Patent Landscape Analysis of Bivalve Mollusc Decontamination Technologies: A Review
Abstract
1. Introduction
2. Materials and Methods
3. Relevant Sections
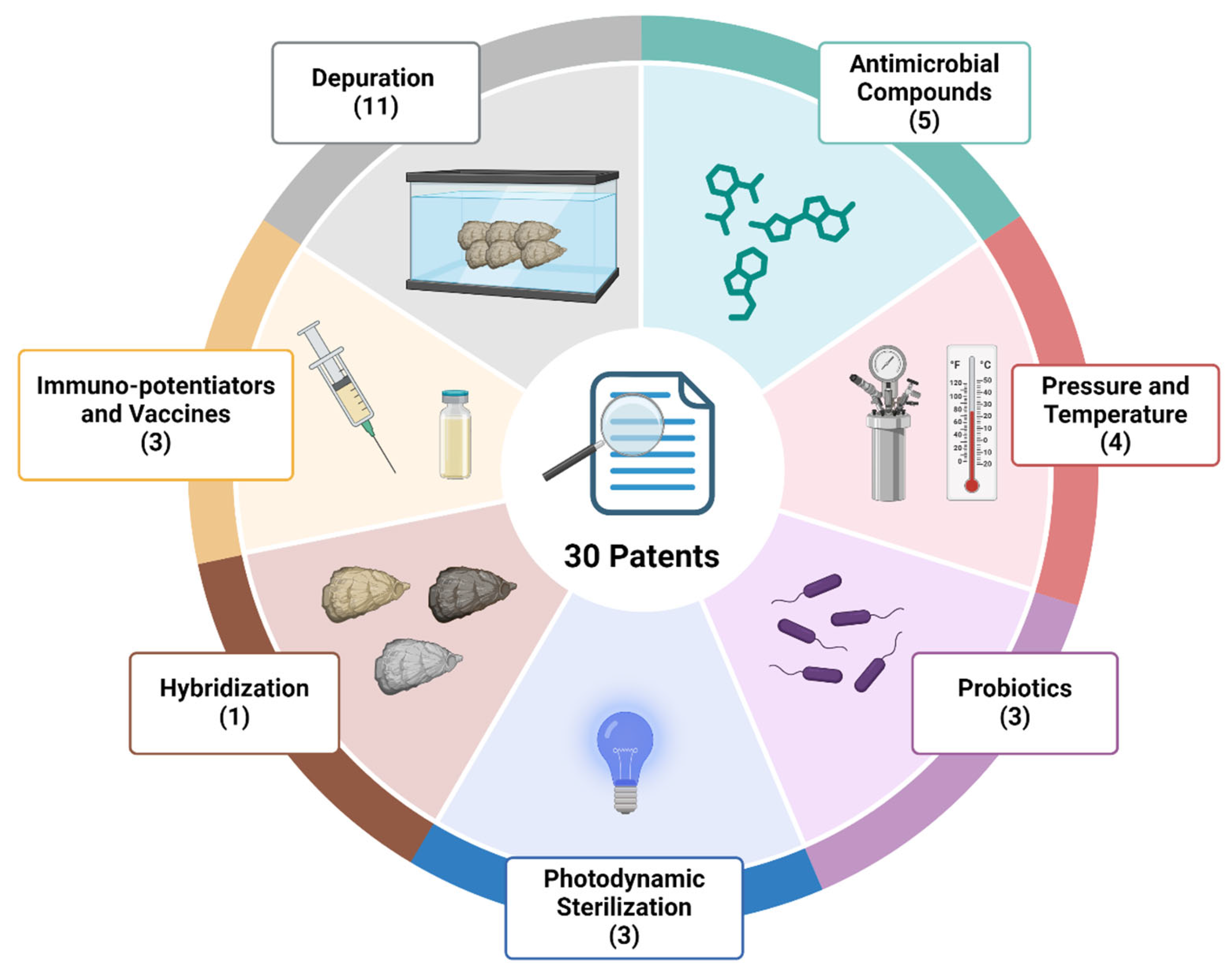
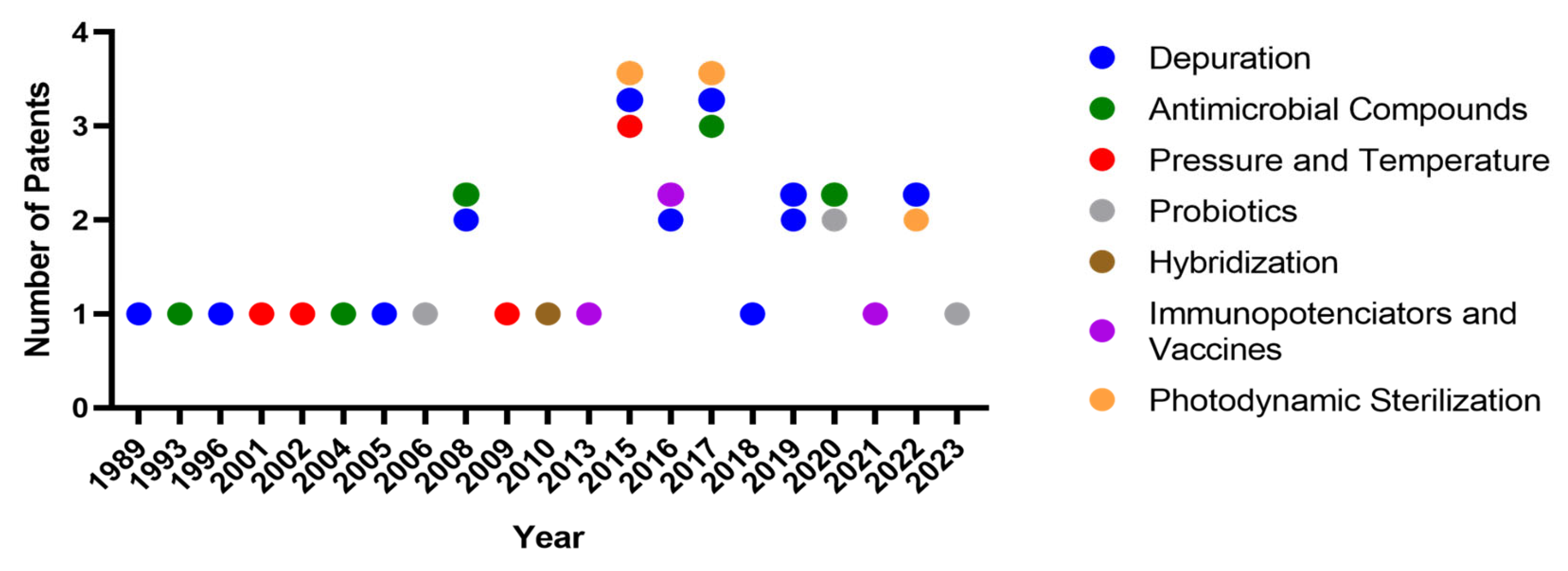
3.1. Information About the Selected Patents
3.2. Depuration
3.3. Pressure and Temperature
3.4. Immuno-Potentiators and Vaccines
3.5. Probiotics
3.6. Antimicrobial Compounds
3.7. Photodynamic Sterilization
3.8. Hybridization
3.9. Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPO | European Patent Organization |
| USA | United States of America |
| WIPO | World Intellectual Property Organization |
| UV | Ultraviolet light |
| pH | Potential of Hydrogen Ion Concentration |
| NoV-GI | Human Norovirus Genogroup I |
| NoV | Human Norovirus |
| EU | European Union |
| EFSA | European Food Safety Authority |
| PC | Performance Criterion |
| HHP | High Hydrostatic Pressure |
| EDTA | Ethylenediaminetetraacetic Acid |
| Fe | Iron |
| Na | Sodium |
| Zn | Zinc |
| OsHV-1 | Ostreid Herpesvirus Type 1 |
| ISAPP | International Scientific Association for Probiotics and Prebiotics |
| PACAP | Pituitary Adenylate Cyclase-Activating Polypeptide |
| DBDP | Dielectric Barrier Discharge Plasma |
| PASW | Plasma-Activated Simulated Seawater |
References
- FAO. The state of world fisheries and aquaculture. In Towards Blue Transformation; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Gawel, J.P.F.; Aldridge, D.C.; Willer, D.F. Barriers and drivers to increasing sustainable bivalve seafood consumption in a mass market economy. Food Front. 2023, 4, 1257–1269. [Google Scholar] [CrossRef]
- Kowalewski, M.; Domènech, R.; Martinell, J. Vanishing clams on an Iberian beach: Local consequences and global implications of accelerating loss of shells to tourism. PLoS ONE 2014, 9, e83615. [Google Scholar] [CrossRef]
- Holovkov, A.M.; Kovalenko, V.F.; Sova, A.M. Application of bivalve molluscs in the biological purification of polluted natural waters. J. Water Chem. Technol. 2023, 45, 481–486. [Google Scholar] [CrossRef]
- Damásio, J.; Navarro-Ortega, A.; Tauler, R.; Lacorte, S.; Barceló, D.; Soares, A.M.; López, M.V.; Riva, M.C.; Barata, C. Identifying major pesticides affecting bivalve species exposed to agricultural pollution using multi-biomarker and multivariate methods. Ecotoxicology 2010, 19, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Khanjani, M.H.; Sharifinia, M.; Mohammadi, A. The impact of microplastics on bivalve molluscs: A bibliometric and scientific review. Mar. Pollut. Bull. 2023, 194, 115271. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, M. EU regulatory risk management of marine biotoxins in the marine bivalve Mollusc Food-Chain. Toxins 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Razafimahefa, R.M.; Ludwig-Begall, L.F.; Thiry, É. Cockles and mussels, alive, alive, oh—The role of bivalve molluscs as transmission vehicles for human norovirus infections. Transbound. Emerg. Dis. 2019, 67, 9–25. [Google Scholar] [CrossRef]
- Zuykov, M.; Pelletier, É.; Harper, D.T. Bivalve molluscs in metal pollution studies: From bioaccumulation to biomonitoring. Chemosphere 2013, 93, 201–208. [Google Scholar] [CrossRef]
- Allam, B.; Raftos, D.A. Immuno responses to infectious diseases in bivalves. J. Invertebr. Pathol. 2015, 131, 121–136. [Google Scholar] [CrossRef]
- Grizzle, J.M.; Brunner, C.J. Infectious diseases of freshwater mussels and other freshwater bivalve molluscs. Rev. Fish. Sci. 2009, 17, 425–467. [Google Scholar] [CrossRef]
- EFSA BIOHAZ. Evaluation of heat treatments, different from those currently established in the EU legislation, that could be applied to live bivalve molluscs from B and C production areas, that have not been submitted to purification or relaying, in order to eliminate pathogenic microorganisms. EFSA J. 2015, 13, 4332. [Google Scholar] [CrossRef]
- Filho, C.E.F.G.; Calixto, F.A.A.; Kasnowski, M.C.; De Fátima, M.M.E. Depuration of bivalve molluscs: A literature review. Food Sci. Technol. 2022, 42, e06622. [Google Scholar] [CrossRef]
- Messens, W.; Escámez, P.S.F.; Lees, D.N.; Lindqvist, R.; O’Mahony, M.; Suffredini, E.; Abrahantes, J.C.; Chantzis, E.; Koutsoumanis, K. Thermal processing of live bivalve molluscs for controlling viruses: On the need for a risk-based design. Crit. Rev. Food Sci. Nutr. 2017, 58, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.C.; Fan, Y.; Baker, G.L. Nutritional value and food safety of bivalve molluscan shellfish. J. Shellfish Res. 2018, 37, 695–708. [Google Scholar] [CrossRef]
- Abbas, A.; Zhang, L.; Khan, S.U. A literature review on the state-of-the-art in patent analysis. World Pat. Inf. 2014, 37, 3–13. [Google Scholar] [CrossRef]
- De Souza, R.V.; Moresco, V.; Miotto, M.; Souza, D.S.M.; De Campos, C.E.M.; Suplicy, F.M. Depuration and heat treatment to reduce pathogen levels in bivalve molluscs produced in Santa Catarina State, Brazil. Agropecu. Catarin. 2022, 35, 78–82. [Google Scholar] [CrossRef]
- Martinez-Albores, A.; López-Santamarina, A.; Rodríguez, J.A.; Ibarra, I.S.; Del Carmen, M.A.; Miranda, J.M.; Lamas, A.; Cepeda, A. Complementary methods to improve the depuration of bivalves: A review. Foods 2020, 9, 129. [Google Scholar] [CrossRef]
- Odeyemi, O.A.; Dabadé, D.; Amin, M.; Dewi, F.R.; Kasan, N.A.; Onyeaka, H.; Dada, A.C.; Stratev, D.; Anyogu, A. Microbial diversity of bivalve shellfish and the use of innovative technologies for preservation, monitoring and shelf-life extension. Food Res. 2023, 7, 209–221. [Google Scholar] [CrossRef]
- Zhang, L.; Qi, F.; Huang, Y.; Van Looy, B.; Chen, L.; Sarıtas, O. Chinese public university patents during 2006–20: A comprehensive investigation and comparative study. Sci. Public Policy 2023, 50, 416–432. [Google Scholar] [CrossRef]
- WIPO—World International Patent Organization. Patents. 2024. Available online: http://www.wipo.int/patents/en/ (accessed on 13 January 2024).
- Richards, G.P. Microbial purification of shellfish: A review of depuration and relaying. J. Food Prot. 1988, 51, 218–251. [Google Scholar] [CrossRef]
- Larsen, A.M.; Rikard, F.S.; Walton, W.C.; Arias, C.R. Effective reduction of Vibrio vulnificus in the eastern oyster (Crassostrea virginica) using high salinity depuration. Food Microbiol. 2013, 34, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, A.A.; Rigotto, C.; Moresco, V.; Kleemann, C.R.; Teixeira, A.L.; Poli, C.R.; Simões, C.M.O.; Barardi, C.R.M. The depuration dynamics of oysters (Crassostrea gigas) artificially contaminated with hepatitis A virus and human adenovirus. Mem. Inst. Oswaldo Cruz 2012, 107, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.R.; Rikard, S.; Arias, C.R. Evaluation of a flow-through depuration system to eliminate the human pathogen Vibrio vulnificus from Oysters. J. Aquac. Res. Dev. 2010, 1, 103. [Google Scholar] [CrossRef]
- McMenemy, P.; Kleczkowski, A.; Taylor, N. Modelling norovirus dynamics within oysters emphasises potential food safety issues associated with current testing & depuration protocols. Food Microbiol. 2023, 116, 104363. [Google Scholar] [CrossRef]
- Barile, N.B.; Scopa, M.; Nerone, E.; Mascilongo, G.; Recchi, S.; Cappabianca, S.; Antonetti, L. Study of the efficacy of a closed cycle depuration system on bivalve molluscs. Vet. Ital. 2009, 45, 555–566. [Google Scholar]
- Campbell, V.; Chouljenko, A.; Hall, S.G. Depuration of live oysters to reduce Vibrio parahaemolyticus and Vibrio vulnificus: A review of ecology and processing parameters. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3480–3506. [Google Scholar] [CrossRef]
- Powell, A.; Scolding, J.W. Direct application of ozone in aquaculture systems. Rev. Aquac. 2016, 10, 424–438. [Google Scholar] [CrossRef]
- Ramos, R.J.; Miotto, M.; Squella, F.J.L.; Cirolini, A.; Ferreira, J.F.; Vieira, C.R.W. Depuration of oysters (Crassostrea gigas) contaminated with Vibrio parahaemolyticus and Vibrio vulnificus with UV light and chlorinated seawater. J. Food Prot. 2012, 75, 1501–1506. [Google Scholar] [CrossRef]
- Sorio, J.C.; Peralta, J.P. Evaluation of a small scale UV-treated recirculating depuration system for oysters (Crassostrea iredalei). Am. J. Food. Sci. Technol. 2017, 5, 117–124. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Wang, J.; Shi, H.; Li, Z.; Gao, C.; Zhang, X.; Xue, Y.; Zhang, H. Investigating comprehensive effects of depuration salinity and duration on posterior anhydrous living-preservation of pacific oyster (Crassostrea gigas). Food Chem. 2024, 435, 137545. [Google Scholar] [CrossRef]
- Chinnadurai, S.; Elavarasan, K.; Geethalakshmi, V.; Kripa, V.; Mohamed, K.S. Evaluation of static and flow-through depuration system on depuration of naturally contaminated farmed edible oyster Crassostrea madrasensis (Preston, 1916). Aquaculture 2021, 545, 737141. [Google Scholar] [CrossRef]
- Younger, A.; Neish, A.; Walker, D.L.; Jenkins, K.L.; Lowther, J.; Stapleton, T.; Alves, M.T. Strategies to reduce norovirus (NoV) contamination from oysters under depuration conditions. Food Chem. Toxicol. 2020, 143, 111509. [Google Scholar] [CrossRef] [PubMed]
- Guyader, F.S.L.; Loisy, F.; Atmar, R.L.; Hutson, A.M.; Estes, M.K.; Ruvoën-Clouet, N.; Pommepuy, M.; Pendu, J.L. Norwalk virus–specific binding to oyster digestive tissues. Emerg. Infect. Dis. 2006, 12, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, H.; Zakhour, M.; Pendu, J.L.; Saux, J.L.; Atmar, R.L.; Guyader, F.S.L. Distribution in tissue and seasonal variation of norovirus genogroup I and II ligands in oysters. Appl. Environ. Microbiol. 2010, 76, 5621–5630. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.; Li, J.; Shi, Z.; An, R.; Wang, Y.; Luo, G.; Wang, D. Identification of potential proteinaceous ligands of GI.1 norovirus in Pacific oyster tissues. Viruses 2023, 15, 631. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martínez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Primers 2018, 4, 1–19. [Google Scholar] [CrossRef]
- Flynn, A.; Davis, B.J.K.; Atherly, E.; Olson, G.; Bowers, J.C.; DePaola, A.; Curriero, F.C. Associations of environmental conditions and Vibrio parahaemolyticus Genetic markers in Washington State pacific oysters. Front. Microbiol. 2019, 10, 2797. [Google Scholar] [CrossRef]
- Froelich, B.; Oliver, J.D. The Interactions of Vibrio vulnificus and the oyster Crassostrea virginica. Microb. Ecol. 2013, 65, 807–816. [Google Scholar] [CrossRef]
- Ndraha, N.; Wong, H.C.; Hsiao, H. Managing the risk of Vibrio parahaemolyticus infections associated with oyster consumption: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1187–1217. [Google Scholar] [CrossRef]
- Jianming, S.; Tianlong, Q.; Wenchao, C.; Jinhu, L.; Yishuai, D.; Li, Z. Tide-simulated bivalve mollusk purification system and purification method. CN115067259A, 20 September 2022. [Google Scholar]
- Qing, L.; Qingtao, M.; Liejin, L.; Wenjun, Y. Ocean Bivalve Mollusk Purifying and Manually Fattening Device. CN204168890U, 25 February 2015. [Google Scholar]
- Li, J.; Duan, Q.; Li, X.; Li, D. Device for Purifying Microbiology in the Body of Seashell Seafood and Method Thereof. CN101180980A, 21 May 2008. [Google Scholar]
- Woodage, C. Shellfish Depuration. US2016100558A1, 14 April 2016. [Google Scholar]
- Junji, N. Shellfish Purification Method and Shellfish Purification System. WO2019138590A1, 18 July 2019. [Google Scholar]
- Xianghu, H.; Danyong, D.; Changling, L. Breeding Method for Lowering Bacterial Quantity and Heavy Metal Content in Bodies of Bivalve Molluscs. CN108668965A, 19 October 2018. [Google Scholar]
- Te’en, F. Method for Preparing Shellfish Purifying Agent and Method for Purifying Shellfishes. CN109819915B, 13 November 2019. [Google Scholar]
- Akira, S.; Mamoru, Y. Method for Purifying Bivalve, Method for Evaluating Purification of Bivalve, and Device for Purifyng Bivalve. JP4393254B2, 21 December 2005. [Google Scholar]
- Robinson, J.R.; William, L. Method for Reducing Contamination of Shellfish. US5482726A, 16 January 1996. [Google Scholar]
- Suplicy, F.M.; de Souza, R.V.; Rosa, E.; Miotto, M.; Tribuzi, G. O desafio de reduzir a carga de bactérias indicadoras fecais e evitar a desova durante a depuração de mexilhões Perna perna. Agropecu. Catarin. 2024, 37, 56–61. [Google Scholar] [CrossRef]
- Murchie, L.; Cruz-Romero, M.C.; Kerry, J.P.; Linton, M.; Patterson, M.F.; Smiddy, M.; Kelly, A.L. High pressure processing of shellfish: A review of microbiological and other quality aspects. Innov. Food Sci. Emerg. Technol. 2005, 6, 257–270. [Google Scholar] [CrossRef]
- Yamamoto, K. Food processing by high hydrostatic pressure. Biosci. Biotechnol. Biochem. 2017, 81, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Bonfim, R.C.; De Oliveira, F.A.; De Oliveira Godoy, R.L.; Rosenthal, A. A review on high hydrostatic pressure for bivalve mollusk processing: Relevant aspects concerning safety and quality. J. Food Sci. Technol. 2019, 39, 515–523. [Google Scholar] [CrossRef]
- Voisin, E.A. Process of Elimination of Bacteria in Shellfish and of Shucking Shellfish. US6426103B2, 30 July 2002. [Google Scholar]
- Hiroshi, U.; Yoshiaki, K.; Hirohisa, K.; Osamu, Y.; Hiroshi, I.; Akira, N.; Yasuyoshi, G.; Masamitsu, M. Virus Inactivation Method in Bivalve. JP2015171323A, 17 September 2015. [Google Scholar]
- Duran, V.S.; Lopez, O.J.C. Procedure for the Treatment of the Seafood (Machine-Translation by Google Translate, not Legally Binding). ES2319037B1, 1 May 2009. [Google Scholar]
- Tadaaki, A.; Ryuhei, U.; Soukai, U. Method for Thermally Bivalve and Bivalve Packed in Container. JP2001029047A, 06 February 2001. [Google Scholar]
- Semple, S.L.; Rodríguez-Ramos, T.; Carpio, Y.; Lumsden, J.S.; Estrada, M.P.; Dixon, B. PACAP Is lethal to Flavobacterium psychrophilum through either direct membrane permeabilization or indirectly, by priming the immuno response in rainbow trout macrophages. Front. Immunol. 2019, 10, 926. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W. The use of immunopotentiators in aquaculture. In Aquaculture Science and Engineering, 2nd ed.; Balasubramanian, B., Liu, W.C., Sattanathan, G., Eds.; Springer: Singapore, 2022; pp. 275–290. [Google Scholar] [CrossRef]
- Lafont, M.; Petton, B.; Vergnes, A.; Pauletto, M.; Segarra, A.; Gourbal, B.; Montagnani, C. Long-lasting antiviral innate immuno priming in the lophotrochozoan pacific oyster, Crassostrea gigas. Sci. Rep. 2017, 7, 13143. [Google Scholar] [CrossRef]
- Bin, Z.; Chuandong, F.; Haixiao, W.; Shanggui, D. Purifying Method for Bivalve Molluscs. CN105248342A, 20 January 2016. [Google Scholar]
- Morga, B.; Montagnani, C.; Renault, T.; Faury, N.; Pepin, J.F.; Degremont, L.; Mege, M. Composition for the Treatment and/or Prevention of Marine Mollusc Viral Infection. WO2021229086A1, 18 November 2021. [Google Scholar]
- Harris, D.L.; Erdman, M.; Kamrud, K.; Smith, J.; Loy, J.D.; Bartholomay, L.; Scura, E. Method of Rapidly Producing Improved Vaccines for Animals. WO2013066665A1, 9 May 2013. [Google Scholar]
- Todorov, S.D.; Carneiro, K.O.; Lipilkina, T.A. Beneficial microorganisms for the health-promoting in oyster aquaculture: Realistic alternatives. Aquac. Int. 2024, 32, 10085–10107. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Prado, S.; Romalde, J.L.; Barja, J.L. Review of probiotics for use in bivalve hatcheries. Vet. Microbiol. 2010, 145, 187–197. [Google Scholar] [CrossRef]
- Sohn, S.; Lundgren, K.M.; Tammi, K.; Smolowitz, R.; Nelson, D.R.; Rowley, D.; Gómez-Chiarri, M. Efficacy of probiotics in preventing vibriosis in the larviculture of different species of bivalve shellfish. J. Shellfish Res. 2016, 35, 319–328. [Google Scholar] [CrossRef]
- Tamilselvan, M.; Raja, S. Exploring the role and mechanism of potential probiotics in mitigating the shrimp pathogens. Saudi J. Biol. Sci. 2024, 31, 103938. [Google Scholar] [CrossRef]
- Elston, R.A.; Gee, A.; Humphrey, K.L. Probiotic System for Aquaculture. WO2006132944A2, 14 December 2006. [Google Scholar]
- Nelson, D.R.; Laporte, J.; Rowley, D.C.; Gomez-Chiarri, M. Marine Bacteria Formulation Useful in Aquaculture. US11851644B2, 2 January 2024. [Google Scholar]
- Pichereau, V.; Paillard, C.; Delavat, F.; Rahmani, A.; Le Chevalier, P. Biological Control of Vibriosis in Aquaculture. WO2023046966A1, 23 March 2023. [Google Scholar]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.; Skubel, S.A.; Patel, H.; Shi, D.C.; Bushek, D.; Chikindas, M.L. From farm to fingers: An exploration of probiotics for oysters, from production to human consumption. Probiotics Antimicrob. Proteins 2020, 12, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Baralla, E.; Demontis, M.P.; Dessì, F.; Varoni, M.V. An overview of antibiotics as emerging contaminants: Occurrence in bivalves as biomonitoring organisms. Animals 2021, 11, 3239. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, L.M.; Nobile, M.; Malandra, R.; Panseri, S.; Arioli, F. Occurrence of antibiotics in mussels and clams from various FAO areas. Food Chem. 2018, 240, 16–23. [Google Scholar] [CrossRef]
- Kijewska, A.; Koroza, A.; Grudlewska-Buda, K.; Kijewski, T.; Wiktorczyk-Kapischke, N.; Zorena, K.; Skowron, K. Molluscs—A ticking microbial bomb. Front. Microbiol. 2023, 13, 1061223. [Google Scholar] [CrossRef]
- Citarasu, T. Natural antimicrobial compounds for use in aquaculture. In Infectious Disease in Aquaculture Prevention and Control, 2nd ed.; Austin, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 419–456. [Google Scholar] [CrossRef]
- Rahimi, N.N.M.N.; Ikhsan, N.F.M.; Loh, J.; Ranzil, F.K.E.; Gina, M.; Lim, S.H.E.; Lai, K.; Chong, C. Phytocompounds as an alternative antimicrobial approach in aquaculture. Antibiotics 2022, 11, 469. [Google Scholar] [CrossRef]
- Vatsos, I.N.; Rebours, C. Seaweed extracts as antimicrobial agents in aquaculture. J. Appl. Phycol. 2015, 27, 2017–2035. [Google Scholar] [CrossRef]
- Bender, F.G.; Brotsky, E. Process for Treating Fish and Shellfish to Control Bacterial Contamination and/or Growth. US5262186A, 16 March 1993. [Google Scholar]
- Riquera, V.R.; Sanchez, L.J.L.; Ben, M.F.F. Novel Antibiotics Against Vibrio anguillarum and the Applications Thereof in Cultures of Fish, Crustaceans, Molluscs and Other Aquaculture Activities. ES2204294B2, 16 July 2004. [Google Scholar]
- García, M.P.E.; González, J.M.L.; González, Y.C.; Piñeiro, C.T. PACAP for the Treatment of Viral Infections in Aquatic Organisms. PT2647369T, 11 April 2017. [Google Scholar]
- Burwell, S.R.; Busch, F. Compositions and Methods for Reducing or Preventing Microorganism Growth or Survival in Aqueous Environments. WO2008008362A2, 17 January 2008. [Google Scholar]
- Cristiano, M.; Cabral, L.; Leite, R.; Leal, J. New Endoperoxide Compounds, Process for Obtaining Them and Uses Thereof for Control of Perkinsiosis in Bivalves. WO2020240266A1, 3 December 2020. [Google Scholar]
- Sheng, L.; Li, X.; Wang, L. Photodynamic inactivation in food systems: A review of its application, mechanisms, and future perspective. Trends Food. Sci. Technol. 2022, 124, 167–181. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Pei, J.; Xue, F.; Cui, X.; Xiong, X.; Li, C. The application of photodynamic inactivation to microorganisms in food. Food Chem. 2021, 12, 100150. [Google Scholar] [CrossRef]
- Chen, B.; Huang, J.; Liu, Y.; Liu, H.; Yong, Z.; Wang, J.J. Effects of the curcumin-mediated photodynamic inactivation on the quality of cooked oysters with Vibrio parahaemolyticus during storage at different temperature. Int. J. Food Microbiol. 2021, 345, 109152. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.; Li, Z.; Zhang, X.; Lu, N.; Xue, C.; Leung, A.W.; Xu, C.; Tang, Q. Curcumin-mediated photodynamic inactivation (PDI) against DH5α contaminated in oysters and cellular toxicological evaluation of PDI-treated oysters. Photodiagn. Photodyn. Ther. 2019, 26, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Gorji, M.E.; Li, D. Photoinactivation of bacteriophage MS2, Tulane virus and Vibrio parahaemolyticus in oysters by microencapsulated rose bengal. Food Qual. Saf. 2022, 6, fyac017. [Google Scholar] [CrossRef]
- Wu, J.; Hou, W.; Cao, B.; Zuo, T.; Xue, C.; Leung, A.W.; Xu, C.; Tang, Q. Virucidal efficacy of treatment with photodynamically activated curcumin on murine norovirus bio-accumulated in oysters. Photodiagn. Photodyn. Ther. 2015, 12, 385–392. [Google Scholar] [CrossRef]
- Tang, Q.; Xue, C.; Cao, B.; Wu, J.; Xue, Y.; Li, Z.; Wang, Y.; Liang, R.; Xu, C. Novel Water Product Photodynamic Cold Sterilization Fresh Keeping Method. CN106857784A, 20 June 2017. [Google Scholar]
- Tang, Q.; Cao, B.; Wu, J.; Liang, R.; Xu, C.; Zuo, T.; Xue, Y.; Li, Z.; Wang, Y.; Xue, C. Photodynamic Cold Sterilizing and Fresh-Keeping Method. CN104304408A, 18 January 2015. [Google Scholar]
- Aboubakr, H.; Goyal, S. Photodynamic Method to Decontaminate Surfaces. US2022023454A1, 27 January 2022. [Google Scholar]
- Liang, Y.; Zhang, G.; Jiang, G.; Hu, Y.; Fang, J.; Chi, Y.; Xu, C.; Liu, W.; Liu, H.; Li, Q. Hybridization between “Haida No. 1” and orange-shell line of the pacific oyster reveals high heterosis in survival. Aquaculture 2024, 551, 737945. [Google Scholar] [CrossRef]
- Meng, L.; Li, Q.; Xu, C.; Liu, S.; Kong, L.; Yu, H. Hybridization improved stress resistance in the pacific oyster: Evidence from physiological and immuno responses. Aquaculture 2021, 545, 737227. [Google Scholar] [CrossRef]
- Guy, L. Method for Obtaining Oysters Resistant to Pathogenic Agents. US2010263600A1, 21 October 2010. [Google Scholar]
- Campbell, V.; Hall, S.G.; Salvi, D. Antimicrobial effects of plasma-activated simulated seawater (PASW) on total coliform and Escherichia coli in live oysters during static depuration. Fishes 2023, 8, 396. [Google Scholar] [CrossRef]
- Pereira, C.; Moreirinha, C.; Teles, L.; Rocha, R.J.M.; Calado, R.; Romalde, J.L.; Nunes, M.L.; Almeida, A. Application of phage therapy during bivalve depuration improves Escherichia coli decontamination. Food Microbiol. 2017, 61, 102–112. [Google Scholar] [CrossRef]
- Song, M.; Kim, J.Y.; Jeon, E.B.; Kim, S.; Heu, M.S.; Lee, J.; Kim, J.; Park, S.Y. Antiviral efficacy of dielectric barrier discharge plasma against Hepatitis A virus in fresh oyster using PMA/RT-qPCR. Appl. Sci. 2023, 13, 3513. [Google Scholar] [CrossRef]
- Fisch, C.; Hassel, T.M.; Sandner, P.; Block, J. University patenting: A comparison of 300 leading universities worldwide. J. Technol. Transf. 2015, 40, 318–345. [Google Scholar] [CrossRef]

| Title | Patent Number | Year | Technology | Target Bivalve Mollusc Species | Recommended or Tested Pathogens | Recommended Depuration Time |
|---|---|---|---|---|---|---|
| Tide-simulated bivalve mollusc purification system and purification method | CN115067259A | 2022 | Depuration system + simulating tides | Clams and oysters | Bacterial microorganism 1 and heavy metal | Not mentioned |
| Shellfish purification method and shellfish purification system | WO2019138590A1 | 2019 | Depuration system + microbubble generator | No defined species 2 | NoV | Not mentioned |
| Method for preparing shellfish purifying agent and method for purifying shellfishes | CN109819915B | 2019 | Depuration system + fermented tea | No defined species 2 | Coliforms, heavy metal, and other substances | 0.5–24 h |
| Breeding method for lowering bacterial quantity and heavy metal content in bodies of bivalve molluscs | CN108668965A | 2018 | Depuration system + chlorinated disinfectant and sodium thiosulfate | No defined species 2 | Coliforms, other bacteria 1, and heavy metal | Not mentioned |
| Shellfish conditioning and depuration system with closed recirculation type | KR101799761B1 | 2017 | Depuration system | Bivalve shellfish | Microorganisms such as NoV and Vibrio | Not mentioned |
| Shellfish depuration | US2016100558A1 | 2016 | Depuration system in artificial reservoir | No defined species 2 | Bacteria 1 and viruses (including NoV) | Approximately 6 days |
| Ocean bivalve mollusc purifying and manually fattening device | CN204168890U | 2015 | Depuration system | No defined species 2 | E. coli | Approximately 24 h |
| Device for purifying microbiology in the body of seashell seafood and method thereof | CN101180980A | 2008 | Depuration system | Bivalve shellfish | Coliforms | 4–40 h |
| Method for purifying bivalve, method for evaluating purification of bivalve, and device for purifying bivalve | JP4393254B2 | 2005 | Electrolytic water | Oysters | NoV/Feline Calicivirus | Not mentioned |
| Method for reducing contamination of shellfish | US5482726A | 1996 | Depuration system + pressurization with ascorbic acid + irradiation | No defined species 2 | Bacterial microorganisms 1 | Not mentioned |
| Molluscs depuration system | ES2009416A6 | 1989 | Depuration system | No defined species 2 | Not mentioned | Not mentioned |
| Title | Patent Number | Year | Technology | Target Bivalve Mollusc Species | Recommended or Tested Pathogens | Pressure, Temperature, and Time |
|---|---|---|---|---|---|---|
| Virus inactivation method in bivalve | JP2015171323A | 2015 | HHP + green tea extract | No defined species 2 | NoV/feline calicivirus | 300–500 MPa 3 min 20 °C |
| Procedure for the treatment of the seafood | ES2319037B1 | 2009 | Pre-cooking + refrigeration + HHP | No defined species 2 | Bacterial microorganism 1 | Pre-cooking: 2–5 min at 85–120 °C HHP: 6000 bar for 5 min |
| Process of elimination of bacteria in shellfish and of shucking shellfish | US6426103B2 | 2002 | HHP | No defined species 2 | Vibrio vulnificus and other bivalve pathogens | 10.000–100.000 psi 1–15 min up to 65.5 °C |
| Method for thermally treating bivalve and bivalve packed in container | JP2001029047A | 2001 | Heat treatment | No defined species 2 | Not mentioned | 120–125 °C for 2–4 min |
| Title | Patent Number | Year | Technology | Target Bivalve Mollusc Species | Recommended or Tested Pathogens | Described Composition of the Formulation |
|---|---|---|---|---|---|---|
| Composition for the treatment and/or prevention of marine mollusk viral infection | WO2021229086A1 | 2021 | Antiviral composition | Oysters | Viruses from the Herpesviridae family | Inactivated viral particle and absorption promoters |
| Purifying method for bivalve molluscs | CN105248342A | 2016 | Immuno-potentiator | Oysters or mussels | V. parahaemolyticus, E. coli, Norwalk virus and astrovirus | Selenomethionine, EDTA-FeNa, EDTA-ZnNa, and β-glucan |
| Methods of rapidly producing improved vaccines for animals | WO2013066665A1 | 2013 | Vaccine formulation | No defined species 2 | Not mentioned 1 | Nucleic acid |
| Title | Patent Number | Year | Technology | Target Bivalve Mollusc Species | Recommended or Tested Pathogens | Probiotic Microorganisms |
|---|---|---|---|---|---|---|
| Biological control of vibriosis in aquaculture | WO2023046966A1 | 2023 | Probiotics | Clams (Venerupis philippinarum) | Pathogenic Vibrio tapetis | Non-pathogenic Vibrio tapetis |
| Marine bacteria formulation useful in aquaculture | US11851644B2 | 2020 | Probiotics | Oysters (Crassostrea virginica) | Vibrio coralliilyticus | Phaeobacter inhibens, Pseudoalteromonas piscicida |
| Probiotic system for aquaculture | WO2006132944A2 | 2006 | Probiotics | Oysters (Crassostrea gigas) | Vibrio tubiashii | Pseudoalteromonas spp. |
| Title | Patent Number | Year | Technology | Target Bivalve Mollusc Species | Recommended or Tested Pathogens | Antimicrobial Compound |
|---|---|---|---|---|---|---|
| New endoperoxide compounds, process for obtaining them and uses thereof for control of perkinsiosis in bivalves | WO2020240266A1 | 2020 | Antiparasitic compounds | No defined species 2 | Perkinsus olseni | Endoperoxide compounds |
| PACAP for the treatment of viral infections in aquatic organisms | PT2647369T | 2017 | Antiviral compound | No defined species 2 | Viral hemorrhagic septicemia virus (VHSV) | Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) |
| Compositions and methods for reducing or preventing microorganism growth or survival in aqueous environments | WO2008008362A2 | 2008 | Antimicrobial water treatment | Not mentioned | Bacterial pathogens 1 | Aliphatic heteroaryl salt, trichloromelamine and other compounds |
| Novel antibiotics against Vibrio anguillarum and the applications thereof in cultures of fish, crustaceans, molluscs and other aquaculture activities | ES2204294B2 | 2004 | Antibiotics | Oyster (Ostrea edulis) and Clam (Ruditapes decussatus) | Vibrio anguillarum | Diketopiperazines |
| Process for treating fish and shellfish to control bacterial contamination and/or growth | US5262186A | 1993 | Decontaminating solution | No defined species 2 | Pseudomonas aeruginosa, Bacillus cereus, Moraxella osloensis | Trialkali metal phosphate |
| Title | Patent Number | Year | Technology | Target Bivalve Mollusc Species | Recommended or Tested Pathogens | Photosensitizer |
|---|---|---|---|---|---|---|
| Photodynamic method to decontaminate surfaces | US2022023454A1 | 2022 | Photodynamic sterilization | Depuration water | Feline Calicivirus, Tulane virus | Rose bengal and phloxine-B |
| Novel water product photodynamic cold sterilization fresh keeping method | CN106857784A | 2017 | Photodynamic sterilization | Oysters | Not mentioned | Curcumin |
| Photodynamic cold sterilizing and fresh-keeping method | CN104304408A | 2015 | Photodynamic sterilization | Oysters | Not mentioned | Curcumin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Provenzi, M.A.; Fongaro, G.; De Dea Lindner, J.; Nunes, I.L.; Savi, B.P.; Zanchetta, L.; Todorov, S.D.; Chikindas, M.L.; Miotto, M. Patent Landscape Analysis of Bivalve Mollusc Decontamination Technologies: A Review. Aquac. J. 2025, 5, 22. https://doi.org/10.3390/aquacj5040022
Provenzi MA, Fongaro G, De Dea Lindner J, Nunes IL, Savi BP, Zanchetta L, Todorov SD, Chikindas ML, Miotto M. Patent Landscape Analysis of Bivalve Mollusc Decontamination Technologies: A Review. Aquaculture Journal. 2025; 5(4):22. https://doi.org/10.3390/aquacj5040022
Chicago/Turabian StyleProvenzi, Marcel Afonso, Gislaine Fongaro, Juliano De Dea Lindner, Itaciara Larroza Nunes, Beatriz Pereira Savi, Lucas Zanchetta, Svetoslav Dimitrov Todorov, Michael Leonidas Chikindas, and Marilia Miotto. 2025. "Patent Landscape Analysis of Bivalve Mollusc Decontamination Technologies: A Review" Aquaculture Journal 5, no. 4: 22. https://doi.org/10.3390/aquacj5040022
APA StyleProvenzi, M. A., Fongaro, G., De Dea Lindner, J., Nunes, I. L., Savi, B. P., Zanchetta, L., Todorov, S. D., Chikindas, M. L., & Miotto, M. (2025). Patent Landscape Analysis of Bivalve Mollusc Decontamination Technologies: A Review. Aquaculture Journal, 5(4), 22. https://doi.org/10.3390/aquacj5040022








