Systematic Review of the Role of Kv4.x Potassium Channels in Neurodegenerative Diseases: Implications for Neuronal Excitability and Therapeutic Modulation
Abstract
1. Introduction
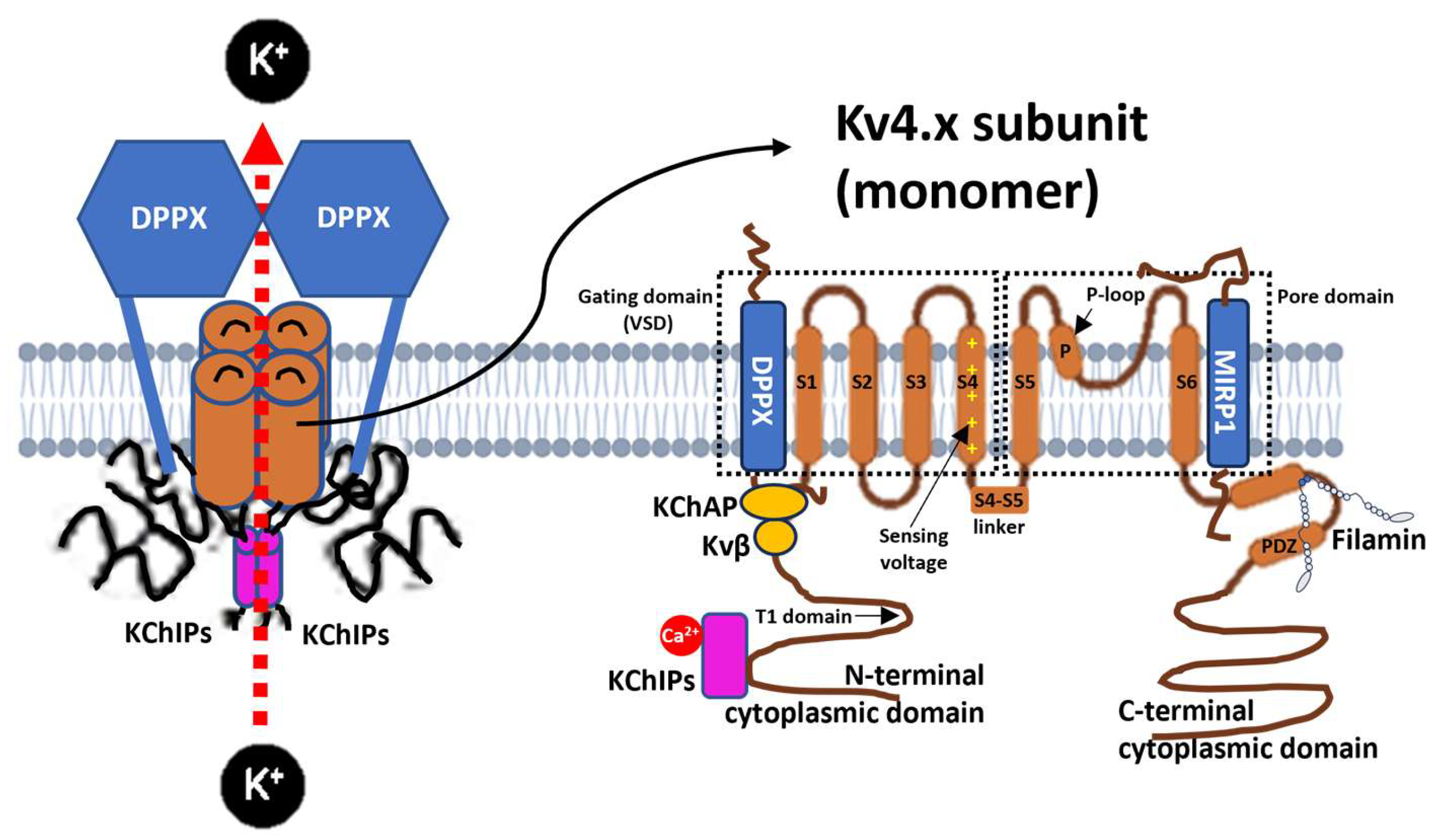
1.1. Molecular Structure of Kv4 Channels
1.2. Subunits and Auxiliary Proteins That Interact with Kv4
1.3. Kv4 Channelopathies and Implications for Neuronal Function
2. Results
2.1. Amyotrophic Lateral Sclerosis (ALS)
2.2. Parkinson’s Disease (PD)
2.3. Alzheimer’s Disease (AD)
2.4. Spinocerebellar and Episodic Ataxias
2.5. Prionopathies (Prion Diseases)
2.6. Integrative Analysis of Mechanisms, Causality, and Clinical Implications
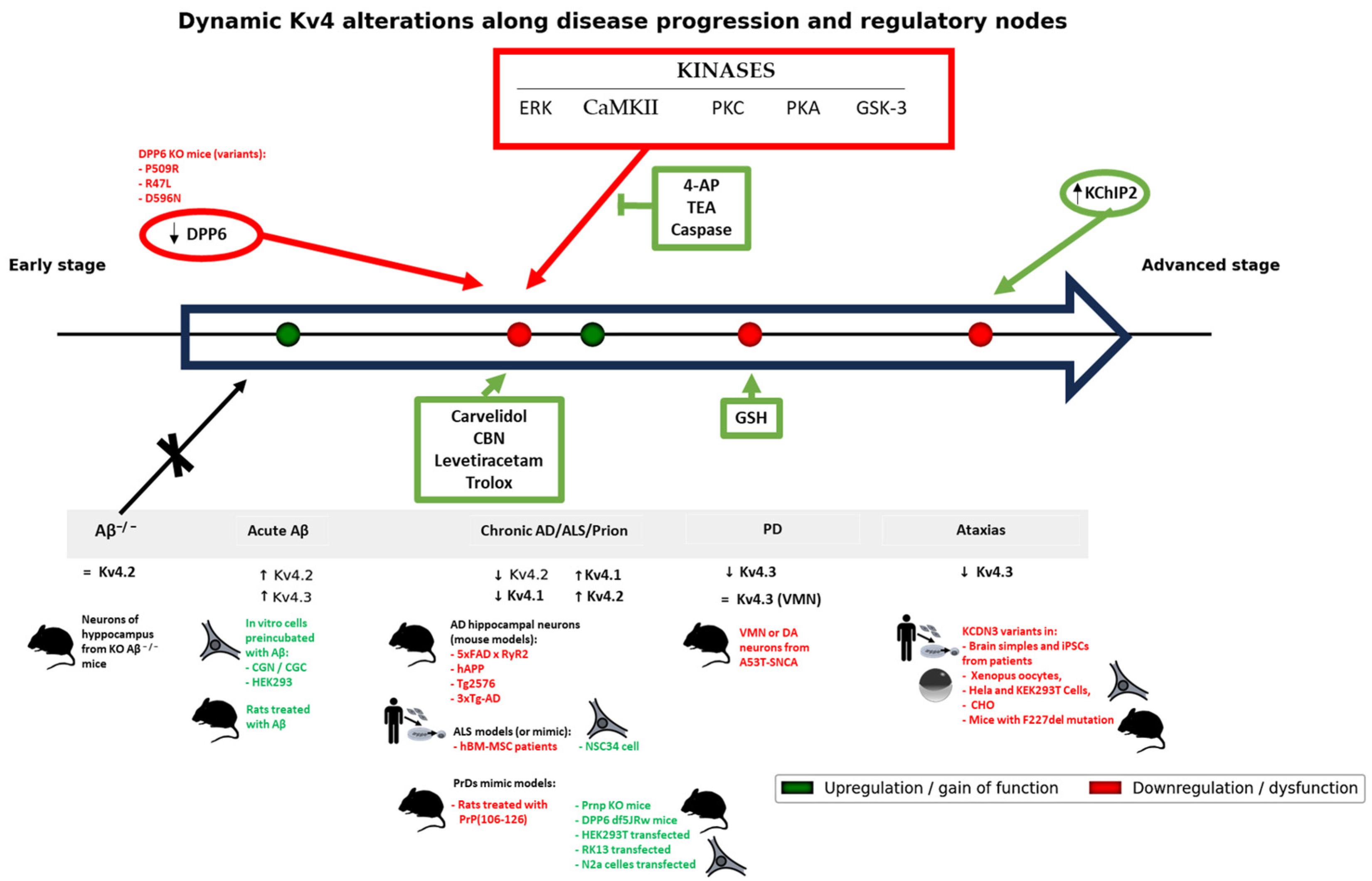
3. Discussion
3.1. Pathophysiological and Clinical Implications of Kv4 Dysfunction in Neurodegenerative Diseases
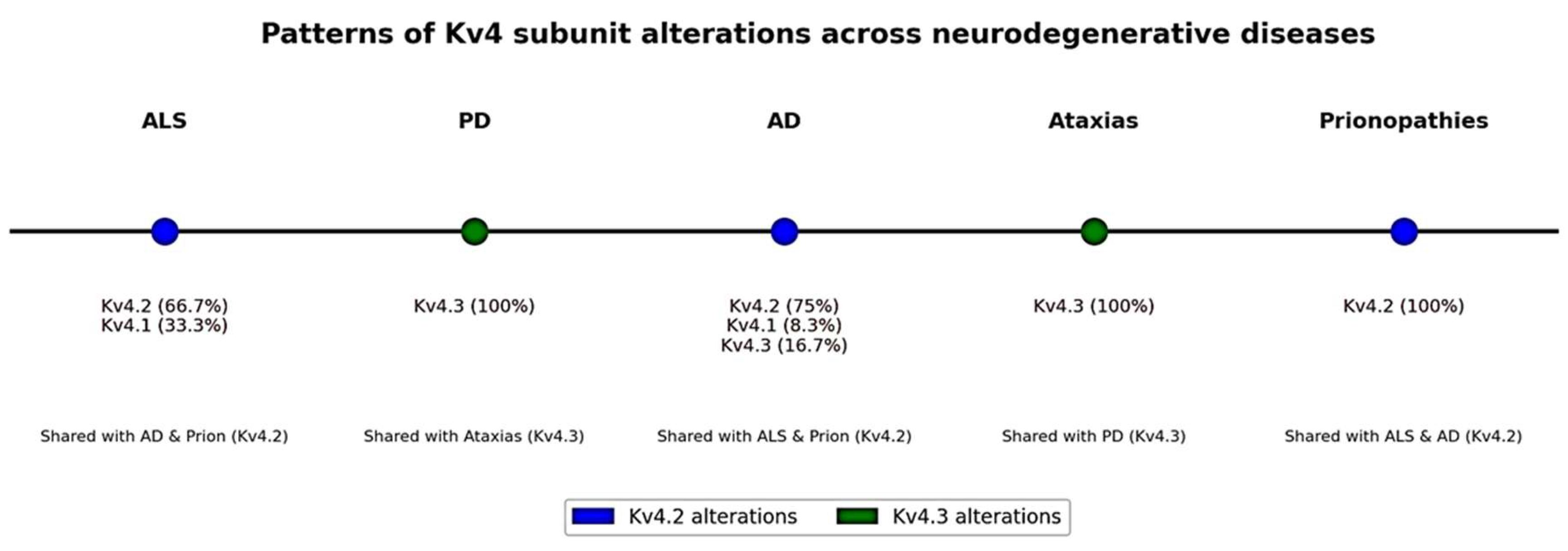
3.2. Common Patterns and Differences Between Diseases and Models
3.3. Integrative Synthesis of Kv4 Alterations
4. Materials and Methods
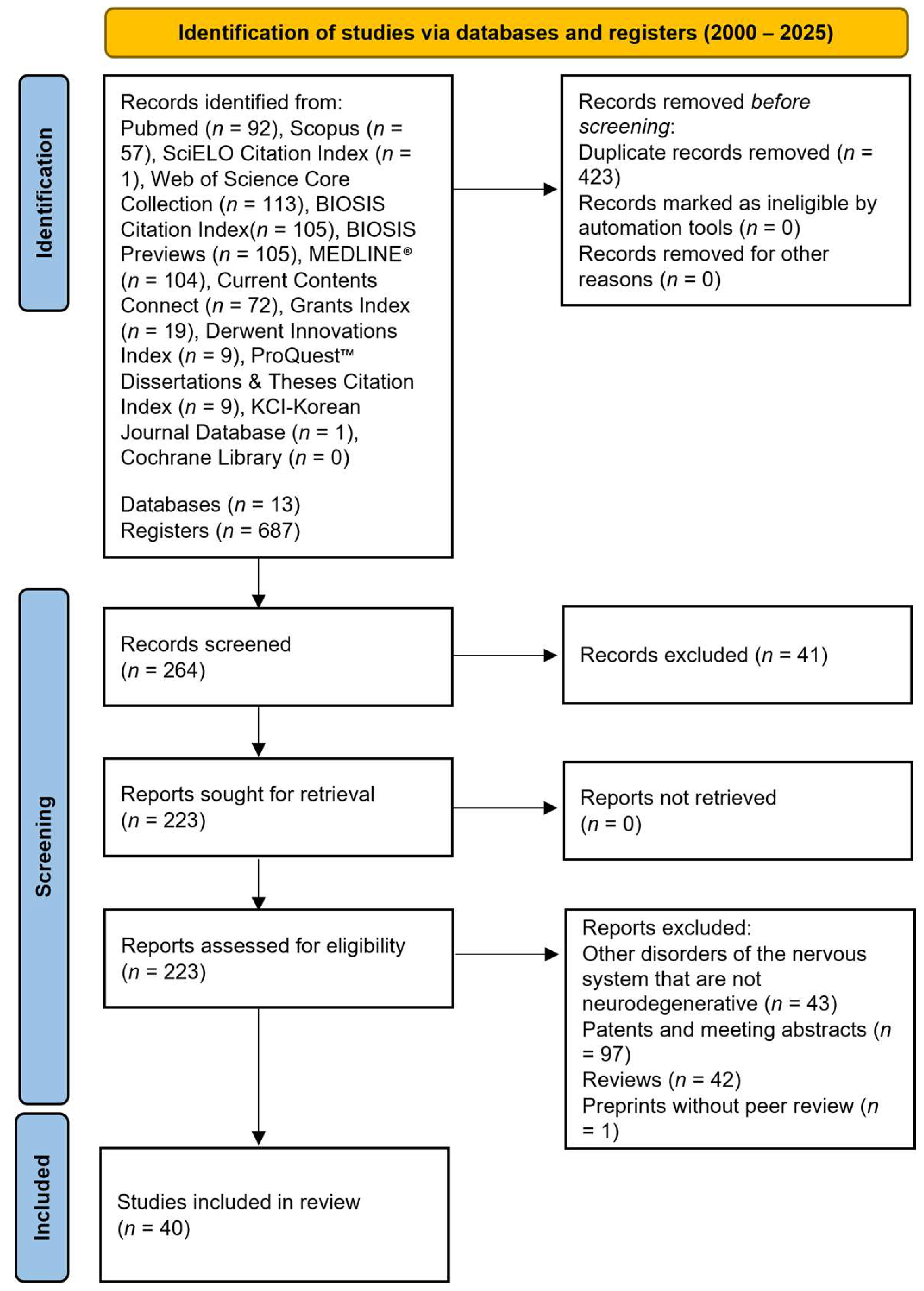
4.1. Literature Search and Selection Criteria
4.2. Assessment of the Methodological Quality and Risk of Bias of the Included Studies
4.3. Verification of Genetic Variants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.B. Channelopathies. Korean J. Pediatr. 2014, 57, 1–18. [Google Scholar] [CrossRef]
- Isbrandt, D.; Leicher, T.; Waldschütz, R.; Zhu, X.; Luhmann, U.; Michel, U.; Sauter, K.; Pongs, O. Gene Structures and Expression Profiles of Three Human KCND (Kv4) Potassium Channels Mediating A-Type Currents I(TO) and I(SA). Genomics 2000, 64, 144–154. [Google Scholar] [CrossRef]
- Decher, N.; Barth, A.S.; Gonzalez, T.; Steinmeyer, K.; Sanguinetti, M.C. Novel KChIP2 Isoforms Increase Functional Diversity of Transient Outward Potassium Currents. J. Physiol. 2004, 557, 761–772. [Google Scholar] [CrossRef]
- Birnbaum, S.G.; Varga, A.W.; Yuan, L.L.; Anderson, A.E.; Swepatt, J.D.; Schrader, L.A. Structure and Function of Kv4-Family Transient Potassium Channels. Physiol. Rev. 2004, 84, 803–833. [Google Scholar] [CrossRef] [PubMed]
- Ruppersberg, J.P.; Schröter, K.H.; Sakmann, B.; Stocker, M.; Sewing, S.; Pongs, O. Heteromultimeric Channels Formed by Rat Brain Potassium-Channel Proteins. Nature 1990, 345, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Isacoff, E.Y.; Jan, Y.N.; Jan, L.Y. Evidence for the Formation of Heteromultimeric Potassium Channels in Xenopus Oocytes. Nature 1990, 345, 530–534. [Google Scholar] [CrossRef]
- Strang, C.; Cushman, S.J.; DeRubeis, D.; Peterson, D.; Pfaffinger, P.J. A Central Role for the T1 Domain in Voltage-Gated Potassium Channel Formation and Function. J. Biol. Chem. 2001, 276, 28493–28502. [Google Scholar] [CrossRef]
- Nanao, M.H.; Zhou, W.; Pfaffinger, P.J.; Choe, S. Determining the Basis of Channel-Tetramerization Specificity by X-Ray Crystallography and a Sequence-Comparison Algorithm: Family Values (FamVal). Proc. Natl. Acad. Sci. USA 2003, 100, 8670–8675. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.; Chikvashvili, D.; Singer-Lahat, D.; Peretz, T.; Thornhill, W.B.; Lotan, I. Phosphorylation of a K+ Channel Alpha Subunit Modulates the Inactivation Conferred by a Beta Subunit. Involv. Cytoskelet. J. Biol. Chem. 1996, 271, 29321–29328. [Google Scholar] [CrossRef]
- Horn, R. A New Twist in the Saga of Charge Movement in Voltage-Dependent Ion Channels. Neuron 2000, 25, 511–514. [Google Scholar] [CrossRef]
- Kong, W.; Po, S.; Yamagishi, T.; Ashen, M.D.; Stetten, G.; Tomaselli, G.F. Isolation and Characterization of the Human Gene Encoding Ito: Further Diversity by Alternative MRNA Splicing. Am. J. Physiol. 1998, 275, H1963–H1970. [Google Scholar] [CrossRef]
- Gonzalez, W.G.; Pham, K.; Miksovska, J. Modulation of the Voltage-Gated Potassium Channel (Kv4.3) and the Auxiliary Protein (KChIP3) Interactions by the Current Activator NS5806. J. Biol. Chem. 2014, 289, 32201–32213. [Google Scholar] [CrossRef]
- Deschênes, I.; Tomaselli, G.F. Modulation of Kv4.3 Current by Accessory Subunits. FEBS Lett. 2002, 528, 183–188. [Google Scholar] [CrossRef]
- England, S.K.; Uebele, V.N.; Shear, H.; Kodali, J.; Bennett, P.B.; Tamkun, M.M. Characterization of a Voltage-Gated K+ Channel Beta Subunit Expressed in Human Heart. Proc. Natl. Acad. Sci. USA 1995, 92, 6309–6313. [Google Scholar] [CrossRef]
- Fink, M.; Duprat, F.; Lesage, F.; Heurteaux, C.; Romey, G.; Barhanin, J.; Lazdunski, M. A New K+ Channel Beta Subunit to Specifically Enhance Kv2.2 (CDRK) Expression. J. Biol. Chem. 1996, 271, 26341–26348. [Google Scholar] [CrossRef]
- Heinemann, S.H.; Rettig, J.; Wunder, F.; Pongs, O. Molecular and Functional Characterization of a Rat Brain Kv Beta 3 Potassium Channel Subunit. FEBS Lett. 1995, 377, 383–389. [Google Scholar] [CrossRef]
- Leicher, T.; Bähring, R.; Isbrandt, D.; Pongs, O. Coexpression of the KCNA3B Gene Product with Kv1.5 Leads to a Novel A-Type Potassium Channel. J. Biol. Chem. 1998, 273, 35095–35101. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, N.; Papazian, D.M. Potassium Channel Alpha and Beta Subunits Assemble in the Endoplasmic Reticulum. J. Biol. Chem. 1997, 272, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, K.; Shi, G.; Rhodes, K.J.; Trimmer, J.S. Selective Interaction of Voltage-Gated K+ Channel Beta-Subunits with Alpha-Subunits. J. Biol. Chem. 1996, 271, 7084–7089. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, M.T.; López-López, J.R.; González, C. Kvbeta1.2 Subunit Coexpression in HEK293 Cells Confers O2 Sensitivity to Kv4.2 but not to Shaker Channels. J. Gen. Physiol. 1999, 113, 897–907. [Google Scholar] [CrossRef]
- Rhodes, K.J.; Monaghan, M.M.; Barrezueta, N.X.; Nawoschik, S.; Bekele-Arcuri, Z.; Matos, M.F.; Nakahira, K.; Schechter, L.E.; Trimmer, J.S. Voltage-Gated K+ Channel Beta Subunits: Expression and Distribution of Kv Beta 1 and Kv Beta 2 in Adult Rat Brain. J. Neurosci. 1996, 16, 4846–4860. [Google Scholar] [CrossRef]
- Shi, G.; Nakahira, K.; Hammond, S.; Rhodes, K.J.; Schechter, L.E.; Trimmer, J.S. Beta Subunits Promote K+ Channel Surface Expression through Effects Early in Biosynthesis. Neuron 1996, 16, 843–852. [Google Scholar] [CrossRef]
- Trimmer, J.S. Regulation of Ion Channel Expression by Cytoplasmic Subunits. Curr. Opin. Neurobiol. 1998, 8, 370–374. [Google Scholar] [CrossRef]
- Yang, E.K.; Alvira, M.R.; Levitan, E.S.; Takimoto, K. Kvbeta Subunits Increase Expression of Kv4.3 Channels by Interacting with Their C Termini. J. Biol. Chem. 2001, 276, 4839–4844. [Google Scholar] [CrossRef] [PubMed]
- An, W.F.; Bowlby, M.R.; Betty, M.; Cao, J.; Ling, H.P.; Mendoza, G.; Hinson, J.W.; Mattsson, K.I.; Strassle, B.W.; Trimme, J.S.; et al. Modulation of A-Type Potassium Channels by a Family of Calcium Sensors. Nature 2000, 403, 553–556. [Google Scholar] [CrossRef]
- Bähring, R.; Dannenberg, J.; Peters, H.C.; Leicher, T.; Pongs, O.; Isbrandt, D. Conserved Kv4 N-Terminal Domain Critical for Effects of Kv Channel-Interacting Protein 2.2 on Channel Expression and Gating. J. Biol. Chem. 2001, 276, 23888–23894. [Google Scholar] [CrossRef]
- Deschênes, I.; DiSilvestre, D.; Juang, G.J.; Wu, R.C.; An, W.F.; Tomaselli, G.F. Regulation of Kv4.3 Current by KChIP2 Splice Variants: A Component of Native Cardiac Ito? Circulation 2002, 106, 423–429. [Google Scholar] [CrossRef]
- Holmqvist, M.H.; Cao, J.; Hernandez-Pineda, R.; Jacobson, M.D.; Carroll, K.I.; Sung, M.A.; Betty, M.; Ge, P.; Gilbride, K.J.; Brown, M.E.; et al. Elimination of Fast Inactivation in Kv4 A-Type Potassium Channels by an Auxiliary Subunit Domain. Proc. Natl. Acad. Sci. USA 2002, 99, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Morohashi, Y.; Hatano, N.; Ohya, S.; Takikawa, R.; Watabiki, T.; Takasugi, N.; Imaizumi, Y.; Tomita, T.; Iwatsubo, T. Molecular Cloning and Characterization of CALP/KChIP4, a Novel EF-Hand Protein Interacting with Presenilin 2 and Voltage-Gated Potassium Channel Subunit Kv4. J. Biol. Chem. 2002, 277, 14965–14975. [Google Scholar] [CrossRef]
- Patel, S.P.; Campbell, D.L.; Morales, M.J.; Strauss, H.C. Heterogeneous Expression of KChIP2 Isoforms in the Ferret Heart. J. Physiol. 2002, 539, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Rosati, B.; Grau, F.; Rodriguez, S.; Li, H.; Nerbonne, J.M.; McKinnon, D. Concordant Expression of KChIP2 MRNA, Protein and Transient Outward Current throughout the Canine Ventricle. J. Physiol. 2003, 548, 815–822. [Google Scholar] [CrossRef]
- Rosati, B.; Pan, Z.; Lypen, S.; Wang, H.S.; Cohen, I.; Dixon, J.E.; McKinnon, D. Regulation of KChIP2 Potassium Channel Beta Subunit Gene Expression Underlies the Gradient of Transient Outward Current in Canine and Human Ventricle. J. Physiol. 2001, 533, 119–125. [Google Scholar] [CrossRef]
- Shibata, R.; Misonou, H.; Campomanes, C.R.; Anderson, A.E.; Schrader, L.A.; Doliveira, L.C.; Carroll, K.I.; Sweatt, J.D.; Rhodes, K.J.; Trimmer, J.S. A Fundamental Role for KChIPs in Determining the Molecular Properties and Trafficking of Kv4.2 Potassium Channels. J. Biol. Chem. 2003, 278, 36445–36454. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, H.; Aimond, F.; Johns, D.C.; Rhodes, K.J.; Trimmer, J.S.; Nerbonne, J.M. Role of Heteromultimers in the Generation of Myocardial Transient Outward K+ Currents. Circ. Res. 2002, 90, 586–593. [Google Scholar] [CrossRef]
- Guo, W.; Malin, S.A.; Johns, D.C.; Jeromin, A.; Nerbonne, J.M. Modulation of Kv4-Encoded K(+) Currents in the Mammalian Myocardium by Neuronal Calcium Sensor-1. J. Biol. Chem. 2002, 277, 26436–26443. [Google Scholar] [CrossRef]
- Nakamura, T.Y.; Pountney, D.J.; Ozaita, A.; Nandi, S.; Ueda, S.; Rudy, B.; Coetzee, W.A. A Role for Frequenin, a Ca2+-Binding Protein, as a Regulator of Kv4 K+-Currents. Proc. Natl. Acad. Sci. USA 2001, 98, 12808–12813. [Google Scholar] [CrossRef]
- Kuryshev, Y.A.; Gudz, T.I.; Brown, A.M.; Wible, B.A. KChAP as a Chaperone for Specific K+ Channels. Am. J. Physiol. Cell Physiol. 2000, 278, C931–C941. [Google Scholar] [CrossRef] [PubMed]
- Kuryshev, Y.A.; Wible, B.A.; Gudz, T.I.; Ramirez, A.N.; Brown, A.M. KChAP/Kvbeta1.2 Interactions and Their Effects on Cardiac Kv Channel Expression. Am. J. Physiol. Cell Physiol. 2001, 281, C290–C299. [Google Scholar] [CrossRef] [PubMed]
- Wible, B.A.; Yang, Q.; Kuryshev, Y.A.; Accili, E.A.; Brown, A.M. Cloning and Expression of a Novel K+ Channel Regulatory Protein, KChAP. J. Biol. Chem. 1998, 273, 11745–11751. [Google Scholar] [CrossRef]
- Nadal, M.S.; Ozaita, A.; Amarillo, Y.; Vega-Saenz De Miera, E.; Ma, Y.; Mo, W.; Goldberg, E.M.; Misumi, Y.; Ikehara, Y.; Neubert, T.A.; et al. The CD26-Related Dipeptidyl Aminopeptidase-like Protein DPPX Is a Critical Component of Neuronal A-Type K+ Channels. Neuron 2003, 37, 449–461. [Google Scholar] [CrossRef]
- Zagha, E.; Ozaita, A.; Chang, S.Y.; Nadal, M.S.; Lin, U.; Saganich, M.J.; McCormack, T.; Akinsanya, K.O.; Qi, S.Y.; Rudy, B. DPP10 Modulates Kv4-Mediated A-Type Potassium Channels. J. Biol. Chem. 2005, 280, 18853–18861. [Google Scholar] [CrossRef] [PubMed]
- Petrecca, K.; Miller, D.M.; Shrier, A. Localization and Enhanced Current Density of the Kv4.2 Potassium Channel by Interaction with the Actin-Binding Protein Filamin. J. Neurosci. 2000, 20, 8736–8744. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Tsaur, M.L.; Nung Jan, Y.; Yeh Jan, L. Subcellular Segregation of Two A-Type K+ Channel Proteins in Rat Central Neurons. Neuron 1992, 9, 271–284. [Google Scholar] [CrossRef]
- Vasilyev, D.V.; Barish, M.E. Regulation of an Inactivating Potassium Current (IA) by the Extracellular Matrix Protein Vitronectin in Embryonic Mouse Hippocampal Neurones. J. Physiol. 2003, 547, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.; Newell, E.W.; Jugloff, D.G.M.; Jones, O.T.; Schlichter, L.C. Cell Surface Targeting and Clustering Interactions between Heterologously Expressed PSD-95 and the Shal Voltage-Gated Potassium Channel, Kv4.2. J. Biol. Chem. 2002, 277, 20423–20430. [Google Scholar] [CrossRef]
- Hsueh, Y.P.; Sheng, M. Requirement of N-Terminal Cysteines of PSD-95 for PSD-95 Multimerization and Ternary Complex Formation, but not for Binding to Potassium Channel Kv1.4. J. Biol. Chem. 1999, 274, 532–536. [Google Scholar] [CrossRef]
- Abbott, G.W.; Butler, M.H.; Bendahhou, S.; Dalakas, M.C.; Ptacek, L.J.; Goldstein, S.A.N. MiRP2 Forms Potassium Channels in Skeletal Muscle with Kv3.4 and Is Associated with Periodic Paralysis. Cell 2001, 104, 217–231. [Google Scholar] [CrossRef]
- Abbott, G.W.; Sesti, F.; Splawski, I.; Buck, M.E.; Lehmann, M.H.; Timothy, K.W.; Keating, M.T.; Goldstein, S.A.N. MiRP1 Forms IKr Potassium Channels with HERG and Is Associated with Cardiac Arrhythmia. Cell 1999, 97, 175–187. [Google Scholar] [CrossRef]
- Schroeder, B.C.; Waldegger, S.; Fehr, S.; Bleich, M.; Warth, R.; Greger, R.; Jentsch, T.J. A Constitutively Open Potassium Channel Formed by KCNQ1 and KCNE3. Nature 2000, 403, 196–199. [Google Scholar] [CrossRef]
- Tinel, N.; Diochot, S.; Borsotto, M.; Lazdunski, M.; Barhanin, J. KCNE2 Confers Background Current Characteristics to the Cardiac KCNQ1 Potassium Channel. EMBO J. 2000, 19, 6326–6330. [Google Scholar] [CrossRef]
- Wulfsen, I.; Hauber, H.P.; Schiemann, D.; Bauer, C.K.; Schwarz, J.R. Expression of MRNA for Voltage-Dependent and Inward-Rectifying K Channels in GH3/B6 Cells and Rat Pituitary. J. Neuroendocr. Neuroendocrinol. 2000, 12, 263–272. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, M.; Tseng, G.N. MinK-Related Peptide 1 Associates with Kv4.2 and Modulates Its Gating Function: Potential Role as Beta Subunit of Cardiac Transient Outward Channel? Circ. Res. 2001, 88, 1012–1019. [Google Scholar] [CrossRef]
- Hall, A.M.; Throesch, B.T.; Buckingham, S.C.; Markwardt, S.J.; Peng, Y.; Wang, Q.; Hoffman, D.A.; Roberson, E.D. Tau-Dependent Kv4.2 Depletion and Dendritic Hyperexcitability in a Mouse Model of Alzheimer’s Disease. J. Neurosci. 2015, 35, 6221–6230. [Google Scholar] [CrossRef] [PubMed]
- Pieri, M.; Amadoro, G.; Carunchio, I.; Ciotti, M.T.; Quaresima, S.; Florenzano, F.; Calissano, P.; Possenti, R.; Zona, C.; Severini, C. SP Protects Cerebellar Granule Cells against Beta-Amyloid-Induced Apoptosis by down-Regulation and Reduced Activity of Kv4 Potassium Channels. Neuropharmacology 2010, 58, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.; Waro, G.; Licursi, A.; Smith, S.; Vo-Ba, D.A.; Tsunoda, S. Shal/K(v)4 Channels Are Required for Maintaining Excitability during Repetitive Firing and Normal Locomotion in Drosophila. PLoS ONE 2011, 6, e16043. [Google Scholar] [CrossRef]
- Park, K.S.; Choi, M.R.; Jung, K.D.; Kim, S.H.; Kim, H.Y.; Kim, K.S.; Cha, E.-J.; Kim, Y.; Chai, Y.G. Diversity of Ion Channels in Human Bone Marrow Mesenchymal Stem Cells from Amyotrophic Lateral Sclerosis Patients. Korean J. Physiol. Pharmacol. 2008, 12, 337–342. [Google Scholar] [CrossRef]
- Lasser-Katz, E.; Simchovitz, A.; Chiu, W.-H.; Oertel, W.H.; Sharon, R.; Soreq, H.; Roeper, J.; Goldberg, J.A. Mutant α-Synuclein Overexpression Induces Stressless Pacemaking in Vagal Motoneurons at Risk in Parkinson’s Disease. J. Neurosci. 2017, 37, 47–57. [Google Scholar] [CrossRef]
- Dabrowska, J.; Rainnie, D.G. Expression and Distribution of Kv4 Potassium Channel Subunits and Potassium Channel Interacting Proteins in Subpopulations of Interneurons in the Basolateral Amygdala. Neuroscience 2010, 171, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Ruíz, R.; Aguado, C.; Martín-Belmonte, A.; Moreno-Martínez, A.E.; Luján, R. Expression, Cellular and Subcellular Localisation of Kv4.2 and Kv4.3 Channels in the Rodent Hippocampus. Int. J. Mol. Sci. 2019, 20, 246. [Google Scholar] [CrossRef]
- Tsaur, M.L.; Sheng, M.; Lowenstein, D.H.; Jan, Y.N.; Jan, L.Y. Differential Expression of K+ Channel MRNAs in the Rat Brain and Down-Regulation in the Hippocampus Following Seizures. Neuron 1992, 8, 1055–1067. [Google Scholar] [CrossRef]
- Breen, M.S.; Dobbyn, A.; Li, Q.; Roussos, P.; Hoffman, G.E.; Stahl, E.; Chess, A.; Sklar, P.; Li, J.B.; Devlin, B.; et al. Global Landscape and Genetic Regulation of RNA Editing in Cortical Samples from Individuals with Schizophrenia. Nat. Neurosci. 2019, 22, 1402–1412. [Google Scholar] [CrossRef]
- Bernard, C.; Anderson, A.; Becker, A.; Poolos, N.P.; Deck, H.; Johnston, D. Acquired Dendritic Channelopathy in Temporal Lobe Epilepsy. Science 2004, 305, 532–535. [Google Scholar] [CrossRef]
- MacDonald, J.F.; Belrose, J.C.; Xie, Y.F.; Jackson, M.F. Nonselective Cation Channels and Links to Hippocampal Ischemia, Aging, and Dementia. Adv. Exp. Med. Biol. 2013, 961, 433–447. [Google Scholar] [CrossRef]
- Tiwari, D.; Rajathi, V.; Rymer, J.K.; Beasley, L.N.; McGann, A.M.; Bunk, A.T.; Parkins, E.V.; Rice, M.F.; Smith, K.E.; Ritter, D.M.; et al. Estradiol- and Progesterone-Associated Changes in MicroRNA-Induced Silencing and Reduced Antiseizure Efficacy of an Antagomir in Female Mice. eNeuro 2023, 10, ENEURO.0047-22.2023. [Google Scholar] [CrossRef]
- Choubey, M.; Bansal, R.; Siddharthan, D.; Naik, N.; Sharma, G.; Saxena, A. Early Repolarization Syndrome, Epilepsy, and Atrial Fibrillation in a Young Girl with Novel KCND3 Mutation Managed with Quinidine. J. Cardiovasc. Electrophysiol. 2022, 33, 1312–1315. [Google Scholar] [CrossRef]
- Tiwari, D.; Schaefer, T.L.; Schroeder-Carter, L.M.; Krzeski, J.C.; Bunk, A.T.; Parkins, E.V.; Snider, A.; Danzer, R.; Williams, M.T.; Vorhees, C.V.; et al. The Potassium Channel Kv4.2 Regulates Dendritic Spine Morphology, Electroencephalographic Characteristics and Seizure Susceptibility in Mice. Exp. Neurol. 2020, 334, 113437. [Google Scholar] [CrossRef]
- Gross, C.; Yao, X.; Engel, T.; Tiwari, D.; Xing, L.; Rowley, S.; Danielson, S.W.; Thomas, K.T.; Jimenez-Mateos, E.M.; Schroeder, L.M.; et al. MicroRNA-Mediated Downregulation of the Potassium Channel Kv4.2 Contributes to Seizure Onset. Cell Rep. 2016, 17, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.H.; Malloy, C.; Hoffman, D.A. P38 Regulates Kainic Acid-Induced Seizure and Neuronal Firing via Kv4.2 Phosphorylation. Int. J. Mol. Sci. 2020, 21, 5921. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, P.J.; Chandler, L.J. Inhibition of Glutamate Transporters Couples to Kv4.2 Dephosphorylation through Activation of Extrasynaptic NMDA Receptors. Neuroscience 2010, 165, 130–137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hong, Y.M.; Jo, D.G.; Lee, M.C.; Kim, S.Y.; Jung, Y.K. Reduced Expression of Calsenilin/DREAM/KChIP3 in the Brains of Kainic Acid-Induced Seizure and Epilepsy Patients. Neurosci. Lett. 2003, 340, 33–36. [Google Scholar] [CrossRef]
- Trainito, A.; Muscarà, C.; Gugliandolo, A.; Chiricosta, L.; Salamone, S.; Pollastro, F.; Mazzon, E.; D’Angiolini, S. Cannabinol (CBN) Influences the Ion Channels and Synaptic-Related Genes in NSC-34 Cell Line: A Transcriptomic Study. Cells 2024, 13, 1573. [Google Scholar] [CrossRef]
- Subramaniam, M.; Althof, D.; Gispert, S.; Schwenk, J.; Auburger, G.; Kulik, A.; Fakler, B.; Roeper, J. Mutant α-Synuclein Enhances Firing Frequencies in Dopamine Substantia Nigra Neurons by Oxidative Impairment of A-Type Potassium Channels. J. Neurosci. 2014, 34, 13586–13599. [Google Scholar] [CrossRef] [PubMed]
- Cacace, R.; Heeman, B.; Van Mossevelde, S.; De Roeck, A.; Hoogmartens, J.; Van Rijk, P.; Gossye, H.; Van Vos, K.; De Coster, W.; Strazisar, M.; et al. Loss of DPP6 in Neurodegenerative Dementia: A Genetic Player in the Dysfunction of Neuronal Excitability. Acta Neuropathol. 2019, 137, 901–918. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Sun, B.; Institoris, A.; Zhan, X.; Guo, W.; Song, Z.; Liu, Y.; Hiess, F.; Boyce, A.K.J.; Ni, M.; et al. Limiting RyR2 Open Time Prevents Alzheimer’s Disease-Related Neuronal Hyperactivity and Memory Loss but Not β-Amyloid Accumulation. Cell Rep. 2020, 32, 108169. [Google Scholar] [CrossRef]
- Plant, L.D.; Webster, N.J.; Boyle, J.P.; Ramsden, M.; Freir, D.B.; Peers, C.; Pearson, H.A. Amyloid Beta Peptide as a Physiological Modulator of Neuronal ‘A’-Type K+ Current. Neurobiol. Aging 2006, 27, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-R.; Kim, Y.Y.; Jeong, H.-J.; Kang, J.-S.; Lee, S.H.S.-H.S.H.; Kim, Y.Y.; Lee, S.H.S.-H.S.H.; Ho, W.-K. Impaired Pattern Separation in Tg2576 Mice Is Associated with Hyperexcitable Dentate Gyrus Caused by Kv4.1 Downregulation. Mol. Brain 2021, 14, 62. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.-Z.; Deng, Y.-M.; Wang, K.; Yang, L.; Long, C. Amyloid-β Protein Precursor Regulates Electrophysiological Properties in the Hippocampus via Altered Kv1.4 Expression and Function in Mice. J. Alzheimers Dis. 2023, 92, 1241–1256. [Google Scholar] [CrossRef]
- Scala, F.; Fusco, S.; Ripoli, C.; Piacentini, R.; Li Puma, D.D.; Spinelli, M.; Laezza, F.; Grassi, C.; D’Ascenzo, M. Intraneuronal Aβ Accumulation Induces Hippocampal Neuron Hyperexcitability through A-Type K+ Current Inhibition Mediated by Activation of Caspases and GSK-3. Neurobiol. Aging 2015, 36, 886–900. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, X.; Tong, X.; Wang, X. Messenger RNA and Protein Expression Analysis of Voltage-Gated Potassium Channels in the Brain of Aβ25-35-Treated Rats. J. Neurosci. Res. 2004, 77, 94–99. [Google Scholar] [CrossRef]
- Kerrigan, T.L.; Atkinson, L.; Peers, C.; Pearson, H.A. Modulation of ‘A’-Type K+ Current by Rodent and Human Forms of Amyloid β Protein. Neuroreport 2008, 19, 839–843. [Google Scholar] [CrossRef]
- Campolongo, P.; Ratano, P.; Ciotti, M.T.; Florenzano, F.; Nori, S.L.; Marolda, R.; Palmery, M.; Rinaldi, A.M.; Zona, C.; Possenti, R.; et al. Systemic Administration of Substance P Recovers Beta Amyloid-Induced Cognitive Deficits in Rat: Involvement of Kv Potassium Channels. PLoS ONE 2013, 8, e78036. [Google Scholar] [CrossRef]
- Ågren, R.; Geerdink, N.; Brunner, H.G.; Paucar, M.; Kamsteeg, E.-J.; Sahlholm, K. An E280K Missense Variant in KCND3/Kv4.3—Case Report and Functional Characterization. Int. J. Mol. Sci. 2023, 24, 10924. [Google Scholar] [CrossRef]
- Duarri, A.; Lin, M.-C.A.; Fokkens, M.R.; Meijer, M.; Smeets, C.J.L.M.; Nibbeling, E.A.R.; Boddeke, E.; Sinke, R.J.; Kampinga, H.H.; Papazian, D.M.; et al. Spinocerebellar Ataxia Type 19/22 Mutations Alter Heterocomplex Kv4.3 Channel Function and Gating in a Dominant Manner. Cell Mol. Life Sci. 2015, 72, 3387–3399. [Google Scholar] [CrossRef]
- Reis, M.C.; Mandler, L.; Kang, J.-S.; Oliver, D.; Halaszovich, C.; Nolte, D. A Novel KCND3 Variant in the N-Terminus Impairs the Ionic Current of Kv4.3 and Is Associated with SCA19/22. J. Cell Mol. Med. 2024, 28, e70039. [Google Scholar] [CrossRef] [PubMed]
- Duarri, A.; Jezierska, J.; Fokkens, M.; Meijer, M.; Schelhaas, H.J.; den Dunnen, W.F.A.; van Dijk, F.; Verschuuren-Bemelmans, C.; Hageman, G.; van de Vlies, P.; et al. Mutations in Potassium Channel Kcnd3 Cause Spinocerebellar Ataxia Type 19. Ann. Neurol. 2012, 72, 870–880. [Google Scholar] [CrossRef]
- Li, M.; Liu, F.; Hao, X.; Fan, Y.; Li, J.; Hu, Z.; Shi, J.; Fan, L.; Zhang, S.; Ma, D.; et al. Rare KCND3 Loss-of-Function Mutation Associated With the SCA19/22. Front. Mol. Neurosci. 2022, 15, 919199. [Google Scholar] [CrossRef]
- Kurihara, M.; Ishiura, H.; Sasaki, T.; Otsuka, J.; Hayashi, T.; Terao, Y.; Matsukawa, T.; Mitsui, J.; Kaneko, J.; Nishiyama, K.; et al. Novel De Novo KCND3 Mutation in a Japanese Patient with Intellectual Disability, Cerebellar Ataxia, Myoclonus, and Dystonia. Cerebellum 2018, 17, 237–242. [Google Scholar] [CrossRef]
- Hsiao, C.-T.; Fu, S.-J.; Cheng, K.-M.; Lo, H.; Tang, C.-Y.; Chan, C.-C.; Jeng, C.-J. Restoration of Shal/K(V)4 Proteostasis and Motor Function in a Drosophila Model of Spinocerebellar Ataxia Type 19/22. Cell Mol. Life Sci. 2025, 82, 181. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, F.; Martin, F.; Ruiz-Fuentes, J.; Diaz, E.; Hermosilla, T.; Gonzalez, W.; Simon, F.; Avila-Jaque, D.; Luna-Álvarez, M.; Dávila Ortiz de Montellano, D.J.; et al. Functional Characterization of Two KCND3 Variants Associated with SCA 19/22 Ataxia in Latin American Families. Biol. Res. 2025, 58, 18. [Google Scholar] [CrossRef] [PubMed]
- Paucar, M.; Ågren, R.; Li, T.; Lissmats, S.; Bergendal, Å.; Weinberg, J.; Nilsson, D.; Savichetva, I.; Sahlholm, K.; Nilsson, J.; et al. V374A KCND3 Pathogenic Variant Associated With Paroxysmal Ataxia Exacerbations. Neurol. Genet. 2021, 7, e546. [Google Scholar] [CrossRef]
- Duarri, A.; Nibbeling, E.; Fokkens, M.R.; Meijer, M.; Boddeke, E.; Lagrange, E.; Stevanin, G.; Brice, A.; Durr, A.; Verbeek, D.S. The L450P Mutation in KCND3 Brings Spinocerebellar Ataxia and Brugada Syndrome Closer Together. Neurogenetics 2013, 14, 257–258. [Google Scholar] [CrossRef]
- Marina, M.L.C.; Fraile, P.Q.; Marmiesse, A.F.; Cruz, N.G.; del Valle, F.M. De novo sporadic mutation in the KCND3 gene in a patient with early onset of chronic ataxia. Rev. Neurol. 2019, 68, 398–399. [Google Scholar] [CrossRef]
- Zanni, G.; Hsiao, C.-T.; Fu, S.-J.; Tang, C.-Y.; Capuano, A.; Bosco, L.; Graziola, F.; Bellacchio, E.; Servidei, S.; Primiano, G.; et al. Novel KCND3 Variant Underlying Nonprogressive Congenital Ataxia or SCA19/22 Disrupt KV4.3 Protein Expression and K plus Currents with Variable Effects on Channel Properties. Int. J. Mol. Sci. 2021, 22, 4986. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.-T.; Fu, S.-J.; Liu, Y.-T.; Lu, Y.-H.; Zhong, C.-Y.; Tang, C.-Y.; Soong, B.-W.; Jeng, C.-J. Novel SCA19/22-Associated KCND3 Mutations Disrupt Human K(V) 4.3 Protein Biosynthesis and Channel Gating. Hum. Mutat. 2019, 40, 2088–2107. [Google Scholar] [CrossRef]
- Contaldi, E.; Gallo, S.; Corrado, L.; D’Alfonso, S.; Magistrelli, L. Parkinsonism in SCA19/22: Dopamine Transporter Imaging in an Italian Family Harboring a Novel Mutation. Cerebellum 2024, 23, 1226–1230. [Google Scholar] [CrossRef]
- Paucar, M.; Bergendal, Å.; Gustavsson, P.; Nordenskjöld, M.; Laffita-Mesa, J.; Savitcheva, I.; Svenningsson, P.; Bergendal, A.; Gustavsson, P.; Nordenskjold, M.; et al. Novel Features and Abnormal Pattern of Cerebral Glucose Metabolism in Spinocerebellar Ataxia 19. Cerebellum 2018, 17, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Avila-Jaque, D.; Martin, F.; Bustamante, M.L.; Álvarez, M.L.; Fernández, J.M.; de Montellano, D.J.D.O.; Pardo, R.; Varela, D.; Miranda, M. The Phenotypic Spectrum of Spinocerebellar Ataxia Type 19 in a Series of Latin American Patients. Cerebellum 2024, 23, 1727–1732. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Durr, A.; Majczenko, K.; Huang, Y.-H.; Liu, Y.-C.; Lien, C.-C.; Tsai, P.-C.; Ichikawa, Y.; Goto, J.; Monin, M.-L.; et al. Mutations in KCND3 Cause Spinocerebellar Ataxia Type 22. Ann. Neurol. 2012, 72, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.-C.; Lin, J.-H.; Teng, Y.-C.; Kao, C.-H.; Wang, P.-Y.; Soong, B.-W.; Tsai, T.-F. A Dominant Negative Kcnd3 F227del Mutation in Mice Causes Spinocerebellar Ataxia Type 22 (SCA22) by Impairing ER and Golgi Functioning. J. Pathol. 2025, 265, 57–68. [Google Scholar] [CrossRef]
- Tada, Y.; Kume, K.; Matsuda, Y.; Kurashige, T.; Kanaya, Y.; Ohsawa, R.; Morino, H.; Tabu, H.; Kaneko, S.; Suenaga, T.; et al. Genetic Screening for Potassium Channel Mutations in Japanese Autosomal Dominant Spinocerebellar Ataxia. J. Hum. Genet. 2020, 65, 363–369. [Google Scholar] [CrossRef]
- Hourez, R.; Servais, L.; Orduz, D.; Gall, D.; Millard, I.; D’EXaerde, A.d.K.; Cheron, G.; Orr, H.T.; Pandolfo, M.; Schiffmann, S.N. Aminopyridines Correct Early Dysfunction and Delay Neurodegeneration in a Mouse Model of Spinocerebellar Ataxia Type 1. J. Neurosci. 2011, 31, 11795–11807. [Google Scholar] [CrossRef]
- Hsiao, C.-T.; Tropea, T.F.; Fu, S.-J.; Bardakjian, T.M.; Gonzalez-Alegre, P.; Soong, B.-W.; Tang, C.-Y.; Jeng, C.-J. Rare Gain-of-Function KCND3 Variant Associated with Cerebellar Ataxia, Parkinsonism, Cognitive Dysfunction, and Brain Iron Accumulation. Int. J. Mol. Sci. 2021, 22, 8247. [Google Scholar] [CrossRef]
- Smets, K.; Duarri, A.; Deconinck, T.; Ceulemans, B.; van de Warrenburg, B.P.; Züchner, S.; Gonzalez, M.A.; Schüle, R.; Synofzik, M.; Van der Aa, N.; et al. First de Novo KCND3 Mutation Causes Severe Kv4.3 Channel Dysfunction Leading to Early Onset Cerebellar Ataxia, Intellectual Disability, Oral Apraxia and Epilepsy. BMC Med. Genet. 2015, 16, 51. [Google Scholar] [CrossRef]
- Choi, K.-D.; Kim, J.-S.; Kim, H.-J.; Jung, I.; Jeong, S.-H.; Lee, S.-H.; Kim, D.U.; Kim, S.-H.; Choi, S.Y.; Shin, J.-H.; et al. Genetic Variants Associated with Episodic Ataxia in Korea. Sci. Rep. 2017, 7, 13855. [Google Scholar] [CrossRef]
- Mercer, R.C.C.; Ma, L.; Watts, J.C.; Strome, R.; Wohlgemuth, S.; Yang, J.; Cashman, N.R.; Coulthart, M.B.; Schmitt-Ulms, G.; Jhamandas, J.H.; et al. The Prion Protein Modulates A-Type K+ Currents Mediated by Kv4.2 Complexes through Dipeptidyl Aminopeptidase-like Protein 6. J. Biol. Chem. 2013, 288, 37241–37255. [Google Scholar] [CrossRef] [PubMed]
- Alier, K.; Li, Z.; MacTavish, D.; Westaway, D.; Jhamandas, J.H. Ionic Mechanisms of Action of Prion Protein Fragment PrP(106–126) in Rat Basal Forebrain Neurons. J. Neurosci. Res. 2010, 88, 2217–2227. [Google Scholar] [CrossRef]
- Baldwin, K.J.; Correll, C.M. Prion Disease. Semin. Neurol. 2019, 39, 428–439. [Google Scholar] [CrossRef]
- Wilke, C.; Pellerin, D.; Mengel, D.; Traschütz, A.; Danzi, M.C.; Dicaire, M.J.; Neumann, M.; Lerche, H.; Bender, B.; Houlden, H.; et al. GAA-FGF14 Ataxia (SCA27B): Phenotypic Profile, Natural History Progression and 4-Aminopyridine Treatment Response. Brain 2023, 146, 4144–4157. [Google Scholar] [CrossRef] [PubMed]
- Clément, G.; Puisieux, S.; Pellerin, D.; Brais, B.; Bonnet, C.; Renaud, M. Spinocerebellar Ataxia 27B (SCA27B), a Frequent Late-Onset Cerebellar Ataxia. Rev. Neurol. 2024, 180, 410–416. [Google Scholar] [CrossRef]
- Jensen, H.B.; Ravnborg, M.; Dalgas, U.; Stenager, E. 4-Aminopyridine for Symptomatic Treatment of Multiple Sclerosis: A Systematic Review. Ther. Adv. Neurol. Disord. 2014, 7, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.; Koska, V.; Hecker, C.; Göttle, P.; Hilla, A.M.; Heskamp, A.; Lepka, K.; Issberner, A.; Hallenberger, A.; Baksmeier, C.; et al. Protective Effects of 4-Aminopyridine in Experimental Optic Neuritis and Multiple Sclerosis. Brain 2020, 143, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanpour, S.; Rahimibarghani, S.; Rohani, S.; Rastkar, M.; Ghajarzadeh, M. Fampridine for Gait Imbalance in Patients with Multiple Sclerosis (MS): A Systematic Review and Meta-Analysis. Neurol. Sci. 2023, 44, 3059–3069. [Google Scholar] [CrossRef]
- Lecat, M.; Decavel, P.; Magnin, E.; Lucas, B.; Gremeaux, V.; Sagawa, Y. Multiple Sclerosis and Clinical Gait Analysis before and after Fampridine: A Systematic Review. Eur. Neurol. 2017, 78, 272–286. [Google Scholar] [CrossRef]
- Elbini, I.; Neili, N.-E. Potassium Channels at the Crossroads of Neuroinflammation and Myelination in Experimental Models of Multiple Sclerosis. Biochem. Biophys. Res. Commun. 2023, 653, 140–146. [Google Scholar] [CrossRef]
- Toljan, K.; Aboseif, A.; Amin, M. Efficacy of Pharmacologic Treatments for Fatigue in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Mult. Scler. Relat. Disord. 2025, 96, 106352. [Google Scholar] [CrossRef]
- Leussink, V.I.; Montalban, X.; Hartung, H.P. Restoring Axonal Function with 4-Aminopyridine: Clinical Efficacy in Multiple Sclerosis and Beyond. CNS Drugs 2018, 32, 637–651. [Google Scholar] [CrossRef]
- Kawaguchi, N.; Nakanishi, T. Animal Disease Models and Patient-IPS-Cell-Derived In Vitro Disease Models for Cardio-vascular Biology—How Close to Disease? Biology 2023, 12, 468. [Google Scholar] [CrossRef]
- Lei, T.; Zhang, X.; Fu, G.; Luo, S.; Zhao, Z.; Deng, S.; Li, C.; Cui, Z.; Cao, J.; Chen, P.; et al. Advances in Human Cellular Mechanistic Understanding and Drug Discovery of Brain Organoids for Neurodegenerative Diseases. Ageing Res. Rev. 2024, 102, 102517. [Google Scholar] [CrossRef] [PubMed]
- Wainger, B.J.; Kiskinis, E.; Mellin, C.; Wiskow, O.; Han, S.S.W.; Sandoe, J.; Perez, N.P.; Williams, L.A.; Lee, S.; Boulting, G.; et al. Intrinsic Membrane Hyperexcitability of ALS Patient-Derived Motor Neurons. Cell Rep. 2014, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Feng, Y.; Quinn, R.J.; Pountney, D.L.; Richardson, D.R.; Mellick, G.D.; Ma, L. Potassium Channels in Parkinson’s Disease: Potential Roles in Its Pathogenesis and Innovative Molecular Targets for Treatment. Pharmacol. Rev. 2023, 75, 758–788. [Google Scholar] [CrossRef]
- Bhoi, R.; Mitra, T.; Tejaswi, K.; Manoj, V.; Ghatak, S. Role of Ion Channels in Alzheimer’s Disease Pathophysiology. J. Membr. Biol. 2025, 258, 187. [Google Scholar] [CrossRef]
- Wulf, M.A.; Senatore, A.; Aguzzi, A. The Biological Function of the Cellular Prion Protein: An Update. BMC Biol. 2017, 15, 34. [Google Scholar] [CrossRef]
- Jerng, H.H.; Pfaffinger, P.J. Modulatory Mechanisms and Multiple Functions of Somatodendritic A-Type K+ Channel Auxiliary Subunits. Front. Cell Neurosci. 2014, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- de Benito-Bueno, A.; Socuellamos, P.G.; Merinero, Y.G.; Cercos, P.; Izquierdo, C.; Daniel-Mozo, M.; Marín-Olivero, I.; Perez-Lara, A.; Gonzalez-Vera, J.A.; Orte, A.; et al. Modulation of KV4.3-KChIP2 Channels by IQM-266: Role of DPP6 and KCNE2. Int. J. Mol. Sci. 2022, 23, 9170. [Google Scholar] [CrossRef] [PubMed]
- Radomski, K.L.; Zi, X.; Lischka, F.W.; Noble, M.D.; Galdzicki, Z.; Armstrong, R.C. Acute Axon Damage and Demyelination Are Mitigated by 4-Aminopyridine (4-AP) Therapy after Experimental Traumatic Brain Injury. Acta Neuropathol. Commun. 2022, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Göbel, K.; Wedell, J.H.; Herrmann, A.M.; Wachsmuth, L.; Pankratz, S.; Bittner, S.; Budde, T.; Kleinschnitz, C.; Faber, C.; Wiendl, H.; et al. 4-Aminopyridine Ameliorates Mobility but not Disease Course in an Animal Model of Multiple Sclerosis. Exp. Neurol. 2013, 248, 62–71. [Google Scholar] [CrossRef]
- Tang, S.L.; Tran, V.; Wagner, E.J. Sex Differences in the Cannabinoid Modulation of an A-Type K+ Current in Neurons of the Mammalian Hypothalamus. J. Neurophysiol. 2005, 94, 2983–2986. [Google Scholar] [CrossRef]
- Angelova, P.R.; Müller, W.S. Arachidonic Acid Potently Inhibits Both Postsynaptic-Type Kv4.2 and Presynaptic-Type Kv1.4 IA Potassium Channels. Eur. J. Neurosci. 2009, 29, 1943–1950. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Lo, C.K.L.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing Reviewers’ to Authors’ Assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological Quality and Synthesis of Case Series and Case Reports. BMJ Evid. Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef] [PubMed]
| Citation | Disease (Study Design) | Participants and/or Model | Comparator | Outcome Variable | Key Findings | RoB |
|---|---|---|---|---|---|---|
| Amyotrophic Lateral Sclerosis (ALS) | ||||||
| Park et al., 2008 [56] | ALS (Experimental study) | hBM-MSC (from ALS patients, in vitro) | K+ blocker (4-AP, TEA) | Kv4.x current and KCND2 mRNA | ↓ KCND2 expression and Ito current detected in hBM-MSCs. Kv4.2-dependent profile varied with passage. | High |
| Trainito et al., 2024 [71] | ALS (Experimental study) | NSC-34 (motor neuron line) | Untreated vs. CBN | KCND1 and KCND2 mRNA | ↑ KCND1 and KCND2 expression. CBN increases KCND1 and KCND2 expression in a dose-dependent manner. | Low |
| Parkinson’s Disease (PD) | ||||||
| Subramaniam et al. (2014) [72] | PD (Experimental study) | DA neurons in the substantia nigra (SN) from A53T-α-syn mouse | WT | Kv4.3 function | ↓ Function of Kv4.3. Mutant α-syn induced Kv4.3 dysfunction and enhanced neuronal excitability in DA neurons. | Some concerns |
| Lasser-Katz et al. (2017) [57] | PD (Experimental study) | DMV neurons (A53T-α-syn mouse) | WT mice and treated with glutation (GSH intervention) | Kv4.3 currents | = Function of Kv4.3. Kv4.3 currents unchanged in A53T-α-syn; GSH rescued currents in WT but not mutant mice, suggesting DMV neurons are less vulnerable to oxidative stress. | Low |
| Alzheimer’s disease (AD) | ||||||
| Cacace et al. (2019) [73] | AD (Observational study of EOAD and FTD) | DPP6 KO mice; patient brain (variants p.R509R, p.R47L, p.D596N) | WT/patient carriers | Kv4.2 protein expression and function | ↓ Kv4.2 expression and function with DPP6 loss. Variants linked to reduced Kv4.2 and impaired excitability. | Low |
| Yao et al. (2020) [74] | AD (Experimental study) | CA1 neurons from 5xFAD × RyR2-E4872Q mice | WT and R-carvedilol intervention | Kv4.2 current and function | ↓ Kv4.2 current. RyR2 hyperactivation showed dysfunction of the Kv4.2 channel. Carvedilol rescued Kv4.2 impairment. | Some concerns |
| Plant et al. (2006) [75] | AD (Experimental study) | Rat cerebellar granule neurons; HEK293 (Aβ1–40/42) | N/A | Kv4.2/Kv4.3 current and protein expression | ↑ Expression and function of Kv4.2 and Kv4.3. The Aβ peptide modulates Kv4 channel activity in culture. | Low |
| Hall et al. (2015) [53] | AD (Experimental study) | CA1/dentate neurons (hAPPJ20, hAPP/PS1dE9 mice) | WT; and hAPP model with levetiracetam intervention | Kv4.2 protein expression | ↓ Kv4.2 expression in dendrites (rather than in somas). Aβ load correlated with reduced Kv4.2. Levetiracetam prevents the loss of Kv4.2 protein. | Some concerns |
| Kim et al. (2021) [76] | AD (Experimental study) | Dentate gyrus neurons (Tg2576 mice) | WT and with Trolox intervention | Kv4.1 mRNA, protein expression and function | ↓ Kv4.1 expression and function. Trolox restores Kv4.1 expression, normalizes excitability, and restores Kv4.1 levels. | Some concerns |
| Li et al. (2023) [77] | AD (Experimental study) | Hippocampal neurons (APP−/− mice) | WT | Kv4.2 protein expression, LFP, glutamatergic neuron firing | = Kv4.2 expression. APP loss did not affect Kv4.2 expression or function; neuronal excitability unaltered. | Low |
| Scala et al. (2015) [78] | AD (Experimental study) | Hippocampal neurons in a 3xTg-AD mice | Intracellular incubation of Aβ42 and treatment with caspase and glycogen synthase kinase 3 (GSK-3) as an intervention | A-type K+ currents and Kv4.2 phosphorylation | ↓ Kv4.2 function. Intracellular Aβ42 accumulation induces hyperexcitability dependent on the inhibition of Ia mediated by Kv4.2 phosphorylation. Dephosphorylation of Kv4.2 by GSK-3 inhibition prevents this increase in excitability. | Low |
| Pan et al. (2004) [79] | AD (Experimental study) | Rats treated with Aβ25-35 | N/A | Kv4.2 and Kv4.3 mRNA and protein expression | ↑ Kv4.2 expression and = Kv4.3 expression. Aβ25-35 increases Kv4.2 mRNA (58%) in cortex. Kv4.2 protein analysis increased (42%) in cortex and (5%) hippocampus. No change in Kv4.3 mRNA. | Some concerns |
| Kerrigan et al. (2008) [80] | AD (Experimental study) | Rat CGN and HEK293 cells treated with Aβ1-40 | CGN WT | K+ currents (IK and Ia) and Kv4.2 mRNA expression | ↑ Kv4.2 expression and function. Aβ1-40 modulates K+ currents, increased Kv4.2 expression and altered Kv4.2 inactivation in CGNs. | Some concerns |
| Pieri et al. (2010) [54] | AD (Experimental study) | Rat CGCs treated with Aβ25-35 | CGC SP intervention | K+ currents and Kv4.2 protein expression | ↑ Kv4.2 and↑Kv4.3 expression and function. Aβ25–35 increased Ia current and the protein expression of Kv4.2 and Kv4.3. SP reversed these effects, normalizing current and expression. | Some concerns |
| Campolongo et al. (2013) [81] | AD (Experimental study) | Rats with intracerebroventricular Aβ25-35 | Intervention with SP | Kv4.2 protein expression | ↑ Kv4.2 expression (only hippocampus). SP treatment did not rescue changes in hippocampus or cortex. | Some concerns |
| Ataxia | ||||||
| Ågren et al. (2023) [82] | SCA19/22 (Case report) | Family clinical case with atypical SCA19/22 and Xenopus oocytes | WT and his variant: Kv4.3 WT, WT/E280K | Family clinical evaluation, KCND3 variant identification, and electrophysiological characterization of the channel in oocytes | Altered Kv4.3 function. The E280K variant causes a positive shift in the activation and inactivation potentials without altering the maximum current amplitude, associated with developmental delay, but without ataxia or parkinsonism in carriers. | High |
| Duarri et al. (2015) [83] | SCA19/22 (Experimental study) | SCA19/22-mutant Kv4.3 HeLa cells | WT | Current amplitude or gating properties, and protein Kv4.3 expression | ↓ Kv4.3 expression and altered function. T352P, M373I, S390N and ΔF227 mutations in Kv4.3 alter its function and localization. | Low |
| Reis et al. (2024) [84] | SCA19/22 (Experimental study) | Patient and CHO cells | WT and variant p.D152G, with or without KChIP2b as intervention | KCND3 variant identification and functional characterization by electrophysiology | ↓ Kv4.3 function. Variant p.D152G does not change gating but reduces ionic current. Coexpression with KChIP2b mitigates negative effect.although it does not reach statistical significance. Voltage dependence unaffected. | High |
| Duarri et al. (2012) [85] | SCA19/22 (Experimental study) | Post-mortem brain tissue of SCA19 patients and HeLa cells (Kv4.3 mutants) | WT and intervention with KChIP2b | KCND3 variant identification, protein expression, trafficking and function | ↓ Kv4.3 expression and function. Kv4.3 mutants (T352P, M373I, S390N) of HeLa cells showed absent or reduced surface expression due to ER retention, protein instability, and loss of function. KChIP2 subunit rescues localization in T352P and M373I, but without restoring function. The mislocalization of the S390N was not rescued by coexpression of KChIP2b. | High |
| Li et al. (2022) [86] | SCA19/22 (Experimental study) | iPSC of SCA19/22 patients | Healthy controls | KCND3 variant identification and Kv4.3 protein expression | ↓ Kv4.3 expression. It was identified T377M mutation in patients. iPSC with T377M reduces functional protein, mRNA unchanged, and associated with enrichment of ER stress. | Some concerns |
| Kurihara et al. (2018) [87] | SCA19/22 (Case report) | Patients with early-onset cerebellar ataxia | N/A | KCND3 variant identification | ? Kv4.3 expression or function. Novel KCND3 mutation (c.1150G > A, p.G384S). | High |
| Hsiao et al. (2025) [88] | SCA19/22 (Preclinical study) | Patient fibroblasts (C317Y, P375S), Drosophila, HEK293T, Xenopus oocytes | Control | Kv4.3 protein and locomotor function | ↓ Expression and function in Kv4.3 variants. Kv4.3 proteostasis defects underlie SCA19/22, and overexpression of human Kv4.3 rescued locomotor impairment. Human Kv4.3 variants (V338E and P375S) reduce Shal expression in flies and decrease K+ currents in Xenopus. | Some concerns |
| Arancibia et al. (2025) [89] | SCA19/22 (Genetic cohort study) | Patients and AD293 cells | WT AD293 and coexpressed with KChIP2 as intervention | KCND3 variant identification and current | ↓Kv4.3 function. Kv4.3 variants (G371R and S357W) either prevent or do not generate current. Coexpression with KChIP2 partially failed to rescue. | High |
| Paucar et al. (2021) [90] | SCA19/22 (Experimental study) | Family case, HEK293T cells and Xenopus oocytes | HEK293T cells transfected with V374A variant as intervention | Clinical evaluations, K+ currents, KCND3 variant identification and membrane expression | ↓ Kv4.3 function and = Kv4.3 expression. V374A mutant reduces K+ current, with normal membrane expression, associated with paroxysmal ataxia. | High |
| Duarri et al. (2013) [91] | SCA19/22 and Brugada syndrome (Case Report) | Patients and in vitro cells (HeLa and HEK293T) | WT and intervention with coexpressed KChIP2 | KCND3 variant identification and current analysis | ↑ Kv4.3 (p.L450P) and ? Kv4.3 (p.P614S) function. Identification of ataxia L450P variant with gain of function in Kv4.3 and a P614S variant not significantly different of WT. | Low |
| Carrasco-Marina et al. (2019) [92] | SCA19/22) (Case Report) | Pediatric patient with early-onset chronic ataxia | N/A | KCND3 variant identification and in silico functional analysis | Altered Kv4.3 function. Gly371Arg alters the function with non-conservative substitution in the channel pore region. | High |
| Zanni et al. (2021) [93] | SCA19/22) (Case Report) | Patients with non-progressive congenital ataxia and in vitro cells (Xenopus oocytes, HEK293T cells) | WT | KCND3 variant identification, protein expression and current | ↓ Kv4.3 expression and function. G345V, S347W, and W359G variants produce loss of function through reduced expression and currents. | Some concerns |
| Hsiao et al. (2019) [94] | SCA19/22) (Case Report) | Patients and in vitro cells (Xenopus oocytes, HEK293T cells) | WT and intervention with KChIP2 coexpressed | KCND3 variant identification, protein expression and current | ↓ Kv4.3 expression and function. New mutations identified: C317Y, P375S, V338E and T377M, with loss of function not restored by coexpression with KChIP2 and reduction in total Kv4.3 protein expression in the variants. | Low |
| Contaldi et al. (2024) [95] | SCA19/22) (Case Report) | Patient | N/A | KCND3 variant identification | ? Kv4.3 expression or function. Identified Ser346Phe mutation. Effect on function unclear. | High |
| Paucar et al. (2018) [96] | SCA19, allelic with SCA22 (Case Report) | Family case | N/A | KCND3 variant identification | ? KCND3 function or expression. Identified T377M mutation. | High |
| Avila-Jaque et al. (2024) [97] | SCA19 (Case Report) | Patients | N/A | KCND3 variant identification | ? Kv4.3 expression and function. Pathogenic variants from different Latin American populations reported: Ser357Trp, Gly384Ser, Gly371Arg. | High |
| Lee et al. (2012) [98] | SCA22 (Experimental study) | Patients and in vitro cells (HEK-293T transfected) | WT | KCND3 variant identification, protein expression and current | ↓ Kv4.3 expression and function. Multiple KCND3 mutations (F227del, G345V, V338E, T377M). F227del disrupted protein localization and decreases K+ currents. | Low |
| Hung et al. (2025) [99] | SCA22 (Experimental study) | Knock-in mice (F227del mutation) | WT | Current Kv4.3 and protein localization | ↓ Kv4.3 expression and function. F227del mutation causes SCA22 through a dominant negative mechanism that reduces function, affecting localization to the plasma membrane, protein accumulation, and causing dysfunctions in the ER and Golgi. | Some concerns |
| Tada et al. (2020) [100] | SCA13, SCA19 (Clinical Trial) | Patients | N/A | KCND3 variant identification | ? Kv4.3 function and 0 pathogenic variants in KCND3. No pathogenic mutations identified. | Low |
| Hourez et al. (2011) [101] | SCA1 (Comparative Study) | SCA1 Transgenic mouse model and in vitro cerebellar slices | WT and with aminopyridines intervention | mRNA expression and currents of Kv4 channels | = Kv4.1, = Kv4.2, = Kv4.3 (10× more in membrane surface) expressions. No increase in the expression of Kv4 in SCA1 mice. Increased Ia in presymptomatic PCs mediated by Kv4.3. Aminopyridines partially reversed electrical dysfunction. | Some concerns |
| Hsiao et al. (2021) [102] | Progressive cerebellar ataxia (Case Report) | Patients and HEK293T cells | WT | KCND3 variant identification, protein expression and current | ↑ Kv4.3 function and = Kv4.3 expression. Identified R419H mutation in the patient, which is predicted to be disease-causing but does not affect Kv4.3 localization or quantity, although increases the K+ current. | High |
| Smets et al. (2015) [103] | Early cerebellar ataxia (Case Report) | Patient and in vitro cells (HeLa and CHO-K1 cells) | WT and intervention with KChIP2 coexpression | KCND3 variant identification, protein expression and current | ↓ Kv4.3 function and = Kv4.3 expression. p.Arg293_Phe295dup mutation causes changes in the gating and inactivation properties of the channel, with reduction in output current. Kv4.3 detected at cell surface but reduced by cycloheximide and rescued by KChIP2 coexpression. | High |
| Choi et al. (2017) [104] | EA (Genetic cohort study) | Patients | N/A | KCND3 variant identification | ? Kv4.3 function. Identified Arg431Cys variant, but functional validation is required. | Some concerns |
| Prion diseases | ||||||
| Mercer et al. (2013) [105] | PrDS (GSS) (Experimental study) | Prnp0/0 KO mice, DPP6 df5J/Rw mice, and in vitro transfected cell lines (HEK293T, RK13, and N2a) | WT | Kv4.2 current and protein interactions | ↑ Kv4.2 function. The PrPC prion modulates Ia through DPP6, but the mutant prion variant (GSS form) loses Kv4.2 function. | Low |
| Alier et al. (2010) [106] | PrDs (Experimental study) | Rats | Control | Kv4.2 current and mRNA Kv4.2 expression | ↓ Kv4.2 function. Application of the PrP(106-126) fragment reduced Ia by 23.5% and IK currents. mRNA Kv4.2 expressed in 75% of cholinergic and 60% of GABAergic neurons, altering excitability. | Some concerns |
| KCND3 Variant | c. Nucleotide Change (HGVS) | p. Amino Acid Change | Accession Number (NM) | dbSNP rsID | Associated Ataxia | Affected Transmembrane Segment |
|---|---|---|---|---|---|---|
| C317Y | c.950G > A | p.Cys317Tyr | NM_001378969.1 | rs1571939905 | SCA19/22 | S4-S5 |
| D152G | c.455A > G | p.Asp455Gly | N/A | N/A | SCA19/22 | NH2 |
| E280K | c.838G > A | p.Glu280Lys | NM_001378969.1 | rs2101995916 | SCA19/22 | S3-S4 |
| F227del | c.679_681delTTC | p.Phe227del | NM_001378969.1 | rs397515475 | SCA19/22 | S2 |
| G345V | c.1034G > T | p.Gly345Val | NM_001378969.1 | rs797045634 | SCA19/22 | S5-S6 |
| G384S | c.1150G > A | p.Gly384Ser | NM_001378969.1 | rs1664632655 | SCA19/22 | S6 |
| G371R | c.1111G > A | p.Gly371Arg | NM_001378969.1 | rs1057521793 | SCA19/22 | S5-S6 |
| L450P | c.1348C > T | p.Leu450Phe | NM_001378969.1 | rs150401343 | SCA19/22 | COOH |
| M373I | c.1119G > A | p.Met373Ile | NM_001378969.1 | rs397515477 | SCA19/22 | S5-S6 |
| P375S | c.1123C > T | p.Pro375Ser | NM_001378969.1 | rs1571636508 | SCA19/22 | S5-S6 |
| P614S | c.1840C > T | p.Pro633Ser | NM_001378970.1 | N/A | SCA19/22 | COOH |
| R419H | c.1256G > A | p.Arg419His | NM_001378969.1 | rs774338559 | Progressive cerebelar ataxia | COOH |
| R431C | c.1291C > T | p.Arg431Cys | NM_001378969.1 | rs777183510 | SCA19/22 | COOH |
| S347W | c.1040C > G | p.Ser347Trp | N/A | N/A | SCA19/22 | S5-S6 |
| S357W | c.1070C > G | p.Ser357Trp | NM_001378969.1 | rs867628133 | SCA19/22 | S5-S6 |
| S390N | c.1169G > A | p.Ser390Asn | NM_001378969.1 | rs397515478 | SCA19/22 | S6 |
| T352P | c.1054A > C | p.Thr352Pro | NM_001378969.1 | rs397515476 | SCA19/22 | S5-S6 |
| T377M | c.1130C > T | p.Thr377Met | NM_001378969.1 | rs1571636501 | SCA19/22 | S5-S6 |
| V338E | c.1013T > C | p.Val338Glu | NM_001378969.1 | rs1571939827 | SCA19/22 | S5 |
| V374A | c.1121T > C | N/A | N/A | N/A | SCA19/22 | S5-S6 |
| W359G | c.1075T > G | p.Trp359Gly | N/A | N/A | SCA19/22 | S5-S6 |
| N/A | c.1037C > T | p.Ser346Phe | N/A | N/A | SCA19/22 | N/A |
| N/A | c.877_885dupCGCGTCTTC | p.Arg293_Phe295dup | NM_001378969.1 | rs2525698722 | SCA19/22 | S4-S5 |
| Neurodegenerative Disease | N Studies with Alterations of Kv | Kv4.1 (n, %) | Kv4.2 (n, %) | Kv4.3 (n, %) |
|---|---|---|---|---|
| ALS | 3 | 1 (33.3%) | 2 (66.7%) | 0 (0%) |
| PD | 1 | 0 (0%) | 0 (0%) | 1 (100%) |
| AD | 12 | 1 (8.3%) | 9 (75.0%) | 2 (%) |
| Ataxias (SCA/EA) | 16 | 0 (0%) | 0 (0%) | 16 (6.7%) |
| Prionopathies | 2 | 0 (0%) | 2 (100%) | 0 (0%) |
| Total | 34 | 2 (5.9%) | 13 (38.2%) | 19 (55.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teruel-Peña, B.; Gómez-Torres, P.; Galarreta-Aperte, S.; Suleiman-Martos, N.; Prieto, I.; Ramírez-Sánchez, M.; Fernández-Martos, C.M.; Domínguez-Vías, G. Systematic Review of the Role of Kv4.x Potassium Channels in Neurodegenerative Diseases: Implications for Neuronal Excitability and Therapeutic Modulation. Physiologia 2025, 5, 31. https://doi.org/10.3390/physiologia5030031
Teruel-Peña B, Gómez-Torres P, Galarreta-Aperte S, Suleiman-Martos N, Prieto I, Ramírez-Sánchez M, Fernández-Martos CM, Domínguez-Vías G. Systematic Review of the Role of Kv4.x Potassium Channels in Neurodegenerative Diseases: Implications for Neuronal Excitability and Therapeutic Modulation. Physiologia. 2025; 5(3):31. https://doi.org/10.3390/physiologia5030031
Chicago/Turabian StyleTeruel-Peña, Bárbara, Piedad Gómez-Torres, Sergio Galarreta-Aperte, Nora Suleiman-Martos, Isabel Prieto, Manuel Ramírez-Sánchez, Carmen M. Fernández-Martos, and Germán Domínguez-Vías. 2025. "Systematic Review of the Role of Kv4.x Potassium Channels in Neurodegenerative Diseases: Implications for Neuronal Excitability and Therapeutic Modulation" Physiologia 5, no. 3: 31. https://doi.org/10.3390/physiologia5030031
APA StyleTeruel-Peña, B., Gómez-Torres, P., Galarreta-Aperte, S., Suleiman-Martos, N., Prieto, I., Ramírez-Sánchez, M., Fernández-Martos, C. M., & Domínguez-Vías, G. (2025). Systematic Review of the Role of Kv4.x Potassium Channels in Neurodegenerative Diseases: Implications for Neuronal Excitability and Therapeutic Modulation. Physiologia, 5(3), 31. https://doi.org/10.3390/physiologia5030031









