1. Introduction
Aquatic foods are the healthiest and largest sources of animal protein for humans. Globally, nearly 185.4 million tons (mt) of aquatic food is produced annually, which is more than chicken (144 mt), beef (76 mt), and lamb (17 mt) combined [
1,
2]. The capture or harvesting of wild fish stocks likely originated as a fundamental activity to obtain nutrient-rich food. After the 1970s, wild fish catching declined, and modern aquaculture started [
2]. Fish farming or aquaculture grew rapidly after the 1980s and has become the fastest-growing food production sector. FAO data show that the production of farmed aquatic foods increased by almost 189-fold over 72 years of farming history, i.e., it increased from 0.5 mt in the 1950s to 94.4 mt in 2022, whereas wild fishing was 91.0 mt [
1]. Approximately 51% of the aquatic food we consume originates from farming. More than 700 finfish, shellfish, and aquatic plants are used in aquaculture. Crustaceans such as shrimp, prawn, and crab, etc., contribute significantly to food and nutrition security. Among them, penaeid shrimps are popular and highly traded seafood items across the globe. There are two major commercially important farmed species of crustaceans, namely the Giant tiger shrimp (
Penaeus monodon) and the Pacific white shrimp (
Litopenaeus vannamei). Total penaeid shrimp production reached nearly 8 mt in 2022 [
2]. The recent shrimp market research report shows that the value of shrimp was over USD 75 billion in 2023. It is expected to grow at about 5% per year and reach more than USD 124 billion by 2032 [
3]. Production increased due to advances in farming, storage, transportation technologies, and trade across countries. Major penaeid shrimp-exporting countries are Ecuador, India, Vietnam, Indonesia, China, Argentina, and Thailand, etc., and importing countries include China, the US, Japan, and European countries [
4].
Commercial aquaculture depends on feed as its main input. Assuming an average feed conversion ratio (FCR) of 1.5 [
5], a total of 12 mt of feed is required globally to support 8 mt production of penaeid shrimp. Various raw materials are utilized in the production of aquaculture feed. Fishmeal is regarded as the best ingredient; however, due to sustainability concerns, efforts have been made to substitute it with plant-based alternatives. Plant-based products have a high chance of developing fungus that produces mycotoxins. Earlier FAO reported that 25% of the global food crops are contaminated by mycotoxins; however, other scholars have pointed out that it should be 60–80% higher than that value [
6]. More importantly, low-quality or animal-grade food crops are utilized as feed ingredients, which implies that nearly all components are likely to be contaminated with mycotoxins. Around 400 mycotoxins have been identified, but only 30 of them are known to be harmful [
7,
8,
9,
10].
Aspergillus,
Fusarium, and
Penicillium are the main genera of fungi capable of producing mycotoxins such as aflatoxins, ochratoxin A, fumonisins, trichothecenes, and zearalenone [
8,
11]. Mycotoxin-contaminated feed negatively affects aquaculture productivity by lowering feed intake, reducing growth, increasing FCR, causing abnormality and cancer, damaging gills and liver, causing toxicity, and causing high mortalities [
12]. It is almost impossible to obtain animal feed without mycotoxins; however, their effects are underestimated and rarely recognized. Some studies have highlighted the occurrence of mycotoxins in aquaculture feeds and feed ingredients in Asia and Europe by Gonçalves et al. [
13,
14]. Consuming fish or other products contaminated with mycotoxins might be one of the sources of entering the human food chain, threatening food safety and public health, causing toxicities and even cancer [
9]. Mycotoxins can remain in the kidney, liver, and the muscles of fish and finally be ingested by humans, which may affect the health of humans as they are genotoxins, nephrotoxins, carcinogens, and immunosuppressors [
8,
12,
15]. Therefore, the European Food Safety Authority (EFSA) panel has established guidelines for assessing additives and reducing the contamination of feed by mycotoxins [
16,
17].
Shrimp farming suffers from recurring slow growth, various diseases, and high mortalities, which have been difficult to relate to any specific causes. Mycotoxins, especially aflatoxins, might be one of the reasons [
18,
19]. As mycotoxins are hard to avoid in feed, the strategy should be to minimize their effects on the animal itself. Various attempts have been made to improve the digestibility and absorption of nutrients, which affects the nutritional value of feeds, making tremendous impacts on the growth and survival [
20]. A number of substances have been tested to reduce the growth of fungus or bind the mycotoxins to make them unavailable for absorption along the gastrointestinal tract. Some anti-mycotoxin products and enzymes can selectively transform mycotoxins into less toxic forms. However, due to a large number of mycotoxins, often present in the feed, many types of enzymes are needed, which is not feasible. A more practical approach is the use of mycotoxin binders, especially natural adsorbents, such as clays and yeast derivatives, combined with phytogenics, which can bind a wide range of mycotoxins compared to synthetic adsorbents, are less toxic, leave no residues, and provide nutritional benefits while preserving the bioavailability of other nutrients of the diet.
BIŌNTE
® QUIMITŌX
® AQUA PLUS, a commercial anti-mycotoxin additive (AMA), from BIŌNTE NUTRITION S.L., has been developed specially for aquaculture to use as a feed supplement. It is an innovative, broad-spectrum mycotoxin detoxifying agent. It performs three modes of action: adsorption, bio-protection, and post-biotic effects specific to aquatic species. The AMA bentonite and sepiolitic clay are able to bind mycotoxins via electrostatic and van der Waals forces along the digestive tract of the animals [
21,
22,
23,
24]. The clays adsorb the mycotoxins, preventing them from being absorbed by the animal’s digestive system and thereby avoiding or reducing health damage. The bioactive plant extracts are curcumin from turmeric (
Curcuma longa), Silymarin from milk thistle, and lysolecithin from sunflower (
Helianthus annuus), which mitigate the oxidative stress caused by mycotoxins [
25,
26,
27,
28,
29]. In addition, the AMA also contains post-biotic compounds from inactivated yeast, such as a mixture of yeast cell wall and hydrolyzed yeast from
Saccharomyces cerevisiae, which agglutinate endotoxins and mycotoxins, improve overall gut health, contribute to enhanced resilience, and increase innate immunity and ammonia nitrogen stress resistance, thereby improving growth performance [
21,
30,
31]. The present study was carried out by designing two consecutive trials to evaluate the efficacy and determine the appropriate dose of BIŌNTE
® QUIMITŌX
® AQUA PLUS, an anti-mycotoxin additive (AMA) designed specifically for aquatic species, on the survival, growth, feed utilization, immune response, and health parameters in Pacific white shrimp (
Litopenaeus vannamei) as no research has been carried out so far.
2. Materials and Methods
2.1. Experimental System and Animals
The experiment was conducted at the Asian Institute of Technology (AIT), Pathum Thani, Thailand (coordinates: 14.08° N, 100.61° E). The post-larvae trial utilized a total of 12 aquaria under a zinc-sheet roof (semi-outdoor conditions with half sunlight allowed through a row of transparent sheets) protected by a wire fence. Each aquarium (100 L) was connected to its own recirculatory aquaculture system (RAS). A complete randomized design (CRD) was used in which each of the four treatments was randomly assigned to three different aquaria as replicates.
The post-larvae (0.2 mg) of Pacific white shrimp (Litopenaeus vannamei) were obtained from a private hatchery located in Chachoengsao province of Thailand. They were acclimatized during a period of 14 days and kept in two fiberglass tanks under the same conditions before the experiment. During this time, they were fed the control diet three times daily to apparent satiation. The experiment was divided into two parts: first 20 days as post-larval rearing in an aquarium of 100 L and 90 days of grow-out period in fiberglass tanks (300 L). During the first trial of 20 days, 300 post-larvae (PLs) were stocked in each replicate aquarium, and during the grow-out phase (90 days), 110 post-larvae were chosen from each corresponding replicate aquarium used in the first phase trial.
2.2. Test Diets and Mycotoxins
Locally available commercial shrimp powder containing 43% crude protein (CP) (Ocean Aquaculture Platinum Plus #4 PL10-15, 400–600 µm (Ocean Nutrution Europe bvba, Rijkmakerlaan 15, 2910 ESSEN, Belgium) was used for the post-larval trial. For the grow-out trial, a commercial pellet containing 40% CP (Feed #1, Charoen Pokphand Co., Ltd., Bangkok, Thailand) was used. A locally sourced corn with observed fungal growth was used as a source of mycotoxins. The corn was processed into a fine powder using a grinder machine called Powder Grinder, ECO (Spring Green Evolution, Bangkok, Thailand), resulting in a particle size range of 44–250 µm. The average concentration of fungus cells that produce mycotoxins in the corn was found to be 2.83 × 105 CFU. Therefore, 10% corn was mixed with the 90% standard shrimp feeds (40–43% CP) to have 2.83 × 104 CFU (toxic level is 1.0 × 104 CFU), which resulted in at least 36.8% CP after dilution due to the added corn, which had approx. 8% crude protein (reasonably good protein level). The two components were thoroughly blended using a feed mixing electric machine (Imarflex, IF-169, Bangkok, Thailand) to ensure homogenous distribution of the contaminated material throughout the feed. Mycotoxin-contaminated feed served as the control treatment in both the post-larvae nursing and grow-out trials to evaluate the protective effects of dietary supplementation of AMA. The test diets were thoroughly dried at 50 °C in an oven overnight and stored in a normal refrigerator at 4–7 °C, protected from light and humidity.
The concentration of specific mycotoxins was determined through standard analytical methods. In order to confirm that the feed had mycotoxins, aflatoxins were identified and quantified using the in-house HPLC method CH-002-TM, which is based on AOAC Official Method 991.31 [
32]. For the detection of fumonisins, the in-house method CH-127-TM was applied, following the protocol outlined in EN 15662:2018. The report showed that the feed had a total of 46.6 µg fumonisins with B1 (35.1 µg/kg) and B2 (11.5 µg/kg), and aflatoxins were below LOD, i.e., <0.1 µg/kg.
During both the trial periods, shrimp were fed to near satiation; three times daily during post-larval nursing and two times during the grow-out trial. Feeding behavior was observed and recorded. Feeding rates were used 4%, 6%, and 8% of the biomass per aquarium each day for the 1st, 2nd, and 3rd weeks, respectively, as suggested by Hung and Quy [
33], Antunes et al. [
34], and Effendi et al. [
35] for the post-larvae. During the grow-out phase, they were fed daily at the rate of 4%, 5%, 6%, 8%, and 8% of the biomass per tank, changing the rate weekly from week 1 to 5. For the remaining grow-out period, the feeding rate was reduced to 5–6% daily. Uneaten feed and feces were removed from tanks weekly and separated by a hand net. They were collected into a deep freezer (−18 °C) for proximate analysis.
2.3. Feed Supplement and Treatments
BIŌNTE
® QUIMITŌX
® AQUA PLUS, a commercial anti-mycotoxin additive (BIŌNTE NUTRITION S.L., Reus, Spain) specially designed for aquatic species, was used as a dietary supplement for both the nursing and grow-out trials. Four experimental diets were prepared. The control diet received only the naturally contaminated commercial feed (0 g/kg), while the three treatment diets were supplemented with low, medium, and high levels, i.e., 1, 2, and 3 g/kg of AMA powder in the diet, respectively, as shown in
Table 1. The AMA powder was mixed homogenously into each diet using an electric blender.
2.4. Water Quality Management
The experiment was conducted using freshwater. Water quality parameters such as temperature, dissolved oxygen (DO), pH, ammonia, and nitrites were carefully monitored and maintained throughout experimental periods of both nursing and grow-out trials. The pH levels ranged from 7.5 to 8.5, while the water temperature was maintained within the optimal range of 27–28 °C. A multiparameter probe (YSI Professional Plus, Yellow Springs, OH, USA) was employed to measure pH and temperature, and DO concentrations were monitored weekly in the early morning at 07:30 hrs. using a DO meter (LAQUA, Horiba, Model 220, Kyoto, Japan). Photoperiod was controlled at a 12 h light and 12 h dark cycle to simulate natural day-night conditions. In both trials, water exchange was performed weekly. Before exchanging water each time, 50% of the water volume in each tank was drained by siphoning to eliminate accumulated wastes, i.e., uneaten feed, feces, and excess organic matter. Freshwater was then added to restore the original volume in each culture tank. Additionally, before each water exchange, water samples (500 mL per replicate) were collected from each tank for analysis of ammonia-nitrogen (NH
3-N) and nitrite-nitrogen (NO
2-N) concentrations. These parameters were determined using the standard titration method [
36].
2.5. Sampling of Feed
About 50 g of each of the test diets was randomly sampled for proximate analysis and performed at the laboratory facility of AIT. Commercial feed without mixing of the supplement as a control treatment, and different mixing ratios of the supplement were collected in three replicates each. The samples were analyzed using the standard protocols [
32]. Crude protein was determined by the micro-Kjeldahl method using FOSS Kjeltec 8100 apparatus (FOSS Analytical AB, Hoganas, Sweden). Crude lipid was tested by the Soxhlet method using FOSS Soxtec 2043 apparatus (FOSS Scino Co., Ltd., Suzhou, China). The crude fiber was estimated by following Weende method using FOSS Fibertec 1020 apparatus (FOSS Scino Co., Ltd., Suzhou, China). Moisture was estimated by air oven Lab Tech (Model LDO-100E, Daihan Labtech Co., Ltd., Namyangju City, Republic of Korea). Ash was determined by burning the samples at 550 °C using a muffle furnace (Lab Tech Model LEF-115S-1 muffle furnace, Namyangju City, Republic of Korea).
2.6. Sampling of Shrimp
At the end of the post-larvae trial of 20 days, 110 juveniles per replicate aquarium were weighed and transferred to a plastic tank for the grow-out trial. The remaining were kept in a deep freezer (−80 °C) for proximate analysis. At the end of the grow-out trial, about 50 g (7–8 shrimps) per tank were randomly selected for sacrifice in the deep freezer for proximate analyses of the whole body. The samples were analyzed in triplicate. Batch weights (total bulk weight of shrimp in each tank) were taken at the end of the nursing period and every 30 days during the grow-out phase. At the final harvest, shrimp were counted to calculate the survival rate. During the experimental period, shrimp health and mortality were observed during each feeding.
2.7. Data Records
Other performance parameters, such as feed intake, weight, length, and survival, were recorded and compiled in an MS Excel file. Feed utilization indicators such as feed conversion ratio (FCR) and protein efficiency rate (PER) were also calculated and compared. For the post-larvae trial as well as the grow-out trial, 20 shrimps were randomly sampled for one initial pooled sample, and 10 were taken to measure length from each replicate aquarium or tank.
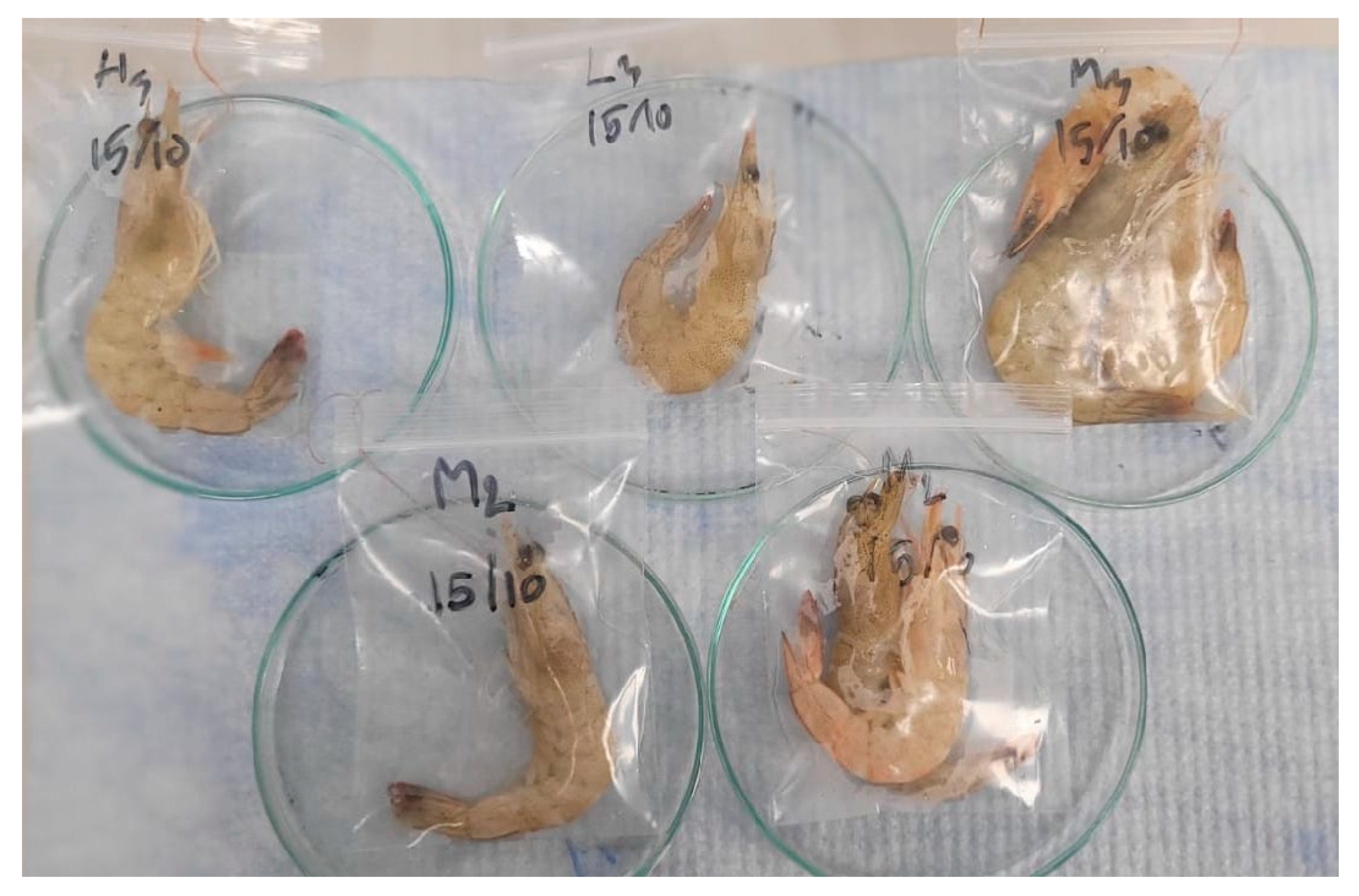
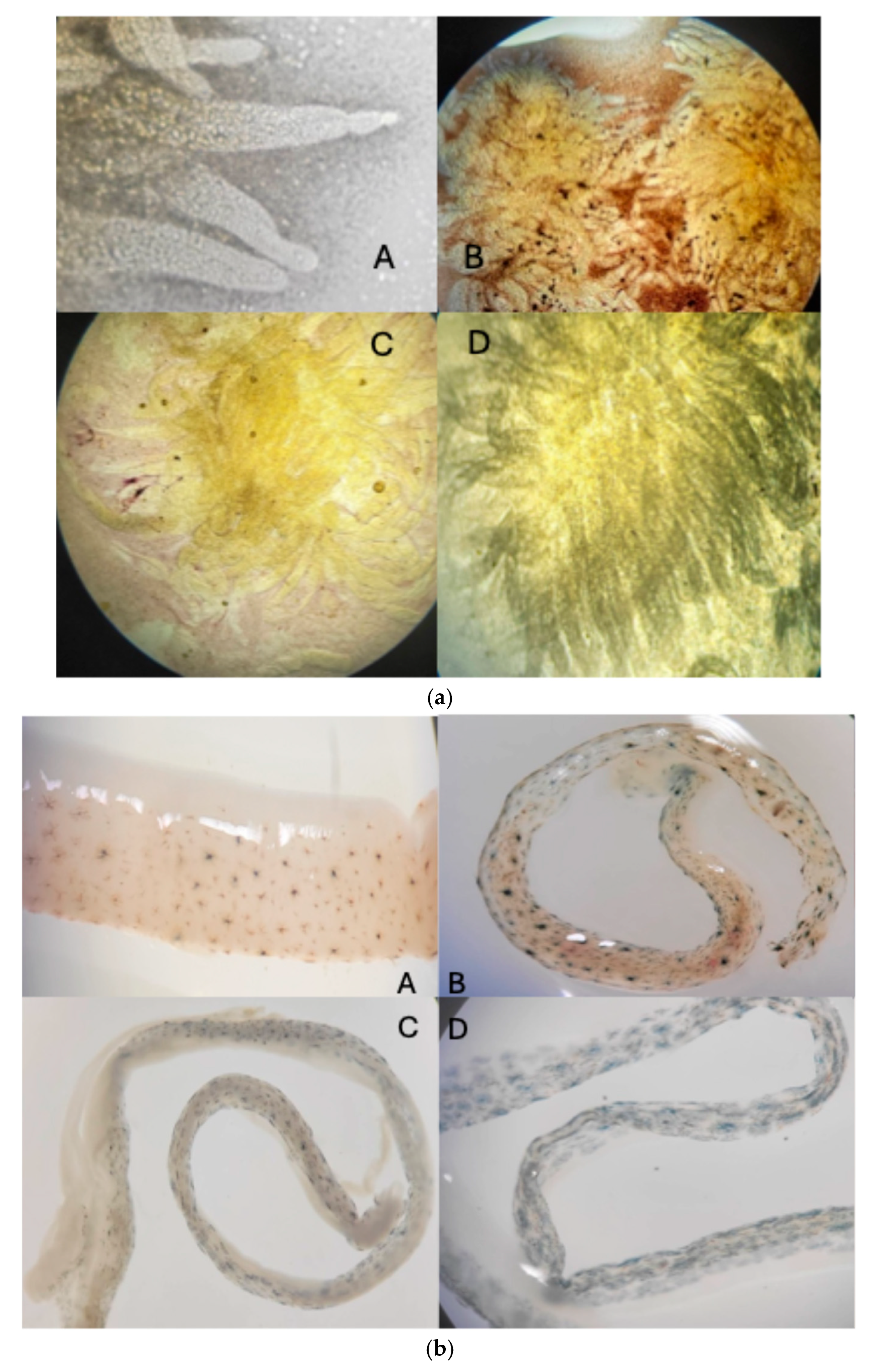
At the final harvest of the grow-out phase, five shrimps were sampled for morphological analysis. Their internal organs, such as the stomach, intestine, and hepatopancreas, were observed both with the naked eye (macroscopically) and under a compound microscope. Photos were taken for treatment demonstration.
Survival, specific growth rate, daily weight gain, daily length gain, feed conversion ratio, and hepatosomatic index were compared among the treatments, calculating the following parameters [
37]:
where
2.8. Bacterial Challenge Test
In order to evaluate the capacity of the AMA to ameliorate immune suppression, a bacterial challenge test against Aeromonas hydrophila was performed after 90 days of grow-out test. The shrimp of all the 12 tanks were divided into two groups: one group for the exposure to bacteria (infected group) and the other for control or mock-infected. Therefore, the challenge test was conducted using 24 plastic tanks (30 L) each stocked with 10 shrimps per tank. A pre-challenge test was performed in order to determine the amount of the CFU of Aeromonas hydrophila used for the immersion method (LD50, i.e., lethal dose that caused 50% mortality). Mortality and external body conditions were closely observed daily during 14 days of the challenge test, and the accumulated mortality was calculated to compare among the treatments.
2.9. Data Analysis
The data are presented as the average of three replicates for each of the four treatments along with their standard errors (Average ± SE). One-way analysis of variance (ANOVA) was used for each growth, survival, and other parameters to test the effects of the factor (feed supplement AMA), followed by Tukey’s HSD-test to compare among treatment groups using SPSS (Version 23). Quadratic regression (polynomial function 2nd degree) was used to determine the relationship between the factor (AMA dose) and variables (survival, weight, length, etc.) Significance level was considered significant at 5% and highly significant at 1%. Data were compiled in Microsoft® Excel for MAC, Microsoft 365 @2025, and statistical analyses were performed using IBM SPSS Statistics (Version 23).
4. Discussion
Shrimp farming is considered a high-risk business due to the occurrence of high mortalities resulting from various causes, including bacterial and viral diseases, poor physical and chemical water properties, and the quality of aquafeed that is often deficient in nutrients and contaminated with mycotoxins and other pollutants. Although high mortalities in shrimp are often untraceable, more recently, problems related to feed and the harms caused by the presence of various mycotoxins in feed and feed ingredients have become more apparent [
8]. About 400 mycotoxins have been identified so far, but only 20–30 of them are well-known and are produced by fungi of three genera:
Aspergillus,
Fusarium, and
Penicilium [
8,
9,
10]. In order to reduce aquatic food production costs, the use of plant-based ingredients has increased to replace fishmeal in diets. However, the risk of exposure of farmed aquatic species to feed pollutants, such as mycotoxins, is increased due to the higher inclusion of potentially contaminated raw materials, including cereals and grains. Then, it is almost impossible to avoid mycotoxins from aquafeed. Therefore, various nutritional strategies such as the supplementation of mycotoxin detoxifiers have been proposed as a solution for aquaculture challenges [
13,
14,
19].
The anti-mycotoxin additive (AMA) investigated is a commercial mycotoxin detoxifier specifically developed for aquatic species, including shrimp. AMA is a broad-spectrum mycotoxin detoxifier that presents three modes of action: adsorption, from a combination of selected binding materials; purified plant extracts from turmeric (
Curcuma Longa) and milk thistle (
Silybum Marianum), and sunflower (
Helianthus annuus) lysolecithin for systemic health enhancement (bioprotection); and inactivated yeast derivates from
Saccharomyces cerevisiae that provide a post-biotic effect in the gastrointestinal tract and support the immune system function [
29]. Specifically, AMA includes a combination of selected natural clays, including bentonite and sepiolitic clay, that can bind mycotoxins through various physicochemical mechanisms, such as electrostatic and van der Waals forces. These adsorbents perform their detoxification activity along the digestive tract of the animals, preventing toxins from being absorbed in the intestine and thus being eliminated through feces [
21,
22,
23,
24]. The purified plant extracts included in AMA contain important levels of bioactive phytogenic compounds, including curcumin from turmeric, silymarin from milk thistle, and lysophospholipids from sunflower lysolechitin, which have been demonstrated to provide antioxidant, anti-inflammatory, and hepatoprotective benefits [
29,
38,
39,
40]. The inactivated yeast products provide a source of post-biotic biopolymers such as β-glucans and mannan-oligosaccharides (MOS) from yeast cell walls, along with highly digestible nutrients from hydrolyzed yeast [
31,
41]. β-glucans, which constitute the insoluble fraction of the inner layer of the yeast cell wall, have demonstrated effective binding properties against some mycotoxins. This process occurs through chemical interactions between the toxins and the hydroxyl, ketone, and lactone groups of the polymers [
42,
43]. Furthermore, MOS may contribute to the reduction of pathogenic bacterial populations and mitigate the detrimental effects of endotoxins (LPS) in the gut. This is accomplished through a competitive mechanism based on the structural similarity of MOS to mannosylated receptors of the epithelial cells [
30,
44]. Hydrolyzed yeast is produced by enzymatic processes to break down the cell wall and other macromolecules into small peptides and amino acids. Moreover, it contains valuable trace minerals and vitamins, such as those from the B-group. These components are highly digestible and bioavailable, thereby enhancing the growth and immune function of aquatic animals [
45,
46]. In this context, the present study was designed to assess the efficacy and determine the optimal dosage of AMA in Pacific white shrimp fed a naturally contaminated diet, in terms of growth performance, survival, bacterial disease resistance, and organ integrity.
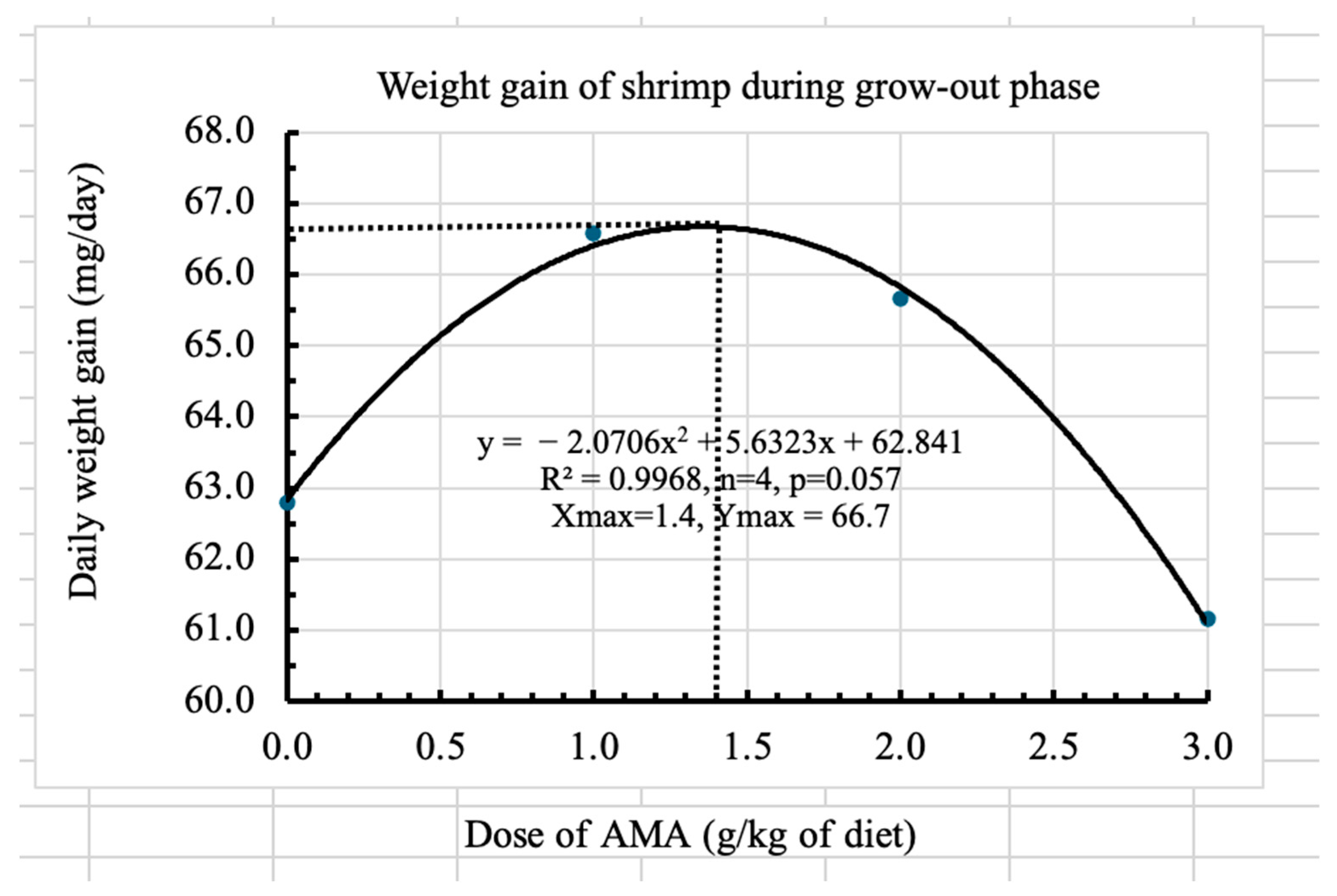
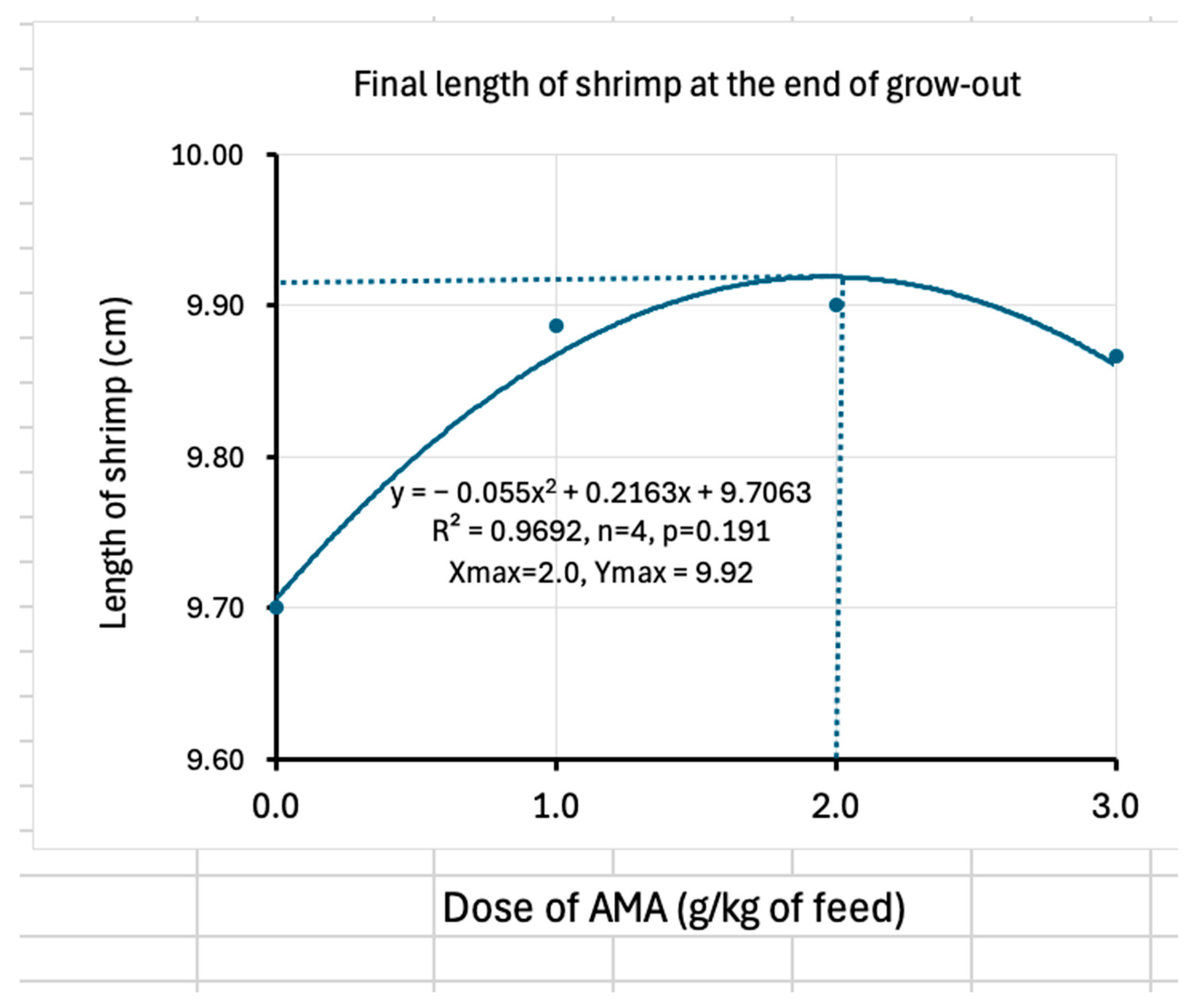
The administration of AMA resulted in a significant increase in length gain during the post-larval phase. However, due to the limited duration of this phase, its potential effects on additional physiological or growth parameters could not be fully assessed. Therefore, a subsequent grow-out trial was conducted to evaluate longer-term impacts. The effect of the supplementation during the larval stage might have accumulated in the grow-out phase. During the grow-out phase, positive effects of AMA were seen in the survival rate, daily weight gain, and length gain. Survival increased with the increase in the dose of the AMA; however, it peaked at 2.5 g/kg of diet and then declined, showing a quadratic relationship. The highest survival rate, which could be achieved, was determined by the regression to be 60.3%, which is more than double that of the survival rate without the supplementation of AMA. Therefore, it clearly showed the benefit of supplementation. However, daily weight gain improved only at the lowest AMA dose, while the medium and high doses did not further enhance growth. The regression analysis indicated that a diet containing 1.4 g of AMA/kg results in the highest weight gain. Therefore, the most suitable dose of AMA would be between 1.4 and 2.5 g/kg of diet. Within this range, lower doses enhance growth, and higher doses increase survival. As a result, the growth or the size of individual shrimp may be compromised due to limitations in space and food availability for a greater number of shrimps. Therefore, a low dose of AMA can be used to produce larger shrimp where demand and prices are high for larger shrimp. A high dose, i.e., 2.5 g/kg diet, would be suitable for those who want to produce more but smaller shrimps. The same dose of another AMA was applied by Kracizy et al. [
19], who found improved growth in Pacific white shrimp. Applying lower stocking densities of shrimp might result in higher survival at low doses and enhanced growth at higher doses of AMA. However, further research is needed to confirm these findings.
The inclusion of AMA in the diet had a clear effect on the feed conversion ratio (FCR). The FCR was reduced from 2.3 ± 0.8 in the control treatment to 1.3 ± 0.1 for the lowest dose of 1 g/kg and up to 1.2 ± 0.1 for the highest dose of 3 g/kg. Similar findings on growth factors have been reported in isolated studies of AMA components in farmed crustaceans. In Pacific white shrimp juveniles challenged by a contaminated diet with 150 µg of aflatoxin B1 (AFB1) per kg of feed, including treatments with the supplementation of bentonite ranging from 0, 2.5, and 5 g/kg; a significant (
p < 0.05) increase in weight gain (WG) and survival rate was observed when the clay was added to the diets, the effect being greater at higher dosages [
23]. Likewise, the effects on performance of supplementing a bentonite-based mycotoxin detoxifier at a 2.5 g/kg dose in
Litopenaeus vannamei juveniles fed diets with total aflatoxin levels of 1067.8 ng/kg and 1715.3 ng/kg of fumonisins (FB1 + FB2) were evaluated. A reduction in total biomass and specific growth rate (SGR) was reported in both contaminated treatments in comparison to the control diet without mycotoxins, and the addition of the clay supplement resulted in a significant (
p < 0.05) improvement in WG, SFR, and FCR [
19].
In regards of turmeric extract, García-Pérez et al. [
26] observed a significant increase (
p < 0.05) in the feed intake and SGR in Pacific white shrimp juveniles fed a contaminated diet with 200 μg/kg of total aflatoxins when turmeric extract (70% w/w curcumin) was included at a rate of 0.15–0.30 g/kg, achieving the maximum benefits against aflatoxin effects at a dosage of 0.20 g of curcumin per kg of finished feed. A similar response to curcumin supplementation (0.1–0.2 g/kg) was observed in Pacific white shrimp challenged with 500 μg/kg of AFB1 for 56 days, with a significant (
p < 0.05) increase in final body weight and numerically lower mortality values [
25]. Correspondingly,
L. vannamei juveniles administered a control diet with the supplementation of 0, 0.075, 0.0150, and 0.300 g/kg of curcumin for 9 weeks, resulting in a significant (
p < 0.05) augmentation of growth parameters, including WG, SGR, and FCR. Interestingly, the inclusion of 0.075 g/kg of curcumin resulted in a greater performance improvement compared to the control diet without the addition of mycotoxins [
28].
Similarly, the effects of the other two components of the AMA, namely, silymarin, an extract of the seeds of the milk thistle, and lysolecithin from sunflower seeds, have also been studied in shrimp species. The inclusion of silymarin in shrimp feed in the range from 0.5 to 1.0 g/kg resulted in an enhancement in growth factors, immune response, antioxidant capacity, and gut morphology in Pacific white shrimp [
38], and in 1.0–2.0 g/kg dosages, it reduced inflammation and lipid peroxidation in the shrimp [
39]. Likewise,
L. vannamei under low salinity stress were supplemented with silymarin at 0.1, 0.2, and 0.4 g/kg, which resulted in a significant (
p < 0.05) improvement in WG, FCR, digestive enzymes activities, hepatopancreas integrity, and alleviated oxidative damage in comparison to the control diet [
47]. Regarding lysolecithin, the effects of dietary lysophospholipids on the growth performance and nutrient utilization, due to its emulsifying properties, have been reported in farmed crustaceans. In dosages of lysophospholipids ranging from 0.3 to 2 g/kg, a significant (
p < 0.05) increase in daily weight gain (DWG), SGR, and FCR has been observed in Pacific white shrimp [
39,
40,
48]. Interestingly, in tiger shrimp (
Penaeus monodon), the feeding of 2 g of lysolecithin per kg of diet for 42 days significantly improved SGR and daily growth coefficient [
49].
Inactivated yeast products from
Saccharomyces cerevisiae, including yeast hydrolysate, have been evaluated in Pacific white shrimp in terms of growth performance and feed utilization. An 8-week feeding trial in
L. vannamei with the supplementation of 10 g/kg of hydrolyzed yeast resulted in higher WG, SGR, and improved FCR [
31]. Controversially, in a 56-day feeding trial with up to 30 g/kg of yeast hydrolysate, no significant improvement was observed in terms of growth. However, significant (
p < 0.05) digestive enzyme activities were reported, indicating a better utilization of nutrients, including carbohydrates and lipids [
41]. The reported data of the isolated materials support the benefits provided by AMA in the growth performance of Pacific white shrimp observed in the present study.
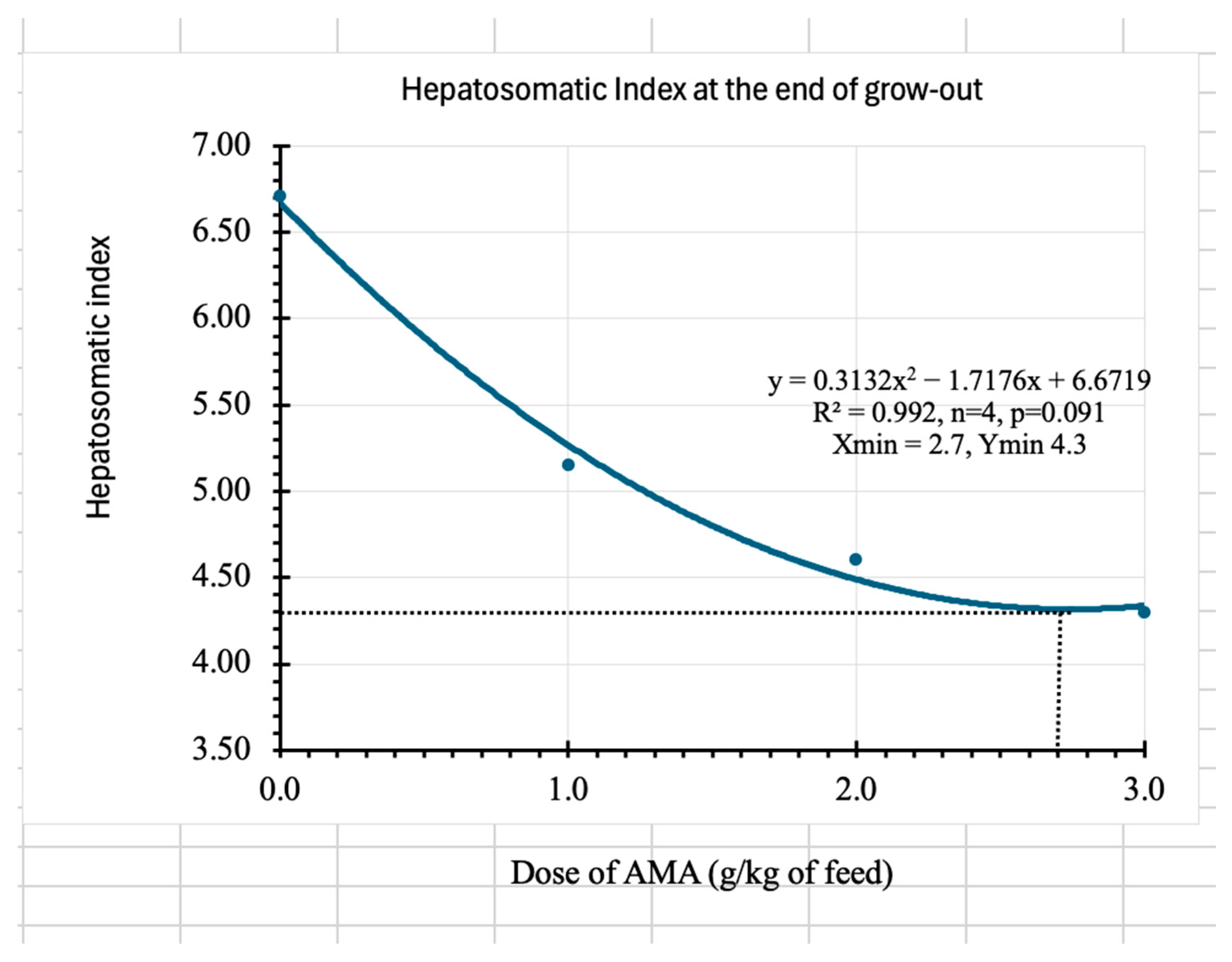
Regarding the protein efficiency ratio, it was found to be 92% in the control, whereas up to 168% in the case of the highest dose of AMA. In the proximate analyses performed, the ameliorating effects of AMA were observed in the chemical composition of feces, especially in decreased lipid content. The low lipid content in the feces of shrimp fed the AMA indicated that the additive might have enhanced lipid digestion and metabolism, which could have been utilized as a source of energy, thereby sparing protein and improving growth and immune response [
50]. This effect has been previously reported in other studies, specifically with the administration of lysophospholipids, which demonstrated enhanced lipid metabolism and, consequently, improved performance in shrimp [
39,
40,
48,
49]. More importantly, the 25% higher ash content in the feces of the AMA-supplemented groups compared to the control indicated that the clays included in AMA to bind mycotoxins must have contributed to binding the mycotoxins in the digestive system [
22,
23,
24]. AMA, especially the clay materials, is not digested and is added to the ash content significantly, as other nutrients are absorbed, e.g., lipids. Moreover, the presence of indigestible clay and yeast products may have served as fiber, supporting the digestion of other nutrients [
41].
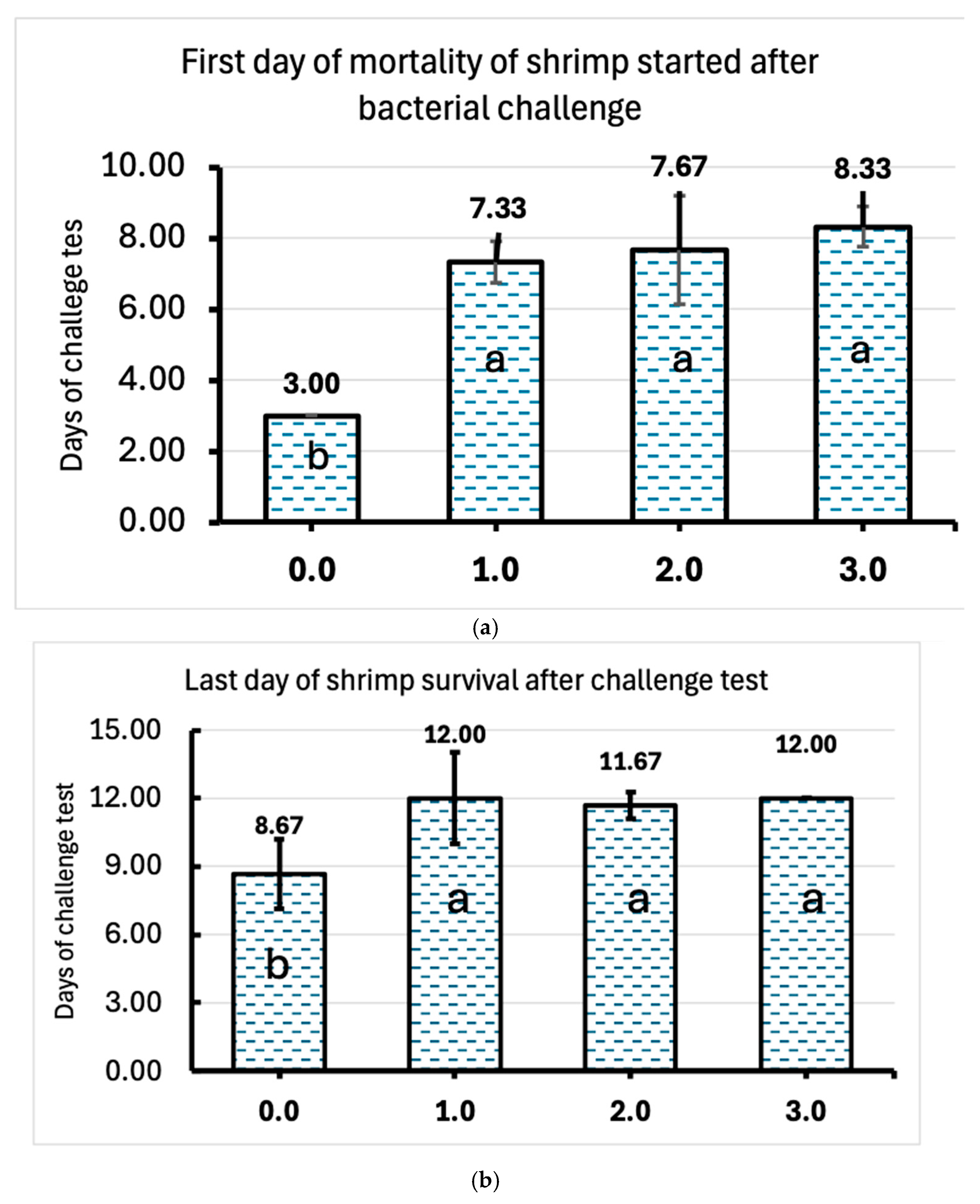
Moreover, the results of the bacterial challenge test showed that the AMA used in this research was able to protect the shrimp and significantly delay the occurrence of mortality. The mortality of shrimp began after 7 days in the case of animals that received AMA supplementation, whereas in the control treatment, it started from the 3rd day post-infection. In addition, the lowest dose of AMA delayed mobility by the same number of days as the medium and high doses. Similarly, all the shrimp in the control group died before the 9th day post-infection, while shrimp supplemented with AMA at all tested dosages were able to survive for 3 additional days, up to 12 days post-infection. These results clearly indicate that the AMA, which contains selected clay adsorbents, curcumin from turmeric extract, silymarin from milk thistle extract, lysophospholipids from sunflower lysolecithin, and inactivated yeast components, presents a significant benefit in enhancing the immunity of shrimp, thereby improving their resistance to bacterial infections. Since mycotoxins’ main adverse effects include the impairment of the immune function in shrimp, the use of dietary strategies that mitigate these toxins by adsorption mechanisms complemented with active substances for the improvement of the overall health status may be able to counteract the higher induced sensitivity to infectious diseases as reported in the literature [
51,
52,
53].
The use of dietary mycotoxin detoxifier bentonite at 2.5–5 g/kg demonstrated a significant (
p < 0.05) increase in
L.Vannamei survival from acute hepatopancreatic necrosis bacterial disease produced by pathogenic strains of
Vibrio parahaemolyticus [
54]. Similarly, the expression of Pacific white shrimp immunity-related genes improved through the supplementation of magnesium aluminosilicate clays at a range of 10–40 g/kg [
55]. In stinging catfish under a bacterial challenge with
Aeromonas hydrophila, sodium bentonite was administered at 5 and 10 g per kg of feed, which resulted in statistically (
p < 0.05) enhanced complement, respiratory burst, and lysozyme activities, consequently, lowering cumulative mortality. In addition, curcumin mitigates the oxidative stress caused by mycotoxins and other stressors, thereby improving innate immune function [
25,
26,
27,
28,
29]. Other phytogenics, such as silymarin and lysolecithin, combined with post-biotic inactivated yeast products, exhibit bio-protective capacities, including antioxidant and anti-inflammatory properties, thereby improving the resilience of shrimp to infectious diseases and other environmental or nutritional stressors [
29,
30,
31,
47,
48]. For instance, in shrimp fed with isonitrogenous and isolipidic diets supplemented with 10 g/kg of yeast hydrolysate challenged by ammonia nitrogen stress, inflammation-related genes
TNF-α and
IL-1β were reduced in the intestine, and the expression level of immune-related genes (
dorsal,
relish, and
proPO) was augmented in comparison to the control diet [
31]. Likewise, Neto and Nunes [
30] observed a significantly (
p < 0.05) enhanced survival of L. vannamei juveniles orally exposed to infectious myonecrosis virus (IMNV) when fed 1 g/kg of β-glucans from yeast cell walls. However, the effectiveness of various combinations of plant and yeast extracts with adsorbent materials is not yet known. More research may be conducted to investigate whether AMA might cause any problems, such as gut dysbiosis and poor growth. Further research should also be conducted to determine the optimal doses of AMA for varying levels of mycotoxin contamination and different commercial farm conditions of Pacific white shrimp, as well as other aquatic species. This study was limited to small aquaria and fiberglass tanks in a research station.