Individual Growth Parameterization Models Using the Observed Variance in Organisms Subject to Aquaculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Bases
2.2. Individual Growth Analysis
2.3. Model Parameterization
2.4. Selecting the Best Structure Error
3. Results
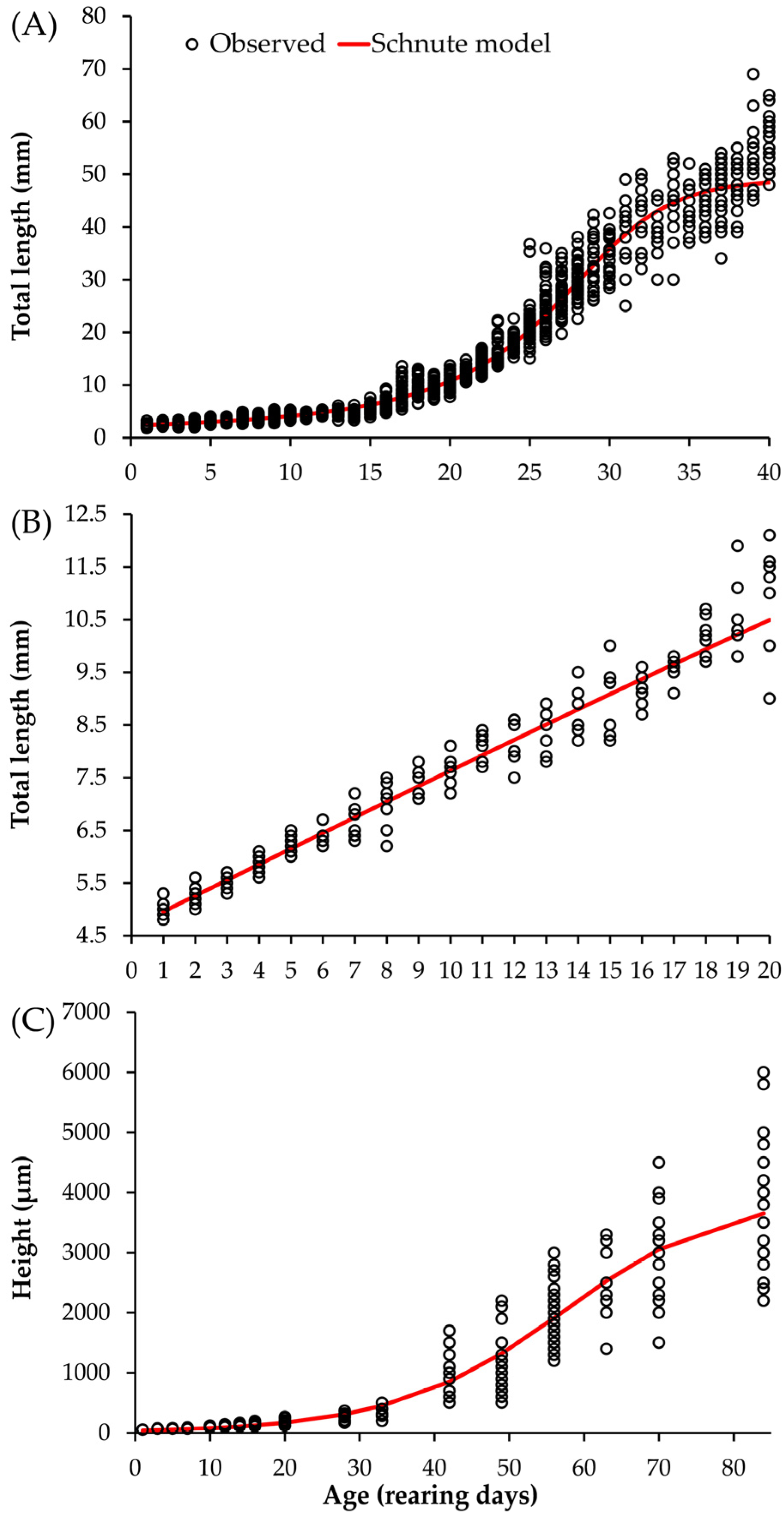
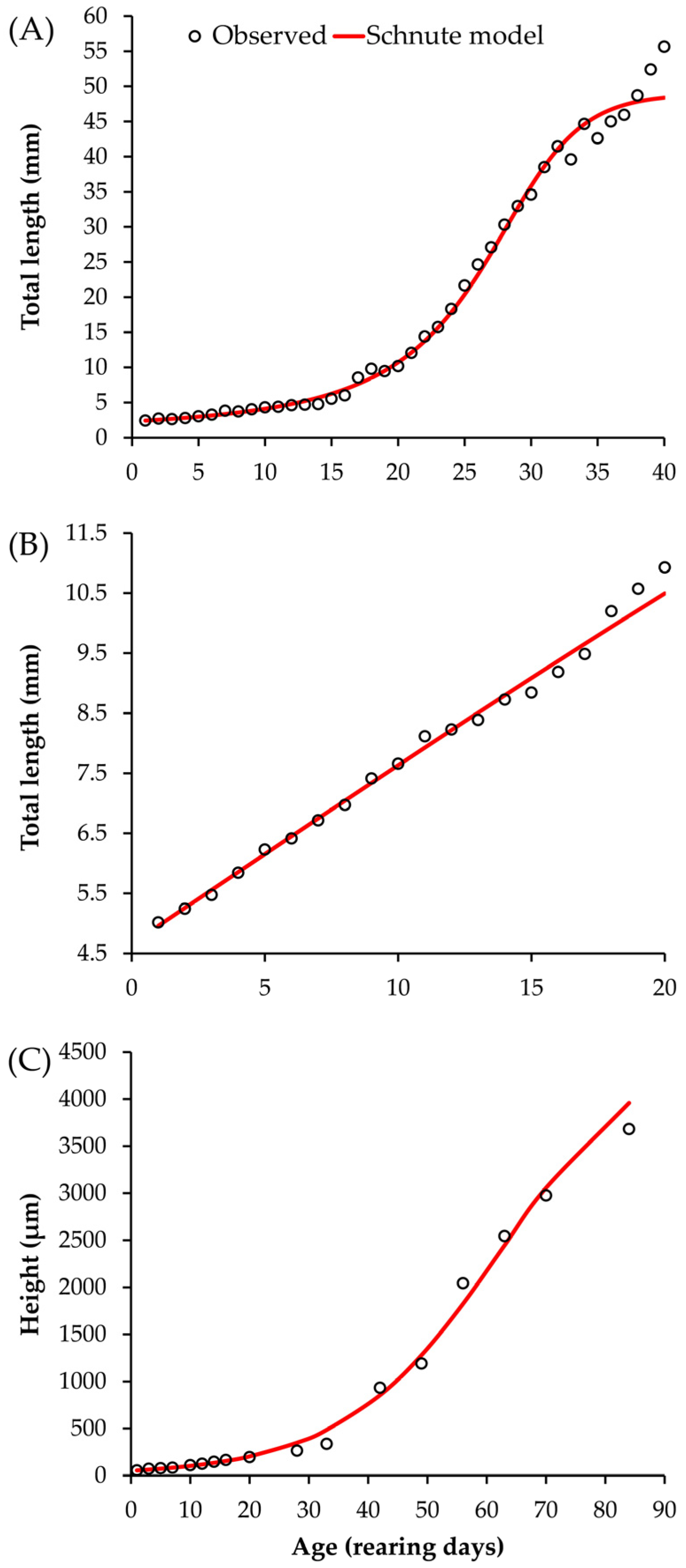
3.1. Development of Organisms in Captivity
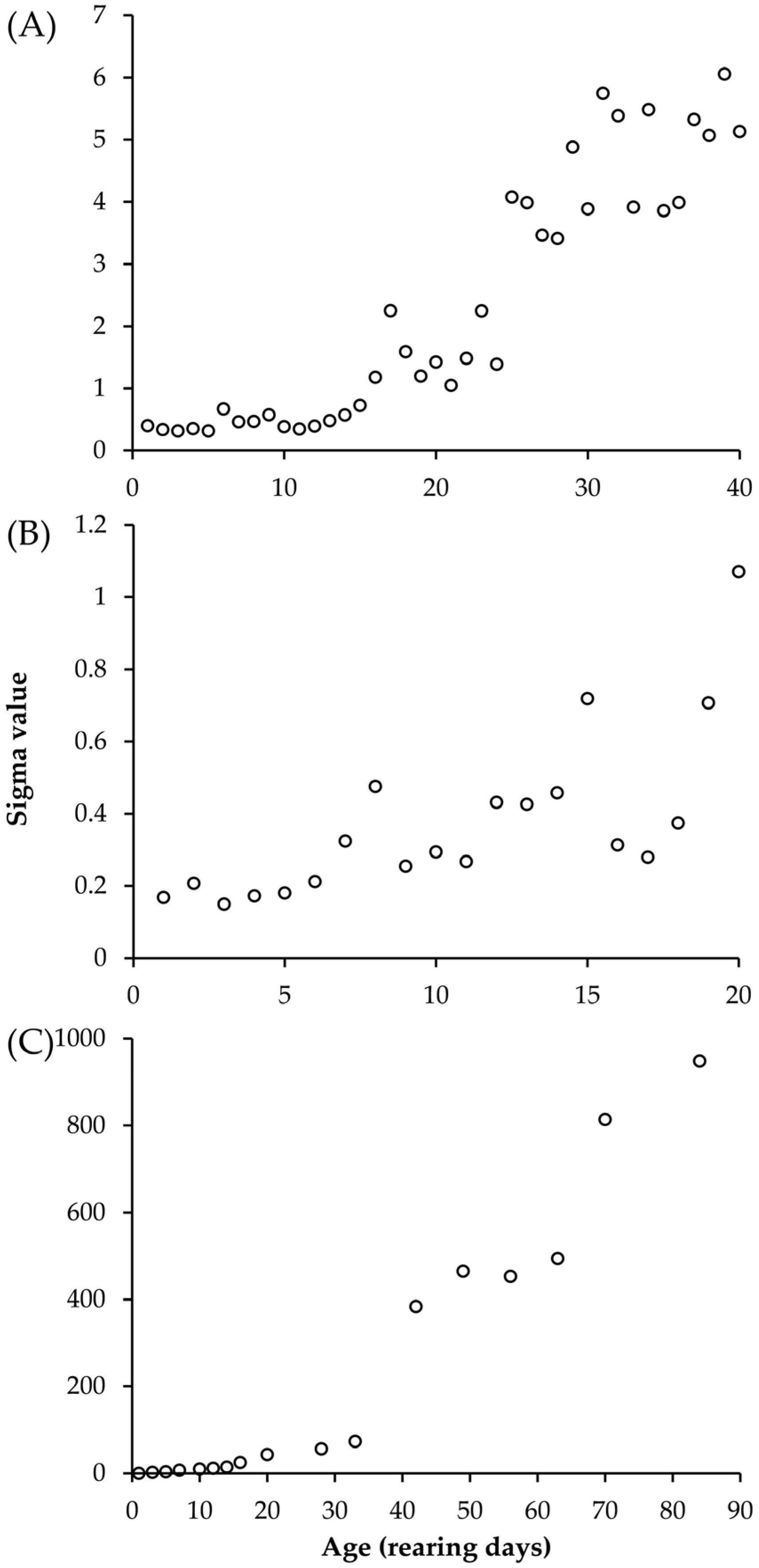
3.2. Observed Variability in Reared Organisms
3.3. Selecting the Appropriate Error Form
3.4. Average Growth of the Three Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BIC | Bayesian Information Criterion |
| CIBNOR | Centro de Investigaciones Biológicas del Noroeste (Northwest Biological Research Center) |
| CONAPESCA | Comisión Nacional de Acuacultura y Pesca (National Fisheries and Aquaculture Commission) |
| FAO | Food and Agricultural Organization |
References
- Urías-Sotomayor, R.; Félix-Ortiz, J.A.; Payán-Alejo, J.; Ramos-Torres, B. El cultivo de camarón Penaeus spp. en México con diferente salinidad en el agua. Rev. Cienc. Mar UAS 2024, 2, 103–121. Available online: https://revistas.uas.edu.mx/index.php/CIMAR/article/view/500 (accessed on 1 September 2025).
- Food and Agricultural Organization (FAO). El Estado Mundial de la Pesca y la Acuicultura. Hacia la Transformación Azul; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Comisión Nacional de Acuacultura y Pesca CONAPESCA. Anuario Estadístico de Acuacultura y Pesca 2023. Available online: https://www.gob.mx/conapesca/documentos/anuario-estadistico-de-acuacultura-y-pesca (accessed on 9 September 2025).
- Rodríguez-Domínguez, G.; Aragón-Noriega, E.A.; Payán-Alejo, J.; Mendivil-Mendoza, J.E.; Curiel-Bernal MVValenzuela-Quiñonez, W.; Urías-Sotomayor, R. The Fractal Approach to Describe Growth of Farmed Marine Species: Using Double and Triple Logistic Models. Fishes 2024, 9, 106. [Google Scholar] [CrossRef]
- Lugert, V.; Thaller, G.; Tetens, J.; Schulz, C.; Krieter, J. A review on fish growth calculation: Multiple functions in fish production and their specific application. Rev. Aquacult. 2016, 8, 30–42. [Google Scholar] [CrossRef]
- Hopkins, K.D. Reporting Fish Growth: A Review of the Basics. J. World Aquac. Soc. 1992, 23, 173–179. [Google Scholar] [CrossRef]
- Mata-Sotres, J.A.; Lazo, J.P.; Baron-Sevilla, B. Effect of age on weaning success in totoaba (Totoaba macdonaldi) larval culture. Aquac. 2015, 437, 292–296. [Google Scholar] [CrossRef]
- von Bertalanffy, L. A quantitative theory of organic growth (Inquires on growth laws II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Gompertz, B. On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. Philos. Trans. R. Soc. Lond. 1825, 115, 513–583. [Google Scholar] [CrossRef]
- Johnson, N.O. A trend line for growth series. J. Am. Stat. Assoc. 1935, 30, 717. [Google Scholar] [CrossRef]
- Richards, F.J. A flexible growth function for empirical use. J. Exp. Bot. 1959, 10, 290–300. [Google Scholar] [CrossRef]
- Ricker, W.E. Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Board Can. 1975, 191, 1–382. [Google Scholar]
- Schnute, J. A versatile growth model with statistically stable parameters. Can. J. Fish. Aquat. Sci. 1981, 38, 1128–1140. [Google Scholar] [CrossRef]
- Restrepo, V.R.; Diaz, G.A.; Walter, J.F.; Neilson, J.D.; Campana, S.E.; Secor, D.; Wingate, R.L. Updated estimate of the growth curve of western Atlantic bluefin tuna. Aquat. Living Resour. 2010, 23, 335–342. [Google Scholar] [CrossRef]
- Félix-Ortiz, J.A.; Aragón-Noriega, E.A.; Castañeda-Lomas, N.; Rodríguez-Domínguez, G.; Valenzuela-Quiñónez, W.; Castillo-Vargasmachuca, S. Individual growth analysis of the Pacific yellowlegs shrimp Penaeus californiensis via multi-criteria approach. Lat. Am. J. Aquat. Res. 2020, 48, 768–778. [Google Scholar] [CrossRef]
- Curiel-Bernal, M.V.; Cisneros-Mata, M.Á.; Rodríguez-Domínguez, G.; Sánchez-Velasco, L.; Jiménez-Rosenberg, S.P.A.; Parés-Sierra, A.; Aragón-Noriega, E.A. Modelling Early Growth of Totoaba macdonaldi (Teleostei: Sciaenidae) under Laboratory Conditions. Fishes 2023, 8, 155. [Google Scholar] [CrossRef]
- Naranjo Páramo, J.; Aragón Noriega, E.A.; Magallón Barajas, F.; Portillo Clark, G. Producción de postlarvas de camarón café Penaeus californiensis en tanques semi comerciales. Oceanología 1996, 10, 73–81. [Google Scholar]
- Hoyos-Chairez, F.; Aragón-Noriega, E.A.; Chávez-Villalba, J. Modelling early growth of the pearl oyster Pteria sterna under pilot-commercial production. Aquac Res. 2020, 41, 5106–5117. [Google Scholar] [CrossRef]
- Neter, J.; Kutner, M.H.; Nachtsheim, C.J.; Wasserman, W. Applied Linear Statistical Models, 4th ed.; McGraw-Hill: New York, NY, USA, 1996; pp. 1–720. [Google Scholar]
- Schnute, J.; Fournier, D. A new approach to length-frequency analysis: Growth structure. Can. J. Fish. Aquat. Res. 1980, 37, 1337–1351. [Google Scholar] [CrossRef]
- Chen, Y.; Fournier, D. Impacts of atypical data on Bayesian inference and robust Bayesian approach in fisheries. Can. J. Fish. Aquat. Sci. 1999, 56, 1525–1533. [Google Scholar] [CrossRef]
- Luquin-Covarrubias, M.A.; Morales-Bojorquez, E.; González-Pelaez, S.S.; Lluch-Cota, D.B. Joint likelihood function based on multinomial and normal distributions for analyzing the phenotypic growth variability of geoduck clam Panopea globosa. CalCOFI Rep. 2016, 57, 151–162. Available online: https://calcofi.org/downloads/publications/calcofireports/v57/Vol57-Luquin_pages.151-162.pdf (accessed on 27 August 2025).
- Curiel-Bernal, M.V.; Aragón-Noriega, E.A.; Cisneros-Mata, M.Á.; Sánchez-Velasco, L.; Jiménez-Rosenberg, S.P.A.; Parés-Sierra, A. Using observed residual error structure yields the best estimates of individual growth parameters. Fishes 2021, 6, 35. [Google Scholar] [CrossRef]
- Castillo-Vargasmachuca, S.G.; Aragón-Noriega, E.A.; Rodríguez-Domínguez, G.; Martínez-Cárdenas, L.; Arámbul-Muñoz, E.; Burgos Arcos, Á.J. The standard deviation structure as a new approach to growth analysis in weight and length data of farmed Lutjanus guttatus. Fishes 2021, 6, 60. [Google Scholar] [CrossRef]
- Abdo-de la Parra, M.I.; Rodríguez-Ibarra, E.; Rodríguez-Montes de Oca, G.; Velasco-Blanco, G.; Ibarra-Casto, L. Estado actual del cultivo de larvas del pargo flamenco (Lutjanus guttatus). Lat. Am. J. Aquat. Res. 2015, 43, 415–423. [Google Scholar] [CrossRef]
- Ibarra-Castro, L.; Ochoa-Bojórquez, M.O.; Sánchez-Téllez, J.L.; Rojo-Cebreros, A.H.; Alvarez-Lajonchere, L. A new efficient method for the mass production of juvenile spotted rose snapper. Lutjanus Guttatus. Aquac. Rep. 2020, 18, 100550. [Google Scholar] [CrossRef]
- Baer, A.; Schulz, C.; Traulsen, I.; Krieter, J. Analysing the growth of turbot (Psetta maxima) in a commercial recirculation system with the use of three different growth models. Aquacult. Int. 2010, 19, 497–511. [Google Scholar] [CrossRef]
- Ansah, Y.B.; Frimpong, E.A. Using model-based inference to select a predictive growth curve for farmed Tilapia. North Amer. J. Aquac. 2015, 77, 281–288. [Google Scholar] [CrossRef]
- Mercier, L.; Panfili, J.; Paillon, C.; N’diaye, A.; Mouillot, D.; Darnaude, A.M. Otolith reading and multi-model inference for improved estimation of age and growth in the gilthead seabream Sparus aurata (L.). Estuar. Coast. Shelf Sci. 2011, 92, 534–545. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; pp. 1–488. [Google Scholar]
| Species | Error Structure | BIC | Δi | Wi (%) |
|---|---|---|---|---|
| Totoaba | Observed | 4740 | 0 | 100 |
| Multiplicative | 4794 | 54 | 0.00 | |
| Additive | 6764 | 2025 | 0.00 | |
| Shrimp | Observed | 108 | 0 | 100 |
| Multiplicative | 143 | 35 | 0.00 | |
| Additive | 182 | 74 | 0.00 | |
| Pearl oyster | Observed | 5873 | 0 | 100 |
| Multiplicative | 5922 | 49 | 0.00 | |
| Additive | 7522 | 1649 | 0.00 |
| Species | Error Structure | Y1 | Y2 | a | b |
|---|---|---|---|---|---|
| Totoaba | Observed | 0.882 | 3.879 | 0.367 | −7.012 |
| Multiplicative | 0.886 | 3.881 | 0.374 | −7.183 | |
| Additive | 0.743 | 3.942 | 0.210 | −3.171 | |
| Shrimp | Observed | 1.601 | 2.351 | 0.024 | 1.979 |
| Multiplicative | 1.600 | 2.375 | −0.070 | 6.092 | |
| Additive | 1.595 | 2.383 | −0.128 | 8.956 | |
| Pearl oyster | Observed | 55.0 | 3958.9 | 0.083 | −1.189 |
| Multiplicative | 59.6 | 3432.3 | 0.225 | −3.646 | |
| Additive | 38.8 | 3652.9 | 0.099 | −1.264 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragón-Noriega, E.A.; Alcántara-Razo, E.; Félix-Ortiz, J.A.; Ayón-Jiménez, S.A. Individual Growth Parameterization Models Using the Observed Variance in Organisms Subject to Aquaculture. Aquac. J. 2025, 5, 21. https://doi.org/10.3390/aquacj5040021
Aragón-Noriega EA, Alcántara-Razo E, Félix-Ortiz JA, Ayón-Jiménez SA. Individual Growth Parameterization Models Using the Observed Variance in Organisms Subject to Aquaculture. Aquaculture Journal. 2025; 5(4):21. https://doi.org/10.3390/aquacj5040021
Chicago/Turabian StyleAragón-Noriega, Eugenio Alberto, Edgar Alcántara-Razo, José Adán Félix-Ortiz, and Samuel Angiee Ayón-Jiménez. 2025. "Individual Growth Parameterization Models Using the Observed Variance in Organisms Subject to Aquaculture" Aquaculture Journal 5, no. 4: 21. https://doi.org/10.3390/aquacj5040021
APA StyleAragón-Noriega, E. A., Alcántara-Razo, E., Félix-Ortiz, J. A., & Ayón-Jiménez, S. A. (2025). Individual Growth Parameterization Models Using the Observed Variance in Organisms Subject to Aquaculture. Aquaculture Journal, 5(4), 21. https://doi.org/10.3390/aquacj5040021