Emotional Salience of Evolutionary and Modern Disgust-Relevant Threats Measured Through Electrodermal Activity
Abstract
1. Introduction
1.1. Psychophysiology of Disgust
1.2. Categorization of Disgust and of Disgust-Evoking Threats
1.3. Aims
2. Results
2.1. Effect of Threat Category on Probability of SR Response
2.2. Effect of Threat Category on Amplitude of SR Response
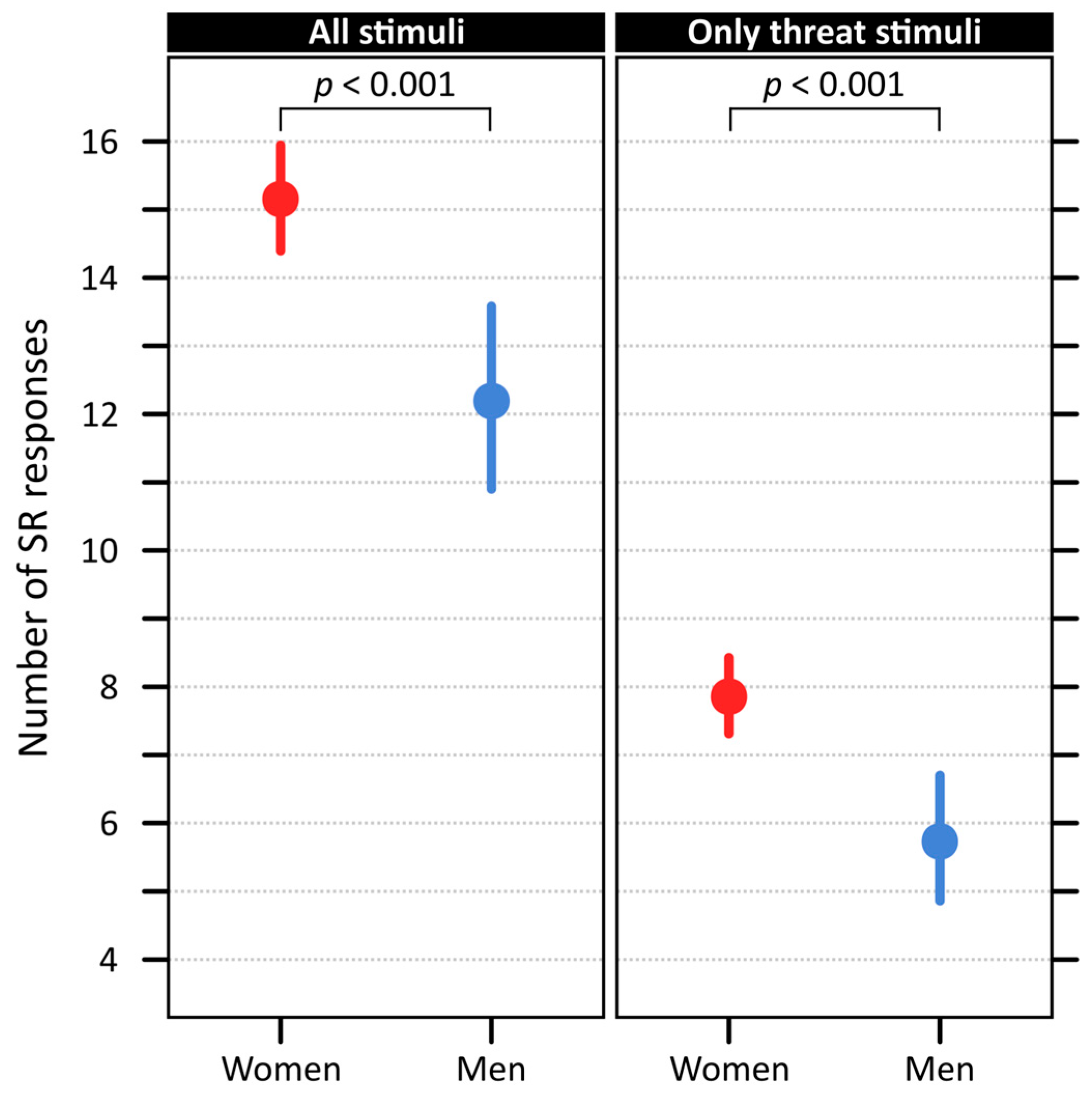
2.3. Effect of General Anxiousness, Sensitivity to Disgust, and Participant Sex on Their Reactivity
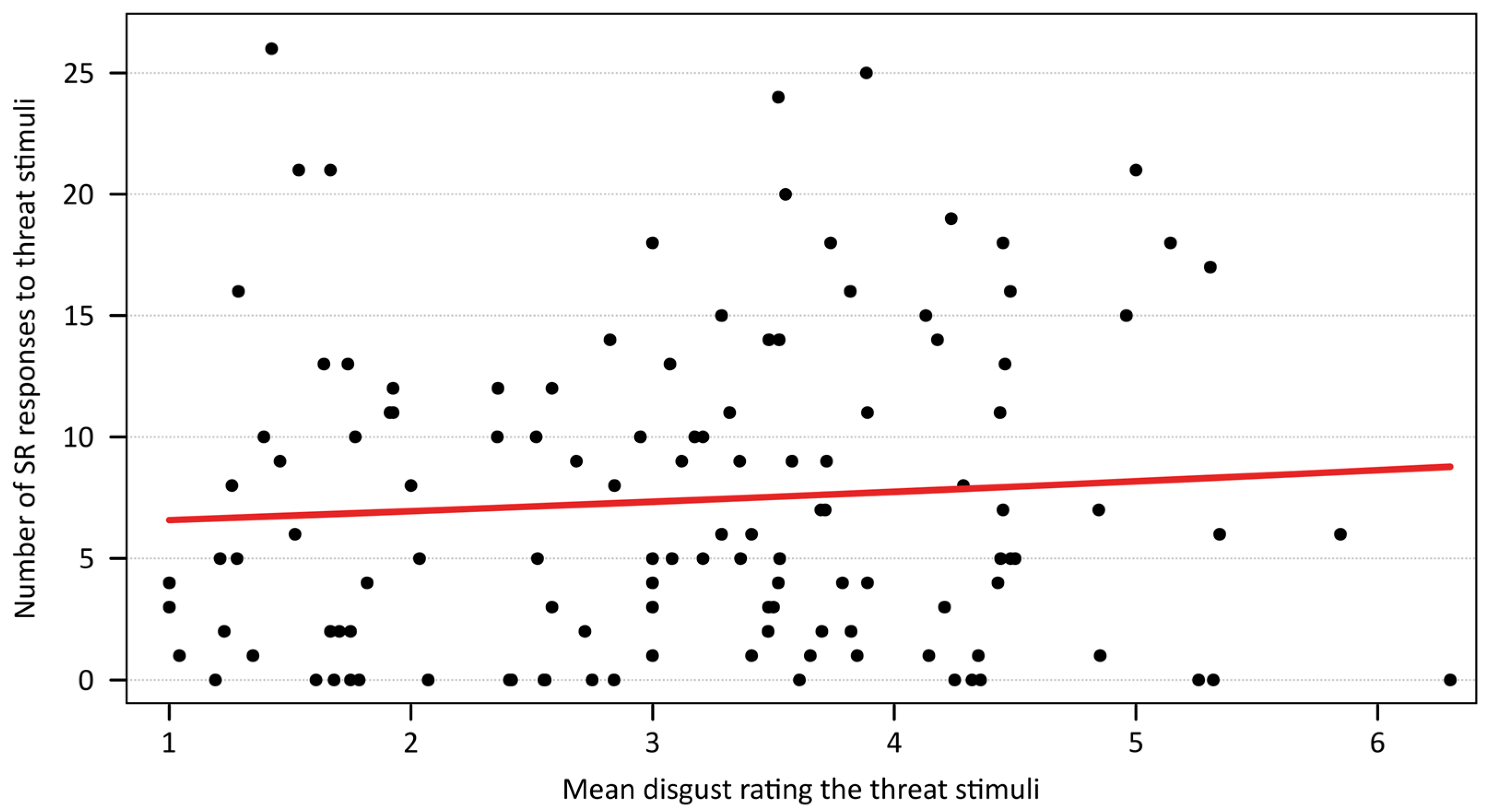
2.4. Effect of Subjective Disgust Saliency on Electrodermal Responses
3. Discussion
3.1. Differences Between Stimulus Categories
3.2. Subjective Disgust Rating and Reactivity
3.3. Participants Characteristics and Reactivity
4. Materials and Methods
4.1. Participants
4.2. Stimuli
4.3. Procedure
4.4. Data Curation
4.5. Statistical Analysis
4.6. Ethical Note
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brosch, T.; Pourtois, G.; Sander, D. The Perception and Categorisation of Emotional Stimuli: A Review. Cogn. Emot. 2010, 24, 377–400. [Google Scholar] [CrossRef]
- Ekman, P.; Levenson, R.W.; Friesen, W.V. Autonomic Nervous System Activity Distinguishes among Emotions. Science 1983, 221, 1208–1210. [Google Scholar] [CrossRef]
- Lang, P.J.; Greenwald, M.K.; Bradley, M.M.; Hamm, A.O. Looking at Pictures: Affective, Facial, Visceral, and Behavioral Reactions. Psychophysiology 1993, 30, 261–273. [Google Scholar] [CrossRef]
- Cannon, W.B. The Emergency Function of the Adrenal Medulla in Pain and the Major Emotions. Am. J. Physiol. Leg. Content 1914, 33, 356–372. [Google Scholar] [CrossRef]
- McCarty, R. Chapter 4—The Fight-or-Flight Response: A Cornerstone of Stress Research. In Stress: Concepts, Cognition, Emotion, and Behavior; Fink, G., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 33–37. ISBN 978-0-12-800951-2. [Google Scholar]
- McCorry, L.K. Physiology of the Autonomic Nervous System. Am. J. Pharm. Educ. 2007, 71, 78. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.M.; Codispoti, M.; Cuthbert, B.N.; Lang, P.J. Emotion and Motivation I: Defensive and Appetitive Reactions in Picture Processing. Emotion 2001, 1, 276–298. [Google Scholar] [CrossRef]
- Collet, C.; Vernet-Maury, E.; Delhomme, G.; Dittmar, A. Autonomic Nervous System Response Patterns Specificity to Basic Emotions. J. Auton. Nerv. Syst. 1997, 62, 45–57. [Google Scholar] [CrossRef]
- Croy, I.; Laqua, K.; Suess, F.; Joraschky, P.; Ziemssen, T.; Hummel, T. The Sensory Channel of Presentation Alters Subjective Ratings and Autonomic Responses toward Disgusting Stimuli—Blood Pressure, Heart Rate and Skin Conductance in Response to Visual, Auditory, Haptic and Olfactory Presented Disgusting Stimuli. Front. Hum. Neurosci. 2013, 7, 510. [Google Scholar] [CrossRef]
- Christie, I.C.; Friedman, B.H. Autonomic Specificity of Discrete Emotion and Dimensions of Affective Space: A Multivariate Approach. Int. J. Psychophysiol. 2004, 51, 143–153. [Google Scholar] [CrossRef]
- Kreibig, S.D. Autonomic Nervous System Activity in Emotion: A Review. Biol. Psychol. 2010, 84, 394–421. [Google Scholar] [CrossRef] [PubMed]
- Stark, R.; Walter, B.; Schienle, A.; Vaitl, D. Psychophysiological Correlates of Disgust and Disgust Sensitivity. J. Psychophysiol. 2005, 19, 50–60. [Google Scholar] [CrossRef]
- Baker, L.B. Physiology of Sweat Gland Function: The Roles of Sweating and Sweat Composition in Human Health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef]
- Harker, M. Psychological Sweating: A Systematic Review Focused on Aetiology and Cutaneous Response. Ski. Pharmacol. Physiol. 2013, 26, 92–100. [Google Scholar] [CrossRef]
- Morris-Jones, R. Neural Control of Sweat Secretion: A Review of the Neurology and Current Treatment Options for Hyperhidrosis. Br. J. Dermatol. 2018, 178, 1233–1234. [Google Scholar] [CrossRef] [PubMed]
- Wilke, K.; Martin, A.; Terstegen, L.; Biel, S.S. A Short History of Sweat Gland Biology. J. Cosmet. Sci. 2007, 29, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Boucsein, W.; Fowles, D.C.; Grimnes, S.; Ben-Shakhar, G.; Roth, W.T.; Dawson, M.E.; Filion, D.L. Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures Publication Recommendations for Electrodermal Measurements. Psychophysiology 2012, 49, 1017–1034. [Google Scholar] [CrossRef]
- Arabian, H.; Schmid, R.; Wagner-Hartl, V.; Moeller, K. Analysis of EDA and Heart Rate Signals for Emotional Stimuli Responses. Curr. Dir. Biomed. Eng. 2023, 9, 150–153. [Google Scholar] [CrossRef]
- Vrana, S.R. The Psychophysiology of Disgust: Differentiating Negative Emotional Contexts with Facial EMG. Psychophysiology 1993, 30, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Rymarczyk, K.; Żurawski, Ł.; Jankowiak-Siuda, K.; Szatkowska, I. Empathy in Facial Mimicry of Fear and Disgust: Simultaneous EMG-fMRI Recordings During Observation of Static and Dynamic Facial Expressions. Front. Psychol. 2019, 10, 701. [Google Scholar] [CrossRef]
- Gläscher, J.; Adolphs, R. Processing of the Arousal of Subliminal and Supraliminal Emotional Stimuli by the Human Amygdala. J. Neurosci. 2003, 23, 10274–10282. [Google Scholar] [CrossRef] [PubMed]
- Gold, A.K.; Kredlow, M.A.; Orr, S.P.; Hartley, C.A.; Otto, M.W. Skin Conductance Levels and Responses in Asian and White Participants during Fear Conditioning. Physiol. Behav. 2022, 251, 113802. [Google Scholar] [CrossRef]
- Sánchez-Navarro, J.P.; Martínez-Selva, J.M.; Maldonado, E.F.; Carrillo-Verdejo, E.; Pineda, S.; Torrente, G. Autonomic Reactivity in Blood-Injection-Injury and Snake Phobia. Psychosom. Res. 2018, 115, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Schienle, A.; Stark, R.; Vaitl, D. Evaluative Conditioning: A Possible Explanation for the Acquisition of Disgust Responses? Learn. Motiv. 2001, 32, 65–83. [Google Scholar] [CrossRef]
- Grus, A.; Hromatko, I. Psychological and Physiological Correlates of Pathogen-Induced Disgust. In Proceedings of the 22nd Psychology Days in Zadar, Zadar, Croatia, 1–3 October 2020; Banai, I.P., Ed.; University of Zadar, Department of Psychology: Zadar, Croatia, 2022; Volume 3, pp. 29–40. [Google Scholar]
- Rozin, P.; Haidt, J.; McCauley, C.R. Disgust: The Body and Soul Emotion. In Handbook of Cognition and Emotion; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1999; pp. 429–445. ISBN 978-0-471-97836-7. [Google Scholar]
- Levenson, R.W. Autonomic Nervous System Differences among Emotions. Psychol. Sci. 1992, 3, 23–27. [Google Scholar] [CrossRef]
- Levenson, R.W.; Ekman, P.; Friesen, W.V. Voluntary Facial Action Generates Emotion-Specific Autonomic Nervous System Activity. Psychophysiology 1990, 27, 363–384. [Google Scholar] [CrossRef]
- Comtesse, H.; Stemmler, G. Cardiovascular Regulation Pattern of Contamination-Related Disgust: Consistency and Context Dependence. Psychophysiology 2016, 53, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- de Jong, P.J.; van Overveld, M.; Peters, M.L. Sympathetic and Parasympathetic Responses to a Core Disgust Video Clip as a Function of Disgust Propensity and Disgust Sensitivity. Biol. Psychol. 2011, 88, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Hopp, H. Cardiovascular Indicators of Disgust. Int. J. Psychophysiol. 2008, 68, 201–208. [Google Scholar] [CrossRef]
- Olatunji, B.O.; Haidt, J.; McKay, D.; David, B. Core, Animal Reminder, and Contamination Disgust: Three Kinds of Disgust with Distinct Personality, Behavioral, Physiological, and Clinical Correlates. J. Res. Pers. 2008, 42, 1243–1259. [Google Scholar] [CrossRef]
- Tybur, J.M.; Lieberman, D.; Griskevicius, V. Microbes, Mating, and Morality: Individual Differences in Three Functional Domains of Disgust. J. Pers. Soc. Psychol. 2009, 97, 103–122. [Google Scholar] [CrossRef]
- Davey, G.C.L. Mechanisms of Disgust in Psychopathology. In The Handbook of Disgust Research: Modern Perspectives and Applications; Powell, P.A., Consedine, N.S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 191–208. ISBN 978-3-030-84486-8. [Google Scholar]
- Ekman, P. An Argument for Basic Emotions. Cogn. Emot. 1992, 6, 169–200. [Google Scholar] [CrossRef]
- Janovcová, M.; Polák, J.; Končická, A.; Chomik, A.; Kaňková, Š.; Frynta, D.; Landová, E. From bugs to sickness: Disgust evaluation of ancestral, modern, and pandemic threats. Evol. Psychol. Sci. 2025, 11, 273–290. [Google Scholar] [CrossRef]
- Rozin, P.; Haidt, J.; McCauley, C. Disgust: The Body and Soul Emotion in the 21st Century. In Disgust and Its Disorders: Theory, Assessment, and Treatment Implications; American Psychological Association: Washington, DC, USA, 2009; pp. 9–29. ISBN 978-1-4338-0397-0. [Google Scholar]
- Bradshaw, H.K.; Gassen, J. The Evolution of Disgust, Pathogens, and the Behavioural Immune System. In The Handbook of Disgust Research: Modern Perspectives and Applications; Powell, P.A., Consedine, N.S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 31–51. ISBN 978-3-030-84486-8. [Google Scholar]
- Schaller, M.; Park, J.H. The Behavioral Immune System (and Why It Matters). Curr. Dir. Psychol. Sci. 2011, 20, 99–103. [Google Scholar] [CrossRef]
- Darwin, C. The Expression of the Emotions in Man and Animals; John Murray: London, UK, 1872; pp. vi, 374. [Google Scholar]
- Olatunji, B.O.; Williams, N.L.; Tolin, D.F.; Abramowitz, J.S.; Sawchuk, C.N.; Lohr, J.M.; Elwood, L.S. The Disgust Scale: Item Analysis, Factor Structure, and Suggestions for Refinement. Psychol. Assess. 2007, 19, 281–297. [Google Scholar] [CrossRef]
- Shapouri, S.; Martin, L.L. Snakes vs. Guns: A Systematic Review of Comparisons Between Phylogenetic and Ontogenetic Threats. Adapt. Hum. Behav. Physiol. 2022, 8, 131–155. [Google Scholar] [CrossRef]
- Landová, E.; Polák, J.; Janovcová, M.; Štolhoferová, I.; Peterková, Š.; Chomik, A.; Frynta, D. Imprint of Ancestral and Modern Threats in Human Mind—Experience of Fear, Disgust, and Anger. Front. Psychol. 2025, 15, 1520224. [Google Scholar] [CrossRef]
- Peléšková, Š.; Polák, J.; Janovcová, M.; Chomik, A.; Sedláčková, K.; Frynta, D.; Landová, E. Human Emotional Evaluation of Ancestral and Modern Threats: Fear, Disgust, and Anger. Front. Psychol. 2024, 14, 1321053. [Google Scholar] [CrossRef]
- Bennett, K. Ancestral Threats vs. Modern Threats. In Encyclopedia of Evolutionary Psychological Science; Shackelford, T.K., Weekes-Shackelford, V.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–3. ISBN 978-3-319-16999-6. [Google Scholar]
- Schaller, M. The Behavioural Immune System and the Psychology of Human Sociality. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 3418–3426. [Google Scholar] [CrossRef]
- Curtis, V.; de Barra, M.; Aunger, R. Disgust as an Adaptive System for Disease Avoidance Behaviour. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 389–401. [Google Scholar] [CrossRef]
- Thiebaut, G.; Méot, A.; Witt, A.; Prokop, P.; Bonin, P. The Behavioral Immune System: How Does It Contribute to Our Understanding of Human Behavior? In Advances in Psychology Research; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2021; pp. 1–59. ISBN 978-1-5361-9542-2. [Google Scholar]
- Schaller, M.; Miller, G.E.; Gervais, W.M.; Yager, S.; Chen, E. Mere Visual Perception of Other People’s Disease Symptoms Facilitates a More Aggressive Immune Response. Psychol. Sci. 2010, 21, 649–652. [Google Scholar] [CrossRef]
- Giner-Sorolla, R.; Kupfer, T.; Sabo, J. Chapter Five—What Makes Moral Disgust Special? An Integrative Functional Review. In Advances in Experimental Social Psychology; Olson, J.M., Ed.; Academic Press: Cambridge, MA, USA; Oxford, UK, 2018; Volume 57, pp. 223–289. [Google Scholar]
- Ottaviani, C.; Mancini, F.; Petrocchi, N.; Medea, B.; Couyoumdjian, A. Autonomic Correlates of Physical and Moral Disgust. Int. J. Psychophysiol. 2013, 89, 57–62. [Google Scholar] [CrossRef]
- Armelagos, G.J.; Goodman, A.H.; Jacobs, K.H. The Origins of Agriculture: Population Growth during a Period of Declining Health. Popul. Environ. 1991, 13, 9–22. [Google Scholar] [CrossRef]
- Latham, K. Human Health and the Neolithic Revolution: An Overview of Impacts of the Agricultural Transition on Oral Health, Epidemiology, and the Human Body. Neb. Anthropol. 2013, 28, 95–102. [Google Scholar]
- Piret, J.; Boivin, G. Pandemics Throughout History. Front. Microbiol. 2021, 11, 631736. [Google Scholar] [CrossRef] [PubMed]
- Davey, G.C.L. Disgust. In Encyclopedia of Human Behavior; Ramachandran, V.S., Ed.; San Diego Press: San Diego, CA, USA, 1994; pp. 135–141. [Google Scholar]
- Rozin, P.; Fallon, A.E. A Perspective on Disgust. Psychol. Rev. 1987, 94, 23–41. [Google Scholar] [CrossRef]
- Prokop, P.; Fančovičová, J.; Šramelová, D.; Thiebaut, G.; Méot, A.; Bonin, P. Mouth Proximity Influences Perceived Disgust of Visual Stimuli. Pers. Individ. Dif. 2023, 207, 112146. [Google Scholar] [CrossRef]
- Ciesielski, B.G.; Armstrong, T.; Zald, D.H.; Olatunji, B.O. Emotion Modulation of Visual Attention: Categorical and Temporal Characteristics. PLoS ONE 2010, 5, e13860. [Google Scholar] [CrossRef]
- Armstrong, T.; Stewart, J.G.; Dalmaijer, E.S.; Rowe, M.; Danielson, S.; Engel, M.; Bailey, B.; Morris, M. I’ve Seen Enough! Prolonged and Repeated Exposure to Disgusting Stimuli Increases Oculomotor Avoidance. Emotion 2022, 22, 1368–1381. [Google Scholar] [CrossRef]
- Fančovičová, J.; Prokop, P.; Šramelová, D.; Thiebaut, G.; Méot, A.; Witt, A.; Bonin, P.; Medina-Jerez, W. Does Food Play a Prominent Role in Visual Attention to Disgusting Stimuli? J. Ethol. 2022, 40, 23–29. [Google Scholar] [CrossRef]
- Schlezingerová, N.; Málková, P.; Kocourek, M.; Telenský, P. Mild Hunger Elicits Attentional Desensitization to Visual Food Cues in Healthy, Non-Obese Individuals. Front. Psychol. 2024, 15, 1441184. [Google Scholar] [CrossRef]
- Gerdes, A.B.M.; Uhl, G.; Alpers, G.W. Spiders Are Special: Fear and Disgust Evoked by Pictures of Arthropods. Evol. Hum. Behav. 2009, 30, 66–73. [Google Scholar] [CrossRef]
- Polák, J.; Rádlová, S.; Janovcová, M.; Flegr, J.; Landová, E.; Frynta, D. Scary and Nasty Beasts: Self-Reported Fear and Disgust of Common Phobic Animals. Br. J. Psychol. 2020, 111, 297–321. [Google Scholar] [CrossRef]
- Arrindell, W.A. Phobic Dimensions: IV. The Structure of Animal Fears. Behav. Res. Ther. 2000, 38, 509–530. [Google Scholar] [CrossRef]
- Davey, G.C.L.; McDonald, A.S.; Hirisave, U.; Prabhu, G.G.; Iwawaki, S.; Jim, C.I.; Merckelbach, H.; de Jong, P.J.; Leung, P.W.L.; Reimann, B.C. A Cross-Cultural Study of Animal Fears. Behav. Res. Ther. 1998, 36, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Prokop, P.; Fančovičová, J. The Association between Disgust, Danger and Fear of Macroparasites and Human Behaviour. Acta Ethologica 2010, 13, 57–62. [Google Scholar] [CrossRef]
- Fox, E.; Griggs, L.; Mouchlianitis, E. The Detection of Fear-Relevant Stimuli: Are Guns Noticed as Quickly as Snakes? Emotion 2007, 7, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Flykt, A.; Esteves, F.; Öhman, A. Skin Conductance Responses to Masked Conditioned Stimuli: Phylogenetic/Ontogenetic Factors versus Direction of Threat? Biol. Psychol. 2007, 74, 328–336. [Google Scholar] [CrossRef]
- Hugdahl, K.; Johnsen, B.H. Preparedness and Electrodermal Fear-Conditioning: Ontogenetic vs Phylogenetic Explanations. Behav. Res. Ther. 1989, 27, 269–278. [Google Scholar] [CrossRef]
- Subra, B.; Muller, D.; Fourgassie, L.; Chauvin, A.; Alexopoulos, T. Of Guns and Snakes: Testing a Modern Threat Superiority Effect. Cogn. Emot. 2018, 32, 81–91. [Google Scholar] [CrossRef]
- Abado, E.; Aue, T.; Okon-Singer, H. Spider vs. Guns: Expectancy and Attention Biases to Phylogenetic Threat Do Not Extend to Ontogenetic Threat. Front. Psychol. 2023, 14, 1232985. [Google Scholar] [CrossRef]
- Sulikowski, D. Are Natural Threats Superior Threats? Evol. Hum. Behav. 2022, 43, 34–43. [Google Scholar] [CrossRef]
- Rozin, P.; Lowery, L.; Imada, S.; Haidt, J. The CAD Triad Hypothesis: A Mapping between Three Moral Emotions (Contempt, Anger, Disgust) and Three Moral Codes (Community, Autonomy, Divinity). J. Pers. Soc. Psychol. 1999, 76, 574–586. [Google Scholar] [CrossRef]
- Miceli, M.; Castelfranchi, C. Contempt and Disgust: The Emotions of Disrespect. J. Theor. Soc. Behav. 2018, 48, 205–229. [Google Scholar] [CrossRef]
- Davey, G.C.L.; Chapman, L. Disgust and Eating Disorder Symptomatology in a Non-Clinical Population: The Role of Trait Anxiety and Anxiety Sensitivity. Clin. Psychol. Psychother. 2009, 16, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.E. William James’s Theory of Emotion. Trans. Charles S Peirce Soc. 1969, 5, 67–89. [Google Scholar]
- Cannon, W.B. The James-Lange Theory of Emotions: A Critical Examination and an Alternative Theory. Am. J. Physiol. 1927, 39, 106–124. [Google Scholar] [CrossRef]
- Taschereau-Dumouchel, V.; Kawato, M.; Lau, H. Multivoxel Pattern Analysis Reveals Dissociations between Subjective Fear and Its Physiological Correlates. Mol. Psychiatry 2020, 25, 2342–2354. [Google Scholar] [CrossRef]
- Rohrmann, S.; Hopp, H.; Schienle, A.; Hodapp, V. Emotion Regulation, Disgust Sensitivity, and Psychophysiological Responses to a Disgust-Inducing Film. Anxiety Stress Coping 2009, 22, 215–236. [Google Scholar] [CrossRef]
- Tuvblad, C.; Gao, Y.; Isen, J.; Botwick, T.; Raine, A.; Baker, L.A. The Heritability of the Skin Conductance Orienting Response: A Longitudinal Twin Study. Biol. Psychol. 2012, 89, 47–53. [Google Scholar] [CrossRef]
- Knopf, K.; Pössel, P. Individual Response Differences in Spider Phobia: Comparing Phobic and Non-Phobic Women of Different Reactivity Levels. Anxiety Stress Coping 2009, 22, 39–55. [Google Scholar] [CrossRef]
- Wendt, J.; Lotze, M.; Weike, A.I.; Hosten, N.; Hamm, A.O. Brain Activation and Defensive Response Mobilization during Sustained Exposure to Phobia-Related and Other Affective Pictures in Spider Phobia. Psychophysiology 2008, 45, 205–215. [Google Scholar] [CrossRef]
- Schaefer, H.S.; Larson, C.L.; Davidson, R.J.; Coan, J.A. Brain, Body, and Cognition: Neural, Physiological and Self-Report Correlates of Phobic and Normative Fear. Biol. Psychol. 2014, 98, 59–69. [Google Scholar] [CrossRef]
- Serrano, M.Á.; Rosell-Clari, V.; García-Soriano, G. The Role of Perceived Control in the Psychophysiological Responses to Disgust of Subclinical OCD Women. Sensors 2019, 19, 4180. [Google Scholar] [CrossRef]
- Whitton, A.E.; Henry, J.D.; Grisham, J.R. Cognitive and Psychophysiological Correlates of Disgust in Obsessive-Compulsive Disorder. Br. J. Clin. Psychol. 2015, 54, 16–33. [Google Scholar] [CrossRef]
- Pruneti, C.; Coscioni, G.; Guidotti, S. A Systematic Review of Clinical Psychophysiology of Obsessive–Compulsive Disorders: Does the Obsession with Diet Also Alter the Autonomic Imbalance of Orthorexic Patients? Nutrients 2023, 15, 755. [Google Scholar] [CrossRef]
- Štolhoferová, I.; Hladíková, T.; Janovcová, M.; Peléšková, Š.; Frynta, D.; Landová, E. Subjective and Psychophysiological Response to Pictures of Ancestral and Modern Threats: Not All Evolutionary Threats Are Alike. PLoS ONE 2025. submitted. [Google Scholar]
- Kopacz II, F.M.; Smith, B.D. Sex Differences in Skin Conductance Measures as a Function of Shock Threat. Psychophysiology 1971, 8, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Hopp, H.; Quirin, M. Gender Differences in Psychophysiological Responses to Disgust. J. Psychophysiol. 2008, 22, 65–75. [Google Scholar] [CrossRef]
- Bari, D.S. Gender Differences in Tonic and Phasic Electrodermal Activity Components. Sci. J. Univ. Zakho 2020, 8, 29–33. [Google Scholar] [CrossRef]
- Kelly, M.M.; Forsyth, J.P.; Karekla, M. Sex Differences in Response to a Panicogenic Challenge Procedure: An Experimental Evaluation of Panic Vulnerability in a Non-Clinical Sample. Behav. Res. Ther. 2006, 44, 1421–1430. [Google Scholar] [CrossRef]
- Wrase, J.; Klein, S.; Gruesser, S.M.; Hermann, D.; Flor, H.; Mann, K.; Braus, D.F.; Heinz, A. Gender Differences in the Processing of Standardized Emotional Visual Stimuli in Humans: A Functional Magnetic Resonance Imaging Study. Neurosci. Lett. 2003, 348, 41–45. [Google Scholar] [CrossRef]
- Druschel, B.A.; Sherman, M.F. Disgust Sensitivity as a Function of the Big Five and Gender. Pers. Individ. Dif. 1999, 26, 739–748. [Google Scholar] [CrossRef]
- Haidt, J.; McCauley, C.; Rozin, P. Individual Differences in Sensitivity to Disgust: A Scale Sampling Seven Domains of Disgust Elicitors. Pers. Individ. Dif. 1994, 16, 701–713. [Google Scholar] [CrossRef]
- Heretik, A.; Ritomský, A.; Novotný, V.; Pečeňák, J. Restandardizace state-trait anxiety inventory x-2—Úzkostnost jako rys—Proquest. Českoslov. psychol. 2009, 53, 587–599. [Google Scholar]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E. Manual for the State-Trait Anxiety Inventory (Self-Evaluation Questionnaire); Consulting Psychologists Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Polák, J.; Landová, E.; Frynta, D. Undisguised Disgust: A Psychometric Evaluation of a Disgust Propensity Measure. Curr. Psychol. 2019, 38, 608–617. [Google Scholar] [CrossRef]
- Haberkamp, A.; Glombiewski, J.A.; Schmidt, F.; Barke, A. The DIsgust-RelaTed-Images (DIRTI) Database: Validation of a Novel Standardized Set of Disgust Pictures. Behav. Res. Ther. 2017, 89, 86–94. [Google Scholar] [CrossRef]
- Kurdi, B.; Lozano, S.; Banaji, M.R. Introducing the Open Affective Standardized Image Set (OASIS). Behav. Res. 2017, 49, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Mikels, J.A.; Fredrickson, B.L.; Larkin, G.R.; Lindberg, C.M.; Maglio, S.J.; Reuter-Lorenz, P.A. Emotional Category Data on Images from the International Affective Picture System. Behav. Res. Methods 2005, 37, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Crone, D.L.; Bode, S.; Murawski, C.; Laham, S.M. The Socio-Moral Image Database (SMID): A Novel Stimulus Set for the Study of Social, Moral and Affective Processes. PLoS ONE 2018, 13, e0190954. [Google Scholar] [CrossRef]
- Kašpar, J.; Hon, Z.; Janatová, M.; Smrčka, P.; Vítězník, M.; Hána, K. RESDB. Available online: https://isdv.upv.gov.cz/webapp/resdb.print_detail.det?pspis=PT/2014-979 (accessed on 27 July 2024).
- Landová, E.; Peléšková, Š.; Sedláčková, K.; Janovcová, M.; Polák, J.; Rádlová, S.; Vobrubová, B.; Frynta, D. Venomous Snakes Elicit Stronger Fear than Nonvenomous Ones: Psychophysiological Response to Snake Images. PLoS ONE 2020, 15, e0236999. [Google Scholar] [CrossRef] [PubMed]
- Kosonogov, V. The Effects of the Order of Picture Presentation on the Subjective Emotional Evaluation of Pictures. Psicologia 2020, 34, 171–178. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
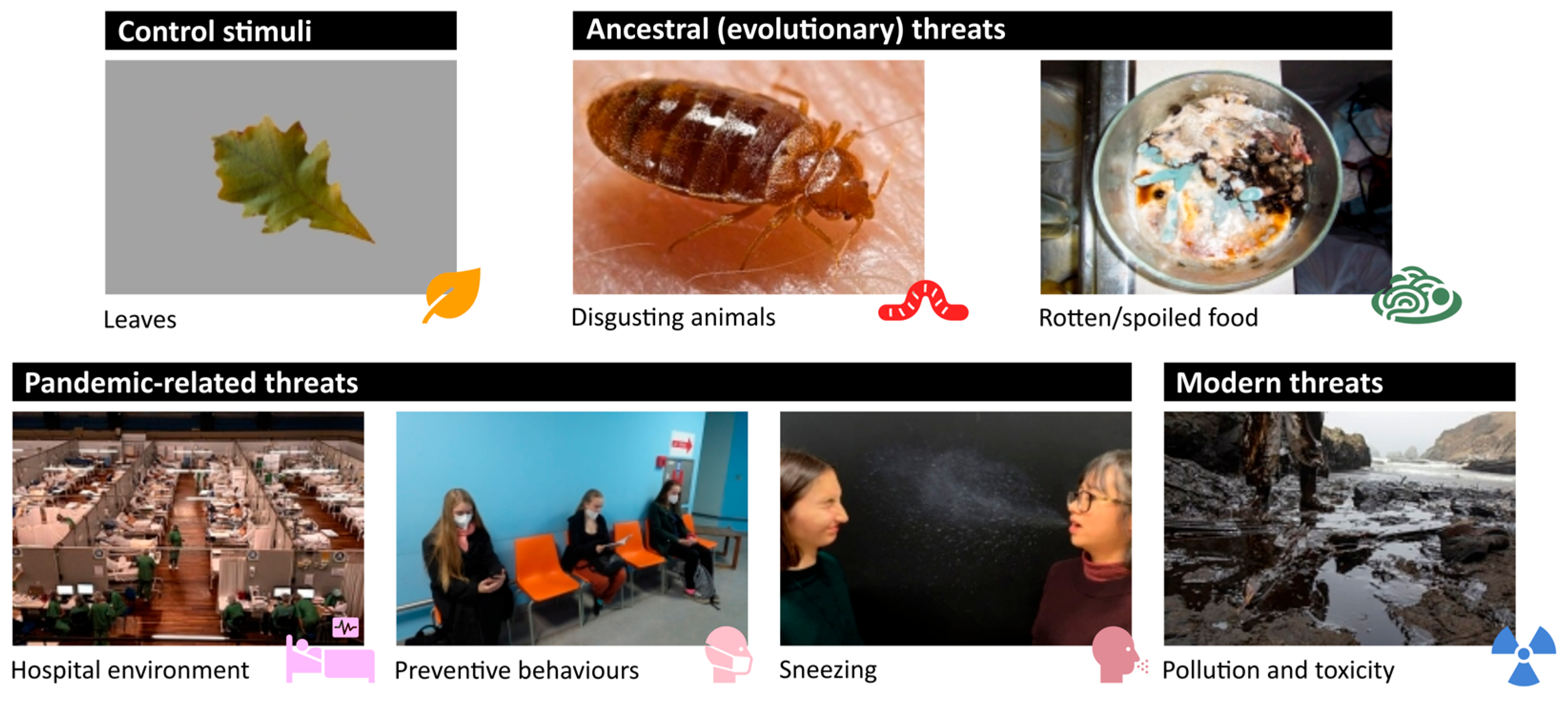

| Category | Mean, 95% CI (Logit Scale) | Mean, 95% CI (Probability Scale) | z-Value | p-Value |
|---|---|---|---|---|
| Control | −0.814 (−1.123, −0.504) | 0.31 (0.24, 0.38) | – | – |
| “Disgusting” animals | −0.504 (−0.881, −0.126) | 0.38 (0.29, 0.47) | 2.39 | 0.017 |
| Spoiled food | −1.062 (−1.459, −0.664) | 0.26 (0.19, 0.34) | −1.69 | 0.092 |
| Hospital environment | −0.638 (−1.071, −0.204) | 0.35 (0.26, 0.45) | 1.04 | 0.299 |
| Preventive behaviours | −0.920 (−1.376, −0.464) | 0.29 (0.20, 0.39) | −0.58 | 0.565 |
| Sneezing | 0.070 (−0.700, 0.840) | 0.52 (0.33, 0.70) | 2.41 | 0.016 |
| Toxicity and pollution | −1.040 (−1.421, −0.660) | 0.26 (0.19, 0.34) | −1.72 | 0.085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hladíková, T.; Štolhoferová, I.; Frynta, D.; Landová, E. Emotional Salience of Evolutionary and Modern Disgust-Relevant Threats Measured Through Electrodermal Activity. Physiologia 2025, 5, 41. https://doi.org/10.3390/physiologia5040041
Hladíková T, Štolhoferová I, Frynta D, Landová E. Emotional Salience of Evolutionary and Modern Disgust-Relevant Threats Measured Through Electrodermal Activity. Physiologia. 2025; 5(4):41. https://doi.org/10.3390/physiologia5040041
Chicago/Turabian StyleHladíková, Tereza, Iveta Štolhoferová, Daniel Frynta, and Eva Landová. 2025. "Emotional Salience of Evolutionary and Modern Disgust-Relevant Threats Measured Through Electrodermal Activity" Physiologia 5, no. 4: 41. https://doi.org/10.3390/physiologia5040041
APA StyleHladíková, T., Štolhoferová, I., Frynta, D., & Landová, E. (2025). Emotional Salience of Evolutionary and Modern Disgust-Relevant Threats Measured Through Electrodermal Activity. Physiologia, 5(4), 41. https://doi.org/10.3390/physiologia5040041






