The Past, Present and Future in Tube- and Paper-Based Colorimetric Gas Detectors
Abstract
1. Introduction
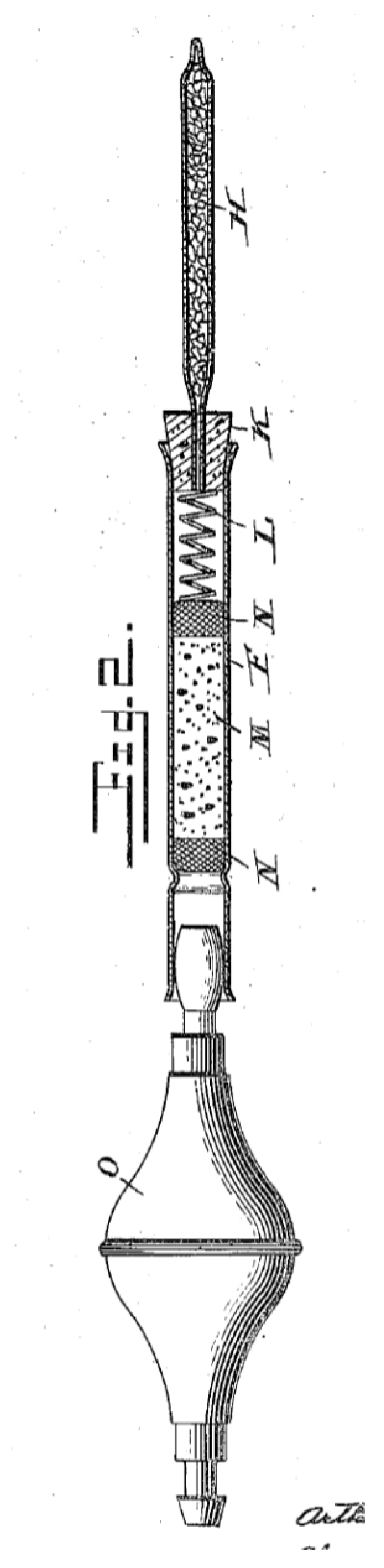
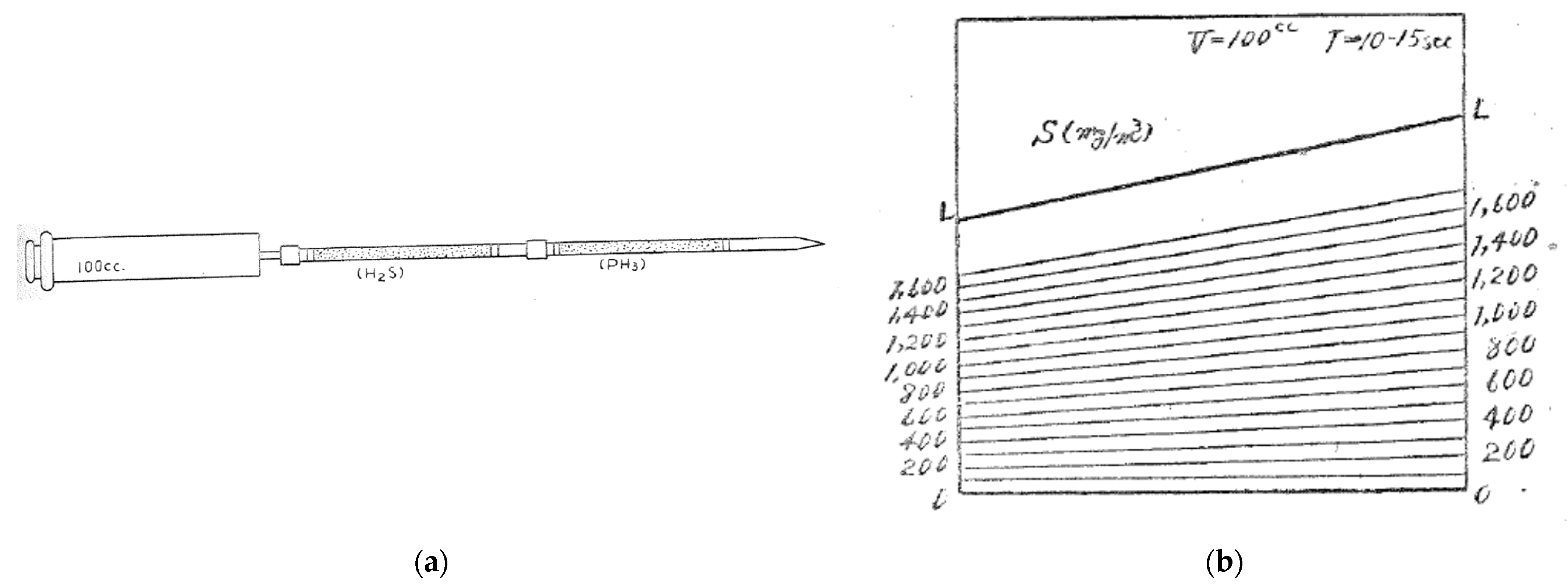
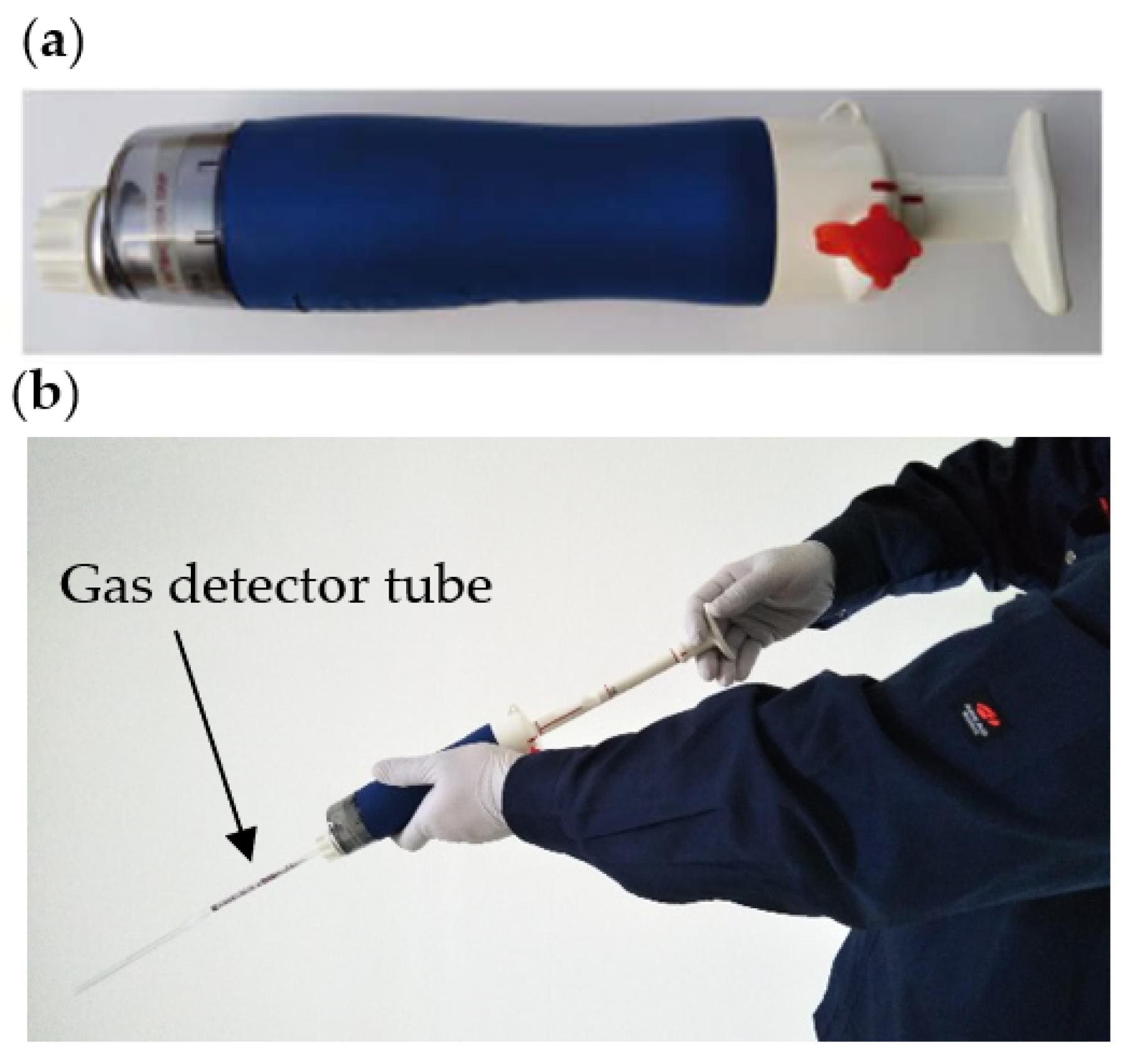
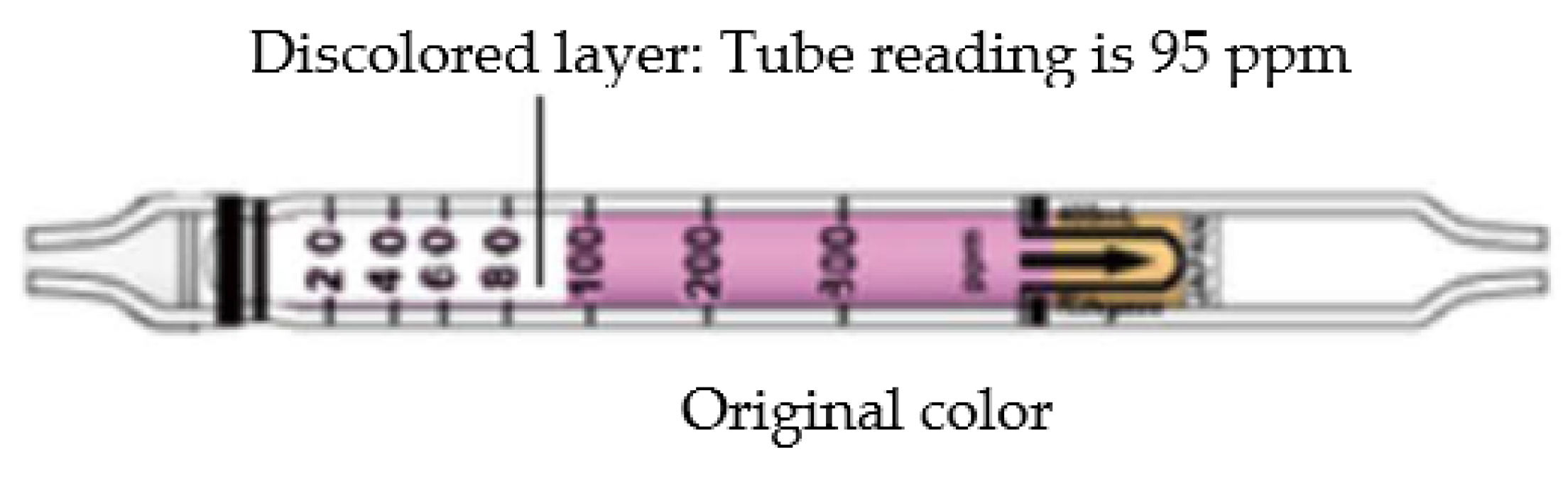
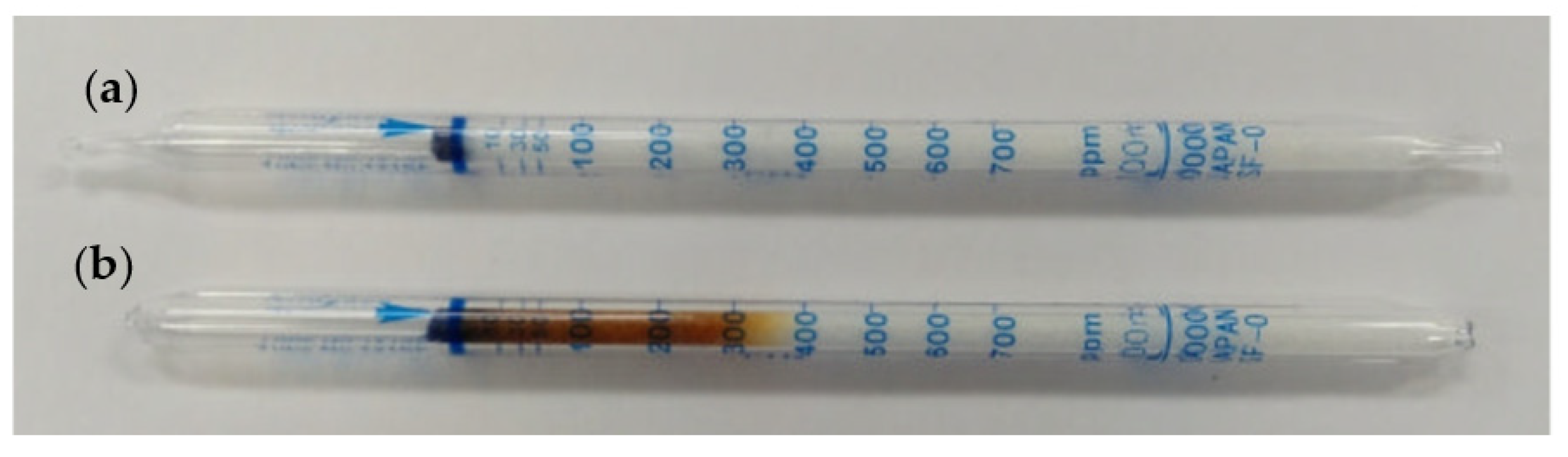
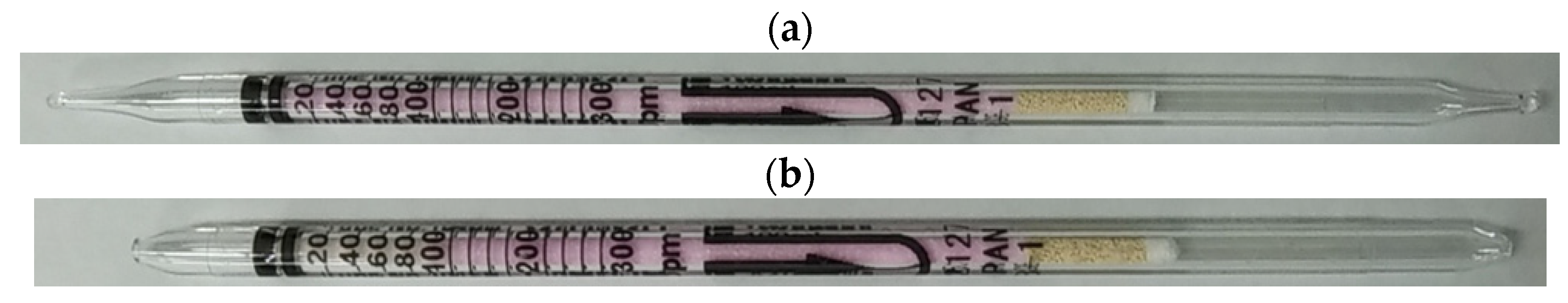
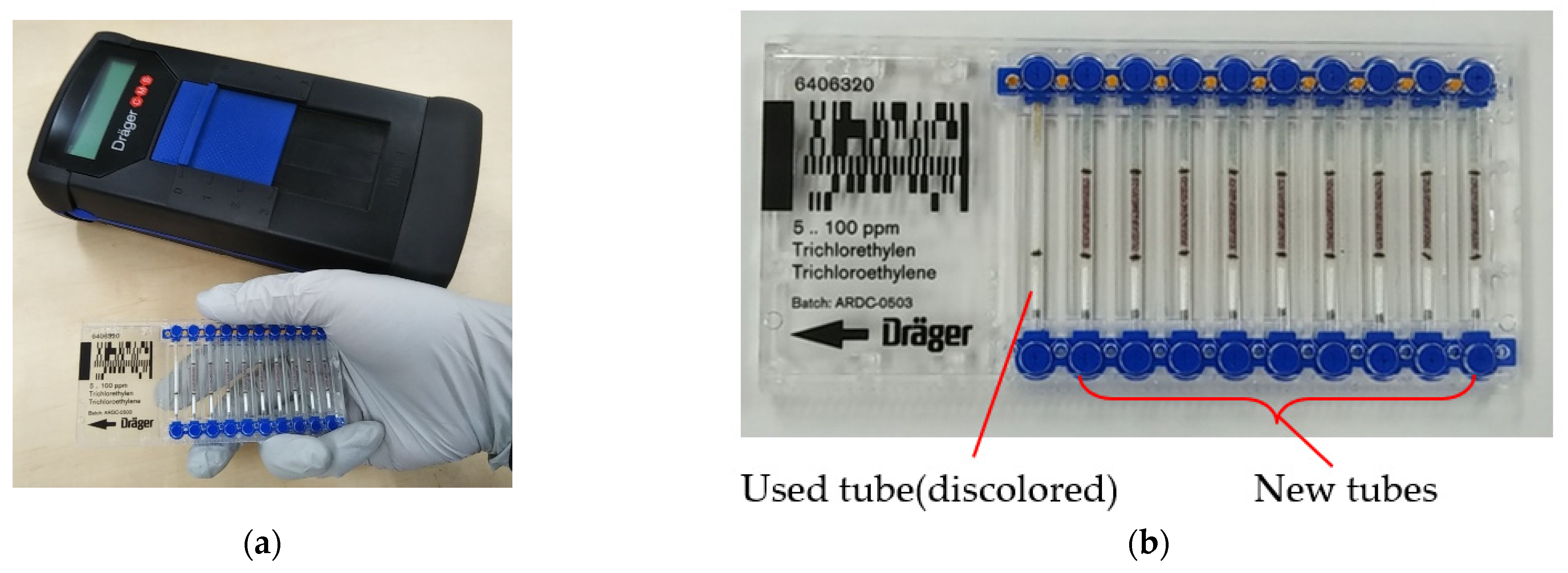
2. Gas Detector Tube Method
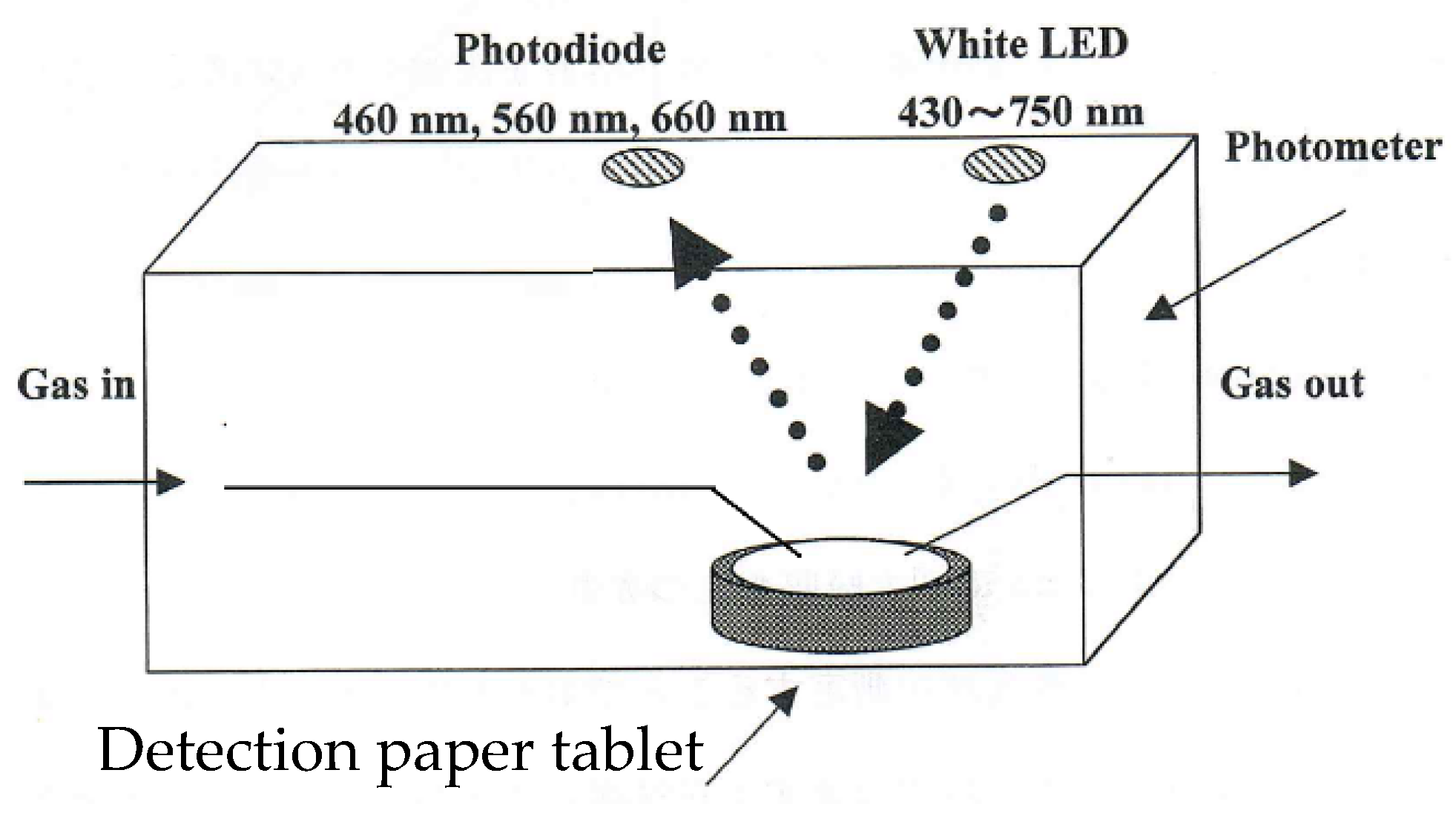
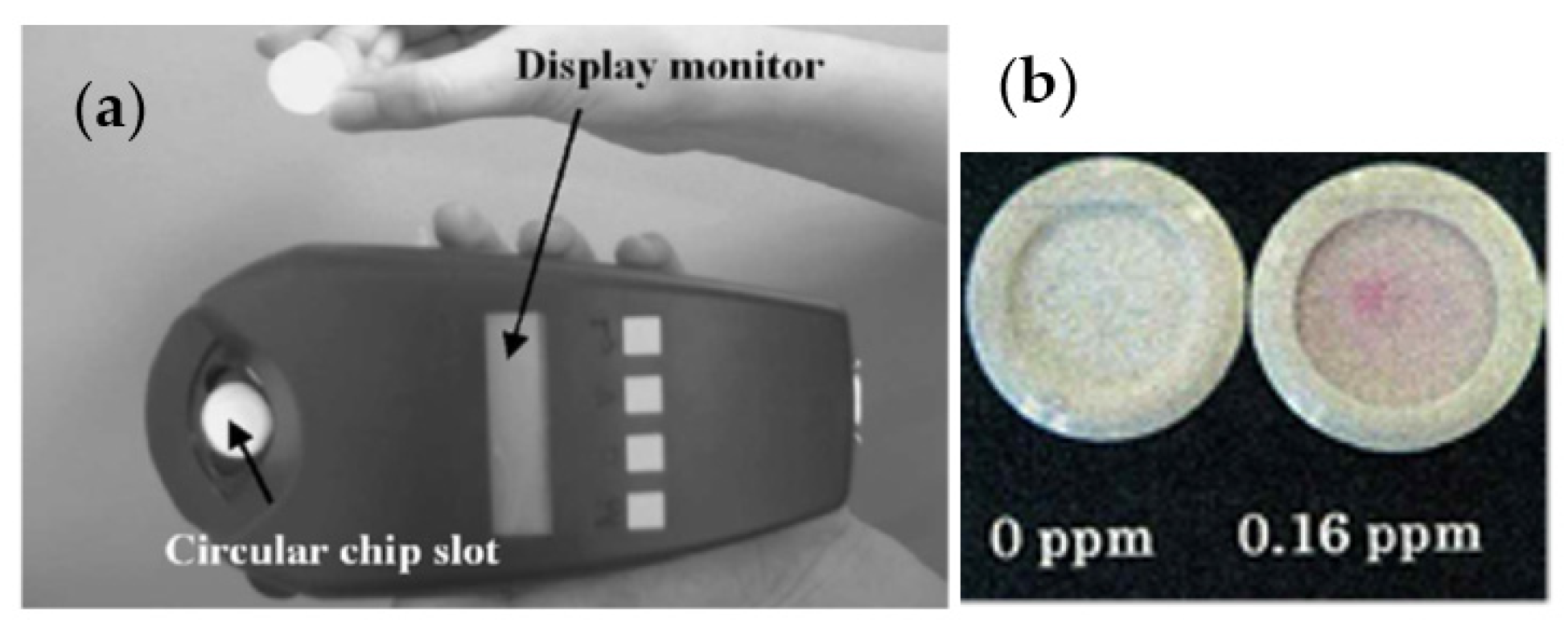
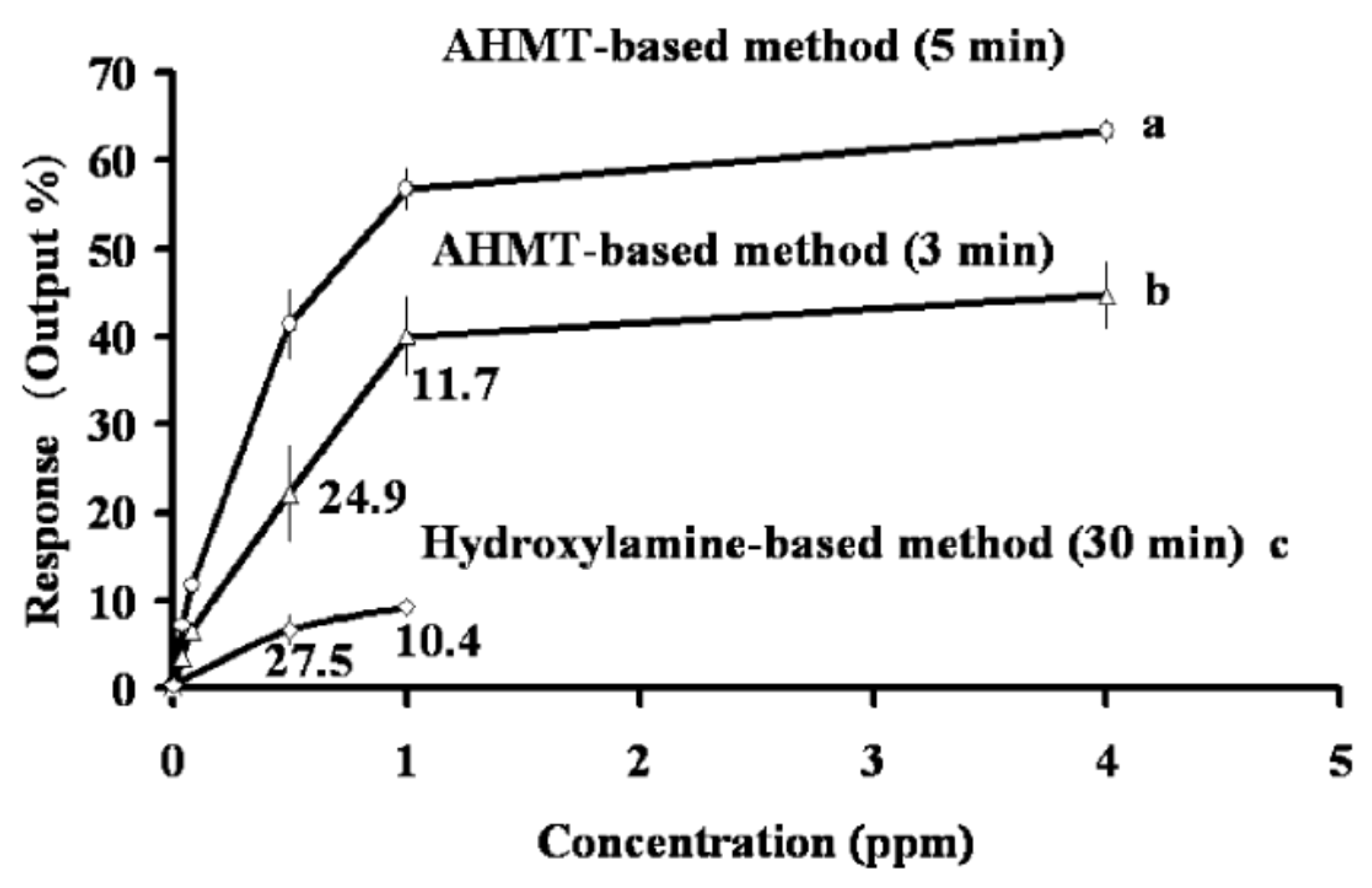
3. Gas Sensing Paper and Color Intensity Reader for a Gas Monitoring System
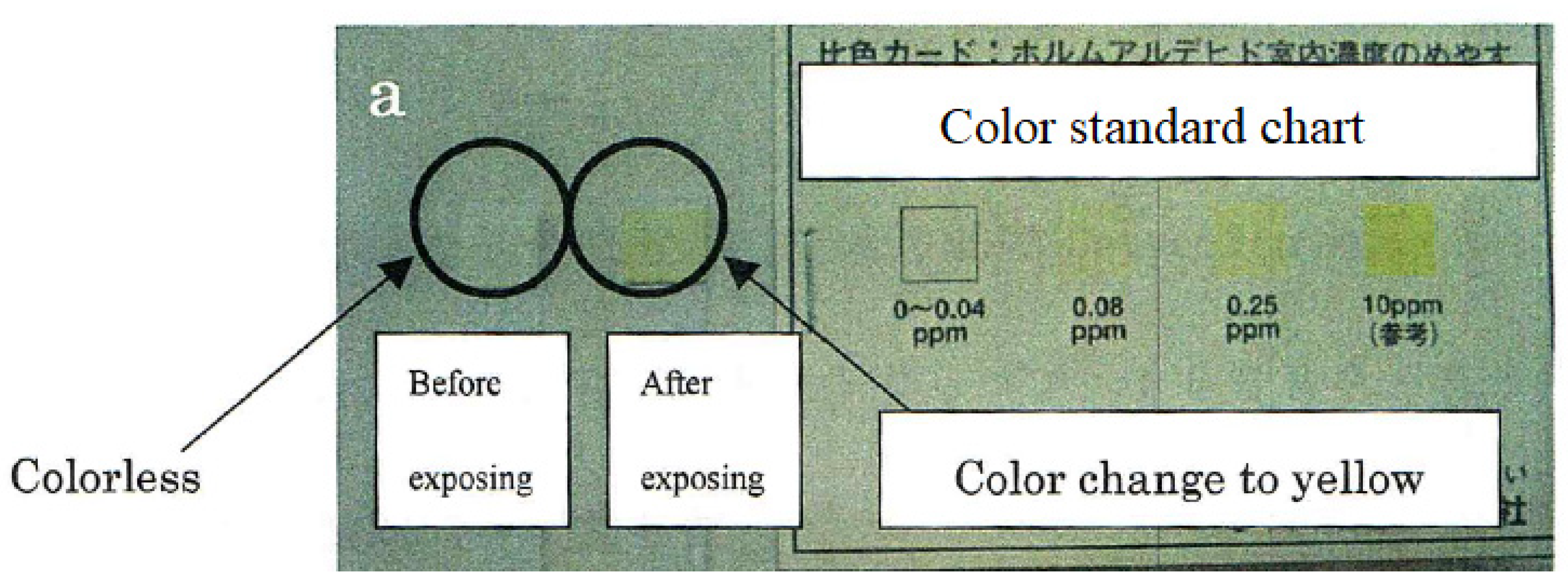
4. Passive Sampling Detection Paper Method
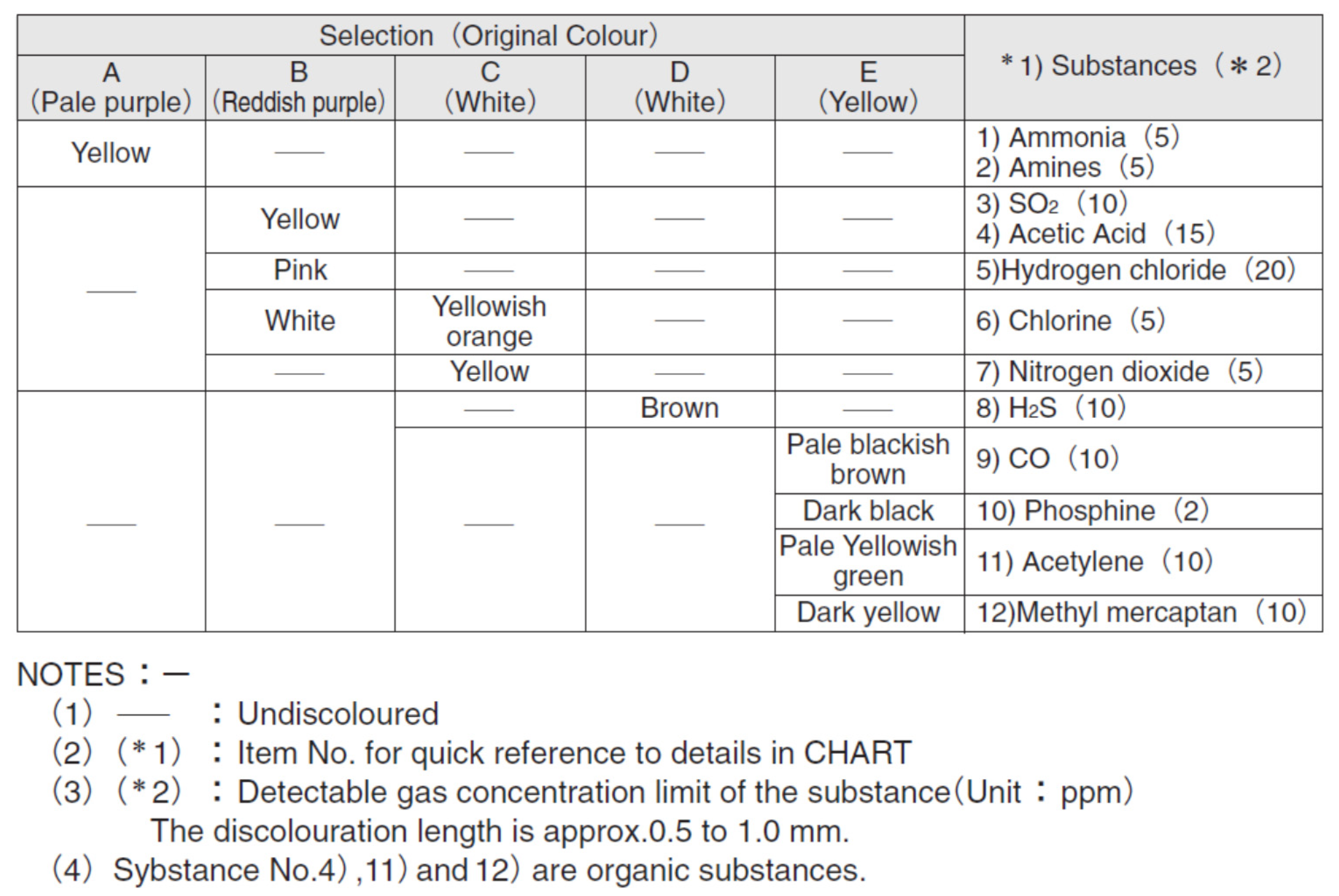
5. Colorimetric Sensor Arrays
6. Colorimetric Gas Detector with Biomaterial
7. Colorimetric Gas Detector and ICT Technology
8. Comparison of Various Gas Detector Technologies
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeffrey, K.S.; World War, I. The History of Chemical and Biological Detectors, Alarms, and Warning Systems; Kathleen, S.C., Ed.; Army Chemical and Biological Defense Command: Aberdeen, MD, USA, 2000. [Google Scholar]
- Angel, A.; Phil, B.; Vincenzo, C.; Christina, F.; Roger, G.; Janusz, L.; Arvid, N.; Milan, P.; Danilo, R. Hazard mitigation and preventive measures. In Hazards of Inert Gases and Oxygen Depletion; Revision of IGC Doc 44/00/E; European Industrial Gases Association Aisbl: Brussels, Belgum, 2009; p. 7. [Google Scholar]
- Lamb, A.B.; Hoover, C.R. Gas Detector. U.S. Patent 1,321,062, 28 December 1918. [Google Scholar]
- Kitagawa, T. Research of rapid gas detection method. J. High. Press. Gas. Saf. Inst. Jpn. 1948, 12, 2–12. [Google Scholar]
- Douglas, V.M.; Lawewnce, T.J.; Charleston, W.V. H2S recorder. U.S. Patent 2,232,622, 23 May 1940. [Google Scholar]
- Hirayama, S.; Nagashima, K.; Nakano, N. A Monitoring Tape for Chlorine Gas with N, N-Diethyl-p-Phenylenediamine Sulfate. J. Environ. Chem. 2000, 10, 867–871. [Google Scholar] [CrossRef]
- Maity, A.; Ghosh, B. Fast response paper based visual color change gas sensor for efficient ammonia detection at room temperature. Sci. Rep. 2018, 8, 16851. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K. Research on the Development and Practical Application of Gas Sensors for Sick Building Syndrome. Ph.D. Dissertation, Japan Advanced Institute of Science and Technology, Ishikawa, Japan, 2004. [Google Scholar]
- Mitsubayashi, K. Bio-sniffers and Sniff-cam based on human disease and metabolism for gaseous chemicals. J. Jpn. Assoc. Odor Environ. 2015, 46, 200–208. [Google Scholar] [CrossRef]
- Shinohara, N.; Kajiwara, T.; Ochiai, S.; Fujii, M.; Kobayashi, T.; Uchi, T. Ynagagisawa. Development of a Detector to Measure Emission Rates of Formaldehyde using Enzyme Reaction and Measurement of Reflectance Indoor Environment. J. Soc. Indoor Environ. Jpn. 2007, 9, 75–81. [Google Scholar]
- Saegusa, S.; Suzuki, M. Gas Detector Tube. Japanese Patent JP5081046, 19 March 2014. [Google Scholar]
- Gomes, J.; Rodrigues, J.J.; Rabêlo, R.A.; Kumar, N.; Kozlov, S. IoT-Enabled Gas Sensors: Technologies, Applications, and Opportunities. J. Sens. Actuator Netw. 2019, 8, 57. [Google Scholar] [CrossRef]
- Kawamura, K.; Okada, S.; Nakamoto, S. Cloud-Based IoT monitoring system for Oxygen. In Proceedings of the RSC Tokyo International Conference, Chiba, Japan, 6–7 September 2018; p. 34. [Google Scholar]
- Kitagawa, T. Rapid analysis of phosphine and hydrogen sulphide in acetylene. J. Japan Chem. Ind. Soc. 1951, 33, 1–10. [Google Scholar]
- Kawamura, K.; Ohkuma, H.; Miyazawa, K. Development of a Chromium-free Gas Detector Tube for Alcohols. Anal. Sci. 2021, 37, 387–391. [Google Scholar] [CrossRef]
- JIS K 0804:2014 Gas Detector Tube Measurement System (Length-of-Stain Type); Japanese Standards Association: Tokyo, Japan, 2014.
- ISO 17621:2015 Workplace Atmospheres—Short Term Detector Tube Measurement Systems—Requirements and Test Methods; The International Organization for Standardization: Geneva, Switzerland, 2015.
- Azuma, M.; Endo, Y.; Miyazaki, T.; Hikita, Y.; Ikeda, H.; Morita, Y.; Hara, I.; Araki, S. Efficacy of a Detector Tube Method in Formaldehyde Measurement. Ind. Health 2003, 41, 306–312. [Google Scholar] [CrossRef]
- Ishizawa, F.; Hirano, H. Development of a New Type Detector Tube for Light Mineral Oils. Bull. Jpn. Assoc. Fire Sci. Eng. 2005, 55, 29–32. [Google Scholar]
- Norimine, E.; Ishiwata, T.; Ishizawa, F.; Saegusa, S. Development of a Biodiesel Fuel Detector Tube. Bull. Jpn. Assoc. Fire Sci. Eng. 2009, 59, 56–60. [Google Scholar]
- Kobayashi, Y.; Abe, T.; Takeno, H.; Kanno, S.; Fukui, S.; Naito, S. Investigation on the contribution of engine exhausts to urban atmospheric pollution at a street of heavy traffic density in Yokohama. Bunseki Kagaku 1963, 12, 1057–1062. [Google Scholar] [CrossRef][Green Version]
- Hori, M.; Uda, K.; Yang, J.P. Determination of low-concentration formaldehyde in indoor air with discriminator and detector tube. Bunseki Kagaku 1998, 47, 405–410. [Google Scholar] [CrossRef][Green Version]
- Kawamura, K. Chapter 3 Gas detector tube. In New Information and Proposal for Modern Control Technology; Korai, H., Takatori, H., Eds.; Technosystems: Tokyo, Japan, 2019; pp. 245–248. [Google Scholar]
- Kawamura, K.; Honma, H.; Iwasaki, T.; Tanisho, S. Development of the Apparatus for Cellulosic Bio-Ethanol learning and Establishment of Its Procedure. Chem. Educ. J. 2009, 12, 2. [Google Scholar]
- Kelimu, A.; Hori, M.; Watanabe, Y.; Takashima, T. Development of an Apparatus for Model Experiment of Ozone Depletion and Establishment of Its Procedure. Chem. Educ. 2006, 54, 106–109. [Google Scholar]
- Kelimu, A.; Hori, M. Study on Application of Model Experiment Device for Ozone Layer Depletion to Classes. Chem. Educ. 2008, 17, 14–24. [Google Scholar]
- Kawamura, K.; Honma, H. Challenge to advance gas detector tube. Safety Digest. 2010, 56, 24–28. [Google Scholar]
- The WHO European Centre for Environment and Health, Bonn Office. Formaldehyde. In WHO Guidelines for Indoor Air Quality: Selected Pollutants; WHO Regional Office for Europe: Copenhagen, Denmark, 2010; pp. 103–142. [Google Scholar]
- Kawamura, K. Gas detector tube and measurement of odor. J. Jpn. Assoc. Odor Environ. 2019, 50, 371. [Google Scholar]
- Katsumata, M. Discharge Control Measures for Volatile Organic Compounds. J. Jpn. Assoc. Odor Environ. 2008, 39, 364–369. [Google Scholar] [CrossRef][Green Version]
- Kawamura, K.; Honma, H. Novel simplified VOC measurement methods for voluntary action on Air Pollution Control Law. Environ. Meas. Technol. 2006, 33, 14–21. [Google Scholar]
- Website of Komyo Rikagaku Kogyo. Available online: https://www.komyokk.co.jp/pdata/tpdf/120SE_1.pdf (accessed on 25 May 2021).
- Kitagawa, T.; Ogawa, T. A rapid method for the determination of phosphine and hydrogen sulfide in acetylene. Denki Kagaku 1951, 19, 258–261. [Google Scholar]
- Kitagawa, T.; Wright, B.M. A quantitative detector-tube method for breath-alcohol estimation. Br. Med. J. 1962, 2, 652–653. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Molecular. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar]
- Kawamura, K.; Saegusa, S.; Miyazawa, K. Development of a Gas Detector tube for Phosphine based on Silver without Mercury. In Proceedings of the RSC Tokyo International Conference, Makuhari, Japan, 5 September 2019; p. 55. [Google Scholar]
- Website of Minamata Convention on Mercury. Available online: http://www.mercuryconvention.org/ (accessed on 20 May 2021).
- Saegusa, S.; Suzuki, M. Detecting Tube. JP Patent 6196803, 4 December 2014. [Google Scholar]
- Kubota, R. Qualitative Toxic Gas Detector Tube. Japanese Patent JP59-194066U1, 14 July 1983. [Google Scholar]
- Website of Komyo Rikagaku Kogyo. Available online: https://www.komyokk.co.jp/pdata/tpdf/131_1.pdf (accessed on 25 May 2021).
- Hartmut, S.; Peter, D. A Device for Colorimetric Detection of Gaseous and/or Vapor Components of a Gas Mixture. Japanese Patent JP06-242008A, 10 February 1994. [Google Scholar]
- Wolfgang, B. Gas Measurement Chip, Transportable Chip Measurement System and Method for Operating a Transportable Chip Measurement System. International Patent WO 2016/074788 AI, 19 May 2016. [Google Scholar]
- Douglas, V.; Moses, J.W.; Brumbaugh, C.W.V. Automatic Gas Analyzer. U.S. Patent 2,551,281, 29 November 1944. [Google Scholar]
- Keinash, G. Record Survey: 11 Chemical Recording. In Recording Surface and Marking Methods; United States Department of Commerce National Bureau of Standards: Washington, DC, USA, 1959; pp. 33–34. [Google Scholar]
- Nakano, N.; Yamamoto, A.; Kawabe, T.; Nagashima, K. An Automatic Measurement of Formaldehyde in Air by a Monitoring Tape Method. Nippon Kagaku Kaishi 1998, 7, 506–510. [Google Scholar] [CrossRef]
- Nakano, N.; Ishikawa, M.; Kobayashi, Y.; Nagashima, K. Development of a Monitoring Tape for Formaldehyde Using Hydroxylamine Sulfate and Methyl Yellow. Anal. Sci. 1994, 10, 641–645. [Google Scholar] [CrossRef]
- Nagashima, K.; Tamura, M.; Ishiguro, G.; Nakano, N. Development of a Monitoring Tape for Hydrogen Cyanide in Air Using Cu (II)-DPD and Eliminating Oxidizing Gases. J. Jpn. Soc. Atmos. Environ. 2005, 40, 165–171. [Google Scholar]
- Nakano, N.; Kawabe, T.; Isaka, M.; Nozima, Y. An automatic monitor of ammonia in air using ammonia-sensitive tape. Bunseki Kagaku 1998, 47, 423–428. [Google Scholar] [CrossRef]
- Nakano, N.; Yabe, H.; Nagashima, K.; Kamaya, M. Development of a monitoring tape for fluorescence detection of hydrogen chloride gas using 6, 9-dichloro-2-methoxyacridine. Bunseki Kagaku 1995, 44, 151–155. [Google Scholar] [CrossRef][Green Version]
- Nakano, N.; Yamamoto, A.; Kobayashi, Y.; Nagashima, K. Development of a monitoring tape for determination of hydrogen sulfide using silver perchlorate. Bunseki Kagaku 1994, 43, 645–648. [Google Scholar] [CrossRef][Green Version]
- Nakano, N.; Kobayashi, Y.; Nagashima, K. Development of a tape for the determination of silane using ethyleneglycol. Bunseki Kagaku 1993, 42, 89–92. [Google Scholar] [CrossRef]
- Nakano, N.; Kobayashi, Y.; Nagashima, K. An improvement of light resistance of the tape for the determination of phosphin and arsine. Bunseki Kagaku 1992, 41, 659–661. [Google Scholar] [CrossRef]
- Terauchi, Y.; Kawabe, T.; Nakano, N.; Suzuki, Y.; Suzuki, K. Development of a Portable Monitor for a Formaldehyde in the Workplace Environment. Bunseki Kagaku 2005, 54, 65–70. [Google Scholar] [CrossRef][Green Version]
- Suzuki, Y.; Fujikawa, T.; Nakano, N.; Terauchi, Y.; Suzuki, K. Development of Colorimetric Reagents and Easy Monitoring Devices for the Selective Detection of Formaldehyde and Their Practical Uses. Bunseki Kagaku 2010, 59, 885–894. [Google Scholar] [CrossRef][Green Version]
- Suzuki, Y.; Nakano, N.; Suzuki, K. Portable Sick House Syndrome Gas Monitoring System Based on Novel Colorimetric Reagents for the Highly Selective and Sensitive Detection of Formaldehyde. Environ. Sci. Technol. 2003, 37, 5695–5700. [Google Scholar] [CrossRef]
- Ishiguro, G.; Kawabe, T.; Nakano, N.; Nagashima, K. Development of a Detection Tablet for a Portable NO2 Monitoring System. Anal. Sci. 2006, 22, 789–791. [Google Scholar] [CrossRef]
- Kawamura, K.; Kerman, K.; Fujihara, M.; Nagatani, N.; Hashiba, T.; Tamiya, E. Development of a novel hand-held formaldehyde gas sensor for the rapid detection of sick building syndrome. Sens. Actuators B Chem. 2005, 105, 495–501. [Google Scholar] [CrossRef]
- Kawamura, K.; Vestergaard, M.; Ishiyama, M.; Nagatani, N.; Hashiba, T.; Tamiya, E. Development of a novel hand-held toluene gas sensor: Possible use in the prevention and control of sick building syndrome. Measurement 2006, 39, 490–496. [Google Scholar] [CrossRef]
- Cook, D.A.; Syracuse, Y.N. Test Strips for Detecting Low Concentrations of Chlorine in Air. U.S. Patent 2,606,102, 3 December 1946. [Google Scholar]
- Website of American Chemistry Council. 4.0 Industrial Hygiene and Personal Protective Equipment. Available online: https://www.americanchemistry.com/Industrial-Hygiene-and-Protective-Equipment/ (accessed on 20 May 2021).
- Diller, W.; Reichold, E. Plaque for Measuring the Dose of Reactive Gases. U.S. Patent 4,271,121, 13 September 1979. [Google Scholar]
- American Conference of Governmental Industrial Hygienists. 2021 Threshold Limit Values (TLVs) and Biological Exposure Indices (BEIs); Signature Publication; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 2021; p. 34.
- Maruo, Y.Y.; Nakamura, J. Indoor air monitoring using newly developed portable formaldehyde monitoring device. Indoor Environ. 2010, 13, 163–172. [Google Scholar] [CrossRef]
- Askim, J.R.; Li, Z.; LaGasse, M.K.; Rankina, J.M.; Suslick, K.S. An optoelectronic nose for identification of explosives. Chem. Sci. 2016, 7, 199–206. [Google Scholar] [CrossRef]
- Li, Z.; Askim, J.R.; Suslick, K.S. The Optoelectronic Nose: Colorimetric and Fluorometric Sensor Arrays. Chem. Rev. 2019, 119, 231–292. [Google Scholar] [CrossRef]
- Lim, S.H.; Feng, L.; Kemling, J.W.; Musto, C.J.; Suslick, K.S. An optoelectronic nose for the detection of toxic gases. Nat. Chem. 2009, 1, 562–567. [Google Scholar] [CrossRef]
- Kangas, M.J.; Burks, R.M.; Atwater, J.; Lukowicz, R.M.; Williams, P.; Holmes, A.E. Colorimetric Sensor Arrays for the Detection and Identification of Chemical Weapons and Explosives. Crit. Rev. Anal. Chem. 2017, 47, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Mitsubayashi, K.; Hashimoto, Y.; Nakayama, Y.; Montesinos, T.; Nouger, T.; Marty, J.L. Gas-sensors with high selectivity using a biological catalytic system. J. Adv. Sci. 2000, 12, 211–216. [Google Scholar] [CrossRef]
- Seto, Y. On-site Detection Method for Biological and Chemical Warfare Agents. Bunseki Kagaku. 2006, 55, 891–906. [Google Scholar] [CrossRef][Green Version]
- Kawamura, K.; Hashiba, T.; Tamiya, E. Phosphate Ester Detection Method, Detection Device and Detection Kit. Japanese Patent JP2003-322619, 2 March 2002. [Google Scholar]
- Owyeung, R.E.; Panzer, M.J.; Sonkusale, S.R. A colorimetric gas sensing washable threads for smart textiles. Sci. Rep. 2019, 9, 5607. [Google Scholar] [CrossRef]
- Wodjenski, M.J.; Arno, J.I. Gas Cabinet Including Integrated Effluent Scrubber. U.S. Patent 7,018,448 B2, 28 October 2003. [Google Scholar]
- Website of Komyo Rikagaku Kogyo. Available online: https://www.komyokk.co.jp/product/001/001/0136.html (accessed on 25 May 2021).
- Cho, S.H.; Suh, J.M.; Eom, T.H.; Kim, T.; Jang, H.W. Colorimetric Sensors for Toxic and Hazardous Gas Detection: A Review. Electron. Mater. Lett. 2021, 17, 1–17. [Google Scholar] [CrossRef]
- Lin, C.; Du, Z.; Tao, N.; Wang, D. Gradient-Based Colorimetric Array Sensor for Continuous Monitoring of Multiple Gas Analytes. ACS Sens. 2021, 6, 439–442. [Google Scholar] [CrossRef]
- Nitta, T.; Takeda, Y.; Haradome, M. 1.3 Type and characteristic of gas sensor. In Gas Sensors and Their Application; Power: Tokyo, Japan, 1987; pp. 8–17. [Google Scholar]
- Oyabu, T.; Kimura, H. Development of a Monitoring System for Indoor Air-Pollution Using a Semiconductive Tin Oxide Gas Sensor. Trans. Soc. Instrum. Control. Eng. 1996, 32, 1121–1128. [Google Scholar] [CrossRef][Green Version]
- Yamazoe, N. Semiconductor type gas sensor. In Chemical Sensor Practical Handbook; Seiyama, T., Ed.; NTS: Tokyo, Japan, 1986; pp. 13–18. [Google Scholar]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Mirzaei, A.; Lee, J.H.; Majhi, S.M.; Weber, M.; Bechelany, M.; Kim, H.W.; Kim, S.S. Resistive gas sensors based on metal-oxide nanowires. J. Appl. Phys. 2019, 126, 241102. [Google Scholar] [CrossRef]
- Feng, S.; Farha, F.; Li, Q.; Wan, Y.; Xu, Y.; Zhang, T.; Ning, H. Review on Smart Gas Sensing Technology. Sensors 2019, 19, 3760. [Google Scholar] [CrossRef]
- Egashira, M. Catalytic Combustion type gas sensor. In Chemical Sensor Practical Handbook; Seiyama, T., Ed.; NTS: Tokyo, Japan, 1986; pp. 61–69. [Google Scholar]
- Ishiji, T. Elecrochemical Gas Sensors for Environmental Monitoring. IEEJ Trans. Sens. Micromach. 1998, 118, 76–79. [Google Scholar] [CrossRef][Green Version]
- Shiokawa, J.; Yamakawa, K. Electrochemical sensor. In Chemical Sensor Practical Handbook; Seiyama, T., Ed.; NTS: Tokyo, Japan, 1986; pp. 61–71. [Google Scholar]
- Hori, H.; Ishimatsu, S.; Fueta, Y.; Hinoue, M.; Ishidao, T. Sensing Characteristics of a Real-time Monitor Using a Photoionization Detector on Organic Solvent Vapors. J. UOEH 2012, 34, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Website of the Department of Homeland Security. Available online: https://www.dhs.gov/sites/default/files/publications/HH-PIDs-MSR_1113-508.pdf (accessed on 20 July 2021).
- RIKEN KEIKI Co., Ltd. 11-3. Kist of gases for reading VOC. In Portable Multi-Gas Monitor GX-6000 Operating Munual; RIKEN KEIKI Co., Ltd.: Tokyo, Japan, 2014; pp. 120–140. [Google Scholar]
- Website of Komyo Rikagaku Kogyo. Available online: https://www.komyokk.co.jp/en/product/001/index.html (accessed on 25 May 2021).
- Website of Gastec. Available online: https://www.gastec.co.jp/product/detector_tube/search/ (accessed on 19 July 2021).
- Drager Safety AG & Co KGaA. Drager-Tube/CMS Handbook: Handbook for Short Term Measurements in Soil, Water, and Air Investigations as Well as a Technical Gas Analysis; Drager Safety AG & Co KGa: Lubeck, Germany, 2004; pp. 59–263. [Google Scholar]
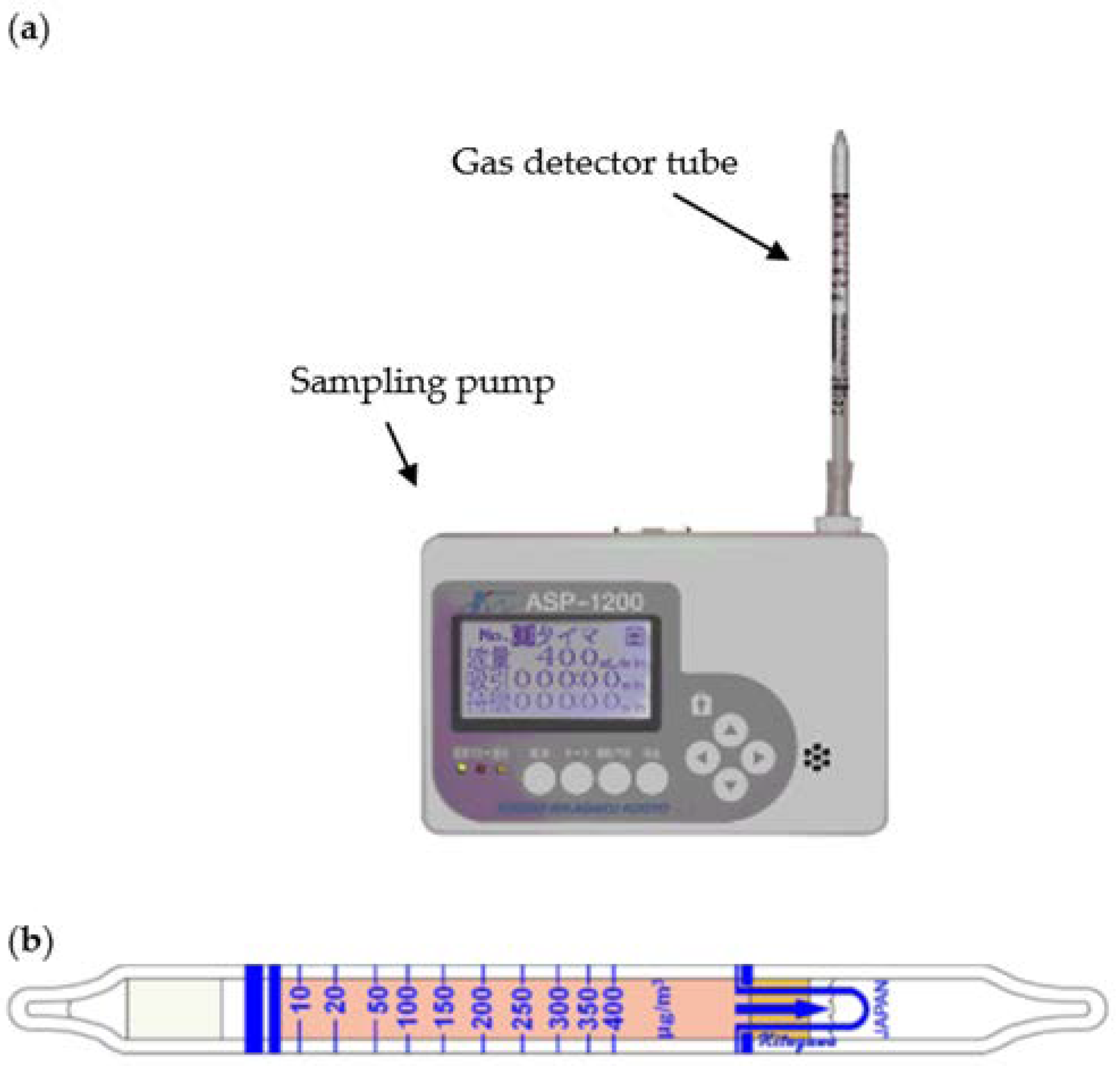
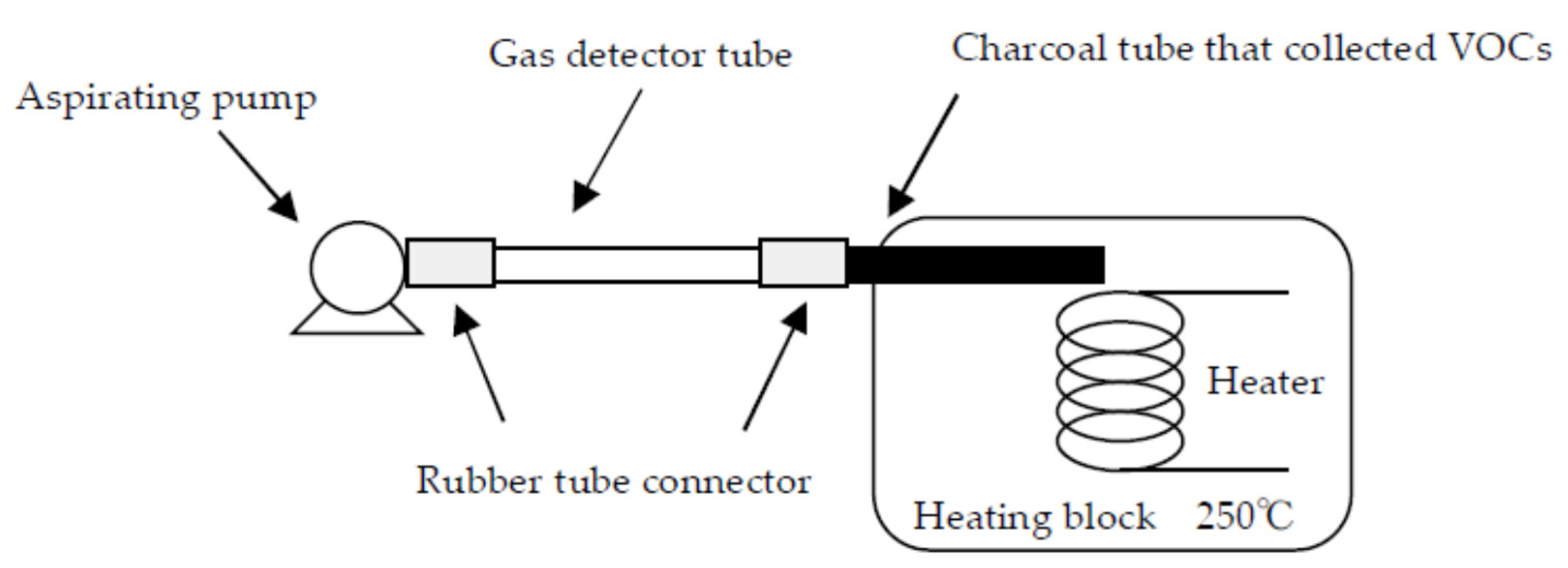

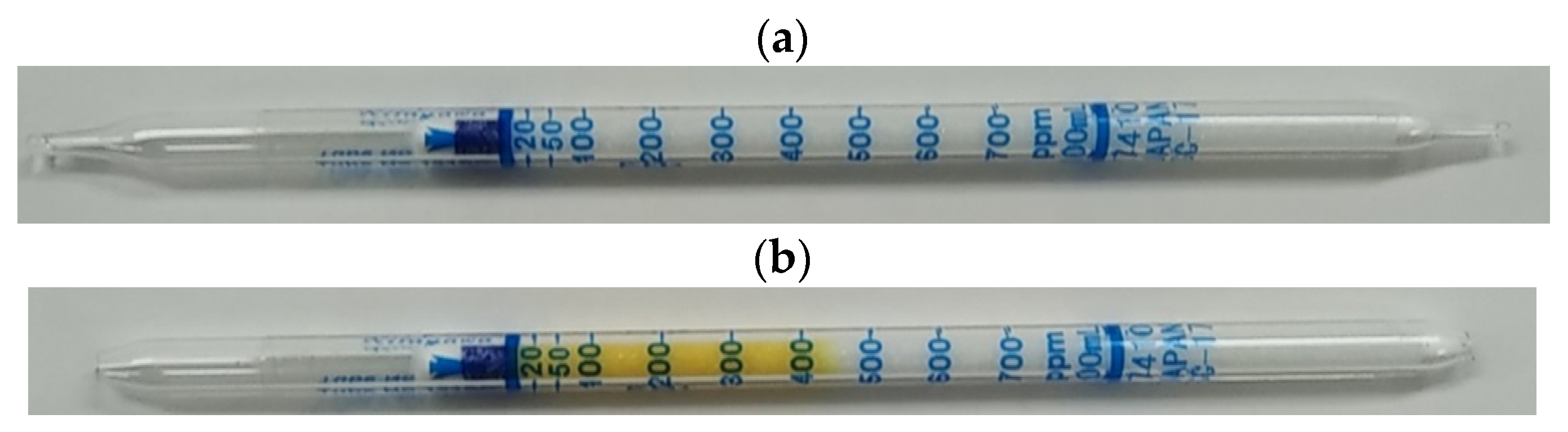
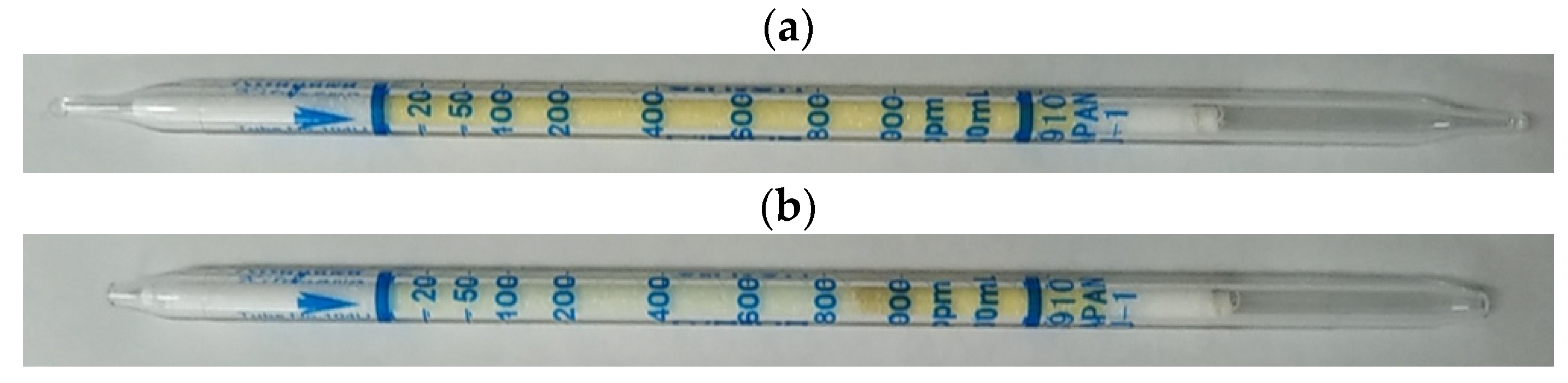






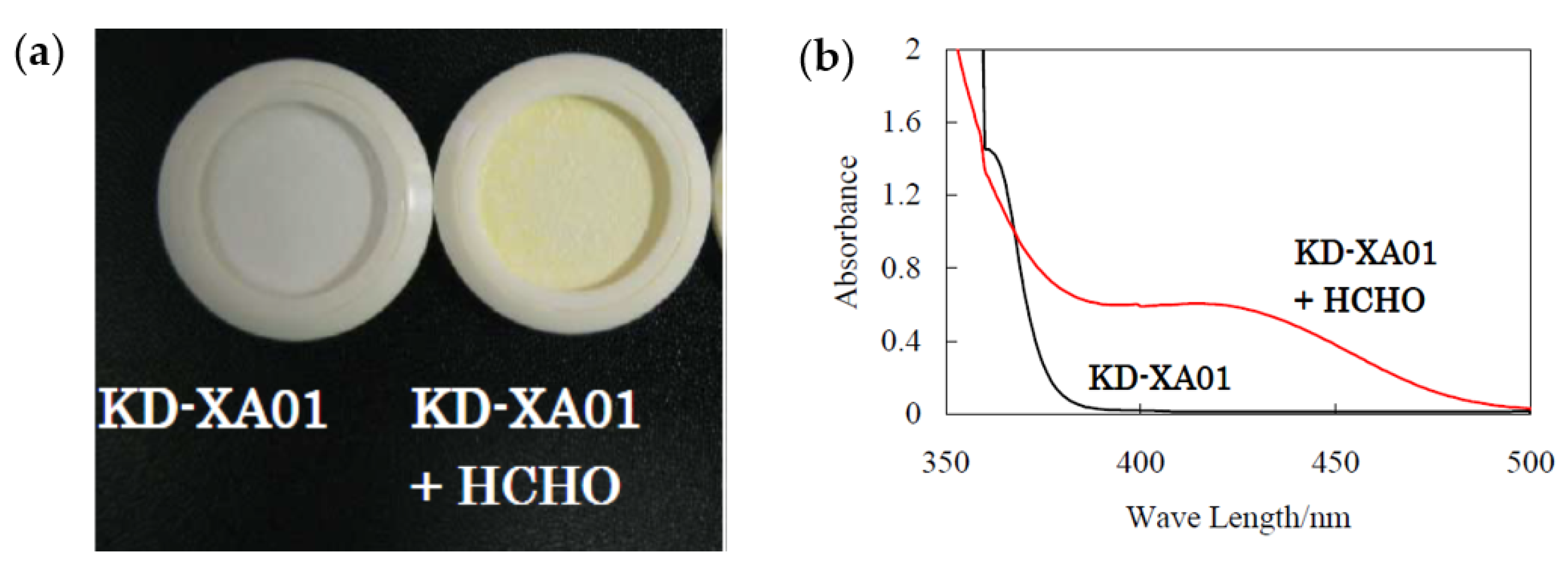


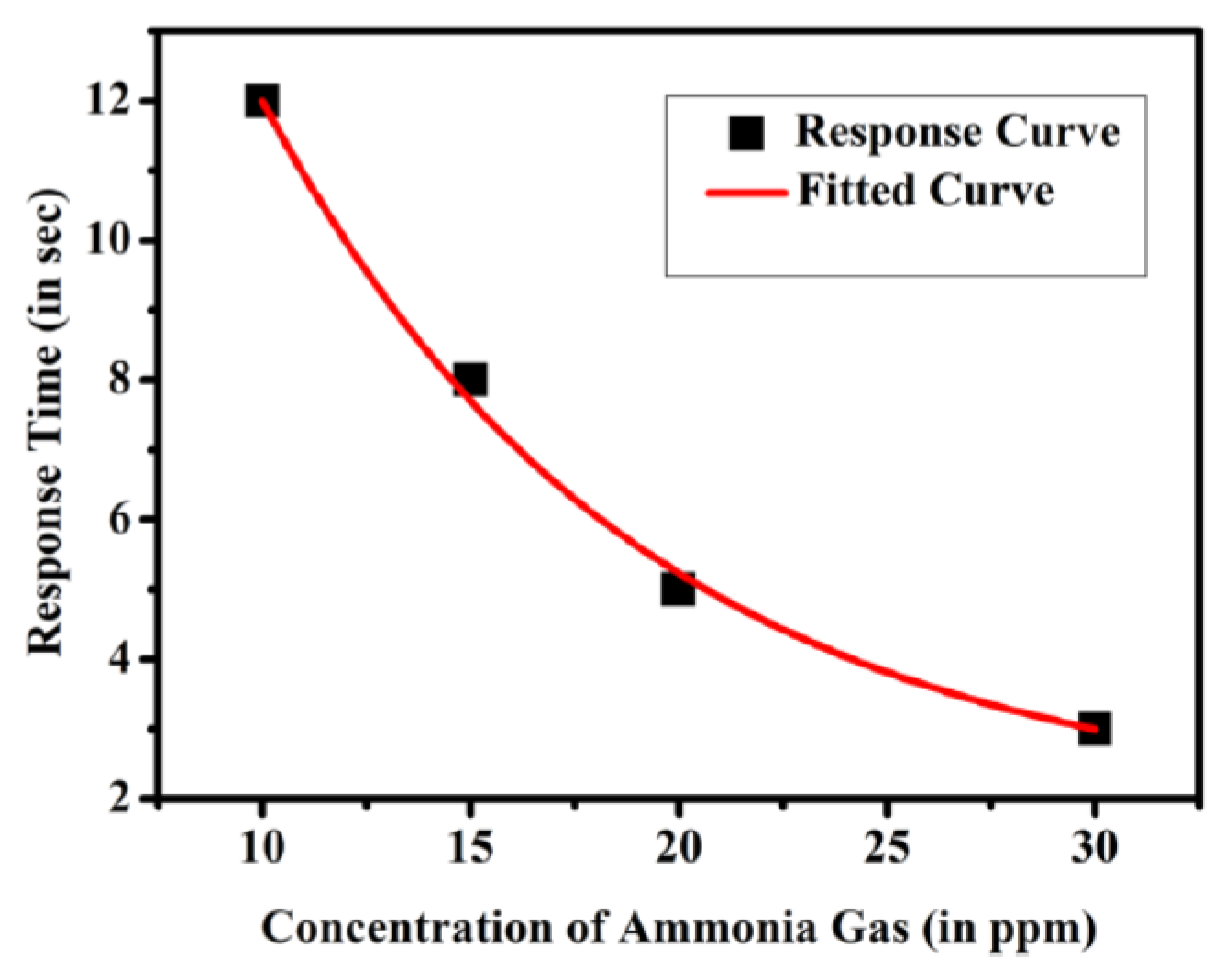

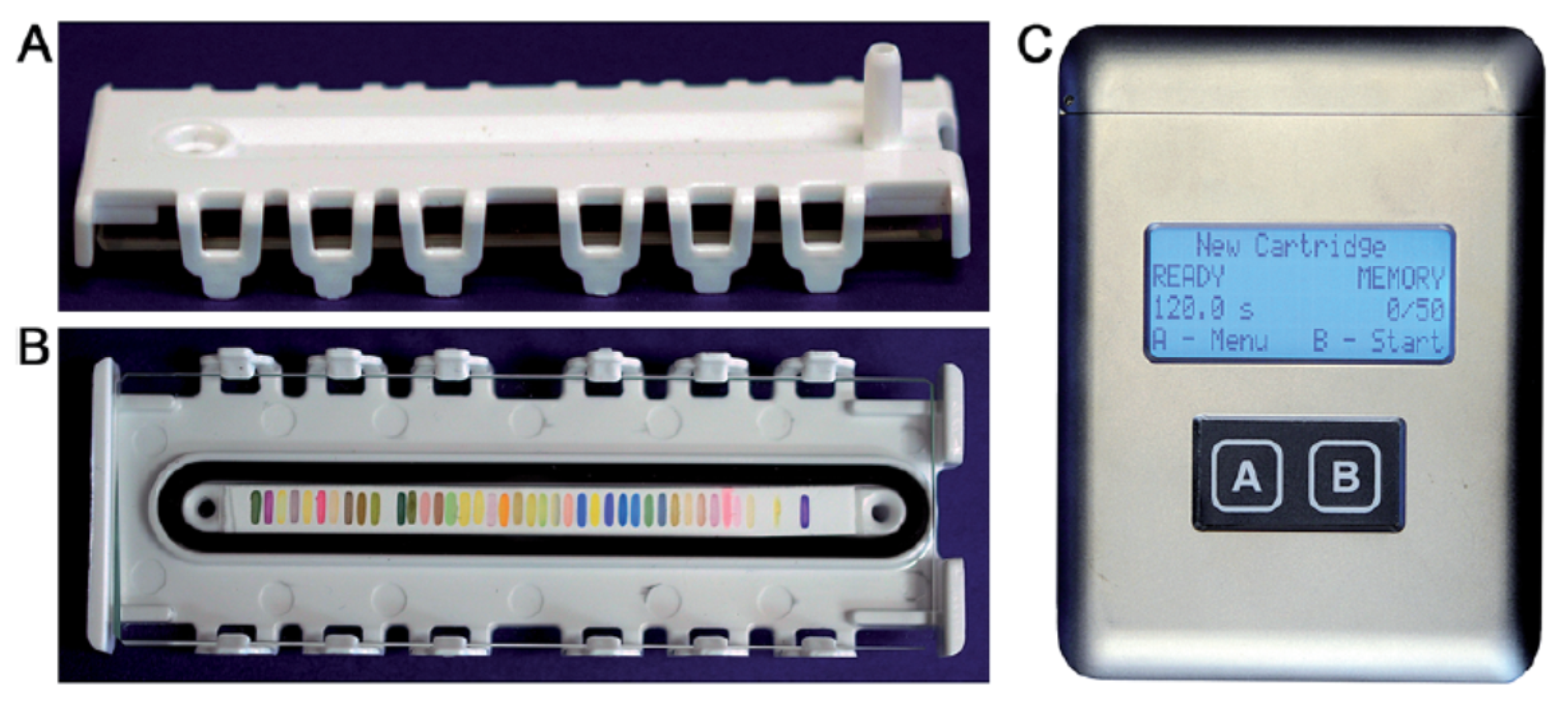
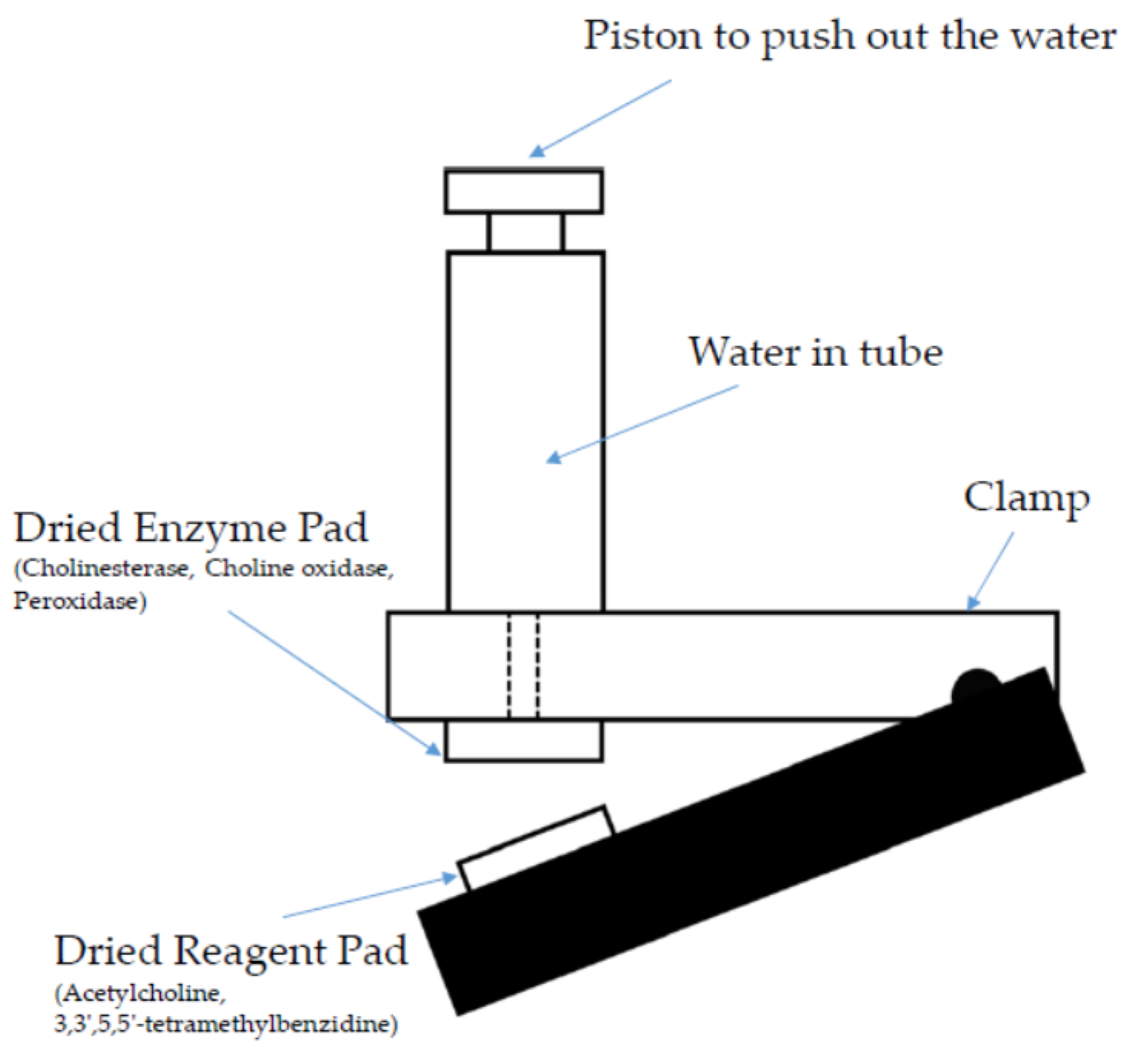



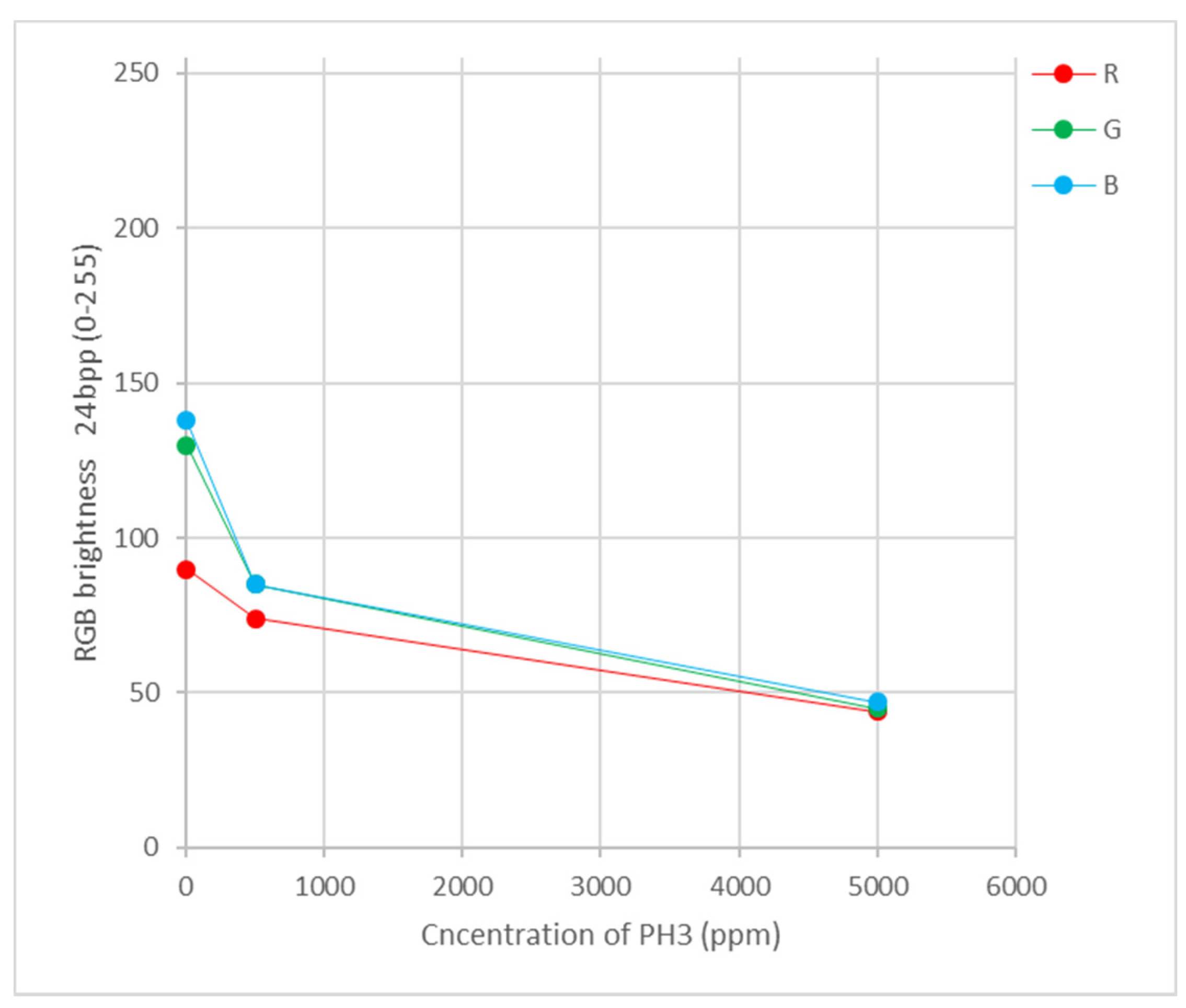
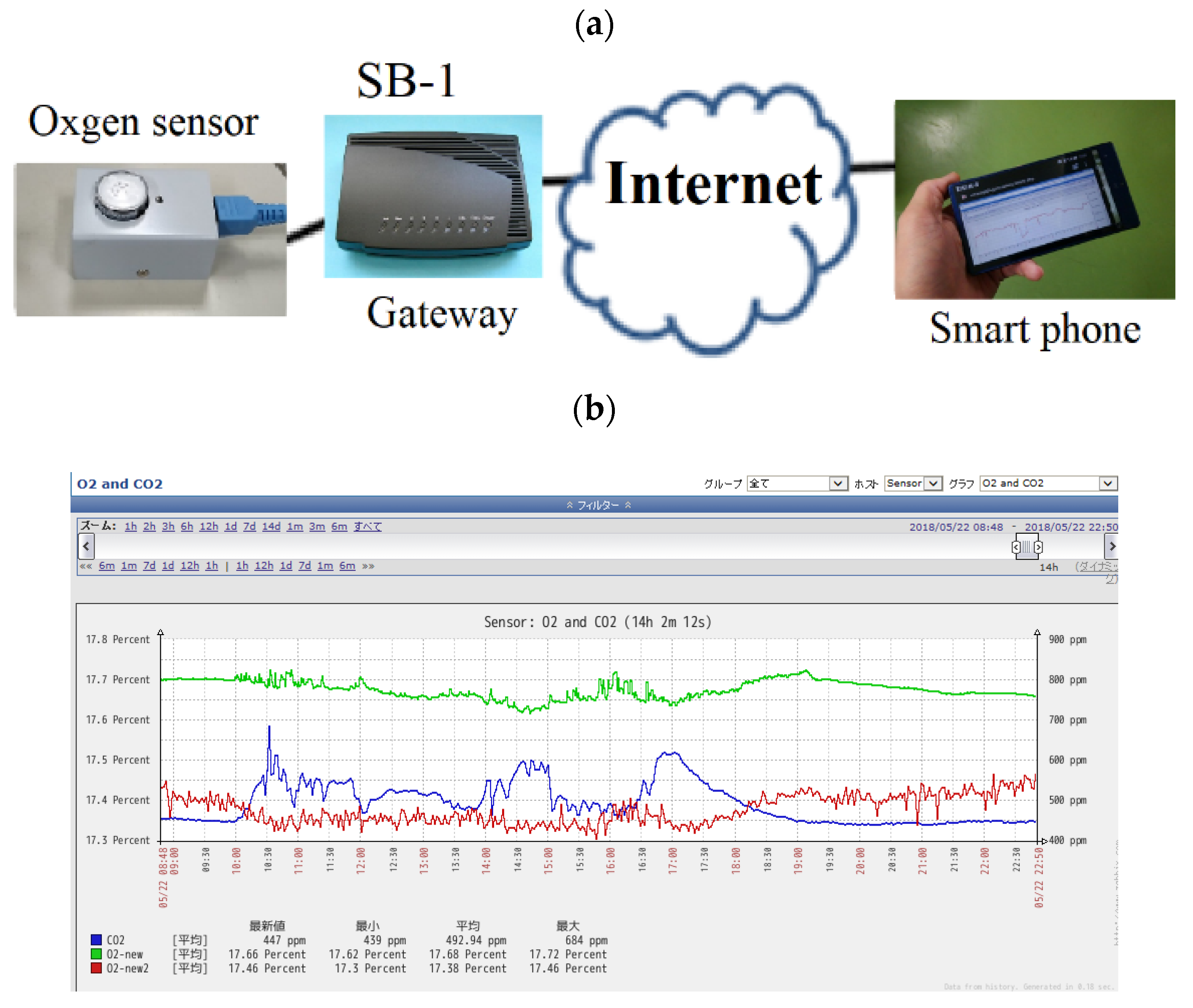
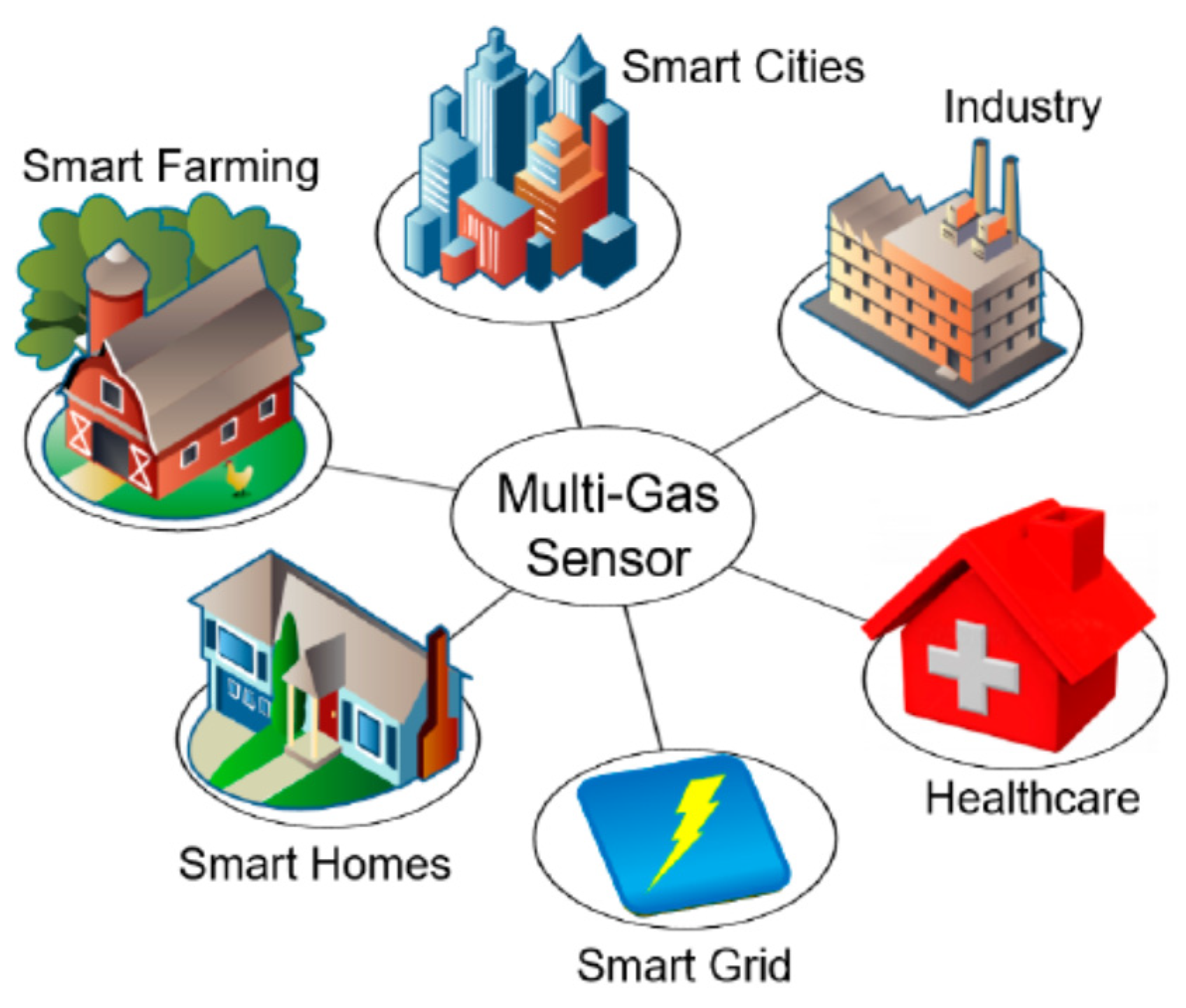
| VOC | CC-GD (ppmC) | GC-FID (ppmC) | Bias of CC-GD for GC-FID (%) 1 |
|---|---|---|---|
| Toluene | 2960 | 2945 | 0.5 |
| Ethyl acetate | 2445 | 2618 | −1.4 |
| Mixed VOC 1 2 | 2660 | 2440 | 9.0 |
| Mixed VOC 2 3 | 420 | 428 | −1.9 |
| NH3Sensors | Response Time (in s) | NH3 Gas Concentration (in ppm) |
|---|---|---|
| rGo 1 | 108 | 10 |
| PANI 2 | 800 | 50 |
| SnO2 | 3000 | 10 |
| CNT–TiO2 | 600 | Not mentioned |
| Semiconductor [76,77,78,79,80] | Catalytic Combustible [76,81,82] | Electrochemical [76,83,84] | Photoionization [85,86,87] | Colorimetric [88,89,90] | |
|---|---|---|---|---|---|
| Sensitivity | O (sub-ppm) | × (sub-%) | △ (ppm) | ◎ (ppb) | ◎ (ppb) |
| Selectivity | × | × | △ | △ | ◎ |
| Continuous monitoring/data transfer | O | O | O | O | △ 1 |
| Oxygen requirement during measurement | Required | Required | Required | Not required | Not required |
| Number of measurable gases for commercial products | × For reducing gas/oxidizing gas | × For combustible gas | △ (less than 100) | ◎ (more than 300) | ◎ (more than 300) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawamura, K.; Miyazawa, K.; Kent, L. The Past, Present and Future in Tube- and Paper-Based Colorimetric Gas Detectors. AppliedChem 2021, 1, 14-40. https://doi.org/10.3390/appliedchem1010003
Kawamura K, Miyazawa K, Kent L. The Past, Present and Future in Tube- and Paper-Based Colorimetric Gas Detectors. AppliedChem. 2021; 1(1):14-40. https://doi.org/10.3390/appliedchem1010003
Chicago/Turabian StyleKawamura, Koji, Kazumasa Miyazawa, and Lloyd Kent. 2021. "The Past, Present and Future in Tube- and Paper-Based Colorimetric Gas Detectors" AppliedChem 1, no. 1: 14-40. https://doi.org/10.3390/appliedchem1010003
APA StyleKawamura, K., Miyazawa, K., & Kent, L. (2021). The Past, Present and Future in Tube- and Paper-Based Colorimetric Gas Detectors. AppliedChem, 1(1), 14-40. https://doi.org/10.3390/appliedchem1010003





