Objective Evaluation of Fatigue-Associated Facial Expressions Using Measurements of Eye-Opening Degree, Motion Capture, and Heart Rate Variability Spectrum Analysis
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Experiment Overview
- (1)
- The participants applied an appropriate amount of makeup remover to their hands, lathered it with lukewarm water, spread it over their faces to remove makeup, washed their faces, and rinsed thoroughly.
- (2)
- The participants completed a questionnaire and then rested for 20 min to acclimate to the temperature and humidity conditions in the environment-control room (room temperature: 24.0 °C; humidity: 50.0% RH).
- (3)
- A digital single-lens reflex camera was used to photograph the participants’ relaxed, full face (straight facial expression, neutral), from which the degree of eye-opening was calculated based on the images (the details are described in Section 2.3.3 “Measurement of the Degree of Eye-Opening”).
- (4)
- An electrocardiogram (ECG) electrode was placed at the V5 chest lead, and an ECG was recorded while the participants were at rest. After data acquisition, power spectrum analysis of heart rate variability was performed offline to assess autonomic nervous activity.
- (5)
- A three-dimensional facial imaging device captured participants’ relaxed, neutral facial expressions. Subsequently, a high-speed camera recorded motion during the intentional smiling process. In the offline analysis, a facial wireframe was aligned with the neutral facial expression image, and markers were placed on the facial surface according to representative grid points. The maximum movement distance (MMD) and speed of these markers were calculated from the motion-captured images and analyzed as indicators of fatigue (the details are described in Section 2.4 “Dynamics of intentional facial expressions”).
2.3. Subjective Assessment of Fatigue
2.3.1. Fatigue Questionnaire
2.3.2. Visual Analog Scale Method
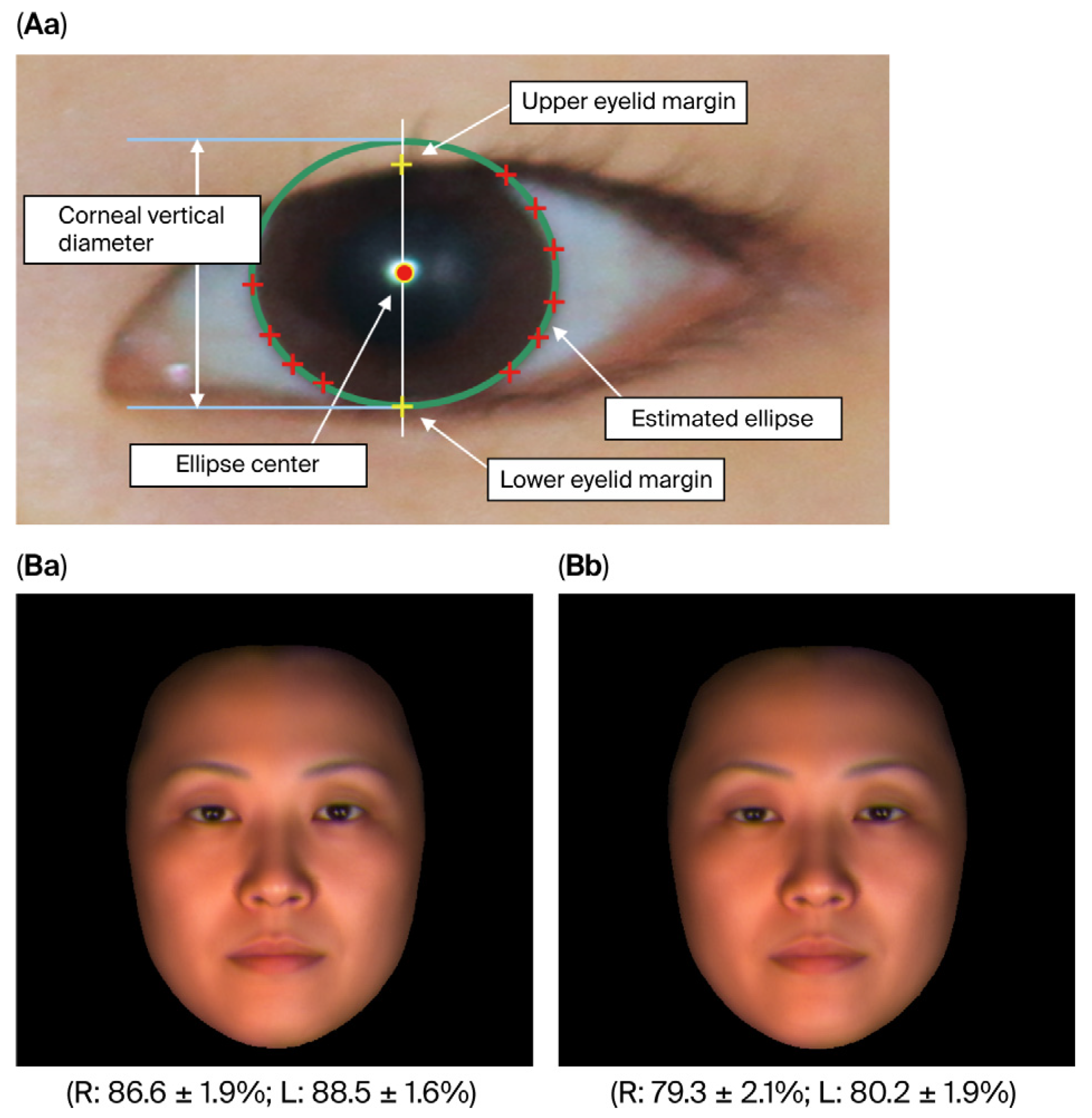
2.3.3. Measurement of the Degree of Eye-Opening
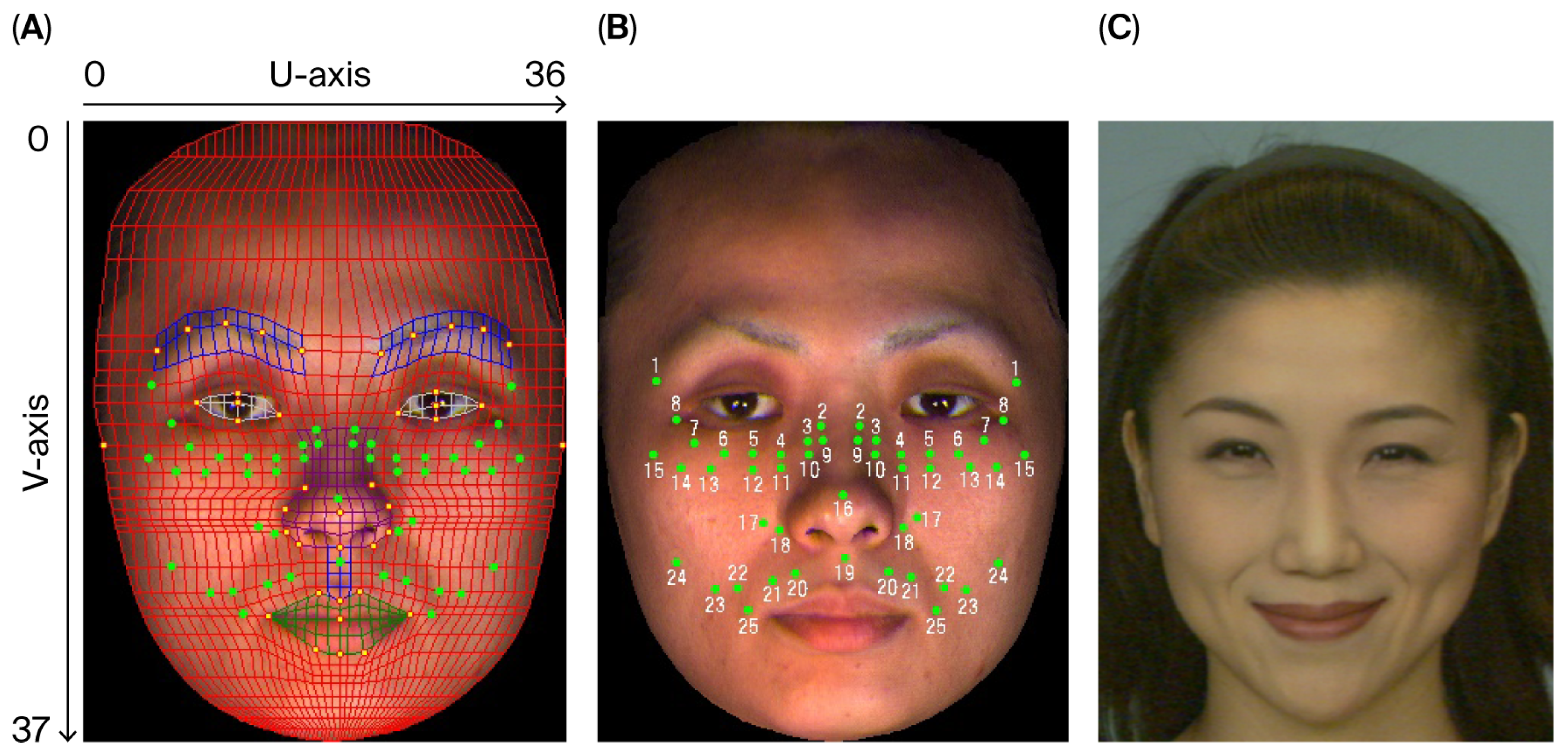
2.4. Dynamics of Intentional Facial Expressions
2.4.1. Experimental Equipment
2.4.2. Marker Placement
2.4.3. Facial Expression Conditions
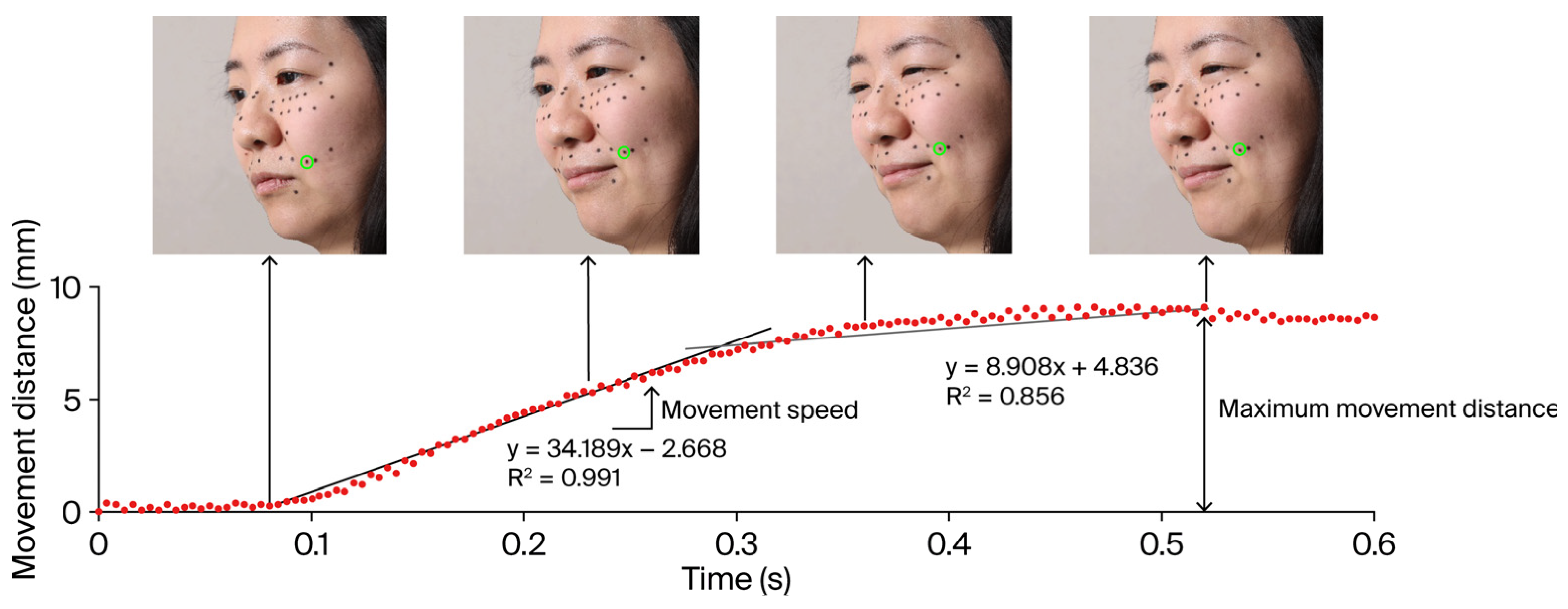
2.4.4. Analytical Methods
2.4.5. Accuracy of the Motion Capture Method
2.5. ECG R-R Interval Variability Spectrum Analysis
2.6. Statistical Analysis
3. Results
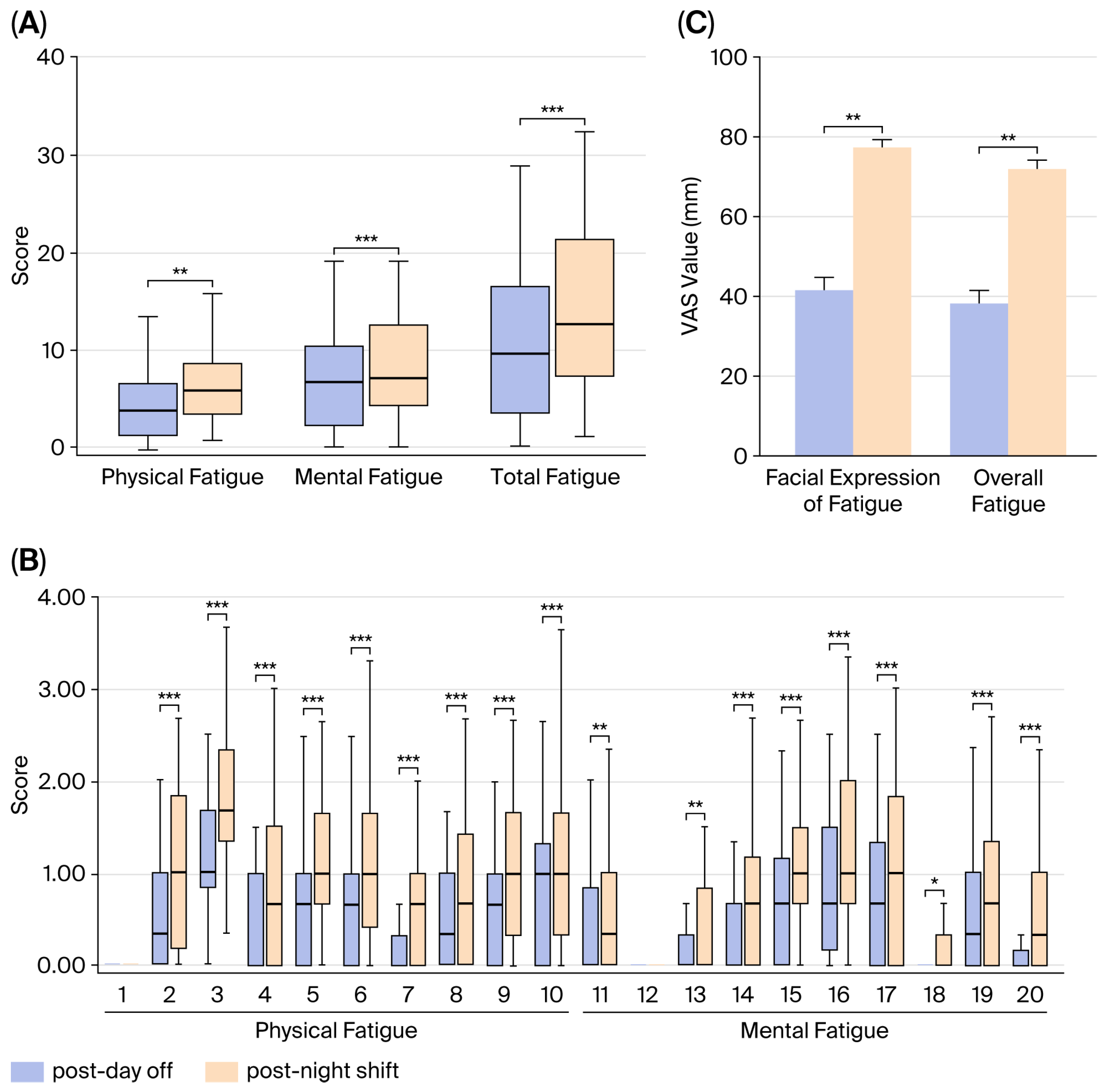
3.1. Evaluation of the Degree of Fatigue
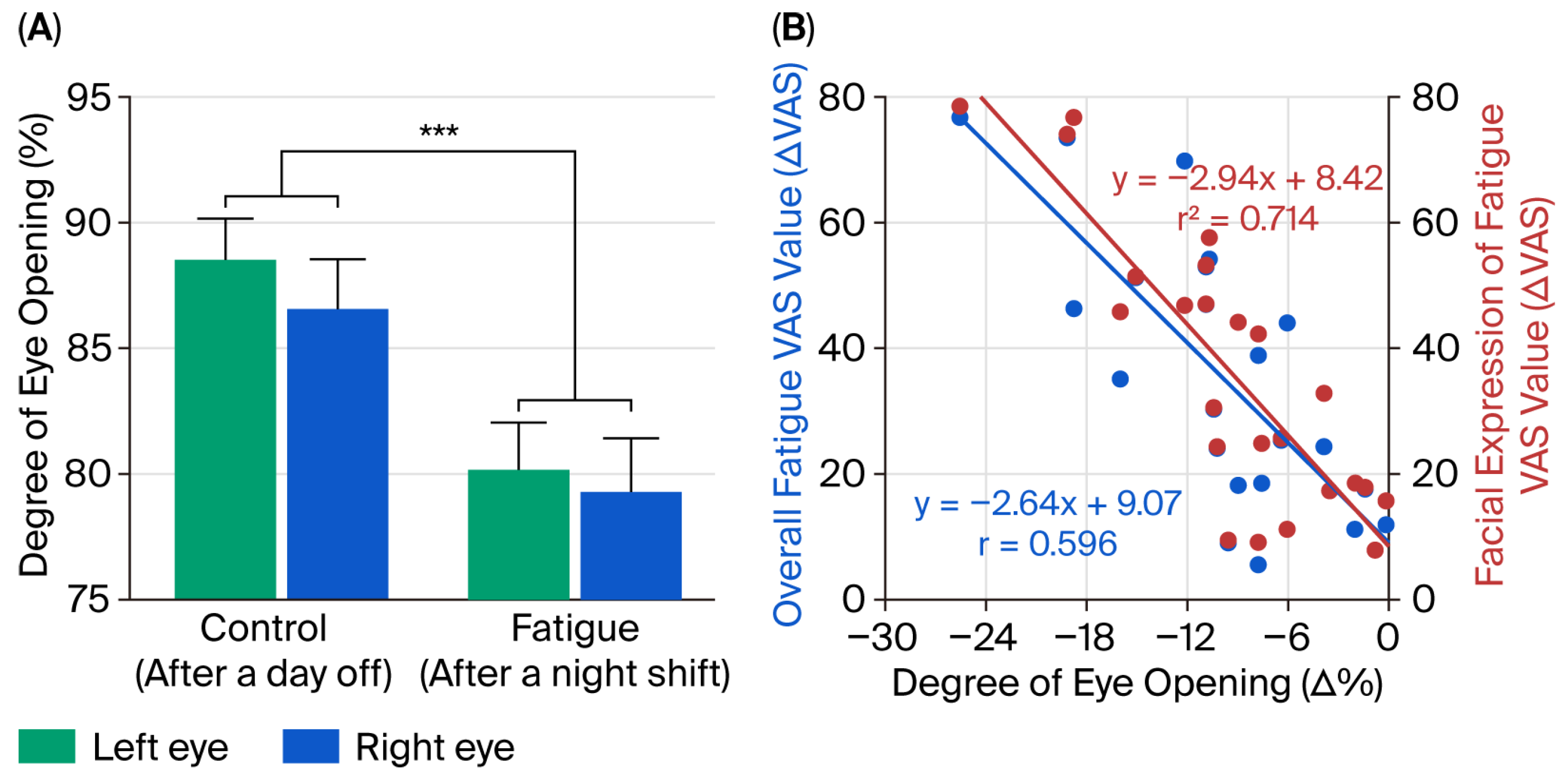
3.2. Comparison of Degree of Eye-Opening
3.3. Relationship Between the Degree of Eye-Opening and Subjective Assessment of Fatigue
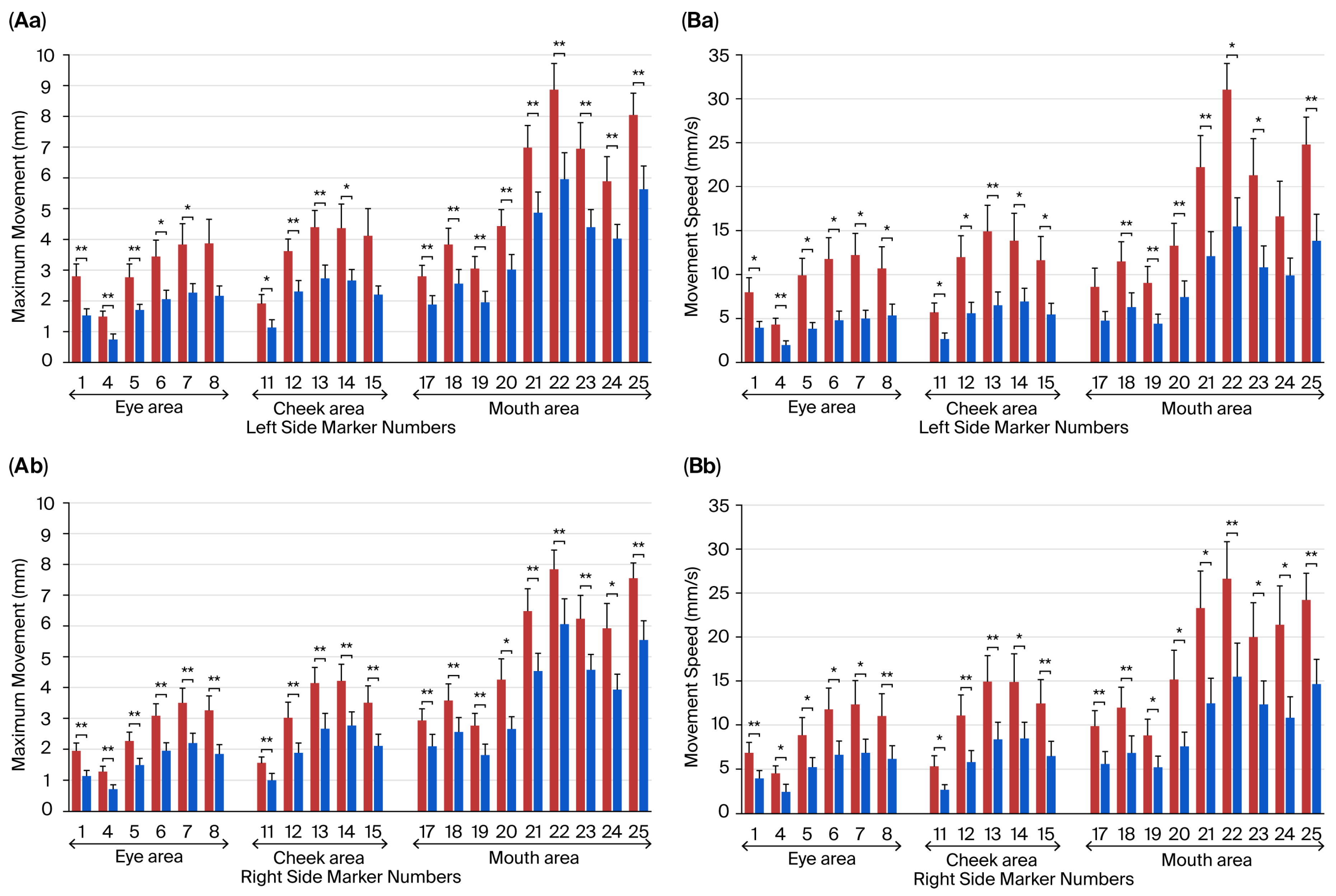
3.4. Effects of the Fatigue State on Movements of Facial Parts During Intentional Smiling
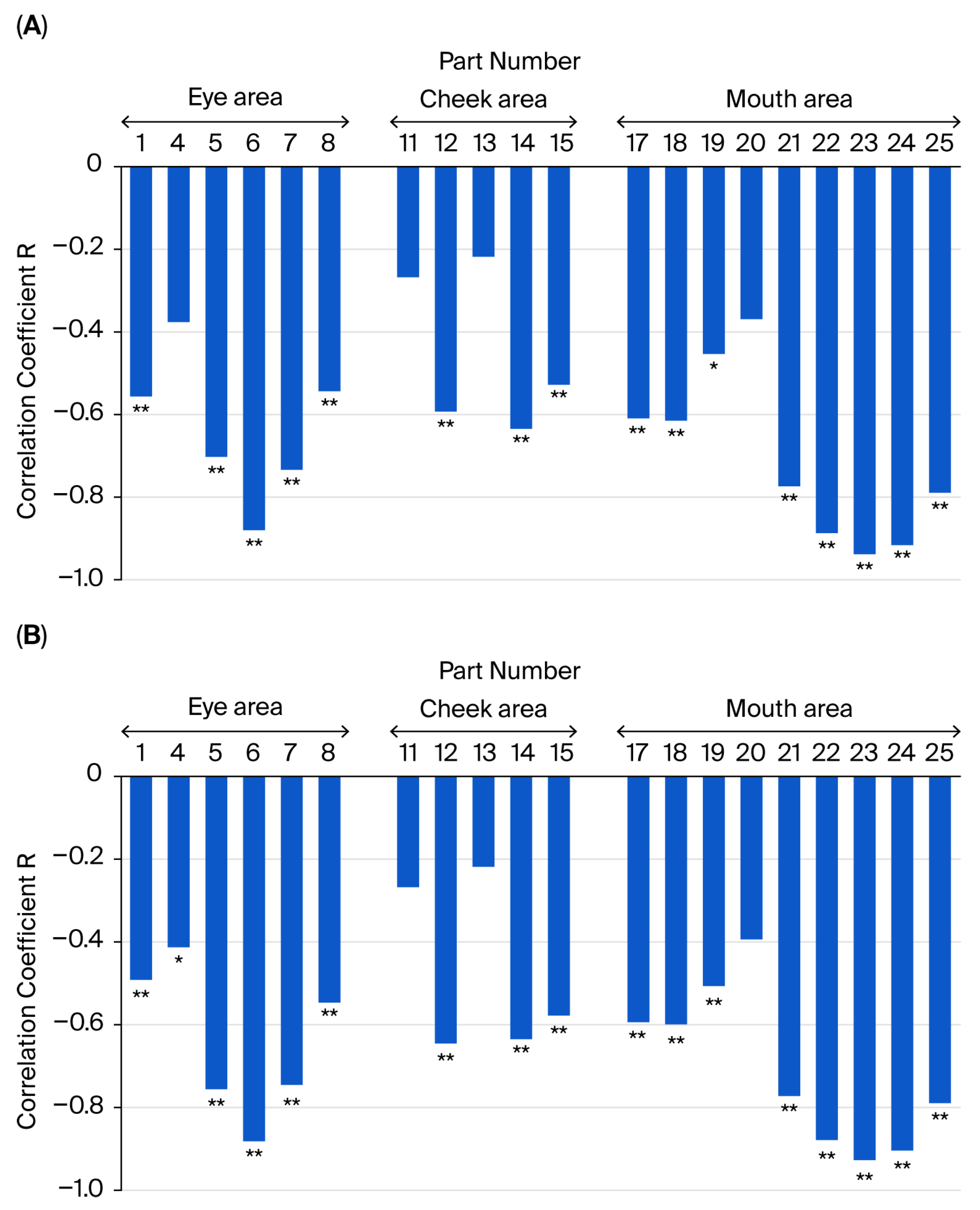
3.5. Relationship Between the Subjective Assessment of Fatigue and Facial Movement Distance and Speed During Intentional Smiling
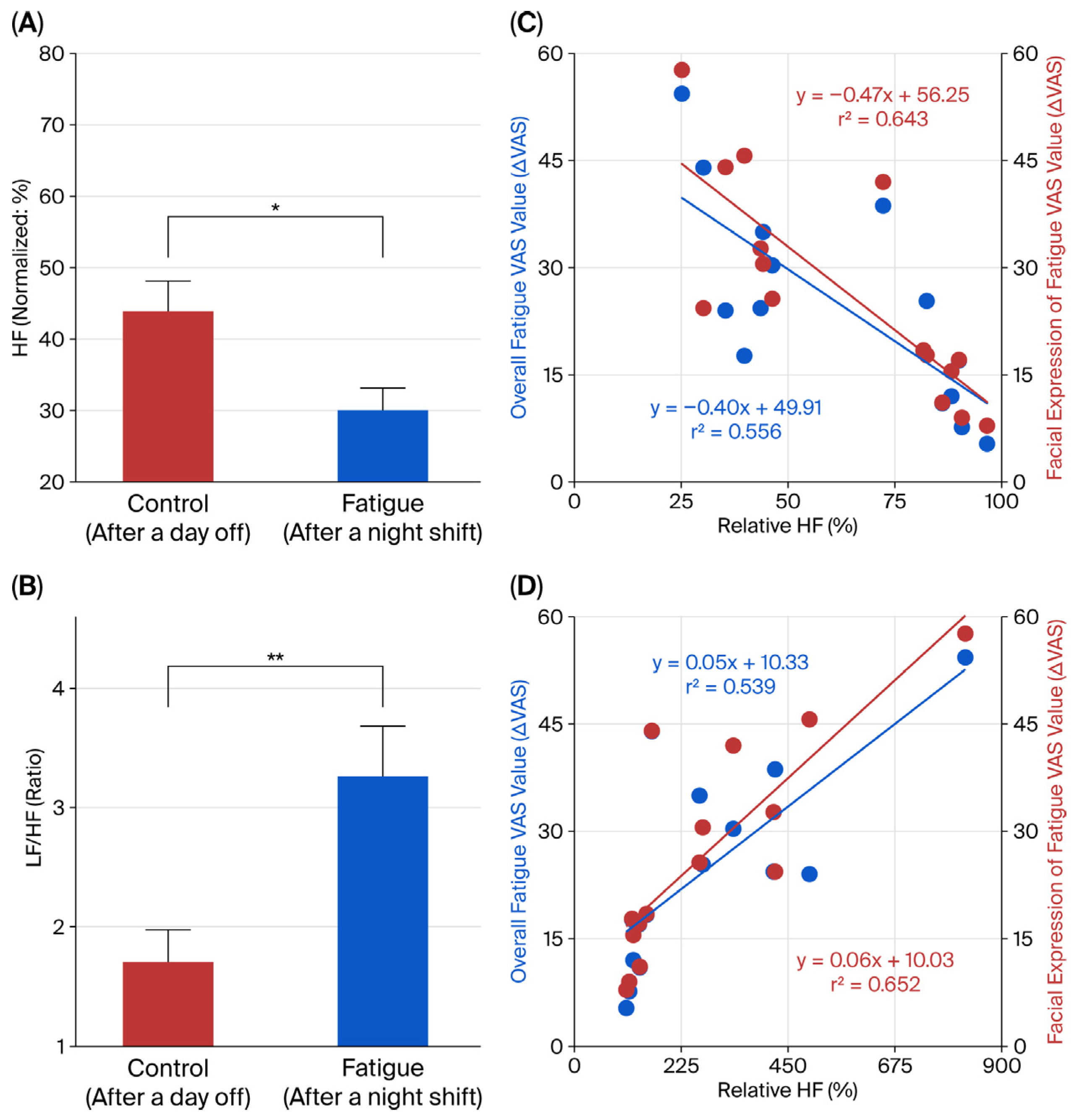
3.6. Evaluation of Autonomic Nervous Activity and Its Relationship with Fatigue State
4. Discussion
4.1. Subjective and Objective Assessment of Fatigue
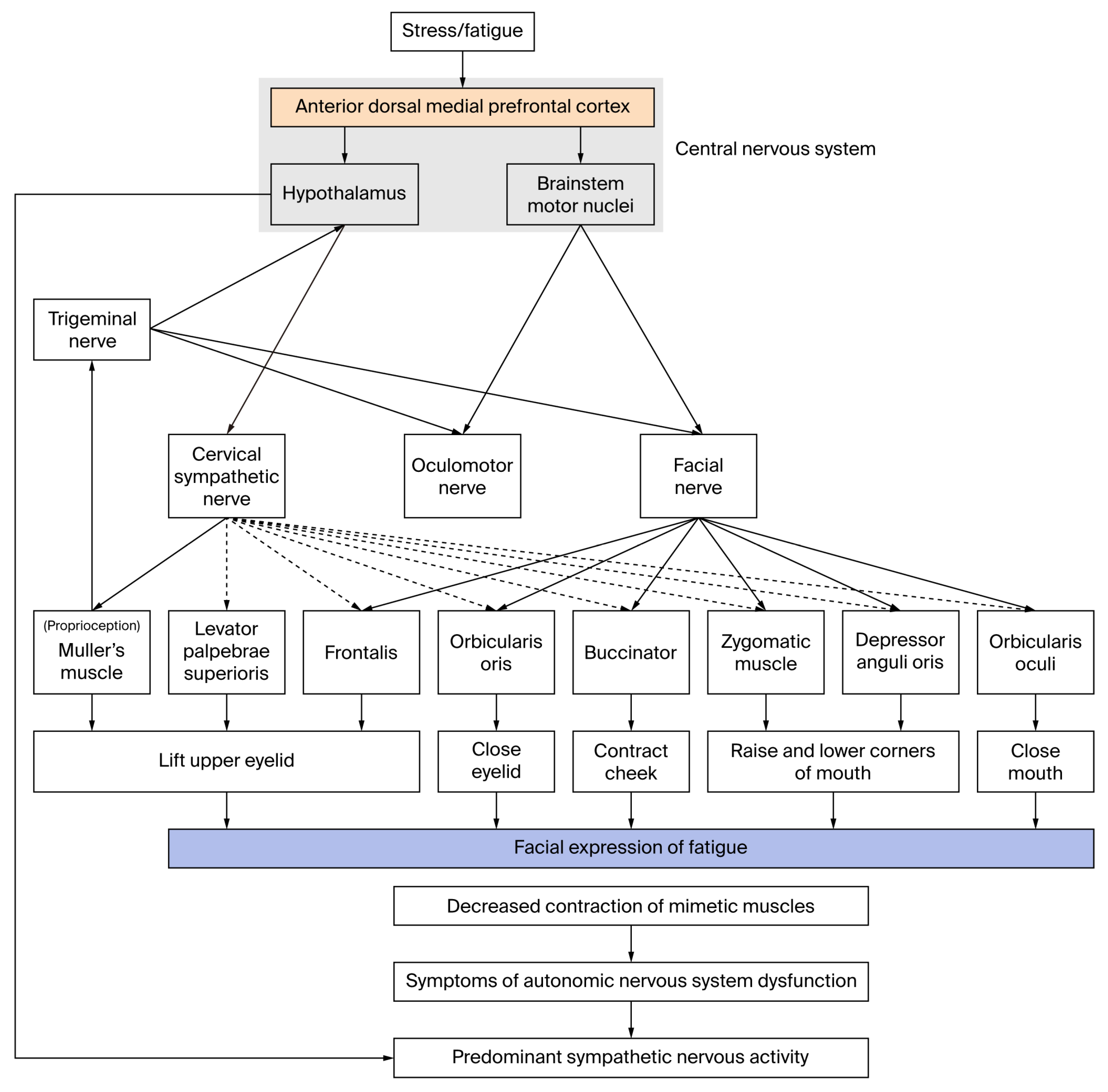
4.2. Physiological Mechanisms of Facial Expressions of Fatigue
4.3. Applications to Cosmetology and Medicine
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| CPG | Central Pattern Generator |
| CFS | Chronic Fatigue Syndrome |
| ECG | Electrocardiogram |
| EMG | Electromyography |
| HF | High Frequency (component of heart rate variability) |
| IBM | International Business Machines |
| LF | Low Frequency (component of heart rate variability) |
| LF/HF | Low Frequency-to-High Frequency Ratio |
| MF | Mental Fatigue |
| MMD | Maximum Movement Distance |
| MS | Movement Speed |
| PF | Physical Fatigue |
| RMS | Root Mean Square |
| SD | Standard Deviation |
| SEM | Standard Error of the Mean |
| TF | Total Fatigue |
| VAS | Visual Analog Scale |
References
- Suzuki, A.; Akamatsu, R. Sex differences in relationship between stress responses and lifestyle in Japanese workers. Saf. Health Work 2014, 5, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N. Mental health of working mothers. Kosodate wo shinagara hataraku jyosei no sutoresu to mentaruherusu. Jpn. J. Occup. Ment. Health 2023, 31, 21–24. (In Japanese) [Google Scholar] [CrossRef]
- Rosendal, M.; Olesen, F.; Fink, P. Management of medically unexplained symptoms. BMJ 2005, 330, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Gender Equality Bureau Cabinet Office. Available online: https://www.gender.go.jp/research/kenkyu/health_ishiki.html (accessed on 2 May 2024).
- Veldhuizen, I.J.; Gaillard, A.W.; de Vries, J. The influence of mental fatigue on facial EMG activity during a simulated workday. Biol. Psychol. 2003, 63, 59–78. [Google Scholar] [CrossRef]
- Iida, T.; Ito, Y.; Kanazashi, M.; Murayama, S.; Miyake, T.; Yoshimaru, Y.; Tatsumi, A.; Ezoe, S. Effects of psychological and physical stress on oxidative stress, serotonin, and fatigue in young females induced by objective structured clinical examination: Pilot study of u-8-OHdG, u-5HT, and s-HHV-6. Int. J. Tryptophan Res. 2021, 14, 11786469211048443. [Google Scholar] [CrossRef]
- Vercoulen, J.H.; Swanink, C.M.; Fennis, J.F.; Galama, J.M.; van der Meer, J.W.; Bleijenberg, G. Dimensional assessment of chronic fatigue syndrome. J. Psychosom. Res. 1994, 38, 383–392. [Google Scholar] [CrossRef]
- Schaufeli, W.B.; Leiter, M.P.; Maslach, C.; Jackson, S.E. Maslach Burnout Inventory—General survey. In Maslach Burnout Inventory Manual, 3rd ed.; Consulting Psychologists Press: Palo Alto, CA, USA, 1996; pp. 19–26. [Google Scholar]
- Smets, E.M.A.; Garssen, B.; Bonke, B.; De Haes, J.C.J.M. The multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995, 39, 315–325. [Google Scholar] [CrossRef]
- Chalder, T.; Berelowitz, G.; Pawlikowska, T.; Watts, L.; Wessely, S.; Wright, D.; Wallace, E.P. Development of a fatigue scale. J. Psychosom. Res. 1993, 37, 147–153. [Google Scholar] [CrossRef]
- Alberts, M.; Smets, E.M.A.; Vercoulen, J.H.M.M.; Garssen, B.; Bleijenberg, G. Verkorte vermoeidheidsvragenlijst: Een praktisch hulpmiddel bij het scoren van vermoeidheid [Abbreviated fatigue questionnaire’: A practical tool in the classification of fatigue]. Ned. Tijdschr. Geneeskd. 1997, 141, 1526–1530. (In German) [Google Scholar]
- Słomko, J.; Estévez-López, F.; Kujawski, S.; Zawadka-Kunikowska, M.; Tafil-Klawe, M.; Klawe, J.J.; Morten, K.J.; Szrajda, J.; Murovska, M.; Newton, J.L.; et al. Autonomic phenotypes in chronic fatigue syndrome (CFS) are associated with illness severity: A cluster analysis. J. Clin. Med. 2020, 9, 2531. [Google Scholar] [CrossRef]
- Jason, L.A.; McGarrigle, W.J.; Vermeulen, R.C.W. The head-up tilt table test as a measure of autonomic functioning among patients with myalgic encephalomyelitis/chronic fatigue syndrome. J. Pers. Med. 2024, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Nojima, J.; Motoki, Y.; Tsuneoka, H.; Kuratsune, H.; Matsui, T.; Yamamoto, M.; Yanagihara, M.; Hinoda, Y.; Ichihara, K. “Oxidation stress index” as a possible clinical marker for the evaluation of non-Hodgkin lymphoma. Br. J. Haematol. 2011, 155, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Sashihara, J.; Shimada, K.; Takemoto, M.; Amo, K.; Miyagawa, H.; Yamanishi, K. Recognition of a novel stage of betaherpesvirus latency in human herpesvirus 6. J. Virol. 2003, 77, 2258–2264. [Google Scholar] [CrossRef]
- Van Boxtel, A.; Jessurun, M. Amplitude and bilateral coherency of facial and jaw-elevator EMG activity as an index of effort during a two-choice serial reaction task. Psychophysiology 1993, 30, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Burton, C. Beyond somatisation: A review of the understanding and treatment of medically unexplained physical symptoms (MUPS). Br. J. Gen. Pract. 2003, 53, 231–239. [Google Scholar]
- Saito, T. Contested illness to solve the social issues in modern medicine: Beyond mind-body dualism. Mitome rare nu yamai to gendaiiryo no shakaiteki kadai: Shinshin ni genron wo koete. Clin. Eval. 2016, 44, 381–386. (In Japanese) [Google Scholar]
- Fujimura, T. The functional role of facial expressions in communication. Jpn. J. Physiol. Psychol. Psychophysiol. 2017, 35, 3–13. (In Japanese) [Google Scholar] [CrossRef]
- Sato, W.; Hyniewska, S.; Minemoto, K.; Yoshikawa, S. Facial expressions of basic emotions in Japanese laypeople. Front. Psychol. 2019, 10, 259. [Google Scholar] [CrossRef]
- Suzuki, A. Emotion elicitation using facial expression images: A perspective from research on facial expression recognition and person perception. Kao hyoujyou gazou ni yoru kanjyou kanki: Kao hyoujyou (taijin) ninchi kenkyuu kara no shiten. Jpn. J. Res. Emot. 2022, 29, 58–63. (In Japanese) [Google Scholar]
- Arai, S. Skin care products for skin darkness or dullness. Kusumi to kiso keshouhin. Jpn. J. Cosmetol. 1994, 18, 149–153. (In Japanese) [Google Scholar]
- Suzuki, M.; Nagashima, Y.; Yada, Y.; Morishima, S.; Yamada, H. Fatigue and an expression of women belong to the same generation. Dou sedai jyosei no hirou kan to hyoujyou no kankei. J. Facial. Stud. 2009, 9, 245. (In Japanese) [Google Scholar]
- Ohjimi, H. Mechanisms of facial aging. Youbou rouka no mekanizumu. J. Anti Aging Med. 2014, 10, 45–52. (In Japanese) [Google Scholar]
- Flament, F.; Pierre, J.; Delhommeau, K.; Adam, A.S. How a working day-induced- tiredness may alter some facial signs in differently-aged Caucasian women. Int. J. Cosmet. Sci. 2017, 39, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Flament, F.; Qiu, H.; Abric, A.; Charbonneau, A. Assessing changes in some facial signs of fatigue in Chinese women, induced by a single working day. Int. J. Cosmet. Sci. 2019, 41, 21–27. [Google Scholar] [CrossRef]
- Kuratsune, H.; Watanabe, Y. Heidelberg chronic fatigue syndrome. In Fatigue Science for Human Health; Watanabe, Y., Evengård, B., Natelson, B.H., Jason, L.A., Kuratsune, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 67–88. [Google Scholar]
- Kuratsune, H.; Kuratsune, K. Industrial fatigue screening. In The Latest Science of Fatigue-from Japan: Proposals for Anti-Fatigue and Anti-Overwork. Sangyou Hirou Tokutei Kenshin; Watanabe, Y., Ed.; Ishiyaku Publishers: Tokyo, Japan, 2010; pp. 148–153. (In Japanese) [Google Scholar]
- Bron, A.J.; Tripathi, R.C.; Tripathi, B.J. The eyeball and its dimensions. In Wolff’s Anatomy of the Eye and Orbit; Chapman & Hall Medical: London, UK, 1997; pp. 211–232. [Google Scholar]
- Dawson, D.G.; Ubels, J.L.; Edelhauser, H.F. Cornea and sclera. In Adler’s Physiology of the Eye; Levin, L.A., Nilsson, S.F.E., Ver Hoeve, J.J., Wu, S., Eds.; Elsevier: Amsterdam, The Netherlands; Saunders: Philadelphia, PA, USA, 2011; pp. 71–130. [Google Scholar]
- Takagi, S.; Ohjimi, H.; Tan, J.; Eto, A. Factors that influence the postoperative upper eyelid position following surgery for involutional blepharoptosis. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 278–285. [Google Scholar] [CrossRef]
- Miyashita, W.; Nakahara, R. Three-dimensional average faces in Japanese with normal occlusion-evaluation of construction method and reproducibility. Nihonjin seijyou kougousha no sanjigenteki heikingao Sakusei houhou oyobi saigensei no kenshou. Orthod. Waves. 2005, 64, 36–43. (In Japanese) [Google Scholar]
- Akselrod, S.; Gordon, D.; Ubel, F.A.; Shannon, D.C.; Berger, A.C.; Cohen, R.J. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science 1981, 213, 220–222. [Google Scholar] [CrossRef]
- Pagani, M.; Lombardi, F.; Guzzetti, S.; Rimoldi, O.; Furlan, R.; Pizzinelli, P.; Sandrone, G.; Malfatto, G.; Dell’Orto, S.; Piccaluga, E. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ. Res. 1986, 59, 178–193. [Google Scholar] [CrossRef]
- Berger, R.D.; Saul, J.P.; Cohen, R.J. Transfer function analysis of autonomic regulation. I. Canine atrial rate response. Am. J. Physiol. 1989, 256, H142–H152. [Google Scholar] [CrossRef]
- Saul, J.P.; Berger, R.D.; Albrecht, P.; Stein, S.P.; Chen, M.H.; Cohen, R.J. Transfer function analysis of the circulation: Unique insights into cardiovascular regulation. Am. J. Physiol. 1991, 261, H1231–H1245. [Google Scholar] [CrossRef]
- Freeman, K.; Smyth, C.; Dallam, L.; Jackson, B. Pain measurement scales: A comparison of the visual analogue and faces rating scales in measuring pressure ulcer pain. J. Wound Ostomy Cont. Nurs. 2001, 28, 290–296. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Ishii, N. The relationship between self-awareness of fatigue symptoms and working conditions in female nurses. Jyosei kangoshi no hirou no jikaku shoujyou to kinmu no kankei. Sangyo Eiseigaku Zasshi 2015, 57, 230–240. (In Japanese) [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Labour and Welfare Science Research Results Database. Available online: https://mhlw-grants.niph.go.jp/project/19790 (accessed on 30 August 2024).
- Takamoto, K.; Hori, E.; Urakawa, S.; Katayama, M.; Nagashima, Y.; Yada, Y.; Ono, T.; Nishijo, H. Thermotherapy to the facial region in and around the eyelids altered prefrontal hemodynamic responses and autonomic nervous activity during mental arithmetic. Psychophysiology 2013, 50, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Wyller, V.B.; Saul, J.P.; Amlie, J.P.; Thaulow, E. Sympathetic predominance of cardiovascular regulation during mild orthostatic stress in adolescents with chronic fatigue. Clin. Physiol. Funct. Imaging 2007, 27, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.R.; Rahman, K.; Kadota, Y.; Lloyd, A.; Vollmer-Conna, U. Reduced heart rate variability predicts poor sleep quality in a case–control study of chronic fatigue syndrome. Exp. Brain Res. 2010, 204, 71–78. [Google Scholar] [CrossRef]
- Tanaka, M.; Mizuno, K.; Yamaguti, K.; Kuratsune, H.; Fujii, A.; Baba, H.; Matsuda, K.; Nishimae, A.; Takesaka, T.; Watanabe, Y. Autonomic nervous alterations associated with daily level of fatigue. Behav. Brain Funct. 2011, 7, 46. [Google Scholar] [CrossRef]
- Yasui, H.; Takamoto, K.; Hori, E.; Urakawa, S.; Nagashima, Y.; Yada, Y.; Ono, T.; Nishijo, H. Significant correlation between autonomic nervous activity and cerebral hemodynamics during thermotherapy on the neck. Auton. Neurosci. 2010, 156, 96–103. [Google Scholar] [CrossRef]
- Passatore, M.; Grassi, C.; Filippi, G.M. Sympathetically-induced development of tension in jaw muscles: The possible contraction of intrafusal muscle fibres. Pflugers Arch. 1985, 405, 297–304. [Google Scholar] [CrossRef]
- Yuzuriha, S.; Matsuo, K.; Hirasawa, C.; Moriizumi, T. Refined distribution of myelinated trigeminal proprioceptive nerve fibres in Mueller’s muscle as the mechanoreceptors to induce involuntary reflexive contraction of the levator and frontalis muscles. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 1403–1410. [Google Scholar] [CrossRef]
- Matsuo, K. Stretching of the Mueller muscle results in involuntary contraction of the levator muscle. Ophthalmic Plast. Reconstr. Surg. 2002, 18, 5–10. [Google Scholar] [CrossRef]
- Ministry of Health, Labor and Welfare. Available online: https://www.mhlw.go.jp/toukei/itiran/roudou/saigai/anzen/08/02.html#6 (accessed on 14 September 2024).
- Bear, M.F.; Connors, B.W.; Paradiso, M.A. Neuroscience: Exploring the Brain; Jones & Bartlett Publishers Learning: Burlington, MA, USA, 2016; pp. 453–482. [Google Scholar]
- Lund, J.P.; Lamarre, Y.; Lavigne, G.; Duquet, G. Human jaw reflexes. Adv. Neurol. 1983, 39, 739–755. [Google Scholar]
- Shepherd, S.V.; Lanzilotto, M.; Ghazanfar, A.A. Facial muscle coordination in monkeys during rhythmic facial expressions and ingestive movements. J. Neurosci. 2012, 32, 6105–6116. [Google Scholar] [CrossRef]
- Grillner, S. Biological pattern generation: The cellular and computational logic of networks in motion. Neuron 2006, 52, 751–766. [Google Scholar] [CrossRef]
- Cosker, D.; Krumhuber, E.; Hilton, A.; Facs, A. Valid 3D dynamic action unit database with applications to 3D dynamic morphable facial modeling. In Proceedings of the 13th IEEE International Conference on Computer Vision (ICCV), Barcelona, Spain, 6–13 November 2011; Metaxas, S., Quan, L., Sanfeliu, A., van Gool, L., Eds.; IEEE Publications: Barcelona, Spain, 2011; pp. 2296–2303. [Google Scholar] [CrossRef]
- Taira, A.; Igarashi, T.; Gyoba, J. Cognitive structure of youthful facial impression in young women—Understanding youthfulness in women in their 30s through comparison with women in their 50s. Jakunen josei ni okeru wakawakashii kao no inshou ninchi kouzou—50 dai josei to no hikaku wo tooshita 30 dai josei ni okeru wakawakashisa no rikai. Trans. Jpn. Soc. Kansei Eng. 2022, 21, 425–430. (In Japanese) [Google Scholar] [CrossRef]
- Kim, M.H.; Cho, J.; Zhao, D.; I Woo, K.; Kim, Y.-D.; Kim, S.; Yang, S.W. Prevalence and associated factors of blepharoptosis in Korean adult population: The Korea National Health and Nutrition Examination survey 2008-2011. Eye 2017, 31, 940–946. [Google Scholar] [CrossRef]
- Morales, J.M.; Díaz-Piedra, C.; Rieiro, H.; Roca-González, J.; Romero, S.; Catena, A.; Fuentes, L.J.; Di Stasi, L.L. Monitoring driver fatigue using a single-channel electroencephalographic device: A validation study by gaze-based, driving performance, and subjective data. Accid. Anal. Prev. 2017, 109, 62–69. [Google Scholar] [CrossRef]
- Makhmudov, F.; Turimov, D.; Xamidov, M.; Nazarov, F.; Cho, Y.I. Real-Time Fatigue Detection Algorithms Using Machine Learning for Yawning and Eye State. Sensors 2024, 24, 7810. [Google Scholar] [CrossRef]
| Items | Physical Symptoms | Items | Mental/Psychological Symptoms |
|---|---|---|---|
| 1 | Slight fever | 11 | Decline in cognitive ability |
| 2 | Feeling tired or sluggish | 12 | Difficulty sleeping |
| 3 | Experiencing significant fatigue even with minimal exercise or work | 13 | Feelings of depression |
| 4 | Muscle pain | 14 | Concern about physical health |
| 5 | Recent loss of bodily strength | 15 | Lack of motivation to work |
| 6 | Swollen lymph nodes | 16 | Trouble remembering minor details |
| 7 | Headache or a heavy feeling in the head | 17 | Occasional dizziness from bright light |
| 8 | Fatigue that persists despite a night’ sleep | 18 | Periods of feeling dazed |
| 9 | Sore throat | 19 | Reduced concentration |
| 10 | Joint pain | 20 | Persistent oversleeping |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagashima, Y.; Takamoto, K.; Hiraishi, M.; Hori, E.; Kataoka, K.; Nishijo, H. Objective Evaluation of Fatigue-Associated Facial Expressions Using Measurements of Eye-Opening Degree, Motion Capture, and Heart Rate Variability Spectrum Analysis. Physiologia 2025, 5, 42. https://doi.org/10.3390/physiologia5040042
Nagashima Y, Takamoto K, Hiraishi M, Hori E, Kataoka K, Nishijo H. Objective Evaluation of Fatigue-Associated Facial Expressions Using Measurements of Eye-Opening Degree, Motion Capture, and Heart Rate Variability Spectrum Analysis. Physiologia. 2025; 5(4):42. https://doi.org/10.3390/physiologia5040042
Chicago/Turabian StyleNagashima, Yoshinao, Kouichi Takamoto, Makiko Hiraishi, Etsuro Hori, Kiyoshi Kataoka, and Hisao Nishijo. 2025. "Objective Evaluation of Fatigue-Associated Facial Expressions Using Measurements of Eye-Opening Degree, Motion Capture, and Heart Rate Variability Spectrum Analysis" Physiologia 5, no. 4: 42. https://doi.org/10.3390/physiologia5040042
APA StyleNagashima, Y., Takamoto, K., Hiraishi, M., Hori, E., Kataoka, K., & Nishijo, H. (2025). Objective Evaluation of Fatigue-Associated Facial Expressions Using Measurements of Eye-Opening Degree, Motion Capture, and Heart Rate Variability Spectrum Analysis. Physiologia, 5(4), 42. https://doi.org/10.3390/physiologia5040042






