Abstract
During pregnancy, physiological adaptations occur in the respiratory and cardiovascular systems to support the increased metabolic needs of both mother and fetus. Key respiratory changes include mechanical adjustments of the chest wall and diaphragm to accommodate the growing uterus; decreases in functional residual capacity and its components—expiratory reserve volume and residual volume—with minimal or no change in total lung capacity; and an increase in minute ventilation. Major cardiovascular adaptations involve elevated cardiac output, stroke volume and heart rate, and decreased mean arterial pressure and systemic vascular resistance. During exercise in pregnancy, there is an increase in ventilation, alveolar diffusion, elevated oxygen consumption, greater carbon dioxide production and changes in respiratory volumes and capacities, as well as increases in cardiac output, stroke volume and heart rate. Understanding these normal physiological changes during pregnancy and exercise in pregnancy is essential for healthcare providers to develop and adapt exercise programs according to the gestational age and physical fitness level of the pregnant woman.
1. Introduction
Pregnancy is a dynamic process associated with physiological changes in the body. Numerous adjustments and adaptations occur in the respiratory and cardiovascular systems during rest and exercise during pregnancy. These changes are mechanisms that the body has adapted to meet the increased metabolic demands of the mother and fetus, thus enabling adequate delivery of oxygenated blood to peripheral tissues and to ensure adequate uteroplacental circulation for fetal growth and development [1].
When prescribing an exercise program for a pregnant woman, it is essential to consider the physiological responses of body systems during exercise, the changes in maternal body systems, the effects of these changes on the fetus, and the fetal responses to maternal exercise during pregnancy. Every movement requires activation and control of the musculoskeletal system, while the cardiovascular and respiratory systems provide the capacity to perform movements over an extended period of time. Regular exercise during pregnancy leads to specific adaptive processes in body systems that enhance physical capabilities. The extent of these adaptations largely depends on the intensity and duration of the exercise.
The primary functions of the cardiovascular and respiratory systems are to supply the body with oxygen and nutrients, remove carbon dioxide and metabolic waste products, maintain body temperature and acid/base balance, and transport hormones from endocrine glands to their target organs [2]. To be effective and efficient, both the cardiovascular and respiratory systems must be able to respond to increased activity of the skeletal muscles.
Exercise places significant demands on the cardiovascular system in order to improve circulation in the skeletal muscles and meet the increased metabolic demands of the body. The degree of adaptation of body systems largely depends on the physical fitness level of the pregnant woman, as well as the mode, intensity, duration and frequency of exercise. The aim of this review is to present cardiovascular and respiratory adaptations during pregnancy and exercise in pregnancy. Knowledge of the adaptations of the cardiovascular and respiratory systems during pregnancy and exercise in pregnancy is necessary for prescribing and modifying an exercise program.
2. Cardiovascular Adaptations Pregnancy
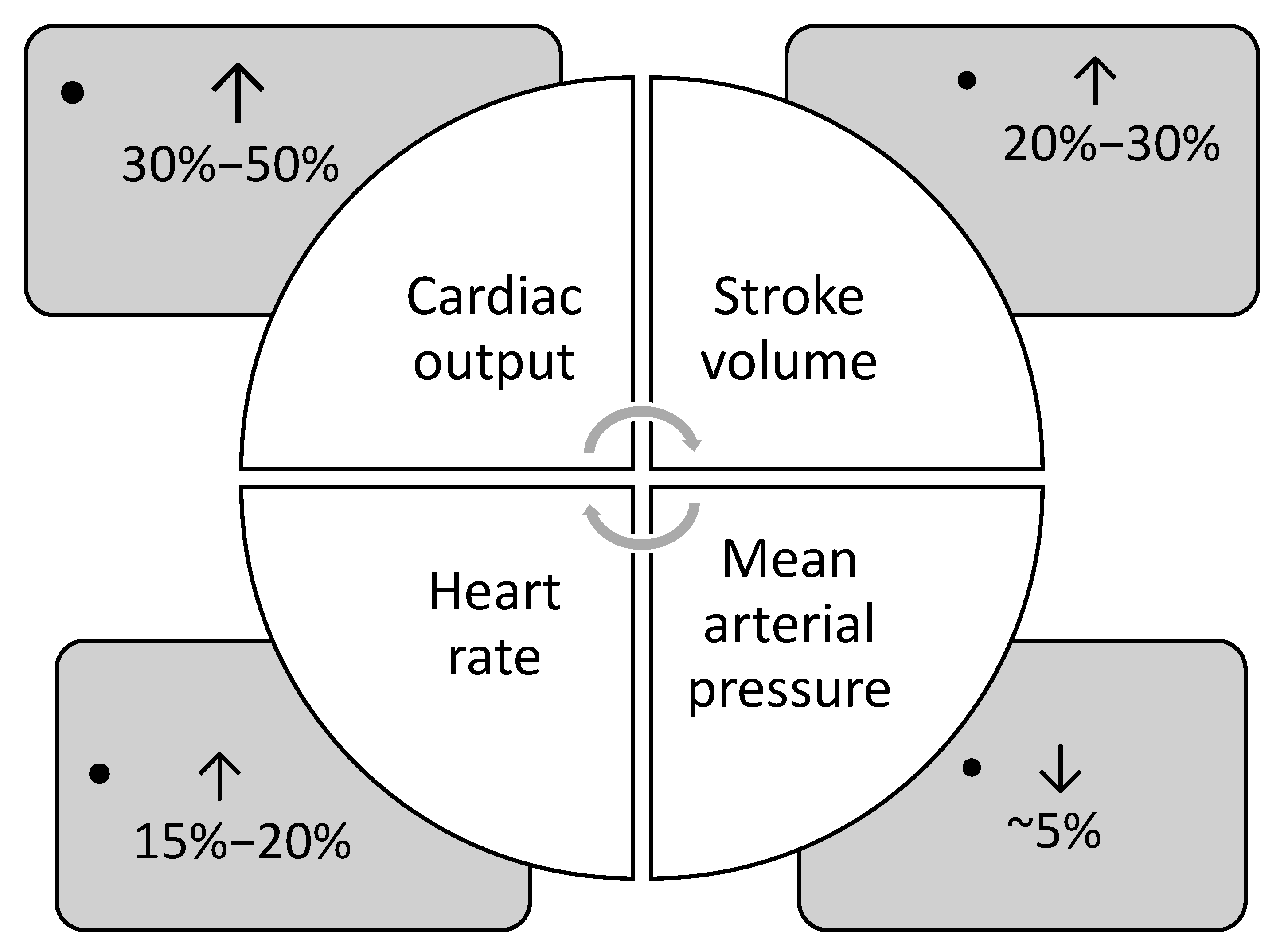
During pregnancy, cardiovascular adaptations are essential to sustain adequate uteroplacental blood flow and ensure favorable pregnancy outcomes [3]. Physiological changes in cardiovascular function occur gradually throughout pregnancy, with the maternal body continuously adapting to these shifts [4]. The main adaptations include elevated cardiac output, stroke volume and heart rate, and decreased mean arterial pressure and systemic vascular resistance (Figure 1).
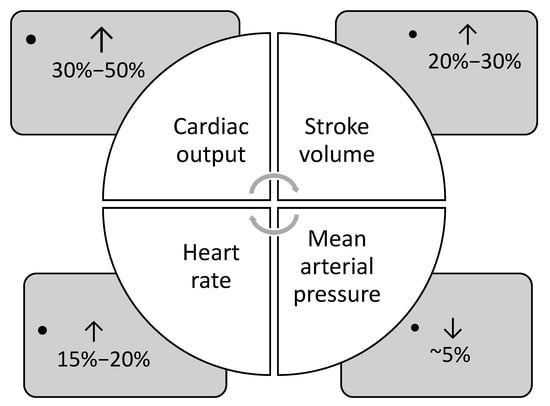
Figure 1.
Main cardiovascular adaptations during pregnancy (data from Refs. [1,4,5]).
Cardiac output begins to rise in the first trimester and continues to rise into the second trimester, by 30–50% [1,5]. By approximately 24 weeks of gestation, cardiac output may rise by as much as 45% in a singleton pregnancy. In twin pregnancies, cardiac output is 15% higher than in singleton pregnancies, accompanied by a significantly greater increase in left atrial diameter, indicative of volume overload [1,6,7]. The rise in cardiac output during early pregnancy is driven by a 20% increase in heart rate and a 20–30% increase in stroke volume [8,9]. The rise in stroke volume is primarily due to an increase in preload, resulting from the expanded plasma volume, as well as a 20–30% reduction in systemic vascular resistance [1,5,6]. Remodeling of the uterine vasculature reduces resistance within the uteroplacental circulation, contributing to the overall decline in systemic vascular resistance during pregnancy [3]. Due to progesterone-induced smooth muscle relaxation, systemic vascular resistance decreases, resulting in a drop in blood pressure during pregnancy [4,10]. Arterial pressures reach their lowest values during the second trimester, with reductions of approximately 5–10 mm Hg from baseline [4,10]. This drop in pressure, alongside increased cardiac output, reflects the overall decline in systemic vascular resistance throughout gestation [4,10]
Maternal heart rate gradually increases throughout pregnancy, peaking in the third trimester, with an overall rise of 25% above pre-pregnancy levels [1,4]. This is largely attributed to heightened sympathetic activity, which likely acts as a compensatory mechanism for widespread peripheral vasodilation [11,12]. Increased estradiol levels stimulate nitric oxide synthesis, leading to β-adrenergic-mediated vasodilation and further contributing to sympathetic overactivity. Instead of increasing end-diastolic volume, the heart meets the increased circulatory demand by accelerating heart rate, thereby boosting cardiac output [4,11].
There are also marked increases in total blood volume and red blood cell mass during pregnancy. Total blood volume usually increases by approximately 45% [1,5,13]. Red blood cell mass rises by up to 40%, leading to hemodilution and the development of physiological anemia during pregnancy [1,5,14].
Maternal posture also affects cardiac output. It is highest in the knee-chest and left lateral positions, and lowest in the standing and supine positions [10,15,16]. In the supine position, cardiac output decreases due to compression of the inferior vena cava by the enlarged uterus, which reduces venous return [4,5,10].
3. Respiratory Adaptations During Pregnancy
Hormonal changes (especially increased estrogen) cause mucosal edema, hyperemia, capillary congestion and increased fragility in the upper airway [9,10]. These changes commonly lead to nasal congestion and reduced nasal airflow, and may result in the development of rhinitis, nosebleeds and rhinitis [5,17]. The incidence of rhinitis in pregnancy has been reported to be between 18% and 42% [5,18,19].
As the uterus expands and the abdomen enlarges, the diaphragm shifts upward by about 4–5 cm, the antero-posterior and transverse diameter of the chest increases by 2 cm, the chest circumference increases by 5–7 cm and the subcostal angle increases from 68° to 103° [5,20,21,22,23]. These structural changes are partially offset by hormonal relaxation of the rib ligaments, but overall, chest wall compliance and total respiratory system compliance decrease, while the lungs themselves retain their normal compliance [8,19,23].
Despite this progressive distortion of the thoracic and abdominal areas, the strength of both the inspiratory and expiratory muscles remains intact, and diaphragmatic movement is not impaired [20,21]. This preserved strength may be due to improved length–tension positioning of the diaphragm as it rises [8,9,20]. However, breathing becomes more energy-demanding during pregnancy [2,23].
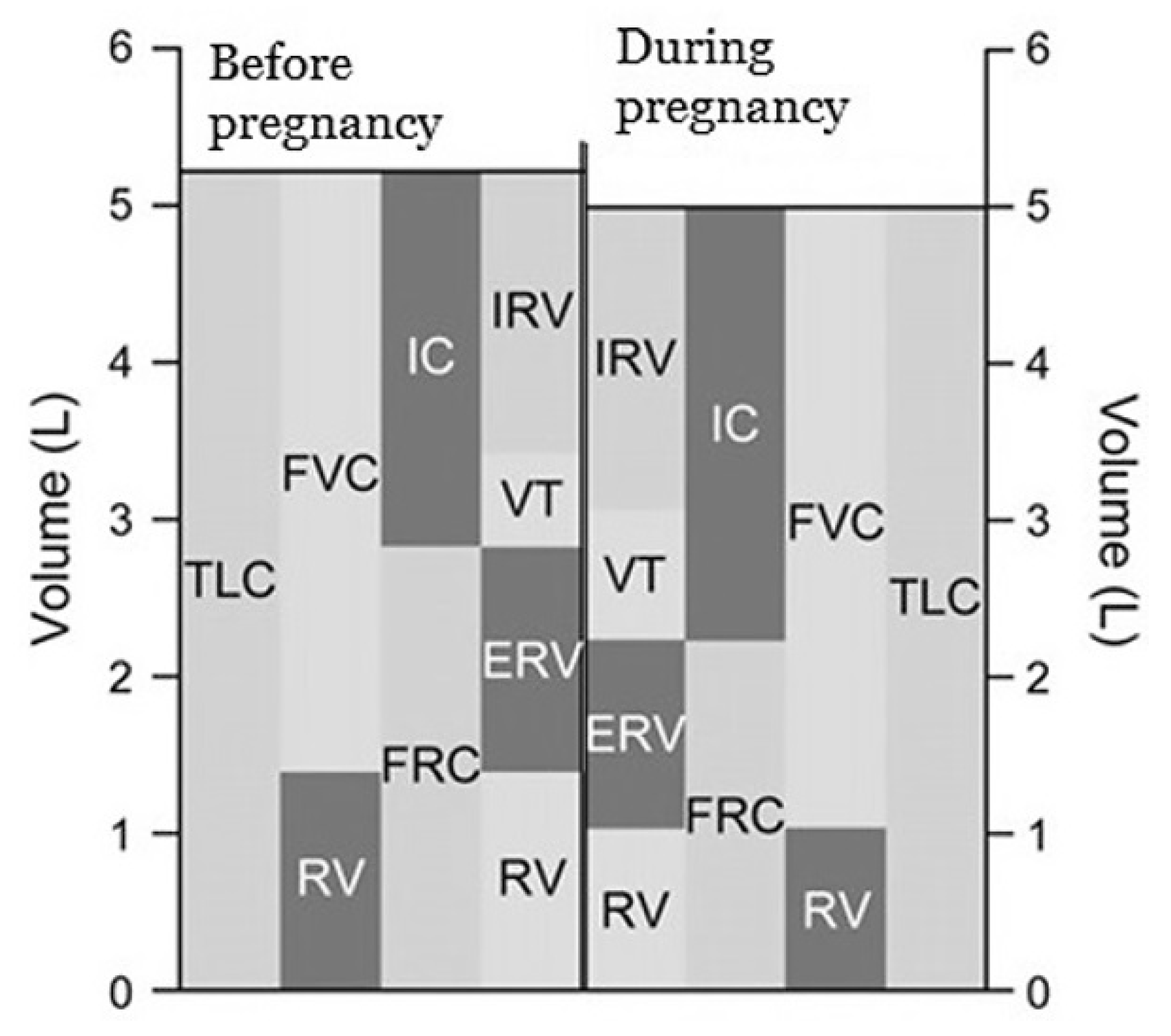
Functional residual capacity (FRC) decreases by about 20% due to diaphragmatic elevation and reduced chest wall compliance [10,11,20]. The decline in expiratory reserve volume (ERV) by 15% to 20% contributes to this reduction [5,18]. Noticeable decreases in FRC are seen at around six months of gestation and continue progressively as pregnancy advances [8,9]. In the near term, FRC decreases an additional 25% when lying down compared to sitting [5,21].
In contrast, inspiratory capacity (IC) increases by 5% to 10% and total lung capacity (TLC) remains unchanged or decreases slightly (less than 5%) in the near term during pregnancy [5,9,21]. Figure 2 presents changes in respiratory volumes and capacities before and during pregnancy.
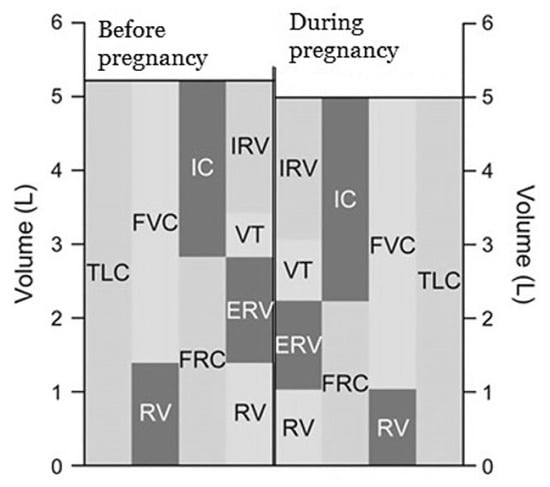
Figure 2.
Changes in respiratory volumes and capacities before and during pregnancy (data from Refs. [5,7]).
Minute ventilation (VE) significantly increases during pregnancy [8,9,10]. VE is elevated by 20% to 50% in the term compared to nonpregnant levels [5,18,21]. This increase is mainly due to a 30% to 50% rise in tidal volume and unchanged or slight changes (by 1–2 breaths per minute) in respiratory rate [5,18,19].
The increase in tidal volume happens without lengthening the inspiratory time or respiratory cycle duration, which means inspiratory flow is faster [2,5,21].
The metabolic demands of the fetus, uterus and maternal organs rise during pregnancy, leading to increased oxygen consumption (VO2), carbon dioxide production (VCO2) and basal metabolic rate [8,9,10]. At term, VO2 and VCO2 are about 20% and 35% higher, respectively, than in nonpregnant women [2,5,20,21]. The respiratory exchange ratio (VCO2/VO2) remains mostly unchanged or shows a slight increase [20,21]. The rise in minute ventilation (VE) surpasses the increases in VO2 and VCO2, which causes alveolar and arterial oxygen partial pressures (PAO2 and PaO2) to increase, while alveolar and arterial CO2 partial pressures (PACO2 and PaCO2) decrease [2,5,8,20].
Maternal arterial partial pressure of carbon dioxide (PaCO2) decreases to between 26 and 32 mm Hg due to increased minute ventilation during pregnancy [5,9,20]. This reduction in PaCO2 establishes a gradient that enables the fetus to transfer CO2 to the maternal circulation for elimination via the respiratory system [20,21]. As alveolar CO2 levels drop, maternal alveolar oxygen tension rises, and PaO2 can reach up to 106 mm Hg in the first trimester and slightly decrease to 102 mmHg in the near term [5,8,20]. The fetus relies on an oxygen gradient to maintain diffusion across the placenta, and the slight rise in maternal PaO2 supports this process [12,21]. Fetal oxygenation also depends on maternal arterial oxygen content, hematocrit levels and uterine artery perfusion [12,14]. Impairment of these components may result in fetal hypoxemia and, eventually, acidemia [2,12,18].
4. Cardiovascular and Respiratory Adaptations During Exercise in Pregnancy
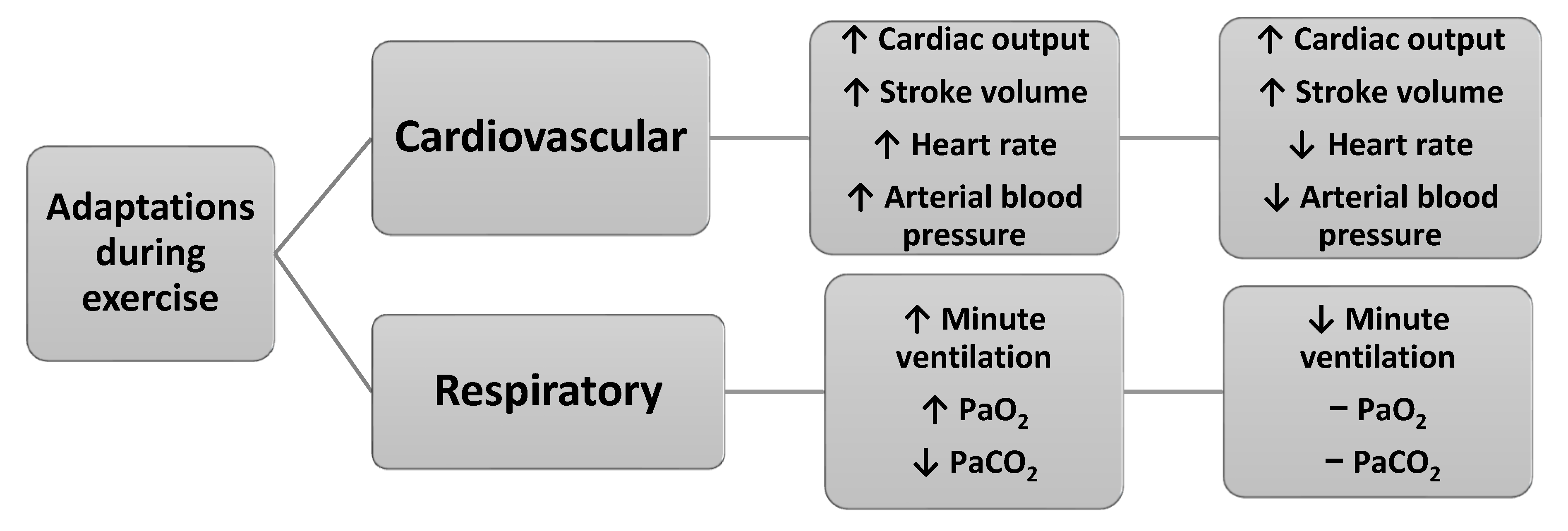
Exercise throughout pregnancy contributes to better pregnancy outcomes, such as reduced risks of gestational diabetes, hypertension, preeclampsia, peripheral edema, varicose veins, deep vein thrombosis, urinary incontinence, spinal and pelvic pain, excessive weight gain and increased cardiorespiratory fitness [5,12,13,14,23]. Figure 3 presents the main cardiovascular and respiratory adaptations during exercise in pregnancy.
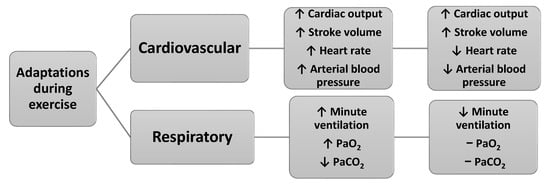
Figure 3.
Main cardiovascular and respiratory adaptations during exercise in pregnancy (data from Refs. [3,7]).
At moderate exercise intensities, pregnant women exhibit different physiological responses compared to nonpregnant women [5,24]. The increase in oxygen consumption (VO2) for a given workload is higher in pregnancy, but VO2 changes depend on the exercise type [5,7]. For example, weight-bearing activities like treadmill walking elicit significantly higher VO2 in pregnant women than cycle ergometry, a difference more pronounced than in nonpregnant women [5,7].
At mild exercise intensities, pregnant women show a significantly higher respiratory rate than nonpregnant women [15]. However, during moderate to maximal exercise, respiratory rate increases are similar between the two groups [18,25].
During pregnancy, both minute ventilation (VE) and alveolar ventilation (VA) are elevated at rest, and their rates of increase during exercise are faster compared to the nonpregnant state [5,15]. At submaximal workloads, VE and VA increase by 20% to 25% relative to nonpregnant values [5,7]. The rise in ventilation exceeds the rise in oxygen consumption, leading to an increased ventilatory equivalent for oxygen (VE/VO2) [5,26].
Cardiac output is also higher in pregnant women during exercise, mainly due to an increase in stroke volume [5,7]. This proportionally larger cardiac output reduces the difference between arterial and mixed venous oxygen content (CaO2–CvO2), enhancing oxygen delivery to the fetus during maternal exercise [5,15,18].
Acid–base balance remains stable during exercise in pregnancy, with no significant changes observed [18,27]. Interestingly, pregnant women preferentially utilize fat as their primary energy source during physical activity, with a reduced reliance on carbohydrates for fuel [18,27].
4.1. Effects of Acute Exercise in Pregnancy
During acute exercise in pregnancy, multiple physiological mechanisms work together to ensure that active tissues receive sufficient oxygen for mitochondrial function. These mechanisms include enhanced alveolar ventilation, improved oxygen diffusion into the bloodstream, increased blood flow to tissues, and more efficient oxygen transport into muscle cells and mitochondria [3,7,28].
A single bout of exercise elevates both oxygen consumption and carbon dioxide production in the working muscles, with oxygen consumption (VO2) increasing proportionally to exercise intensity [3,12,27]. Consequently, alveolar ventilation (VA) also rises in accordance with the intensity of the activity [12]. At the same time, pulmonary blood flow increases significantly, primarily driven by elevated left ventricular filling pressure [12]. This pressure enhances capillary recruitment and distension within the lungs, thereby expanding both surface area and volume for gas exchange [3,29].
The cardiovascular system responds to exercise demands in direct proportion to the oxygen requirements of skeletal muscle [7,13]. Cardiac output increases due to a rise in both stroke volume and heart rate. Stroke volume climbs with power output to approximately 40–60% of VO2max, after which it plateaus, while further increases in heart rate continue to elevate cardiac output [3,24,28]. Blood flow is redistributed from areas with low metabolic demand (e.g., splanchnic organs) toward high-demand regions such as the heart, active skeletal muscles and the skin (due to thermoregulatory needs) [3,23,29].
Acute exercise during pregnancy also induces a shift of interstitial fluid into the plasma, expanding circulating blood volume [15]. Vascular adaptations include increased endothelial shear stress, which promotes flow-mediated vasodilation [3,13,27]. This response, along with the recruitment of additional microvascular units, increases the surface area for exchange and facilitates nutrient and oxygen delivery to muscle tissue [12,13]. Additionally, vasodilation in active muscles is triggered by local metabolites such as adenosine, lactate, potassium ions (K+), hydrogen ions (H+) and carbon dioxide (CO2) [3,26,30].
As exercise intensity increases, both mean arterial and systolic blood pressure rises, while diastolic pressure tends to remain stable [7,15]. This is a normal adaptation attributed to the resetting of the arterial baroreflex at a higher-pressure threshold, which helps maintain perfusion without inducing hypotension [3,15,25].
Acute exercise also activates the sympathetic nervous system, leading to the release of catecholamines (adrenaline, noradrenaline and dopamine) into circulation and in target tissues [3,13,23]. These hormones stimulate cardiovascular, respiratory, metabolic and thermoregulatory responses that support sustained exercise [15]. Examples include increased heart rate and cardiac output, vasoconstriction in renal and splanchnic arteries, and vasodilation in skeletal muscle [3,24,26].
4.2. Effects of Regular Exercise in Pregnancy
Engaging in regular exercise during pregnancy leads to significant metabolic, structural and functional adaptations across multiple physiological systems. These long-term changes include lowered resting heart rate and blood pressure, improved vasodilatory function of the muscle microvasculature, and an expanded microvascular network through angiogenesis and arteriogenesis—resulting in increased muscle capillary density [3,27,31].
With consistent endurance training, pulmonary ventilation (VE) decreases during submaximal exercise but increases during maximal exercise [3,12,24]. Tidal volume (VT) increases and breathing frequency decreases during submaximal activity; however, both rise during maximal exertion [3,12]. Regular exercise also enhances ventilatory efficiency, reflected in a lower ventilatory equivalent for oxygen (VE/VO2), and reduces the proportion of total body oxygen cost for breathing [12]. Furthermore, maximal oxygen uptake (VO2max) increases, improving aerobic capacity [3,12,28].
To manage the hemodynamic demands of repeated endurance exercise (whether pressure or volume overload), the heart undergoes structural adaptations, including increased ventricular wall thickness and chamber dilation [3,7,27]. These changes boost contractile strength, stroke volume and ejection fraction at rest and during exercise [13]. Resting heart rate declines due to enhanced parasympathetic tone, though maximal heart rate generally remains stable. In parallel, plasma volume and total blood volume expand, elevating cardiac output [3,24,27]. The rise in plasma volume may cause relative hemodilution, which facilitates smoother capillary blood flow [3,5,15]. Although hemoglobin concentration may appear reduced, this is counterbalanced by increases in total red blood cell volume and higher levels of red cell 2,3-diphosphoglycerate (2,3-DPG), which improve oxygen delivery to tissues [3,27,29].
While acute exercise elevates mean arterial pressure, regular exercise leads to reductions in resting and submaximal systolic and diastolic blood pressure [3,7,28]. This effect is attributed to chronic reductions in systemic vascular resistance [15]. Enhanced endothelial shear stress and the metabolic activity of skeletal muscle promote the release of nitric oxide and prostacyclin, which relax vascular smooth muscle, lowering vascular tone [3,13,30]. These vascular benefits are reinforced by increased arteriole density and vasodilatory capacity in skeletal muscle, improving blood flow and nutrient delivery [13,15].
Additional contributors to reduced blood pressure include diminished sympathetic nerve activity, reduced arterial stiffness and decreased systemic inflammation [3,7,31]. Endurance training also promotes angiogenesis, raising muscle capillary density, which prolongs mean blood transit time and enhances oxygen extraction [3,13,30]. This allows the body to meet oxygen demands with lower blood flow during rest and submaximal activity [15].
5. Discussion
Regular exercise during pregnancy has been shown to improve or maintain physical and cardiorespiratory fitness [32,33], which is associated with less bodily pain, lumbar and sciatic pain, and reduced pain disability [34]. Observational studies of women who exercise during pregnancy have shown benefits such as decreased gestational diabetes [35,36], gestational hypertension [35], cesarean birth [37] and operative vaginal delivery [33,37,38], and postpartum recovery time [33]. Fetal benefits include decreased fat mass, improved stress tolerance and advanced neurobehavioral maturation [39]. Multiple professional societies and healthcare organizations, including the American College of Obstetricians and Gynecologists, recommend that pregnant women exercise for at least 150 min a week at moderate intensity, a combination of aerobic exercise and resistance exercises at least twice a week [40,41,42]. Also, they suggest that the principles of exercise prescription for pregnant women do not differ from the nonpregnant population [6,40]. When prescribing exercise during pregnancy, it is necessary to consider cardiovascular adaptations and adjust the type of exercise, intensity, duration and frequency of exercise according to the level of the physical condition of the pregnant woman and the week of pregnancy [6,40]. According to the American College of Obstetricians and Gynecologists, exercise in pregnancy is associated with minimal risks and has been shown to benefit most women, although some modifications to exercise routines may be necessary because of normal anatomic and physiologic changes and fetal requirements [40]. A thorough clinical evaluation should be conducted before recommending an exercise program to ensure that a pregnant woman does not have a medical reason to avoid exercise [40].
The body’s mechanisms operate at a minimal level, requiring a certain basic amount of energy to maintain the fundamental functioning of cells, organs, organ systems and the organism as a whole [8]. In contrast, exercise (physical work) during pregnancy represents a change in the state of the organism that forces it—through a range of functional and regulatory deviations—to provide, on one hand, an amount of energy proportional to the physical effort and, on the other hand, enough energy to eliminate excess metabolites and thermal energy [9].
Regardless of a certain number of anaerobic energy sources stored in the muscles, the ultimate provision and restoration of energy in tissues, as well as the removal of metabolites, depend on aerobic energy sources, which can release energy only through oxidative cellular respiration processes [24]. In other words, the additional supply of oxygen is proportional to the intensity and duration of exercise in pregnancy [22,29]. Since there is virtually no oxygen reserve in the body, it must be continuously transported from the atmosphere into the cells—both at rest and during exercise [22,29]. This task is carried out by the body’s oxygen transport system, which includes the respiratory and cardiovascular systems, as well as the blood [22,29].
The functional capacity of the oxygen transport system—its maximal ability to deliver oxygen to active tissues per unit of time—determines the level of aerobic work capacity and, consequently, various physiological indicators of functional fitness of pregnant women [29,31]. In order to meet the increased demand for oxygen during exercise in pregnancy, the transport system must adapt its functions to a higher level of activity proportional to the current demands [22,31]. This is reflected, among other things, in increased ventilation, alveolar diffusion, an increased volume of blood ejected by the heart per unit time and the redirection of a larger portion of blood flow to active tissues [28,30]. All these contribute to more efficient oxygen uptake, transport and release into the tissues [22,30].
For a trained muscle to perform higher-intensity work, an increased supply of energy (oxygen) is necessary, which is possible only through an appropriate increase in blood flow through the muscle [9,22]. However, certain adaptations to exercise also occur in the periphery. These primarily include increased capillarization of trained muscles, better opening of existing capillaries in trained muscles and more efficient blood redistribution [26]. Strength training and aerobic exercise stimulate the development of new capillaries in the muscles, increasing the density of the local capillary network and thus the perfusion capacity of muscle tissue [25]. In trained muscles, even existing capillaries open more effectively during exercise, ensuring and contributing to better blood flow [24,25]. Increased blood flow to active muscles also results from the redistribution of blood from inactive regions [25].
Therefore, it can be said that the oxygen transport system is not only capable of adapting to the organism’s current oxygen demands but also, under the influence of exercise, increases its transport capacity [24,28]. This ensures an increase in maximal oxygen uptake (aerobic capacity) in a pregnant woman who exercises [22,28]. In this way, not only does the potential intensity of exercise increase but also the overall aerobic endurance and fitness of the pregnant woman increase [9,22].
Bgeginski et al. reported that blood pressure is reduced in the pregnant group during exercise compared to the nonpregnant group, which showed that pregnancy is responsible for a reduction in blood pressure due to decreased peripheral vascular resistance [31]. This response represents a protective effect on cardiovascular health in the pregnant woman [31]. Also, Bgeginski et al. point out that the respiratory variables are higher with increasing exercise volume achieved by pregnant and nonpregnant groups, which shows that exercise results in an instantaneous increase in the energy demand of exercised muscles [31]. Pulmonary ventilation and VO2 increase during exercise in direct proportion to the body’s metabolic needs [43]. The extent of this response during resistance exercise appears to be influenced by the number of sets performed and is further modulated by training variables, including exercise intensity, the amount of muscle mass engaged and the interval between repetitions [43]. Finkelsten et al. did not find significant differences in the behavior of VO2 in pregnant women exercising on a cycle ergometer, in submaximal intensities on land, when compared to the nonpregnant state [44]. But Jensen et al. reported significant differences in VO2 at the maximum equivalent load between pregnant and nonpregnant women [45]. Also, Finkelsten et al. indicate that pregnant women, at the beginning of the last trimester of pregnancy, presented cardiovascular responses similar to the nonpregnant women during exercise, when performed at the intensity corresponding to the ventilatory threshold (VO2VT) [44]. Wolfe and Weissgerber have shown that oxygen uptake at the ventilatory threshold is not significantly affected by pregnancy or advancing gestational age [8]. These findings indicate that pregnant women are able to perform submaximal work without accumulating lactic acid in blood and becoming fatigued [8]. However, peak blood lactate levels following strenuous exercise testing have been reported to be lower during pregnancy than in the nonpregnant state, due possibly to dilution of the lactate produced in an expanded maternal blood volume [46]. Also, respiratory gas exchange measurements show reduced carbon dioxide output at work rates above the point of respiratory compensation, suggesting that the capacity for anaerobic metabolism may be reduced in late pregnancy [8].
Changes in submaximal VO2 during pregnancy depend on the type of exercise performed [8]. During maternal rest or submaximal weight-bearing exercise (e.g., walking, stepping, treadmill exercise), absolute maternal VO2 is significantly increased compared with the nonpregnant state [8]. Furthermore, Christou et al. point out that endurance exercise training in nonpregnant athletes can induce an early increase in VO2peak with subsequent leveling off or even no significant change in VO2peak during a macrocycle [46]. However, VO2VT1 and VO2VT2 appear to rise continuously from the beginning of exercise training and even when VO2peak has reached a plateau [46]. Moreover, peak performance of nonpregnant athletes in endurance events is more strongly related to VO2VT1 or VO2VT2, rather than VO2peak [46]. However, Cai et al. emphasize that no submaximal exercise test protocol has been validated for use in pregnancy [47], and Moholdt et al. emphasize that, due to the safety and functional relevance of VO2VT1, an increase in VO2VT1 is likely the more sensitive marker to detect endurance capacity improvements after training in pregnant women but a direct sensitivity comparison study is still an evidence gap [48].
Maximal heart rate has been reported to be approximately 4 beats per minute lower during pregnancy compared to the postpartum period [49]. This attenuated heart rate response to maximal exercise is thought to result from a diminished sympathoadrenal response to heavy exertion during pregnancy [50]. Consequently, due to an elevated resting heart rate and a reduced maximal heart rate, heart rate reserve is decreased [50]. This leads to a less pronounced increase in heart rate relative to rising oxygen consumption (VO2), lowering the slope of the heart rate–VO2 relationship during pregnancy compared to the nonpregnant state [8]. Notably, apart from the increase in resting heart rate, the overall alteration in the heart rate–VO2 relationship does not appear to be significantly influenced by a woman’s exercise throughout pregnancy [8].
Regular exercise in pregnancy induces a series of physiological changes in the transport and higher regulatory systems, improving their reactivity and functional capacity [27,30]. This enhances the body’s energy and work-related capabilities [28]. Adaptation is a biological response through which an organism aims to ensure its survival and more efficient functioning under different conditions and demands. A limitation of this review is that it has only considered studies in English. Mainly, narrative and systematic reviews of randomized controlled trials were included, which may have limited its comprehensiveness. This review provides insight into cardiovascular and respiratory responses during pregnancy and exercise in pregnancy, and contributes significantly to the scientific knowledge in the field of clinical physiology of exercise (particularly in the context of pregnancy), which is important for exercise prescription in clinical practice. It reassures and increases confidence in the prescription of exercise during pregnancy. It highlights awareness of the importance of exercising during pregnancy and prescribes regular exercise for women during pregnancy.
6. Conclusions
Pregnancy induces a range of physiological adaptations that affect maternal cardiovascular and respiratory function. A comprehensive understanding of these gestational and exercise-related changes is fundamental to prescribing exercise and optimizing care for pregnant women. These adaptations are necessary to meet the increased metabolic demands of the mother and fetus and maintain performance under physical strain. Exercise during pregnancy has beneficial effects on both pregnant women and the fetus. Knowing these expected changes to the cardiovascular and respiratory systems will help healthcare professionals to better manage exercise in pregnancy and enhance the quality of perinatal care.
Author Contributions
Both authors contributed equally in the conceptualization, writing and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sanghavi, M.; Rutherford, R.D. Cardiovascular physiology of pregnancy. Circulation 2014, 130, 1003–1008. [Google Scholar] [CrossRef]
- LoMauro, A.; Aliverti, A. Respiratory physiology of pregnancy. Breathe 2015, 11, 297–301. [Google Scholar] [CrossRef] [PubMed]
- van Poppel, M.N.M.; Kruse, A.; Carter, A.M. Maternal physical activity in healthy pregnancy: Effect on fetal oxygen supply. Acta Physiol. 2024, 240, e14229. [Google Scholar] [CrossRef] [PubMed]
- Taranikanti, M. Physiological Changes in Cardiovascular System during Normal Pregnancy: A Review. Indian J. Cardiovasc. Dis. Women 2018, 3, 62–67. [Google Scholar] [CrossRef][Green Version]
- Hegewald, M.J.; Crapo, R.O. Respiratory physiology in pregnancy. Clin. Chest Med. 2011, 32, 1–13. [Google Scholar] [CrossRef]
- Melzer, K.; Schutz, Y.; Boulvain, M.; Kayser, B. Physical Activity and Pregnancy Cardiovascular Adaptations, Recommendations and Pregnancy Outcomes. Sports Med. 2010, 40, 493–507. [Google Scholar] [CrossRef]
- Perales, M.; Santos-Lozano, A.; Sanchis-Gomar, F.; Luaces, M.; Pareja-Galeano, H.; Garatachea, N.; Barakat, R.; Lucia, A. Maternal Cardiac Adaptations to a Physical Exercise Program during Pregnancy. Med. Sci. Sports Exerc. 2016, 48, 896–906. [Google Scholar] [CrossRef]
- Wolfe, L.A.; Weissgerber, T.L. Clinical physiology of exercise in pregnancy: A literature review. J. Obstet. Gynaecol. Can. 2003, 25, 473–483. [Google Scholar] [CrossRef]
- Talbot, L.; Maclennan, K. Physiology of pregnancy. Anaesth. Intensive Care Med. 2016, 17, 341–345. [Google Scholar] [CrossRef]
- Ouzounian, J.G.; Elkayam, U. Physiologic changes during normal pregnancy and delivery. Cardiol. Clin. 2012, 30, 317–329. [Google Scholar] [CrossRef]
- Jain, P.G.; Patil, S.D.; Chaudhari, H.; Pawar, A.R.; Patil, A.B. Physiological and Anatomical changes during Pregnancy. Asian J. Res. Chem. 2020, 13, 279–282. [Google Scholar] [CrossRef]
- Witvrouwen, I.; Mannaerts, D.; Van Berendoncks, A.M.; Jacquemyn, Y.; Van Craenenbroeck, E.M. The Effect of Exercise Training During Pregnancy to Improve Maternal Vascular Health: Focus on Gestational Hypertensive Disorders. Front. Physiol. 2020, 11, 450–460. [Google Scholar] [CrossRef]
- Melzer, K.; Schutz, Y.; Soehnchen, N.; Othenin-Girard, V.; Martinez de Tejada, B.; Irion, O.; Boulvain, M.; Kayser, B. Effects of recommended levels of physical activity on pregnancy outcomes. Am. J. Obstet. Gynecol. 2010, 202, 266.e1–266.e6. [Google Scholar] [CrossRef]
- Guinhouya, B.C.; Duclos, M.; Enea, C.; Storme, L. Beneficial Effects of Maternal Physical Activity during Pregnancy on Fetal, Newborn, and Child Health: Guidelines for Interventions during the Perinatal Period from the French National College of Midwives. J. Midwifery Womens Health 2022, 67, S149–S157. [Google Scholar] [CrossRef] [PubMed]
- Newton, E.R.; May, L. Adaptation of Maternal-Fetal Physiology to Exercise in Pregnancy: The Basis of Guidelines for Physical Activity in Pregnancy. Clin. Med. Insights Womens Health 2017, 10, 1179562X17693224. [Google Scholar] [CrossRef] [PubMed]
- Gascoigne, E.A.; Webster, C.M.; West Honart, A.; Wang, P.; Smith-Ryan, A.; Manuck, T.A. Physical activity and pregnancy outcomes: An expert review. Am. J. Obstet. Gynecol. MFM 2023, 5, 100758. [Google Scholar] [CrossRef]
- Kodali, B.S.; Segal, S. Maternal Physiological Changes During Pregnancy, Labor, and the Postpartum Period. In Datta’s Obstetric Anesthesia Handbook, 5th ed.; Segal, S., Kodali, B.S., Eds.; Springer: Cham, Switzerland, 2023; pp. 1–17. [Google Scholar]
- Bobrowski, R.A. Pulmonary physiology in pregnancy. Clin. Obstet. Gynecol. 2010, 53, 285–300. [Google Scholar] [CrossRef]
- Ejikeme, C.; Nandakumar, V.; Gotur, D. Respiratory physiological changes in pregnancy. Respir. Med. 2025, 246, 108245. [Google Scholar] [CrossRef]
- Murphy, V.E.; Jensen, M.E. Longitudinal Changes in Upper and Lower Airway Function in Pregnancy. Immunol. Allergy Clin. N. Am. 2023, 43, 17–26. [Google Scholar] [CrossRef]
- Okrzymowska, P.; Kurtys, M.; Smolarek, N.; Kurzaj, M.; Slopien, R.; Rozek-Piechura, K. Lung ventilation and the strength of the respiratory muscles of women in the third trimester of pregnancy in the aspect of physical activity. Clin. Exp. Obstet. Gynecol. 2020, 47, 324–328. [Google Scholar] [CrossRef]
- Anitha, O.R.; Johncy, S.; Bondade, S.Y.; Thomas, C. Respiratory Responses to Exercise in Pregnancy. J. Evol. Med. Dent. Sci. 2014, 3, 10127–10133. [Google Scholar]
- Jensen, D.; Webb, K.A.; O’Donnell, D.E. Chemical and mechanical adaptations of the respiratory system at rest and during exercise in human pregnancy. Appl. Physiol. Nutr. Metab. 2007, 32, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Elsisi, H.F.; Aneis, Y.M.; El Refaye, G.E.; Ghareeb, H.O. Blood oxygenation response to aerobic exercise combined with breathing exercises in pregnant women: A randomized controlled trial. Bull. Fac. Phys. Ther. 2022, 27, 16–23. [Google Scholar] [CrossRef]
- Ribeiro, M.M.; Andrade, A.; Nunes, N. Physical exercise in pregnancy: Benefits, risks and prescription. J. Perinat Med. 2021, 50, 4–17. [Google Scholar] [CrossRef]
- Lavie, C.J.; Arena, R.; Swift, D.L.; Johannsen, N.M.; Sui, X.; Lee, D.C.; Earnest, C.P.; Church, T.S.; O’Keefe, J.H.; Milani, R.V.; et al. Exercise and the cardiovascular system: Clinical science and cardiovascular outcomes. Circ. Res. 2015, 117, 207–219. [Google Scholar] [CrossRef]
- Jin, L.; Diaz-Canestro, C.; Wang, Y.; Tse, M.A.; Xu, A. Exerkines and cardiometabolic benefits of exercise: From bench to clinic. EMBO Mol. Med. 2024, 16, 432–444. [Google Scholar] [CrossRef]
- Ardavani, A.; Aziz, H.; Phillips, B.E.; Doleman, B.; Ramzan, I.; Mozaffar, B.; Atherton, P.J.; Idris, I. Indicators of response to exercise training: A systematic review and meta-analysis. BMJ Open. 2021, 11, e044676. [Google Scholar] [CrossRef]
- Miyamoto, T.; Sotobayashi, D.; Ito, G.; Kawai, E.; Nakahara, H.; Ueda, S.; Toyama, T.; Saku, K.; Nakanishi, Y.; Kinoshita, H. Physiological role of anticipatory cardiorespiratory responses to exercise. Physiol. Rep. 2022, 10, e15210. [Google Scholar] [CrossRef]
- Kang, J.; Ratamess, N.A.; Faigenbaum, A.D.; Bush, J.A.; Finnerty, C.; DiFiore, M.; Garcia, A.; Beller, N. Time-of-day effects of exercise on cardiorespiratory responses and endurance performance-A systematic review and meta-analysis. J. Strength Cond. Res. 2023, 37, 2080–2090. [Google Scholar] [CrossRef] [PubMed]
- Bgeginski, R.; Almada, B.P.; Martins Kruel, L.F. Cardiorespiratory responses of pregnant and nonpregnant women during resistance exercise. J. Strength Cond. Res. 2015, 29, 596–603. [Google Scholar] [CrossRef]
- de Oliveria Melo, A.S.; Silva, J.L.; Tavares, J.S.; Barros, V.O.; Leite, D.F.; Amorim, M.M. Effect of a physical exercise program during pregnancy on uteroplacental and fetal blood flow and fetal growth: A randomized controlled trial. Obstet. Gynecol. 2012, 120, 302–310. [Google Scholar] [CrossRef]
- Price, B.B.; Amini, S.B.; Kappeler, K. Exercise in pregnancy: Effect on fitness and obstetric outcomes-a randomized trial. Med. Sci. Sports Exerc. 2012, 44, 2263–2269. [Google Scholar] [CrossRef]
- Marin-Jimenez, N.; Acosta-Manzano, P.; Borges-Cosic, M.; Baena-Garcia, L.; Coll-Risco, I.; Romero-Gallardo, L.; Apricio, V.A. Association of self-reported physical fitness with pain during pregnancy: The GESTAFIT Project. Scand. J. Med. Sci. Sports 2019, 29, 1022–1030. [Google Scholar] [CrossRef]
- Cordero, Y.; Mottola, M.F.; Vargas, J.; Blanco, M.; Barakat, R. Exercise is associated with a reduction in gestational diabetes mellitus. Med. Sci. Sports Exerc. 2015, 47, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Laditka, J.N.; Mayer-Davis, E.J.; Pate, R.R. Does physical activity during pregnancy reduce the risk of gestational diabetes among previously inactive women? Birth 2008, 35, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Pelaez, M.; Lopez, C.; Montejo, R.; Coteron, J. Exercise during pregnancy reduces the rate of cesarean and instrumental deliveries: Results of a randomized controlled trial. J. Matern. Fetal. Neonatal Med. 2012, 2, 2372–2376. [Google Scholar] [CrossRef]
- Pennick, V.; Liddle, S.D. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database Syst. Rev. 2013, CD001139. [Google Scholar] [CrossRef]
- Clapp, J.F., III; Lopez, B.; Harcar-Sevcik, R. Neonatal behavioral profile of the offspring of women who continued to exercise regularly throughout pregnancy. Am. J. Obstet. Gynecol. 1999, 180, 91. [Google Scholar] [CrossRef]
- Physical Activity and Exercise During Pregnancy and the Postpartum Period: ACOG Committee Opinion Summary, Number 804. Obstet. Gynecol. 2020, 135, 991–993. [CrossRef]
- Mottola, M.F.; Davenport, M.H.; Ruchat, S.M.; Davies, G.A.; Poitras, V.J.; Gray, C.E.; Jaramillo Garcia, A.; Barrowman, N.; Adamo, K.B.; Duggan, M.; et al. 2019 Canadian guideline for physical activity throughout pregnancy. Br. J. Sports Med. 2018, 52, 1339–1346. [Google Scholar] [CrossRef]
- Evenson, K.R.; Barakat, R.; Brown, W.J.; Dargent Molina, P.; Haruna, M.; Mikkelsen, E.M.; Mottola, M.F.; Owe, K.M.; Rousham, E.K.; Yeo, S.A. Guidelines for Physical Activity during Pregnancy: Comparisons From Around the World. Am. J. Lifestyle Med. 2014, 8, 102–121. [Google Scholar] [CrossRef]
- Neto, A.G.C.; Silva, N.L.; Farinatti, P.T.V. Influence of resistance training variables on post-exercise oxygen consumption: A systematic review. Rev. Bras. Med. Esporte 2009, 15, 70–78. [Google Scholar]
- Finkelsten, I.d.; Figueiredo, P.A.; Alberton, C.L.; Bgeginski, R.; Stein, R.; Kruel, L.F. Cardiorespiratory responses during and after water exercise in pregnant and non-pregnant women. Rev. Bras. Ginecol. Obstet. 2011, 33, 388–394. [Google Scholar]
- Jensen, D.; Webb, K.A.; Wolfe, L.A.; O’Donnell, D.E. Effects of human pregnancy and advancing gestation on respiratory discomfort during exercise. Respir. Physiol. Neurobiol. 2007, 156, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Christou, G.A.; Christou, M.A.; Davos, C.H.; Markozannes, G.; Christou, K.A.; Mantzoukas, S.; Christodoulou, D.K.; Kiortsis, D.N.; Christou, P.A.; Tigas, S.; et al. Ergophysiological evaluation of heart failure patients with reduced ejection fraction undergoing exercise-based cardiac rehabilitation: A systematic review and meta-analysis. Hell. J. Cardiol. 2024, 77, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Ruchat, S.M.; Sivak, A.; Davenport, M.H. Prenatal Exercise and Cardiorespiratory Health and Fitness: A Meta-analysis. Med. Sci. Sports Exerc. 2020, 52, 1538–1548. [Google Scholar] [CrossRef]
- Moholdt, T.; Garnæs, K.K.; Vik, I.P.; Mørkved, K.; Salvesen, K.J.; Ingul, C.H. Cardiovascular effects of exercise training in pregnant people with a high body mass index: Secondary results from a randomised controlled trial (ETIP). BMJ Open. Sport Exerc. Med. 2024, 10, e002099. [Google Scholar] [CrossRef]
- Bonen, A.; Campagna, P.; Gilchrist, L.; Young, D.C.; Beresford, P. Substrate and endocrine responses during exercise at selected stages of pregnancy. J. Appl. Physiol. 1992, 73, 134–142. [Google Scholar] [CrossRef]
- Avery, N.D.; Wolfe, L.A.; Amara CEo Davies, G.A.L.; McGrath, M.J. Effects of human pregnancy on autonomic function above and below the ventilatory threshold. J. Appl. Physiol. 2001, 90, 321–328. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).