Abstract
The lack of an established seaweed aquaculture industry in the Atlantic Southeast reflects the persistent challenges in identifying macroalgal species that can consistently produce year-round under regional environmental conditions. As a result, in this study, locally abundant Charlestonian Ulva spp. were selected as sustainable algal candidates for a pilot investigation, due to their resilience to abiotic (e.g., seasonal changes in temperature and nutrients) and biotic (e.g., predation and epiphytes) factors, thus allowing for practical land-based aquaculture. Ulva spp. were analyzed for their seasonal biomass and potential bioremediation applications using the existing land-based aquaculture infrastructure of the SCDNR in Charleston, South Carolina. The biomass of tank-cultivated Ulva spp. was monitored on a biweekly basis for 16 months and was found to be highest (31.8 kg) in the spring, increasing by 22% in just two weeks as water temperatures rose. A synthetic nutrient fertilizer was incorporated into aquaculture at the latter stages of this study to observe the effects on algal biomass while simulating an anthropogenic event. Interestingly, inorganic supplementation did not induce growth but was absorbed by the algal tissue, significantly lowering the δ15N to <7‰. Additionally, Vibrio spp. bacteria proliferated following the inorganic nutrient spike, while coliform populations decreased. Biochemical composition analyses comparing tank-cultivated and wild in situ Ulva spp. revealed variations in essential trace element (e.g., potassium: tank—19,530; wild—5520 mg/kg) concentrations, yet shared similar trace metal (e.g., arsenic: tank—4.47; wild—4.52 mg/kg) and pesticide (e.g., DEET: tank—0.048; wild—0.040 mg/kg) concentrations. This is the first reported macroalgal aquaculture research in South Carolina and serves as a pilot study for future research or commercialization in the Lowcountry and the greater southeastern coastal communities of the United States.
1. Introduction
Macroalgal cultivation in the Southeastern United States is limited, particularly in the Carolinas, suggesting that challenges exist in either aquaculture resources or the sustainability of macroalgae species, or both. This may explain the lack of research on macroalgal aquaculture in the Carolinas, since the only previous studies from either North or South Carolina have focused on using freshwater microalgae (phytoplankton) for wastewater treatment and for mitigation strategies to eliminate harmful algae blooms (HABs) in brackish ponds [1,2]. In the research presented here, locally abundant Ulva spp. Chlorophyta (green macroalgae) from Charleston, South Carolina, were selected for a pilot study among Rhodophyta (red) and Phaeophyta (brown) species, due to their observed sustainability in outdoor land-based aquaculture. Sustainability refers to the practicality and minimum input required for maintenance (e.g., an outdoor tank using sunlight, as opposed to an indoor tank using artificial light, in terms of cost expenditure), due to both abiotic and biotic factors. Based on the results of previous outdoor aquarium test runs, as well as a 6-month pre-pilot multi-species study, Ulva spp. were identified as the most optimal candidate for a longer-term 16-month pilot investigation into biomass and bioremediation potential.
Ulva, commonly known as sea lettuce, is a genus of green macroalgae in the phylum Chlorophyta, order Ulvophyceae, with a cosmopolitan distribution along intertidal and estuarine shorelines [3,4]. Several studies have demonstrated the exceptional ability of Ulva to accumulate nutrients and contaminants from various wastewater sources, including land-based aquaculture and industrial byproducts [5]. For instance, de Oliveira et al. (2016) demonstrated the bioremediation potential of employing Ulva lactuca to treat nitrogenous waste derived from oilfield wastewater [6]. In another study, Nielsen et al. (2011) used livestock manure as the primary nutrient source for cultivating U. lactuca in multitrophic aquaculture, which produced algal tissue high in protein ([total amino acid (TAA) content]) and nutrients (nitrogen and phosphorus) ideal for biomass production [7]. Macroalgal biomass production has multifaceted applications, extending beyond wastewater remediation, and ranges from sourcing unique bioactive compounds for pharmaceuticals and cosmetics to serving as a potential feedstock for biorefineries [7]. For instance, Ulva reticulata has been reported as a vegan protein source, comprising nearly 40% of the total essential amino acids found in eggs and soy-based protein, thus serving as a potential alternative to meat products [8,9]. Ulva’s ability to rapidly absorb nutrients demonstrates its potential as a natural nutrient sink, while highlighting its ability to produce algal biomass rich in proteins, carbohydrates, and lipids [10].
A suitability assessment model to determine the ‘best-suited’ locations for expanding commercial Ulva cultivation suggests that the Southeastern United States holds the most potential, with South Carolina amongst the top optimal economic zones due to environmental suitability and lower production costs [11]. There is considerable potential in cultivating Ulva to produce sustainable and renewable algal biomass for animal feed, fertilizers, bioenergy, and biomaterials, as well as to mitigate human-induced anthropogenic pollution [12,13,14,15,16]. The genus Ulva is largely tolerant of wide ranges of salinity (<0.5–49 PSU), temperature, and desiccation, which are favorable traits for both land-based and open-water macroalgal aquaculture [12,17,18,19]. Interestingly, there is a lack of reported studies on land-based continuous flow systems cultivating Ulva in the southeastern U.S. However, international studies have been reported, including those on Ulva prolifera, Ulva reticulata, and Ulva lactuca from Indonesia, Tanzania, and Saudi Arabia, respectively [20,21,22]. Ulva’s characteristic resilience, rapid growth rate, and year-round availability make it an ideal and sustainable candidate for exploring biomass production and potential bioremediation using land-based flow-through aquaculture in Charleston, SC.
2. Materials and Methods
2.1. Assembly of Tumble Culture Setup for Land-Based Tanks
The South Carolina Department of Natural Resources (SCDNR) granted permission to use their available infrastructure, including outdoor land-based flow-through aquaculture tanks that pump unfiltered seawater directly from the Charleston Harbor into a seawater settlement basin. Seawater from the basin was then pumped into 450-gallon (1700 L) tanks located in the historic Fort Johnson complex on James Island. Smaller land-based tanks #2, #4, and #5 were modified for tumble culture by constructing a standpipe and a cross-shaped air diffuser (Figures S1 and S2—Supplemental Information). A nested PVC standpipe setup consisted of 0.25 in (0.64 cm) hardware mesh covering an external standpipe layer and an internal standpipe layer permitting drainage by overflow without loss of macroalgae. The standpipe height of 21.5 in (54.6 cm) regulated the tank’s volume capacity to 1382 L. A perforated cross-shaped PVC (¾-inch pipes drilled with holes arranged at 90° angles) was submerged to the bottom of each tank, which supplied compressed air, generating bubbles that induced a continuous tumble culture. The flow rate of the harbor water intake line, which pumps unfiltered seawater into the tank, was approximately 8 seconds per gallon, or 7.5 gallons per minute (8 s/3.8 L or 28.4 L/1 min).
2.2. Macroalgal Selection
Locally abundant green (Ulva spp.), red (Gracilaria tikvahiae, Gracilaria vermiculophylla, Phyllymenia [Grateloupia] gibbesii, Agardhiella subulata, and Rhodymenia pseudopalmata), and brown (Petalonia fascia and Sargassum filipendula) macroalgae were collected and monitored for their biomass in the land-based aquaculture tanks. This served as a preliminary investigation to identify an optimal macroalgal candidate for this research (Table S1). Biomass was measured biweekly for tank-cultivated Ulva spp., and weekly for all other macroalgae (Figures S3–S10). A digital food scale (Etekcity; Anaheim, CA, USA; [1.0–5.0 kg]) was used to obtain wet weight (WW) for these species. Over the course of 6 months, all tested species, except Ulva spp., experienced heavy epiphyte loading and exhibited negative growth rates due to variable conditions, including sediment buildup from the raw, unfiltered seawater, as well as fluctuations in salinity, temperature, and nutrient levels. Similar results were observed for an outdoor test run in 40-gallon glass aquariums with aeration (Aqueon, Franklin, WI, USA) using artificial seawater reconstituted according to the manufacturer’s instructions (Instant Ocean®, Spectrum Brands, Blacksburg, VA, USA) on various species from the Gracilaria genus (Figure S11). Based on these results, Ulva spp. were selected as a sustainable and low-maintenance locally abundant macroalgae for this study.
2.3. Biomass Determination
The seasonal biomass of the locally abundant Charlestonian Ulva spp. was studied for 16 months, from 3 February 2023 to 14 June 2024, on a biweekly basis, in a modified land-based flow-through aquaculture system (Tank #5). Biomass measurements were performed on a biweekly basis via the following steps (Figure 1): Harvesting of Ulva spp. using a hand-held net (Ozark Trail; Bentonville, AR, USA; [23]) by circulating the tank perimeter three consecutive times, and then raising the net above the tank to drain water (gravity) for 60 s (timed via stopwatch). Drained algal biomass was centrifuged (25 revolutions per aliquot) via a large commercial salad spinner (Elixum; Mannheim, Germany; [6.6 gal/30 L]). The centrifuged biomass was then transferred to tared 5-gallon (19 L) buckets. A handheld digital scale (Dr. Meter; Shenzhen, China; [0.1–50 kg]) was then used to measure the WW (kg; centrifuged) of biomass from each bucket. After the measurements were recorded, Ulva spp. were gently returned to their tank.

Figure 1.
Biweekly biomass measurements (“weigh-ins”) for the tank-cultivated Ulva spp. consisted of the following steps: (A) Harvest using a hand-held net. (B) Drainage of water via centrifugation. (C) Transfer of centrifuged biomass to buckets. (D) Measurement of Ulva spp. biomass (WW; kg).
2.4. Aquaculture Tank Measurements, Station Data, and Collection Sites
Tank water temperature and salinity of SCDNR Tanks #2, #4, and #5 were measured weekly using a handheld digital salinity meter (Orapxi; Jinan, China; [temp. (°C), salinity (0–200 ppt)]). Tank water pH was measured weekly using a handheld digital pH meter (Ruolan, 0–14 pH) (Figures S12 and S13, Tables S2 and S3). These data were compared to temperature and salinity recorded by the public USGS Water Station Data (https://waterdata.usgs.gov/, accessed on 3 February 2023–14 June 2024) at the Cooper River at U.S. Hwy 17 at Charleston, SC—021720709 (Figures S14–S16, Table S4). The collection sites for the tank-cultivated vs. wild in situ Ulva spp. experiments were conducted on 31 May 2024 at the following coordinates: 32°45′07″ N, 70°53′56″ W (outdoor DNR aquaculture tanks) and 32°45′03″ N, 79°54′12″ W (adjacent Fort Johnson intertidal mudflats) (Figure 2).
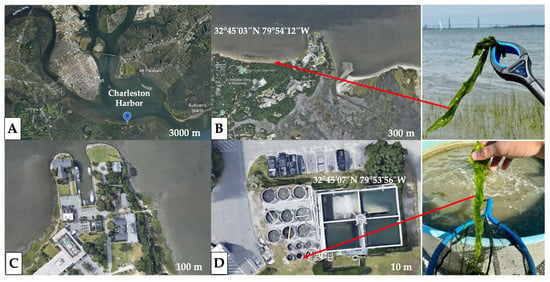
Figure 2.
(A) Satellite aerial view at 3000 m—Fort Johnson, James Island, and the coastal communities surrounding the Charleston Harbor. (B) 300 m—Fort Johnson intertidal mudflats for in situ collection of locally abundant wild Ulva; 32°45′03″ N 79°54′12″ W. (C) 100 m—SCDNR site. (D) 10 m—land-based aquaculture tanks for collection of tank-cultivated Ulva spp.; 32°45′07” N, 70°53′56” W).
2.5. Nutrient Spike of Ulva spp. Tank
Plant Food Water & Vegetable fertilizer (Osmocote® Smart-Release®; Marysville, OH, USA), with 14% nitrogen (8.2% ammoniacal nitrogen, 5.8% nitrate nitrogen), 14% phosphorus, 14% potassium (14-14-14 NPK), was added to aquarium mesh zipper bags (6″ × 4″) [23]. The dose for this experiment, determined based on the suggested dose (1 cap/4 sq. ft.; 0.37 sq. m), the Ulva tank’s surface area (27.50 sq. ft.; 2.55 sq. m), and the amount per cap (42.84 g), was calculated to be 295 g. A total of two mesh bags, weighted down with zip-tied fishing weights (12 oz), were placed into the tank for 2 weeks (Figure S17). Tank-cultivated Ulva spp. were collected for the pre- and post-spike analyses on 31 May and 14 June 2024, respectively.
2.6. Chemical and Biological Analyses
2.6.1. Carbon-Nitrogen (C/N) Isotopes
For the stable isotope and tissue nutrient analysis of tank-cultivated Ulva spp., samples were collected on 31 May (pre-spike) and 14 June 2024 (post-spike) and processed following the methods demonstrated by Strait et al. (2022) [24]. Samples were rinsed in deionized water, weighed (40 g WW), and dried at 60 °C in an oven. Once dried, the samples (n = 3) were homogenized into a powder using a mortar and pestle and prepared for carbon-nitrogen (C/N) isotope analyses in 1.5 mL microcentrifuge tubes (Figure S18A–C). Samples were analyzed on a Costech ECS 4010 Elemental Combustion System (NC Technologies; Bussero, Italy) using a Zero Blank Autosampler (Costech Analytical Technologies Inc.; Valencia, CA, USA) and coupled to a ThermoFinnigan Delta Plus XP (Thermo Electron Corporation; Bremen, Germany) or a Thermo Scientific Delta V Advantage via a Thermo Scientific Conflo IV (Thermo Fisher Scientific; Waltham, MA, USA). Quantification and isotopic corrections were calculated using a glycine standard characterized against international reference materials (USGS 32, USGS 34, USGS 35, and IAEA N3 for δ15N, and NBS 18, NBS 19, and NIST 1547 for δ13C). The weights of the glycine and/or acetanilide standards analyzed vary to bracket the amount of N and C expected to be generated from the samples being analyzed (isotope values for any samples that fall outside the range of standards are not reported). For quantification, a standard curve of peak area vs. expected μg N or C is calculated and applied to the peak areas generated by the analyzed samples. For isotopic correction due to instrument linearity, correction factors are generated using plots of measured δ15N vs. 1/peak area and measured δ13C vs. peak area. These correction factors are then applied to the measured isotope ratios of the samples. All quantitative data are presented as mean ± standard deviation (SD) between replicates. Statistical analyses were not performed due to the absence of biological and analytical replicates.
2.6.2. Essential Trace Element, Trace Metal, and Pesticide Screenings
Samples from tank-cultivated and wild in situ Ulva spp. were collected on 31 May 2024, prior to the nutrient supplementation experiment. Specimens were immediately rinsed with deionized water and lyophilized using a commercial freeze dryer (Harvest Right, Home Pro Freeze Dryer; Salt Lake City, UT, USA). Dried samples were weighed (dry weight, DW): 50.1 g (tank-cultivated) and 42.1 g (wild in situ). They were then vacuum-sealed, labeled accordingly, and shipped to AGQ Labs USA (Oxnard, CA, USA) (https://www.agqlabs.us.com/ [URL accessed on 31 May 2024]) for screening of essential trace elements, trace metals, and pesticides (Figure S18D–F). The essential trace element composition (total N, P, K, Ca, Mg, and S) was measured using ICP-OES (Revvity, Inc.; Waltham, MA, USA) after acid digestion, with total N quantified by Kjeldahl analysis. Trace metals (As, Cd, Pb, and Hg) were quantified using inductively coupled plasma mass spectrometry (ICP-MS) according to US EPA 6020/3052 protocols. Pesticide residues were analyzed using a multi-residue screening method with liquid chromatography-tandem mass spectrometry (LC-MS/MS) and gas chromatography-tandem mass spectrometry (GC-MS/MS), validated against AOAC and EPA guidelines. All analyses were performed under ISO/IEC 17025-accredited procedures with appropriate quality control standards and detection limits. It is important to note that no replicates (n = 1) were performed at the third-party AGQ Labs.
2.6.3. Coliform and Vibrio spp. Bacteria CFUs
Approximately 25 g of tank-cultivated Ulva spp. were collected on 31 May (pre-spike) and 14 June 2024 (post-spike) using gloved hands and placed into sterile resealable freezer bags. Sealed bags of algae were then transported in a cooler on ice to a microbiology laboratory for processing and plating. Within 12 h, in the laboratory, 2.5 g WW of an Ulva spp. sample (n = 1) was gently rinsed with water from the tank to remove any sediment or fouling entities. Rinsed macroalgae were placed into sterile 50 mL conical tubes, which were then filled with 25 mL of autoclaved 1x phosphate-buffered saline (PBS) [25]. Filled tubes were then capped and vortexed for five minutes. After vortexing, multiple dilutions of the aqueous solutions were made by pipetting volumes ranging from 1 mL to 8 mL of the macroalgal solutions into an autoclaved vacuum filter. Various volumes of autoclaved 1xPBS were added to each dilution of the macroalgal solution to bring the total volume to 10 mL. Solutions were vacuum-filtered through sterile 0.45 µM cellulose membrane filters [26]. Inoculated filters were transferred to differential and selective media using flame-sterilized forceps. CompactDryTM EC Plates (Hardy Diagnostics; Santa Maria, CA, USA) were used to enumerate coliform bacteria and E. coli, while CHROMagarTM Vibrio (CHROMagar; Saint-Denis, Île-de-France, France) was used to enumerate Vibrio parahaemolyticus, V. cholerae/V. vulnificus. Inoculated plates were incubated for 24 h, with CompactDryTM EC Plates being incubated at 35 °C and CHROMagar Vibrio plates being incubated at 27 °C. After incubation, plates were removed, and the resulting colonies were counted to determine the colony-forming units (CFU) per 100 mL of sample for each dilution using the following equation, used by the South Carolina Department of Health and Environmental Control (SCDHEC):
All quantitative data are presented as mean ± SD between dilutions. Statistical analyses were not performed due to the absence of biological and analytical replicates.
2.7. Taxonomic Identification
Tank-cultivated Ulva spp. were morphologically identified as Ulva rotundata using the classification system of Schneider & Searles (1991) [27]. Diagnostic traits included blades attached by a distinct median stipe with rounded to deeply lobed margins; surface cells were polygonal to angular; plastids were discoid with two pyrenoids per cell, consistent with published species descriptions [27,28]. The lack of data on whether tank-cultivated Ulva belongs to one or multiple species has led to the conservative labeling of Ulva spp. for this study. Next steps would entail a molecular approach via DNA barcoding-based identification with the tufA and rbcL genes [29,30]. A sample of tank-cultivated Ulva spp. has been retained and is stored at −80 °C for future taxonomic or genetic identification.
2.8. Statistical Analysis
Given the exploratory nature of this pilot study, formal statistical tests were not applied. Reported values represent means ± SD derived from single-tank measurements or individual sample analyses. Because no biological or analytical replicates were available, results should be interpreted as descriptive indicators rather than inferential statistics.
3. Results and Discussion
3.1. Seasonal Biomass
Throughout the study, tank-cultivated Ulva spp. biomass fluctuated in response to seasonal variations in environmental conditions, including temperature, day length, and dissolved nutrients. Higher biomass levels were observed during the warmer spring months (April–May), whereas lower biomass levels were observed during the colder winter months (December–February) (Figure 3 and Figure 4). The highest recorded biomass for the Ulva spp. tank was 31.8 kg, recorded during the spring (28 April 2023), and the lowest was 7.0 kg, recorded during the winter (9 February 2024). In 2023, the highest peaks in growth occurred during the spring between 14 and 28 April 2023, with a 22% increase in biomass from the two weeks prior (30 March 2023). The average water temperature and day length during this time were 18.3 °C and 12.85 h, respectively. In 2024, biomass increased by 66% during the spring, from 19 April to 3 May, with an average water temperature of 22.9 °C and a day length of 13.34 h. Similarly to the results observed in this study, Gadberry et al. (2018) reported that the daylight period between March and April resulted in a notable surge in yields and growth rate of Ulva spp. from Port Orchard, Washington [31]. In addition, they demonstrated that day length was the primary factor influencing yields (kg wet weight d−1 m−2) and growth rate (% WW d−1) [31]. Spring and summer are generally seasons of high light intensity and maximum photosynthetic capacity, and in a tumble culture setup, biomass is dependent on algal exposure to sunlight (surface irradiance) and a tank’s characteristics (size, depth, circulation) [32,33]. The average hours of light in which Ulva spp. tank was exposed to per day was determined based on the average seasonal day length and limited shading (~15% from surrounding tank infrastructure)– Winter: ~9 h/day; Spring: ~11 h/day; Summer: ~12.5 h/day; and Fall: ~10 h/day.
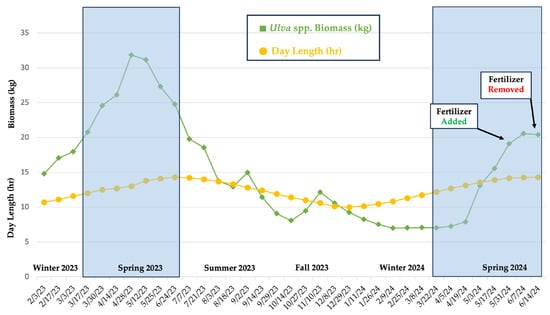
Figure 3.
Biweekly Ulva spp. biomass * vs. daylength over a 16-month period. (* Starting biomass in the existing tank was ~15 kg). The shaded region represents comparisons in surges in % increases in biomass during the spring.
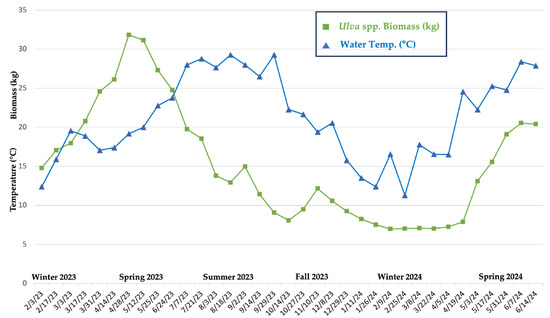
Figure 4.
Biweekly Ulva spp. biomass * (kg) vs. tank water temperatures (°C) over a 16-month period. (* Starting biomass in the existing tank was ~15 kg).
Biomass stagnated between 9 February and 5 April 2024, which was attributed to shorter day lengths (reduced photoperiods for photosynthesis) and lower water temperatures (Figure 3 and Figure 4). The seasonal decline in biomass observed in the tank-cultivated Ulva spp. was presumably due to the death and fragmentation of the macroalgae into small fragments that were able to escape through the mesh of the standpipe. In addition to colder temperatures, low salinity levels, as recorded by the nearby Ashley River USGS monitoring station (Figures S14–S16, Table S4), may have led to physiological and biochemical stress, slowing algal metabolism and other cellular processes [10,18]. Jansen et al. (2022) investigated the seasonal variations in Ulva spp. productivity in land-based aquaculture in the Netherlands and revealed similar trends in growth, with significant decreases during the summer and fall [34]. Furthermore, a commercial pilot facility for cultivating Ulva sp. via pond culture on the temperate Pacific coast of Mexico revealed that production was highest in the spring (up to 290 g m−2 day−1) and the lowest in the winter (as low as 40 g m−2 day−1) [35].
3.2. Nutrient Supplementation
3.2.1. Carbon and Nitrogen Isotopes
Plant fertilizer was used to introduce inorganic nutrients into the Ulva spp. tank to mimic an anthropogenic eutrophication event from a synthetic or manufactured source. This was conducted using inorganic nutrient granules (295 g/27.5 sq. ft) encapsulating 14-14-14 NPK, which were incorporated into the latter stages (the last two weeks: 31 May to 14 June 2024) of the study. The estimated nutrient load introduced into a submerged tumble-culture environment was estimated as ~28 g each for N, P, and K over 14 days. This was determined using a Fickian diffusion-controlled release model, as employed by Trientini and Fisher (2020) and Adams et al. (2023) in their studies on controlled-release polymer-coated fertilizers [36,37,38]. The following tank parameters are important to note as variables that affect the rate of diffusion: tank water temperature (28 °C), tank volume (1382 L), and constant tumble culture (via compressed aeration).
Algal biomass stalled during this nutrient spike period, despite an anticipated surge in growth. Due to the lack of a control tank (resulting from limited aquaculture infrastructure), no statistical comparison could be made with a non-dosed (spiked) tank. Nevertheless, it was apparent that the macroalgae metabolized fertilizer-sourced nutrients (e.g., inorganic N): supplementation resulted in a decrease in the algal tissue N isotope ratio (δ15N) from 7.6 to 4.9 (‰ vs. AIR) (Table 1). Pre- vs. post-spike Ulva spp. samples revealed that stable algal carbon-to-nitrogen (C:N) isotope ratios were higher for pre-spike (9.9) in comparison to post-spike (8.6), indicating that Ulva spp. metabolized higher concentrations of inorganic N. C:N ratios are indicative of inorganic nutrient concentrations, and can be used to infer if an alga has undergone N-limitation. C:N values >20 indicate that an alga is limited in N, whereas those <20 indicate that N is not limiting [39].

Table 1.
Stable carbon and nitrogen isotopes (δ13C/δ15N) of pre- vs. post-spike tank-cultivated Ulva spp. tissues following inorganic nutrient supplementation. δ15N- the ratio of 14N to 15N isotopes in a sample; ‰N- units of per mille of N in a sample, specifically in algal tissues; C:N- algal ratio of μg C to μg N isotopes in a sample (DW). Atmospheric nitrogen (AIR); Vienna Pee Dee Belemnite (VPDB). Samples are an average of triplicates (n = 3). Standard deviations (SD ±).
A decrease in the C:N ratio marked a shift from a higher trophic source (e.g., Charleston Harbor water abundant in potentially wastewater-derived N; δ15N ≥ 7‰) to a lower trophic source with isotopically lighter forms of N (e.g., inorganic N from synthetic fertilizers or natural N sources; δ15N < 7‰). Unlike elemental C or N estimations, measurements of isotopic δ15N can indicate the trophic origin of the nutrients metabolized by macroalgae, with levels exceeding 7‰ commonly being accepted as being indicative of wastewater N [40,41,42]. In circumstances when macroalgae are enriched with inorganic N, as with synthetic fertilizer runoff, or from lower trophic levels, δ15N values can decrease, while the %N in an alga’s tissue increases. This inverse relationship is due to these inorganic or natural N sources containing little to no δ15N [41]. This was observed as %N increased from 30 to 31%, which indicated that fertilizer-derived δ14N was metabolized. In other enrichment experiments, Fujita (1985) found that species, such as U. lactuca, with high N requirements grew with dissolved inorganic N, whereas species with low N requirements did not [43]. The lack of an increase in biomass suggests that inorganic nutrient supplementation may not be responsible for stimulating subsequent vegetative growth in tank-cultivated Ulva spp. [44].
On the other hand, the source of inorganic N to which Ulva species are exposed in aquaculture can influence algal photosynthesis, growth, C:N ratios, N and P uptake, as well as lipid and P content in biomass [45]. This was demonstrated by Shahar et al. (2020), who reported that Ulva fasciata acclimated with nitrate (NO3−) exhibited no significant changes in nutrient uptake, photosynthesis, or growth rate, but showed shifts in tissue biochemical composition, including a relatively higher C:N ratio [45]. In contrast, ammonium (NH4+) promoted rapid short-term growth and elevated tissue N content, leading to a lower C:N ratio; however, prolonged exposure induced metabolic imbalances and stress effects on photosynthesis [45]. This may explain the stall in biomass for nutrient-dosed tank-cultivated Ulva spp., during the last two weeks of this study (31 May to 14 June 2024), where the synthetic fertilizer used was composed of both NH4+ (8.2%) and NO3− (5.8%).
3.2.2. Bacterial Proliferation
Comparisons were made between bacterial populations of tank-cultivated Ulva spp. and water samples, both before and after nutrient supplementation, to investigate the presence of Gram-negative bacteria associated with fecal contamination (e.g., coliform bacteria) and seafood consumption (e.g., Vibrio spp. bacteria). Bacterial colony-forming units per 100 mL of sample (CFUs/100 mL) inversely fluctuated with nutrient supplementation (Table 2). Specifically, coliform CFUs decreased for the tank-cultivated Ulva spp. and tank water samples after two weeks of inorganic nutrient supplementation, whereas Vibrio spp. CFUs increased in both. In tank aquaculture, a dose of inorganic nitrogen may shift bacterial composition by promoting heterotrophic proliferation in the water column. Similar inorganic nutrient-driven increases in Vibrio CFUs were observed in an integrated seaweed–abalone system, underscoring the strong coupling between dissolved inorganic nutrients and microbial dynamics in aquaculture environments [46]. On the other hand, the rapid increase in Vibrio spp. abundance may be attributed to its ability to proliferate in the presence of dissolved organic matter (DOM) released from Ulva spp., resulting from an influx of inorganic nutrients into the ecosystem [47]. DOM is a major biodegradable component released from macroalgae, which is crucial for balancing the carbon cycle and microbial communities in coastal estuaries [48].

Table 2.
Coliform and Vibrio spp. bacteria from pre- vs. post-spike tank-cultivated Ulva spp. Unit: average colony-forming unit (CFU)/100 mL. Measured values are the average CFUs/100 mL between 0.25 and 8.0 mL dilutions. There were no replicates (n = 1). Standard deviations (SD ±).
Additionally, there is a strong correlation between Vibrio abundance and increases in water temperatures [49]. The timing of these increases poses a public health concern, particularly in the consumption of seafood (e.g., oysters), which can lead to Vibrio-related gastrointestinal and skin infections (vibriosis) that, if severe enough, may cause sepsis [50,51]. This is especially relevant given the average increase in estuary water temperatures and the reported occurrence of Vibrio spp. isolates with antibiotic resistance in aquaculture [52,53,54,55]. This is also true for coliform bacteria, which, although they did not proliferate after nutrient dosage in Ulva spp. tank, are a serious public health and environmental concern as antibiotic-resistant bacteria (ARB) [56]. For instance, Emery et al. (2022), in collaboration with the non-profit organization Charleston Waterkeeper, noted that ARB fecal coliforms proliferated in abundance in response to increased rainfall in Shem Creek, SC [57]. They also raised concerns about antibiotic runoff into waterways from local hospitals, residential areas, and industrial sites, which are notoriously susceptible to flooding in Charleston [57]. The temperature- and nutrient-driven proliferation in Vibrio spp. observed in this pilot study highlight the potential risk of pathogenic bacteria in future land-based aquaculture systems, such as those in an outdoor, unfiltered flow-through system.
3.3. Tank-Cultivated vs. Wild In Situ Ulva spp.
Growing macroalgae in land-based aquaculture has advantages and disadvantages. Advantages include simpler monitoring and harvesting, reduced predation by marine animals, and the potential for incorporating specific wastewater streams [58,59]. Disadvantages include expenditures for operating the tank and seawater intake infrastructure, as well as the power required to continuously run an intake, along with compressed air to maintain the tumble culture [58]. The use of a continuous flow-through system, such as that employed in this study, could also result in increased exposure to toxic metals or pesticides in tank-cultivated Ulva spp. due to the accumulation of sediment over time. To determine whether this was the case, tank-cultivated Ulva spp. were compared with wild in situ Ulva spp. from the adjacent Fort Johnson shore for differences in tissue trace elements, trace metals, and pesticides. This aspect of the study was essential for evaluating the nutrient and toxicity profiles of locally abundant Ulva spp. from two different ecosystems: aquaculture and intertidal.
3.3.1. Algal Tissue Analysis for Essential Trace Elements
Tank-cultivated and wild in situ Ulva spp. were collected on 31 May 2024, prior to the nutrient supplementation of the tank. Algal tissues (n = 1) were evaluated for the following essential trace elements: nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and sulfur (S) (Table 3). Limits of quantification (LOQ) were as follows: 5.00 mg/kg for P, K, Ca, and Mg; 500 mg/100 g for N, and 6.70 mg/kg for S. N and P concentrations were similar between tank-cultivated and wild in situ Ulva spp., whereas K, Ca, Mg, and S fluctuated greatly between the two. The trace elements K, Mg, and S were nearly four, five, and two times as concentrated, respectively, in the tank-cultivated Ulva spp. compared the wild in situ Ulva spp. Wild in situ Ulva spp. were also approximately twice as concentrated in Ca. Higher concentrations of K, Mg, and S in the tank-cultivated Ulva may be attributed to sediment buildup resulting from the flow-through aquaculture system, which continuously pumps raw and unfiltered harbor water. The higher Ca concentrations of the Ulva spp. collected from the intertidal Fort Johnson shoreline may be explained by biogenic calcium carbonate (CaCO3), such as from bivalve shells (e.g., oysters), which often compose about 50% of coastal ocean sediments [60].
3.3.2. Algal Tissue Analyses for Trace Metals and Pesticides
Tank-cultivated and wild in situ Ulva spp. were collected on 31 May 2024 prior to nutrient supplementation of the tank. Algal tissues (n = 1) were evaluated for four major trace metals: arsenic (As), cadmium (Cd), lead (Pb), and mercury (Hg), as well as potential pesticides from a 354-compound screen (Table 3). The LOQ for trace metals and pesticides was 0.010 mg/kg. It was determined that As and Pb levels are roughly equivalent between tank-cultivated and wild in situ Ulva spp., yet Cd and Hg are approximately 1.5 and 4.5 times higher in tank-cultivated Ulva spp., respectively. Diethyltoluamide (DEET), the active ingredient in insect repellants, was the only quantified pesticide, just barely above the LOQ. The total As, consisting of both organic and inorganic As (iAs), was measured at 4.47 and 4.52 mg/kg for the tank-cultivated and wild in situ Ulva spp., respectively. In New England, USA, the iAs in Chlorophyta and Rhodophyta comprise ~5% and 6% of total As, respectively, which is notably higher in Phaeophyta, comprising around 22% [61]. For instance, the iAs of Ulva prolifera harvested from the Great Bay tidal estuary in New Hampshire is ~0.82% of total As, whereas Laminaria digitata from Maine is ~16.5% [62].
Given that the Provisional Tolerable Weekly Intake (PTWI) of the World Health Organization (WHO) of inorganic As is 15 µg/kg of body weight, an individual (e.g., 70 kg) would have to consume ~2.35 kg DW (1050 μg/week ÷ 4.47 mg/kg) of the tank-cultivated Ulva spp. to exceed the safe dose, assuming that inorganic As is 10% (high end) of the total content [63]. To put this in proportion, a consumer would have to ingest over 750 seaweed snack sheets (~3 g each) per week to reach the PTWI for inorganic As. The similar As values between tank-cultivated and wild in situ Ulva spp. suggest that there is no increased risk in As accumulation in land-based tank cultivation. Dietary risk assessments based solely on total As concentrations may overestimate or underestimate actual risk, depending on the iAs present. Therefore, the robust evaluation of Ulva as a food source requires an As speciation analysis (e.g., HPLC–ICP-MS) to quantify iAs. Until such data are available, only provisional comparisons can be made, and dietary exposure estimates must be interpreted with caution.
Conversely, the increased concentration of Cd (~79% difference), Pb (~17% difference), and Hg (~130% difference) in the tank-cultivated Ulva spp., in comparison to the wild in situ Ulva spp., suggests that land-based aquaculture may be an option for wastewater management for treating anthropogenic contaminants, such as sewage discharges [64]. Cd (0.205 mg/kg–tank; 0.089 mg/kg–wild) and Pb (0.103 mg/kg–tank; 0.022 mg/kg–wild) in Ulva were far below EU maximum levels (3.0 and 1.0 mg/kg, respectively) and WHO/JECFA intake limits (0.0058 and 0.004 mg/kg/week). Pb (1.35; 1.14 mg/kg) was below the EU limit (3.0 mg/kg) and lower than the WHO intake value (0.025 mg/kg/week). Overall, Cd, Pb, and Hg occurred at minute levels, remaining safely within international standards.

Table 3.
Tank-cultivated vs. wild in situ Ulva spp. biochemical composition: tissue essential trace elements analysis (K, Ca, Mg, and S), trace metals (As, Cd, Pb, and Hg), and pesticide screen (DEET). Samples collected 31 May 2024 (pre-spike). Unit: mg/kg (DW); Limit of Quantification (LOQ): trace metals and pesticides–0.010 mg/kg; essential trace elements–5.00 mg/kg for Ca, Mg, K, and P; 6.70 mg/kg for S; 500 mg/100 g for N. There were no replicates (n = 1). European Food Safety Authority (EFSA), European Union (EU), and WHO weekly intake limits (mg/kg/bw/week, 70 kg) are included for reference [63,65,66,67,68,69].
Table 3.
Tank-cultivated vs. wild in situ Ulva spp. biochemical composition: tissue essential trace elements analysis (K, Ca, Mg, and S), trace metals (As, Cd, Pb, and Hg), and pesticide screen (DEET). Samples collected 31 May 2024 (pre-spike). Unit: mg/kg (DW); Limit of Quantification (LOQ): trace metals and pesticides–0.010 mg/kg; essential trace elements–5.00 mg/kg for Ca, Mg, K, and P; 6.70 mg/kg for S; 500 mg/100 g for N. There were no replicates (n = 1). European Food Safety Authority (EFSA), European Union (EU), and WHO weekly intake limits (mg/kg/bw/week, 70 kg) are included for reference [63,65,66,67,68,69].
| Tank-Cultivated Ulva spp. (mg/kg) | Wild In Situ Ulva spp. (mg/kg) | EFSA Intake Limits (mg/kg/week) EU Maximum Levels (mg/kg/week) | WHO Intake Limits (mg/kg/week) | ||
|---|---|---|---|---|---|
| Essential Trace Elements | Nitrogen (N) | 22,870 | 29,680 | N/A | N/A |
| Phosphorus (P) | 1326 | 1323 | N/A | N/A | |
| Potassium (K) | 19,530 | 5520 | 250 (EFSA) | N/A | |
| Calcium (Ca) | 4841 | 8103 | 250 (EFSA) | N/A | |
| Magnesium (Mg) | 30,383 | 10,530 | 25 (EFSA) | N/A | |
| Sulfur (S) | 52,618 | 24,288 | N/A | N/A | |
| Trace Metals | Arsenic (As) | 4.47 | 4.52 | 0.50 (EU) | 0.015 |
| Cadmium (Cd) | 0.205 | 0.089 | 3.0 (EU) | 0.0058 | |
| Lead (Pb) | 1.35 | 1.14 | 3.0 (EU) | 0.025 | |
| Mercury (Hg) | 0.103 | 0.022 | 1.0 (EU) | 0.004 | |
| Pesticide | DEET | 0.048 | 0.040 | 0.01 (EU) | N/A |
3.4. Limitations
Several limitations should be considered when interpreting these findings. This pilot study utilized a single aquaculture tank to examine the locally abundant Ulva spp. and was limited to 16 months due to scheduled construction at the outdoor SCDNR tank site. It should also be noted that no control or independent replicate tanks were available to account for environmental or system-level variability. Fertilizer supplementation was evaluated over a relatively short two-week period, offering only a limited view of inorganic nutrient release dynamics and algal physiological responses through time. Biochemical composition analyses conducted by a third-party laboratory, AGQ Labs, were not replicated, constraining the statistical robustness of the trace element, trace metal, and pesticide data. Moreover, water-column monitoring was not comprehensive, as parameters such as dissolved inorganic N speciation and temperature fluctuations were not continuously measured. Collectively, these factors underscore the need for longer-term, replicated experiments with comprehensive water-quality monitoring to elucidate better the biomass and bioremediation potential of Ulva spp. in land-based cultivation systems. Finally, results may reflect tank- or site-specific conditions, as this represents the first documented macroalgal aquaculture study from Charleston Harbor (Fort Johnson) and possibly the first of its kind in South Carolina.
4. Conclusions
Locally abundant Ulva spp. from Charleston, SC, were determined as optimal candidates for a pilot aquaculture study due to their sustainability: practicality as self-sufficient and cost-effective macroalgae, and their established resilience to abiotic and biotic factors. As a result, a 16-month investigation into seasonal biomass and potential bioremediation was conducted using SCDNR’s outdoor, land-based flow-through tanks, which were modified for tumble culture. Seasonal observations in biomass, temperature, and day length were made, indicating high productivity during the spring as water temperatures increased. Year-to-year variations (e.g., comparing the high-productivity spring months between 2023 and 2024) in biomass were observed throughout the seasons; however, an overall correlation with temperature and day length was determined. Unpredictable factors, such as the influx or lack of nutrients, temperature fluctuations related to the weather, and potential predation or epiphytes in the Ulva spp. tank, were reminiscent of variables that farmers or gardeners may encounter with terrestrial plants during a growth season.
Fertilizer supplementation was incorporated to spike the Ulva spp. tank with inorganic nutrients to mimic an anthropogenic event, such as from industrial runoff. Interestingly, this resulted in no increase in productivity in terms of biomass, yet it revealed that the macroalgae metabolized inorganic N (δ15N < 7‰) in their tissues. Additionally, δ15N served as an indicator of the potential trophic origin of the nutrients metabolized by the tank-cultivated Ulva spp. Thus, demonstrating the utility of tank-cultivated Ulva spp. as natural indicators for anthropogenic contaminants in the Charleston Harbor and surrounding estuaries [16]. The synthetic fertilizer also resulted in an uptick of Vibrio spp. bacterial populations, raising safety concerns when cultivating macroalgae for potential human or animal consumption. Lastly, tank-cultivated and wild in situ Ulva spp. were chemically analyzed for their biochemical composition before nutrient supplementation, revealing similar low concentrations of trace metals and the DEET pesticide, as well as notable variations in the concentration of essential trace elements. Land-based cultivation of Ulva spp. may offer solutions to the impending wastewater from the rapidly growing metropolitan coastal areas around Charleston, SC.
Future studies investigating tank-cultivated Ulva spp. as potential bioindicators of anthropogenic pollution—through biomarkers such as δ15N and C:N isotope ratios—would provide valuable insights for public health monitoring, particularly in relation to local fisheries and recreational waters. Expanding the system to include additional replicate tanks will be crucial for assessing reproducibility and scaling up production under controlled conditions (e.g., optimal temperatures for biomass growth). Moreover, comprehensive water-quality monitoring should be incorporated into future aquaculture designs to detect antibiotic-resistant bacteria beyond Vibrio and coliforms (e.g., E. coli), as well as trace metals and urban-derived contaminants associated with stormwater and flooding events [70,71]. Collectively, these efforts will optimize the application of Ulva spp. or other locally abundant macroalgae for sustainable biomass production and as a natural tool for monitoring coastal water quality.
To the best of our knowledge, the pilot study presented here is the first reported research on macroalgal aquaculture in South Carolina. It serves as a foundation for cultivating Ulva spp. sustainably for the seasonal production of biomass and potential bioremediation via sequestration of essential trace elements, trace metals, and pesticides from local water systems. Hence, this research serves as a preliminary framework for commercial or publicly supported land-based aquaculture sectors interested in cultivating macroalgae along the coast of South Carolina.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/aquacj5040023/s1, Table S1: Red (Rhodophyta), brown (Phaeophyta), and green (Chlorophyta) macroalgae species were attempted in aquaculture; Figure S1. Outdoor SCDNR aquaculture tanks at Fort Johnson; Figure S2: Assembly of a flow-through standpipe and cross-shaped airline; Figure S3: Biomass data for Ulva spp., a green alga (Chlorophyta), over a 16-month period; Figure S4: Biomass data for Gracilaria tikvahiae, a red alga (Rhodophyta), over a 6-month period; Figure S5: Biomass data for Gracilaria vermiculophyla, a red alga (Rhodophyta), over a 2-month period; Figure S6: Biomass data for Petalonia fascia, a brown alga (Phaeophyta), over a 6-month period; Figure S7: Biomass data for Sargassum filipendula, a brown alga (Phaeophyta), over a 6-month period; Figure S8: Biomass data for Phyllymenia [fmr. Grateloupia] gibbesii, a red alga (Rhodophyta), over a 6-month period; Figure S9: Biomass data for Agardhiella subulata, a red alga (Rhodophyta), over a 6-month period; Figure S10: Biomass data for Agardhiella subulata, a red alga (Rhodophyta), over a 6-month period; Figure S11: An outdoor aquaculture test run was conducted using 40-gallon glass aquarium tanks to culture locally abundant, invasive, and commercial-sourced (AlgaeBarn) species of various Gracilaria macroalgae; Figure S12: Handheld meters were used to measure the following water parameters in the Ulva spp. tank: (A) salinity and water temperature. (B) pH; Figure S13: Ulva spp. tank water temperature, salinity, and pH data throughout the 16-month study; Table S2: Temperature, salinity, and pH data of Tank #5 between 6 January 2023 and 14 June 2024; Table S3: Temperature, salinity, and pH data of DNR Tanks #2 and #4 for the other macroalgae cultivated during the early stages of the study from 6 January to 24 June 2023; Figure S14: Map of the USGS Ashley River monitoring locations at the Charleston Harbor; Figure S15: Water and air temperature data from the USGS Ashley River monitoring station and The Weather Channel, respectively, during the 16-month study; Figure S16: Salinity and dissolved O2 data from the USGS Ashley River monitoring station during the 16-month study; Table S4: Tide, temperature, salinity, and dissolved oxygen data from the USGS Ashley River monitoring station were recorded weekly; Figure S17: Nutrient spike of Ulva spp. tank; Figure S18: Pre- and post-spike Ulva spp. prepared for carbon-nitrogen (C/N) isotope analyses.
Author Contributions
Conceptualization, M.M.B. and L.M.K.; methodology, M.M.B., L.M.K., C.J.C., H.L.S. and A.W.; investigation, M.M.B., L.M.K. and C.J.C.; resources, L.M.K., H.L.S. and A.W.; data curation, M.M.B., L.M.K. and C.J.C.; writing—original draft preparation, M.M.B. and L.M.K.; writing—review and editing, M.M.B., L.M.K., C.J.C. and G.S.H.; supervision, L.M.K. and H.L.S.; project administration, L.M.K.; funding acquisition, L.M.K., H.L.S. and G.S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of South Carolina (MUSC) Department of Pharmacology and Immunology—L.M.K.; the Department of Biology at the College of Charleston (CofC)—H.L.S.; the 2023 Center for Resilience Excellence (CORE SC) Innovation Grant (grant index # 631239)—H.L.S., and the National Center for Complementary and Integrative Health: (F31AT011158)—G.S.H.
Institutional Review Board Statement
This study involved only marine macroalgae and did not include human participants or vertebrate animals. Therefore, ethical approval was not required under U.S. federal regulations governing research with human subjects (45 CFR 46—Protection of Human Subjects) or vertebrate animals (9 CFR Parts 1–3—Animal Welfare Act Regulation).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We would like to thank the SCDNR for their infrastructure support and for allowing us to utilize their outdoor aquaculture tanks for our research project. Lastly, we would like to thank the Biogeochemical Stable Isotope Facility at SOEST, University of Hawaiʻi-Mānoa, for processing the samples for the macroalgal stable isotope and tissue nutrient data.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| HAB | harmful algae blooms |
| TAA | total amino acid |
| CFU | colony-forming unit |
| NPK | nitrogen/phosphorus/potassium |
| SCDNR | South Carolina Department of Natural Resources |
| USGS | United States Geological Survey |
| δ13C | Ratio of 13C:12C in a sample |
| δ15N | Ratio of 15N:14N in a sample |
| DOM | dissolved organic matter |
| CaCO3 | calcium carbonate |
| WW | wet weight |
| PVC | polyvinyl chloride |
| N | nitrogen |
| P | phosphorus |
| K | potassium |
| Ca | calcium |
| Mg | magnesium |
| S | sulfur |
| As | arsenic |
| Pb | lead |
| LOQ | limit of quantification |
| EU | European Union |
| WHO | World Health Organization |
| PTWI | Provisional Tolerable Weekly Intake |
| DEET | diethyltoluamide |
| AIR | atmospheric nitrogen |
| VPDB | Vienna Pee Dee Belemnite |
| ARB | antibiotic-resistant bacteria |
| SD | standard deviation |
References
- Lewitus, A.J.; Schmidt, L.B.; Mason, L.J.; Kempton, J.W.; Wilde, S.B.; Wolny, J.L.; Williams, B.J.; Hayes, K.C.; Hymel, S.N.; Keppler, C.J. Harmful algal blooms in South Carolina residential and golf course ponds. Popul. Environ. 2003, 24, 387–413. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Riddicka, B.A.; Li, R.; Able, J.R.; Boakye-Boaten, N.A.; Shahbazi, A. Sustainable production of algal biomass and biofuels using swine wastewater in North Carolina, US. Sustainability 2016, 8, 477. [Google Scholar] [CrossRef]
- Linnaeus, C. Species Plantarum, Exhibentes Plantas Rite Cognitas ad Genera Relatas, Cum Differentiis Specificis, Nominibus Trivialibus, Synonymis Selectis, Locis Natalibus, Secundum Systema Sexuale Digestas; De Trattnern: Vienna, Austria, 1764; Volume 4. [Google Scholar]
- AlgaeBase (Ulva Linnaeus, 1753, nom. et typ. cons.) [Internet]. National University of Ireland, Galway. 1753. Available online: https://www.algaebase.org/search/genus/detail/?genus_id=33 (accessed on 10 August 2025).
- Neveux, N.; Bolton, J.J.; Bruhn, A.; Roberts, D.A.; Ras, M. The bioremediation potential of seaweeds: Recycling nitrogen, phosphorus, and other waste products. In Blue Biotechnology: Production and Use of Marine Molecules; Wiley: Weinheim, Germany, 2018; Volume 1, pp. 217–239. [Google Scholar]
- de Oliveira, V.P.; Martins, N.T.; Guedes, P.d.S.; Pollery, R.C.G.; Enrich-Prast, A. Bioremediation of nitrogenous compounds from oilfield wastewater by Ulva lactuca (Chlorophyta). Bioremediat. J. 2016, 20, 1–9. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Bruhn, A.; Rasmussen, M.B.; Olesen, B.; Larsen, M.M.; Møller, H.B. Cultivation of Ulva lactuca with manure for simultaneous bioremediation and biomass production. J. Appl. Phycol. 2012, 24, 449–458. [Google Scholar] [CrossRef]
- Samarathunga, J.; Wijesekara, I.; Jayasinghe, M. Seaweed proteins as a novel protein alternative: Types, extractions, and functional food applications. Food Rev. Int. 2023, 39, 4236–4261. [Google Scholar] [CrossRef]
- Espinosa-Ramírez, J.; Mondragón-Portocarrero, A.C.; Rodríguez, J.A.; Lorenzo, J.M.; Santos, E.M. Algae as a potential source of protein meat alternatives. Front. Nutr. 2023, 10, 1254300. [Google Scholar] [CrossRef]
- Bews, E.; Booher, L.; Polizzi, T.; Long, C.; Kim, J.-H.; Edwards, M.S. Effects of salinity and nutrients on metabolism and growth of Ulva lactuca: Implications for bioremediation of coastal watersheds. Mar. Pollut. Bull. 2021, 166, 112199. [Google Scholar] [CrossRef]
- Geddie, A.W.; Hall, S.G. Development of a suitability assessment model for the cultivation of intertidal macroalgae in the United States. Sci. Total Environ. 2020, 699, 134327. [Google Scholar] [CrossRef]
- Moreira, A.; Cruz, S.; Marques, R.; Cartaxana, P. The underexplored potential of green macroalgae in aquaculture. Rev. Aquac. 2022, 14, 5–26. [Google Scholar] [CrossRef]
- Benjamin, M.M.; Hanna, G.S.; Hamann, M.T. Marine Algae’s Major Role in Carbon Sequestration and Petroleum Product Independence. Int. J. Oceanogr. Aquac. Res. 2021, 1. [Google Scholar] [CrossRef]
- Baharlooeian, M.; Benjamin, M.M.; Choudhary, S.; Hosseinian, A.; Hanna, G.S.; Hamann, M.T. Marine Metabolites for the Sustainable and Renewable Production of Key Platform Chemicals. Processes 2025, 13, 2685. [Google Scholar] [CrossRef]
- Pereira, L.; Cotas, J.; Gonçalves, A.M. Seaweed Proteins: A Step towards Sustainability? Nutrients 2024, 16, 1123. [Google Scholar] [CrossRef]
- Troell, M.; Henriksson, P.J.; Buschmann, A.; Chopin, T.; Quahe, S. Farming the ocean–seaweeds as a quick fix for the climate? Rev. Fish. Sci. Aquac. 2023, 31, 285–295. [Google Scholar] [CrossRef]
- Holzinger, A.; Herburger, K.; Kaplan, F.; Lewis, L.A. Desiccation tolerance in the chlorophyte green alga Ulva compressa: Does cell wall architecture contribute to ecological success? Planta 2015, 242, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, X.; Gao, C.; Jiang, M.; Li, R.; Wang, Z.; Li, Y.; Fan, S.; Zhang, X. Effect of temperature, salinity and irradiance on growth and photosynthesis of Ulva prolifera. Acta Oceanol. Sin. 2016, 35, 114–121. [Google Scholar] [CrossRef]
- Rybak, A.S. Species of Ulva (Ulvophyceae, Chlorophyta) as indicators of salinity. Ecol. Indic. 2018, 85, 253–261. [Google Scholar] [CrossRef]
- Diamahesa, W.A.; Masumoto, T.; Jusadi, D.; Setiawati, M. Growth and protein content of Ulva prolifera maintained at different flow rates in integrated aquaculture system. J. Ilmu Dan. Teknol. Kelaut. Trop. 2017, 9, 429–441. [Google Scholar] [CrossRef]
- Msuya, F.E.; Kyewalyanga, M.S.; Salum, D. The performance of the seaweed Ulva reticulata as a biofilter in a low-tech, low-cost, gravity generated water flow regime in Zanzibar, Tanzania. Aquaculture 2006, 254, 284–292. [Google Scholar] [CrossRef]
- Al-Hafedh, Y.S.; Alam, A.; Buschmann, A.H. Bioremediation potential, growth and biomass yield of the green seaweed, Ulva lactuca in an integrated marine aquaculture system at the Red Sea coast of Saudi Arabia at different stocking densities and effluent flow rates. Rev. Aquacult. 2015, 7, 161–171. [Google Scholar] [CrossRef]
- Everris. Osmocote Classic 14-14-14 SKU# E90550. Available online: https://www.domyown.com/msds/Osmocote_14_14_14_label_opt.pdf?srsltid=AfmBOopI7C4GcOOo98aUI68MrYNxzIcbjUZPeSLHRlx0lTXpHrC22hLq (accessed on 10 August 2025).
- Strait, N.; Williams, T.M.; Sherwood, A.R.; Kosaki, R.K.; Giuseffi, L.; Smith, C.M.; Spalding, H.L. Nitrogen stable isotopes (δ 15N) and tissue nitrogen in shallow—water and mesophotic macroalgae differ between the Main Hawaiian Islands and the Northwestern Hawaiian Islands. Limnol. Oceanogr. 2022, 67, 1211–1226. [Google Scholar] [CrossRef]
- Gonzalez, D.J.; Gonzalez, R.A.; Froelich, B.A.; Oliver, J.D.; Noble, R.T.; McGlathery, K.J. Non-native macroalga may increase concentrations of Vibrio bacteria on intertidal mudflats. Mar. Ecol. Prog. Ser. 2014, 505, 29–36. [Google Scholar] [CrossRef]
- Dufour, A.P.; Strickland, E.R.; Cabelli, V.J. Membrane filter method for enumerating Escherichia coli. Appl. Environ. Microbiol. 1981, 41, 1152–1158. [Google Scholar] [CrossRef]
- Craig, W.; Schneider, R.B.S. Seaweeds of the Southeastern United States Cape Hatteras to Cape Canaveral; Duke University Press: Durham, NC, USA, 1991. [Google Scholar]
- Ulva Lactuca Linnaeus 1753 [Internet]. National University of Ireland. [Cited 2025]. 2010. Available online: https://www.algaebase.org/search/species/detail/?species_id=39 (accessed on 10 August 2025).
- Guidone, M.; Thornber, C.; Wysor, B.; O’Kelly, C.J. Molecular and morphological diversity of Narragansett Bay (RI, USA) Ulva (Ulvales, Chlorophyta) populations. J. Phycol. 2013, 49, 979–995. [Google Scholar] [CrossRef] [PubMed]
- Kuba, G.M.; Carpio-Aguilar, B.; Eklund, J.; Freshwater, D.W. A demonstration of DNA Barcoding-based identification of blade-form Ulva (Ulvophyceae, Chlorophyta) species from three site in the San Juan islands, Washington, USA. Diversity 2022, 14, 899. [Google Scholar] [CrossRef]
- Gadberry, B.C.J.; Maynard, D.; Boratyn, D.C. Intensive land-based production of red and green macroalgae for human consumption in the Pacific Northwest: An evaluation of seasonal growth, yield, nutritional composition, and contaminant levels. Algae 2018, 33, 109–125. [Google Scholar] [CrossRef]
- Brinkhuis, P. Seasonal variations in salt-march macroalgae photosynthesis. II. Fucus vesiculosus and Ulva lactuca. Mar. Biol. 1977, 44, 177–186. [Google Scholar] [CrossRef]
- Pang, S.; Lüning, K. Tank cultivation of the red alga Palmaria palmata: Effects of intermittent light on growth rate, yield and growth kinetics. J. Appl. Phycol. 2004, 16, 93–99. [Google Scholar] [CrossRef]
- Jansen, H.M.; Bernard, M.S.; Nederlof, M.A.; van der Meer, I.M.; van der Werf, A. Seasonal variation in productivity, chemical composition and nutrient uptake of Ulva spp. (Chlorophyta) strains. J. Appl. Phycol. 2022, 34, 1649–1660. [Google Scholar] [CrossRef]
- Zertuche-González, J.A.; Sandoval-Gil, J.M.; Rangel-Mendoza, L.K.; Gálvez-Palazuelos, A.I.; Guzmán-Calderón, J.M.; Yarish, C. Seasonal and interannual production of sea lettuce (Ulva sp.) in outdoor cultures based on commercial size ponds. J. World Aquac. Soc. 2021, 52, 1047–1058. [Google Scholar] [CrossRef]
- Fick, A.V. On liquid diffusion. Lond. Edinb. Dubl. Phil. Mag. 1855, 10, 30–39. [Google Scholar] [CrossRef]
- Trientini, F.; Fisher, P.R. Hydroponic fertilizer supply for basil using controlled-release fertilizer. HortScience 2020, 55, 1683–1691. [Google Scholar] [CrossRef]
- Adams, C.; Frantz, J.; Bugbee, B. Macro-and micronutrient-release characteristics of three polymer-coated fertilizers: Theory and measurements. J. Plant Nutri. Soil. Sci. 2013, 176, 76–88. [Google Scholar] [CrossRef]
- Sheppard, E.J.; Hurd, C.L.; Britton, D.D.; Reed, D.C.; Bach, L.T. Seaweed biogeochemistry: Global assessment of C:N and C:P ratios and implications for ocean afforestation. J. Phycol. 2023, 59, 879–892. [Google Scholar] [CrossRef]
- Thornber, C.S.; DiMilla, P.; Nixon, S.W.; McKinney, R.A. Natural and anthropogenic nitrogen uptake by bloom-forming macroalgae. Mar. Pollut. Bull. 2008, 56, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Amato, D.W.; Smith, C.M.; Duarte, T.K. Submarine groundwater discharge differentially modifies photosynthesis, growth, and morphology for two contrasting species of Gracilaria (Rhodophyta). Hydrology 2018, 5, 65. [Google Scholar] [CrossRef]
- Barr, N.G.; Dudley, B.D.; Rogers, K.M.; Cornelisen, C.D. Broad-scale patterns of tissue-δ15N and tissue-N indices in frondose Ulva spp.; developing a national baseline indicator of nitrogen-loading for coastal New Zealand. Mar. Pollut. Bull. 2013, 67, 203–216. [Google Scholar] [CrossRef]
- Fujita, R.M. The role of nitrogen status in regulating transient ammonium uptake and nitrogen storage by macroalgae. J. Exp. Mar. Biol. Ecol. 1985, 92, 283–301. [Google Scholar] [CrossRef]
- Teichberg, M.; Fox, S.E.; Aguila, C.; Olsen, Y.S.; Valiela, I. Macroalgal responses to experimental nutrient enrichment in shallow coastal waters: Growth, internal nutrient pools, and isotopic signatures. Mar. Ecol. Prog. Ser. 2008, 368, 117–126. [Google Scholar] [CrossRef]
- Shahar, B.; Shpigel, M.; Barkan, R.; Masasa, M.; Neori, A.; Chernov, H.; Salomon, E.; Kiflawi, M.; Guttman, L. Changes in metabolism, growth and nutrient uptake of Ulva fasciata (Chlorophyta) in response to nitrogen source. Algal Res. 2020, 46, 101781. [Google Scholar] [CrossRef]
- Pang, S.J.; Xiao, T.; Bao, Y. Dynamic changes of total bacteria and Vibrio in an integrated seaweed–abalone culture system. Aquaculture 2006, 252, 289–297. [Google Scholar] [CrossRef]
- Liang, J.; Liu, J.; Zhan, Y.; Zhou, S.; Xue, C.-X.; Sun, C.; Lin, Y.; Luo, C.; Wang, X.; Zhang, X.-H. Succession of marine bacteria in response to Ulva prolifera-derived dissolved organic matter. Environ. Int. 2021, 155, 106687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, X. Release and microbial degradation of dissolved organic matter (DOM) from the macroalgae Ulva prolifera. Mar. Pollut. Bull. 2017, 125, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Froelich, B.A.; Daines, D.A. In hot water: Effects of climate change on Vibrio–human interactions. Environ. Microbiol. 2020, 22, 4101–4111. [Google Scholar] [CrossRef] [PubMed]
- Hazards, E.P.O.B.; Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F. Public health aspects of Vibrio spp. related to the consumption of seafood in the EU. EFSA J. 2024, 22, e8896. [Google Scholar] [CrossRef]
- DePaola, A.; Nordstrom, J.L.; Bowers, J.C.; Wells, J.G.; Cook, D.W. Seasonal abundance of total and pathogenic Vibrio parahaemolyticus in Alabama oysters. Appl. Environ. Microbiol. 2003, 69, 1521–1526. [Google Scholar] [CrossRef]
- Prum, P.; Harris, L.; Gardner, J. Widespread warming of Earth’s estuaries. Limnol. Oceanogr. 2024, 9, 268–275. [Google Scholar] [CrossRef]
- Climate Trends. Chapter 4. In South Carolina State Climate Assessment South Carolina Office of Resilience, Columbia, SC 2023. Available online: https://scor.sc.gov/sites/scor/files/Documents/Chapter%204%20Climate%20Trends.pdf (accessed on 10 August 2025).
- Igbinosa, E.O. Detection and Antimicrobial Resistance of Vibrio Isolates in Aquaculture Environments: Implications for Public Health. Microb. Drug Resist. 2016, 22, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Vezzulli, L.; Pezzati, E.; Brettar, I.; Höfle, M.; Pruzzo, C. Effects of global warming on Vibrio ecology. Microbiol. Spectr. 2015, 3, 10–1128. [Google Scholar] [CrossRef]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding human health risks caused by antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARG) in water environments: Current knowledge and questions to be answered. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2016–2059. [Google Scholar] [CrossRef]
- Emery, B.; Fullerton, H.; Bossak, B. Resistance on the rise: Assessment of antibiotic-resistant indicator organisms in Shem Creek, Charleston, South Carolina. Dialogues Health 2022, 1, 100063. [Google Scholar] [CrossRef]
- Rickard, L.N.; Houston, C.L.; McGreavy, B.; Johnson, B.B.; Gurney, G. Fish prisons and bluehouses: Perceived risks and benefits of land-based aquaculture in four US communities. Environ. Commun. 2023, 17, 930–946. [Google Scholar] [CrossRef]
- Castine, S.A.; McKinnon, A.D.; Paul, N.A.; Trott, L.A.; de Nys, R. Wastewater treatment for land-based aquaculture: Improvements and value-adding alternatives in model systems from Australia. Aquacu. Environ. Interact. 2013, 4, 285–300. [Google Scholar] [CrossRef]
- Drylie, T.P.; Needham, H.R.; Lohrer, A.M.; Hartland, A.; Pilditch, C.A. Calcium carbonate alters the functional response of coastal sediments to eutrophication-induced acidification. Sci. Rep. 2019, 9, 12012. [Google Scholar] [CrossRef]
- Ma, Z.; Lin, L.; Wu, M.; Yu, H.; Shang, T.; Zhang, T.; Zhao, M. Total and inorganic arsenic contents in seaweeds: Absorption, accumulation, transformation and toxicity. Aquaculture 2018, 497, 49–55. [Google Scholar] [CrossRef]
- Taylor, V.F.; Jackson, B.P. Concentrations and speciation of arsenic in New England seaweed species harvested for food and agriculture. Chemosphere 2016, 163, 6–13. [Google Scholar] [CrossRef]
- Safety Evaluation of Certain Contaminants in Food: Food and Agriculture Organization of the United Nations (FAO). 2011. Available online: https://iris.who.int/bitstream/handle/10665/43406/9241660554_eng.pdf (accessed on 10 August 2025).
- Cabral-Oliveira, J.; Coelho, H.; Pratas, J.; Mendes, S.; Pardal, M.A. Arsenic accumulation in intertidal macroalgae exposed to sewage discharges. J. Appl. Phycol. 2016, 28, 3697–3703. [Google Scholar] [CrossRef]
- Overview on Tolerable Upper Intake Levels as Derived by the Scientific Committee on Food (SCF) and the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Available online: https://www.efsa.europa.eu/sites/default/files/2024-05/ul-summary-report.pdf (accessed on 10 August 2025).
- Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX%3A32023R0915 (accessed on 10 August 2025).
- Cadmium—Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). 2021. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/1376? (accessed on 10 August 2025).
- Lead—Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). 2011. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/3511? (accessed on 10 August 2025).
- Mercury—Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). 2011. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/1806 (accessed on 10 August 2025).
- Fullerton, H.; Vulava, V.M.; Dunn, E.; Caspino, H.; Webb, J.; Gamble, C. The Presence of Antibiotic-Resistant E. coli and Coliforms in Urban Floodwaters of Charleston, SC. In Proceedings of the AGU Fall Meeting Abstracts, Washington, DC, USA, 9–13 December 2024. [Google Scholar]
- Squiggins, K.T.; Fullerton, H.; Vulava, V.M. The presence of ampicillin-resistant coliforms in urban floodwaters of a coastal city in the southeastern United States. Front. Water 2024, 6, 1359196. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).