Abstract
Background/Objectives: This study investigates whether systemic ovalbumin–aluminum sensitization followed by cutaneous challenge induces thermal hyperalgesia and spinal cord gliosis in mice. Methods: Adult female ICR-CD1 mice received ovalbumin–aluminum salt solution intraperitoneally on days 0, 7 and 14, and subcutaneously with daily skin application via brush during days 15–21, 28–35 and 42–49. Control animals received saline. Plantar thermal hyperalgesia was assessed on days 21, 35 and 49. On day 49, tissues (dorsal skin, spinal cord, footpads) were harvested after perfusion and processed histologically to detect mast cells, astrocytes, microglia, afferent fibers and intraepidermal nerve profiles. Results: Ovalbumin-treated mice displayed thermal hyperalgesia, increased astrogliosis, and reactive microglia in the spinal cord, and expanded CGRP- and IB4-immunoreactive fiber areas. In footpads, CGRP-positive intraepidermal nerve profile density was elevated, and dorsal skin showed increased mast cell density compared to controls. Conclusions: Combined systemic and cutaneous ovalbumin–aluminum sensitization induces skin inflammation, spinal gliosis, and nociceptive fiber sprouting in both central and peripheral sites, which likely contribute to the observed thermal hyperalgesia.
1. Introduction
The cutaneous administration of ovalbumin solution is a chemical method used for inducing atopic dermatitis in mice [1,2], rats [3], and dogs [4]. In this chemical model of murine atopic dermatitis, like what is observed in humans, there is an increase in epidermal thickness (epidermal hyperplasia), an abundance of eosinophils, mast cells, and T-cell lymphocytes, overexpression of inflammatory cytokines and chemokines, sensory hyperinnervation, and scratching behavior [2]. These findings suggest that this murine model reproduces approximately 85–90% of the signs and symptoms observed in human atopic dermatitis. Likewise, it has recently been reported that subcutaneous injection and topical application (with a brush) of an ovalbumin and aluminum salt solution can induce dermatitis-like changes in mice [5]. The overall prevalence of atopic dermatitis is estimated to be 15–20% in children and up to 10% in adults [6,7].
Patients with atopic dermatitis experience skin pruritus or itching, leading to scratching that exacerbates the infectious and inflammatory processes of the condition [8,9]. However, between 55–60% of patients with atopic dermatitis also report experiencing skin pain [10,11,12]. The plantar, palmar, and thoracic skin areas are the regions where skin pain is most frequently observed in these patients [13]. Patients with atopic dermatitis describe the pain as burning, itchy, electric shock-like, or cold, often characterizing it as sharp and bothersome [12].
Compounds such as complete Freund’s adjuvant (CFA) [14,15], carrageenan [16,17,18,19], formalin [20], and histamine [21,22] are known to trigger inflammatory pain when administered cutaneously. In contrast, the intracutaneous injection of ovalbumin induces an inflammatory response in animals pre-sensitized with ovalbumin and aluminum salts [23,24]. Furthermore, animals sensitized with 200 µL of ovalbumin solution and aluminum salts in saline, administered subcutaneously into the skin of the dorsum, exhibit paw-licking responses when ovalbumin is injected into the footpads, suggesting nocifensive behavior [25]. These findings suggest that all these compounds, including ovalbumin injected into the skin, induce both inflammation and pain.
In experimental models of atopic dermatitis induced by treatment with ovalbumin and aluminum salts in phosphate-buffered saline (PBS) or saline solution, administered via epicutaneous application with a patch impregnated with the solutions [26,27,28,29], by subcutaneous injection and brush application [5], or by subcutaneous injection alone [30], an increase in mast cells has been observed in the treated skin areas. Mast cells, during degranulation, synthesize and release a wide variety of chemical mediators including: (i) biogenic amines (histamine, serotonin, dopamine), (ii) lipid metabolites (platelet-activating factor, prostaglandins, leukotrienes), (iii) peptides (bradykinin, VIP, CRH), (iv) growth factors (NGF, bFGF, VEGF), (v) peptidoglycans (heparin, chondroitin sulfate), (vi) cytokines (IL1, IL3, IL4, IL6, IL8, IL9, IL17, IL33, Interferon gamma, TNF-α, TGF-β, (vii) chemokines (MCP-1, eotaxin, TARC, RANTES), (viii) enzymes (chymase, tryptase, serine proteases, aspartic acid proteases, cysteine proteases, metalloproteinases), and (ix) nitric oxide [31,32,33,34,35,36].
Some of these chemical mediators interact with transmembrane proteins (membrane receptors) on cutaneous nociceptors in the dermis and epidermis. These mediator-receptor interactions alter ionic conductance in nociceptive nerve fibers, generating action potentials that propagate through the somatosensory pain system, and give rise to the sensation and perception of pain [37,38,39]. Through a similar mechanism, these mediators can also activate pruritogenic nerve endings in the epidermis and dermis, generating action potentials that lead to the sensation and perception of itch or pruritus [39,40,41]. These chemical mediators released by mast cells also induce peripheral sensitization of nociceptors and pruritogenic receptors in the skin [42,43,44,45]. Taken together, this evidence suggests that mast cells, through the release of chemical mediators during degranulation, play a role in activating and sensitizing nociceptors and pruritogenic receptors (specifically those on C- and A-delta fiber nerve endings) that innervate the skin.
Intraplantar injection of carrageenan induces hyperalgesia and microgliosis in the dorsal horn of the spinal cord [46,47]. Activated microglial cells have also been observed in the thalamus and parietal cortex in response to carrageenan-induced inflammation in the hind paw [48]. CFA-induced pain hypersensitivity and peripheral inflammation, along with strong microglial activation in the spinal cord, have also been reported [49]. CFA-injection into the plantar surface of the hind paw causes thermal hyperalgesia and mechanical allodynia, as well as microgliosis and astrogliosis in the spinal cord, midbrain and thalamus [50]. Subcutaneous formalin injection into the hind paw similarly produces mechanical allodynia, thermal hyperalgesia, and gliosis in the dorsal horn of the spinal cord [51]. Microgliosis has also been observed in the dorsal horn of the spinal cord and the gracile nucleus of the brainstem following formalin injection into the hind paw [52]. These findings suggest that peripheral skin inflammation leads to pain responses associated with gliosis in both the spinal cord and supraspinal structures.
Furthermore, in an experimental model of rhinitis induced by treatment with ovalbumin, microgliosis was observed in the olfactory bulb [53]. Intrathecal injection of an ovalbumin solution (20 µg/mL in PBS) has also been shown to induce microgliosis in the hippocampus [54]. In another study, adult rats were sensitized with ovalbumin and treated intranasally on gestational day 15 with either an ovalbumin solution or vehicle. The offspring of rats exposed to ovalbumin showed microgliosis in the hippocampus [55]. These experimental findings indicate that microglial changes can occur in the central nervous system following exposure to ovalbumin.
Taken together, this evidence suggests that the cutaneous application of ovalbumin with aluminum salts in saline solution induces skin inflammation, which can lead to pain and activation of glial cells in the spinal cord. This animal model is intended to reflect human dermatitis, a cutaneous inflammation that causes nociceptive sensitization. The objectives of the present study are (1) to determine whether cutaneous application of saline solution containing ovalbumin and aluminum salts induces pain, as assessed by the plantar thermal algesimetry (or plantar) test; (2) to investigate, using immunohistochemical techniques, whether changes occur in microglial and astroglial cells in the spinal cord; (3) to assess histological changes in nociceptive afferent fibers in the dorsal horn of the spinal cord; and (4) to evaluate whether this treatment alters skin innervation in the foot pads.
2. Results
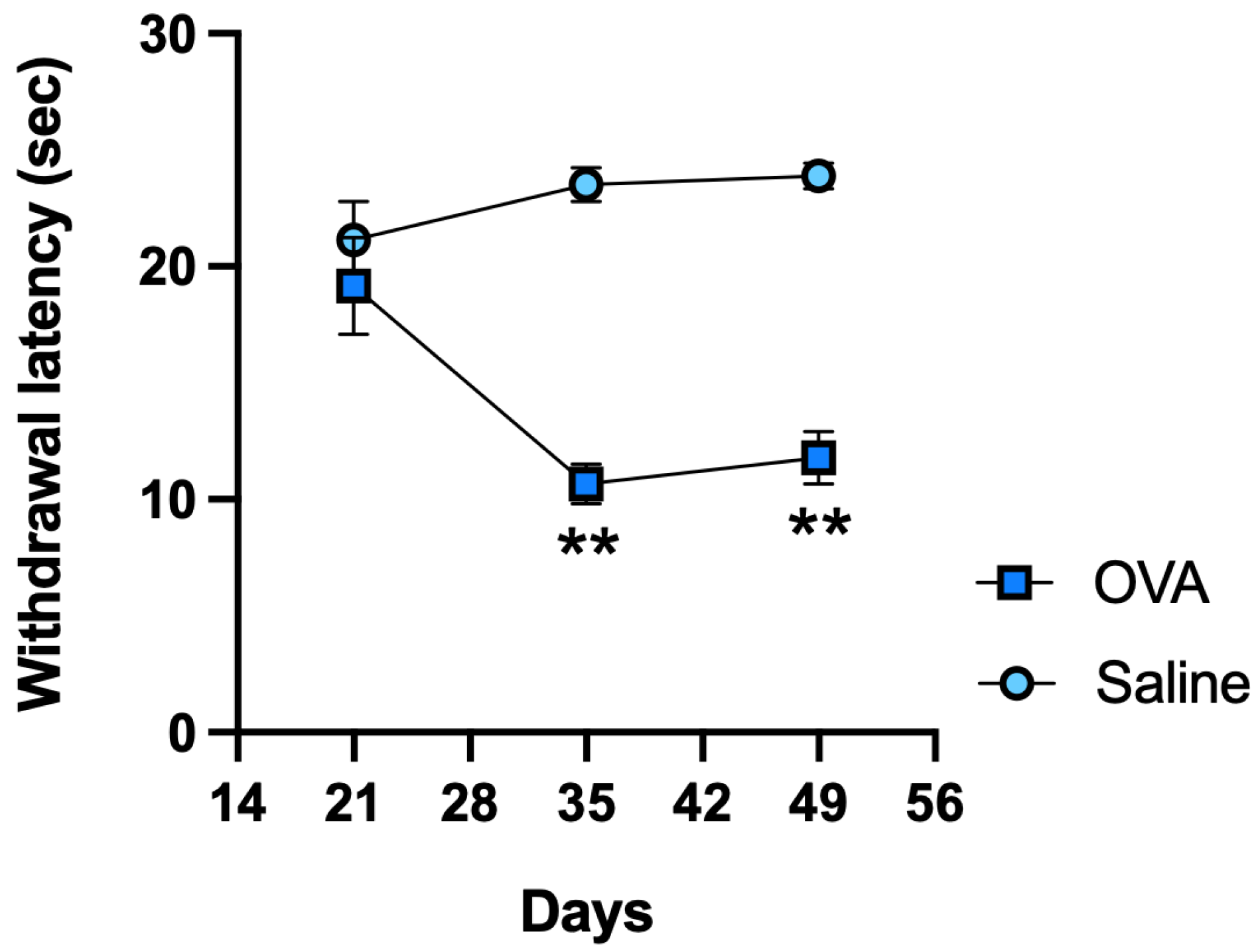
In response to noxious thermal stimulation, OVA-treated animals exhibited significantly shorter withdrawal latencies on days 35 and 49 compared to the control (saline) group, indicating the development of thermal hyperalgesia (Figure 1).
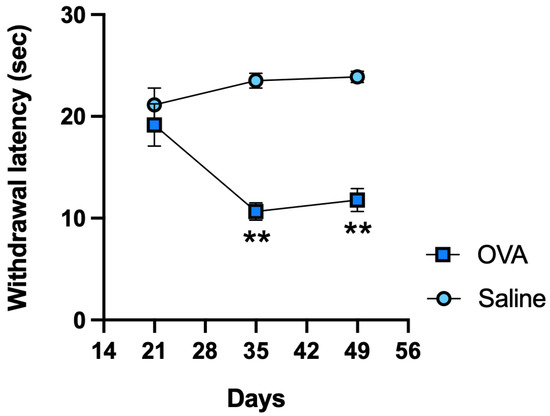
Figure 1.
Functional results in ICR-CD1 mice treated with OVA or saline over 49 days. Plantar thermal test withdrawal latency measured on days 21, 35, and 49. Values are expressed as mean ± standard error of the mean (SEM). The number of animals per experimental group is 6 (n = 6). ** p < 0.01 compared to the control group (saline).
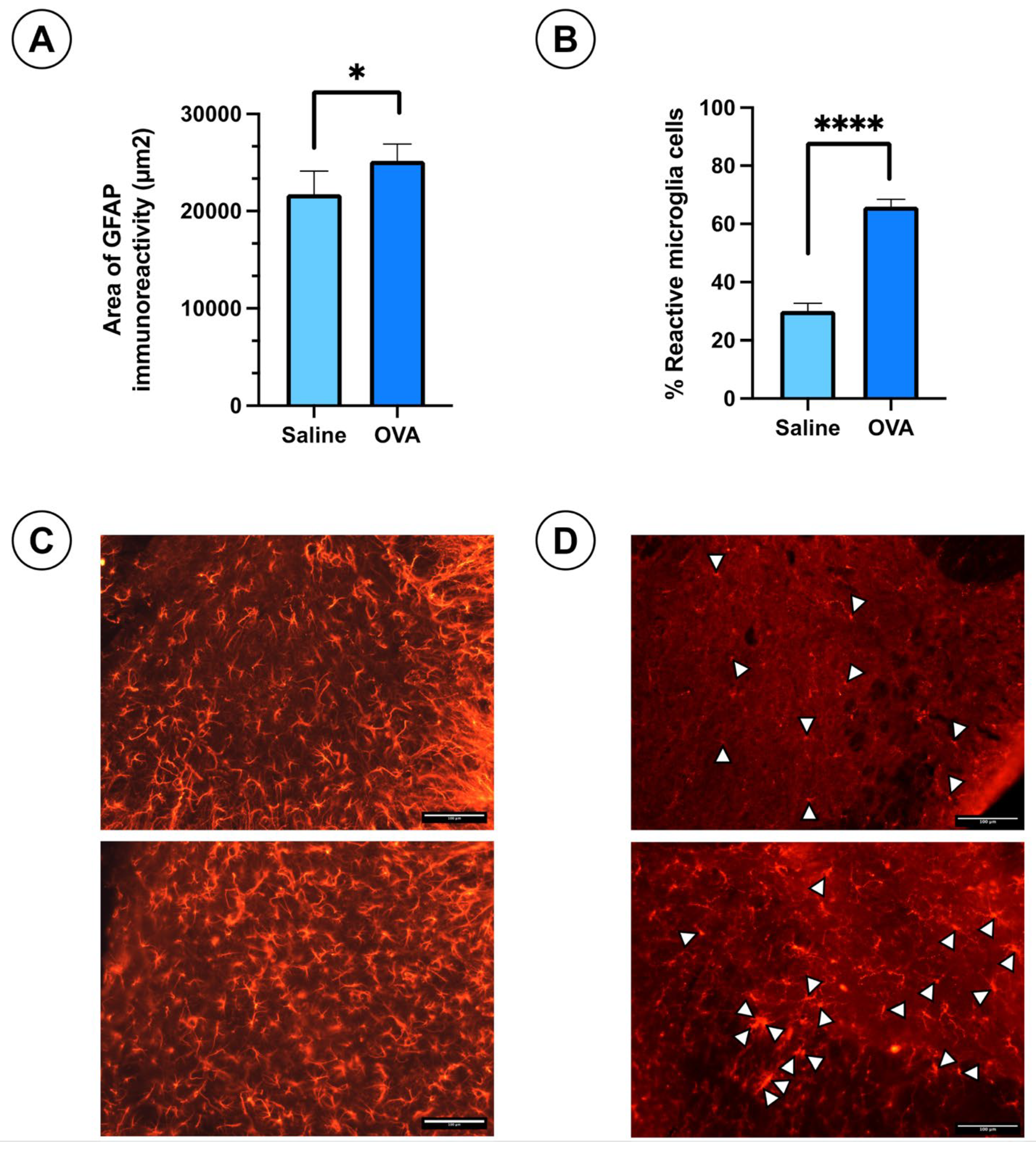
Immunohistochemical analysis of the spinal cord tissue sections at day 49 of follow-up revealed slightly hypertrophic astrocytes stained with GFAP antibodies in both OVA and saline-treated groups. The astrocytes appeared slightly more reactive in the OVA group (Figure 2C). However, the GFAP immuno-reactivity area was significantly larger in the OVA group compared to controls (Figure 2A). Non-reactive microglial cells exhibit small somas with highly branched cytoplasmic processes, whereas reactive microglia display retracted processes, an enlarged soma, and an amoeboid shape, with phagocytic capacity. In the IBA1-stained spinal cord sections, reactive microglia were identified by white arrowheads (Figure 2D). The percentage of reactive microglia was significantly higher in OVA-treated animals compared to saline-treated mice (Figure 2B). These results suggest that OVA treatment induces gliosis in the spinal cord, including both astrogliosis and microgliosis.
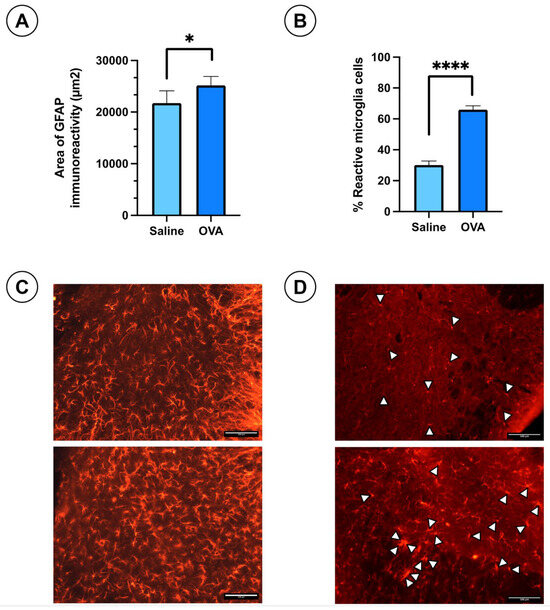
Figure 2.
Histological results in ICR-CD1 mice treated with OVA or saline over 49 days. (A) Area of immunoreactivity to glial fibrillary acidic protein (GFAP) in spinal cord sections from animals in both experimental groups. (B) Percentage of reactive microglia in IBA1-stained spinal cord histological sections for both groups. (C) Histological images showing GFAP immunoreactivity in spinal cord sections from both groups. (D) Histological images showing IBA1 immunoreactivity in spinal cord sections from both groups. White arrowheads indicate reactive microglia cells. Values are expressed as mean ± standard error of the mean (SEM). The number of animals per experimental group is 6 (n = 6). * p < 0.05 and **** p < 0.0001 compared to the control group (saline). Scale bar = 100 µm.
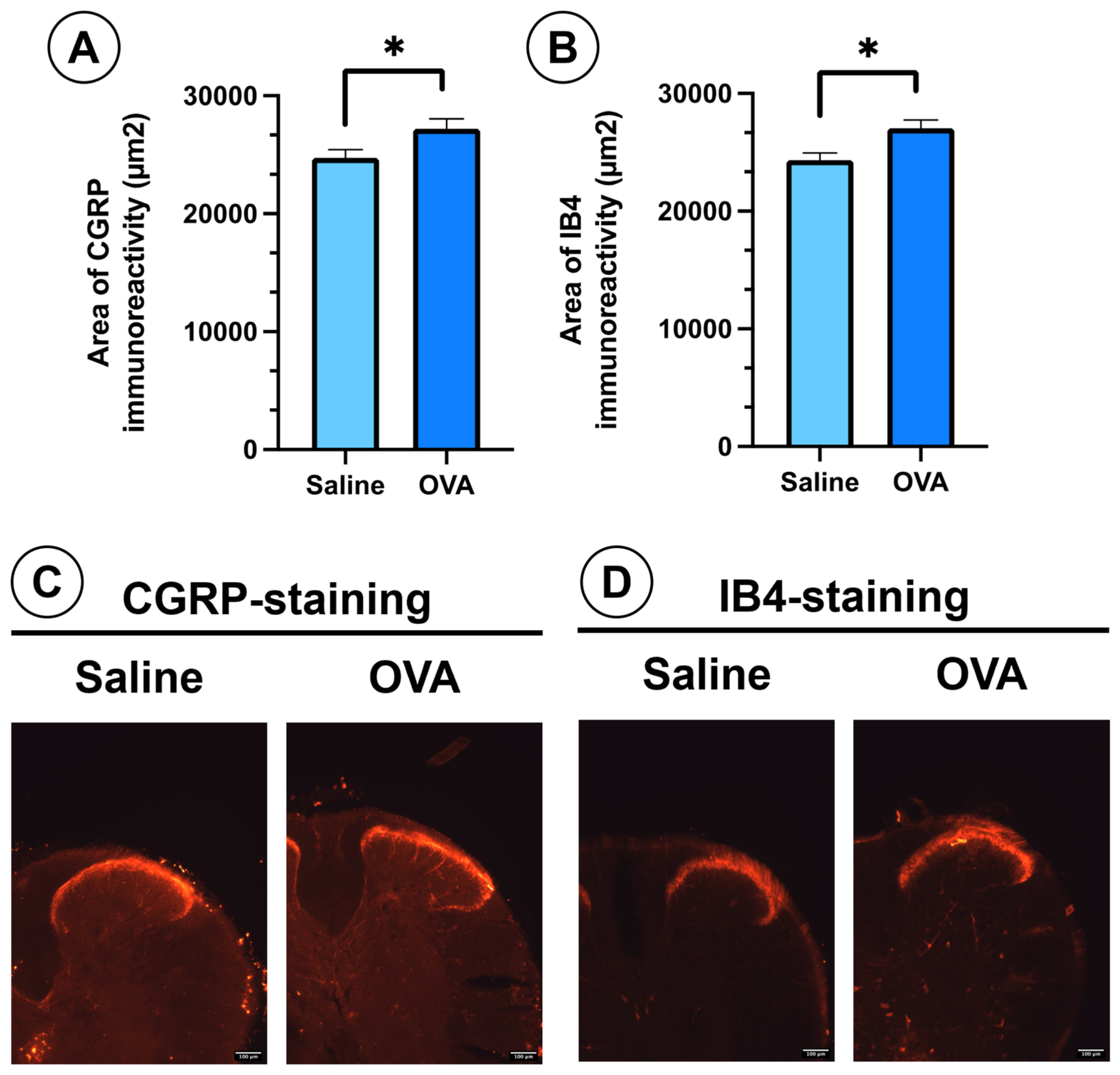
In spinal cord histological sections, OVA treatment also promoted the sprouting of peptidergic (CGRP-positive) and non-peptidergic (IB4-positive) nociceptive afferent fibers in the dorsal horn of the spinal cord. This was evidenced by a significant increase in the immunoreactivity area for these two markers in the dorsal horn (Figure 3A,B). These peptidergic (CGRP) and non-peptidergic (IB4) afferent nerve fibers project in the dorsal horn of the spinal cord onto the most superficial laminae of Rexed of the dorsal horn (laminae I, II) (Figure 3C,D).
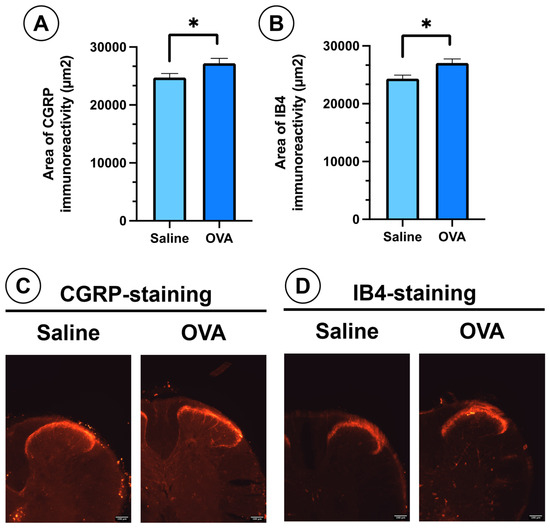
Figure 3.
CGRP and IB4 immunoreactivity in spinal cord histological sections from different experimental groups at day 49. (A) Area of immunoreactivity to CGRP in the dorsal horn across experimental groups. (B) Area of immunoreactivity to IB4 in the dorsal horn across experimental groups. (C) Histological images showing nociceptive afferent fibers in the dorsal horn, immunolabelled for CGRP (peptidergic nociceptive fibers). (D) Histological images showing nociceptive afferent fibers in the dorsal horn, immunolabelled for IB4 (non-peptidergic fibers). Data are presented as mean ± SEM (n = 6). * p < 0.05 vs. saline control. Scale bar = 100 µm.
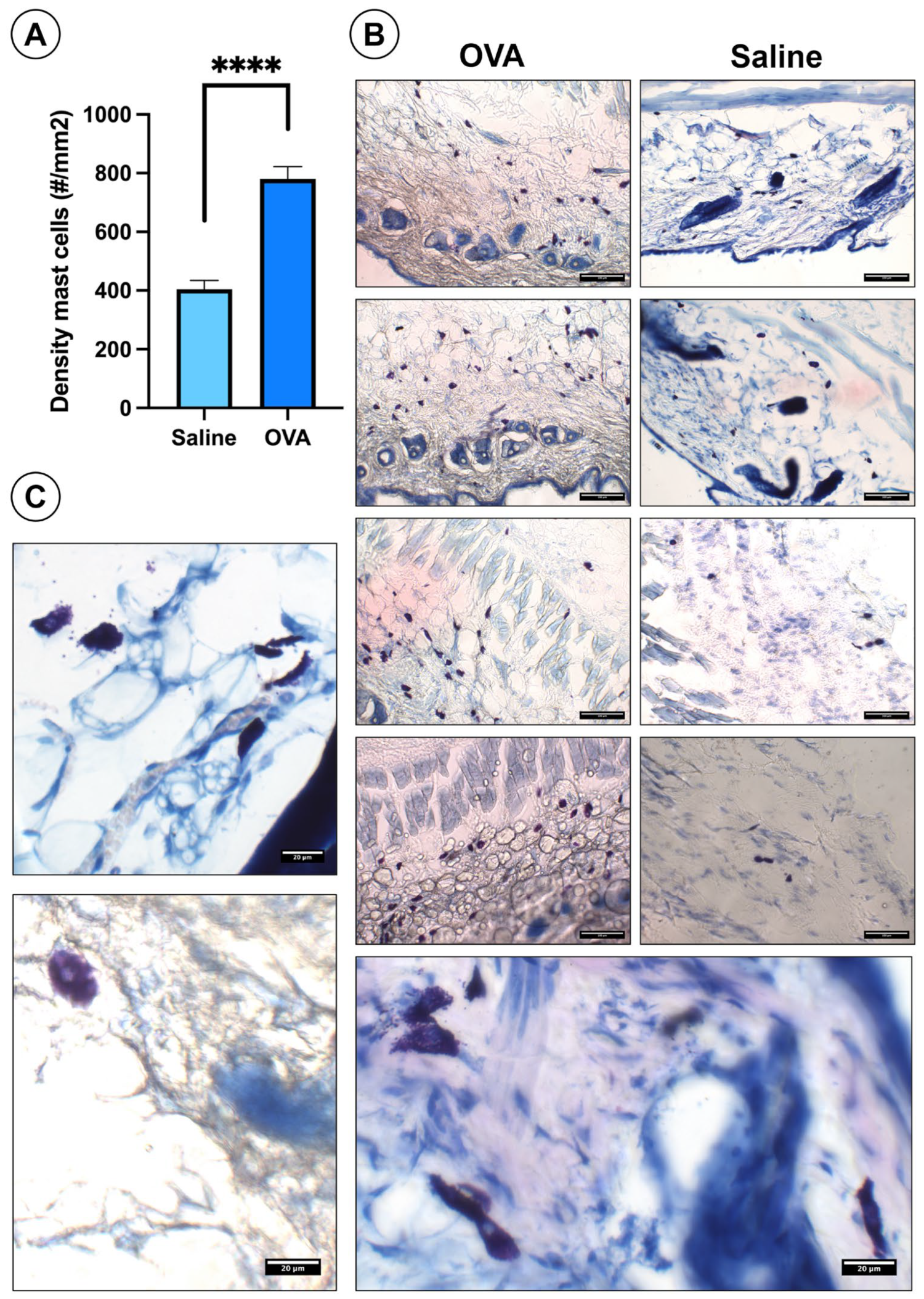
In skin histological sections, OVA treatment significantly increased mast cell density compared to the saline controls (Figure 4A). OVA-treated sections showed more mast cells throughout the dermis of the dorsal skin. (Figure 4B). Mast cells appear as bluish-violet cells distributed throughout the dermis of the dorsal skin (Figure 4B). Under higher magnification, many mast cells displayed degranulation (Figure 4C).
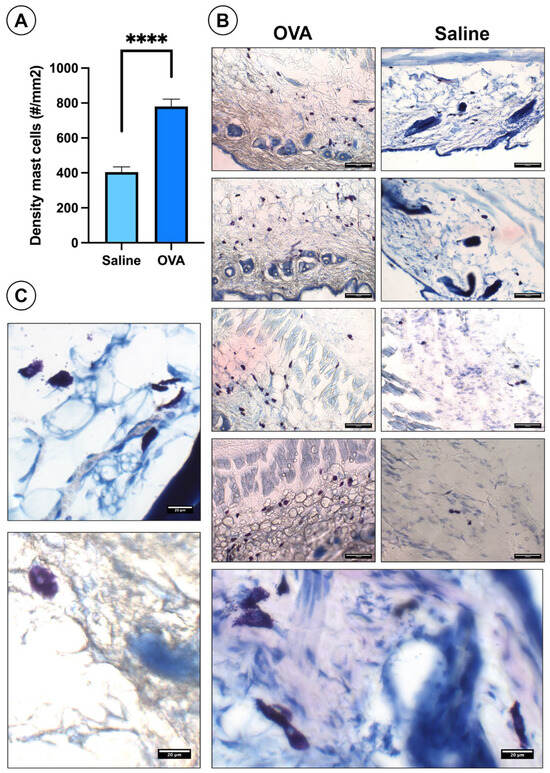
Figure 4.
Histological results of mast cells in the dorsal skin at day 49. (A) Histogram showing mast cell density in the dorsal skin across experimental groups. (B) Histological images of the dorsal skin from both experimental groups highlighting the presence of mast cells. (C) High-magnification images of degranulated mast cells. Data are presented as mean ± SEM. (n = 6). **** p < 0.0001 vs. saline control. Scale bars: 100 µm (B), 20 µm (C).
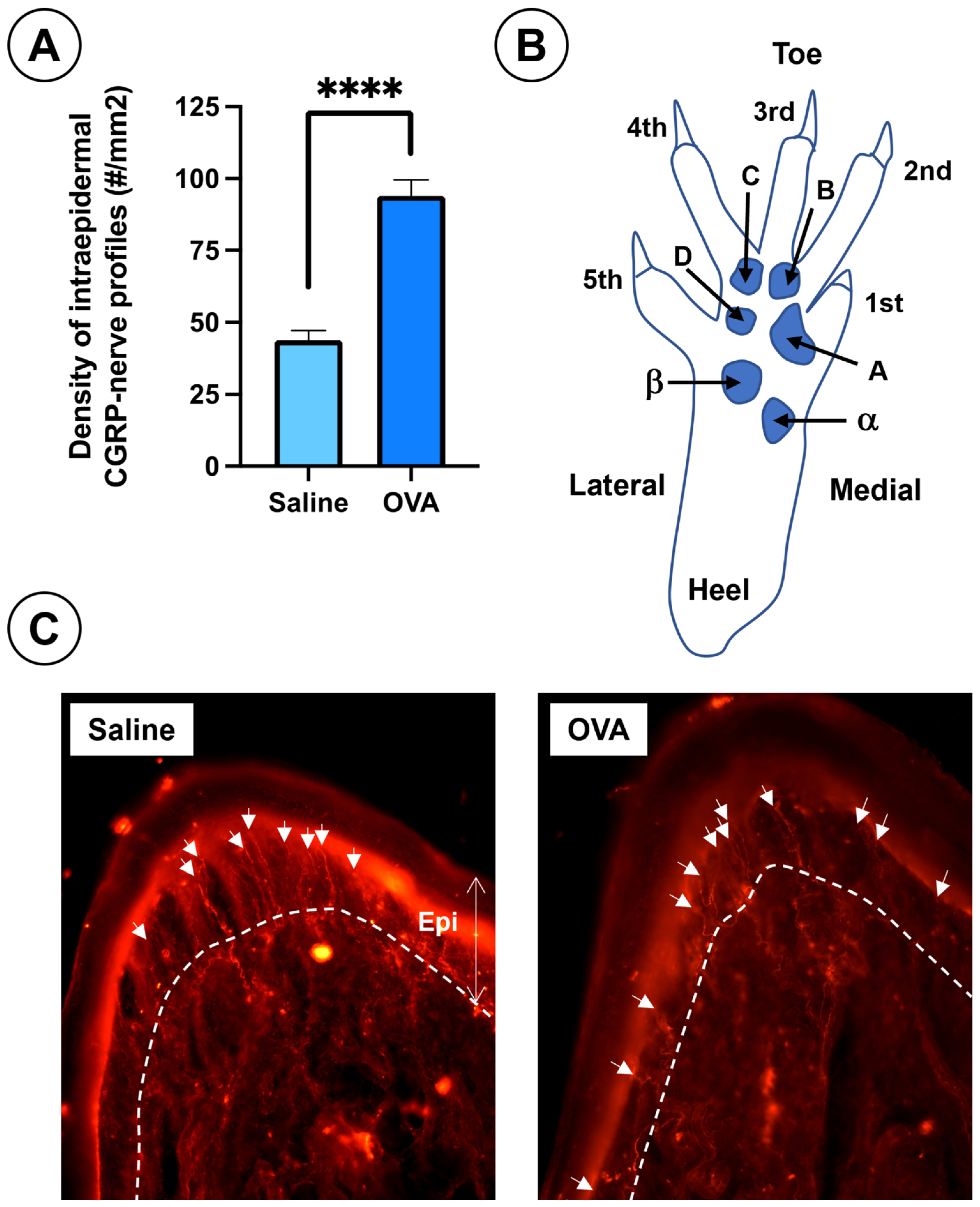
In addition, OVA treatment caused a significant increase in CGRP-positive intraepidermal nerve fiber density in the footpads, compared to saline-treated animals (Figure 5).
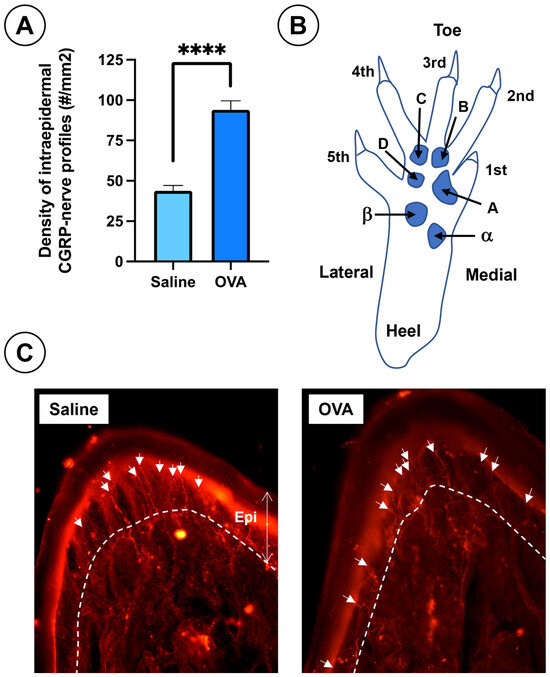
Figure 5.
Results of nociceptive afferent fibers in the skin of the footpads at day 49 across the experimental groups. (A) Density of intraepidermal nerve profiles immunoreactive to CGRP in the plantar pads from both experimental groups. (B) Diagram of the mouse hind paw, (right side frontal and plantar view) showing toes (1st to 5th), plantar pads (alpha, beta, A, B, C, D), and heel. Plantar pad nomenclature follows Kennedy et al. [56,57]. (C) Histological sections of footpads from saline and OVA groups at day 49. The dermal-epidermal junction is indicated by a dashed white line and white arrows point to CGRP- immunolabelled intraepidermal nerve profiles. Data are expressed as mean ± SEM (n = 6); **** p < 0.0001 vs. saline control. Scale bar = 100 µm.
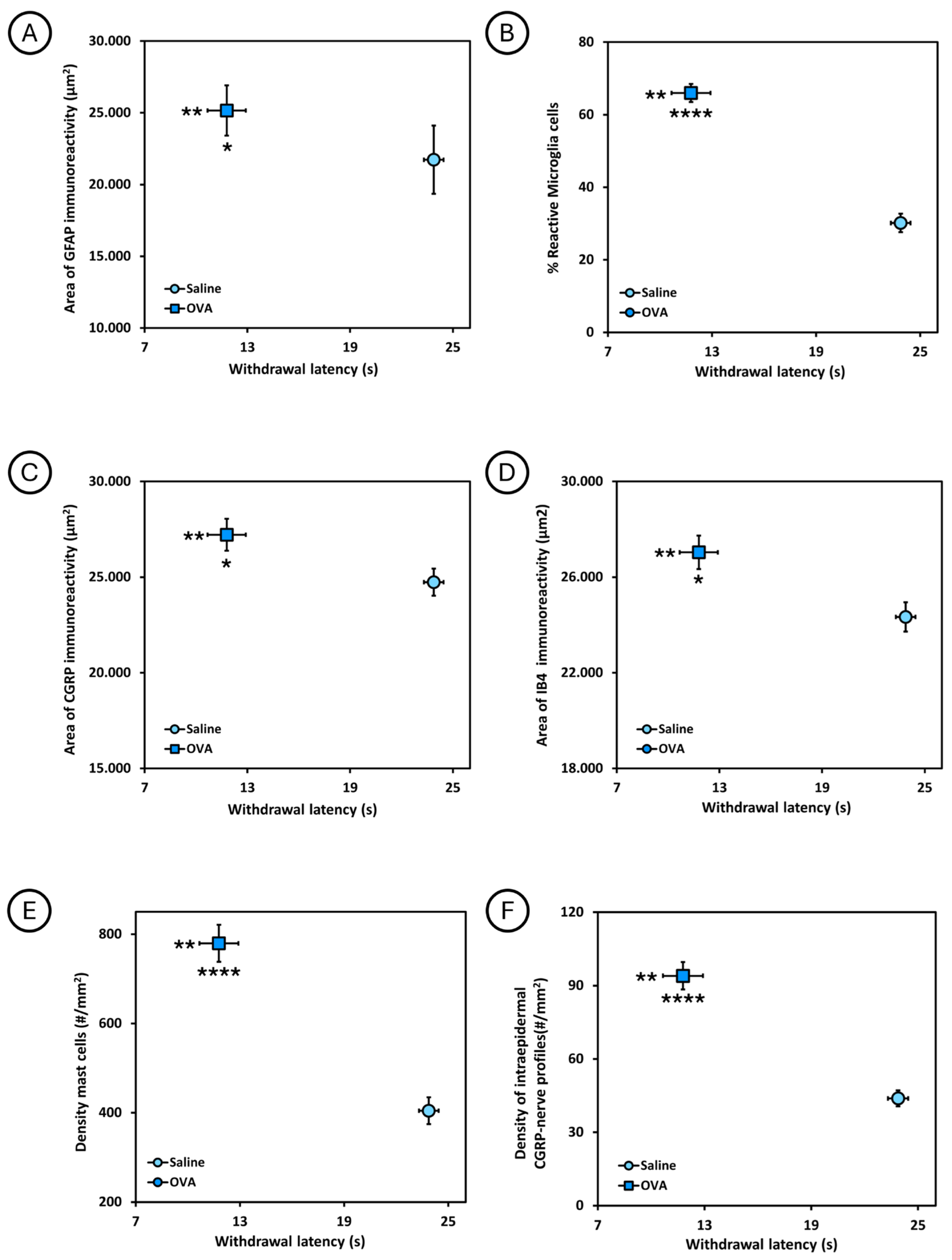
The relationship between the withdrawal latency to the thermal stimulus in the plantar test and the different histological parameters analyzed is shown in Figure 6. Animals treated with ovalbumin (OVA) had a shorter withdrawal latency and, at the same time, a larger area of GFAP immunoreactivity and percentage of reactive microglia in the spinal cord, as well as a larger area of CGRP and IB4 immunoreactivity in the dorsal horn of the spinal cord, and a higher density of mast cells in the dorsal skin, as well as a higher density of intraepidermal CGRP nerve profiles in the footpads, compared to animals treated with saline solution (saline). Significant differences were observed between these two experimental groups.
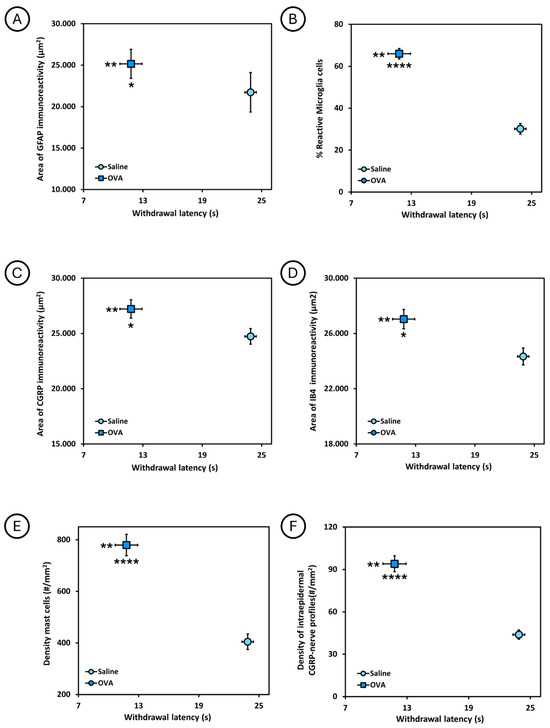
Figure 6.
Relationship between the withdrawal latency to thermal stimulus in the plantar test and the different histological parameters analyzed at 49 days of follow-up, in the two experimental groups (saline and OVA). (A) Relationship between withdrawal latency and area of GFAP immunoreactivity (µm2) in the spinal cord. (B) Relationship between withdrawal latency and the percentage of reactive microglia cells in the spinal cord. (C) Relationship between withdrawal latency and the area of CGRP immunoreactivity (µm2) in the spinal cord. (D) Relationship between withdrawal latency and the area of IB4 immunoreactivity (µm2) in the spinal cord. (E) Relationship between withdrawal latency and mast cell density (#/mm2) in dorsal skin. (F) Relationship between withdrawal latency and the density of intraepidermal CGRP nerve profiles (#/mm2) in the skin of the footpads. * p < 0.05, ** p < 0.01, **** p < 0.0001. In each graph, asterisks to the left of the symbol indicate withdrawal to heat noxious stimuli, whereas asterisks below refer to immunohistological data.
Likewise, the percentage of mast cells in degranulation was 37.83 ± 2.39% in the OVA group and 21.22 ± 1.28% in the saline group, observing significant differences (p < 0.0001) between both experimental groups.
3. Discussion
In this study, OV-AL-treated mice exhibited significantly higher mast cell density in the dorsal skin, along with gliosis in the spinal cord. Additionally, there was an increased density of peptidergic (CGRP) and non-peptidergic (IB4) nociceptive afferent fibers as well as elevated CGRP-positive intraepidermal profiles in the foot pads. Functionally, animals treated with OVA-AL developed thermal hyperalgesia. These findings suggest that subcutaneous OV-AL sensitization coupled with topical application induces thermal hyperalgesia, associated with inflammation in the dorsal skin and gliosis in spinal cord.
Previous studies using similar OVA protocols have also reported increased mast cells in the skin [1,28,58,59,60]. There is evidence that cutaneous injection of ovalbumin promotes mast cell degranulation [61] and intraperitoneal injection of ovalbumin also facilitates mast cell degranulation [62]. Furthermore, in OVA-induced allergic airway inflammation, mast cell degranulation has been observed [63]. Collectively, these findings suggest that ovalbumin treatment promotes mast cell degranulation. Mast cell granules contain a wide variety of chemicals including biogenic amines (histamine, serotonin, dopamine), eicosanoids (prostaglandins, leukotrienes, thromboxanes), cytokines/chemokines (IL1, IL3, IL4, IL6, IL8, IL9, IL17, IL33, TNF-α, Interferon gamma, TGF-β, MIP-1, GM-CSF, eotaxin, TARC, RANTES, MCP-1, CXCL8, CCL2, CCL5), peptides/neuropeptides (bradykinin, VIP, CRH), growth factors (NGF, NT3/NT4, bFGF, VEGF, BDNF), enzymes/proteases (chymase, tryptase, serine proteases, aspartic acid proteases, cysteine proteases, metalloproteinases) and nitric oxide [31,32,33,34,35,36,64,65,66,67]. Some of these mediators activate and depolarize the nociceptors and pruritoceptors that innervate the skin, generating action potentials in these nerve fibers that propagate toward the spinal cord and brain, giving the sensation/perception of pain and itch-scratching [8,37,38,39,40]. These mediators enhance afferent fiber excitation by interacting with membrane receptors of nociceptors/pruritoreceptors, such as serotonin by binding to 5-HT3 receptors [68], histamine by binding to H1 receptors [69], and bradykinin by binding to B1 receptors [70]. In addition, other chemical mediators also contribute to the sensitization of nociceptors/pruritoreceptors including bradykinin, prostaglandins, and histamine [42,43,44,45,71]. Finally, other chemical mediators, such as prostaglandins (PGE2), contribute to the hyperexcitability of nociceptive afferent fibers by promoting ionic currents through voltage-gated sodium ion channels [72].
Peripheral sensitization, excitation, and hyperexcitation of nociceptors/pruritoreceptors that innervate the skin leads to the release of neurotransmitters and neuromodulators in the spinal cord dorsal horn affecting both neurons and glial cells. Nociceptive afferent fibers primarily release glutamate while A-delta afferent fibers also release CGRP and ATP, whereas peptidergic C fibers release CGRP, SP and ATP, while non-peptidergic C fibers also release ATP [73,74,75,76,77,78]. Itch-sensitive afferent nerve fibers release SP, natriuretic polypeptide B, gastrin releasing peptide (GRP), and glutamate [79,80]. These mediators activate second-order neurons, spinal interneurons, and glial cells, including microglia. It is well known that CNS microglial cells express a variety of receptors on their membrane including glutamate receptors [81,82], CGRP receptors [83,84], substance P receptors [85,86,87], purinergic receptors [88,89,90,91], and natriuretic peptide receptors [92]. This triggers microglial reactivation, shifting them from a quiescent state to a reactive state marked by the release of cytokines (IL-1β, TNF, IL-6), chemokines (CXCL10, CXCL12), and neurotrophins (BDNF, NGF, NT3) [93,94,95,96,97,98]. Similarly, astrocytes express glutamatergic, purinergic, and natriuretic peptide receptors [92,99], enabling them to respond to neurotransmitters released by nociceptive/pruritogenic afferent fibers, transforming into reactive astrocytes that secrete cytokines/chemokines (IL1-beta, IL6, TNF-alpha, MCP1/CCL2, CXCL8, MIF) [100], and neurotrophins (GDNF, NGF) [101,102]. These cytokines and chemokines released by reactive glial cells contribute to central sensitization of nociceptive neurons in the dorsal horn of the spinal cord [103,104,105] and promote long-term potentiation of synapses between nociceptive afferent fibers and spinal nociceptive neurons [103,106,107]. Certain neurotrophins such as BDNF released by reactive glial cells also promote long-term potentiation of synaptic transmission in the dorsal horn of the spinal cord [108]. Other neurotrophins, like NGF, induce the sprouting of nociceptive afferent fibers in the spinal cord [109]. In addition, CGRP-positive afferent nerve fibers express the TRKA receptor [110], while IB4-positive afferent nerve fibers express the GDNF receptor [111]. Thus, CGRP-positive peptidergic afferent fibers are sensitive to the action of NGF [112,113], while IB4-positive non-peptidergic afferent fibers are sensitive to GDNF [114,115,116], and both neurotrophins favor the sprouting of nerve fibers [117,118,119,120]. These findings suggest that the sensitization and excitation of cutaneous nociceptors and pruritoreceptors by chemical mediators from degranulated mast cells lead to an increased release of neurotransmitters in the dorsal horn. This, in turn, results in enhanced excitation of spinal nociceptive neurons and reactivation of glial cells. Chemical mediators facilitate central sensitization of nociceptive neurons, enhance synaptic transmission and sprouting of nociceptive afferent fibers in the dorsal horn, causing pain and hyperalgesia, as observed in the present study.
The preceding paragraphs indicate that ovalbumin treatment causes mast cell degranulation, which triggers activation and sensitization of nociceptors and pruritoreceptors in the skin. Understanding how ovalbumin induces degranulation is key. Mast cells present several receptors on their plasma membrane that are involved in their activation and degranulation, such as high-affinity IgE receptor (FcεRI), low-affinity IgG receptors (FcγRs), mast-related G-protein-coupled receptor X2 (MRGPRX2), P2X Purinoceptor 7 (P2RX7), and adhesion G-protein-coupled receptor E2 (ADGRE2) [121]. Treatment with ovalbumin induces the generation of IgE antibodies [122,123,124,125,126], which bind to high-affinity FcεRI on mast cells, triggering their activation and degranulation. FcεRI’s extracellular alpha chain binds IgE, while the beta and gamma chains are responsible for intracellular transduction. These intracellular chains present an immunoreceptor tyrosine-based activation motif (ITAM). When IgE binds to the receptor, tyrosine residues in the ITAM are phosphorylated by the Src family protein tyrosine kinase (SfK). This recruits another tyrosine kinase, Syk, and all of this allows the phosphorylation of adaptors and signaling enzymes in mast cells, such as PLC, which generates IP3 and DAG, which in turn activate PKC and mobilize calcium from the endoplasmic reticulum. This calcium outflow from the reticulum is detected by the STIM1 and 2 sensors, allowing STIM1/2 to bind to the plasma membrane Ca2+ channel Orai-1 (calcium release-activated channel [CRAC]), leading to activation of the Orai1 channel and the subsequent influx of Ca2+ into the mast cell. This increase in calcium in the mast cell promotes fusion of the granules with the membrane, i.e., mast cell degranulation [127]. The granule-membrane fusion machinery involves the N-ethylmaleimide-sensitive factor-binding protein receptor (SNARE) protein cluster. The first SNARE identified in mast cells is SNAP-23. During stimulation, SNAP-23 relocates from the plasma membrane to form cytoplasmic degranulation channels. A second identified t-SNARE is STX3, which reverse-localizes from secretory granules to the plasma membrane. Together, these recognition proteins between secretory granules and the plasma membrane allow the release of granule contents by exocytosis [127,128,129].
Treatment with ovalbumin induces the generation of IgG antibodies [130,131,132,133,134], which can bind to the low-affinity IgG receptor on mast cells. Activation of these receptors by IgG antibodies triggers an intracellular signaling pathway involving ITAM tyrosine phosphorylation, followed by dynamic protein phosphorylation cascades, including the SRC family of tyrosine kinases (Lyn), Syk, raft-associated transmembrane adaptors (LAT and NTAL), and phosphatidylinositol 3-kinase, among others [135]. There is evidence to suggest that activation of these low-affinity IgG receptors also induces calcium ion influxes [136]. Consequently, the cytosolic increase in calcium ions favors the activation of protein complexes that help fuse the granules with the plasma membrane, promoting the release of their contents through exocytosis.
On the other hand, peptides derived from human albumin could cause mast cell degranulation by binding to the MRGPRX2 receptor [137,138]. Other peptides (e.g., Substance P, VIP) are also ligands for MRGPRX2 receptors [121,138]. Nociceptive terminals in the skin can secrete substance P [139,140], and cutaneous nociceptors have high-affinity IgE receptors [141], as well as low-affinity IgG receptors [142]. Therefore, the IgE and IgG antibodies generated after ovalbumin administration can induce an increase in calcium ions in the nociceptive nerve terminal, which favors the fusion of vesicles loaded with substance P with the nociceptor plasma membrane, releasing this neuropeptide into the skin [139,140]. Now, this release of substance P in the skin can interact with the MRGPRX2 receptors of mast cells, facilitating their degranulation [121,138]. Like what has been described above, the binding of the ligand to the mast cell MRGPRX2 receptor activates the intracellular SRC, LYN, and PLC-gamma cascade. The latter factor favors the activation of Orai channels, allowing an influx of calcium ions, which activate protein complexes that promote the fusion of granules with the mast cell plasma membrane [138,143].
Overall, mast cells have different membrane receptors that, when activated by ligands that appear after treatment with ovalbumin, promote the entry of calcium ions into these cells, which in turn allows the fusion of the granules with the mast cell membrane and the release of their contents into the extracellular space.
On the other hand, hyperalgesia and pain responses have also been observed in animals treated with OVA. In an animal model of allergic inflammation induced by inhalation of OVA after sensitization with this same product, animals develop mechanical allodynia, categorized as a neuropathic pain response, due to the reactivation of microglial cells in the spinal cord [144]. In the experimental model of ocular allergy induced by OVA, increased scratching behavior is accompanied by heightened electrophysiological activity in ocular nociceptive fibers, indicating that OVA promotes nociceptor activation [145]. Additionally, the intraplantar subcutaneous injection of OVA and CFA induces mechanical hyperalgesia, due to elevated levels of inflammatory cytokines (IL1beta, TNF-alpha) in the plantar skin [146]. Mice sensitized with OVA plus aluminum also exhibit a significant increase in licking behavior in response to intraplantar injection [25]. Consistent with these findings, in the present study OVA-treated ICR-CD1 mice displayed significant plantar thermal hyperalgesia.
Mast cell degranulation releases biogenic amines (e.g., histamine, serotonin, dopamine), cytokines (e.g., TNF-α, IL-4, TGFβ, bFGF-2, IL-15), growth factors (e.g., NGF, VEGF), lysosomal enzymes (e.g., cathepsins, galactosidase, glucuronidase, hexosaminidase), and proteases (e.g., chymase, tryptase, cathepsin G, MMP9) [147]. These mediators sensitize and nociceptive terminals in the skin [44,148,149], contributing to hyperalgesia [150,151,152].
In OVA-treated animals, the density of CGRP-positive intraepidermal nerve profiles in footpad skin was significantly increased compared to saline controls. The increased density of intraepidermal profiles in the plantar skin could also be explained by the sprouting changes induced by the release of NGF by reactive glial cells in the spinal cord of OVA-treated mice, that is, the sprouting of CGRP-positive fibers in the dorsal horn of the spinal cord also causes sprouting of these same nerve terminals in the plantar skin, with an increased number and density of intraepidermal CGRP-positive nerve profiles. In this sense, CGRP-positive intraepidermal nerve fibers are also sensitive to the action of NGF [153,154]. These intraepidermal CGRP-positive afferent fibers located in mouse footpads or human skin have been identified as nociceptors [155,156,157]. Preclinical and clinical studies link cutaneous nerve fiber sprouting with increased pain sensitivity [158,159,160], while local injection of capsaicin triggers a loss of CGRP-positive epidermal nerve fibers that is accompanied by a reduction in pain [156,161]. Taken together, these mechanisms likely underlie the plantar thermal hyperalgesia observed in OVA-treated animals in this study.
All the previous paragraphs have presented scientific evidence that, in part, explains the results obtained in this study. However, it should be noted that the present study aims to reflect human dermatitis: a cutaneous inflammation related to allergens and adjuvants that causes nociceptive sensitization. In this context, there are different animal models of active sensitization to induce dermatitis, including repeated exposure to trinitrochlorobenzene (TNCB), dinitrofluorobenzene (DNFB), dinitrochlorobenzene (DNCB), ovalbumin (OVA), oxazolone (OXA), and calcipotriol (MC903) [2,162,163,164,165,166]. In the DNFB-induced dermatitis model, animals were initially systemically sensitized with intravenous injections of IgE antibodies. Subsequently, the skin was painted with DNFB 48 h post-sensitization. This caused IgE- and mast cell-dependent skin swelling. This is known as the first and second phases [167]. A third phase of skin swelling occurs after 8 days, characterized by an accumulation of eosinophils, mast cells, and T cells [168,169]. Repeated epicutaneous application of 2,4,6-trinitrochlorobenzene (TNCB) induces epidermal hyperplasia, accumulation of CD4-positive T cells and mast cells, and elevated plasma levels of IgE. Type 2 helper cells are responsible for the cutaneous response and IgE production. TNCB triggers a cytokine response from type 2 helper cells [170,171]. Topical application of dinitrochlorobenzene (DNCB) causes modification of macromolecules that are internalized by antigen-presenting cells in the skin (e.g., Langerhans cells, dermal dendritic cells, macrophages), processed, and presented to T lymphocytes, triggering their activation. Twenty-four hours after exposure to DNCB, macrophage accumulation is observed in the exposed area, contributing to the development of skin swelling and inflammation. Animals treated with DNCB show increased serum IgG1 levels, much higher than other immunoglobulins, as well as activation of type 2 helper lymphocytes with the production of cytokines (e.g., IL4, IL5, IL10) [172,173,174]. Repeated application of oxazolone (OXA) to the skin causes edema and the influx of inflammatory cells, especially mononuclear and polynuclear cells, into the area of application, which persists for 15 h. At this time, edema persists, but the cellular infiltrate, especially mast cells, decreases. After 48 h, the edema disappears, and the infiltrated cells decrease [175]. It is now known that OXA induces the influx of T and B lymphocytes, as well as neutrophils, and increases the levels of cytokines/interleukins (e.g., IL1β, TNF-α, IL4, IL10, IL13) [176,177]. Animals treated with MC903 exhibit dermatitis, with type 2 inflammation, skin barrier dysfunction characterized by increased trans-epidermal water loss (TEWL), scratching associated with pruritus, and increased serum IgE levels [178,179]. Histological analysis of MC903-treated skin reveals infiltration of granulocytes (e.g., eosinophils) and CD3-positive T cells, as well as an increase in dermal mast cells. Increased expression of interleukins (IL4, IL13, IL33) and serum IgE levels are also observed. Many of these changes persist long-term [178,179,180]. Finally, ovalbumin (OVA)-induced dermatitis triggers a type 2 helper T-cell-dependent immune response, with production of interleukin (IL4, IL5, IL13) and elevated serum IgE levels. Histological analysis of OVA-treated skin shows lymphocytes (CD4 positive), eosinophils, mast cells, and thickening of the dermis and epidermis. The animals exhibit scratching behavior in response to itching or pruritus [25,26,27,28,29,181]. In most of these animal models of dermatitis, an elevated presence of granulocytes (e.g., eosinophils, neutrophils), mast cells, and T lymphocytes is observed in the treated skin. These cells synthesize and secrete a variety of chemical mediators, which in addition to inducing swelling, skin redness, and inflammation [182,183,184,185], also trigger the excitation and sensitization of cutaneous nociceptors and pruritoreceptors [186,187,188,189,190,191], triggering itching and scratching behaviors of the skin [184,185,191,192,193]. The increased excitability of skin nociceptors/itch receptors causes these sensory nerve fibers to release increased numbers of neurotransmitters in the spinal cord. These neurotransmitters not only excite second-order spinal nociceptive neurons [194,195,196] but also activate spinal cord microglial cells, which are also capable of responding to these neurotransmitters [197]. In turn, these reactive microglial cells secrete increased chemical mediators into the spinal cord parenchyma, which facilitate chemical neurotransmission through sensory afferent fibers reaching the dorsal horn and central sensitization of second-order nociceptive neurons [198,199]. These chemical mediators released by reactive microglia cells diffuse through the parenchyma and can reach the lumbosacral regions, where more microglia cells can also be reactivated [200,201], contributing to more chemical mediators that can sensitize lumbosacral nociceptive neurons, and this in turn can promote hyperalgesia [202,203].
Although animal models for human diseases are very important for examining the mechanisms involved, as well as for developing treatment strategies for diseases, there are increasingly attempts to use alternative methods to the use of laboratory animals, with the aim of reducing their use. In this context, 2D cell cultures, 3D cells cultures, cell co-cultures, ex vivo skin organ culture, “skin on a chip” and skin tissue engineering can be used to study dermatitis or other dermatological diseases. In this context, it has been shown that 2D co-cultures between immune cells (e.g., eosinophils, basophils, T cells) and structural cells of the skin (e.g., keratinocytes, fibroblasts) stimulated by pruritogenic cytokines (e.g., IL31, IL33) mimic the environment of atopic dermatitis, with secretion of cytokines (IL1β, IL6) and chemokines (CXCL1, CXCL8, CCL2, CCL18) [204,205,206,207]. There are two alternative models to laboratory animals that adequately mimic the characteristics of the human epidermis: the reconstituted human epidermis (RHE) model and the full-thickness human skin equivalent (FTHE) model. These models reproduce epidermal morphology, differentiation, and barrier function [208,209,210,211,212]. Using the FTHE model, the application of interleukins (e.g., IL4, IL13) triggers changes like atopic dermatitis, allowing the testing of the effects of various treatments, such as indole-3-lactic acid [213] and Tofacitinib [214]. In the RHE model, the effect of various metal allergens (K2Cr2O7, CoCl2, NiCl2) has been tested [215]. This model has also been used to study the influence of cytokines on the organization and differentiation of the epidermis [216], as well as to study the changes in the skin barrier under the influence of Th2-derived cytokines [217]. The ex vivo skin organ culture (EVSOC) model consists of using small pieces of skin explants to perform various experimental studies. This model has been used to evaluate the effects of ultraviolet radiation on the skin [218], test the effects of various skin creams [219], and analyze hair growth [220], among others. The EVSOC model has been used to induce dermatitis upon contact with Staphylococcus aureus, and to analyze the production of cytokines [221]. The “skin-on-a-chip” (SoC) model has been used for a wide variety of dermatological studies including the development of strategies to promote hair follicle regeneration [222], to study the degree of absorption of creams [223], and to assess inflammation and edema, and the use of treatments [224]. An epidermis-on-a-chip model has also been tested to evaluate the effects of various irritants [225], and this technology has been used to test treatment with Propionibacterium acnes as an acne inducer [226]. More recently, SoC has been used to test skin cancer treatments [227]. Taken together, these findings suggest that there are in vitro alternatives to the use of animal models that mimic the structure and function of the skin, and that they can be used to study cutaneous physiological and pathophysiological processes, as well as for the screening of cutaneous treatments.
To our knowledge, this is the first report showing that systemic OVA-aluminum sensitization followed by cutaneous challenge in mice induces (1) thermal hyperalgesia, (2) sprouting of nociceptive endings in the foot pads, (3) increased peptidergic and non-peptidergic fiber density in the spinal dorsal horn and (4) astrogliosis and microgliosis, when compared to saline-treated controls. Moreover, these findings not only demonstrate the robust effects of combining systemic and cutaneous ovalbumin–aluminum sensitization but also provide a solid foundation for future studies aimed at elucidating the specific inflammatory and neuro-immune mechanisms underlying the observed functional and histological changes.
This study has some limitations that should be acknowledged. First, the number of animals per experimental group was relatively small. Although this warrants caution in data interpretation, the consistent trends observed clearly support the conclusions presented. Future studies with larger sample sizes will help further validate these findings. Second, only plantar thermal hyperalgesia was assessed, whereas mechanical sensitivity (e.g., von Frey filaments) was not evaluated. Thus, it cannot be excluded that the animals developed mechanical allodynia in addition to thermal hyperalgesia. Including additional behavioral assays in future studies could provide a more comprehensive assessment of pain-related responses. Finally, the estrous cycle of female mice was not monitored. Because hormonal fluctuations may influence neuroimmune interactions and pain responses, monitoring the estrous cycle in future studies could add valuable insight. Importantly, none of these limitations undermine the main conclusions of this study but rather highlight useful directions for further research.
4. Materials and Methods
4.1. Animals, Study Design, and Ethical Considerations
Adult female mice of the ICR-CD1 strain, aged 8-weeks and weighing 25–30 g, were used (Janvier Laboratories, Le-Genest-Saint Isle, France). The sample size was calculated using the GRANMO software for comparison of two independent means. Accepting an alpha risk of 0.05 and a statistical power of 0.5 in a one-tailed test, six animals per group were required to detect a statistically significant a difference greater than or equal to 1 unit. In this study, six mice per experimental group were assigned to each experimental group.
Upon arrival at the Animal Care Unit of the University of Barcelona (Bellvitge campus), the mice were randomly allocated to different cages. Each cage was designated as an experimental group. It is important to note that the experimental unit was each individual animal.
A maximum of six mice were housed in each standard mouse cage (#1145T; 369 × 165 × 132 mm, floor area: 435 cm2; Tecniplast, Madrid, Spain). All cages were maintained in the same animal facility room, at a stable temperature of 21 ± 1 °C, with a humidity between 40 and 60%, and with a 12:12 h light–dark cycle. In these cages, the animals had access to food and drink ad libitum. Only the study animals were housed in the animal room, with no other types of animals or mouse strains other than those used in this experiment. All experiments were performed between 8:00 a.m. and 18:00 p.m., under light conditions, in silence, and with the minimum possible ambient noise.
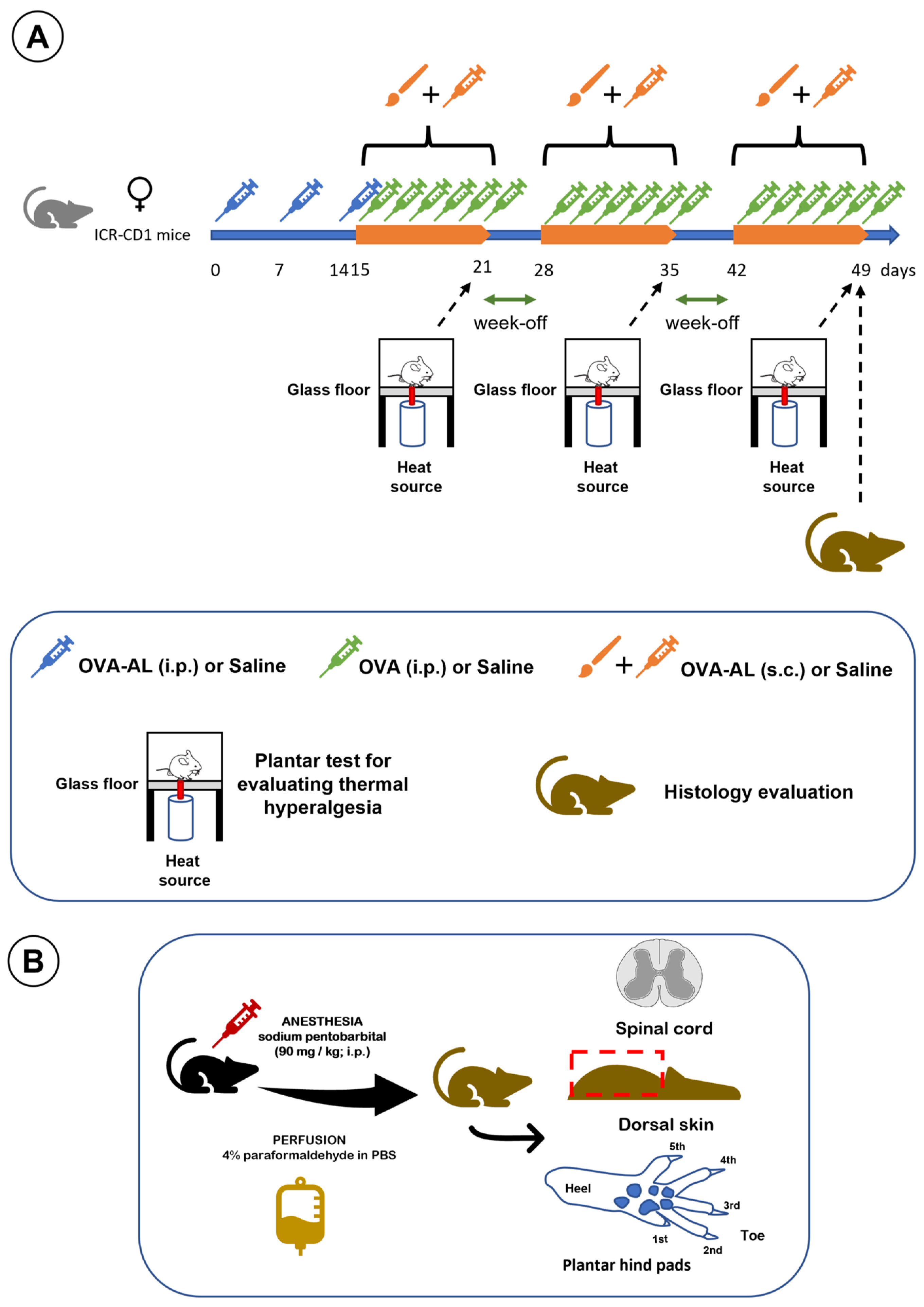
This study employed a model of atopic dermatitis based on the subcutaneous injection of ovalbumin and aluminum salts in saline solution, combined with topical application of the same solution using a brush, as previously described [5]. Sensitization occurred on days 0, 7, and 14 via intraperitoneal injection of the ovalbumin–aluminum solution (OVA-AL). On day 14, after the intraperitoneal injection of OVA-AL and shaving and depilating the dorsal skin, the animals also received skin impregnation with OVA-AL solution using a brush. Daily, on days 15–21, 28–35, and 42–49, the animals received OVA solution intraperitoneally (i.p.), as well as OVA-AL solution subcutaneously (s.c.), and the dorsal skin was impregnated with OVA-AL solution using a brush. Plantar thermal hyperalgesia was assessed on days 21, 35, and 49. At the end of the study, dorsal skin, plantar pads, and spinal cord were collected and processed for histological analysis [5]. The experimental design of this study is illustrated in Figure 7.
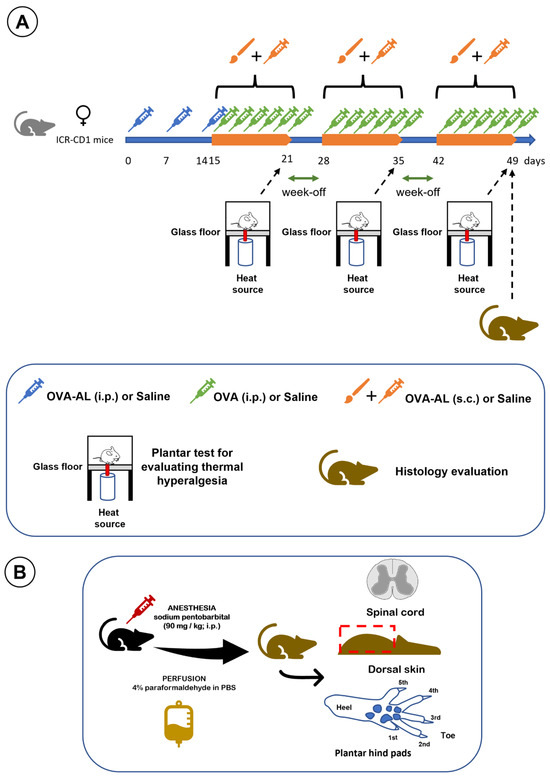
Figure 7.
Illustrates the study design. (A) Timeline of OVA, OVA + aluminum, and saline applications, with thermal hyperalgesia assessments on days 21, 35, and 49, followed by histological analysis at study end. (B) Schematic of tissue collection and histological processing: after anesthesia and perfusion, the spinal cord, dorsal skin, and footpads were dissected and processed for analyses. See main text for details.
All in vivo experimental procedures were performed at the Bellvitge Animal Facility of the University of Barcelona, in accordance with the ARRIVE 2.0 guidelines, the ethical principles of the International Association for the Study of Pain (IASP) for the use of animals in pain research animals [228], and the Directive of the European Parliament and of the Council of 22 September 2010 (2010/63/UE). The Animal Experimentation Ethics Committee of the University of Barcelona (CEEA, ref: 50/19; approved on 11 April 2019) and the Ethics Committee of the Department of Agriculture, Livestock, Fisheries, Food, and the Natural Environment of the Government of Catalonia (DAAMM, ref: 10672, approved on 22 November 2019) approved the animal experimentation procedures for this study.
4.2. Preparation of Ovalbumin Solutions and Skin Treatment
The animals were treated with two types of ovalbumin (OVA) solutions: one containing aluminum salts (OVA-AL solution) and one without (OVA solution). The OVA-AL solution consisted of 0.5 g of ovalbumin grade V (#A5503 Sigma-Aldrich-Merck, Darmstadt, Germany) and 0.2 g of aluminum hydroxide hydrate, (#A1577 Sigma-Aldrich-Merck, Germany) dissolved in 500 mL of 0.9% saline solution (Vitulia Physiological Serum, Barcelona, Spain) The OVA solution contained 0.5 g of ovalbumin dissolved in 500 mL of saline solution. Both solutions were filtered through 0.22 µm syringe filters (#SFNY-122-100; Labbox, Barcelona, Spain), aliquoted into 50 mL tubes (# PTSP-E50-025; Labbox) and stored at 4 °C until use.
Mice were injected intraperitoneally (i.p.) with 0.2 mL of OVA-AL solution on days 0, 7, and 14. On day 14, after the i.p. administration of the OVA-AL solution, the mice were anesthetized with sodium pentobarbital (#Y000219450 Sigma-Aldrich-Merck; 50 mg/kg, 10 mg/mL, i.p.). The dorsal skin was shaved using an electric shaver and then depilated using a depilatory cream (Silky fresh sensitive skin; Veet, Reckitt Benckiser Healthcare, Slough, UK). The depilated area was subsequently treated with OVA-AL solution using a bristle brush (n° 10; Pelikan 721431; Hannover, Germany).
From days 15 to 21, 28 to 35, and 42 to 49, the mice received daily treatments consisting of (i) 0.2 mL of OVA solution intraperitoneally, (ii) 0.2 mL of OVA-AL solution subcutaneously, and (iii) topical application of OVA-AL solution to the dorsal skin using a brush. These mice constituted the OVA group. Control animals (saline group) were treated exclusively with saline solution (0.9% Vitulia Physiological Serum, Barcelona, Spain), following the same schedule and procedures as the OVA group. For repeated application of OVA or saline solutions, the procedure described above was followed with minor modifications [29,229]. Two experimental groups were used in this study: (i) the saline group, treated with saline solution only, and (ii) the OVA group, sensitized with OVA-AL (i.p.) and subsequently treated with OVA (OVA i.p.+ OVA-AL s.c. + OVA-AL with a brush) during the periods of days 15–21, 28–35, 42–49, as described above.
4.3. Thermal Hyperalgesia Evaluation
Thermal hyperalgesia was assessed on days 21, 35, and 49 following the administration of OVA or saline solutions, using a plantar-test apparatus (#37370; Ugo Basile, 21036 Gemonio VA, Italy). Mice were placed in methacrylate boxes with glass floors and allowed to acclimatize for 60 min. A beam of incandescent light from a 100 W bulb was then directed at the plantar surface of the hind paws, and the withdrawal latency to this painful stimulus was determined. The exposure time was limited to a maximum of 30 s to prevent burns. The mean withdrawal latency for both hind paws was calculated as the average of three separate trials, conducted at 5-min intervals. It should be noted that the animals stand directly on the glass floor, which is 2 mm thick, and immediately below, also touching the glass, is the incandescent light outlet [230,231,232].
4.4. Histological Evaluation
On day 49, after completing the functional assessments, the animals were deeply anesthetized with sodium pentobarbital (90–100 mg/kg; 10 mg/mL; i.p.) and perfused intraventricularly with 4% paraformaldehyde solution in phosphate-buffered saline (PBS; 0.1 M, pH = 7.4). Subsequently, the dorsal skin was then excised and transferred to a jar containing the same fixative. Following a dorsal laminectomy, the spinal cord was removed and placed in Eppendorf tubes with fixative. Finally, the plantar pads (B and C) of both hind legs were also excised and placed in Eppendorf tubes with fixative. All tissues were fixed at 4 °C for 15 days. After fixation, samples were transferred to a cryo-protective solution (30% sucrose in PBS) and stored at 4 °C for an additional 15–20 days.
4.4.1. Skin Processing
Dorsal skin samples were stretched on cork boards and secured with needles. From the center, a 2 cm2 section was excised and embedded in tissue freezing medium (Ref: 0201-08-926; Leica, Barcelona, Spain), by using a special mold. Blocks were frozen in a cryostat (CM1520, Leica, Barcelona, Spain) at −24 °C. Skin was cryosectioned at 20 µm, and 8–10 sections per animal were mounted on pregelatinized slides. Sections were stained with Giemsa (azur-eosin-methylene blue; #EOMB-MSD-1K0; Labbox, Barcelona, Spain) diluted 1:4 in distilled water (60 mL Giemsa solution and 240 mL distilled water) for 90 min. Afterwards, the sections were rinsed in 0.1% acetic acid for 15–20 s, followed by 96% ethanol for another 15–20 s, and then washed in methanol (three baths, 2 min per bath). Finally, the skin sections were rinsed three times in xylene (2 min per bath) and coverslipped with DPX (# 1.01.979.500; Merck, Germany).
4.4.2. Spinal Cord Processing
Spinal cord segments (2–3 mm, thoracic region) were embedded in the same freezing medium, frozen at −24 °C, and sectioned at 60 µm. Sections (8–10 per animal) were collected in 6-well porcelain plates (#SPPC-006-001; Labbox, Barcelona, Spain) and washed at room temperature with agitation in PBS (10 min), PBS + 0.3% Triton-X-100 (10 min), and PBS-Triton + 1% fetal bovine serum (30–45 min).
Sections were incubated for 48 h at 4 °C in a humid chamber with constant agitation in the following primary antibodies: (i) Rabbit anti-glial fibrillary acidic protein (GFAP; 1:200; #ab7260, ABCAM, Cambridge, UK) for astrocytes, (ii) Rabbit anti-ionized calcium-binding adapter molecule type 1 (Iba1; 1: 200; # 019-19741; WAKO, Richmond, VA, USA) for microglia, (iii) Goat anti-calcitonin gene-related protein (CGRP; 1:200; #ab36001, ABCAM, UK) for peptidergic nociceptive afferent fibers, and (iv) Biotinylated Bandeiraea simplicifolia Isolectin (IB4; 1:200; # L2140, Sigma-Aldrich-Merck, Germany) for non-peptidergic nociceptive afferent fibers.
After the primary antibody incubation, sections were washed three times for 10 min each with PBS-Triton and then incubated for 24 h at 4 °C in a humidified chamber with constant agitation in the following secondary antibody solution: donkey anti-goat and donkey anti-rabbit, and avidin, all conjugated to cyanine 3.18 (Cy3; 1:200; Jackson Immunoresearch, West Grove, PA, USA). After several additional washes with PBS-Triton and PBS, the sections were mounted on pregelatinized slides, dehydrated through graded in ethanol (70°, 96°, then absolute) and coverslipped with DPX [233].
Footpads were similarly embedded in tissue freezing medium, frozen at −24 °C and cryosectioned at a thickness of 60 µm. All sections from each footpad were collected in the same well of a 6-well porcelain plate and processed using the same immunohistochemical protocol with goat anti-CGRP as primary antibody and Cy3-conjugated donkey anti-goat as secondary, to visualize intraepidermal CGRP-positive nociceptor profiles [155,234]. To verify antibody specificity, some spinal cord and footpad samples) were processed as described, except the primary antisera was omitted.
Spinal cord and footpad sections were examined under a Leica DMRXA epifluorescence microscope (Leica Microsistemas S.L.U., Hospitalet de Llobregat, Spain) equipped with a digital camera (FMVU-13S2C-CS). Using Image-J software (NIH, Bethesda, MD, USA; version 2.14.0/1.54f), CGRP and IB4 immunolabelling areas were quantified from spinal cord images at ×100 magnification. GFAP immunoreactivity was quantified at ×200 and served as an index of astrogliosis [166]. Iba1-immunostained sections (×200) were used to count reactive versus non-reactive microglial cells expressed as a percentage to quantify microgliosis [232,235].
Dorsal skin sections stained with Giemsa were analyzed under the Leica DMRXA optical microscope (without epifluorescence), and images were captured using the FMVU-13S2C-CS camera. Mast cells were manually counted in Image-J (same version) and density was expressed as cells/mm2. The percentage of degranulating mast cells was also determined.
4.5. Statistical Analysis
Functional and histological analyses were performed blinded, with each animal assigned a numerical code. The researcher assessing thermal hyperalgesia was unaware of the experimental groups. Histological analyses were independently performed by two other researchers who were also blinded to group assignment.
Statistical comparisons between the different experimental groups were conducted using Kruskal–Wallis and Mann–Whitney U non-parametric statistical tests. Results are presented as mean ± standard error of the mean (SEM), and a significance level of p < 0.05 was applied. All analyses were conducted using GraphPad Prism 9.0 for Macintosh.
Author Contributions
All authors have contributed sufficiently to be included as authors. Con-ceptualization, G.B., P.B.-V., and E.V.; methodology, G.S.-D., J.G.-B., P.B.-V., and E.V.; validation, P.B.-V., and E.V.; formal analysis, G.S.-D., P.B.-V., and E.V.; investigation, G.S.-D., J.G.-B., P.B.-V., and E.V.; resources, P.B.-V., and E.V.; data curation, G.S.-D., J.G.-B., P.B.-V., and E.V.; writing—original draft preparation, E.V., and P.B.-V.; writing—review and editing, G.S.-D., J.G.-B., G.B., P.B.-V., and E.V.; visualization, G.S.-D., P.B.-V., and E.V.; supervision and project administration, P.B.-V., and E.V.; funding acquisition, G.S.-D., P.B.-V., and E.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by IBSA Farmaceutici Italia (S.r.l), Via della Filanda 30, 26900 Lodi, Italy (grant number 019/19).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee for Animal Experimentation of the University of Barcelona (CEEA; CEEA number 50/19; approved on 11 April 2019), and the Department of Agriculture, Livestock, Fisheries, Food and the Natural Environment of the Generalitat de Catalunya, Generalitat de Catalunya (DAAM number 10672; approved on 22 November 2019), Government of Catalonia (Spain).
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
The authors thank the staff of animal care facility from University of Barcelona (Campus Bellvitge), and Daniel Reyes and Vicenç Oliveras from the Research Technical Services of University of Girona for their skillful technical assistance.
Conflicts of Interest
Gilberto Bellia works full-time at IBSA Farmaceutici Italia, Lodi, Italy. He has no other relevant affiliations or financial involvement. He has received no payment for the preparation of this manuscript and has no conflicts of interest with the subject matter or materials discussed herein other than those declared. The remaining authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 5-HT3 | 5-hydroxytryptamine receptor 3 |
| ADGRE2 | Adhesion G-protein-coupled receptor E2 |
| ATP | Adenosine triphosphate |
| BDNF | Brain-derived nerve factor |
| bFGF | basic fibroblast growth factor |
| CCL2 | Chemokine (C-C motif) ligand 2 |
| CCL5 | Chemokine (C-C motif) ligand 5 |
| CCL18 | Chemokine (C-C motif) ligand 18 |
| CFA | Complete Freund’s adjuvant |
| CGRP | Calcitonin gene-related protein |
| CNS | Central nervous system |
| CoCl2 | Cobalt chloride |
| CRH | Corticotropin-releasing hormone |
| CXCL1 | C-X-C motif chemokine ligand 1 |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| CXCL12 | C-X-C motif chemokine ligand 10 |
| CXCL8 | C-X-C motif chemokine ligand 8 |
| DAG | Diacylglycerol |
| DNCB | Dinitrochlorobenzene |
| DNFB | Dinitrofluorobenzene |
| DPX | Dibutylphthalate polystyrene xylene |
| EVSOC | Ex vivo skin organ culture |
| FcεRI | High-affinity IgE receptor |
| FTHE | Full-thickness human skin equivalent |
| GDNF | Glial cell line-derived neurotrophic factor |
| GFAP | Glial fibrillary acidic protein |
| GM-CSF | Granulocyte macrophage colony-stimulating factor |
| GRP | Gastrin releasing peptide |
| H1 | Histamine receptor type 1 |
| IB4 | Biotinylated Bandeiraea simplicifolia Isolectin |
| IBA1 | Ionized calcium-binding adapter molecule type 1 |
| ICR-CD1 | Institute of Cancer Research (ICR)-CD1 mice |
| IgE | Immunoglobulin E |
| IgG | Immunoglobulin G |
| IL1β | Interleukin 1 beta |
| IL4 | Interleukin 4 |
| IL5 | Interleukin 5 |
| IL6 | Interleukin 6 |
| IL8 | Interleukin 8 |
| IL10 | Interleukin 10 |
| IL13 | Interleukin 13 |
| IL15 | Interleukin 15 |
| IL17 | Interleukin 17 |
| IL31 | Interleukin 31 |
| IL33 | Interleukin 33 |
| ITAM | Immunoreceptor tyrosine-based activation motif |
| IP3 | Inositol triphosphate |
| K2Cr2O7 | Potassium dichromate |
| LAT | Linker for activation of T cells |
| LYN | Tyrosine-protein kinase Lyn |
| MC903 | Calcipotriol |
| MCP-1 | Monocyte chemotactic protein 1 |
| MIF | Macrophage migration inhibitory factor |
| MIP-1 | Macrophage inflammatory protein 1 |
| MMP9 | Matrix metalloproteinase-9 |
| MRGPRX2 | Mast-related G-protein-coupled receptor X2 |
| NiCl2 | Nickel chloride |
| NGF | Nerve growth factor |
| NT3/NT4 | Neurotrophin-3/Neurotrophin-4 |
| NTAL | Non-T-cell activation linker |
| Orai-1/CRAC | Calcium release-activated calcium channel protein 1 |
| OVA | Ovalbumin |
| OVA-AL | Ovalbumin–aluminum salts solution |
| OXA | Oxazolone |
| P2RX7 | P2X Purinoceptor 7 |
| PBS | Phosphate buffer saline |
| PGE2 | Prostaglandin E2 |
| PKC | Protein Kinase C |
| PLC | Phospholipase C |
| RANTES | Regulated on activation, normal T-cell expressed and secreted |
| RHE | Reconstituted human epidermis |
| SEM | Standard error of the mean |
| SfK | Src family protein tyrosine kinase |
| SoC | Skin on a chip |
| SP | Substance P |
| SNARE | N-ethylmaleimide-sensitive factor-binding protein receptor |
| SNAP-23 | Synaptosomal-associated protein 23 |
| SRC | Proto-oncogene tyrosine-protein kinase Src |
| STIM1/2 | Stromal interaction molecule 1 and 2 |
| STX3 | Syntaxin 3 |
| Syk | Spleen tyrosine kinase |
| TARC | Thymus and activation-regulated chemokine |
| TEWL | Trans-epidermal water loss |
| TGF-beta | Transforming growth factor beta |
| TNF-α | Tumor necrosis factor alpha |
| TNCB | Trinitrochlorobenzene |
| TRKA | Tropomyosin receptor kinase A |
| VIP | Vasoactive intestinal peptide |
| VEGF | Vascular endothelial growth factor |
References
- Wang, G.; Savinko, T.; Wolff, H.; Dieu-Nosjean, M.C.; Kemeny, L.; Homey, B.; Lauerma, A.I.; Alenius, H. Repeated epicutaneous exposures to ovalbumin progressively induce atopic dermatitis-like skin lesions in mice. Clin. Exp. Allergy 2007, 37, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Gilhar, A.; Reich, K.; Keren, A.; Kabashima, K.; Steinhoff, M.; Paus, R. Mouse models of atopic dermatitis: A critical reappraisal. Exp. Dermatol. 2021, 30, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Kigasawa, K.; Kajimoto, K.; Hama, S.; Saito, A.; Kanamura, K.; Kogure, K. Noninvasive delivery of siRNA into the epidermis by iontophoresis using an atopic dermatitis-like model rat. Int. J. Pharm. 2010, 383, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.Y.; Jin, H.J.; Tae, H.J.; Oh, H.G.; Hwang, J.H. Establishment of an experimental model of ovalbumin-induced atopic dermatitis in canines. Front. Vet. Sci. 2024, 11, 1296138. [Google Scholar] [CrossRef]
- Siquier-Dameto, G.; Iguaran-Pérez, A.; Gimeno-Beltrán, J.; Bellia, G.; Giori, A.M.; Boadas-Vaello, P.; Verdú, E. Subcutaneous Injection and Brush Application of Ovalbumin-Aluminum Salt Solution Induces Dermatitis-like Changes in Mice. J. Clin. Med. 2025, 14, 1701. [Google Scholar] [CrossRef]
- Sacotte, R.; Silverberg, J.I. Epidemiology of adult atopic dermatitis. Clin. Dermatol. 2018, 36, 595–605. [Google Scholar] [CrossRef]
- Laughter, M.R.; Maymone, M.B.C.; Mashayekhi, S.; Arents, B.W.M.; Karimkhani, C.; Langan, S.M.; Dellavalle, R.P.; Flohr, C. The global burden of atopic dermatitis: Lessons from the Global Burden of Disease Study 1990–2017. Br. J. Dermatol. 2021, 184, 304–309. [Google Scholar] [CrossRef]
- Ständer, S.; Steinhoff, M. Pathophysiology of pruritus in atopic dermatitis: An overview. Exp. Dermatol. 2002, 11, 12–24. [Google Scholar] [CrossRef]
- Ständer, S.; Luger, T.A. Itch in atopic dermatitis—Pathophysiology and treatment. Acta Dermatovenerol. Croat. 2010, 18, 289–296. [Google Scholar]
- Vakharia, P.P.; Chopra, R.; Sacotte, R.; Patel, K.R.; Singam, V.; Patel, N.; Immaneni, S.; White, T.; Kantor, R.; Hsu, D.Y.; et al. Burden of skin pain in atopic dermatitis. Ann. Allergy Asthma Immunol. 2017, 119, 548–552.e3. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Gelfand, J.M.; Margolis, D.J.; Boguniewicz, M.; Fonacier, L.; Grayson, M.H.; Chiesa Fuxench, Z.C.; Simpson, E.L.; Ong, P.Y. Pain Is a Common and Burdensome Symptom of Atopic Dermatitis in United States Adults. J. Allergy Clin. Immunol. Pract. 2019, 7, 2699–2706. [Google Scholar] [CrossRef] [PubMed]
- Pojawa-Gołąb, M.; Reich, A. Skin Pain in Patients with Atopic Dermatitis or Psoriasis: A Web-based Survey. Acta Derm. Venereol. 2020, 100, adv00258. [Google Scholar] [CrossRef] [PubMed]
- Thyssen, J.P.; Halling-Sønderby, A.S.; Wu, J.J.; Egeberg, A. Pain severity and use of analgesic medication in adults with atopic dermatitis: A cross-sectional study. Br. J. Dermatol. 2020, 182, 1430–1436. [Google Scholar] [CrossRef]
- Hassan, A.H.; Pzewłocki, R.; Herz, A.; Stein, C. Dynorphin, a preferential ligand for kappa-opioid receptors, is present in nerve fibers and immune cells within inflamed tissue of the rat. Neurosci. Lett. 1992, 140, 85–88. [Google Scholar] [CrossRef]
- Carlton, S.M.; Coggeshall, R.E. Inflammation-induced changes in peripheral glutamate receptor populations. Brain Res. 1999, 820, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Schrier, D.J.; Moniot, S.; Gluckman, M.I.; Gilbertsen, R.B. The topical anti-inflammatory effects of a topical preparation of meclofenamic acid on carrageenan-induced footpad swelling in mice. J. Pharm. Pharmacol. 1987, 39, 57–59. [Google Scholar] [CrossRef]
- Kirchhoff, C.; Jung, S.; Reeh, P.W.; Handwerker, H.O. Carrageenan inflammation increases bradykinin sensitivity of rat cutaneous nociceptors. Neurosci. Lett. 1990, 111, 206–210. [Google Scholar] [CrossRef]
- Welsh, E.M.; Nolan, A.M. Repeated intradermal injection of low-dose carrageenan induces tachyphylaxis to evoked hyperalgesia. Pain 1994, 59, 415–421. [Google Scholar] [CrossRef]
- Chakrabarty, A.; McCarson, K.E.; Smith, P.G. Hypersensitivity and hyperinnervation of the rat hind paw following carrageenan-induced inflammation. Neurosci. Lett. 2011, 495, 67–71. [Google Scholar] [CrossRef][Green Version]
- Coderre, T.J.; Abbott, F.V.; Melzack, R. Behavioral evidence in rats for a peptidergic-noradrenergic interaction in cutaneous sensory and vascular function. Neurosci. Lett. 1984, 47, 113–118. [Google Scholar] [CrossRef]
- Kjaerheim, V.; Barkvoll, P.; Waaler, S.M.; Rölla, G. Triclosan inhibits histamine-induced inflammation in human skin. J. Clin. Periodontol. 1995, 22, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Heyer, G.; Koppert, W.; Martus, P.; Handwerker, H.O. Histamine and cutaneous nociception: Histamine-induced responses in patients with atopic eczema, psoriasis and urticaria. Acta Derm. Venereol. 1998, 78, 123–126. [Google Scholar] [PubMed]
- Sjögren, F.; Anderson, C.; Groth, O. The cellular dermal infiltrate in experimental immediate type cutaneous hypersensitivity. Acta Derm. Venereol. 1995, 75, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Nagai, H.; Basaki, Y.; Yamaya, H.; Ikizawa, K.; Watanabe, M.; Kojima, M.; Matsuura, N.; Kiniwa, M. The expression of murine cutaneous late phase reaction requires both IgE antibodies and CD4 T cells. Clin. Exp. Allergy 1997, 27, 225–231. [Google Scholar] [CrossRef]
- Piovezan, A.P.; D’Orléans-Juste, P.; Frighetto, M.; Souza, G.E.; Henriques, M.G.; Rae, G.A. Endothelins contribute towards nociception induced by antigen in ovalbumin-sensitised mice. Br. J. Pharmacol. 2004, 141, 755–763. [Google Scholar] [CrossRef]
- Spergel, J.M.; Mizoguchi, E.; Brewer, J.P.; Martin, T.R.; Bhan, A.K.; Geha, R.S. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J. Clin. Investig. 1998, 101, 1614–1622. [Google Scholar] [CrossRef]
- Savinko, T.; Lauerma, A.; Lehtimäki, S.; Gombert, M.; Majuri, M.L.; Fyhrquist-Vanni, N.; Dieu-Nosjean, M.C.; Kemeny, L.; Wolff, H.; Homey, B.; et al. Topical superantigen exposure induces epidermal accumulation of CD8+ T cells, and mixed Th1/Th2-type dermatitis and vigorous production of IgE antibodies in the murine model of atopic dermatitis. J. Immunol. 2005, 175, 8320–8326. [Google Scholar] [CrossRef]
- Lehto, M.; Savinko, T.; Wolff, H.; Kvist, P.H.; Kemp, K.; Lauerma, A.; Alenius, H. A murine model of epicutaneous protein sensitization is useful to study efficacies of topical drug in atopic dermatitis. Int. Immunopharmacol. 2010, 10, 377–384. [Google Scholar] [CrossRef]
- Kopecki, Z.; Stevens, N.E.; Chong, H.T.; Yang, G.N.; Cowin, A.J. Flightless I alters the inflammatory response and autoantibody profile in an OVA-induced atopic dermatitis skin-like disease. Front. Immunol. 2018, 9, 1833. [Google Scholar] [CrossRef]
- Killoran, K.E.; Kropp, L.E.; Lindrose, A.R.; Curtis, H.E.; Cook, D.; Mitre, E. Rush desensitization with a single antigen induces subclinical activation of mast cells and protects against bystander challenge in dually sensitized mice. Clin. Exp. Allergy 2019, 49, 484–494. [Google Scholar] [CrossRef]
- Soter, N.A.; Austen, K.F. The diversity of mast cell-derived mediators: Implications for acute, subacute, and chronic cutaneous inflammatory disorders. J. Investig. Dermatol. 1976, 67, 313–319. [Google Scholar] [CrossRef]
- Johnson, D.; Krenger, W. Interactions of mast cells with the nervous system—Recent advances. Neurochem. Res. 1992, 17, 939–951. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, X.; Qian, Y. Mast cells and neuroinflammation. Med. Sci. Monit. Basic Res. 2014, 20, 200–206. [Google Scholar] [CrossRef]
- Numata, T.; Harada, K.; Nakae, S. Roles of Mast Cells in Cutaneous Diseases. Front. Immunol. 2022, 13, 923495. [Google Scholar] [CrossRef] [PubMed]
- Dileepan, K.N.; Raveendran, V.V.; Sharma, R.; Abraham, H.; Barua, R.; Singh, V.; Sharma, R.; Sharma, M. Mast cell-mediated immune regulation in health and disease. Front. Med. 2023, 10, 1213320. [Google Scholar] [CrossRef] [PubMed]
- Voelker, D.; Pongdee, T. Biomarkers in the diagnosis of mast cell activation. Curr. Opin. Allergy Clin. Immunol. 2025, 25, 27–33. [Google Scholar] [PubMed]
- Johansson, O.; Virtanen, M.; Hilliges, M. Histaminergic nerves demonstrated in the skin. A new direct mode of neurogenic inflammation? Exp. Dermatol. 1995, 4, 93–96. [Google Scholar] [CrossRef]
- Paterson, K.J.; Zambreanu, L.; Bennett, D.L.; McMahon, S.B. Characterisation and mechanisms of bradykinin-evoked pain in man using iontophoresis. Pain 2013, 154, 782–792. [Google Scholar] [CrossRef]
- Ständer, S.; Luger, T.; Kim, B.; Lerner, E.; Metz, M.; Adiri, R.; Canosa, J.M.; Cha, A.; Yosipovitch, G. Cutaneous Components Leading to Pruritus, Pain, and Neurosensitivity in Atopic Dermatitis: A Narrative Review. Dermatol. Ther. 2024, 14, 45–57. [Google Scholar] [CrossRef]
- Jurcakova, D.; Ru, F.; Undem, B.J. Allergen-induced histaminergic and non-histaminergic activation of itch C-fiber nerve terminals in mouse skin. Neuroscience 2019, 410, 55–58. [Google Scholar] [CrossRef]
- Ständer, S.; Steinhoff, M.; Schmelz, M.; Weisshaar, E.; Metze, D.; Luger, T. Neurophysiology of pruritus: Cutaneous elicitation of itch. Arch. Dermatol. 2003, 139, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Mendell, L.M.; Albers, K.M.; Davis, B.M. Neurotrophins, nociceptors, and pain. Microsc. Res. Tech. 1999, 45, 252–261. [Google Scholar] [CrossRef]
- Mayer, S.; Izydorczyk, I.; Reeh, P.W.; Grubb, B.D. Bradykinin-induced nociceptor sensitisation to heat depends on cox-1 and cox-2 in isolated rat skin. Pain 2007, 130, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.C.; Strassman, A.M.; Burstein, R.; Levy, D. Sensitization and activation of intracranial meningeal nociceptors by mast cell mediators. J. Pharmacol. Exp. Ther. 2007, 322, 806–812. [Google Scholar] [CrossRef]
- Fattori, V.; Hohmann, M.S.N.; Rossaneis, A.C.; Manchope, M.F.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q.; Verri, W.A., Jr. Targeting IL-33/ST2 signaling: Regulation of immune function and analgesia. Expert Opin. Ther. Targets 2017, 21, 1141–1152. [Google Scholar] [CrossRef]
- Schreiber, K.L.; Beitz, A.J.; Wilcox, G.L. Activation of spinal microglia in a murine model of peripheral inflammation-induced, long-lasting contralateral allodynia. Neurosci. Lett. 2008, 440, 63–67. [Google Scholar] [CrossRef]
- Vega-Avelaira, D.; Ballesteros, J.J.; López-García, J.A. Inflammation-induced hyperalgesia and spinal microglia reactivity in neonatal rats. Eur. J. Pain 2013, 17, 1180–1188. [Google Scholar] [CrossRef]
- Huber, J.D.; Campos, C.R.; Mark, K.S.; Davis, T.P. Alterations in blood-brain barrier ICAM-1 expression and brain microglial activation after lambda-carrageenan-induced inflammatory pain. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, 732–740. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, Y.; Wang, L.; Huang, J.; Wen, J.; Lv, H.; Wu, X.; Wan, C.; Yu, C.; Zhang, W.; et al. Thymosin Alpha-1 Inhibits Complete Freund’s Adjuvant-Induced Pain and Production of Microglia-Mediated Pro-inflammatory Cytokines in Spinal Cord. Neurosci. Bull. 2019, 35, 637–648. [Google Scholar] [CrossRef]
- Raghavendra, V.; Tanga, F.Y.; DeLeo, J.A. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur. J. Neurosci. 2004, 20, 467–473. [Google Scholar] [CrossRef]
- Wu, Y.; Willcockson, H.H.; Maixner, W.; Light, A.R. Suramin inhibits spinal cord microglia activation and long-term hyperalgesia induced by formalin injection. J. Pain 2004, 5, 48–55. [Google Scholar] [CrossRef]
- Fu, K.Y.; Light, A.R.; Matsushima, G.K.; Maixner, W. Microglial reactions after subcutaneous formalin injection into the rat hind paw. Brain Res. 1999, 825, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Mou, Y.K.; Song, X.Y.; Wei, S.Z.; Wang, H.R.; Wang, Y.; Zhang, W.B.; Li, B.; Song, X.C. P2X7 receptor of microglia in olfactory bulb mediates the pathogenesis of olfactory dysfunction in a mouse model of allergic rhinitis. FASEB J. 2023, 37, e22955. [Google Scholar] [CrossRef]
- Win-Shwe, T.T.; Yanagisawa, R.; Lwin, T.T.; Kawakami, F.; Koike, E.; Takano, H. Dietary Exposure to Flame Retardant Tris (2-Butoxyethyl) Phosphate Altered Neurobehavior and Neuroinflammatory Responses in a Mouse Model of Allergic Asthma. Int. J. Mol. Sci. 2022, 23, 655. [Google Scholar] [CrossRef] [PubMed]
- Breach, M.R.; Dye, C.N.; Joshi, A.; Platko, S.; Gilfarb, R.A.; Krug, A.R.; Franceschelli, D.V.; Galan, A.; Dodson, C.M.; Lenz, K.M. Maternal allergic inflammation in rats impacts the offspring perinatal neuroimmune milieu and the development of social play, locomotor behavior, and cognitive flexibility. Brain Behav. Immun. 2021, 95, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, W.R.; Navarro, X.; Kamei, H. Reinnervation of sweat glands in the mouse: Axonal regeneration versus collateral sprouting. Muscle Nerve 1988, 11, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Navarro, X.; Kennedy, W.R. Effect of age on collateral reinnervation of sweat glands in the mouse. Brain Res. 1988, 463, 174–181. [Google Scholar] [CrossRef]
- Katagiri, K.; Arakawa, S.; Hatano, Y.; Fujiwara, S. Tolerogenic antigen-presenting cells successfully inhibit atopic dermatitis-like skin lesion induced by repeated epicutaneous exposure to ovalbumin. Arch. Dermatol. Res. 2008, 300, 583–593. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.J.; Kang, M.J.; Seo, J.H.; Kim, H.Y.; Jeong, S.K.; Lee, S.H.; Kim, J.M.; Hong, S.J. A novel mouse model of atopic dermatitis with epicutaneous allergen sensitization and the effect of Lactobacillus rhamnosus. Exp. Dermatol. 2012, 21, 672–675. [Google Scholar] [CrossRef]
- Rong, J.; Liu, S.; Hu, C.; Jin, F.; Wang, L. Oral Intake of Lactobacillus helveticus NS8 Alleviates Ovalbumin-Induced Atopic Dermatitis in SKH-1 Hairless Mice. Indian J. Microbiol. 2018, 58, 312–318. [Google Scholar] [CrossRef]
- Inoue, T.; Katoh, N.; Kishimoto, S. Prolonged topical application of tacrolimus inhibits immediate hypersensitivity reactions by reducing degranulation of mast cells. Acta Derm. Venereol. 2006, 86, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Gulati, K.; Ray, A. Effects of chelidonic acid, a secondary plant metabolite, on mast cell degranulation and adaptive immunity in rats. Int. Immunopharmacol. 2016, 40, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Lee, S.; Kim, M.J.; Rho, M.C.; Jang, Y.H.; Kim, S.H. Oleanolic Acid Acetate Inhibits Mast Cell Activation in Ovalbumin-Induced Allergic Airway Inflammation. Allergy Asthma Immunol. Res. 2023, 15, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, G.S.; Anogeianaki, A.; Orso, C.; Tetè, S.; Salini, V.; Antinolfi, P.L.; Sabatino, G. Mast cells and chemokines. J. Biol. Regul. Homeost. Agents 2008, 22, 145–151. [Google Scholar]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef]
- Leon, A.; Buriani, A.; Dal Toso, R.; Fabris, M.; Romanello, S.; Aloe, L.; Levi-Montalcini, R. Mast cells synthesize, store, and release nerve growth factor. Proc. Natl. Acad. Sci. USA 1994, 91, 3739–3743. [Google Scholar] [CrossRef]
- Skaper, S.D.; Pollock, M.; Facci, L. Mast cells differentially express and release active high molecular weight neurotrophins. Brain Res. Mol. Brain Res. 2001, 97, 177–185. [Google Scholar] [CrossRef]
- Zeitz, K.P.; Guy, N.; Malmberg, A.B.; Dirajlal, S.; Martin, W.J.; Sun, L.; Bonhaus, D.W.; Stucky, C.L.; Julius, D.; Basbaum, A.I. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J. Neurosci. 2002, 22, 1010–1019. [Google Scholar] [CrossRef]
- Han, S.K.; Mancino, V.; Simon, M.I. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron 2006, 52, 691–703. [Google Scholar] [CrossRef]
- Dray, A. Kinins and their receptors in hyperalgesia. Can. J. Physiol. Pharmacol. 1997, 75, 704–712. [Google Scholar] [CrossRef]
- Mizumura, K. Natural history of nociceptor sensitization: The search for a peripheral mechanism of hyperalgesia. Pain Rev. 1998, 5, 59–82. [Google Scholar] [CrossRef]
- Rush, A.M.; Waxman, S.G. PGE2 increases the tetrodotoxin-resistant Nav1.9 sodium current in mouse DRG neurons via G-proteins. Brain Res. 2004, 1023, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.J.; Kavookjian, A.M.; Light, A.R. Ultrastructural morphology, synaptic relationships, and CGRP immunoreactivity of physiologically identified C-fiber terminals in the monkey spinal cord. J. Comp. Neurol. 1993, 329, 472–490. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Calejesan, A.A.; Zhuo, M. ATP P2x receptors and sensory synaptic transmission between primary afferent fibers and spinal dorsal horn neurons in rats. J. Neurophysiol. 1998, 80, 3356–3360. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhuo, M. Cholinergic, noradrenergic, and serotonergic inhibition of fast synaptic transmission in spinal lumbar dorsal horn of rat. Brain Res. Bull. 2001, 54, 639–647. [Google Scholar] [CrossRef]
- Lawson, S.N. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Exp. Physiol. 2002, 87, 239–244. [Google Scholar] [CrossRef]
- Li, J.L.; Fujiyama, F.; Kaneko, T.; Mizuno, N. Expression of vesicular glutamate transporters, VGluT1 and VGluT2, in axon terminals of nociceptive primary afferent fibers in the superficial layers of the medullary and spinal dorsal horns of the rat. J. Comp. Neurol. 2003, 457, 236–249. [Google Scholar] [CrossRef]
- Clarke, J.N.; Anderson, R.L.; Haberberger, R.V.; Gibbins, I.L. Non-peptidergic small diameter primary afferents expressing VGluT2 project to lamina I of mouse spinal dorsal horn. Mol. Pain 2011, 7, 95. [Google Scholar] [CrossRef]
- Akiyama, T.; Tominaga, M.; Takamori, K.; Carstens, M.I.; Carstens, E. Roles of glutamate, substance P, and gastrin-releasing peptide as spinal neurotransmitters of histaminergic and nonhistaminergic itch. Pain 2014, 155, 80–92. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wan, L.; Huo, F.Q.; Barry, D.M.; Li, H.; Zhao, Z.Q.; Chen, Z.F. B-type natriuretic peptide is neither itch-specific nor functions upstream of the GRP-GRPR signaling pathway. Mol. Pain 2014, 10, 4. [Google Scholar] [CrossRef]
- Murugan, M.; Ling, E.A.; Kaur, C. Glutamate receptors in microglia. CNS Neurol. Disord. Drug Targets 2013, 12, 773–784. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Zhang, B.; Zhu, J.; Zhou, Z.; Cui, L. Regulation of microglia by glutamate and its signal pathway in neurodegenerative diseases. Drug Discov. Today 2020, 25, 1074–1085. [Google Scholar] [CrossRef]
- Priller, J.; Haas, C.A.; Reddington, M.; Kreutzberg, G.W. Calcitonin gene-related peptide and ATP induce immediate early gene expression in cultured rat microglial cells. Glia 1995, 15, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Sardi, C.; Zambusi, L.; Finardi, A.; Ruffini, F.; Tolun, A.A.; Dickerson, I.M.; Righi, M.; Zacchetti, D.; Grohovaz, F.; Provini, L.; et al. Involvement of calcitonin gene-related peptide and receptor component protein in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2014, 271, 18–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lai, J.P.; Zhan, G.X.; Campbell, D.E.; Douglas, S.D.; Ho, W.Z. Detection of substance P and its receptor in human fetal microglia. Neuroscience 2000, 101, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Qu, C.; Lu, X.; Zhang, S. Activation of microglia by histamine and substance P. Cell Physiol. Biochem. 2014, 34, 768–780. [Google Scholar] [CrossRef]
- Zieglgänsberger, W. Substance P and pain chronicity. Cell Tissue Res. 2019, 375, 227–241. [Google Scholar] [CrossRef]
- Inoue, K. ATP receptors of microglia involved in pain. Novartis Found. Symp. 2006, 276, 263–272. [Google Scholar]
- Koizumi, S.; Ohsawa, K.; Inoue, K.; Kohsaka, S. Purinergic receptors in microglia: Functional modal shifts of microglia mediated by P2 and P1 receptors. Glia 2013, 61, 47–54. [Google Scholar] [CrossRef]
- Calovi, S.; Mut-Arbona, P.; Sperlágh, B. Microglia and the Purinergic Signaling System. Neuroscience 2019, 405, 137–147. [Google Scholar] [CrossRef]
- Illes, P.; Rubini, P.; Ulrich, H.; Zhao, Y.; Tang, Y. Regulation of Microglial Functions by Purinergic Mechanisms in the Healthy and Diseased CNS. Cells 2020, 9, 1108. [Google Scholar] [CrossRef]
- Prado, J.; Baltrons, M.A.; Pifarré, P.; García, A. Glial cells as sources and targets of natriuretic peptides. Neurochem. Int. 2010, 57, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Elkabes, S.; DiCicco-Bloom, E.M.; Black, I.B. Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J. Neurosci. 1996, 16, 2508–2521. [Google Scholar] [CrossRef] [PubMed]
- Heese, K.; Hock, C.; Otten, U. Inflammatory signals induce neurotrophin expression in human microglial cells. J. Neurochem. 1998, 70, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, U.K. Microglia as a source and target of cytokines. Glia 2002, 40, 140–155. [Google Scholar] [CrossRef]
- Gomes, C.; Ferreira, R.; George, J.; Sanches, R.; Rodrigues, D.I.; Gonçalves, N.; Cunha, R.A. Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. J. Neuroinflamm. 2013, 10, 16. [Google Scholar] [CrossRef]
- Donnelly, C.R.; Andriessen, A.S.; Chen, G.; Wang, K.; Jiang, C.; Maixner, W.; Ji, R.R. Central Nervous System Targets: Glial Cell Mechanisms in Chronic Pain. Neurotherapeutics 2020, 17, 846–860. [Google Scholar] [CrossRef]
- Lindhout, I.A.; Murray, T.E.; Richards, C.M.; Klegeris, A. Potential neurotoxic activity of diverse molecules released by microglia. Neurochem. Int. 2021, 148, 105117. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, H.J.; Lim, I.; Satoh, J.; Kim, S.U. Human astrocytes: Secretome profiles of cytokines and chemokines. PLoS ONE 2014, 9, e92325. [Google Scholar]
- Appel, E.; Kolman, O.; Kazimirsky, G.; Blumberg, P.M.; Brodie, C. Regulation of GDNF expression in cultured astrocytes by inflammatory stimuli. Neuroreport 1997, 8, 3309–3312. [Google Scholar] [CrossRef]
- Kuno, R.; Yoshida, Y.; Nitta, A.; Nabeshima, T.; Wang, J.; Sonobe, Y.; Kawanokuchi, J.; Takeuchi, H.; Mizuno, T.; Suzumura, A. The role of TNF-alpha and its receptors in the production of NGF and GDNF by astrocytes. Brain Res. 2006, 1116, 12–18. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Xu, Z.Z.; Wang, X.; Park, J.Y.; Zhuang, Z.Y.; Tan, P.H.; Gao, Y.J.; Roy, K.; Corfas, G.; Lo, E.H.; et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat. Med. 2008, 14, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Donnelly, C.R.; Nedergaard, M. Astrocytes in chronic pain and itch. Nat. Rev. Neurosci. 2019, 20, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.K.; Old, E.A.; Malcangio, M. Neuropathic pain and cytokines: Current perspectives. J. Pain Res. 2013, 6, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Tsuda, M.; Inoue, K.; Murase, K. Long-term potentiation of neuronal excitation by neuron-glia interactions in the rat spinal dorsal horn. Eur. J. Neurosci. 2007, 25, 1297–1306. [Google Scholar] [CrossRef]
- Gruber-Schoffnegger, D.; Drdla-Schutting, R.; Hönigsperger, C.; Wunderbaldinger, G.; Gassner, M.; Sandkühler, J. Induction of thermal hyperalgesia and synaptic long-term potentiation in the spinal cord lamina I by TNF-α and IL-1β is mediated by glial cells. J. Neurosci. 2013, 33, 6540–6551. [Google Scholar] [CrossRef]
- Zhou, L.J.; Zhong, Y.; Ren, W.J.; Li, Y.Y.; Zhang, T.; Liu, X.G. BDNF induces late-phase LTP of C-fiber evoked field potentials in rat spinal dorsal horn. Exp. Neurol. 2008, 212, 507–514. [Google Scholar] [CrossRef]
- Romero, M.I.; Rangappa, N.; Li, L.; Lightfoot, E.; Garry, M.G.; Smith, G.M. Extensive sprouting of sensory afferents and hyperalgesia induced by conditional expression of nerve growth factor in the adult spinal cord. J. Neurosci. 2000, 20, 4435–4445. [Google Scholar] [CrossRef]
- Evans, L.; Andrew, D.; Robinson, P.; Boissonade, F.; Loescher, A. Increased cutaneous NGF and CGRP-labelled trkA-positive intra-epidermal nerve fibres in rat diabetic skin. Neurosci. Lett. 2012, 506, 59–63. [Google Scholar] [CrossRef]
- Salio, C.; Ferrini, F.; Muthuraju, S.; Merighi, A. Presynaptic modulation of spinal nociceptive transmission by glial cell line-derived neurotrophic factor (GDNF). J. Neurosci. 2014, 34, 13819–13833. [Google Scholar] [CrossRef]
- Takahashi, K.; Aoki, Y.; Ohtori, S. Resolving discogenic pain. Eur. Spine J. 2008, 17, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.L.; French, J.; Priestley, J.V.; McMahon, S.B. NGF but not NT-3 or BDNF prevents the A fiber sprouting into lamina II of the spinal cord that occurs following axotomy. Mol. Cell. Neurosci. 1996, 8, 211–220. [Google Scholar] [CrossRef]
- Molliver, D.C.; Wright, D.E.; Leitner, M.L.; Parsadanian, A.S.; Doster, K.; Wen, D.; Yan, Q.; Snider, W.D. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron 1997, 19, 849–861. [Google Scholar] [CrossRef]
- Bennett, D.L.; Michael, G.J.; Ramachandran, N.; Munson, J.B.; Averill, S.; Yan, Q.; McMahon, S.B.; Priestley, J.V. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J. Neurosci. 1998, 18, 3059–3072. [Google Scholar] [CrossRef] [PubMed]
- Akkina, S.K.; Patterson, C.L.; Wright, D.E. GDNF rescues nonpeptidergic unmyelinated primary afferents in streptozotocin-treated diabetic mice. Exp. Neurol. 2001, 167, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Mearow, K.M. The effects of NGF and sensory nerve stimulation on collateral sprouting and gene expression in adult sensory neurons. Exp. Neurol. 1998, 151, 14–25. [Google Scholar] [CrossRef]
- Blits, B.; Carlstedt, T.P.; Ruitenberg, M.J.; de Winter, F.; Hermens, W.T.; Dijkhuizen, P.A.; Claasens, J.W.; Eggers, R.; van der Sluis, R.; Tenenbaum, L.; et al. Rescue and sprouting of motoneurons following ventral root avulsion and reimplantation combined with intraspinal adeno-associated viral vector-mediated expression of glial cell line-derived neurotrophic factor or brain-derived neurotrophic factor. Exp. Neurol. 2004, 189, 303–316. [Google Scholar] [CrossRef]
- Hannila, S.S.; Kawaja, M.D. Nerve growth factor-mediated collateral sprouting of central sensory axons into deafferentated regions of the dorsal horn is enhanced in the absence of the p75 neurotrophin receptor. J. Comp. Neurol. 2005, 486, 331–343. [Google Scholar] [CrossRef]
- Love, S.; Plaha, P.; Patel, N.K.; Hotton, G.R.; Brooks, D.J.; Gill, S.S. Glial cell line-derived neurotrophic factor induces neuronal sprouting in human brain. Nat. Med. 2005, 11, 703–704. [Google Scholar] [CrossRef]
- Yang, B.G.; Kim, A.R.; Lee, D.; An, S.B.; Shim, Y.A.; Jang, M.H. Degranulation of Mast Cells as a Target for Drug Development. Cells 2023, 12, 1506. [Google Scholar] [CrossRef] [PubMed]
- Perini, A.; Mota, I. The production of IgE and IgG1 antibodies in guinea-pigs immunized with antigen and bacterial lipopolysaccharides. Immunology 1973, 25, 297–305. [Google Scholar] [PubMed]
- Bazin, H.; Platteau, B. Production of circulating reaginic (IgE) antibodies by oral administration of ovalbumin to rats. Immunology 1976, 30, 679–684. [Google Scholar] [PubMed]
- Talbott, M.W.; Strausser, H.R. Increase in serum IgE levels of ovalbumin-sensitized cats and the detection of elastase and collagenase activities in secretions of sensitized feline alveolar macrophages challenged in vitro. Int. Arch. Allergy Appl. Immunol. 1977, 54, 198–204. [Google Scholar] [CrossRef]
- Salgado, J.; Gilabert, A.; Castell, M.; Castellote, C.; Queralt, J. ELISA for quantification of specific IgG and IgE antibodies to ovalbumin. Allergol. Immunopathol. 1988, 16, 95–98. [Google Scholar]
- Honma, K.; Kohno, Y.; Saito, K.; Shimojo, N.; Tsunoo, H.; Niimi, H. Specificities of IgE, IgG and IgA antibodies to ovalbumin. Comparison of binding activities to denatured ovalbumin or ovalbumin fragments of IgE antibodies with those of IgG or IgA antibodies. Int. Arch. Allergy Immunol. 1994, 103, 28–35. [Google Scholar] [CrossRef]
- Blank, U.; Huang, H.; Kawakami, T. The high affinity IgE receptor: A signaling update. Curr. Opin. Immunol. 2021, 72, 51–58. [Google Scholar] [CrossRef]
- Guo, Z.; Turner, C.; Castle, D. Relocation of the t-SNARE SNAP-23 from lamellipodia-like cell surface projections regulates compound exocytosis in mast cells. Cell 1998, 94, 537–548. [Google Scholar] [CrossRef]
- Castle, J.D.; Guo, Z.; Liu, L. Function of the t-SNARE SNAP-23 and secretory carrier membrane proteins (SCAMPs) in exocytosis in mast cells. Mol. Immunol. 2002, 38, 1337–1340. [Google Scholar] [CrossRef]
- Hall, J.M.; Pribnow, J.F. An ELISA test to detect IgG antibody production by rabbit ocular cells. Curr. Eye Res. 1982, 2, 459–463. [Google Scholar] [CrossRef]
- Beck, L.; Spiegelberg, H.L. The polyclonal and antigen-specific IgE and IgG subclass response of mice injected with ovalbumin in alum or complete Freund’s adjuvant. Cell. Immunol. 1989, 123, 1–8. [Google Scholar] [CrossRef]
- Uchida, T.; Naito, S.; Horino, A.; Taneichi, M.; Mizuguchi, J.; Nakano, Y.; Oka, T.; Ookuma, K.; Morokuma, K.; Sakurai, S.; et al. Ovalbumin coupled either with murine red blood cells or liposome induces IgG but not IgE antibody production. Dev. Biol. Stand. 1998, 92, 353–363. [Google Scholar]
- Van der Maaden, K.; Varypataki, E.M.; Romeijn, S.; Ossendorp, F.; Jiskoot, W.; Bouwstra, J. Ovalbumin-coated pH-sensitive microneedle arrays effectively induce ovalbumin-specific antibody and T-cell responses in mice. Eur. J. Pharm. Biopharm. 2014, 88, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Majewska-Szczepanik, M.; Askenase, P.W.; Lobo, F.M.; Marcińska, K.; Wen, L.; Szczepanik, M. Epicutaneous immunization with ovalbumin and CpG induces TH1/TH17 cytokines, which regulate IgE and IgG2a production. J. Allergy Clin. Immunol. 2016, 138, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Katz, H.R.; Raizman, M.B.; Gartner, C.S.; Scott, H.C.; Benson, A.C.; Austen, K.F. Secretory granule mediator release and generation of oxidative metabolites of arachidonic acid via Fc-IgG receptor bridging in mouse mast cells. J. Immunol. 1992, 148, 868–871. [Google Scholar] [CrossRef]
- Floto, R.A.; Somasundaram, B.; Allen, J.M.; Mahaut-Smith, M.P. Fcgamma receptor I activation triggers a novel Ca2+-activated current selective for monovalent cations in the human monocytic cell line, U937. J. Biol. Chem. 1997, 272, 4753–4758. [Google Scholar] [CrossRef][Green Version]
- Karhu, T.; Akiyama, K.; Vuolteenaho, O.; Bergmann, U.; Naito, T.; Tatemoto, K.; Herzig, K.H. Mast cell degranulation via MRGPRX2 by isolated human albumin fragments. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2530–2534. [Google Scholar] [CrossRef]
- Al Hamwi, G.; Riedel, Y.K.; Clemens, S.; Namasivayam, V.; Thimm, D.; Müller, C.E. MAS-related G protein-coupled receptors X (MRGPRX): Orphan GPCRs with potential as targets for future drugs. Pharmacol. Ther. 2022, 238, 108259. [Google Scholar] [CrossRef]
- Holzer, P. Neurogenic vasodilatation and plasma leakage in the skin. Gen. Pharmacol. 1998, 30, 5–11. [Google Scholar] [CrossRef]
- Baluk, P. Neurogenic inflammation in skin and airways. J. Investig. Dermatol. Symp. Proc. 1997, 2, 76–81. [Google Scholar] [CrossRef]
- Crosson, T.; Wang, J.C.; Doyle, B.; Merrison, H.; Balood, M.; Parrin, A.; Pascal, M.; Mindt, B.C.; Seehus, C.R.; Ozcan, A.; et al. FcεR1-expressing nociceptors trigger allergic airway inflammation. J. Allergy Clin. Immunol. 2021, 147, 2330–2342. [Google Scholar] [CrossRef]
- Jiang, H.; Shen, X.; Chen, Z.; Liu, F.; Wang, T.; Xie, Y.; Ma, C. Nociceptive neuronal Fc-gamma receptor I is involved in IgG immune complex induced pain in the rat. Brain Behav. Immun. 2017, 62, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Lerner, L.; Babina, M.; Zuberbier, T.; Stevanovic, K. Beyond Allergies-Updates on The Role of Mas-Related G-Protein-Coupled Receptor X2 in Chronic Urticaria and Atopic Dermatitis. Cells 2024, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Yamasaki, R.; Kira, J.I. Novel Neuropathic Pain Mechanisms Associated With Allergic Inflammation. Front. Neurol. 2019, 10, 1337. [Google Scholar] [CrossRef]
- Acosta, C.M.; Luna, C.; Quirce, S.; Belmonte, C.; Gallar, J. Changes in sensory activity of ocular surface sensory nerves during allergic keratoconjunctivitis. Pain 2013, 154, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.M.; Sachs, D.; Canetti, C.A.; Poole, S.; Ferreira, S.H.; Cunha, F.Q. The critical role of leukotriene B4 in antigen-induced mechanical hyperalgesia in immunised rats. Br. J. Pharmacol. 2003, 139, 1135–1145. [Google Scholar] [CrossRef]
- Lundequist, A.; Pejler, G. Biological implications of preformed mast cell mediators. Cell. Mol. Life Sci. 2011, 68, 965–975. [Google Scholar] [CrossRef]
- Undem, B.J.; Taylor-Clark, T. Mechanisms underlying the neuronal-based symptoms of allergy. J. Allergy Clin. Immunol. 2014, 133, 1521–1534. [Google Scholar] [CrossRef]
- Aich, A.; Afrin, L.B.; Gupta, K. Mast Cell-Mediated Mechanisms of Nociception. Int. J. Mol. Sci. 2015, 16, 29069–29092. [Google Scholar] [CrossRef]
- Chatterjea, D.; Wetzel, A.; Mack, M.; Engblom, C.; Allen, J.; Mora-Solano, C.; Paredes, L.; Balsells, E.; Martinov, T. Mast cell degranulation mediates compound 48/80-induced hyperalgesia in mice. Biochem. Biophys. Res. Commun. 2012, 425, 237–243. [Google Scholar] [CrossRef]
- Zuo, Y.; Perkins, N.M.; Tracey, D.J.; Geczy, C.L. Inflammation and hyperalgesia induced by nerve injury in the rat: A key role of mast cells. Pain 2003, 105, 467–479. [Google Scholar] [CrossRef]
- Mai, L.; Liu, Q.; Huang, F.; He, H.; Fan, W. Involvement of Mast Cells in the Pathophysiology of Pain. Front. Cell. Neurosci. 2021, 15, 665066. [Google Scholar] [CrossRef]
- Davis, B.M.; Fundin, B.T.; Albers, K.M.; Goodness, T.P.; Cronk, K.M.; Rice, F.L. Overexpression of nerve growth factor in skin causes preferential increases among innervation to specific sensory targets. J. Comp. Neurol. 1997, 387, 489–506. [Google Scholar] [CrossRef]
- Yamaoka, J.; Di, Z.H.; Sun, W.; Kawana, S. Changes in cutaneous sensory nerve fibers induced by skin-scratching in mice. J. Dermatol. Sci. 2007, 47, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Navarro, X.; Verdú, E.; Wendelscafer-Crabb, G.; Kennedy, W.R. Innervation of cutaneous structures in the mouse hind paw: A confocal microscopy immunohistochemical study. J. Neurosci. Res. 1995, 41, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Simone, D.A.; Nolano, M.; Johnson, T.; Wendelschafer-Crabb, G.; Kennedy, W.R. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: Correlation with sensory function. J. Neurosci. 1998, 18, 8947–8959. [Google Scholar] [CrossRef] [PubMed]
- Nolano, M.; Simone, D.A.; Wendelschafer-Crabb, G.; Johnson, T.; Hazen, E.; Kennedy, W.R. Topical capsaicin in humans: Parallel loss of epidermal nerve fibers and pain sensation. Pain 1999, 81, 135–145. [Google Scholar] [CrossRef]
- Gopinath, P.; Wan, E.; Holdcroft, A.; Facer, P.; Davis, J.B.; Smith, G.D.; Bountra, C.; Anand, P. Increased capsaicin receptor TRPV1 in skin nerve fibres and related vanilloid receptors TRPV3 and TRPV4 in keratinocytes in human breast pain. BMC Womens Health 2005, 5, 2. [Google Scholar] [CrossRef]
- Bechakra, M.; Nieuwenhoff, M.D.; Rosmalen, J.V.; Groeneveld, G.J.; Huygen, F.J.; Zeeuw, C.I.; Doorn, P.A.V.; Jongen, J.L.M. Pain-related changes in cutaneous innervation of patients suffering from bortezomib-induced, diabetic or chronic idiopathic axonal polyneuropathy. Brain Res. 2020, 1730, 146621. [Google Scholar] [CrossRef]
- Bechakra, M.; Nieuwenhoff, M.D.; van Rosmalen, J.; Groeneveld, G.J.; Scheltens-de Boer, M.; Sonneveld, P.; van Doorn, P.A.; de Zeeuw, C.I.; Jongen, J.L. Clinical, electrophysiological, and cutaneous innervation changes in patients with bortezomib-induced peripheral neuropathy reveal insight into mechanisms of neuropathic pain. Mol. Pain 2018, 14, 1744806918797042. [Google Scholar] [CrossRef]
- Wallengren, J.; Chen, D.; Sundler, F. Neuropeptide-containing C-fibres and wound healing in rat skin. Neither capsaicin nor peripheral neurotomy affect the rate of healing. Br. J. Dermatol. 1999, 140, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, N.; Nagai, H. Analysis of the mechanism for the development of allergic skin inflammation and the application for its treatment:mouse models for the development of remedies for human allergic dermatitis. J. Pharmacol. Sci. 2009, 110, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; He, R.; Oyoshi, M.; Geha, R.S. Animal models of atopic dermatitis. J. Investig. Dermatol. 2009, 129, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Amagai, Y.; Oida, K.; Matsuda, H. Recent findings in mouse models for human atopic dermatitis. Exp. Anim. 2012, 61, 77–84. [Google Scholar] [CrossRef][Green Version]
- Martel, B.C.; Lovato, P.; Bäumer, W.; Olivry, T. Translational Animal Models of Atopic Dermatitis for Preclinical Studies. Yale J. Biol. Med. 2017, 90, 389–402. [Google Scholar]
- Ye, S.; Zhu, L.; Ruan, T.; Jia, J.; Mo, X.; Yan, F.; Liu, J.; Zhang, Y.; Chen, D. Comparative study of mouse models of atopic dermatitis. Heliyon 2025, 11, e41989. [Google Scholar] [CrossRef]
- Katayama, I.; Tanei, R.; Yokozeki, H.; Nishioka, K.; Dohi, Y. Induction of eczematous skin reaction in experimentally induced hyperplastic skin of Balb/C mice by monoclonal anti-DNP IgE antibody: Possible implications for skin lesion formation in atopic dermatitis. Int. Arch. Allergy Appl. Immunol. 1990, 93, 148–154. [Google Scholar] [CrossRef]
- Tahara, E.; Satoh, T.; Watanabe, C.; Shimada, Y.; Itoh, T.; Nagai, H.; Terasawa, K.; Saiki, I. A third-phase cutaneous (very late phase) response after elicitation with dinitrofluorobenzene in passively or actively sensitized mice. Allergol. Int. 1999, 48, 265–273. [Google Scholar] [CrossRef]
- Nakamura, N.; Ochi, T.; Sawada, M.; Tanaka, H.; Inagaki, N.; Saiki, I.; Nagai, H. Role of T cells in IgE-dependent triphasic cutaneous reaction caused by dinitrofluorobenzene in the mouse ear: Participation of CD8+ T cells. Allergol. Int. 2003, 52, 31–36. [Google Scholar] [CrossRef]
- Kitagaki, H.; Fujisawa, S.; Watanabe, K.; Hayakawa, K.; Shiohara, T. Immediate-type hypersensitivity response followed by alate reaction is induced by repeated epicutaneous application of contact sensitizing agents in mice. J. Investig. Dermatol. 1995, 105, 749–755. [Google Scholar] [CrossRef]
- Kitagaki, H.; Ono, N.; Hayakawa, K.; Kitazawa, T.; Watanabe, K.; Shiohara, T. Repeated elicitation of contact hypersensitivity induces a shift in cutaneous cytokine milieu from a T helper cell type 1 to a T helper cell type 2 profile. J. Immunol. 1997, 159, 2484–2491. [Google Scholar] [CrossRef]
- Garrigue, J.L.; Nicolas, J.F.; Fraginals, R.; Benezra, C.; Bour, H.; Schmitt, D. Optimization of the mouse ear swelling test for in vivo and in vitro studies of weak contact sensitizers. Contact Dermat. 1994, 30, 231–237. [Google Scholar] [CrossRef]
- Zhang, E.Y.; Chen, A.Y.; Zhu, B.T. Mechanism of dinitrochlorobenzene-induced dermatitis in mice: Role of specific antibodies in pathogenesis. PLoS ONE 2009, 4, e7703. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.; Mirkov, I.; Miljković, D.; Belij, S.; Zolotarevski, L.; Kataranovski, D.; Kataranovski, M. Contact allergic response to dinitrochlorobenzene (DNCB) in rats: Insight from sensitization phase. Immunobiology 2011, 216, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.R.; Ruben, Z.; Butchko, G.M. Histology of and quantitative assays for oxazolone-induced allergic contact dermatitis in mice. Am. J. Dermatopathol. 1986, 8, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tinkle, S.S. Chemical activation of innate and specific immunity in contact dermatitis. J. Investig. Dermatol. 2000, 115, 168–176. [Google Scholar] [CrossRef]
- Amarnani, D.; Sanchez, A.V.; Wong, L.L.; Duffy, B.V.; Ramos, L.; Freitag, S.K.; Bielenberg, D.R.; Kim, L.A.; Lee, N.G. Characterization of a Murine Model of Oxazolone-Induced Orbital Inflammation. Transl. Vis. Sci. Technol. 2020, 9, 26. [Google Scholar] [CrossRef]
- Li, M.; Hener, P.; Zhang, Z.; Kato, S.; Metzger, D.; Chambon, P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc. Natl. Acad. Sci. USA 2006, 103, 11736–11741. [Google Scholar] [CrossRef]
- Moosbrugger-Martinz, V.; Schmuth, M.; Dubrac, S. A Mouse Model for Atopic Dermatitis Using Topical Application of Vitamin D3 or of Its Analog MC903. Methods Mol. Biol. 2017, 1559, 91–106. [Google Scholar]
- Hoshino, Y.; Kirima, K.; Arichika, N.; Kakumoto, Y.; Shibamori, M.; Matsumoto, S.; Hiyama, H. Long-term application of MC903 in mice prolongs the characteristic symptoms of atopic dermatitis, such as inflammation, skin barrier dysfunction, and itching. Exp. Anim. 2025, 74, 276–285. [Google Scholar] [CrossRef]
- Spergel, J.M.; Mizoguchi, E.; Oettgen, H.; Bhan, A.K.; Geha, R.S. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J. Clin. Investig. 1999, 103, 1103–1111. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Kempuraj, D.; Tagen, M.; Conti, P.; Kalogeromitros, D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol. Rev. 2007, 217, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Zhang, G.; Wang, L.; Lu, Q. Eosinophilic Skin Diseases: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 189–213. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Rothenberg, M.E.; Sun, X.; Bachert, C.; Artis, D.; Zaheer, R.; Deniz, Y.; Rowe, P.; Cyr, S. Neuroimmune interplay during type 2 inflammation: Symptoms, mechanisms, and therapeutic targets in atopic diseases. J. Allergy Clin. Immunol. 2024, 153, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Chovatiya, R.; Hawkes, J.E.; DiRuggiero, D.; Pansch, L.A.; Simcox, E.; Gonzalez, T. Type 2 Inflammation and Its Role in Dermatologic Diseases. Int. J. Dermatol. 2025, 64, 978–991. [Google Scholar] [CrossRef]
- Kessler, W.; Kirchhoff, C.; Reeh, P.W.; Handwerker, H.O. Excitation of cutaneous afferent nerve endings in vitro by a combination of inflammatory mediators and conditioning effect of substance P. Exp. Brain Res. 1992, 91, 467–476. [Google Scholar] [CrossRef]
- Maingret, F.; Coste, B.; Padilla, F.; Clerc, N.; Crest, M.; Korogod, S.M.; Delmas, P. Inflammatory mediators increase Nav1.9 current and excitability in nociceptors through a coincident detection mechanism. J. Gen. Physiol. 2008, 131, 211–225. [Google Scholar] [CrossRef]
- Steen, K.H.; Steen, A.E.; Reeh, P.W. A dominant role of acid pH in inflammatory excitation and sensitization of nociceptors in rat skin, in vitro. J. Neursosci. 1995, 15, 3982–3989. [Google Scholar] [CrossRef]
- Petho, G.; Reeh, P.W. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol. Rev. 2012, 92, 1699–1775. [Google Scholar] [CrossRef]
- Cunha, T.M.; Verri, W.A., Jr.; Silva, J.S.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 1755–1760. [Google Scholar] [CrossRef]
- Tominaga, M.; Takamori, K. Peripheral itch sensitization in atopic dermatitis. Allergol. Int. 2022, 71, 265–277. [Google Scholar] [CrossRef]
- Steinhoff, M.; Ahmad, F.; Pandey, A.; Datsi, A.; AlHammadi, A.; Al-Khawaga, S.; Al-Malki, A.; Meng, J.; Alam, M.; Buddenkotte, J. Neuroimmune communication regulating pruritus in atopic dermatitis. J. Allergy Clin. Immunol. 2022, 149, 1875–1898. [Google Scholar] [CrossRef] [PubMed]
- Escalante, A.; Serra-Baldrich, E. Pathogenic mechanisms underlying itch in atopic dermatitis: The emerging role of neuroimmune interactions. Eur. J. Dermatol. 2023, 33, 343–349. [Google Scholar] [CrossRef] [PubMed]
- McHugh, J.M.; McHugh, W.B. Pain: Neuroanatomy, chemical mediators, and clinical implications. AACN Clin. Issues 2000, 11, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. The induction of pain: An integrative review. Prog. Neurobiol. 1999, 57, 1–164. [Google Scholar] [CrossRef]
- Chen, X.J.; Sun, Y.G. Central circuit mechanisms of itch. Nat. Commun. 2020, 11, 3052. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Boakye, P.A.; Tang, S.J.; Smith, P.A. Mediators of Neuropathic Pain; Focus on Spinal Microglia, CSF-1, BDNF, CCL21, TNF-α, Wnt Ligands, and Interleukin 1β. Front. Pain Res. 2021, 2, 698157. [Google Scholar] [CrossRef]
- Taves, S.; Berta, T.; Chen, G.; Ji, R.R. Microglia and spinal cord synaptic plasticity in persistent pain. Neural Plast. 2013, 2013, 753656. [Google Scholar] [CrossRef]
- Gwak, Y.S.; Unabia, G.C.; Hulsebosch, C.E. Activation of p-38alpha MAPK contributes to neuronal hyperexcitability in caudal regions remote from spinal cord injury. Exp. Neurol. 2009, 220, 154–161. [Google Scholar] [CrossRef]
- Detloff, M.R.; Fisher, L.C.; McGaughy, V.; Longbrake, E.E.; Popovich, P.G.; Basso, D.M. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp. Neurol. 2008, 212, 337–347. [Google Scholar] [CrossRef]
- Watkins, L.R.; Milligan, E.D.; Maier, S.F. Glial proinflammatory cytokines mediate exaggerated pain states: Implications for clinical pain. Adv. Exp. Med. Biol. 2003, 521, 1–21. [Google Scholar] [PubMed]
- Cao, H.; Zhang, Y.Q. Spinal glial activation contributes to pathological pain states. Neurosci. Biobehav. Rev. 2008, 32, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Wong, C.K.; Qiu, H.N.; Dong, J.; Cai, Z.; Chu, M.; Hon, K.L.; Tsang, M.S.; Lam, C.W. NOD2 and TLR2 ligands trigger the activation of basophils and eosinophils by interacting with dermal fibroblasts in atopic dermatitis-like skin inflammation. Cell. Mol. Immunol. 2016, 13, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.F.; Wong, C.K.; Ho, A.W.; Hu, S.; Chen, D.P.; Lam, C.W. Activation of human eosinophils and epidermal keratinocytes by Th2 cytokine IL-31: Implication for the immunopathogenesis of atopic dermatitis. Int. Immunol. 2010, 22, 453–467. [Google Scholar] [CrossRef]
- Wong, C.K.; Leung, K.M.; Qiu, H.N.; Chow, J.Y.; Choi, A.O.; Lam, C.W. Activation of eosinophils interacting with dermal fibroblasts by pruritogenic cytokine IL-31 and alarmin IL-33: Implications in atopic dermatitis. PLoS ONE 2012, 7, e29815. [Google Scholar] [CrossRef]
- Renne, J.; Schäfer, V.; Werfel, T.; Wittmann, M. Interleukin-1 from epithelial cells fosters T cell-dependent skin inflammation. Br. J. Dermatol. 2010, 162, 1198–1205. [Google Scholar] [CrossRef]
- Pham, M.A.; Magdalou, J.; Siest, G.; Lenoir, M.C.; Bernard, B.A.; Jamoulle, J.C.; Shroot, B. Reconstituted epidermis: A novel model for the study of drug metabolism in human epidermis. J. Investig. Dermatol. 1990, 94, 749–752. [Google Scholar] [CrossRef]
- Garcia, N.; Doucet, O.; Bayer, M.; Fouchard, D.; Zastrow, L.; Marty, J.P. Characterization of the barrier function in a reconstituted human epidermis cultivated in chemically defined medium. Int. J. Cosmet. Sci. 2002, 24, 25–34. [Google Scholar] [CrossRef]
- Sriram, G.; Bigliardi, P.L.; Bigliardi-Qi, M. Full-Thickness Human Skin Equivalent Models of Atopic Dermatitis. Methods Mol. Biol. 2019, 1879, 367–383. [Google Scholar]
- Reuter, C.; Walles, H.; Groeber, F. Preparation of a Three-Dimensional Full Thickness Skin Equivalent. Methods Mol. Biol. 2017, 1612, 191–198. [Google Scholar] [PubMed]
- Niehues, H.; Bouwstra, J.A.; Ghalbzouri, E.I.; Brandner, J.M.; Zeeuwen, P.L.J.M.; Bogaard, E.H. 3D skin models for 3R research: The potential of 3D reconstructed skin models to study skin barrier function. Exp. Dermatol. 2018, 27, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, H.; Sung, G.Y. Effects of Indole-3-Lactic Acid, a Metabolite of Tryptophan, on IL-4 and IL-13-Induced Human Skin-Equivalent Atopic Dermatitis Models. Int. J. Mol. Sci. 2022, 23, 13520. [Google Scholar] [CrossRef] [PubMed]
- Clarysse, K.; Pfaff, C.M.; Marquardt, Y.; Huth, L.; Kortekaas Krohn, I.; Kluwig, D.; Lüscher, B.; Gutermuth, J.; Baron, J. JAK1/3 inhibition preserves epidermal morphology in full-thickness 3D skin models of atopic dermatitis and psoriasis. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 367–375. [Google Scholar] [CrossRef]
- Frings, V.G.; Müller, D.; Storz, G.; Rossi, A.; Sennefelder, H.; Adam, C.; Goebeler, M.; Groeber-Becker, F.K.; Schmidt, M. Improved metal allergen reactivity of artificial skin models by integration of Toll-like receptor 4-positive cells. Contact Dermat. 2019, 81, 254–261. [Google Scholar] [CrossRef]
- Danso, M.O.; van Drongelen, V.; Mulder, A.; van Esch, J.; Scott, H.; van Smeden, J.; El Ghalbzouri, A.; Bouwstra, J.A. TNF-α and Th2 cytokines induce atopic dermatitis-like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J. Investig. Dermatol. 2014, 134, 1941–1950. [Google Scholar] [CrossRef]
- Cadau, S.; Gault, M.; Berthelemy, N.; Hsu, C.Y.; Danoux, L.; Pelletier, N.; Goudounèche, D.; Pons, C.; Leprince, C.; André-Frei, V.; et al. An Inflamed and Infected Reconstructed Human Epidermis to Study Atopic Dermatitis and Skin Care Ingredients. Int. J. Mol. Sci. 2022, 23, 12880. [Google Scholar] [CrossRef]
- Bäckvall, H.; Wassberg, C.; Berne, B.; Ponten, F. Similar UV responses are seen in a skin organ culture as in human skin in vivo. Exp. Dermatol. 2002, 11, 349–356. [Google Scholar] [CrossRef]
- Sidgwick, G.P.; McGeorge, D.; Bayat, A. Functional testing of topical skin formulations using an optimised ex vivo skin organ culture model. Arch. Dermatol. Res. 2016, 308, 297–308. [Google Scholar] [CrossRef]
- Tobin, D.J. Ex vivo organ culture of human hair follicles: A model epithelial-neuroectodermal-mesenchymal interaction system. Methods Mol. Biol. 2011, 695, 213–227. [Google Scholar]
- Al Kindi, A.; Williams, H.; Matsuda, K.; Alkahtani, A.M.; Saville, C.; Bennett, H.; Alshammari, Y.; Tan, S.Y.; O’Neill, C.; Tanaka, A.; et al. Staphylococcus aureus second immunoglobulin-binding protein drives atopic dermatitis via IL-33. J. Allergy Clin. Immunol. 2021, 147, 1354–1368. [Google Scholar] [CrossRef]
- Jeong, S.; Na, Y.; Nam, H.M.; Sung, G.Y. Skin-on-a-chip strategies for human hair follicle regeneration. Exp. Dermatol. 2023, 32, 13–23. [Google Scholar] [CrossRef]
- Govey-Scotland, J.; Johnstone, L.; Myant, C.; Friddin, M.S. Towards skin-on-a-chip for screening the dermal absorption of cosmetics. Lab Chip 2023, 23, 5068–5080. [Google Scholar] [CrossRef]
- Wufuer, M.; Lee, G.; Hur, W.; Jeon, B.; Kim, B.J.; Choi, T.H.; Lee, S. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci. Rep. 2016, 6, 37471. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Zhang, Y.; Wang, X.; Ouyang, J.; Zhu, J.; Yan, Y.; Sun, X.; Wang, F.; Li, X.; et al. Construction of a high fidelity epidermis-on-a-chip for scalable in vitro irritation evaluation. Lab Chip 2021, 21, 3804–3818. [Google Scholar] [CrossRef]
- Quan, Q.; Weng, D.; Li, X.; An, Q.; Yang, Y.; Yu, B.; Ma, Y.; Wang, J. Analysis of drug efficacy for inflammatory skin on an organ-chip system. Front. Bioeng. Biotechnol. 2022, 10, 939629. [Google Scholar] [CrossRef] [PubMed]
- Barros, N.R.; Kang, R.; Kim, J.; Ermis, M.; Kim, H.J.; Dokmeci, M.R.; Lee, J. A human skin-on-a-chip platform for microneedling-driven skin cancer treatment. Mater. Today Bio 2024, 30, 101399. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Bartnikas, L.M.; Gurish, M.F.; Burton, O.T.; Leisten, S.; Janssen, E.; Oettgen, H.C.; Beaupré, J.; Lewis, C.N.; Austen, K.F.; Schulte, S.; et al. Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 451–460. [Google Scholar] [CrossRef]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Álvarez-Pérez, B.; Homs, J.; Bosch-Mola, M.; Puig, T.; Reina, F.; Verdú, E.; Boadas-Vaello, P. Epigallocatechin-3-gallate treatment reduces thermal hyperalgesia after spinal cord injury by down-regulating RhoA expression in mice. Eur. J. Pain 2016, 20, 341–352. [Google Scholar] [CrossRef]
- Bagó-Mas, A.; Korimová, A.; Deulofeu, M.; Verdú, E.; Fiol, N.; Svobodová, V.; Dubový, P.; Boadas-Vaello, P. Polyphenolic grape stalk and coffee extracts attenuate spinal cord injury-induced neuropathic pain development in ICR-CD1 female mice. Sci. Rep. 2022, 12, 14980. [Google Scholar] [CrossRef]
- Soler-Martínez, R.; Deulofeu, M.; Bagó-Mas, A.; Dubový, P.; Verdú, E.; Fiol, N.; Boadas-Vaello, P. Central Neuropathic Pain Development Modulation Using Coffee Extract Major Polyphenolic Compounds in Spinal-Cord-Injured Female Mice. Biology 2022, 11, 1617. [Google Scholar] [CrossRef]
- Verdú, E.; Navarro, X. Comparison of immunohistochemical and functional reinnervation of skin and muscle after peripheral nerve injury. Exp. Neurol. 1997, 146, 187–198. [Google Scholar] [CrossRef]
- Toledano-Martos, R.; Bagó-Mas, A.; Deulofeu, M.; Homs, J.; Fiol, N.; Verdú, E.; Boadas-Vaello, P. Natural polyphenolic coffee extract administration relieves chronic nociplastic pain in a reserpine-induced fibromyalgia-like female mouse model. Brain Behav. 2024, 14, 3386. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).