Intermolecular Interactions and In Vitro Performance of Methyl Anthranilate in Commercial Sunscreen Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
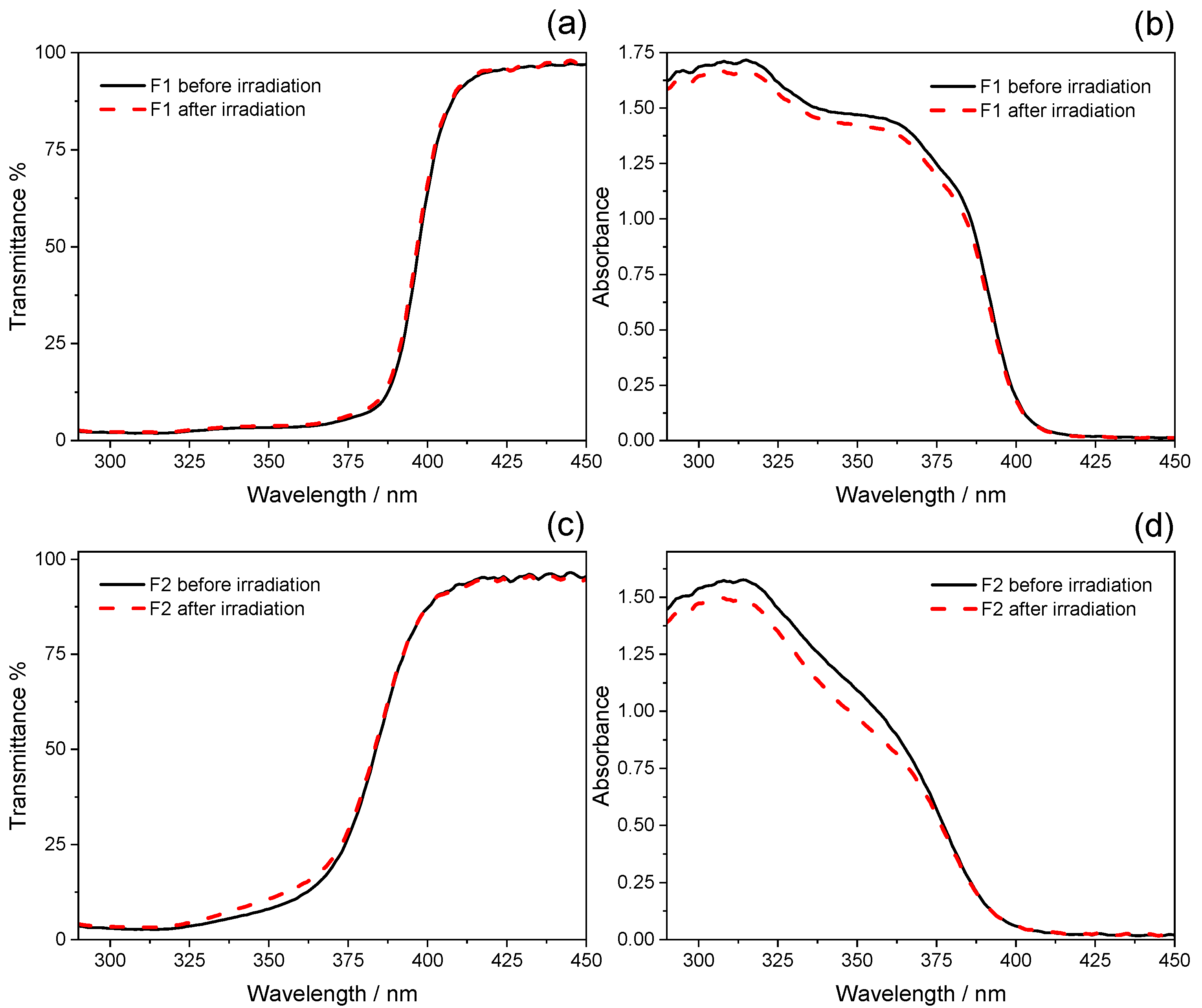
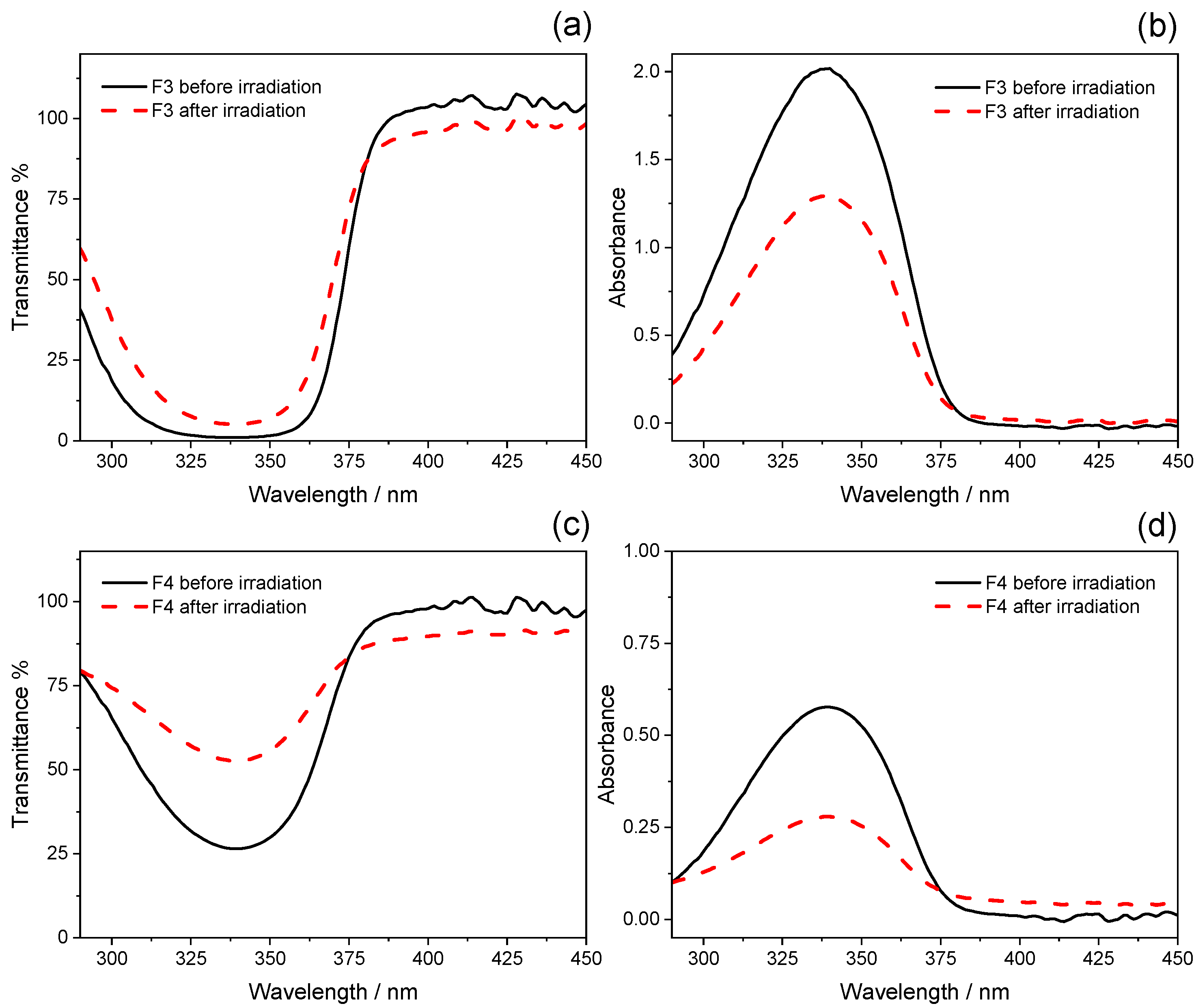
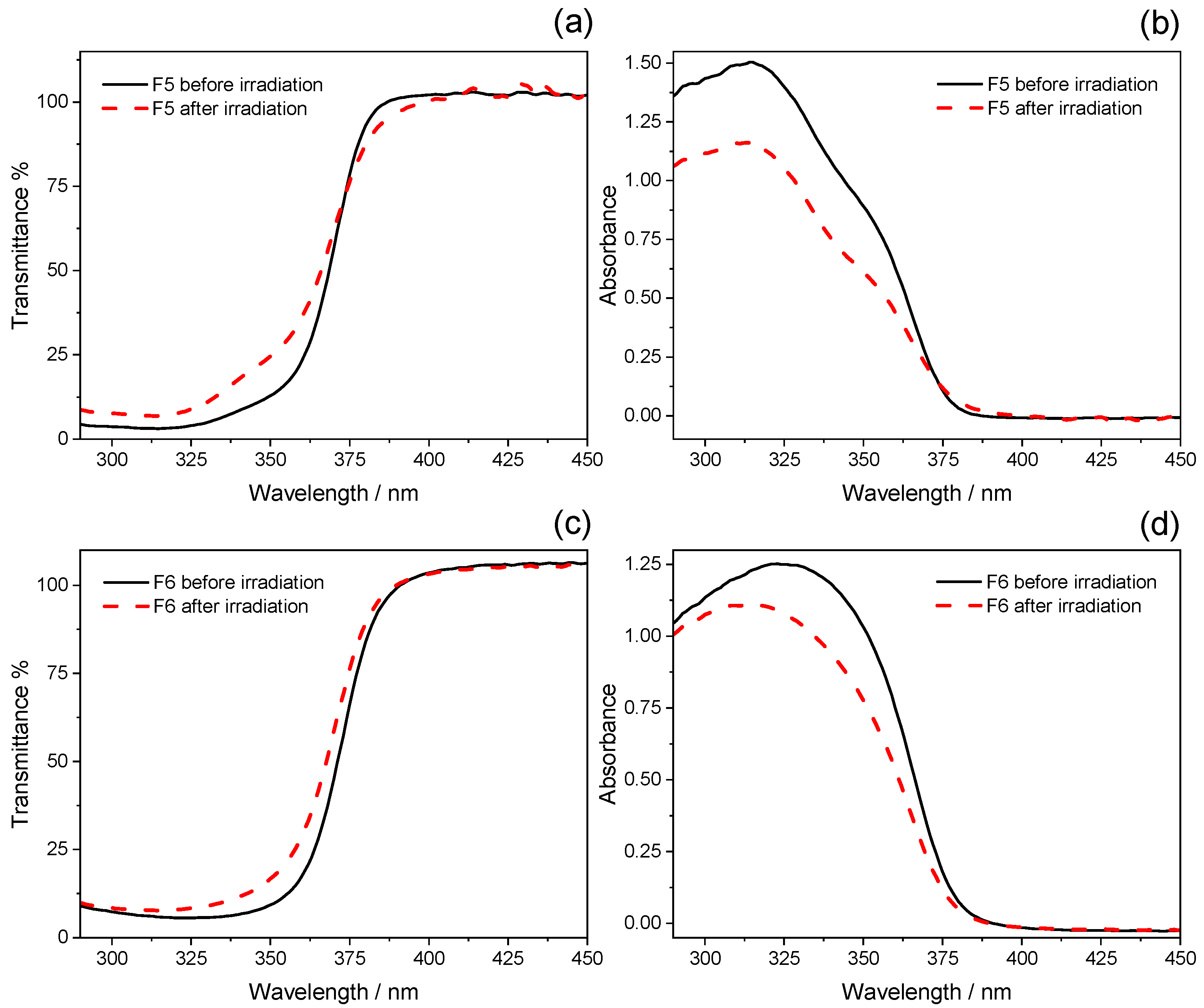
2.2. Photostability Tests
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallagher, R.P.; Lee, T.K. Adverse effects of ultraviolet radiation: A brief review. Prog. Biophys. Mol. Biol. 2006, 92, 119–131. [Google Scholar] [CrossRef]
- Wang, S.Q.; Balagula, Y.; Osterwalder, U. Photoprotection: A Review of the Current and Future Technologies. Dermatol. Ther. 2010, 23, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, J.B.; Maruthi, R.; Wang, S.Q.; Lim, H.W. Sunscreens: An Update. Am. J. Clin. Dermatol. 2017, 18, 643–650. [Google Scholar] [CrossRef]
- Ma, Y.; Yoo, J. History of sunscreen: An updated view. J. Cosmet. Dermatol. 2021, 20, 1044–1049. [Google Scholar] [CrossRef]
- Frederick, J.E.; Snell, H.E.; Haywood, E.K. Solar ultraviolet radiation at the earth’s surface. Photochem. Photobiol. 1989, 50, 443–450. [Google Scholar] [CrossRef]
- Cadet, J.; Mouret, S.; Ravanat, J.-L.; Douki, T. Photoinduced Damage to Cellular DNA: Direct and Photosensitized Reactions†. Photochem. Photobiol. 2012, 88, 1048–1065. [Google Scholar] [CrossRef] [PubMed]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [Green Version]
- Bens, G. Sunscreens. In Sunlight, Vitamin D and Skin Cancer; Springer: New York, NY, USA, 2008; pp. 137–161. [Google Scholar]
- Geoffrey, K.; Mwangi, A.N.; Maru, S.M. Sunscreen products: Rationale for use, formulation development and regulatory considerations. Saudi Pharm. J. 2019, 27, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Young, A.R.; Claveau, J.; Rossi, A.B. Ultraviolet radiation and the skin: Photobiology and sunscreen photoprotection. J. Am. Acad. Dermatol. 2017, 76, S100–S109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, N.d.N.; Stavros, V.G. From Fundamental Science to Product: A Bottom-up Approach to Sunscreen Development. Sci. Prog. 2018, 101, 8–31. [Google Scholar] [CrossRef] [Green Version]
- Serpone, N. Sunscreens and their usefulness: Have we made any progress in the last two decades? Photochem. Photobiol. Sci. 2021, 20, 189–244. [Google Scholar] [CrossRef]
- Gonzalez, H.; Tarras-Wahlberg, N.; Strömdahl, B.; Juzeniene, A.; Moan, J.; Larkö, O.; Rosén, A.; Wennberg, A.-M. Photostability of commercial sunscreens upon sun exposure and irradiation by ultraviolet lamps. BMC Dermatol. 2007, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Mturi, G.J.; Martincigh, B.S. Photostability of the sunscreening agent 4-tert-butyl-4′-methoxydibenzoylmethane (avobenzone) in solvents of different polarity and proticity. J. Photochem. Photobiol. A Chem. 2008, 200, 410–420. [Google Scholar] [CrossRef]
- Vallejo, J.J.; Mesa, M.; Gallardo, C. Evaluation of the avobenzone photostability in solvents used in cosmetic formulations. Vitae 2011, 18, 63–71. [Google Scholar]
- Dunkelberger, A.D.; Kieda, R.D.; Marsh, B.M.; Crim, F.F. Picosecond Dynamics of Avobenzone in Solution. J. Phys. Chem. A 2015, 119, 6155–6161. [Google Scholar] [CrossRef] [PubMed]
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Photostability of sunscreens. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 91–110. [Google Scholar] [CrossRef]
- Yamaji, M.; Kida, M. Photothermal Tautomerization of a UV Sunscreen (4-tert-butyl-4′-methoxydibenzoylmethane) in Acetonitrile Studied by Steady-State and Laser Flash Photolysis. J. Phys. Chem. A 2013, 117, 1946–1951. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Shedding More Light on Sunscreen Absorption: New Research Adds to Our Understanding of Sunscreens. Available online: https://www.fda.gov/news-events/fda-voices/shedding-more-light-sunscreen-absorption (accessed on 10 June 2021).
- Chatelain, E.; Gabard, B. Photostabilization of Butyl methoxydibenzoylmethane (Avobenzone) and Ethylhexyl methoxycinnamate by Bis-ethylhexyloxyphenol methoxyphenyl triazine (Tinosorb S), a New UV Broadband Filter. Photochem. Photobiol. 2007, 74, 401–406. [Google Scholar] [CrossRef]
- Gaspar, L.R.; Maia Campos, P.M.B.G. Evaluation of the photostability of different UV filter combinations in a sunscreen. Int. J. Pharm. 2006, 307, 123–128. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, M.J.; Im, N.R.; Park, S.N. Photolysis of the organic UV filter, avobenzone, combined with octyl methoxycinnamate by nano-TiO2 composites. J. Photochem. Photobiol. B Biol. 2015, 149, 196–203. [Google Scholar] [CrossRef]
- Dondi, D.; Albini, A.; Serpone, N. Interactions between different solar UVB/UVA filters contained in commercial suncreams and consequent loss of UV protection. Photochem. Photobiol. Sci. 2006, 5, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Lhiaubet-Vallet, V.; Marin, M.; Jimenez, O.; Gorchs, O.; Trullas, C.; Miranda, M.A. Filter–filter interactions. Photostabilization, triplet quenching and reactivity with singlet oxygen. Photochem. Photobiol. Sci. 2010, 9, 552. [Google Scholar] [CrossRef] [PubMed]
- Pirotta, G. Sunscreen Regulation in the World. In Sunscreens in Coastal Ecosystems: Occurrence, Behavior, Effect and Risk; Springer: Cham, Switzerland, 2020; pp. 15–35. [Google Scholar]
- Yadav, G.D.; Krishnan, M.S. An Ecofriendly Catalytic Route for the Preparation of Perfumery Grade Methyl Anthranilate from Anthranilic Acid and Methanol. Org. Process Res. Dev. 1998, 2, 86–95. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Browne, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Dagli, M.L.; Date, M.; Dekant, W.; et al. RIFM fragrance ingredient safety assessment, methyl anthranilate, CAS Registry Number 134-20-3. Food Chem. Toxicol. 2017, 110, S290–S298. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.D.N.; Cole-Filipiak, N.C.; Horbury, M.D.; Staniforth, M.; Karsili, T.N.V.; Peperstraete, Y.; Stavros, V.G. Photophysics of the sunscreen ingredient menthyl anthranilate and its precursor methyl anthranilate: A bottom-up approach to photoprotection. J. Photochem. Photobiol. A Chem. 2018, 353, 376–384. [Google Scholar] [CrossRef]
- Heerfordt, I.M.; Torsnes, L.R.; Philipsen, P.A.; Wulf, H.C. Photoprotection by sunscreen depends on time spent on application. Photodermatol. Photoimmunol. Photomed. 2018, 34, 117–121. [Google Scholar] [CrossRef]
- Afonso, S.; Horita, K.; Sousa e Silva, J.P.; Almeida, I.F.; Amaral, M.H.; Lobão, P.A.; Costa, P.C.; Miranda, M.S.; Esteves da Silva, J.C.G.; Lobo, J.S. Photodegradation of avobenzone: Stabilization effect of antioxidants. J. Photochem. Photobiol. B Biol. 2014, 140, 36–40. [Google Scholar] [CrossRef]
- Beeby, A.; Jones, A.E. The Photophysical Properties of Menthyl Anthranilate: A UV-A Sunscreen. Photochem. Photobiol. 2007, 72, 10–15. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kumasaka, R.; Yagi, M.; Kikuchi, A. Triplet–triplet energy transfer between UV absorbers in solutions at room temperature. J. Photochem. Photobiol. A Chem. 2017, 346, 396–400. [Google Scholar] [CrossRef]
- Peperstraete, Y.; Staniforth, M.; Baker, L.A.; Rodrigues, N.D.N.; Cole-Filipiak, N.C.; Quan, W.-D.; Stavros, V.G. Bottom-up excited state dynamics of two cinnamate-based sunscreen filter molecules. Phys. Chem. Chem. Phys. 2016, 18, 28140–28149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herzog, B.; Amorós-Galicia, L.; Sohn, M.; Hofer, M.; Quass, K.; Giesinger, J. Analysis of photokinetics of 2′-ethylhexyl-4-methoxycinnamate in sunscreens. Photochem. Photobiol. Sci. 2019, 18, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Lanzafame, G.; Sarakha, M.; Fabbri, D.; Vione, D. Degradation of Methyl 2-Aminobenzoate (Methyl Anthranilate) by H2O2/UV: Effect of Inorganic Anions and Derived Radicals. Molecules 2017, 22, 619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronov, E.V.; Clark, L. Degradation Studies of the Non-lethal Bird Repellent, Methyl Anthranilate. Pestic. Sci. 1996, 47, 355–362. [Google Scholar] [CrossRef]
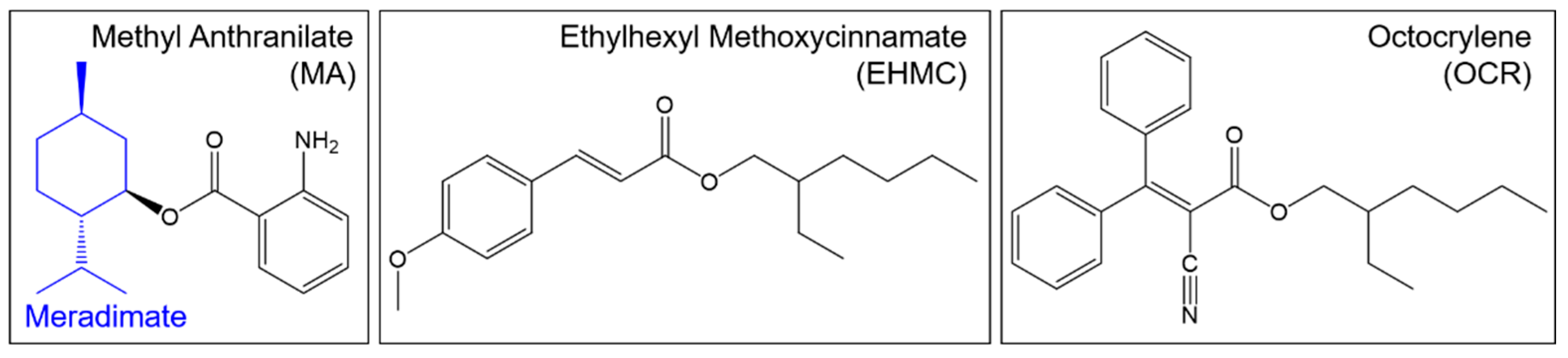
| UV Filter | Maximum Permissible Concentration (EU) * | Maximum Permissible Concentration (USA) ** |
|---|---|---|
| Ethylhexyl Triazone (Uvinul® T150) | 5% | Not approved as an UV filter |
| Bis-ethylhexylxyphenol Methoxyphenyl Triazine (Tinosorb® S) | 10% | Not approved as an UV filter |
| Ethylhexyl Methoxycinnamate (Neo Heliopan® AV) | 10% | 7.5% |
| Avobenzone (Butyl Methoxydibenzoylmethane, Parsol® 1789) | 5% | 3% |
| Octocrylene (Neo Heliopan® 303) | 10% | 10% |
| Methyl Anthranilate (CAS 134-20-3) | Not approved as an UV filter | Not approved as an UV filter |
| Phase | Raw Material | Supplier, City, Country | Formula Number | |||||
|---|---|---|---|---|---|---|---|---|
| F1 w/w % | F2 w/w % | F3 w/w % | F4 w/w % | F5 w/w % | F6 w/w % | |||
| Aqueous | Deionized water | - | 68.45 | 68.45 | 68.45 | 68.45 | 68.45 | 68.45 |
| Disodium EDTA | Ricardo Molina S.A.U., Barcelona, Spain | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | |
| PEMULENTM * EZ-4U | Lubrizol Advanced Materials, Barcelona, Spain | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | |
| CARBOPOL® * ULTREZ 30 | Lubrizol Advanced Materials, Barcelona, Spain | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | |
| GLUCAMTM * E-20 | Lubrizol Advanced Materials, Barcelona, Spain | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | |
| Oil | GLUCAMATETM * SSE-20 | Lubrizol Advanced Materials, Barcelona, Spain | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 |
| Ethylhexyl Triazone (Uvinul® T150) | BASF Española S. L., Barcelona, Spain | 1.00 | 1.00 | - | - | - | - | |
| Bis-ethylhexylxyphenol Methoxyphenyl Triazine (Tinosorb® S) | BASF Europe GmbH, Berlin, Germany | 2.50 | 2.50 | - | - | - | - | |
| Ethylhexyl Methoxycinnamate (Neo Heliopan® AV) | Symrise, Inc., Rennes, France | 4.00 | 4.00 | - | - | 9.70 | - | |
| Avobenzone (Butyl Methoxydibenzoylmethane) (Parsol® 1789) | DSM Nutritional Products Ltd., Heerlen, the Netherlands | 4.00 | - | - | - | - | - | |
| SCHERCEMOLTM * LL | Lubrizol Advanced Materials, Barcelona, Spain | 7.00 | 7.00 | 7.00 | 22.5 | 7.00 | 7.00 | |
| Octocrylene (Neo Heliopan® 303) | Symrise, Inc., Rennes, France | 8.00 | 8.00 | - | - | - | 9.70 | |
| Methyl Anthranilate (CAS 134-20-3) | Merck, Sigma-Adlrich, Madrid, Spain | - | 4.00 | 19.50 | 4.00 | 9.80 | 9.80 | |
| Other | Sodium Hydroxide (NaOH, 18%) | - | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Phenoxyethanol and ethylhexylglycerin (Euxyl® PE 9010) | Schülke and Mayr GmbH, Norderstedt, Germany | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | |
| Formula | SPF | UVAPF | Critical Wavelength/nm | |||
|---|---|---|---|---|---|---|
| Before Irradiation | After Irradiation | Before Irradiation | After Irradiation | Before Irradiation | After Irradiation | |
| F1 | 48 ± 1 | 43 ± 1 | 23.4 ± 0.1 | 21 ± 1 | 379.4 ± 0.3 | 378.8 ± 0.3 |
| F2 | 31 ± 2 | 25 ± 1 | 6.7 ± 0.2 | 5.9 ± 0.1 | 367.5 ± 0.4 | 368 ± 0 |
| F3 | 12 ± 1 | 4.8 ± 0.5 | 5.3 ± 0.2 | 3.7 ± 0.2 | 360 ± 0 | 360.5 ± 0.1 |
| F4 | 2.01 ± 0.04 | 1.48 ± 0.01 | 2.09 ± 0.04 | 1.52 ± 0.01 | 361.7 ± 0.1 | 370.7 ± 0.2 |
| F5 | 22 ± 1 | 11.6 ± 0.3 | 3.05 ± 0.03 | 2.47 ± 0.05 | 354.9 ± 0.1 | 355.5 ± 0.4 |
| F6 | 14 ± 1 | 11.3 ± 0.5 | 3.69 ± 0.05 | 2.81 ± 0.04 | 358.7 ± 0.2 | 356.3 ± 0.5 |
| Formula | % Change in Transmittance | % Change in Absorbance |
|---|---|---|
| F1 | 1.88 | −3.44 |
| F2 | 1.79 | −6.77 |
| F3 | 5.92 | −36.12 |
| F4 | 9.50 | −38.37 |
| F5 | 5.70 | −24.86 |
| F6 | 5.55 | −15.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, N.d.N.; Cebrián, J.; Montané, A.; Mendez, S. Intermolecular Interactions and In Vitro Performance of Methyl Anthranilate in Commercial Sunscreen Formulations. AppliedChem 2021, 1, 50-61. https://doi.org/10.3390/appliedchem1010005
Rodrigues NdN, Cebrián J, Montané A, Mendez S. Intermolecular Interactions and In Vitro Performance of Methyl Anthranilate in Commercial Sunscreen Formulations. AppliedChem. 2021; 1(1):50-61. https://doi.org/10.3390/appliedchem1010005
Chicago/Turabian StyleRodrigues, Natércia d. N., Juan Cebrián, Anna Montané, and Sandra Mendez. 2021. "Intermolecular Interactions and In Vitro Performance of Methyl Anthranilate in Commercial Sunscreen Formulations" AppliedChem 1, no. 1: 50-61. https://doi.org/10.3390/appliedchem1010005
APA StyleRodrigues, N. d. N., Cebrián, J., Montané, A., & Mendez, S. (2021). Intermolecular Interactions and In Vitro Performance of Methyl Anthranilate in Commercial Sunscreen Formulations. AppliedChem, 1(1), 50-61. https://doi.org/10.3390/appliedchem1010005






