Evaluation of an Organically Modified Clinoptilolite (OMC) and a Multi-Component Mycotoxin Detoxifying Agent (MMDA) on Survival, Growth, Feed Utilization and Disease Resistance of Nile Tilapia (Oreochromis niloticus) Fingerlings Fed with Low Aflatoxin
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Fish and System
2.2. Experimental Diet
2.3. Growth and Feed Utilization
- ▪
- Fish survival (%) = (Final fish number/Initial fish number) × 100.
- ▪
- Biomass gain = Final batch weight (g)—Initial batch weight (g).
- ▪
- Specific growth rate (SGR, %/day) = [Ln (Weight at harvest − [Ln (Weight at stocking)] × 100/no. of days.
- ▪
- Feed conversion ratio (FCR) = Feed intake (dry matter)/Wet weight gain.
- ▪
- Feed conversion efficiency (FCE) = Wet weight gain/Feed intake on a dry matter basis.
- ▪
- Protein efficiency ratio (PER) = Wet weight gain/Protein intake.
2.4. Proximate Analysis of Diets and Fish
2.5. Histology Analysis
2.6. Blood Sampling
2.7. Water Quality Analysis
2.8. Bacterial Challenge Test
2.9. Data Analysis
3. Results
3.1. Performance during the Feeding Period
3.2. Proximate Analysis
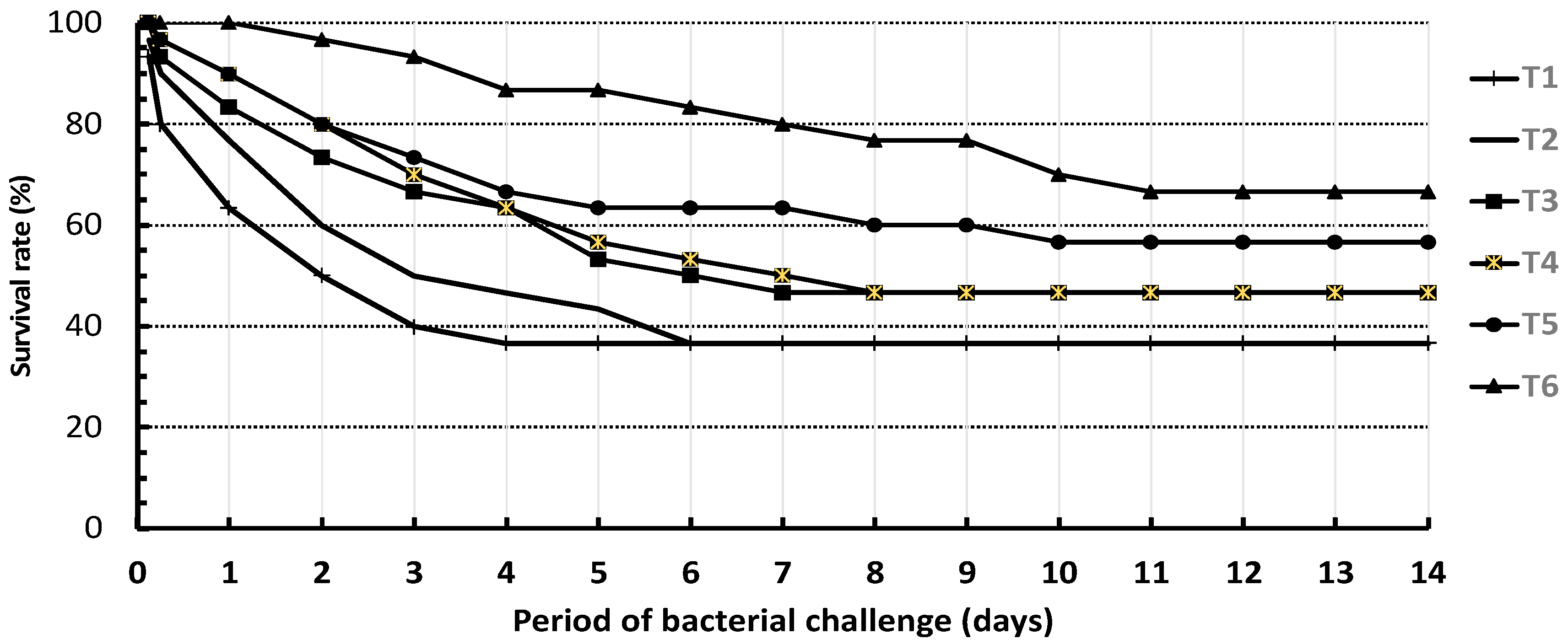
3.3. Bacterial Challenge Test with Aeromonas Hydrophila
3.4. Hematological Results
3.5. Histology Analysis of Intestine Samples
3.6. Water Quality Results
4. Discussion
5. Conclusions and Recommendations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, D.A.; Sookying, D. Strategies for reducing and/or replacing fishmeal in production diets for the Pacific white shrimp, Litopenaeus vannamei. In The Rising Tide, Proceedings of the Special Session on Sustainable Shrimp Farming; Browdy, C.L., Jory, D.E., Eds.; World Aquaculture: Baton Rouge, LA, USA, 2019; pp. 108–114. [Google Scholar]
- Magbanua, T.O.; Ragaza, J.A. Selected dietary plant-based proteins for growth and health response of Nile tilapia. Oreochromis niloticus. Aquac. Fish 2022. [Google Scholar] [CrossRef]
- Krogdahl, A.; Penn, M.; Thorsen, J.; Refstie, S.; Bakke, A.M. Important antinutrients in plant feedstuffs for aquaculture: An update on recent findings regarding responses in salmonids. Aquac. Res. 2010, 41, 333–344. [Google Scholar] [CrossRef]
- Bhujel, R.C. Mycotoxins in Feed—An Underestimated Challenge for the Aquafeed Industry. 2021. Available online: https://mycotoxinsite.com/mycotoxins-feed-underestimated-challenge-aquafeed-industry/?lang=en&fbclid=IwAR1LawrpbuSoMu8eFRYgTeer-gOWTx9WTLJgTlZXkeZV7HrnqFF9k7jeIos (accessed on 12 February 2023).
- Eskola, M.; Kos, G.; Elliott, C.T.; Sultan Mayar, J.H.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, W.V.; de Oliveira, F.K.; Sibaja, K.V.M.; Garcia, S.D.O.; Kupski, L.; Souza, M.M.D.; Tesser, M.B.; Garda-Buffon, J. Occurrence and bioaccessibility of mycotoxins in fish feed. Food Addit. Contam. Part B Surveill. 2020, 13, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, K.; Naehrer, K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Schatzmayr, D.; Albalat, A.; Mackenzie, S. Mycotoxins in aquaculture: Feed and food. Rev. Aquac. 2020, 12, 145–175. [Google Scholar] [CrossRef]
- Goncalves, R.A.; Schatzmayr, D.; Hofstetter, U.; Santos, G.A. Occurrence of mycotoxins in aquaculture: Preliminary overview of Asian and European plant ingredients and finished feeds. World Mycotoxin J. 2017, 10, 183–194. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Naehrer, K.; Santos, G.A. Occurrence of mycotoxins in commercial aquafeeds in Asia and Europe: A real risk to aquaculture? Rev. Aquac. 2016, 10, 263–280. [Google Scholar] [CrossRef]
- García-Pérez, O.D.; Tapia-Salazar, M.; Nieto-López, M.G. Effects of conjugated linoleic acid and curcumin on growth performance and oxidative stress enzymes in juvenile Pacific white shrimp (Litopenaeus vannamei) feed with aflatoxins. Aquac. Res. 2020, 51, 1051–1060. [Google Scholar] [CrossRef]
- Michelin, E.C.; Massocco, M.M.; Godoy, S.H.S.; Baldin, J.C.; Yasui, G.S.; Lima, C.G. Carryover of aflatoxins from feed to lambari fish (Astyanax altiparanae) tissues. Food Addit. Contam. Part A 2017, 34, 265–272. [Google Scholar] [CrossRef]
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism and impact of mycotoxins on humans and animals. Toxicol. Appl. Pharmacol. 2001, 167, 101–134. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, Y.S.; Khalil, R.H. Toxicity, biochemical effects and residue of aflatoxin B1 in marine water-reared sea bass (Dicentrarchus labrax L.). Food Chem. Toxicol. 2009, 47, 1606–1609. [Google Scholar] [CrossRef] [PubMed]
- Hooft, J.M.; Elmor, A.; Ibraheem, E.H.; Encarnação, P.; Bureau, D.P. Rainbow trout (Oncorhynchus mykiss) is extremely sensitive to the feed-borne Fusarium mycotoxin deoxynivalenol (DON). Aquaculture 2011, 311, 224–232. [Google Scholar] [CrossRef]
- McKean, C.; Tang, L.; Tang, M.; Billam, M.; Wang, Z.; Theodorakis, C.W.; Kendall, R.J.; Wang, J.S. Comparative acute and combinative toxicity of aflatoxin B1 and fumonisin B1 in animals and human cells. Food Chem. Toxicol. 2006, 44, 868–876. [Google Scholar] [CrossRef] [PubMed]
- CAST (Council of Agriculture and Technology). Mycotoxins: Risks in Plant, Animal and Human Systems; Task Force Report 139; CAST: Ames, IA, USA, 2003; pp. 1–199. [Google Scholar]
- Shane, S.H.; Eaton, D.L. Economic issues associated with aflatoxins. In Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance; Academic Press: San Diego, CA, USA, 1994; pp. 513–527. [Google Scholar]
- Vasanthi, S.; Bhat, R.V. Mycotoxins in foods-occurrence, health & economic significance & food control measures. Ind. J. Med. Res. 1998, 108, 212–224. [Google Scholar]
- Kabak, B.; Dobson, A.D.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef]
- Leung, M.C.K.; Díaz-Llano, G.; Smith, T.K. Mycotoxins in pet food: A review on worldwide prevalence and preventative strategies. J. Agric. Food Chem. 2006, 54, 9623–9635. [Google Scholar] [CrossRef]
- FAO. The State of Aquaculture and Fisheries—Towards Blue Revolution; FAO: Rome, Italy, 2022; pp. 1–236. [Google Scholar] [CrossRef]
- Martínez-Cordero, F.J.; Delgadillo, T.S.; Sanchez-Zazueta, E.; Cai, J. Tilapia Aquaculture in Mexico: Assessment with a Focus on Social and Economic Performance; FAO Fisheries and Aquaculture Circular No. 1219; FAO: Rome, Italy, 2021. [Google Scholar]
- Norman-López, A.; Bjørndal, T. Markets for Tilapia Globefish Research Programme; FAO: Rome, Italy, 2010; pp. 1–37. [Google Scholar]
- Pietsch, C.; Kersten, S.; Burkhardt-Holm, P.; Valenta, H.; Danicke, S. Occurrence of deoxynivalenol and zearalenone in commercial fish feed: An initial study. Toxins 2013, 5, 184. [Google Scholar] [CrossRef]
- Dirican, S. A review of effects of aflatoxins in aquaculture. Appl. Res. J. 2015, 1, 192–196. [Google Scholar]
- Santacroce, M.; Conversano, M.C.; Casalino, E.; Lai, O.; Zizzadoro, C.; Centoducati, G.; Crescenzo, G. Aflatoxins in aquatic species: Metabolism, toxicity and perspectives. Rev. Fish. Biol. Fish. 2008, 18, 99–130. [Google Scholar] [CrossRef]
- Anh Tuan, N.; Grizzle, J.M.; Lovell, R.T.; Manning, B.B.; Rottinghaus, G.E. Growth and hepatic lesions of Nile tilapia (Oreochromis niloticus) fed diets containing aflatoxin B1. Aquaculture 2002, 212, 311–319. [Google Scholar] [CrossRef]
- Chávez-Sánchez, M.C.; Martínez Palacios, C.A.; Osorio, M.I. Pathological effects of feeding young Oreochromis niloticus diets supplemented with different levels of aflatoxin B1. Aquaculture 1994, 127, 49–60. [Google Scholar] [CrossRef]
- Deng, S.-X.; Tian, L.-X.; Liu, F.-J.; Jin, S.-J.; Liang, G.-Y.; Yang, H.-J. Toxic effects and residue of aflatoxinAFB1 B1 in tilapia (Oreochromis niloticus & O. aureus) during long-term dietary exposure. Aquaculture 2010, 307, 233–240. [Google Scholar]
- Hassan, A.M.; Kenawy, A.M.; Abbas, W.T.; Abdel-Wahhab, M.A. Prevention of cytogenetic, histochemical and biochemical alterations in Oreochromis niloticus by dietary supplement of sorbent materials. Ecotoxicol. Environ. Saf. 2010, 73, 1890–1895. [Google Scholar] [CrossRef]
- Hendricks, J.D. Carcinogenicity of aflatoxins in nonmammalian organisms. In Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance; Eaton, D.L., Groopman, J.D., Eds.; Academic Press: San Diego, CA, USA, 1994. [Google Scholar]
- El-Banna, R.; Teleb, H.M.; Hadi, M.M.; Fakhry, F.M. Performance and tissue residue of tilapias fed dietary aflatoxin. Vet. Med. J. 1992, 40, 17–23. [Google Scholar]
- Tuan, N.A.; Manning, B.B.; Lovell, R.T.; Rottinghaus, G.E. Responses of Nile tilapia (Oreochromis niloticus) fed diets containing different concentrations of moniliformin or fumonisin B1. Aquaculture 2003, 217, 515–528. [Google Scholar] [CrossRef]
- Raj, J.; Farkaš, H.; Jakovčević, Z.; Medina, A.; Magan, A.; Čepela, R.; Vasiljević, M. Comparison of multiple mycotoxins in harvested maize samples in three years (2018–2020) on four continents. Food Addit. Contam. Part A 2022, 39, 599–608. [Google Scholar] [CrossRef]
- Raj, J.; Vasiljević, M.; Farkaš, H.; Bošnjak-Neumüller, J.; Männer, K. Effects of a modified clinoptilolite zeolite on growth performance, health status and detoxification of aflatoxin B1 and ochratoxin A in male broiler chickens. Br. Poult. Sci. 2021, 62, 601–610. [Google Scholar] [CrossRef]
- Riahi, I.; Ramos, A.J.; Raj, J.; Jakovčević, Z.; Farkaš, H.; Vasiljević, M.; Pérez-Vendrell, A.P. Effect of a mycotoxin adsorbent (MMDA) on the growth performance, blood and carcass characteristics of broilers fed ochratoxin A and T-2 mycotoxin contaminated diets. Animals 2021, 11, 3205. [Google Scholar] [CrossRef]
- Kaya, D.; Genc, E.; Palić, D.; Genc, M.A.; Todorović, N.; Sevgili, H.; Vasiljević, M.; Kanyılmaz, M.; Guroy, D. Effect of dietary modified zeolite (clinoptilolite) on growth performance of gilthead sea bream (Sparus aurata) in the recirculating aquaculture system. Aquac. Res. 2021. [Google Scholar] [CrossRef]
- Vasiljevic, M.; Marinković, D.; Milicević, D.; Pleadin, J.; Stefanović, S.; Trialovic, S.; Raj, J.; Petrujkic, B.; Trialovic, J.N. Efficacy of a modified clinoptilolite based adsorbent in reducing detrimental effects of ochratoxin A in laying hens. Toxins 2021, 13, 469. [Google Scholar] [CrossRef] [PubMed]
- Tecator Manual, Tecator Kjeldahl Method800. Manual for Nitrogen/Protein Analysis. 1987. Available online: https://byjus.com/chemistry/kjeldahl-method/ (accessed on 12 February 2023).
- T43ecator Manual, 1980. Fat determination by solvent extraction with Soxtec System. Available online: https://www.labmakelaar.com/fjc_documents/tecator-soxtec-vetbepaling-manual1.pdf (accessed on 12 February 2023).
- Tecator Manual, 1978. Fibertec™ 1023—Semi-Automatic Crude Fiber Analyzer. Available online: https://www.fossanalytics.com/en/products/fibertec1023 (accessed on 12 February 2023).
- American Public Health Association; American Water Works Association; Water Pollution Control Federation. Standard Methods for the Examination of Water and Wastewater, 18th ed.; APHA: Washington, DC, USA, 1992. [Google Scholar]
- Hussain, D.; Mateen, A.; Gatlin, D.M. Alleviation of aflatoxin B 1 (AFB1) toxicity by calcium bentonite clay: Effects on growth performance, condition indices and bioaccumulation of AFB1 residues in Nile tilapia (Oreochromis niloticus). Aquaculture 2017, 475, 8–15. [Google Scholar] [CrossRef]
- Zahran, E.; Risha, E.; Hamed, M.; Ibrahim, T.; Palić, D. Dietary mycotoxicosis prevention with modified zeolite (Clinoptilolite) feed additive in Nile tilapia (Oreochromis niloticus). Aquaculture 2020, 115, 734562. [Google Scholar] [CrossRef]
- Zeng, Z.; Jiang, W.; Wu, P.; Liu, Y.; Zeng, Y.; Jiang, J.; Kuang, S.; Tang, L.; Zhou, X.; Feng, L. Dietary aflatoxin B1 decreases growth performance and damages the structural integrity of immune organs in juvenile grass carp (Ctenopharyngodon Idella). Aquaculture 2019, 500, 1–17. [Google Scholar] [CrossRef]
- Bautista, M.; Lavilla-Pitogo, C.; Subosa, P.; Begino, E. Aflatoxin B1 contamination of shrimp feeds and its effect on growth and hepatopancreas and pre-adult Penaeus monodon. J. Sci. Food Agric. 1994, 65, 5–11. [Google Scholar] [CrossRef]
- Jamshidizadeh, S.; Amrollahi Biuki, N.; Yousefzadi, M.; Aramideh, A. Response of Pacific white leg shrimp (Litopenaeus vannamei) on exposure to aflatoxin in feed. Aquac. Res. 2019, 50, 1973–1984. [Google Scholar] [CrossRef]
- Alinezhad, S.; Tolouee, M.; Kamalzadeh, A.; Motalebi, A.A.; Nazeri, M.; Yasemi, M. Mycobiota and AFB1 contamination of rainbow trout (Oncorhinchus mykiss) feed with emphasis to Aspergillus section Flavi. Iran. J. Fish. Sci. 2011, 10, 363–374. [Google Scholar]
- Greco, M.; Pardo, A.; Pose, G. Mycotoxigenic fungi and natural co-occurrence of mycotoxins in rainbow trout (Oncorhynchus mykiss) feeds. Toxins 2015, 7, 4595–4609. [Google Scholar] [CrossRef]
- Martínez, M.P.; Pereyra, M.L.G.; Juri, M.G.F.; Poloni, V.; Cavaglieri, L. Probiotic characteristics and aflatoxin B1 binding ability of Debaryomyces hansenii and Kazaschtania exigua from rainbow trout environment. Aquac. Res. 2018, 49, 1588–1597. [Google Scholar] [CrossRef]
- Huang, Y.; Han, D.; Xiao, X.; Zhu, X.; Yang, Y.; Jin, J.; Chen, Y.; Xie, S. Effect of dietary aflatoxin B1 on growth, fecundity and tissue accumulation in gibel carp during the stage of gonad development. Aquaculture 2014, 428–429, 236–242. [Google Scholar] [CrossRef]
- Almeida, I.F.M.; Martins, H.M.L.; Santos, S.M.O.; Freitas, M.S.; da Costa, J.M.G.N.; d’Almeida Bernardo, F.M. Microbiota and AFB1 in feed for farmed sea bass (Dicentrarchus labrax). Toxins 2011, 3, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Marijani, E.; Charo-Karisa, H.; Gnonlonfin, G.J.B.; Kigadye, E.; Okoth, S. Effects of aflatoxin B1 on reproductive performance of farmed Nile tilapia. Int. J. Vet. Sci. Med. 2019, 7, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.A.; Dawood, M.A.O.; AlKahtane, A.A.; Abdeen, A.; Abdel-Latif, H.M.R.; Senous, H.H.; Aleya, L.; Alkahtani, S. Spirulina platensis mediated the biochemical indices and antioxidative function of Nile tilapia (Oreochromis niloticus) intoxicated with aflatoxin b1. Toxicon 2020, 184, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Yardimci, B.; Aydin, Y. Pathological findings of experimental Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus). A letter to the editor. Ankara Universitesi Veteriner Fakultesi Dergisi 2011, 58, 47–54. [Google Scholar] [CrossRef]
- Tobback, E.; Decostere, A.; Hermans, K.; Haesebrouck, F.; Chiers, K. Yersinia ruckeri infections in salmonid fish. J. Fish Dis. 2007, 30, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Mehrim, A.I.; Abdelhamid, A.M.; Shosha, A.A.M.; Salem, M.F.I.; El-Sharawy, M.A.M.M. Nutritious attempts to detoxify aflatoxic diets of tilapia fish 2-clinical, biochemical, and histological parameters. J. Arabian Aquac. Soc. 2006, 1, 69–90. [Google Scholar]



| Ingredients | C | OMC | MMDA | AF | AFOMC | AFMMDA |
|---|---|---|---|---|---|---|
| FM | 150 | 150 | 150 | 150 | 150 | 150 |
| SB | 460 | 460 | 460 | 460 | 460 | 460 |
| CF | 72 | 70 | 70 | 44.6 | 53.3 | 53.3 |
| Corn-AFB1 | 0 | 0 | 0 | 27.3 | 16.6 | 16.6 |
| RB | 200 | 200 | 200 | 200 | 200 | 200 |
| Cassava | 50 | 50 | 50 | 50 | 50 | 50 |
| Canola oil | 40 | 40 | 40 | 40 | 40 | 40 |
| Vitamin C | 15 | 15 | 15 | 15 | 15 | 15 |
| Mineral mix ** | 10 | 10 | 10 | 10 | 10 | 10 |
| Vitamin mix * | 3 | 3 | 3 | 3 | 3 | 3 |
| OMC | 0 | 2 | 0 | 0 | 2 | 0 |
| NOMC | 0 | 0 | 2 | 0 | 0 | 2 |
| Total weight/g | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
| Proximate composition | ||||||
| DM (%) | 95.6 b ± 0.0 | 95.0 c ± 0.1 | 96.5 a ± 0.1 | 96.5 a ± 0.1 | 95.0 c ± 0.0 | 94.6 c ± 0.1 |
| Ash (%) | 24.4 ± 0.1 | 23.8 ± 0.2 | 24.2 ± 0.8 | 24.4 ± 0.2 | 23.9 ± 0.7 | 24.6 ± 0.5 |
| Lipid (%) | 12.6 b ± 0.6 | 8.8 a ± 0.3 | 8.2 a ± 0.1 | 7.9 a ± 0.1 | 7.9 a ± 0.1 | 12.8 b ± 0.7 |
| Protein (%) | 35.8 ± 0.2 | 35.9 ± 0.3 | 35.5 ± 0.3 | 36.0 ± 0.2 | 35.8 ± 0.6 | 35.5 ± 0.4 |
| NFE (%) | 26.8 a ± 0.6 | 30.3 ab ±1.4 | 32.6 b ± 0.5 | 31.1 ab ± 0.5 | 31.9 b ± 0.7 | 29.6 ab ± 1.9 |
| GE kcal/kg | 4317 ab ± 38 | 4102 ab ± 63 | 4122 ab ± 44 | 4065 a ± 26 | 4082 ab ± 20 | 4434 b ± 165 |
| Parameters | Growth, Survival and Feed Conversion | |||||
|---|---|---|---|---|---|---|
| Control | OMC | MMDA | AF | AFOMC | AFMMDA | |
| IW (g) | 1.98 a ± 0.01 | 1.95 a ± 0.03 | 1.97 a ± 0.02 | 1.98 a ± 0.01 | 1.96 a ± 0.02 | 1.96 a ± 0.02 |
| FW (g) | 18.8 a ± 1.59 | 19.2 a ± 1.4 | 19.8 a ± 2.0 | 18.2 a ± 0.1 | 18.2 a ± 0.6 | 20.8 a ± 1.2 |
| WG (g/fish) | 16.84 a ± 1.4 | 17.27 a ± 1.3 | 17.79 a ± 2.0 | 16.22 a ± 0.8 | 16.21 a ± 0.6 | 18.83 a ± 1.2 |
| Survival (%) | 58.7 a ± 5.2 | 61.0 a ± 2.5 | 74.7 b ± 2.9 | 76.7 b ± 2.9 | 72.0 ab ± 3.5 | 68.7 ab ± 3.7 |
| FCR | 1.24 a ± 0.1 | 1.22 a ± 0.1 | 1.18 a ± 0.1 | 1.29 a ± 0.1 | 1.32 a ± 0.0 | 1.21 a ± 0.1 |
| FCE | 0.48 a ± 0.1 | 0.82 a ± 0.0 | 0.86 a ± 0.1 | 0.78 a ± 0.0 | 0.76 a ± 0.0 | 0.84 a ± 0.1 |
| PER | 3.4 a ± 0.4 | 3.2 a ± 0.1 | 4.4 ab ± 0.3 | 4.0 ab ± 0.2 | 4.3 ab ± 0.2 | 4.8 b ± 0.4 |
| SGR | 3.21 a ± 0.1 | 3.26 a ± 0.11 | 3.28 a ± 0.14 | 3.16 a ± 0.6 | 3.18 a ± 0.1 | 3.37 a ± 0.1 |
| PI | 5.0 b ± 0.1 | 5.2 b ± 0.2 | 4.03 a ± 0.2 | 4.09 a ± 0.6 | 3.74 a ± 0.1 | 4.0 a ± 0.1 |
| Nutrient | Proximate Composition of the Fry Carcass | |||||
|---|---|---|---|---|---|---|
| Control | OMC | MMDA | AF | AFOMC | AFMMDA | |
| DM (%) | 21.3 ± 0.7 | 21.2 ± 0.4 | 22.6 ± 1.1 | 21.1 ± 0.7 | 20.5 ± 0.3 | 20.8 ± 1.1 |
| Protein (%) | 68.5 ab ± 0.5 | 77.3 d ± 1.2 | 80.5 d ± 1.1 | 64.4 b ± 1.3 | 58.3 a ± 1.1 | 72.1 c ± 0.3 |
| Lipid (%) | 17.4 ± 0.6 | 18.9 ± 1.0 | 18.2 ± 0.9 | 19.1 ± 0.2 | 19.0 ± 0.5 | 18.2 ± 0.9 |
| Ash (%) | 12.3 a ± 1.5 | 11.3 a ± 0.3 | 17.4 b ± 0.9 | 12.0 a ± 0.8 | 13.8 ab ± 1.3 | 17.4 b ± 0.4 |
| GE | 5590 ± 98 | 5848 ± 74 | 5607 ± 26 | 5625 ± 20 | 5455 ± 46 | 5475 ± 59 |
| Treatments | ||||||
|---|---|---|---|---|---|---|
| Control | OMC | MMDA | AF | AFOMC | AFMMDA | |
| WBC 1 | 19,827 ± 1770 b | 9909 ± 1138 a | 12,707 ± 1828 ab | 16,520 ± 1323 ab | 18,267 ± 2662 ab | 19,200 ± 1695 b |
| RBC 2 | 1.71 ± 0.15 | 1.91 ± 0.12 | 1.77 ± 0.08 | 2.05 ± 0.13 | 1.81 ± 0.13 | 1.68 ± 0.10 |
| Hct 3 | 25.9 ± 2.3 | 28.8 ± 1.8 | 27.5 ± 1.2 | 32.0 ± 2.0 | 27.8 ± 1.9 | 26.0 ± 1.6 |
| MCV 4 | 151.7 ± 1.2 | 152.0 ± 2.3 | 155.7 ± 1.8 | 156.3 ± 2.2 | 154.5 ± 1.9 | 154.4 ± 2.1 |
| ALT 5 | 8.9 ± 0.6 | 12.6 ± 1.5 | 6.1 ± 0.7 | 12.3 ± 2.7 | 23.0 ± 9.5 | 16.6 ± 2.4 |
| AST 6 | 70.5 ± 15.1 c | 78.3 ± 20.5 ab | 42.2 ± 8.0 a | 68.9 ± 15.9 a | 59.5 ± 7.6 abc | 97.2 ± 25.9 bc |
| LDH 7 | 1554 ± 481 | 1854 ± 395 | 1031 ± 233 | 1277 ± 244 | 1358 ± 151 | 2209 ± 604 |
| VH | VW | CD | VH/CD | Goblet Cells/0.01 mm2 | |
|---|---|---|---|---|---|
| Control | 92.51 ± 7.52 a | 49.7 ± 2.77 a | 46.57 ± 6.54 ab | 2.87 ± 0.64 a | 31 ± 2 b |
| OMC | 189.5 ± 24.0 a | 62.9 ± 3.9 b | 29.5 ± 2.2 ab | 6.9 ± 1.2 b | 14 ± 4.5 a |
| MMDA | 102.2 ± 5.1 a | 57.8 ± 6.9 a | 51.2 ± 0.2 b | 2.17 ± 7.7 a | NV |
| AF | 189.1 ± 36.8 ab | 61.3 ± 6.6 ab | 47.2 ± 6.6 ab | 3.9 ± 0.5 ab | 16 ± 3 a |
| AFOMC | 85.1 ± 14.7 a | 56.3 ± 4.8 ab | 24.8 ± 1.9 a | 3.5 ± 0.6 ab | 14 ± 1.5 a |
| AFMMDA | 282.2 ± 66.7 b | 66.5 ± 4.9 b | 55.3 ± 15.9 ab | 5.81 ± 1.1 ab | 16 ± 1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhujel, R.C.; Perera, A.D.; Todorović, N.; Raj, J.; Gonçalves, R.A.; Vasiljević, M. Evaluation of an Organically Modified Clinoptilolite (OMC) and a Multi-Component Mycotoxin Detoxifying Agent (MMDA) on Survival, Growth, Feed Utilization and Disease Resistance of Nile Tilapia (Oreochromis niloticus) Fingerlings Fed with Low Aflatoxin. Aquac. J. 2023, 3, 56-69. https://doi.org/10.3390/aquacj3010007
Bhujel RC, Perera AD, Todorović N, Raj J, Gonçalves RA, Vasiljević M. Evaluation of an Organically Modified Clinoptilolite (OMC) and a Multi-Component Mycotoxin Detoxifying Agent (MMDA) on Survival, Growth, Feed Utilization and Disease Resistance of Nile Tilapia (Oreochromis niloticus) Fingerlings Fed with Low Aflatoxin. Aquaculture Journal. 2023; 3(1):56-69. https://doi.org/10.3390/aquacj3010007
Chicago/Turabian StyleBhujel, Ram C., Anusha D. Perera, Nemanja Todorović, Jog Raj, Rui A. Gonçalves, and Marko Vasiljević. 2023. "Evaluation of an Organically Modified Clinoptilolite (OMC) and a Multi-Component Mycotoxin Detoxifying Agent (MMDA) on Survival, Growth, Feed Utilization and Disease Resistance of Nile Tilapia (Oreochromis niloticus) Fingerlings Fed with Low Aflatoxin" Aquaculture Journal 3, no. 1: 56-69. https://doi.org/10.3390/aquacj3010007
APA StyleBhujel, R. C., Perera, A. D., Todorović, N., Raj, J., Gonçalves, R. A., & Vasiljević, M. (2023). Evaluation of an Organically Modified Clinoptilolite (OMC) and a Multi-Component Mycotoxin Detoxifying Agent (MMDA) on Survival, Growth, Feed Utilization and Disease Resistance of Nile Tilapia (Oreochromis niloticus) Fingerlings Fed with Low Aflatoxin. Aquaculture Journal, 3(1), 56-69. https://doi.org/10.3390/aquacj3010007







