Inulin Supplementation in Diets for Tropical Gar (Atractosteus tropicus) Larvae: Effects on Growth, Survival, and Digestive and Antioxidant Enzyme Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Reproduction and Larviculture
2.2. Experimental Design
2.3. Formulation and Preparation for Experimental Diets
2.4. Growth and Survival Rates
2.5. Sampling
2.6. Digestive Enzyme Activity
2.7. Antioxidant Enzyme Activities
2.8. Statistical Analysis
3. Results
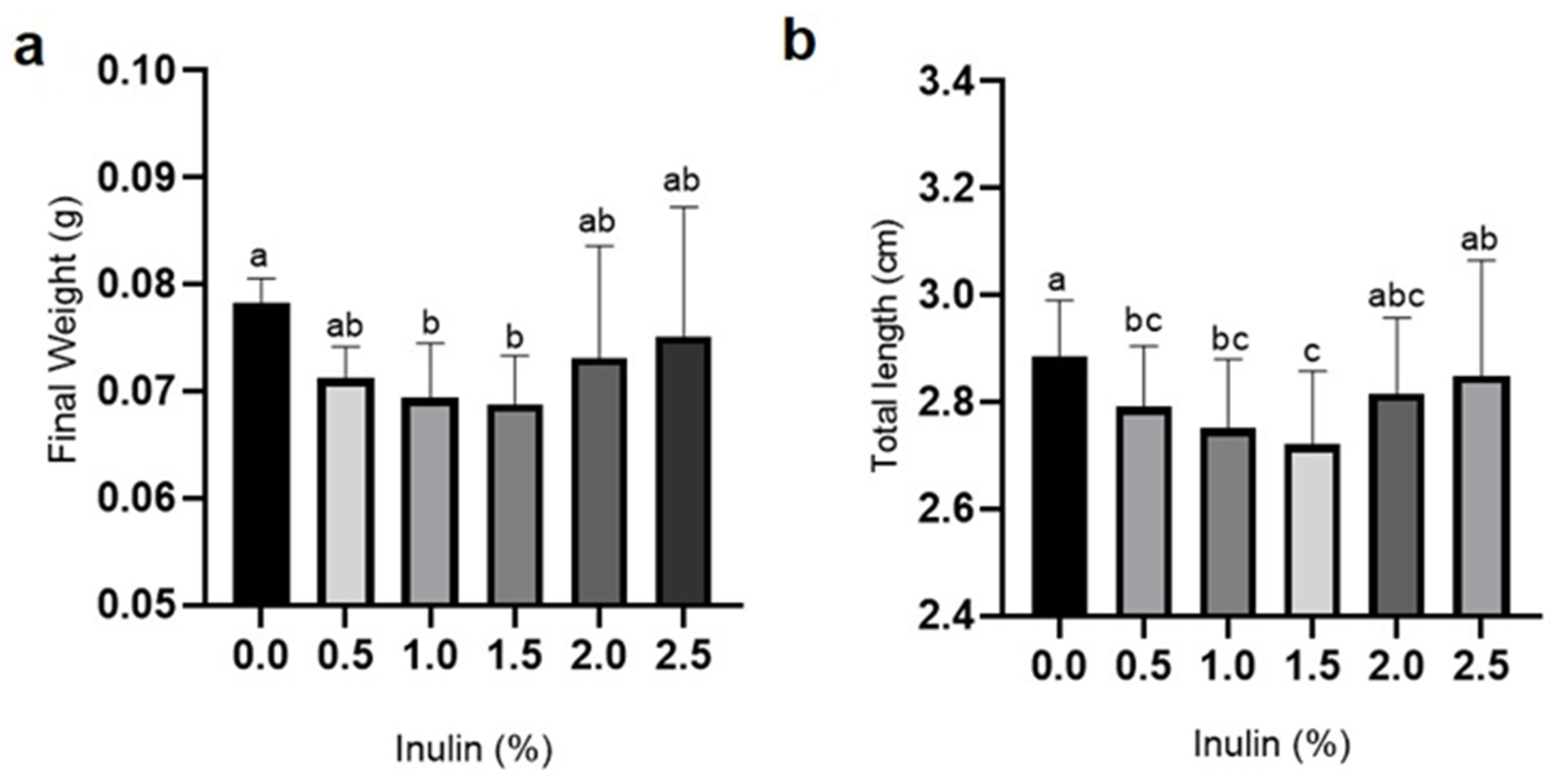
3.1. Growth and Survival Rates
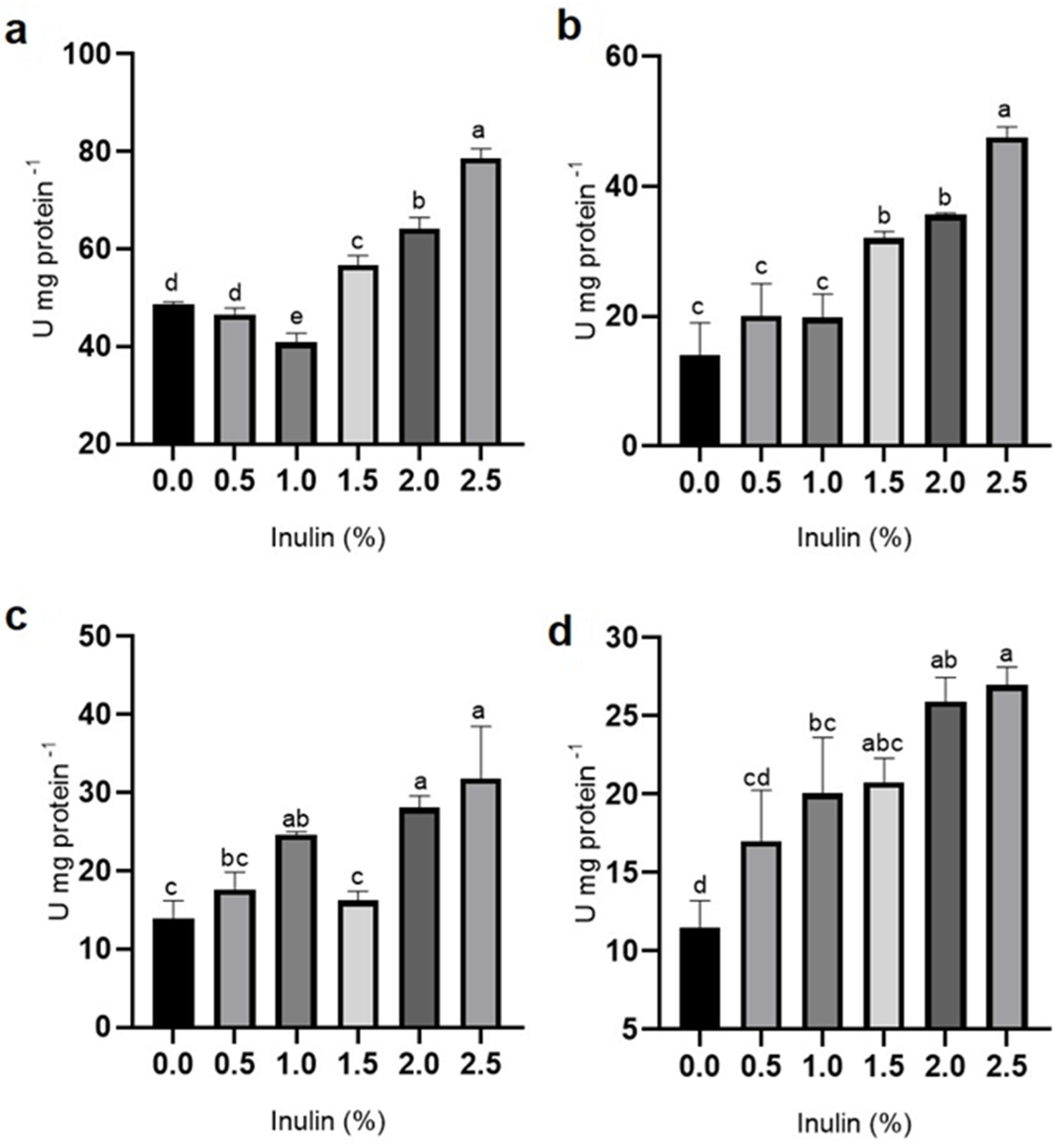
3.2. Digestive Enzyme Activity
3.3. Antioxidant Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobayashi, M.; Msangi, S.; Batka, M.; Vannuccini, S.; Dey, M.M.; Anderson, J.L. Fish to 2030: The Role and Opportunity for Aquaculture. Aquac. Econ. Manag. 2015, 19, 282–300. [Google Scholar] [CrossRef]
- Oliva-Teles, A. Nutrition and health of aquaculture fish. J. Fish Dis. 2012, 35, 83–108. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.; Faggio, C. Importance of prebiotics in aquaculture as immunostimulants. Effects on immune system of Sparus aurata and Dicentrarchus labrax. Fish Shellfish. Immunol. 2016, 54, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Song, S.K.; Beck, B.R.; Kim, D.; Park, J.; Kim, J.; Kim, H.D.; Ringø, E. Prebiotics as immunostimulants in aquaculture: A review. Fish Shellfish. Immunol. 2014, 40, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Mancilha, I.M. Non-digestible oligosaccharides: A review. Carbohydr. Polym. 2007, 68, 587–597. [Google Scholar] [CrossRef]
- Akhter, N.; Wu, B.; Memon, A.M.; Mohsin, M. Probiotics and prebiotics associated with aquaculture: A review. Fish Shellfish Immunol. 2015, 45, 733–741. [Google Scholar] [CrossRef]
- Gatesoupe, F.J. Probiotics and prebiotics for fish culture, at the parting of the ways. Aqua Feed. Formul. Beyond 2005, 2, 3–5. [Google Scholar]
- Iwashita, M.; Addo, S.; Terhune, J.S. Use of pre- and probiotics in finfish aquaculture. In Feed and Feeding Practices in Aquaculture; Davis, D.A., Ed.; Series in Food Science, Technology and Nutrition; Woodhead Publishing: Soston, UK, 2015; pp. 35–249. [Google Scholar] [CrossRef]
- Ringø, E.; Olsen, R.E.; Gifstad, T.Ø.; Dalmo, R.A.; Amlund, H.; Hemre, G.-I.; Bakke, A.M. Prebiotics in aquaculture: A review. Aquac. Nutr. 2010, 16, 117–136. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Aachary, A.A.; Prapulla, S.G. Xylooligosaccharides (XOS) as an Emerging prebiotic: Microbial synthesis, utilization, structural characterization, bioactive properties, and applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 16. [Google Scholar] [CrossRef]
- Kumar, R.; Manjunatha, S.; Kathiravan, T.; Vijayalakshmi, S.; Nadanasabapathi, S.; Raju, P.S. Rheological characteristics of inulin solution at low concentrations: Effect of temperature and solid content. J. Food Sci. Technol. 2015, 52, 5611–5620. [Google Scholar] [CrossRef] [PubMed]
- Kolida, S.; Tuohy, K.; Gibson, G.R. Prebiotic effects of inulin and oligofructose. Braz. J. Nutr. 2002, 87, S193–S197. [Google Scholar] [CrossRef]
- Meyer, D.; Stasse-Wolthuis, M. The bifidogenic effect of inulin and oligofructose and its consequences for gut health. Eur. J. Clin. Nutr. 2009, 63, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Ibrahem, M.D.; Fathi, M.; Mesalhy, S.; Abd El-Aty, A.M. Effect of dietary supplementation of inulin and vitamin C on the growth, hematology, innate immunity, and resistance of Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2010, 29, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Eshaghzadeh, H.; Hoseinifar, S.H.; Vahabzadeh, H.; Ringø, E. The effects of dietary inulin on growth performance, survival and digestive enzyme activities of common carp (Cyprinus carpio) fry. Aquac. Nutr. 2015, 21, 242–247. [Google Scholar] [CrossRef]
- Weiss, M.; Steiner, D.F.; Philipson, L.H. Insulin biosynthesis, secretion, structure, and structure-activity relationships. In EndoText; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2014. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279029/ (accessed on 9 May 2022).
- Mo, W.Y.; Cheng, Z.; Choi, W.M.; Lun, C.H.I.; Man, Y.B.; Wong, J.T.F.; Chen, X.W.; Lau, S.C.K.; Wong, M.H. Use of food waste as fish feeds: Effects of prebiotic fibers (inulin and mannanoligosaccharide) on growth and non-specific immunity of grass carp (Ctenopharyngodon idella). Environ. Sci. Pollut. Res. Int. 2015, 22, 17663–17671. [Google Scholar] [CrossRef]
- Hunt, A.; Çetinkaya, M.; Ozkan Yılmaz, F.; Yildirim, M.; Berkoz, M.; Yalın, S. Effect of dietary supplementation of inulin on growth performance, digestion enzyme activities and antioxidant status of rainbow trout (Oncorhynchus mykiss). Turk. J. Agric. Food Sci. Technol. 2019, 7, 1344–1353. [Google Scholar] [CrossRef]
- Ahmdifar, E.; Akrami, R.; Ghelichi, A.; Mohammadi Zarejabad, A. Effects of different dietary prebiotic inulin levels on blood serum enzymes, hematologic, and biochemical parameters of great sturgeon (Huso huso) juveniles. Comp. Clin. Pathol. 2010, 20, 447–451. [Google Scholar] [CrossRef]
- Ai, Q.; Xu, H.; Mai, K.; Xu, W.; Wang, J.; Zhang, W. Effects of dietary supplementation of Bacillus subtilis and fructooligosaccharide on growth performance, survival, non-specific immune response and disease resistance of juvenile large yellow croaker, Larimichthys Crocea. Aquaculture 2011, 317, 155e61. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Sourinejad, I.; Ashori, S.; Moradinasab, A.A. Effect of different levels of dietary inulin supplementation on growth performance, survival and some hematologic indices in Red Pacu Piaractus brachypomus fry. J. Aquat. Ecol. 2014, 4, 50–44. Available online: http://jae.hormozgan.ac.ir/article-1-38-en.html (accessed on 1 March 2023).
- Mousavi, E.; Mohammadiazarm, H.; Seied Mohammad Mousavi, M.S.; Ghatrami, R.E. Effects of inulin, savory and onion powders in diet of juveniles carp Cyprinus carpio (Linnaeus 1758) on gut micro flora, immune response and blood biochemical parameters. Turk. J. Fish. Aquat. Sci. 2016, 16, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Couturier, G.; Álvarez-González, C.A.; Contreras, W.; Hernández, U.; Hernández, A.; Mendoza, R.; Aguilera, C.; García, T.; Civera, R.; Goytortua, E. Avances en la alimentación y nutrición de pejelagarto Atractosteus tropicus. In Memorias del VIII Simposium Internacional de Nutrición Acuícola; Cruz Suárez, L.E., Ricque Marie, D., Tapia Salazar, M., Nieto López, M.G., Villarreal Cavazos, D.A., Puello Cruz, A.C., García Ortega, A., Eds.; UANL: Monterrey, México, 2006; pp. 446–523. Available online: https://www.uanl.mx/utilerias/nutricion_acuicola/VIII/archivos/28Alvarez.pdf (accessed on 1 March 2023).
- Márquez-Couturier, G.; Vázquez-Navarrete, C.J. Empoderamiento de las organizaciones sociales en el cultivo de pejelagarto (Atractosteus tropicus) en el sureste de Mexico. Agroproductividad 2015, 8, 36–43. Available online: https://biblat.unam.mx/hevila/Agroproductividad/2015/vol8/no3/7.pdf (accessed on 1 March 2023).
- Márquez-Couturier, G.; Vázquez-Navarrete, C.J. Estado del arte de la biología y cultivo de pejelagarto (Atractosteus tropicus). Agroproductividad 2015, 8, 44–51. Available online: https://biblat.unam.mx/hevila/Agroproductividad/2015/vol8/no3/8.pdf (accessed on 1 March 2023).
- Guerrero-Zarate, R.; Álvarez-González, C.A.; Olvera-Novoa, M.A.; Perales-García, N.; Frías-Quintana, C.A.; Martínez-García, R.; Contreras-Sanchez, W.M. Partial characterization of digestive proteases in tropical gar Atractosteus tropicus juveniles. Fish Physiol. Biochem. 2013, 40, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Frías-Quintana, C.A.; Márquez-Couturier, G.; Álvarez-González, C.A.; Tovar-Ramírez, D.; Nolasco-Soria, H.; Galaviz-Espinosa, M.A.; Martínez-García, R.; Camarillo-Coop, S.; Martínez-Yáñez, R.; Gisbert, E. Development of digestive tract and enzyme activities during the early ontogeny of the tropical gar Atractosteus tropicus. Fish Physiol. Biochem. 2015, 41, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Frías-Quintana, C.A.; Domínguez-Lorenzo, J.; Álvarez-González, C.A.; Tovar-Ramírez, D.; Martínez-García, R. Using cornstarch in microparticulate diets for larvicultured tropical gar (Atractosteus tropicus). Fish Physiol. Biochem. 2016, 42, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Frías-Quintana, C.A.; Álvarez-González, C.A.; Tovar-Ramírez, D.; Martínez-García, R.; Camarillo-Coop, S.; Peña-Marín, E.S.; Galaviz, M. Use of potato starch in diets of tropical gar (Atractosteus tropicus, Gill 1863) larvae. Fishes 2017, 2, 3. [Google Scholar] [CrossRef]
- Palma-Cancino, D.J.; Martínez-García, R.; Alvarez-González, C.A.; Camarillo-Coop, S.; Peña-Marín, E.S. Esquemas de alimentación para larvicultura de pejelagarto (Atractosteus tropicus Gill): Crecimiento, supervivencia y canibalismo. Ecosistemas Y Recur. Agropecu. 2019, 6, 273–281. [Google Scholar] [CrossRef]
- Nájera-Arzola, I.C.; Álvarez-González, C.A.; Frías-Quintana, C.A.; Peña, E.; Martínez-García, R.; Camarillo-Coop, S.; Méndez-Marín, O.; Gisbert, E. Evaluación de oligosacáridos de manano (MOS) en dietas balanceadas para juveniles de pejelagarto (Atractosteus tropicus). Hidrobiológica 2018, 28, 239–246. [Google Scholar] [CrossRef]
- Nieves-Rodríguez, K.N.; Álvarez-González, C.A.; Peña-Marín, E.S.; Vega-Villasante, F.; Martínez-García, R.; Camarillo-Coop, S.; Tovar-Ramírez, D.; Guzmán-Villanueva, L.T.; Andree, K.; Gisbert, E. Effect of β-glucans in diets on growth, survival, digestive enzyme activity, and immune system and intestinal barrier gene expression for tropical gar (Atractosteus tropicus) juveniles. Fishes 2018, 3, 27. [Google Scholar] [CrossRef]
- Sepúlveda-Quiroz, C.A.; Peña-Marín, E.S.; Pérez-Morales, A.; Martínez-García, R.; Alvarez-Villagómez, C.S.; Maytorena-Verdugo, C.I.; Camarillo-Coop, S.; Vissio, G.; Pérez Sirkin, D.; Tovar-Ramírez, D.; et al. Fructooligosaccharide supplementation in diets for tropical gar (Atractosteus tropicus) juvenile: Effects on morphophysiology and intestinal barrier function. Aquac. Res. 2021, 52, 37–50. [Google Scholar] [CrossRef]
- Maytorena-Verdugo, C.I.; Peña-Marín, E.S.; Alvarez-Villagómez, C.S.; Pérez-Jiménez, G.M.; Sepúlveda-Quiroz, C.A.; Alvarez-González, C.A. Inclusion of mannan-oligosaccharides in diets for tropical gar Atractosteus tropicus larvae: Effects on growth, digestive enzymes, and expression of intestinal barrier genes. Fishes 2022, 7, 127. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Anson, M.L. The estimation of pepsin, trypsin, papain and cathepsin with hemoglobin. J. Gen. Physiol. 1938, 22, 79–89. [Google Scholar] [CrossRef]
- Walter, H.E. Proteinases: Methods with hemoglobin, casein and azocoll as substrates. In Methods of Enzymatic Analysis; Bergmeyern, H.J., Ed.; Verlag Chemie: Weinheim, Germany, 1984; Volume 1, pp. 270–277. Available online: https://epub.ub.uni-muenchen.de/9220/1/9220.pdf (accessed on 9 May 2022).
- Versaw, W.K.; Cuppett, S.L.; Winters, D.D.; Williams, L.E. An improved colorimetric assay for bacterial lipase in nonfat dry milk. J. Food Sci. 1989, 54, 232–254. [Google Scholar] [CrossRef]
- Robyt, J.F.; Whelan, W.J. General Aspects of Amylase Action, Chap. 13 cr-Amylases, Chap. 14/J-Amylases. In Starch and Its Derivatives, 4th ed.; Radley, J.A., Ed.; Chapman and Hall: London, UK, 1968; pp. 477–497. [Google Scholar]
- Olsen, R.E.; Myklebust, R.; Kryvi, H.; Mayhew, T.M.; Ringø, E. Damaging effect of dietary inulin on intestinal enterocytes in Arctic charr (Salvelinus alpinus L.). Aquac. Res. 2001, 32, 931–934. [Google Scholar] [CrossRef]
- Akrami, R.; Iri, Y.; Rostami, H.K.; Mansour, M.R. Effect of dietary supplementation of fructooligosaccharide (FOS) on growth performance, survival, lactobacillus bacterial population and hemato-immunological parameters of stellate sturgeon (Acipenser stellatus) juvenile. Fish Shellfish. Immunol. 2013, 35, 1235–1239. [Google Scholar] [CrossRef]
- Reza, A.; Abdolmajid, H.; Abbas, M.; Abdolmohammad, A.K. Effect of Dietary Prebiotic Inulin on Growth Performance, Intestinal Microflora, Body Composition and Hematological Parameters of Juvenile Beluga, Huso huso (Linnaeus, 1758). J. World Aquac. Soc. 2009, 40, 771–779. [Google Scholar] [CrossRef]
- Soleimani, N.; Hoseinifar, S.H.; Merrifield, D.L.; Barati, M.; Abadi, Z.H. Dietary supplementation of fructooligosaccharide (FOS) improves the innate immune response, stress resistance, digestive enzyme activities and growth performance of Caspian roach (Rutilus rutilus) fry. Fish Shellfish. Immunol. 2012, 32, 316–321. [Google Scholar] [CrossRef]
- Boonanuntanasarn, S.; Tiengtam, N.; Pitaksong, T.; Piromyou, P.; Teaumroong, N. Effects of dietary inulin and Jerusalem artichoke (Helianthus tuberosus) on intestinal microbiota community and morphology of Nile tilapia (Oreochromis niloticus) fingerlings. Aquac. Nutr. 2018, 24, 712–722. [Google Scholar] [CrossRef]
- Tiengtam, N.; Paengkoum, P.; Sirivoharn, S.; Phonsiri, K.; Boonanuntanasarn, S. The effects of dietary inulin and Jerusalem artichoke (Helianthus tuberosus) tuber on the growth performance, haematological, blood chemical and immune parameters of Nile tilapia (Oreochromis niloticus) fingerlings. Aquac. Res. 2017, 48, 5280–5288. [Google Scholar] [CrossRef]
- Yones, A.M.A.S.M.; Eissa, I.A.M.M.; Ghobashy, M.A.E.F.A.; Marzok, S.S. Effects of dietary inulin as prebiotic on growth performance, immuno-haematological indices and ectoparasitic infection of fingerlings Nile tilapia, Oreochromis niloticus. Natl. Inst. Oceanogr. Fish. 2020, 43, 88–103. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Ahmadi, A.; Khalili, M.; Raeisi, M.; Van Doan, H.; Caipang, C.M. The study of antioxidant enzymes and immune-related genes expression in common carp (Cyprinus carpio) fingerlings fed different prebiotics. Aquac. Res. 2017, 48, 5447–5454. [Google Scholar] [CrossRef]
- Tiengtam, N.; Khempaka, S.; Paengkoum, P.; Boonanuntanasarn, S. Effects of inulin and Jerusalem artichoke (Helianthus tuberosus) as prebiotic ingredients in the diet of juvenile Nile tilapia (Oreochromis niloticus). Anim. Feed. Sci. Technol. 2015, 207, 120–129. [Google Scholar] [CrossRef]
- Ganguly, S.; Dora, K.C.; Sarkar, S.; Chowdhury, S. Supplementation of prebiotics in fish feed: A review. Rev. Fish Biol. Fish. 2012, 23, 195–199. [Google Scholar] [CrossRef]
- Mahious, A.S.; Gatesoupe, F.J.; Hervi, M.; Metailler, R.; Ollevier, F. Effect of dietary inulin and oligosaccharides as prebiotics for weaning turbot, Psetta maxima (Linnaeus, C. 1758). Aquac. Int. 2006, 14, 219–229. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Yusefian, M.; Yavari, V.; Mohamadian, T.; Abhari, H.; Goran, H. Modulation of rainbow trout immune system and enhance resistance against streptococcosis using dietary inulin. In The First National Conference on Caspian Sea Fisheries Resources; Gorgan University: Gorgan, Iran, 2007; p. 12. [Google Scholar]
- Salze, G.; McLeana, E.; Schwarz, M.H.; Craig, S.R. Dietary mannan oligosaccharide enhances salinity tolerance and gut development of larval cobia. Aquaculture 2008, 274, 148–152. [Google Scholar] [CrossRef]
- Levrat, M.A.; Rémésy, C.; Demigné, C. High propionic acid fermentations and mineral accumulation in the cecum of rats adapted to different levels of inulin. J. Nutr. 1991, 121, 1730–1737. [Google Scholar] [CrossRef]
- Trinidad, T.P.; Wolever, T.M.S.; Tompson, L.V. Interactive effects of calcium and short-chain fatty acids on absorption in the distal colon of man. Nutr. Res. 1993, 13, 417–425. [Google Scholar] [CrossRef]
- Ortiz, L.T.; Rebolé, A.; Velasco, S.; Rodríguez, M.L.; Treviño, J.; Tejedor, J.L.; Alzueta, C. Effects of inulin and fructooligosaccharides on growth performance, body chemical composition and intestinal microbiota of farmed rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2012, 19, 475–482. [Google Scholar] [CrossRef]
- Refstie, S.; Bakke-McKellep, A.M.; Penn, M.H.; Sundby, A.; Shearer, K.D.; Krogdahl, Å. Capacity for digestive hydrolysis and amino acid absorption in Atlantic salmon (Salmo salar) fed diets with soybean meal or inulin with or without addition of antibiotics. Aquaculture 2006, 261, 392–406. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. BBr. J. Nutr. 2010, 104 (Suppl. 2), S1–S63. [Google Scholar] [CrossRef] [PubMed]
- Walton, G.E.; Swann, J.R.; Gibson, G.R. Prebiotics. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef]
- Baumgärtner, S.; James, J.; Ellison, A. The supplementation of a prebiotic improves the microbial community in the gut and the skin of Atlantic salmon (Salmo salar). Aquac. Rep. 2022, 25, 101204. [Google Scholar] [CrossRef]
- Wu, P.; Liu, Y.; Jiang, W.D.; Jiang, J.; Zhao, J.; Zhang, Y.A.; Zhou, X.Q.; Feng, L. A Comparative study on antioxidant system in fish hepatopancreas and intestine affected by choline deficiency: Different change patterns of varied antioxidant enzyme genes and nrf2 signaling factors. PLoS ONE 2017, 12, e0169888. [Google Scholar] [CrossRef] [PubMed]
- Rault-Nania, M.H.; Demougeot, C.; Gueux, E.; Berthelot, A.; Dzimira, S.; Rayssiguier, Y.; Rock, E.; Mazur, A. Inulin supplementation prevents high fructose diet-induced hypertension in rats. Clin. Nutr. 2008, 27, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.D.; Souza, C.F.; Parmeggiani, B.; Leipnitz, G.; Verdi, C.M.; Santos, R.V.; Stefani, L.M.; Baldisserotto, B. The disturbance of antioxidant/oxidant balance in fish experimentally infected by Aeromonas caviae: Relationship with disease pathophysiology. Microb. Pathog. 2018, 122, 53–57. [Google Scholar] [CrossRef]
- Guerreiro, I.; Couto, A.; Machado, M.; Castro, C.; Pousão-Ferreira, P.; Oliva-Teles, A.; Enes, P. Prebiotics effect on immune and hepatic oxidative status and gut morphology of white sea bream (Diplodus sargus). Fish Shellfish Immunol. 2016, 50, 168–174. [Google Scholar] [CrossRef]
- Olsvik, P.A.; Kristensen, T.; Waagbø, R.; Rosseland, B.O.; Tollefsen, K.E.; Baeverfjord, G.; Berntssen, M.H. mRNA expression of antioxidant enzymes (SOD, CAT and GSH-Px) and lipid peroxidative stress in liver of Atlantic salmon (Salmo salar) exposer to hyperoxic water during smoltification. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2005, 141, 314–323. [Google Scholar] [CrossRef]
- Butt, R.L.; Volkoff, H. Gut microbiota and energy homeostasis in fish. Front. Endocrinol. 2019, 10, 9. [Google Scholar] [CrossRef]
- Blottiere, H.M.; Buecher, B.; Galmiche, J.-P.; Cherbut, C. Molecular analysis of the effect of short-chain fatty acids on intestinal cell proliferation. Proc. Nutr. Soc. 2003, 62, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.; Hellweg, P.; Taras, D.; Zentek, J. Effects of Dietary Inulin on the Intestinal Short Chain Fatty Acids and Microbial ecology in broiler chickens as revealed by denaturing gradient gel electrophoresis. Poult. Sci. 2008, 87, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Nabizadeh, A. The effect of inulin on broiler chicken intestinal microflora, gut morphology, and performance. J. Anim. Feed. Sci. 2012, 21, 725–734. [Google Scholar] [CrossRef]
- Johnson-Henry, K.C.; Pinnell, L.J.; Waskow, A.M.; Irrazabal, T.; Martin, A.; Hausner, M.; Sherman, P.M. Short-chain fructo-oligosaccharide and inulin modulate inflammatory responses and microbial communities in Caco2-bbe cells and in a mouse model of intestinal injury. J. Nutr. 2014, 144, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Cerezuela, R.; Cuesta, A.; Meseguer, J.; Esteban, M.A. Effects of inulin on gilthead seabream (Sparus aurata L.) innate immune parameters. Fish Shellfish. Immunol. 2008, 24, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, G.M.; Peña-Marín, E.S.; Maytorena-Verdugo, C.I.; Sepúlveda-Quiroz, C.A.; Jiménez-Martínez, L.D.; De la Rosa-García, S.C.; Asencio-Alcudia, G.G.; Martínez-García, R.; Tovar-Ramírez, D.; Galaviz, M.A.; et al. Incorporation of fructooligosaccharides in diets influence growth performance, digestive enzyme activity, and expression of intestinal barrier function genes in tropical gar (Atractosteus tropicus) larvae. Fishes 2022, 7, 137. [Google Scholar] [CrossRef]


| Inulin (%) | ||||||
|---|---|---|---|---|---|---|
| Ingredients (g kg−1) | 0 | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 |
| Fish meal a | 297.9 | 297.9 | 297.9 | 297.9 | 297.9 | 297.9 |
| Poultry meal a | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 |
| Pork meal a | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 |
| Starch b | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Soybean meal c | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Inulin d | 0.0 | 5.0 | 10.0 | 15.0 | 20.0 | 25.0 |
| Sorghum flour c | 86.6 | 81.6 | 76.6 | 71.6 | 66.6 | 61.6 |
| Fish oil a | 40.5 | 40.5 | 40.5 | 40.5 | 40.5 | 40.5 |
| soy lecithin e | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 |
| Grenetin f | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Mixture Vit-Min g | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Vitamin C h | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Proximal composition g/100 g dry matter | ||||||
| Protein | 45.11 | 44.92 | 45.04 | 44.83 | 45.25 | 45.16 |
| Lipid | 15.21 | 14.93 | 15.10 | 14.82 | 14.93 | 15.07 |
| Ash | 11.94 | 12.35 | 12.01 | 11.99 | 12.29 | 12.19 |
| NFE 1 | 27.74 | 27.22 | 27.85 | 28.36 | 27.53 | 27.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De La Cruz-Marín, E.; Martínez-García, R.; López-Hernández, J.F.; Méndez-Marín, O.; De la Rosa-García, S.C.; Peña-Marín, E.S.; Tovar-Ramírez, D.; Sepúlveda-Quiroz, C.A.; Pérez-Jiménez, G.M.; Jiménez-Martínez, L.D.; et al. Inulin Supplementation in Diets for Tropical Gar (Atractosteus tropicus) Larvae: Effects on Growth, Survival, and Digestive and Antioxidant Enzyme Activities. Aquac. J. 2023, 3, 43-55. https://doi.org/10.3390/aquacj3010006
De La Cruz-Marín E, Martínez-García R, López-Hernández JF, Méndez-Marín O, De la Rosa-García SC, Peña-Marín ES, Tovar-Ramírez D, Sepúlveda-Quiroz CA, Pérez-Jiménez GM, Jiménez-Martínez LD, et al. Inulin Supplementation in Diets for Tropical Gar (Atractosteus tropicus) Larvae: Effects on Growth, Survival, and Digestive and Antioxidant Enzyme Activities. Aquaculture Journal. 2023; 3(1):43-55. https://doi.org/10.3390/aquacj3010006
Chicago/Turabian StyleDe La Cruz-Marín, Eduardo, Rafael Martínez-García, Jenny F. López-Hernández, Otilio Méndez-Marín, Susana C. De la Rosa-García, Emyr S. Peña-Marín, Dariel Tovar-Ramírez, Cesar A. Sepúlveda-Quiroz, Graciela M. Pérez-Jiménez, Luis D. Jiménez-Martínez, and et al. 2023. "Inulin Supplementation in Diets for Tropical Gar (Atractosteus tropicus) Larvae: Effects on Growth, Survival, and Digestive and Antioxidant Enzyme Activities" Aquaculture Journal 3, no. 1: 43-55. https://doi.org/10.3390/aquacj3010006
APA StyleDe La Cruz-Marín, E., Martínez-García, R., López-Hernández, J. F., Méndez-Marín, O., De la Rosa-García, S. C., Peña-Marín, E. S., Tovar-Ramírez, D., Sepúlveda-Quiroz, C. A., Pérez-Jiménez, G. M., Jiménez-Martínez, L. D., Asencio-Alcudia, G. G., & Álvarez-González, C. A. (2023). Inulin Supplementation in Diets for Tropical Gar (Atractosteus tropicus) Larvae: Effects on Growth, Survival, and Digestive and Antioxidant Enzyme Activities. Aquaculture Journal, 3(1), 43-55. https://doi.org/10.3390/aquacj3010006








