The Influence of Soaking and Sprouting on the Physicochemical Characteristics of Tigernut Tubers (Cyperus esculentus L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Origin and Treatments
2.2. Germination Capacity and Energy and Tigernut Tuber Sprouting
2.3. Physicochemical and Functional Analysis
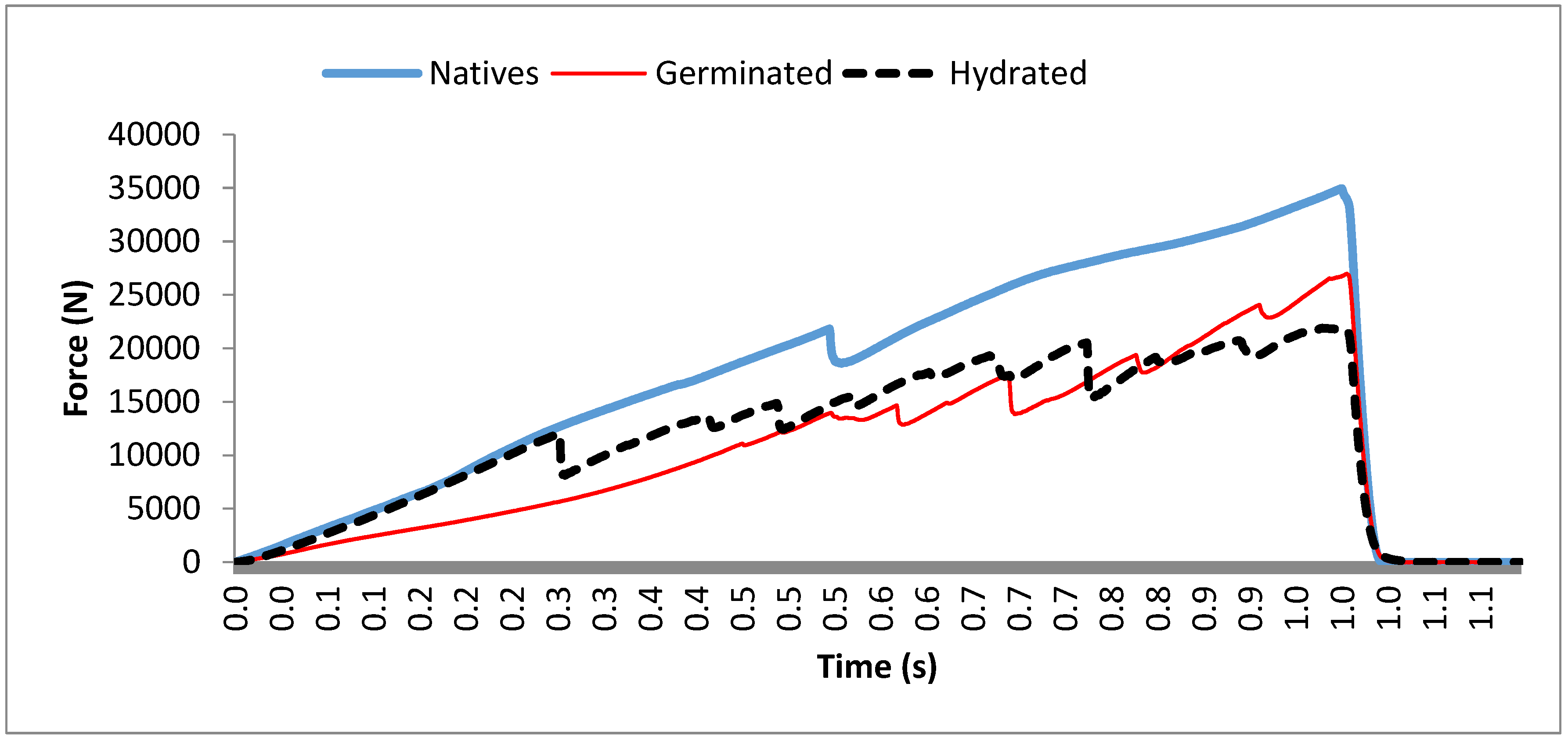
2.3.1. Textural Profile of Tigernut Tubers
2.3.2. Biochemical Assays
Alpha Amylase Activity
2.4. Statistical Analysis
3. Results
3.1. Behavior of Tigernut Tubers at Germination
3.2. Textural Profile of Tigernut Tubers
3.3. Impact of Germination on the Biochemical Composition of Tigernut Tubers
3.4. Influence of Germination on the Amino Acid Profile of Tigernut Tubers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez-Zapata, E.; Fernández-Lopez, J.; Pérez-Alvarez, J.A. Tigernut (Cyperus esculentus) commercialization: Health aspects, composition, properties and food applications. Compr. Rev. Food Sci. Food Saf. 2012, 11, 366–377. [Google Scholar] [CrossRef]
- Djomdi, D.; Kramer, J.K.G.; VanderJagt, D.J.; Ejoh, R.; Ndjouenkeu, R.; Glew, R.H. Influence of soaking on biochemical components of tiger nut (Cyperus esculentus) tubers cultivated in Cameroon. Int. J. Food Process Eng. 2013, 1, 16–28. [Google Scholar]
- Maduka, N.; Ire, F.S. A Review of Some Prevention Strategies against Contamination of Cyperus esculentus and Tigernut Derived Products of Economic Importance. Asian J. Adv. Res. Rep. 2019, 3, 1–13. [Google Scholar] [CrossRef]
- Djomdi, D.; Hamadou, B.; Gibert, O.; Tran, T.; Delattre, C.; Pierre, G.; Michaud, P.; Ejoh, R.; Ndjouenkeu, R. Innovation in Tigernut (Cyperus Esculentus L.) Milk Production: In Situ Hydrolysis of Starch. Polymers 2020, 12, 1404. [Google Scholar] [CrossRef]
- Nwobosi, P.N.U.; Isu, N.R.; Agarry, O.O. Influence of pasteurization and use of natural tropical preservatives on the quality attributes of tigernut drink during storage. Int. J Food Nutri. Sci. 2013, 2, 27–32. [Google Scholar]
- Ukwuru, M.U.; Ogbodo, A.C. Effect of processing treatment on the quality of tiger nut milk. Pak. J. Nutr. 2011, 10, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Codina, I.; Trujillo, A.J.; Ferragut, V.H. Traditional Foods, Integrating Food Science and Engineering Knowledge into the Food Chain; Springer Science + Business Media: New York, NY, USA, 2016; p. 348. [Google Scholar]
- Lorougnon, G. Etude morphologique et biologique de deux variétés de C. esculentus Linn. (Cyperacées) Cah. ORSTOM Ser. Biol. 1969, 10, 35–63. [Google Scholar]
- Umerie, S.C.; Enebeli, J.N. Malt caramel from tubers of Cyperus esculentus. Bioressource Technol. 1996, 57, 215–216. [Google Scholar] [CrossRef]
- Dodet, M.; Petit, R.J.; Gasquez, J. Local spread of the invasive Cyperus esculentus (Cyperaceae) interfered using molecular genetic markers. Weed Res. 2008, 48, 19–27. [Google Scholar] [CrossRef]
- Garcıa-Jimenez, J.; Busto, J.; Vicent, A.; Armengol, J. Control of Dematophora necatrix on Cyperus esculentus tubers by hotwater treatment. Crop Prot. 2004, 23, 619–623. [Google Scholar] [CrossRef]
- Maduka, N.; Ire, F.S. Tigernut plant and useful application of tigernut tubers (Cyperus esculentus)—A review. Current J. Appli. Sci. Technol. 2018, 29, 1–23. [Google Scholar] [CrossRef]
- Ejoh, R.; Djomdi, D.; Ndjouenkeu, R. Characteristics of tigernuts (Cyperus esculentus) tubers and their performance in the production of a milky drink. J. Food Processing Preserv. 2006, 30, 145–163. [Google Scholar] [CrossRef]
- Djomdi, D.; Ejoh, R.; Ndjouenkeu, R. Soaking behaviour and milky extraction performance of tiger nut (Cyperus esculentus) tubers. J. Food Eng. 2007, 78, 546–550. [Google Scholar] [CrossRef]
- European Brewery Convention Analysis Committee. European Brewing Convention, Analysis Committee; Fachverlag Hans Carl: Nürnberg, Germany, 1998. [Google Scholar]
- AFNOR. Recueil des normes françaises. In Produit Dérivé des Fruits et Légumes; AFNOR: Paris, France, 2002; 260p. [Google Scholar]
- Edem, D.O.; Ekwere, E.S.; Eke, O.U. Chemical evaluation of the effect of cooking on the nutritive value Conophor seed (Tetraca rpidium conophor). Trop. Sci. 1994, 34, 377–380. [Google Scholar]
- UICPA (Union Internationale de Chimie Pure et Appliquée). Méthodes D’analyse des Matières Grasses et Dérivés, 6th ed.; ETIG: Paris, France, 1979; 238p. [Google Scholar]
- Mestres, L.; Mestres, C. Détermination De La Teneur en Amidon Des Produits Céréaliers Fermentés. Afr. Food Tradit. Revisit. By Res.. 2011. Available online: https://www.after-fp7.eu/produits/produits-cerealiers (accessed on 15 February 2022).
- Chase, G.; Long, A.R. Liquid chromatographic analysis of all-rac-alpha-tocopheryl acetate, tocopherols, and retinyl palmitate in SRM 1846. J. Liq. Chromatogr. Relat. Technol. 1998, 20, 3317–3327. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; AOACP: Washington, DC, USA, 2000. [Google Scholar]
- Cohen, S.A.; Strydom, D.J. The pico-tag method: A manual of advanced techniques for amino acid analysis. Anal. Biochem. 1988, 174, 1–16. [Google Scholar] [CrossRef]
- Glew, R.H.; Kramer, J.K.G.; Hernandez, M.; Pastuszyn, A.; Ernst, J.; Djomdi, D.; VanderJagt, D.J. The Amino Acid, Mineral and Fatty Acid Content of Three Species of Human Plant Foods in Cameroun. Food 2010, 4, 1–6. [Google Scholar]
- Hugli, T.E.; Moore, S. Determination of the tryptophan content of proteins by ion exchange chromatography of alkaline hydrolysates. J. Biol. Chem. 1972, 247, 2828–2834. [Google Scholar] [CrossRef]
- Buzzigoli, G.; Natali, A.; Taddei, S. Haemodynamics and metabolism in human forearm: Effects of insulin. Diabetes 1990, 39, 490–500. [Google Scholar]
- Mc Grance, S.J.; Cornell, H.J.; Rix, J.C. A simple and rapid colorimetric method for the determination of amylose in starch products. Starch/Stärke 1998, 50, 158–163. [Google Scholar] [CrossRef]
- Briggs, D.E. Malts and Malting, 1st ed.; Blackie Academic & Professional, An Imprint of Thompson Science: London, UK, 1998; pp. 133–218. [Google Scholar]
- Desobgo, Z.S. Modélisation et Optimisation de L’action Des Enzymes Exogènes (Hitempase, Bioglucanase et Brewer Protéase) sur Quelques Caractéristiques Physico-Chimiques des Mouts: Application a Deux Variétés de Sorghos (Safrari et Madjeru). Ph.D. Thesis, ENSAI,IUT,ESMV, Ngaoundéré, Cameroon, 2012. [Google Scholar]
- Obeng-Koranteng, G.; Kavi, R.K.; Bugyei, K.A.; Anafo, P. Information sources used by tiger nut (Cyperus esculentus) farmers for improved sustainable agriculture development in Aduamoa Ghana. J. Sustainable. Dev. Afr. 2017, 19, 84102. [Google Scholar]
- Kaur, M.; Kaushal, P.; Sandhu, S.K. Studies on physicochemical and pasting properties of Taro (Colocasia esculenta L.) flour in comparison with a cereal, tuber and legume flour. J. Food Sci. 2011, 50, 94–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, Z.Y.; Gu, Z.B.; Tong, Q.Y.; Yang, R.J. Carbohydrate Chemistry, 1st ed.; Chemistry Factory Press: Beijing, China, 2008; pp. 306–347. [Google Scholar]
- Michalinos, A.-K. The nutrient content of tigernut (Cyperus esculentus) tubers. J. Food Compos. Anal. 1963, 10, 205–217. [Google Scholar]
- Buléon, A.; Colona, P.; Leloup, V.; Balls, S. Starch granules: Structure and biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112. [Google Scholar] [CrossRef] [Green Version]
- Costa Neto, J.; Silva, R.; Amaral, P.; Leão, M.R.; Gomes, T.; Sant’Ana, G. Extraction, chemical modification by octenyl succinic and characterization of cyperus esculentus starch. Polímeros 2018, 28, 319–322. [Google Scholar] [CrossRef] [Green Version]
- Yudkin, J. The Penguin Encyclopedia of Nutrition; By Penguin Books Ltd.: Harmondsworth, UK, 1988. [Google Scholar]
- Traoré, T.; Mouquet, C.; Icard-Vernière, C.; Traoré, A.S.; Trecie, S. Changes in nutrient composition phytate and cyanide contents and a-amylase activity during cereal malting in small production units in Ouagadougou (Burkina Faso). Food Chem. 2003, 88, 105–114. [Google Scholar] [CrossRef]
- Noort, M.W.J.; Renzetti, S.; Linderhof, V.; Du Rand, G.E.; Marx-Pienaar, N.J.M.M.; De Kock, H.L.; Magano, N.; Taylor, J.R.N. Towards Sustainable Shifts to Healthy Diets and Food Security in Sub-Saharan Africa with Climate-Resilient Crops in Bread-Type Products: A Food System Analysis. Foods 2022, 11, 135. [Google Scholar] [CrossRef]
- FAO/WHO. Energy and protein requirements. In Report of a joint FAO/WHO/UNU Expert Consultation; WHO Technical Reports Series No. 724; Food and Agriculture Organization of the United Nations: Rome, Italy, 2005. [Google Scholar]
- Jira-Anunkul, W.; Pattanagul, W. Seed priming with hydrogen peroxide alleviates the the effects of drought stress in rice (Oryza sativa L.) seedlings. Not. Bot. Horti. Agrobo. 2020, 48, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Kerchev, P.; Van der Meer, T.; Sujeeth, N.; Verlee, A.; Stevens, C.V.; Van Breusegem, F.; Gechev, T. Molecular priming as an approach to induce tolerance against abiotic and oxidative stresses in crop plants. Biotechnol. Adv. 2020, 31, 107503. [Google Scholar] [CrossRef]
- Amritha, M.S.; Sridharan, K.; Puthur, J.T.; Dhankher, O.P. Priming with Nanoscale Materials for Boosting Abiotic Stress Tolerance in Crop Plants. J. Agric. Food Chem. 2021, 69, 10017–10035. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C. Fotopoulos, V. Biostimulants for the Regulation of Reactive Oxygen Species Metabolism in Plants under Abiotic Stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef] [PubMed]
- Hübner, F.; Arendt, E.K. Germination of cereal grains as a way to improve the nutritional value: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Nam, D.S.; Kong, C. Variability in nutrient composition of cereal grains from different origins. Springer Plus 2016, 5, 419. [Google Scholar] [CrossRef] [Green Version]
- Jribi, S.; Sassi, K.; Sfayhi, D.; Debbabi, H. Sprouting, an Eco-Friendly Technology for Improving Nutritional Quality of Tunisian Wheat Cultivar “Khiar”. In Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions. EMCEI 2017. Advances in Science, Technology & Innovation (IEREK Interdisciplinary Series for Sustainable Development); Kallel, A., Ksibi, M., Dhia, H.B., Khélifi, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Singkhornart, S.; Edou-ondo, S.; Ryu, G.H. Influence of germination and extrusion with CO2 injection on physicochemical properties of wheat extrudates. Food Chem. 2013, 143, 122–131. [Google Scholar] [CrossRef]
- Donkor, O.N.; Stojanovska, L.; Ginn, P.; Ashton, J.; Vailjevic, T. Germinated grains–Sources of bioactive compounds. Food Chem. 2012, 135, 950–959. [Google Scholar] [CrossRef]


| Parameters | Values (%) |
|---|---|
| Water content | 7.542 ± 0.483 |
| Germination capacity (H202, 7.5 g/L) | 0 |
| Germination capacity (ascorbic acid, 1 g/L) | 94.740 ± 2.322 |
| Germination energy (24 mL) | 76.180 ± 0.541 |
| Germination energy (48 mL) | 79.482 ± 0.790 |
| Processes | DL (mm) | NPm | A (mm2) |
|---|---|---|---|
| NT | 39,650.74 a | 2.29 a | 9751.61 a |
| GT | 49,500.63 b | 10.20 b | 14,120.03 b |
| ST | 62,207.53 c | 14.77 c | 16,731.64 b |
| Characteristics (g/100 g DM) | NT (Control) | ST | GT |
|---|---|---|---|
| Water content (%) | 7.38 ± 0.14 c | 57.34 ± 0.23 a | 54.38 ± 0.54 b |
| Protein (g/100 g DM) | 7.62 ± 0.11 b | 7.42 ± 0.42 b | 8.82 ± 1.31 a |
| Total carbohydrates (g/100 g DM) | 49.92 ± 0.12 a | 47.52 ± 1.84 a | 48.93 ± 1.18 a |
| Reducing sugars (g/100 g DM) | 20.12 ± 1.11 c | 23.74 ± 1.74 b | 33.36 ± 0.35 a |
| Starch (g/100 g DM) | 26.14 ± 0.27 a | 25.13 ± 0.10 a | 16.63 ± 0.50 b |
| Amyloidosis (%) | 14.15 ± 0.72 a | 13.83 ± 1.21 a | 9.98 ± 0.15 b |
| Amylopectin (%) | 85.95 ± 0.73 b | 86.17 ± 1.27 b | 90.02 ± 0.13 a |
| Lipids (g/100 g DM) | 25.56 ± 0.41 a | 26.25 ± 0.53 a | 24.15± 0.02 b |
| Fibers (g/100 g DM) | 15.56 ± 0,12 a | 12.03 ± 0.94 b | 15.72 ± 0.09 a |
| Ashes (g/100 g DM) | 2.73 ± 0.31 a | 1.84 ± 0.07 b | 3.84 ± 0.18 a |
| Ascorbic acid (mg/100 g) | 252 ± 0.39 c | 328 ± 4.37 a | 275.39 ± 3.41 b |
| Vitamin E (mg/100 g) | 123 ± 0.18 a | 118.79 ± 3.26 b | 118.73 ± 0.55 b |
| Caloric value (kcal/100 g DM) | 445 | 450 | 462 |
| AA * (U/mL) | 3 ± 1.69 c | 15 ± 0.58 b | 60 ± 3.72 a |
| Amino Acids | NT (Control) | ST | GT | |
| Essential amino acids (mg/g DM) | His | 0.98 ± 0.07 a | 0.94 ± 0.16 a | 1.04 ± 0.11 a |
| Ile | 1.32 ± 0.07 a | 1.44 ± 0.22 a | 1.49 ± 0.05 a | |
| Leu | 2.41 ± 0.13 b | 2.73 ± 0.48 a | 2.88 ± 0.15 a | |
| Lys | 2.37 ± 0.14 a | 2.48 ± 0.43 a | 2.40 ± 0.19 a | |
| Met | 0.71 ± 0.01 a | 0.57 ± 0.18 a | 0.87 ± 0.03 a | |
| Phe | 1.45 ± 0.06 a | 1.63 ± 0.28 a | 1.72 ± 0.08 a | |
| Thr | 1.44 ± 0.17 c | 1.99 ± 0.21 b | 2.62 ± 0.23 a | |
| Trp | 0.92 ± 0.04 a | 0.85 ± 0.02 b | 0.95 ± 0.02 a | |
| Val | 1.90 ± 0.12 b | 2.10 ± 0.25 b | 2.88 ± 0.16 a | |
| Chemical index | 106 | 102 | 115 | |
| Banal amino acids (mg/g DM) | Ala | 2.30 ± 0.14 a | 2.34 ± 0.31 a | 2.37 ± 0.20 a |
| Arg | 6.89 ± 0.61 a | 4.74 ± 0.41 b | 7.26 ± 1.07 a | |
| Asp | 4.27 ± 0.56 a | 4.11 ± 0.85 a | 4.63 ± 0.39 a | |
| Cys | 0.75 ± 0.12 a | 0.63 ± 0.04 a | 0.88 ± 0.05 a | |
| Glu | 7.37 ± 0.73 a | 6.09 ± 1.02 a | 7.55 ± 0.58 a | |
| Gly | 1.54 ± 0.06 b | 1.67 ± 0.31 a | 1.76 ± 0.08 a | |
| Pro | 1.78 ± 0.06 a | 1.73 ± 0.34 a | 1.84 ± 0.19 a | |
| Ser | 1.88 ± 0.11 a | 2.04 ± 0.43 a | 1.92 ± 0.13 a | |
| Tyr | 1.04 ± 0.07 b | 1.12 ± 0.20 b | 1.24 ± 0.18 a | |
| Total | 75.4 ± 0.22 b | 76.2 ± 0.15 b | 88.2 ± 0.48 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djomdi; Bakari, H.; Gibert, O.; Tran, T.; Ejoh, R.; Christophe, G.; Michaud, P.; Ndjouenkeu, R. The Influence of Soaking and Sprouting on the Physicochemical Characteristics of Tigernut Tubers (Cyperus esculentus L.). AppliedChem 2022, 2, 48-58. https://doi.org/10.3390/appliedchem2020003
Djomdi, Bakari H, Gibert O, Tran T, Ejoh R, Christophe G, Michaud P, Ndjouenkeu R. The Influence of Soaking and Sprouting on the Physicochemical Characteristics of Tigernut Tubers (Cyperus esculentus L.). AppliedChem. 2022; 2(2):48-58. https://doi.org/10.3390/appliedchem2020003
Chicago/Turabian StyleDjomdi, Hamadou Bakari, Olivier Gibert, Thierry Tran, Richard Ejoh, Gwendoline Christophe, Philippe Michaud, and Robert Ndjouenkeu. 2022. "The Influence of Soaking and Sprouting on the Physicochemical Characteristics of Tigernut Tubers (Cyperus esculentus L.)" AppliedChem 2, no. 2: 48-58. https://doi.org/10.3390/appliedchem2020003
APA StyleDjomdi, Bakari, H., Gibert, O., Tran, T., Ejoh, R., Christophe, G., Michaud, P., & Ndjouenkeu, R. (2022). The Influence of Soaking and Sprouting on the Physicochemical Characteristics of Tigernut Tubers (Cyperus esculentus L.). AppliedChem, 2(2), 48-58. https://doi.org/10.3390/appliedchem2020003








