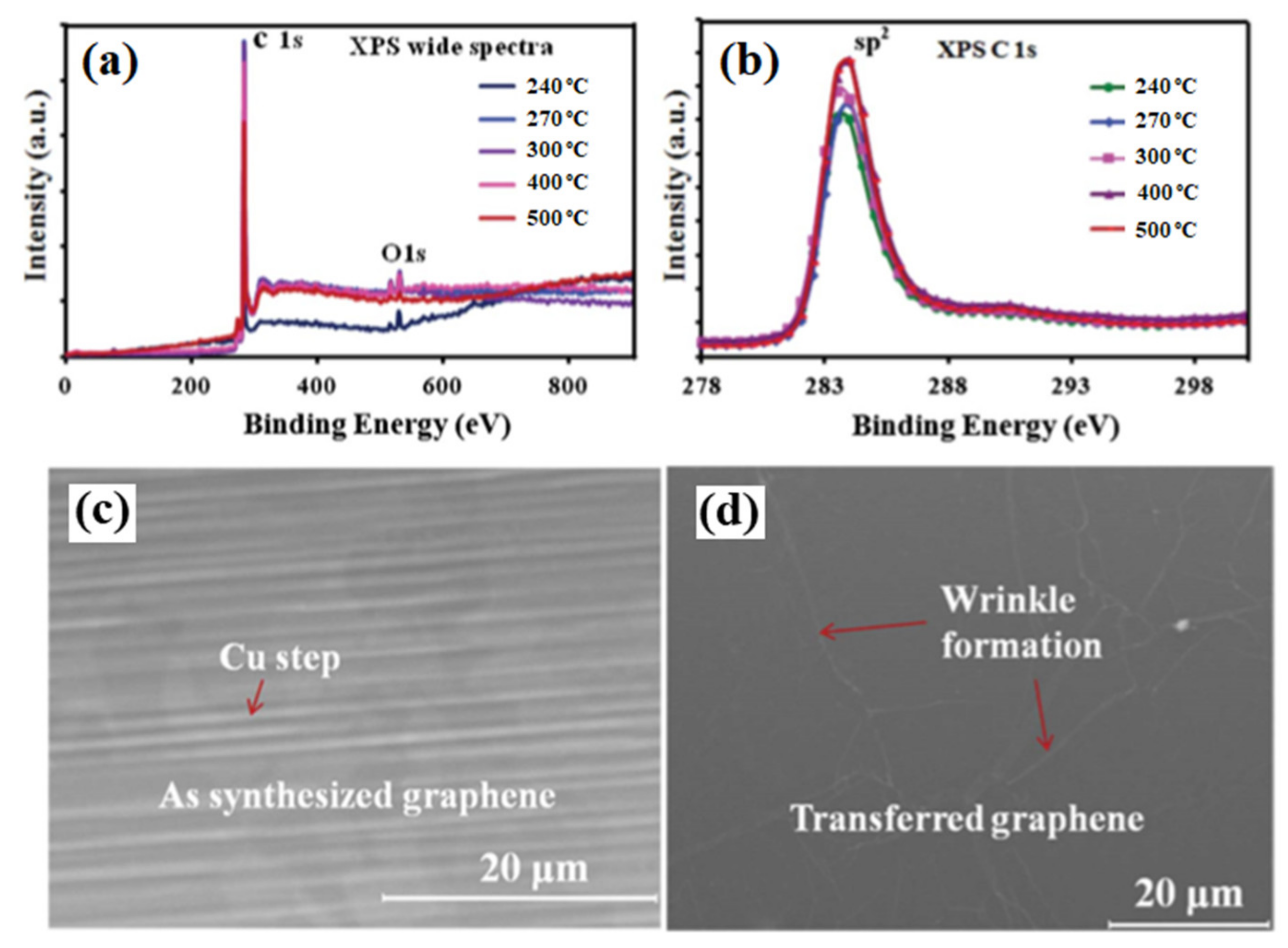
3.1. Growth of Graphene Films on Catalytic Substrates
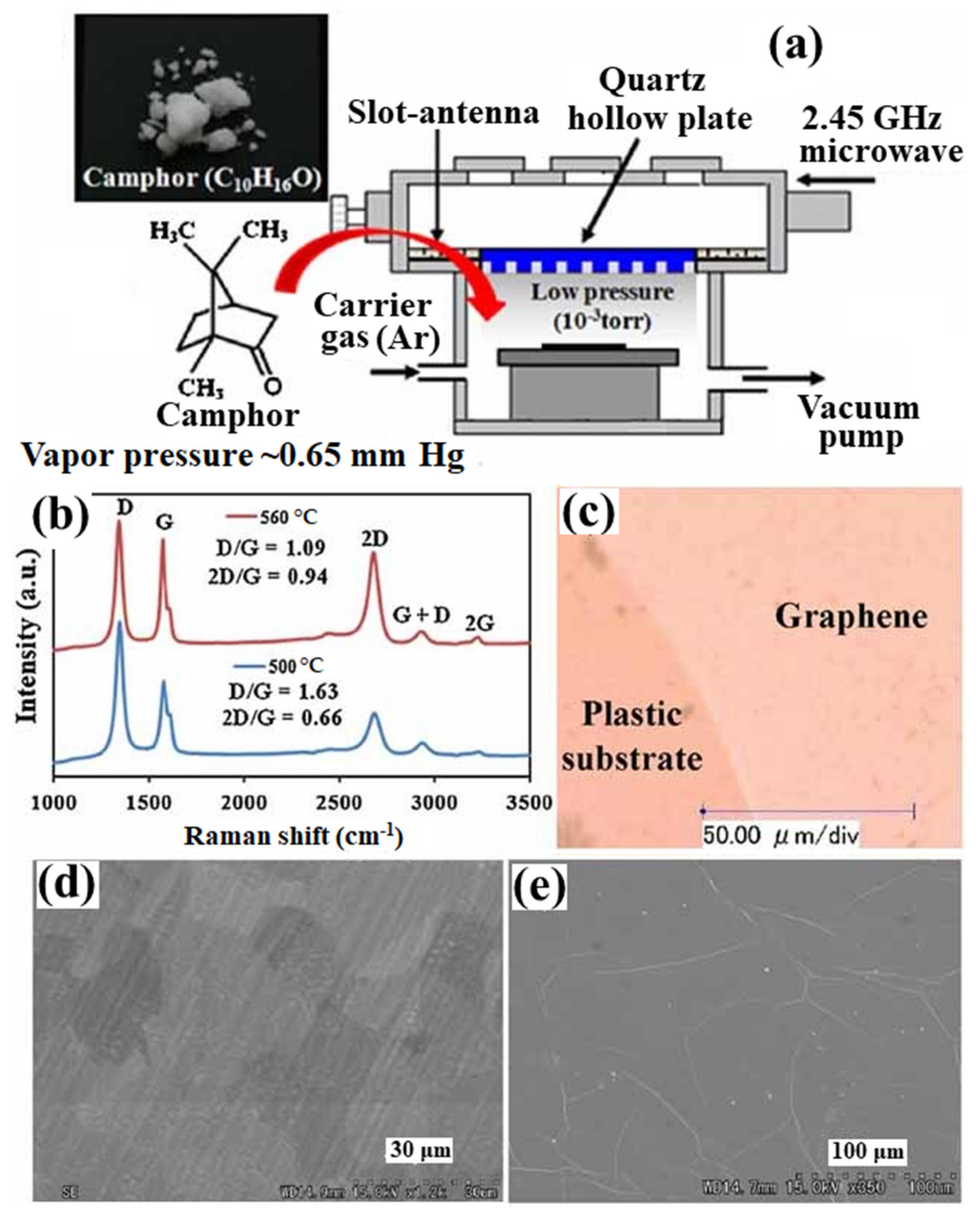
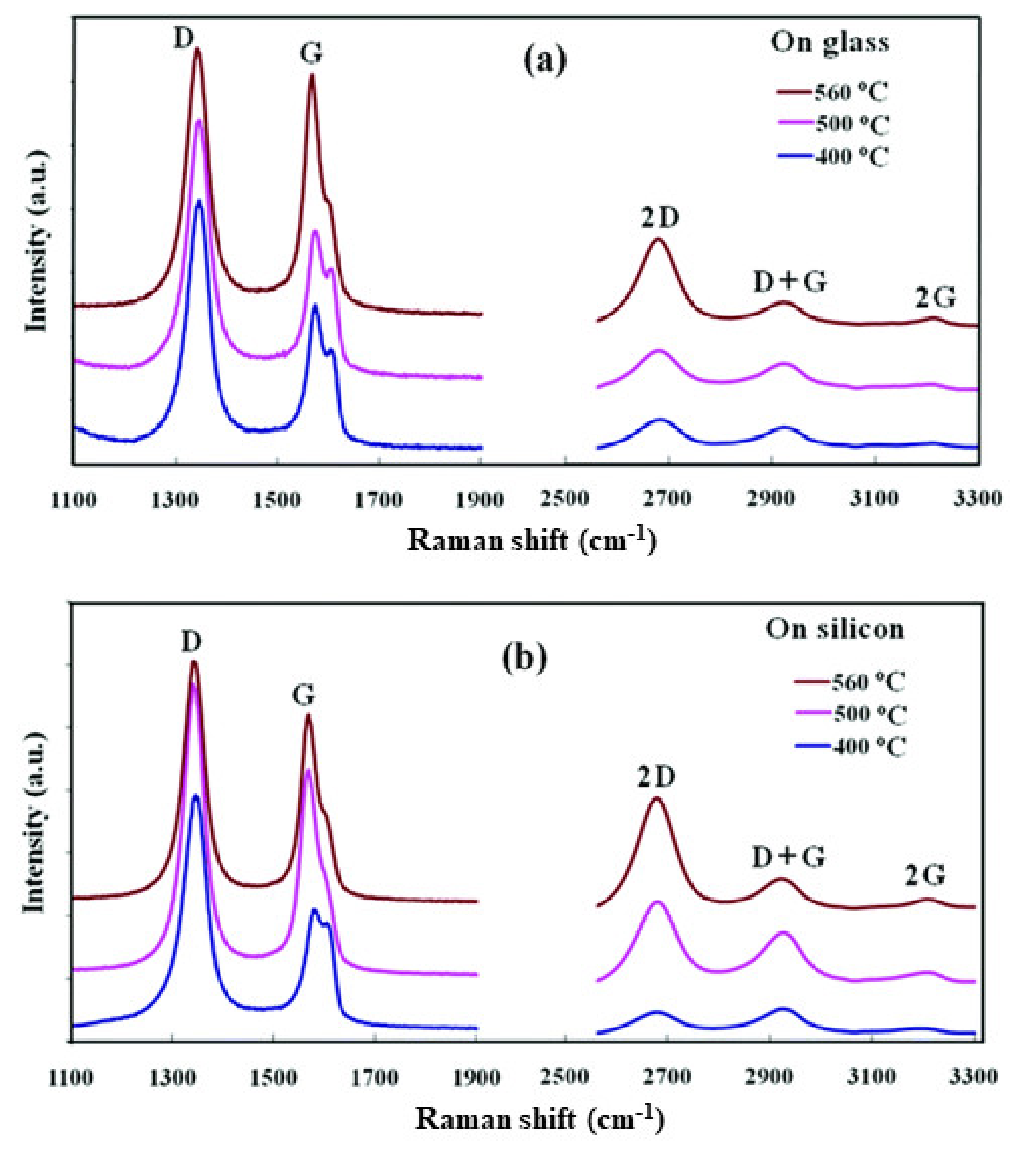
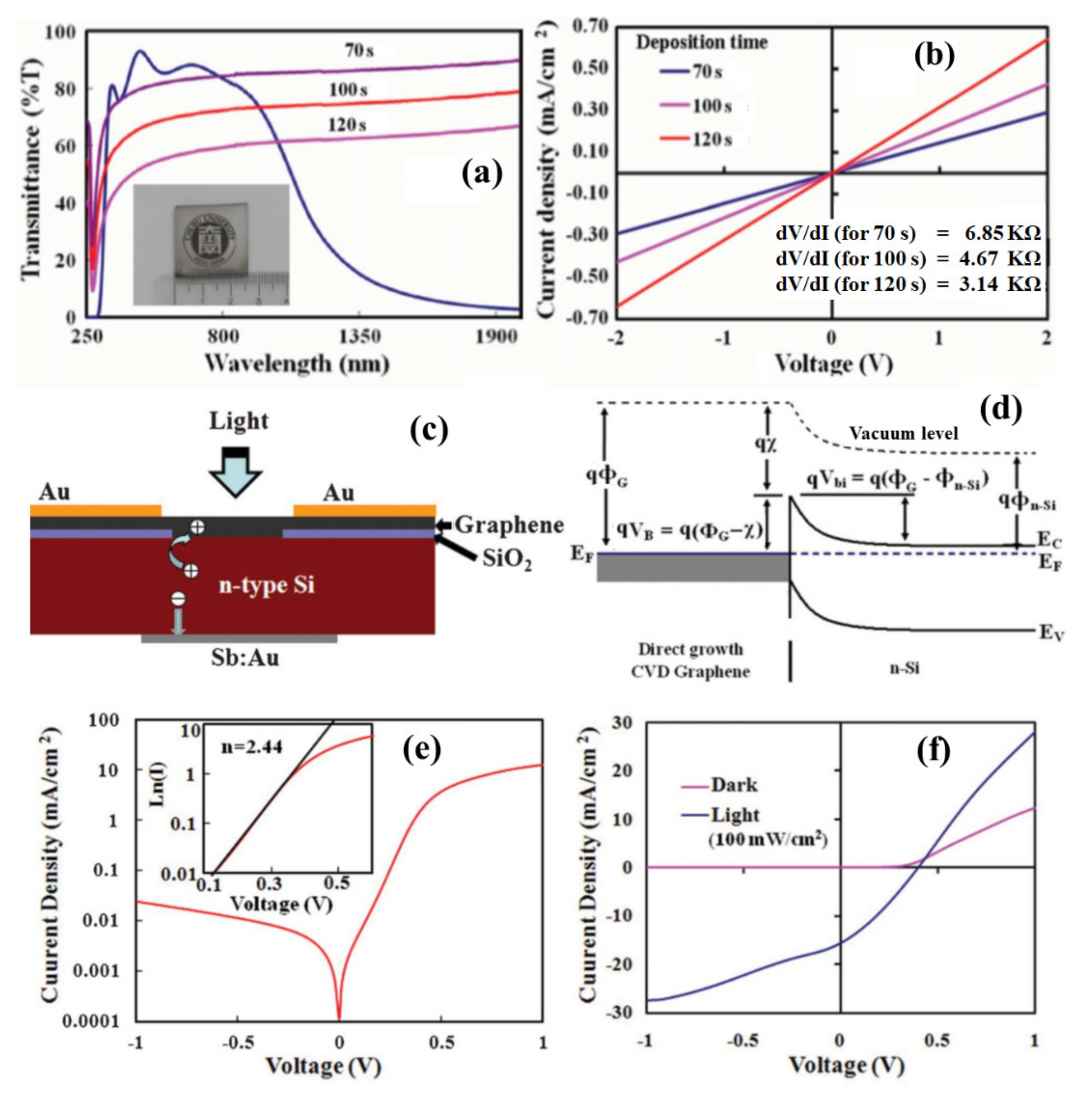
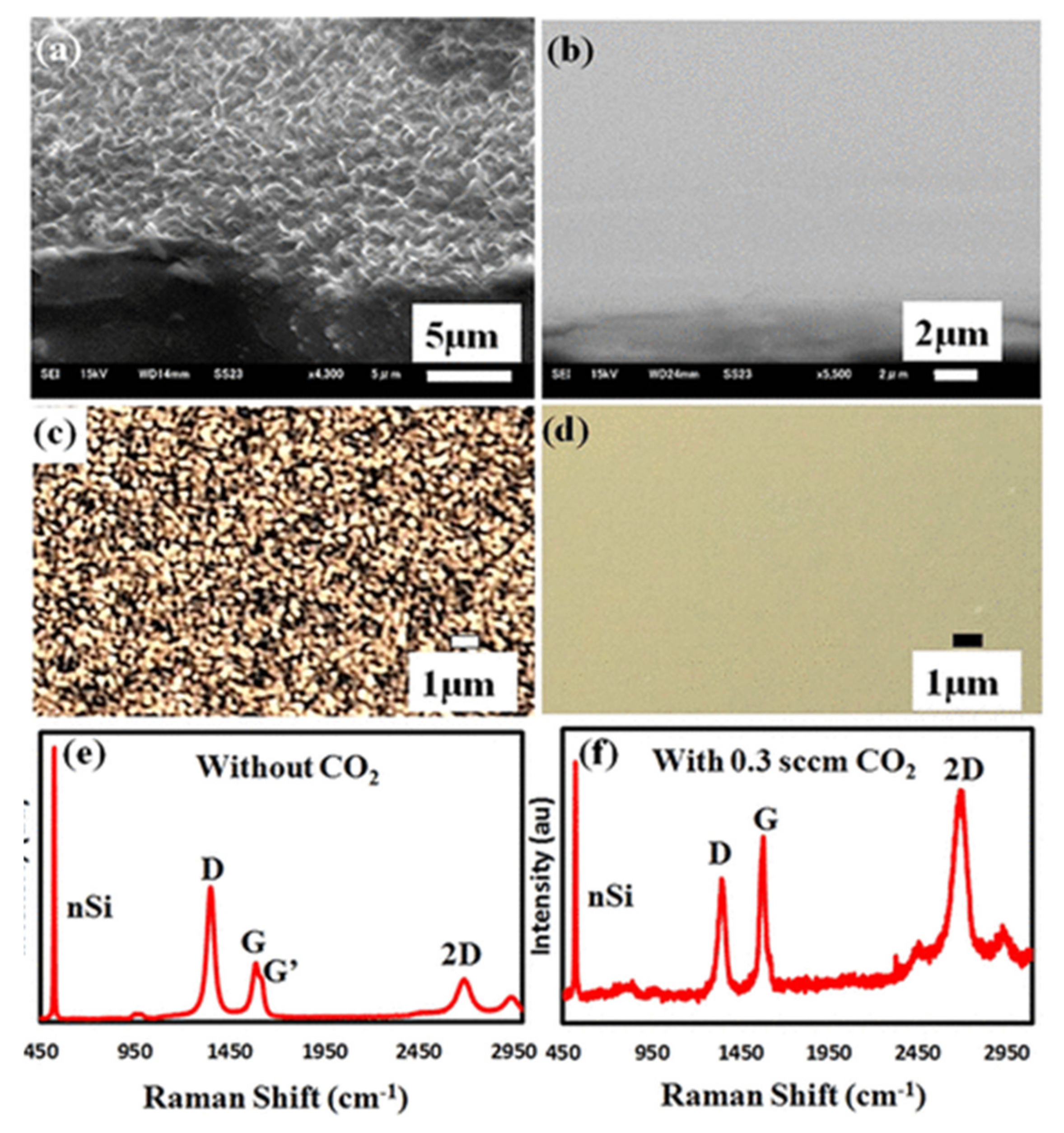
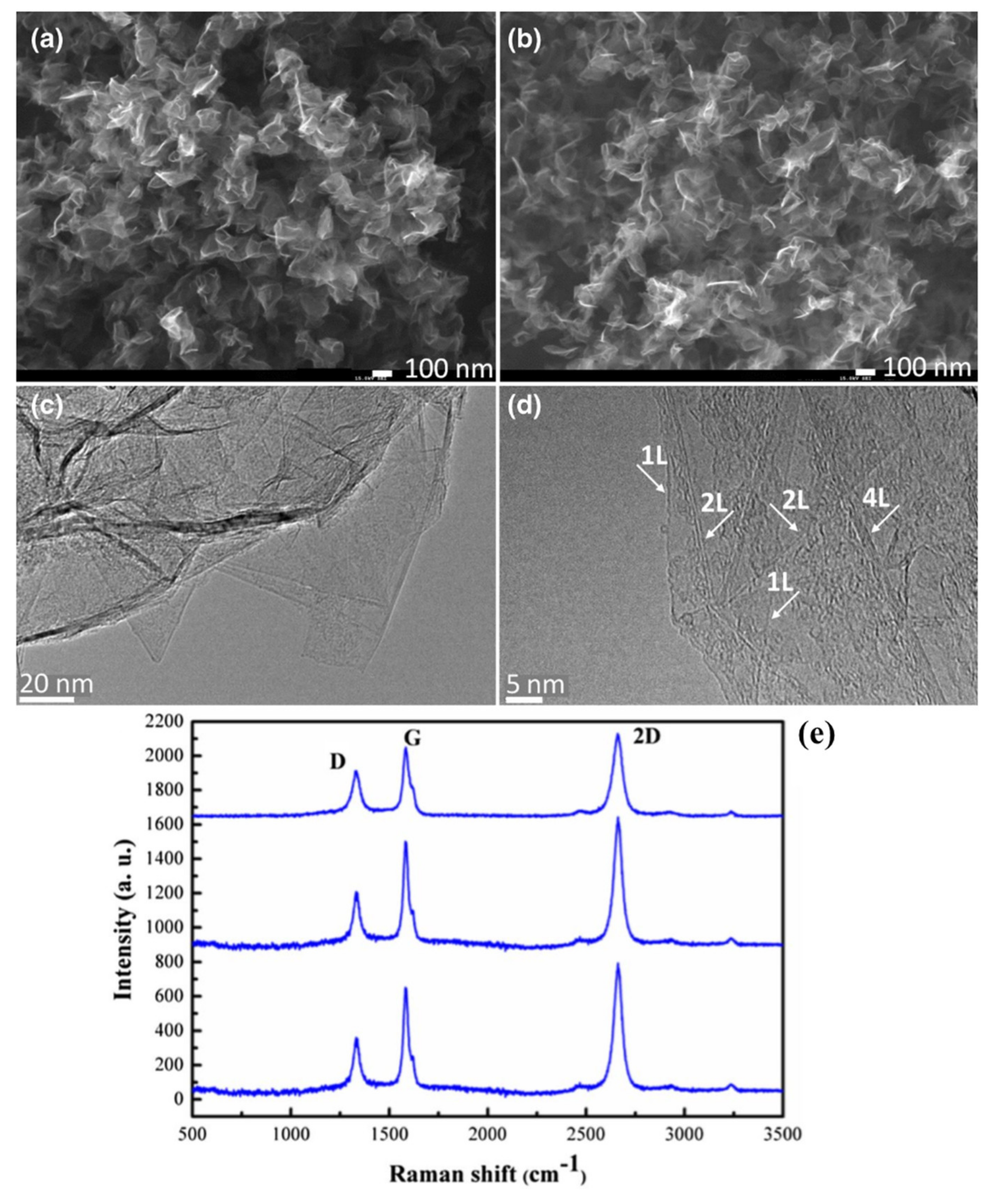
3.2. Direct Growth of Graphene on Arbitrary Substrates
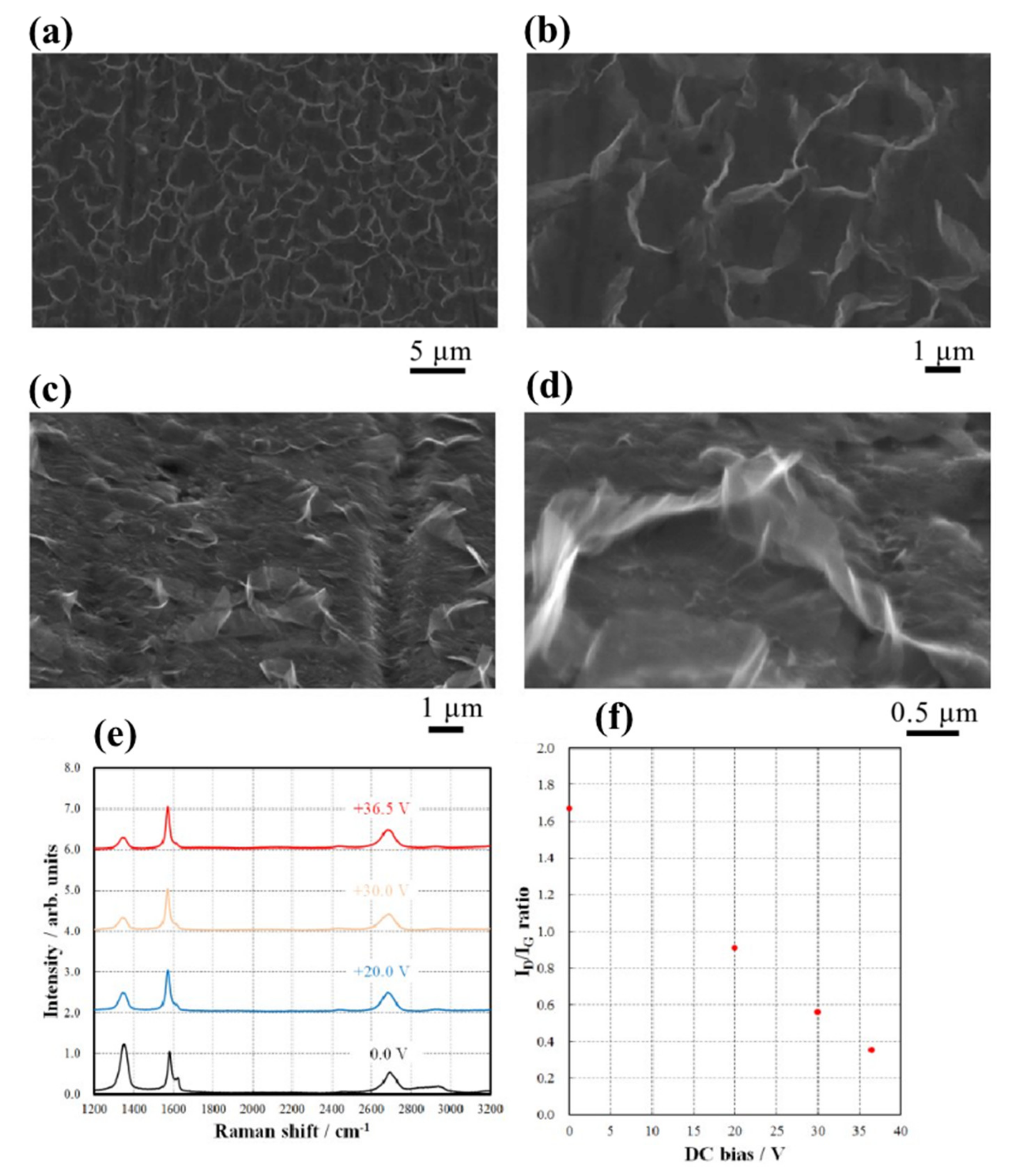
3.3. Growth of Vertically-Oriented Graphene
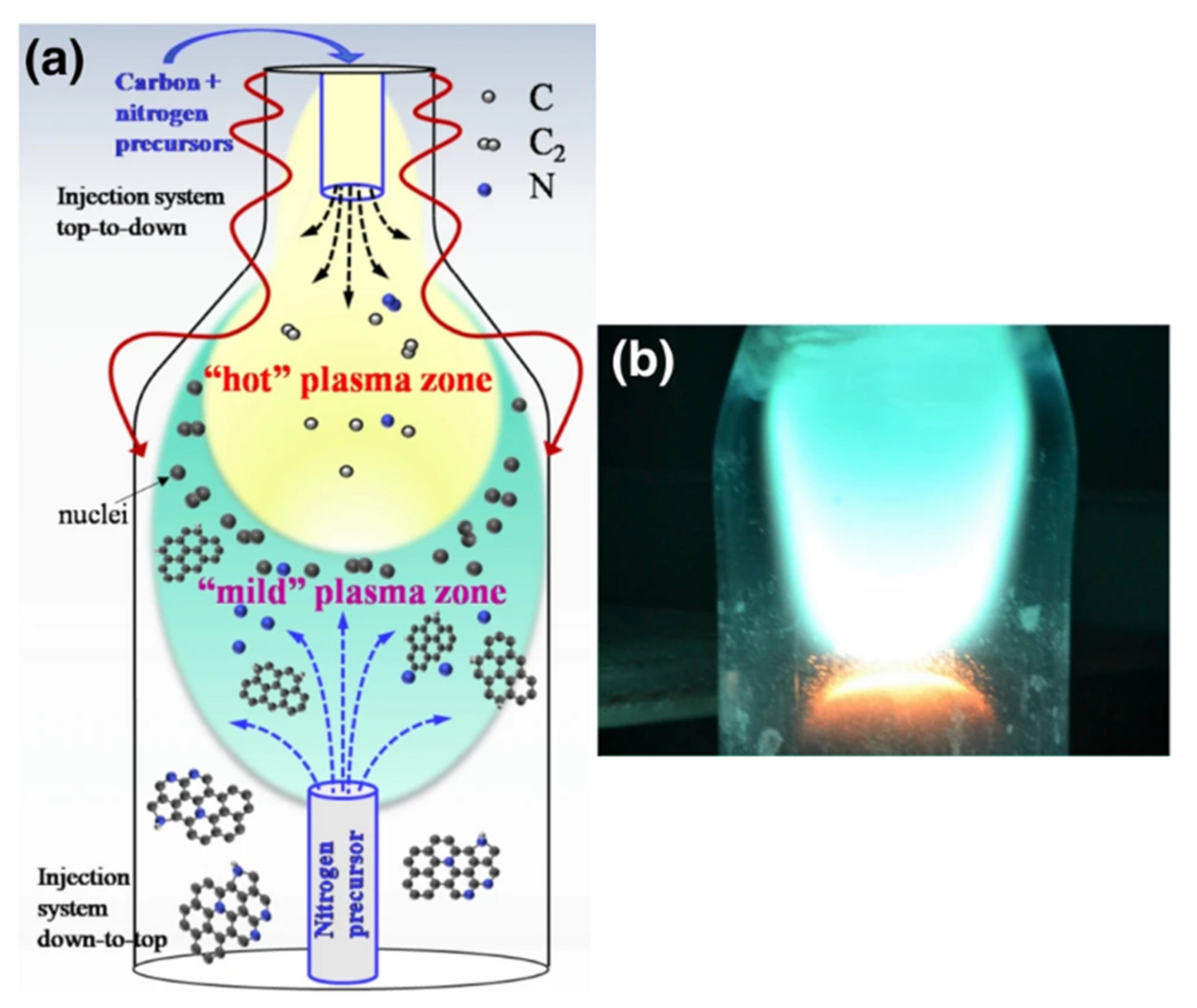
3.4. Doping of Graphene with Heteroatoms
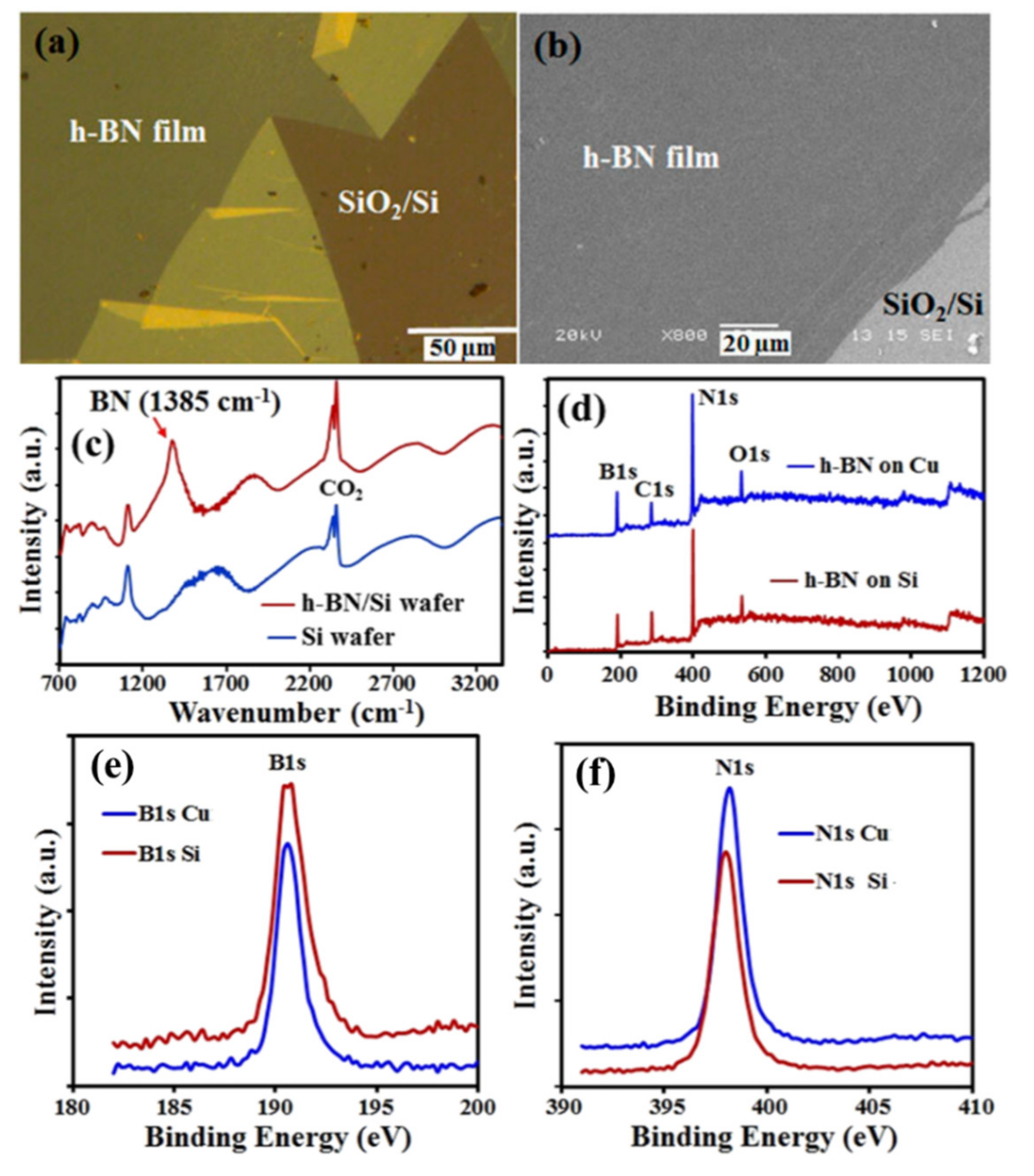
3.5. Growth of Boron Nitride (BN) Related Materials
4. Future Prospects of MW-SWP CVD for 2D Materials Synthesis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, Y.W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2009, 6, 183–191. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Morozov, S.V.; Novoselov, K.S.; Katsnelson, M.I.; Schedin, F.; Elias, D.C.; Jaszczak, J.A.; Geim, A.K. Giant intrinsic carrier mobilities in graphene and its bilayer. Phys. Rev. Lett. 2008, 100, 016602. [Google Scholar] [CrossRef]
- Du, X.; Skachko, I.; Andrei, E.Y.; Barker, A. Approaching ballistic transport in suspended graphene. Nat. Nanotechnol. 2008, 3, 491–495. [Google Scholar] [CrossRef]
- Lin, Y.M.; Dimitrakopoulos, C.; Jenkins, K.A.; Farmer, D.B.; Chiu, H.Y.; Grill, A.; Avouris, P. 100-GHz transistors from wafer-scale epitaxial graphene. Science 2010, 327, 662. [Google Scholar] [CrossRef]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef]
- Cai, D.; Song, M.; Xu, C. Highly conductive carbon-nanotube/graphite-oxide hybrid films. Adv. Mater. 2008, 20, 1706. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H.; Wang, K.; Cao, A.; Wei, J.; Li, C.; Jia, Y.; Li, Z.; Li, X.; Wu, D. Graphene-on-silicon schottky junction solar cells. Adv. Mater. 2010, 22, 2743–2748. [Google Scholar] [CrossRef]
- Sun, X.; Gong, F.; Hao, M.; Wu, L.; Yin, C.; Sun, Z.; Xiao, R. Enhanced thermal transport and corrosion resistance by coating vertically-aligned graphene on zirconium alloy for nuclear reactor applications. Appl. Surf. Sci. 2022, 582, 152484. [Google Scholar] [CrossRef]
- Sadighbayan, D.; Khan, A.M.; Zadeh, E.G. Laser-induced graphene-functionalized field-effect transistor-based biosensing: A potent candidate for COVID-19 detection. IEEE Trans. Nanobiosci. 2022, 21, 232–245. [Google Scholar] [CrossRef]
- Vineesh, T.V.; Kumar, M.P.; Takahashi, C.; Kalita, G.; Alwarappan, S.; Pattanayak, D.K.; Narayanan, T.N. Bifunctional electrocatalytic activity of boron-doped graphene derived from boron carbide. Adv. Energy Mater. 2015, 5, 1500658. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef]
- Heersche, H.B.; Jarillo-Herrero, P.; Oostinga, J.B.; Vandersypen, L.M.K.; Morpurgo, A.F. Bipolar supercurrent in graphene. Nature 2007, 446, 56. [Google Scholar] [CrossRef]
- Ma, K.Y.; Zhang, L.; Jin, S.; Wang, Y.; Yoon, S.I.; Hwang, H.; Oh, J.; Jeong, D.S.; Wang, M.; Chatterjee, S.; et al. Epitaxial single-crystal hexagonal boron nitride multilayers on Ni (111). Nature 2022, 606, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS₂: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Colema, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Desai, P.; Ranade, A.K.; Mahyavanshi, R.; Tanemura, M.; Kalita, G. Influence of MoS2-silicon interface states on spectral photoresponse characteristics. Phys. Status Solidi A 2019, 216, 1900349. [Google Scholar] [CrossRef]
- Mahyavanshi, R.D.; Kalita, G.; Sharma, K.P.; Kondo, M.; Dewa, T.; Kawahara, T.; Tanemura, M. Synthesis of MoS2 ribbons and their branched structures by chemical vapor deposition in sulfur-enriched environment. Appl. Surf. Sci. 2017, 409, 396–402. [Google Scholar] [CrossRef]
- Thangaraja, A.; Shinde, S.M.; Kalita, G.; Tanemura, M. An effective approach to synthesize monolayer tungsten disulphide crystals using tungsten halide precursor. Appl. Phys. Lett. 2016, 108, 053104. [Google Scholar] [CrossRef]
- Wang, X.; Meng, L.; Li, B.; Gong, Y. Heteroatom doped graphene engineering for energy storage and conversion. Mater. Today 2021, 47, 108–130. [Google Scholar] [CrossRef]
- Zhua, H.; Gan, X.; McCreary, A.; Lv, R.; Lin, Z.; Terrones, M. Heteroatom doping of two-dimensional materials. Nano Today 2020, 30, 100829. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, N.J.; Watanabe, K.; Taniguchi, T.; Li, J.I.A. Isospin order in superconducting magic-angle twisted trilayer graphene. Nat. Phys. 2022, 18, 522–527. [Google Scholar] [CrossRef]
- Mahyavanshi, R.D.; Kalita, G.; Ranade, A.; Desai, P.; Kondo, M.; Dewa, T.; Tanemura, M. Photovoltaic action with broadband photoresponsivity in germanium-MoS2 ultrathin heterojunction. IEEE Trans. Electron. Devices 2018, 65, 4434–4440. [Google Scholar] [CrossRef]
- Turkel, S.; Swann, J.; Zhu, Z.; Christos, M.; Watanabe, K.; Taniguchi, T.; Sachdev, S.; Scheurer, M.S.; Kaxiras, E.; Dean, C.R.; et al. Orderly disorder in magic-angle twisted trilayer graphene. Science 2022, 376, 193–199. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Li, Y.; Cai, J.; Liu, Y.; Watanabe, K.; Taniguchi, T.; Xu, X.; Yankowitz, M. Symmetry breaking in twisted double bilayer graphene. Nat. Phys. 2021, 17, 26–30. [Google Scholar] [CrossRef]
- Lyu, J.; Pei, J.; Guo, Y.; Gong, J.; Li, H. A New opportunity for 2D van der Waals heterostructures: Making steep-slope transistors. Adv. Mater. 2020, 32, 1906000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, T.T.; Girit, C.; Hao, Z.; Martin, M.C.; Zettl, A.; Crommie, M.F.; Shen, Y.R.; Wang, F. Direct observation of a widely tunable bandgap in bilayer graphene. Nature 2009, 459, 820–823. [Google Scholar] [CrossRef]
- Rickhaus, P.; Zheng, G.; Lado, J.L.; Lee, Y.; Kurzmann, A.; Eich, M.; Pisoni, R.; Tong, C.; Garreis, R.; Gold, C.; et al. Gap opening in twisted double bilayer graphene by crystal fields. Nano Lett. 2019, 19, 8821–8828. [Google Scholar] [CrossRef]
- Mills, S.; Mizuno, N.; Wang, P.; Lyu, J.; Watanabe, K.; Taniguchi, T.; Camino, F.; Zhang, L.; Du, X. Contact transparency in mechanically assembled 2D material devices. J. Phys. Mater. 2019, 2, 035003. [Google Scholar] [CrossRef]
- Kalita, G.; Sharma, S.; Wakita, K.; Umeno, M.; Hayashi, Y.; Tanemura, M. A photoinduced charge transfer composite of graphene oxide and ferrocene. Phys. Chem. Chem. Phys. 2013, 15, 1271–1274. [Google Scholar] [CrossRef]
- Kalita, G.; Jaisi, B.P.; Umeno, M. Effective reduction and doping of graphene oxide films at near-room temperature by microwave-excited surface-wave plasma process. Diam. Relat. Mater. 2022, 126, 109066. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotech. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Mahyavanshi, R.D.; Kalita, G.; Singh, R.; Kondo, M.; Dewa, T.; Kawahara, T.; Umeno, M.; Tanemura, M. Encapsulation of transition metal dichalcogenides crystals with room temperature plasma deposited carbonaceous films. RSC Adv. 2017, 7, 41136–41143. [Google Scholar] [CrossRef]
- Kalantar-zadeh, K.; Ou, J.Z.; Daeneke, T.; Mitchell, A.; Sasaki, T.; Fuhrer, M.S. Two dimensional and layered transition metal oxides. Appl. Mater. Today 2016, 5, 73–89. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Anasori, B. The rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef]
- Yu, Q.; Lian, J.; Siriponglert, S.; Li, H.; Chen, Y.P.; Pei, S.-S. Graphene segregated on Ni surfaces and transferred to insulators. Appl. Phys. Lett. 2008, 93, 113103. [Google Scholar] [CrossRef]
- Kalita, G.; Masahiro, M.; Uchida, H.; Wakita, K.; Umeno, M. Few layers of graphene as transparent electrode from botanical derivative camphor. Mater. Lett. 2010, 64, 2180–2183. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.; Kim, H.P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Li, X.S.; Cai, W.W.; An, J.H.; Kim, S.; Nah, J.; Yang, D.X.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Kim, J.; Itagaki, H.; Sakakita, H. Graphene creating next-generation electronic devices: Challenges to the low temperature growth using plasmas. J. Inst. Electr. Eng. Jpn. 2021, 141, 219–222. [Google Scholar] [CrossRef]
- Santhosh, N.; Filipič, G.; Tatarova, E.; Baranov, O.; Kondo, H.; Sekine, M.; Hori, M.; Ostrikov, K.; Cvelbar, U. Oriented carbon nanostructures by plasma processing: Precent advances and future challenges. Micromachines 2018, 9, 565. [Google Scholar] [CrossRef]
- Lin, H.C.; Chen, Y.Z.; Wang, Y.C.; Chueh, Y.L. The essential role of Cu vapor for the self-limit graphene via the Cu catalytic CVD method. J. Phys. Chem. C 2015, 119, 6835–6842. [Google Scholar] [CrossRef]
- Sharma, S.; Kalita, G.; Hirano, R.; Hayashi, Y.; Tanemura, M. Influence of gas composition on the formation of graphene domain synthesized from camphor. Mater. Lett. 2013, 93, 258–262. [Google Scholar] [CrossRef]
- Wu, T.; Ding, G.; Shen, H.; Wang, H.; Sun, L.; Jiang, D.; Xie, X.; Jiang, M. Triggering the continuous growth of graphene toward millimeter-sized grains. Adv. Funct. Mater. 2013, 23, 198–203. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.-Y.; Liu, X.-R.; Jin, Z.; Wang, D.; Wan, L.-J. Facile growth of centimeter-sized single-crystal graphene on copper foil at atmospheric pressure. J. Mater. Chem. C 2015, 3, 3530. [Google Scholar] [CrossRef]
- Shinde, S.M.; Kano, E.; Kalita, G.; Takeguchi, M.; Hashimoto, A.; Tanemura, M. Grain structures of nitrogen-doped graphene synthesized by solid source-based chemical vapor deposition. Carbon 2016, 96, 448–453. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Dai, X.; Xiao, J.; Long, M.; Xu, L. The effects of vacancy and heteroatoms-doping on the stability, electronic and magnetic properties of blue phosphorene. Nanotechnology 2021, 32, 135702. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Butler, D.; Lucking, M.C.; Zhang, F.; Xia, T.; Fujisawa, K.; Nakajima, T.G.; Silva, R.C.; Endo, M.; Terrones, H.; et al. Single-atom doping of MoS2 with manganese enables ultrasensitive detection of dopamine: Experimental and computational approach. Sci. Adv. 2020, 6, eabc4250. [Google Scholar] [CrossRef] [PubMed]
- Ngidi, N.P.D.; Ollengo, M.A.; Nyamori, V.O. Heteroatom-doped graphene and its application as a counter electrode in dye-sensitized solar cells. Int. J. Energy Res. 2019, 43, 1702–1734. [Google Scholar] [CrossRef]
- Kalita, G.; Tanemura, M. Fundamentals of chemical vapor deposited graphene and emerging applications. In Graphene Materials-Advanced Applications; George, K., Athanasios, M., Eds.; IntechOpen: London, UK, 2017. [Google Scholar]
- Kalita, G.; Wakita, K.; Takahashi, M.; Umeno, M. Iodine doping in solid precursor-based CVD growth graphene film. J. Mater. Chem. 2011, 21, 15209–15213. [Google Scholar] [CrossRef]
- Kalita, G.; Matsushima, M.; Uchida, H.; Wakita, K.; Umeno, M. Graphene constructed carbon thin films as transparent electrodes for solar cell applications. J. Mater. Chem. 2010, 20, 9713–9717. [Google Scholar] [CrossRef]
- Thangaraja, A.; Shinde, S.M.; Kalita, G.; Tanemura, M. Effect of WO3 precursor and sulfurization process on WS2 crystals growth by atmospheric pressure CVD. Mater. Lett. 2015, 156, 156–160. [Google Scholar] [CrossRef]
- Ayhan, M.E.; Kalita, G.; Sharma, S.; Tanemura, M. Chemical vapor deposition of graphene on silver foil as a tarnish-resistant coating. Phys. Status Solidi RRL 2013, 7, 1076–1079. [Google Scholar] [CrossRef]
- Shinde, S.M.; Kalita, G.; Sharma, S.; Papon, R.; Yusop, M.Z.; Tanemura, M. Synthesis of a three-dimensional structure of vertically aligned carbon nanotubes and graphene from a single solid carbon source. RSC Adv. 2014, 4, 13355–13360. [Google Scholar] [CrossRef]
- Ahmed, K.; Dahal, R.; Weltz, A.; Lu, J.-Q.; Danon, Y.; Bhat, I.B. Growth of hexagonal boron nitride on (111) Si for deep UV photonics and thermal neutron detection. Appl. Phys. Lett. 2016, 109, 113501. [Google Scholar] [CrossRef]
- Jaisi, B.P.; Sharma, K.P.; Sharma, S.; Mahyavanshi, R.D.; Kalita, G.; Tanemura, M. Switching isotropic and anisotropic graphene growth in a solid source CVD system. CrystEngComm 2016, 20, 5356–5363. [Google Scholar] [CrossRef]
- Matsushima, M.; Noda, M.; Yoshida, T.; Kato, H.; Kalita, G.; Kizuki, T.; Uchida, H.; Umeno, M.; Wakita, K. Formation of graphene nano-particle by means of pulsed discharge to ethanol. J. Appl. Phys. 2013, 113, 114304. [Google Scholar] [CrossRef]
- Jafari, A.; Ghoranneviss, M.; Hantehzadeh, M.R.; Boochani, A. Effect of plasma power on growth of multilayer graphene on copper using plasma enhanced chemical vapour deposition. J. Chem. Res. 2016, 40, 40–43. [Google Scholar] [CrossRef]
- Sakai, Y.; Takeda, K.; Hiramatsu, M. Graphene growth in microwave-excited atmospheric pressure remote plasma enhanced chemical vapor deposition. Jpn. J. Appl. Phys. 2022, 61, SA1018. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z. Chemical vapour deposition. Nat. Rev. Methods Primers 2021, 1, 5. [Google Scholar] [CrossRef]
- Han, Z.J.; Murdock, A.T.; Seo, D.H.; Bendavid, A. Recent progress in plasma-assisted synthesis and modification of 2D materials. 2D Mater. 2018, 5, 032002. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Z.; Sun, H.; Dai, D.; Cui, J.; Li, M.; Xu, Y.; Xu, M.; Du, Y.; Jiang, N.; et al. Direct formation of wafer-scale single-layer graphene films on the rough surface substrate by PECVD. Carbon 2018, 129, 456–461. [Google Scholar] [CrossRef]
- Kim, J.; Sakakita, H.; Itagaki, H. Low-temperature graphene growth by forced convection of plasma-excited radicals. Nano Lett. 2019, 19, 739–746. [Google Scholar] [CrossRef]
- Naghdi, S.; Rhee, K.Y.; Park, S.J.A. Catalytic, catalyst-free, and roll-to-roll production of graphene via chemical vapor deposition: Low temperature growth. Carbon 2018, 127, 1–12. [Google Scholar] [CrossRef]
- Cuxart, M.G.; Šics, I.; Goñi, A.R.; Pach, E.; Sauthier, G.; Paradinas, M.; Foerster, M.; Aballe, L.; Fernandez, H.M.; Carlino, V.; et al. Inductively coupled remote plasma-enhanced chemical vapor deposition (rPE-CVD) as a versatile route for the deposition of graphene micro- and nanostructures. Carbon 2017, 117, 331–342. [Google Scholar] [CrossRef]
- Nang, L.V.; Kim, D.-O.; Trung, T.N.; Arepalli, V.K.; Kim, E.-T. Understanding the growth kinetics of graphene on Cu and Fe2O3 using inductively-coupled plasma chemical vapor deposition. Appl. Microsc. 2017, 47, 13–18. [Google Scholar] [CrossRef]
- Li, M.; Liu, D.; Wei, D.; Song, X.; Wei, D.; Wee, A.T.S. Controllable synthesis of graphene by plasma-enhanced chemical vapor deposition and its related applications. Adv. Sci. 2016, 3, 1600003. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhong, G.; Wu, X.; D’Arsiè, L.; Robertson, J. Metal-catalyst-free growth of graphene on insulating substrates by ammonia-assisted microwave plasma-enhanced chemical vapor deposition. RSC Adv. 2017, 7, 33185–33193. [Google Scholar] [CrossRef]
- Hayashi, Y.; Ishidoshiro, S.; Yamada, S.; Kawamura, Y. Ellipsometric monitoring of first stages of graphene growth in plasma-enhanced chemical vapor deposition. J. Vac. Soc. Jpn. 2017, 60, 135–138. [Google Scholar] [CrossRef][Green Version]
- Nonomura, A.; Kawakami, K.; Ishidoshiro, S.; Kawamura, Y.; Hayashi, Y. Synthesis of graphene by magnetron-plasma-enhanced chemical vapor deposition on different substrate materials. J. Vac. Soc. Jpn. 2017, 60, 459–462. [Google Scholar] [CrossRef][Green Version]
- Gottlieb, S.; Wöhrl, N.; Schulz, S.; Buck, V. Simultaneous synthesis of nanodiamonds and graphene via plasma enhanced chemical vapor deposition (MW PE-CVD) on copper. SpringerPlus 2016, 5, 568. [Google Scholar] [CrossRef]
- Kim, H.-U.; Seok, H.; Kanga, W.S.; Kim, T. The first progress of plasma-based transition metal dichalcogenide synthesis: A sTable 1T phase and promising applications. Nanoscale Adv. 2022, 4, 2962–2972. [Google Scholar] [CrossRef]
- Nang, L.V.; Kim, E.-T. Controllable synthesis of high-quality graphene using inductively-coupled plasma chemical vapor deposition. J. Electrochem. Soc. 2012, 159, K93–K96. [Google Scholar] [CrossRef]
- Chugh, S.; Mehta, R.; Lu, N.; Dios, F.D.; Kim, M.J.; Chen, Z. Comparison of graphene growth on arbitrary non-catalytic substrates using low-temperature PECVD. Carbon 2015, 93, 393–399. [Google Scholar] [CrossRef]
- Yamada, T.; Kato, H.; Okigawa, Y.; Ishihara, M.; Hasegawa, M. Electrical properties of bilayer graphene synthesized using surface wave microwave plasma techniques at low temperature. Nanotechnology 2017, 28, 025705. [Google Scholar] [CrossRef]
- Ichimura, S.; Hayashia, Y.; Umeno, M. Effect of ultraviolet light irradiation and ion collision on the quality of multilayer graphene prepared by microwave surface-wave plasma chemical vapor deposition. Diam. Relat. Mater. 2016, 66, 157–162. [Google Scholar] [CrossRef]
- Sugai, H.; Ghanashev, I.; Nagatsu, M. High-density flat plasma production based on surface waves. Plasma Sources Sci. Technol. 1998, 7, 192–205. [Google Scholar] [CrossRef]
- Beaudette, C.A.; Held, J.T.; Mkhoyan, K.A.; Kortshagen, U.R. Nonthermal plasma-enhanced chemical vapor deposition of two-dimensional molybdenum disulfide. ACS Omega 2020, 5, 21853–21861. [Google Scholar] [CrossRef]
- Tian, B.; Li, J.; Chen, M.; Dong, H.; Zhang, X. Synthesis of AAB-stacked single-crystal graphene/hBN/graphene trilayer van der waals heterostructures by in situ CVD. Adv. Sci. 2022, 9, 2201324. [Google Scholar] [CrossRef]
- Wang, K.; Tai, G.; Wong, K.H.; Lau, S.P.; Guo, W. Ni induced few-layer graphene growth at low temperature by pulsed laser deposition. AIP Adv. 2011, 1, 022141. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, Z.; Yu, J.; Yin, H.; Wang, X.; Peng, L.; Wang, Y.; Wang, X.; Jiang, T.; Cao, L.; et al. Epitaxial growth of graphene thin film by pulsed laser deposition. Micro Nano Lett. 2015, 10, 649–652. [Google Scholar] [CrossRef]
- Stankus, V.; Vasiliauskas, A.; Guobienė, A.; Andrulevičius, M.; Meškinis, Š. Direct synthesis of graphene on silicon by reactive magnetron sputtering deposition. Surf. Coat. Technol. 2022, 437, 128361. [Google Scholar] [CrossRef]
- Nakajima, Y.; Murata, H.; Saitoh, N.; Yoshizawa, N.; Suemasu, T.; Toko, K. Low-temperature (400 °C) synthesis of multilayer graphene by metal-assisted sputtering deposition. ACS Omega 2019, 4, 6677–6680. [Google Scholar] [CrossRef]
- Ionescu, M.I.; Sun, X.; Luan, B. Multilayer graphene synthesized using magnetron sputtering for planar supercapacitor application. Can. J. Chem. 2015, 93, 2. [Google Scholar] [CrossRef]
- Lin, M.-Y.; Guo, W.-C.; Wu, M.-H.; Wang, P.-Y.; Liu, T.-H.; Pao, C.-W.; Chang, C.-C.; Lee, S.-C.; Lin, S.-Y. Low-temperature grown graphene films by using molecular beam epitaxy. Appl. Phys. Letts. 2012, 101, 22. [Google Scholar]
- Lopes, J.M.J.; Vignaud, D. Molecular beam epitaxy of graphene and hexagonal boron nitride. In Molecular Beam Epitaxy; Elsevier: Amsterdam, The Netherlands, 2018; pp. 487–513. [Google Scholar]
- Albar, J.D.; Summerfield, A.; Cheng, T.S.; Davies, A.; Smith, E.F.; Khlobystov, A.N.; Mellor, C.J.; Taniguchi, T.; Watanabe, K.; Foxon, C.T.; et al. An atomic carbon source for high temperature molecular beam epitaxy of graphene. Sci. Rep. 2017, 7, 6598. [Google Scholar] [CrossRef] [PubMed]
- Bo, Z.; Yang, Y.; Chen, J.; Yu, K.; Yana, J.; Cen, K. Plasma-enhanced chemical vapor deposition synthesis of vertically oriented graphenenanosheets. Nanoscale 2013, 5, 5180–5204. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Ishihara, M.; Kim, J.; Hasegawa, M.; Iijima, S. A roll-to-roll microwave plasma chemical vapor deposition process for the production of 294 mm width graphene films at low temperature. Carbon 2012, 50, 2615–2619. [Google Scholar] [CrossRef]
- Ichimura, S.; Umeno, M. Synthesis of thin graphite film by microwave surface-wave plasma chemical vapor deposition. Trans. Mater. Res. Soc. Jpn. 2016, 41, 379–383. [Google Scholar] [CrossRef]
- Yamada, T.; Ishihara, M.; Hasegawa, M. Large-area coating of graphene at low temperature using a roll-to-roll microwave plasma chemical vapor deposition. Thin Solid Films 2013, 532, 89–93. [Google Scholar] [CrossRef]
- Yamada, T.; Ishihara, M.; Hasegawa, M. Low temperature graphene synthesis from poly (methyl methacrylate) using microwave plasma treatment. Appl. Phys. Express 2013, 6, 115102. [Google Scholar] [CrossRef]
- Kato, R.; Tsugawa, K.; Yamada, T.; Ishihara, M.; Hasegawa, M. Improvement of multilayer graphene synthesis on copper substrate by microwave plasma process using helium at low temperatures. Jpn. J. Appl. Phys. 2014, 53, 015505. [Google Scholar] [CrossRef]
- Suzuki, N.; Kitagawa, H.; Uchiyama, S. Stable surface-wave plasma through excitation of standing surface wave using plane multislot antenna. Jpn. J. Appl. Phys. 2002, 41, 3930–3935. [Google Scholar] [CrossRef]
- Ishijima, T.; Toyoda, H.; Takanishi, Y.; Sugai, H. Design of large-area surface wave plasma excited by slotted waveguide antennas with novel power divider. Jpn. J. Appl. Phys. 2011, 50, 036002. [Google Scholar] [CrossRef]
- Hasegawa, I.; Yamauchi, T.; Sugai, H. Mechanism of oxidation of Si surfaces exposed to O2/Ar microwave-excited plasma. Jpn. J. Appl. Phys. 2007, 46, 98–104. [Google Scholar] [CrossRef]
- Nakamura, K.; Zhang, Q.; Sugai, H. Plane-type microwave resonator probe for electron density measurement and the sheath effects in reactive processing plasmas. IEEJ Trans. Fundam. Mater. 2010, 130, 930–934. [Google Scholar] [CrossRef]
- Nakao, S.; Sugai, H. Multi-hollow plasma production along dielectric plate in microwave discharge. Jpn. J. Appl. Phys. 2007, 46, L1039–L1041. [Google Scholar] [CrossRef]
- Moisan, M.; Zakrzewski, Z. Plasma sources based on the propagation of electromagnetic surface waves. J. Phys. D: Appl. Phys. 1991, 24, 1025. [Google Scholar] [CrossRef]
- Ishijima, T.; Nojiri, Y.; Toyoda, H.; Sugai, H. A high-speed photoresist removal process using multibubble microwave plasma under a mixture of multiphase plasma environment. Jpn. J. Appl. Phys. 2010, 49, 086002. [Google Scholar] [CrossRef]
- Kimura, Y.; Kawaguchi, H.; Kagami, S.; Furukawa, M.; Shindo, H. A new method of line plasma production by microwave in a narrowed rectangular waveguide. Appl. Phys. Express 2009, 2, 126002. [Google Scholar] [CrossRef]
- Zalieckas, J.; Pobedinskas, P.; Greve, M.M.; Eikehaug, K.; Haenen, K.; Holst, B. Large area microwave plasma CVD of diamond using composite right/left-handed materials. Diamond. Relat. Mater. 2021, 116, 108394. [Google Scholar] [CrossRef]
- Kousaka, H.; Xu, J.; Umehara, N. Generation of long and high-density plasma column using metal-antenna surface wave-excited plasma source. Jpn. J. Appl. Phys. 2005, 44, L1052. [Google Scholar] [CrossRef]
- Ishijima, T.; Nojiri, Y.; Toyoda, H.; Sugai, H. Novel antenna coupler design for production of meter-scale high-density planar surface wave plasma. Jpn. J. Appl. Phys. 2010, 49, 086002. [Google Scholar] [CrossRef]
- Nakamura, K.; Kuwashita, Y.; Sugai, H. New inductive rf discharge using an internal metal antenna. Jpn. J. Appl. Phys. 1995, 34, L1686. [Google Scholar] [CrossRef]
- Nagatsu, M.; Ito, A.; Toyoda, N.; Sugai, H. Characteristics of ultrahigh-frequency surface-wave plasmas excited at 915 MHz. Jpn. J. Appl. Phys. 1999, 38, L679. [Google Scholar] [CrossRef]
- Nagatsu, M.; Xu, G.; Ghanashev, I.; Kanoh, M.; Sugai, H. Mode identification of surface waves excited in a planar microwave discharge. Plasma Sources Sci. Technol. 1997, 6, 427. [Google Scholar] [CrossRef]
- Odrobina, I.; Kudela, J.; Kando, M. Characteristics of the planar plasma source sustained by microwave power. Plasma Sources Sci. Technol. 1998, 7, 238. [Google Scholar] [CrossRef]
- Shirai, H.; Yoshino, K.; Ohkawara, G.; Ueyama, H. Novel high-density microwave plasma. Jpn. J. Appl. Phys. 2001, 40, L701. [Google Scholar] [CrossRef]
- Shimitani, K.; Okamoto, T.; Okamoto, Y. Production of a large-area argon microwave plasma by a ring slot antenna. Vacuum 2002, 66, 359–364. [Google Scholar] [CrossRef]
- Nagatsu, M.; Naito, K.; Ogino, A.; Nanko, S. Production of large-area surface-wave plasmas with an internally mounted planar cylindrical launcher. Plasma Sources Sci. Technol. 2006, 15, 37. [Google Scholar] [CrossRef]
- Gu, J.D.; Chen, P.L. Low-temperature fabrication of silicon films by large-area microwave plasma enhanced chemical vapor deposition. Thin Solid Films 2006, 498, 14. [Google Scholar] [CrossRef]
- Yasaka, Y.; Hojo, H. Enhanced power absorption in planar microwave discharges. Phys. Plasmas 2000, 7, 1601. [Google Scholar] [CrossRef]
- Toba, T.; Katsurai, M. Modeling of argon discharge characteristics of planar-type surface wave plasmas in an electron fluid model. IEEE Trans. Plasma Sci. 2002, 30, 2095. [Google Scholar] [CrossRef]
- Yamauchi, T.; Abdel-Fattah, E.; Sugai, H. Dramatic improvement of surface wave plasma performance using a corrugated dielectric plate. Jpn. J. Appl. Phys. 2001, 40, L1176–L1178. [Google Scholar] [CrossRef]
- Sugai, H. Forefront of large area plasma CVD process development. J. Plasma Fusion Res. 2010, 86, 28–32. (In Japanese) [Google Scholar]
- Chakrabarty, K.; Baker, P.A.; Vijayan, V.M.; Catledge, S.A. Bias-enhanced formation of metastable and multiphase boron nitride coating in microwave plasma chemical vapor deposition. Materials 2021, 14, 7167. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Han, Y.; Luo, P.; Zhou, Y.; Chen, Q.; Zhu, H.; Yang, Y.; Zhang, B.; Huang, K. Advances in microwave-enhanced chemical vapor deposition for graphene synthesis. ChemistrySelect 2022, 7, e202200103. [Google Scholar] [CrossRef]
- Kim, Y.; Song, W.; Lee, S.Y.; Jeon, C.; Jung, W.; Kim, M.; Park, C.-Y. Low-temperature synthesis of graphene on nickel foil by microwave plasma chemical vapor deposition. Appl. Phys. Lett. 2011, 98, 263106. [Google Scholar] [CrossRef]
- Kim, J.; Ishihara, M.; Koga, Y.; Tsugawa, K.; Hasegawa, M.; Iijima, S. Low-temperature synthesis of large-area graphene-based transparent conductive films using surface wave plasma chemical vapor deposition. Appl. Phys. Lett. 2011, 8, 091502. [Google Scholar] [CrossRef]
- Kalita, G.; Wakita, K.; Umeno, M. Low temperature growth of graphene film by microwave assisted surface wave plasma CVD for transparent electrode application. RSC Adv. 2012, 2, 2815–2820. [Google Scholar] [CrossRef]
- Kalita, G.; Sharma, S.; Wakita, K.; Umeno, M.; Hayashi, Y.; Tanemura, M. Synthesis of graphene by surface wave plasma chemical vapor deposition from camphor. Phys. Status Solidi A 2012, 209, 2510–2513. [Google Scholar] [CrossRef]
- Kalita, G.; Ayhan, M.E.; Sharma, S.; Shinde, S.M.; Ghimire, D.; Wakita, K.; Umeno, M.; Tanemura, M. Low temperature deposited graphene by surface wave plasma CVD as effective oxidation resistive barrier. Corros. Sci. 2014, 78, 183–187. [Google Scholar] [CrossRef]
- Mewada, A.; Vishwakarma, R.; Zhu, R.; Umeno, M. Carbon-dot doped, transfer-free, low-temperature, high mobility graphene using microwave plasma CVD. RSC Adv. 2022, 12, 20610–20617. [Google Scholar] [CrossRef]
- Ichimura, S.; Hayashi, Y.; Umeno, M. DC biasing effects on properties of carbon nanowalls by microwave surface-wave plasma chemical vapor deposition and towards transparent electrode. Trans. Mater. Soc. Japan 2016, 41, 229–233. [Google Scholar] [CrossRef][Green Version]
- Okigawa, Y.; Kato, R.; Yamada, T.; Ishihara, M.; Hasegawa, M. Electrical properties and domain sizes of graphene films synthesized by microwave plasma treatment under a low carbon concentration. Carbon 2015, 82, 60–66. [Google Scholar] [CrossRef]
- Kalita, G.; Kayastha, M.S.; Uchida, H.; Wakita, K.; Umeno, M. Direct growth of nanographene films by surface wave plasma chemical vapor deposition and their application in photovoltaic devices. RSC Adv. 2012, 2, 3225. [Google Scholar] [CrossRef]
- Li, X.; Lv, Z.; Zhu, H. Carbon/silicon heterojunction solar cells: State of the art and prospects. Adv. Mater. 2015, 27, 6549–6574. [Google Scholar] [CrossRef]
- Chen, C.C.; Aykol, M.; Chang, C.C.; Levi, A.F.J.; Cronin, S.B. Graphene-silicon schottky diodes. Nano Lett. 2011, 11, 1863–1867. [Google Scholar] [CrossRef]
- Adhikari, S.; Zhu, R.; Umeno, M. Direct synthesis of graphene on silicon at low temperature for Schottky junction solar cells. J. Mater. Sci. Chem. Eng. 2021, 9, 10. [Google Scholar] [CrossRef]
- Vishwakarma, R.; Zhu, R.; Mewada, A.; Umeno, M. Laser-assisted graphene growth directly on silicon. Nanotechnology 2021, 32, 305601. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Hori, M. Carbon Nanowalls: Synthesis and Emerging Applications; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-211-99718-5. [Google Scholar]
- Ichikawa, T.; Shimizu, N.; Ishikawa, K.; Hiramatsu, M.; Hori, M. Synthesis of isolated carbon nanowalls via high-voltage nanosecond pulses in conjunction with CH4/H2 plasma enhanced chemical vapor deposition. Carbon 2020, 161, 403–412. [Google Scholar] [CrossRef]
- Wu, Z.; Yu, Y.; Zhang, G.; Zhang, Y.; Guo, R.; Li, L.; Zhao, Y.; Wang, Z.; Shen, Y.; Shao, G. In situ monitored (N, O)-doping of flexible vertical graphene films with high-flux plasma enhanced chemical vapor deposition for remarkable metal-free redox catalysis essential to alkaline zinc-air batteries. Adv. Sci. 2022, 9, 2200614. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Chai, Z.; Hu, W. Low-temperature plasma synthesis of carbon nanotubes and graphene-based materials and their fuel cell applications. Chem. Soc. Rev. 2013, 42, 8821. [Google Scholar] [CrossRef]
- Vishwakarma, R.; Zhu, R.; Abuelwafa, A.A.; Mabuchi, Y.; Adhikar, S.; Ichimura, S.; Soga, T.; Umeno, M. Direct synthesis of large-area graphene on insulating substrates at low temperature using microwave plasma CVD. ACS Omega 2019, 4, 11263–11270. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Hatakeyama, R. Direct growth of doping-density-controlled hexagonal graphene on SiO2 substrate by rapid-heating plasma CVD. ACS Nano 2012, 6, 8508–8515. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Shen, H.; Chen, J.; Lia, X.; Jiang, Y. Direct growth of nitrogen-doped graphene films on glass by plasma-assisted hot filament CVD for enhanced electricity generation. J. Mater. Chem. A 2019, 7, 12038–12049. [Google Scholar] [CrossRef]
- Bundaleska, N.; Dias, A.; Bundaleski, N.; Felizardo, E.; Henriques, J.; Tsyganov, D.; Abrashev, M.; Valcheva, E.; Kissovski, J.; Ferraria, A.M.; et al. Prospects for microwave plasma synthesized N-graphene in secondary electron emission mitigation applications. Sci. Rep. 2020, 10, 13013. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Bundalesk, N.; Abrashev, M.; Bundaleski, N.; Teodoro, O.M.N.D.; Fonseca, I.; de Ferro, A.M.; Silva, R.P.; Tatarova, E.; Montemor, M.F. Free-standing N-graphene as conductive matrix for Ni(OH)2 based supercapacitive electrodes. Electrochimica Acta 2020, 334, 135592. [Google Scholar] [CrossRef]
- Tsyganov, D.; Bundaleska, N.; Dias, A.; Henriques, J.; Felizardo, E.; Abrashev, M.; Kissovski, J.; do Rego, A.M.B.; Ferraria, A.M.; Tatarova, E. Microwave plasma-based direct synthesis of free-standing N-graphene. Phys. Chem. Chem. Phys. 2020, 22, 4772–4787. [Google Scholar] [CrossRef]
- Watanabe, K.; Taniguchi, T.; Kanda, H. Direct-bandgap properties and evidence for ultraviolet lasing of hexagonal boron nitride single crystal. Nat. Mater. 2004, 3, 404. [Google Scholar] [CrossRef]
- Dean, C.R.; Young, A.F.; Meric, I.; Lee, C.; Wang, L.; Sorgenfrei, S.; Watanabe, K.; Taniguchi, T.; Kim, P.; Shepard, K.L.; et al. Boron nitride substrates for high-quality graphene electronics. Nat. Nanotechnol. 2010, 5, 722. [Google Scholar] [CrossRef]
- Ramkorun, B.; Chakrabarty, K.; Catledge, S.A. Effects of direct current bias on nucleation density of superhard boron-rich boron carbide films made by microwave plasma chemical vapor deposition. Mater. Res. Express 2021, 8, 046401. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, K.; Rosmi, M.S.; Yaakob, Y.; Araby, M.I.; Ohtani, H.; Kalita, G.; Tanemura, M. Morphology-controlled synthesis of hexagonal boron nitride crystals by chemical vapor deposition. Cryst. Growth Des. 2016, 16, 6440–6445. [Google Scholar] [CrossRef]
- Sharma, S.; Kalita, G.; Vishwakarma, R.; Zulkifli, Z.; Tanemura, M. Opening of triangular hole in triangular-shaped chemical vapor deposited hexagonal boron nitride crystal. Sci. Rep. 2015, 5, 10426. [Google Scholar] [CrossRef]
- Sharma, K.P.; Sharma, S.; Sharma, A.K.; Jaisi, B.P.; Kalita, G.; Tanemura, M. Edge controlled growth of hexagonal boron nitride crystals on copper foil by atmospheric pressure chemical vapor deposition. CrystEngComm 2018, 20, 550–555. [Google Scholar] [CrossRef]
- Singh, R.; Kalita, G.; Mahyavanshi, R.D.; Adhikari, S.; Uchida, H.; Tanemura, M.; Umeno, M.; Kawahara, T. Low temperature wafer-scale synthesis of hexagonal boron nitride by microwave assisted surface wave plasma chemical vapour deposition. AIP Adv. 2019, 9, 035043. [Google Scholar] [CrossRef]


| Methods | Graphene | Hexagonal Boron Nitride (hBN) | Transition Metal Dichalcogenides (TMDCs) Layers |
|---|---|---|---|
| Pulsed-Laser Deposition (PLD) | Monolayer/Bilayer/Fewlayers (Catalytic growth; Temperature: 300~1200 °C) | Monolayer/Fewlayers (Catalytic & noncatalytic growth; Temperature: 200~1000 °C) | Fewlayers/Multilayers (Noncatalytic growth; Temperature: 300~800 °C) |
| Magnetron Sputtering | Monolayer/Fewlayers(Catalytic growth; Temperature: 300~1000 °C) | Fewlayers/Multilayers (Catalytic growth & noncatalytic growth; Temperature: 400~1000 °C) | Monolayer/Fewlayers (Noncatalytic growth; Temperature: 300~800 °C) |
| Molecular Beam Epitaxy (MBE) | Monolayer/Bilayer/Fewlayers (Catalytic growth; Temperature: 500~1000 °C) | Monolayer/Bilayer/Fewlayers (Catalytic growth; Temperature: 400~1000 °C) | Monolayer/Fewlayers (Noncatalytic growth; Temperature: 600~1000 °C) |
| Thermal Chemical Vapor Deposition (CVD) | Monolayer/Bilayer/Fewlayers (Catalytic growth; Temperature: >1000 °C) | Monolayer/Bilayer/Fewlayers (Catalytic growth; Temperature: >1000 °C) | Monolayer/Bilayer/Fewlayers (Noncatalytic growth; Temperature: 600~1000 °C) |
| Plasma Chemical Vapor Deposition (CVD) | Monolayer/Bilayer/Fewlayers (Catalytic & noncatalytic growth; Low temperature growth) | Monolayer/Fewlayers/Multilayers (Catalytic & noncatalytic growth; Low temperature growth) | Monolayer/Fewlayers/Multilayers (Noncatalytic growth; Low temperature growth) |
| Dispersion of Solution (Graphene Oxide Solution) | Chemical Vapor Deposition (CVD) | |||
|---|---|---|---|---|
| Thermal CVD | Plasma CVD | |||
| RF Plasma CVD | Microwave-Excited Surface Wave Plasma CVD | |||
| Formation temperature of films | Room temperature (°C) | ~1100 °C | 400~1000 °C | 200~700 °C |
| Layer thickness | Monolayer~Few-layers * Assembly of graphene layers | Monolayer~Few-layers | Monolayer~Few-layers * Lateral growth * Vertical-oriented growth | Monolayer~Few-layers * Lateral growth * Vertical-oriented growth |
| Film formation area | Meter scale (~1 m) | ~0.1 m | Meter scale (~1 m) | Meter scale (~1 m) |
| Features | Room temperature film formation | High quality; Controllable growth of monolayer/bilayers | Low temperature deposition | Low temperature deposition, direct film formation on various substrates (independent of catalytic substrates) |
| Applications | Coating materials, composites, membranes, sensors, energy storage/conversion devices etc. | Electronic devices, sensors, energy storage/conversion devices, oxidation resistance barriers etc. | Electronic devices, sensors, membranes, energy storage/conversion devices, encapsulation layer etc. | Electronic devices, sensors, membranes, energy storage/conversion devices, oxidation resistance barriers, encapsulation layer etc. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalita, G.; Umeno, M. Synthesis of Graphene and Related Materials by Microwave-Excited Surface Wave Plasma CVD Methods. AppliedChem 2022, 2, 160-184. https://doi.org/10.3390/appliedchem2030012
Kalita G, Umeno M. Synthesis of Graphene and Related Materials by Microwave-Excited Surface Wave Plasma CVD Methods. AppliedChem. 2022; 2(3):160-184. https://doi.org/10.3390/appliedchem2030012
Chicago/Turabian StyleKalita, Golap, and Masayoshi Umeno. 2022. "Synthesis of Graphene and Related Materials by Microwave-Excited Surface Wave Plasma CVD Methods" AppliedChem 2, no. 3: 160-184. https://doi.org/10.3390/appliedchem2030012
APA StyleKalita, G., & Umeno, M. (2022). Synthesis of Graphene and Related Materials by Microwave-Excited Surface Wave Plasma CVD Methods. AppliedChem, 2(3), 160-184. https://doi.org/10.3390/appliedchem2030012







