Abstract
Maintenance of skeletal muscle mass and function is critical to health and wellbeing throughout the lifespan. However, disuse through reduced physical activity (e.g., sedentarism), immobilisation, bed rest or microgravity has significant adverse effects on skeletal muscle health. Conversely, resistance exercise training (RET) induces positive muscle mass and strength adaptations. Several studies have employed microarray technology to understand the transcriptional basis of muscle atrophy and hypertrophy after disuse and RET, respectively, to devise fully effective therapeutic interventions. More recently, rapidly falling costs have seen RNA-sequencing (RNA-seq) increasingly applied in exploring muscle adaptations to RET and disuse. The aim of this review is to summarise the transcriptional responses to RET or disuse measured via RNA-seq in young and older adults. We also highlight analytical considerations to maximise the utility of RNA-seq in the context of skeletal muscle research. The limited number of muscle transcriptional signatures obtained thus far with RNA-seq are generally consistent with those obtained with microarrays. However, RNA-seq may provide additional molecular insight, particularly when combined with data-driven approaches such as correlation network analyses. In this context, it is essential to consider the most appropriate study design parameters as well as bioinformatic and statistical approaches. This will facilitate the use of RNA-seq to better understand the transcriptional regulators of skeletal muscle plasticity in response to increased or decreased use.
1. Introduction
Skeletal muscle represents the largest tissue in the human body, providing structural support, facilitating locomotion and regulating metabolism of glucose, lipids [1,2] and amino acids for systemic release in times of need [3]. Skeletal muscle is highly dynamic and capable of rapid changes in size and function. For example, skeletal muscle can lose up to 3.5% of its mass and 9% of its maximum strength after only 5 days of unloading [4], and 90 days of bed rest causes an 18–29% reduction in lower limb muscle volume [5]. This loss of mass and function is accompanied by increased fatigability and insulin resistance, and a shift toward a faster muscle fibre phenotype [5,6,7]. Conversely, resistance exercise training (RET) induces muscle hypertrophy in younger adults [8]. For example, ≥3 weeks RET can increase muscle mass by >5% [9] and strength by >20% [10], whilst improving insulin sensitivity [9,11] and mitochondrial function [12]. RET also remains the most effective non-pharmacological intervention to minimise disuse- [13] and age-related [10] declines in muscle mass and strength.
Given the adaptability of skeletal muscle (albeit highly variable [14]), numerous studies have attempted to delineate the molecular mechanisms underpinning disuse muscle atrophy and RET-induced hypertrophy [15,16,17]. Microarray investigations laid the foundations for understanding the transcriptional basis of muscle adaptation [18]. For example, microarray-based analysis combined with co-expression network analysis (which accounts for the intrinsic organisation of the transcriptome by placing focus on the co-regulation of genes as a function of expression similarity [19,20,21]) has highlighted the role of endothelial-, angiogenesis- and mitochondria-related genes in regulating muscle plasticity [22]. Furthermore, we reported transcriptional profiles implicated in opposing disuse atrophy vs. RET hypertrophy phenotypes [17]. Specifically, genes related to translational regulation were upregulated during disuse and downregulated post-RET, whereas mitochondrial function genes were downregulated in disuse but upregulated in RET [17], implicating these processes in the divergent regulation of muscle mass.
Although microarrays may be more suitable for certain experimental purposes, e.g., gene network reverse engineering [23], RNA-sequencing (RNA-seq) has emerged as an alternative technology for gene expression profiling. RNA-seq permits sequencing of the entire transcriptome, facilitating identification of novel transcripts and single nucleotide variants, whereas microarrays are confined to detecting predefined transcripts via hybridisation [24,25,26]. Additionally, RNA-seq can detect a higher number of differentially expressed genes and low abundance transcripts and can quantify expression across a larger dynamic range than microarrays [25,26]. Nonetheless, direct comparisons show that microarray and RNA-seq platforms perform comparably when predicting cancer associated clinical endpoints [27], although RNA-seq may more accurately identify disease (cancer [27]) and tissue injury [28] transcriptomic characteristics. As the cost of RNA-seq continues to fall [28], it is quickly becoming a cost-effective method to probe transcriptional responses. Indeed, RNA-seq has been employed to examine muscle transcriptional basis of ageing [29], sexual dimorphism [30] and responses to acute resistance and aerobic exercise [20,31,32]. It is, therefore, a powerful tool for probing the precise molecular underpinnings of muscle adaptations to disuse and RET.
Despite previous reviews addressing the transcriptional basis of muscle adaptation, these have focused exclusively on microarray analysis and exercise interventions (i.e., omitting disuse) (e.g., [18]). Therefore, the aim of this review is to summarise the transcriptional responses, exclusively measured using RNA-seq, to RET or disuse in youth and older age. Due to the narrative nature of this review, we apologise to those authors whose work we may have unintentionally omitted.
2. Transcriptional Responses to RET
Since microarray can provide powerful transcriptional insight and RNA-seq is only recently becoming more cost accessible, only a handful of independent studies have utilised RNA-seq to probe the transcriptional responses to RET to date. Due to this, we opted to include in our discussion studies that have investigated long-term resistance trained adults, in addition to studies that have performed controlled RET interventions lasting ≥3 weeks (due to the associated hypertrophic phenotype [8]) (Table 1).

Table 1.
A summary of transcriptional responses to resistance exercise training or disuse in young and older adults. F = females; M = males; yr = years.
Table 1.
A summary of transcriptional responses to resistance exercise training or disuse in young and older adults. F = females; M = males; yr = years.
| Reference | Population | Study Design | Transcriptomic Responses | Phenotypic Adaptations |
|---|---|---|---|---|
| RET | ||||
| Robinson et al. [11] | Young adults (n = 11, 5 M/6 F, 23.7 ± 3.5 yr). Older adults (70.3 ± 3.9 yr, n = 9, 5 M/4 F). | 12 weeks RET. (another group: 12 weeks high intensity interval training) (another group: 12 weeks combined aerobic and resistance exercise). | Regulation of mitochondrial, muscle growth and insulin-related genes (albeit to a lesser extent than high intensity interval training). Upregulation of genes pertaining to angiogenesis and regulation of angiogenesis. | Significant improvements in fat free mass, muscle strength and insulin sensitivity in both age groups. No change in mitochondrial respiration in either age group. |
| Lim et al. [33] | Young males (n = 21, 23.7 ± 2.5 yr). | 10 weeks RET. | Up-regulation in genes related to muscle development, stress response, metabolism, tran-scription factor and cell death (albiet to a lesser extent than acute RE). | Increase in muscle strength and fibre cross-sectional area [34]. |
| Chapman et al. [35] | RET-trained males (n = 7, 42.1 ± 5.8 yr). Age-matched untrained females (n = 8) and males (n = 7). | +15 yr RET experience | Upregulation in genes related to cellular respiration pathways compared to untrained controls. Downregulation in pathways associated with the negative regulation of cell proliferation compared untrained controls. | Greater muscle fibre cross sectional area in RET trained adults (vs. untrained controls). |
| Kulkarni et al. [36] | Older males and females with placebo (n = 48, ≥65 yr). Older males and females with metformin (n = 46, ≥65 yr). | 14 weeks RET. | Increased expression of genes related to extracellular matrix remodelling pathways (compared to baseline). Downregulation in genes related to RNA processing pathways (compared to baseline). | Increased lean body mass, thigh muscle mass and muscle strength [37]. |
| Lavin et al. [38] | Older adults (n = 31, 18 F/13 M, 70 ± 4 yr) | 14 weeks RET. | Two modules of genes significantly and positively related to the change in mid-thigh muscle cross-sectional area, of which the hubs were related to immune and inflammatory processes, specifically: defence response to virus, regulation of leukocyte activation, positive regulation of defence response, positive regulation of cytokine production, and negative regulation of immune system processes (part of “prediction analysis”). | Decreased percentage body fat, increased mid-thigh muscle cross-sectional area and thigh muscle mass. |
| Bolotta et al. [39] | Life-long exercise trained older adults (n = 9, 65–80 yr). Sedentary older adults (n = 5, 70–76 yr). | Life-long RET (n = 4) or aerobically exercise trained (n = 5). | Upregulation in genes related to insulin signalling, energy production (e.g., TCA cycle), mTOR signalling, mitochondria, calcium-regulated energy processes and the cytoskeleton/focal adhesions (compared to sedentary controls). | Fast type fibres were larger in RET versus aerobically trained adults. |
| Disuse | ||||
| Willis et al. [40] | Healthy young (22 yr) males (n = 8). | Four-day unilateral lower limb immobilisation. | Downregulation of mitochondrial and myogenesis. Upregulation of ribosome biogenesis, UPS catabolism, and ribonucleoprotein complex organization/mRNA processing. | Decreased muscle mass (−1.7%) and MPS (−16.2%), with high inter-individual variability. Associations between gene networks phenotypic changes. |
| Sarto et al. [41] | Active young (22 yr) male adults (n = 12). Focus on NMJ. | Ten-day unilateral lower limb suspension (ULLS) followed by a 21-day readaptation program. | Upregulation of ACh receptor subunits genes. Downregulation of Homer proteins genes. Changes in expression of other genes (e.g., neuregulings, neurotrophins, ErbBs, Wnts) indicative of NMJ molecular instability. Downregulation of ion channels gene set. Most ULLS-induced transcrioptional changes were restored after the readapatation program. | Deacreased muscle volume (−4.5%). NMJ transmission stability was unchanged after ULLS. Increased motor unit potential complexity and decreased motor unit firing rates after ULLS. Most ULLS-induced phenotypic changes were restored after the readapatation program. |
| McFarland et al. [42] | Healthy men (n = 22) and women (n = 3) (20–54 yrs) randomized in two groups. | Five-week −6° head down tilt bed rest. Two parallel groups: bed rest only (n = 9) and bed rest with exercise (n = 16). | Downregulation of virtually all aspects of mitochondrial activity. Upregulation of ligands with a key role in pain. The exercise countermeasure normalized most of the genes related to mitochondrial activity. | Reported in other manuscript [43]. Decreased muscle volume (quadriceps; −9%). Exercise during bed rest reduced the muscle atrophy (quadriceps; −5%) |
| Mahmassani et al. [44] | Healthy older and younger male (n = 13) and female (n = 13) adults (mean age; ~52 yrs). | Five-day bed rest. Participants were categorized into high or low susceptibility for insuling resistance after bed rest. | Gene ontologies (GO) that changed in both high and low susceptibility groups: muscle contraction, muscle filament sliding, mitonchondrial ATP synthesis. GOs altered only in high-susceptibility group: lipid metabolic processes, lipid storage, protein homotetramerization. | Both high and low susceptibility groups become insulin-resistant after bed rest but the “High” group had 49% lower insulin sensitivity after bed rest, versus 15% in the “Low” group. |
| Mahmassani et al. [45] | Healthy young (23 yr; n = 9) and older (68 yr, n = 18) participants (13 men and 14 women). | Five-day bed rest. | Common pathways altered in both young and older: Acting cytoskeleton signaling, ILK and RhoA signaling, Mitochondrial dysfunction and calcium signaling. Increased inflammation and fibrotic gene expression in older group only. 51 genes changed in young but not older; after bed rest, the expression of these genes in young nearly matched that in older participants. | Leg lean mass decreased 3.4% in the older group, but did not change in the young group (similar results for total lean mass and myofiber CSA). Leg strength decreased after bed rest in both groups. |
| Standley et al. [46] | Healthy older (between 60–79 yrs) men (n = 11) and women (n = 10). | Ten-day bed rest. Two groups; bed rest only (n = 9), and bed rest with nutritional supplementation (n = 12). | Downregulation of genes related to mitochondria, ribosomes, and oxidative metabolism. Upregulation of genes involved in extracellular matrix, focal adhesion, and collagen. The nutritional supplementation offset some of these changes. | CSA of type IIa fibers decreased in the bed rest group. |
| Mahmassani et al. [47] | Healthy older adults (~72 yrs; n = 8, 6 females and 2 males). | Two-week reduced activity period (from 11,000 steps/day to 2200 steps/day). RNA-seq of muscle and ribosomal profiling. | Altered response for several transcripts (e.g., PFKFB3, GADD45A, NMRK2) in response to leucine stimulation. Uncoupled translation for mTORC1 pathway. Reduction in genes related with ribosomal proteins and alteration of circadian regulators | Unchanged leg lean mass. Tendency for reduced Type I fiber size. Glucose tolerance and insulin sensitivity did not change. |
In a comprehensive study, Robinson and co-authors [11] aimed to identify the transcriptomic (and proteomic) differences between RET, combined training (aerobic and resistance exercise) and high intensity interval training, in both young and older adults. The authors demonstrated that 12 weeks RET regulated a number of genes including mitochondrial-, muscle growth-, and insulin-related genes, albeit to a lesser extent than high intensity interval training. These transcriptional changes were coupled with significant improvements in fat free mass, muscle strength and insulin sensitivity in the absence of enhanced mitochondrial respiration, in both age groups [11]. Two interesting findings are highlighted from these results: (i) the mitochondrial transcriptional response seems not to translate to mitochondrial function and (ii) the lack of blunted age-related responses in functional outcomes (e.g., muscle strength) contradicts previous work (e.g., [10]). The lack of correspondence between mitochondrial gene and organelle adaptations may be explained by the fact that only a small number of mitochondrial genes were regulated. Nonetheless, it is interesting to note the lack of mitochondrial functional adaptation to RET, contradicting previous work in young adults [12]. Similarly, comparable functional gains across age could be seen as unexpected due to the known age-related anabolic resistance to RET [48], but could reflect the long-term RET protocol employed by Robinson et al. The authors also demonstrated that RET (and all other exercise modalities) upregulated genes pertaining to angiogenesis and regulation of angiogenesis, suggesting that cardiovascular remodelling is a global adaptation to exercise regardless of age and exercise type [11].
A subsequent study in young adults demonstrated that 10-weeks lower-limb RET, which was associated with an increase in muscle strength and fibre cross-sectional area [34], significantly up-regulated genes related to muscle development, stress response, metabolism, transcription factor and cell death [33], indicating that these pathways are likely to be involved in the RET-induced hypertrophic response. Interestingly, the authors compared the transcriptional response to acute resistance exercise (RE) versus RET and noted that, while acute RE elicited drastic changes in gene expression, gene regulation following RET was considerably attenuated, denoted by the far fewer differentially expressed genes. It is worth highlighting that in this particular study the authors performed RNA-seq on nine biopsy samples chosen at random from a larger cohort, from which the phenotypic data is reported [34]. Thus, a caveat here is that the authors do not report the phenotypic data for the nine volunteers who had RNA-seq performed and so there is no confirmation that a phenotypic response is present in this smaller collective.
Supporting the previous work, Chapman and colleagues [35] found that long-term RET (+15 years) did not have a sizeable influence on the resting muscle transcriptome (i.e., muscle biopsy taken in the rested state, not after exercise), with only 26 genes regulated in middle aged adults compared to age-matched untrained controls. It should be noted that this study did not implement a RET programme; rather it recruited resistance trained volunteers and thus the “post-RET” samples were compared to untrained “control” volunteers [35]. Validating the volunteers’ exercise history, resistance trained individuals displayed greater muscle fibre cross sectional area compared to controls. Nonetheless, despite the low number of regulated genes by long-term RET, pathway analysis revealed an upregulation in cellular respiration pathways and a downregulation in pathways associated with the negative regulation of cell proliferation [35]. Indeed, this work corroborates microarray-based analysis demonstrating an upregulation in mitochondrial respiration [17] and more targeted work which demonstrated enhanced mitochondrial respiration/function following RET in young adults [12]. In an attempt to explain the low number of regulated genes by long-term RET, which is also supported at the microarray level [49], the authors speculate that many of the accumulated differences that accrue due to RET are at the protein level—as opposed to the transcriptomic level—although this requires extensive proteomic investigations, in which some recent progress has been made (e.g., [50]).
In another study, progressive RET in older adults significantly increased expression of genes related to extracellular matrix remodelling pathways and downregulated genes related to RNA processing pathways [36]. Interestingly, the authors noted an attenuated extracellular matrix transcriptional response in a parallel group taking metformin, who also displayed attenuated gains in muscle growth, perhaps indicating extracellular matrix processes as important for the hypertrophic response [36]. While the functional/phenotypic consequences of downregulated RNA processing pathways remain unknown, it could promote a reduction in the number of genes that are differentially spliced and the number of splicing errors that produce non-functional proteins [36], which may perhaps lead to more effective muscle remodelling/adaptation. The same authors later probed transcriptional responses in order to better understand individual variability to RET [38]. To do this, thigh muscle biopsies obtained from older adults pre- and post-14 weeks of RET were subjected to RNA-seq and subsequent data-driven network and pathway-level information extractor analyses [38]. Of note, prediction analysis (correlating baseline gene expression to change in functional outcomes) identified two modules of genes significantly and positively related to the change in mid-thigh muscle cross-sectional area, of which the hubs were related to immune and inflammatory processes, specifically defence response to virus, regulation of leukocyte activation, positive regulation of defence response, positive regulation of cytokine production and negative regulation of immune system processes. Thus, immune and inflammatory processes may underpin the ability to adapt to RET.
In a recent study comparing life-long exercise trained older adults to sedentary controls, the authors found that training upregulated genes related to insulin signalling, energy production (e.g., TCA cycle), mTOR signalling, mitochondria, calcium-regulated energy processes and the cytoskeleton/focal adhesions [39]. Although the life-long exercise trained older group represents both resistance trained and aerobically-trained volunteers, when comparing the gene profiles independently the authors only identified 20 genes differentiating RET versus aerobically trained volunteers and none of the genes appeared to be functionally related to the specific characteristics of the exercise training modality [39]. This may imply that exercise modality is not relevant for the pathways leading to the prevention of sarcopenia, although this could be specific to the highly trained cohort recruited [39]. Thus, at the transcriptional level, long-term (resistance) exercise training counteracted many molecular pathways that are associated with age-related muscle decline such as mitochondrial dysfunction, impairment of proteo-static mechanisms and metabolic inefficiency.
3. Transcriptional Responses to Disuse
Similar to the RET literature, RNA-seq investigations into the molecular basis of muscle disuse are only starting to emerge, with microarray technology still being commonly utilised. In light of this, herein we highlight studies in young and middle-aged adults followed by investigations in older adults involving ≥4 days disuse/immobilisation (due to the associated atrophy phenotype [40,51]) (Table 1).
Willis et al. [40] employed a short-term (4-day) unilateral lower limb immobilisation protocol to examine the associations between transcriptional changes and muscle protein synthesis (MPS) and atrophy. The differentially expressed genes induced by immobilisation represented biological processes such as energy metabolism and muscle development/contraction. At the network-level, they reported downregulation of mitochondrial function and myogenesis and upregulation of proteasomal ubiquitin-(in)dependent protein catabolism. These results are consistent with previous signatures of muscle disuse detected by microarrays [15,17]. An interesting aspect of this study is that the authors were able to associate molecular networks with the phenotype of short-term disuse. In doing so, they found several networks associated with both immobilisation-induced loss of muscle mass and decreased MPS. Their analysis indicated that extracellular matrix organisation and protein folding networks may be two transcriptional processes that play critical roles in muscle atrophy and decreased MPS, an idea supported by the highly mechanosensitive nature of these processes [52,53].
Two other studies have used RNA-seq of skeletal muscle during unloading in young and/or middle-aged adults with a very specific focus [41,42]. Sarto and co-workers [41] were interested in neuromuscular junction (NMJ) and motor unit changes induced by 10 days of unilateral lower limb suspension (ULLS). The data reported in this study included only the RNA-seq results related to the regulation of NMJ and ion channels, which were considered a possible cause of the changes in motor unit potential. ULLS induced upregulation of acetylcholine (ACh) receptor subunits (CHRNA1, CHRNB1, CHRND, CHRNG), which may indicate an altered innervation pattern, along with downregulation of Homer proteins, which are involved in synaptic-related gene expression. In addition, genes and pathways related to NMJ instability were differentially expressed after ULLS (e.g., neuregulins, Wnts family, epidermal growth factor receptors and neurotrophins). Despite these transcriptional changes, NMJ transmission stability remained unaltered after ULLS, a result supported by a somewhat longer (i.e., 15 days) unilateral lower limb unloading intervention [54]. The authors then examined 33 genes associated with skeletal muscle ion channels and reported that ULLS caused a general downregulation of this group of genes, particularly those associated with voltage-gated potassium channels. These results resemble reports on animal models of unloading [55] and suggest unloading-induced transcriptional regulation of ion channels that ultimately influence motor unit potential. Finally, Sarto et al. [41] pointed out that most of the transcriptional changes triggered by ULLS returned to normal after a period of active recovery.
Moving on to a systemic and longer model of unloading (i.e., 5-week bed rest), McFarland and colleagues performed skeletal muscle RNA-seq with the specific aim of investigating possible unloading-induced changes in pain perception [42]. This study observed a downregulation of virtually all aspects of mitochondrial activity after bed rest that was normalized by exercise performed during the unloading intervention, confirming previous microarray data [15,17]. They also reported that ligands that play a key role in the detection of pain and that are sensitized in chronic pain conditions were upregulated after bed rest. Interestingly, this study attempted to use an unbiased approach to predict the two intervention groups (i.e., bed rest only vs. bed rest and exercise) [42]. This approach was successful in dividing the volunteers into the two groups but failed to predict the intervention. This was because the unbiased analysis assumed that exercise would release only anti-inflammatory factors. However, it is known that skeletal muscle also secretes pro-inflammatory molecules during exercise [56,57] and therefore the blinded analysis failed. These results demonstrate the importance of a deep understanding of muscle and exercise physiology to prepare RNA-seq analyses and to correctly contextualize the results provided by this transcriptional platform.
Only young and middle-aged adults (20–49 years old) participated in the aforementioned studies. In contrast, Mahmassani and co-authors recruited both young and older adults for a 5-day bed rest study and presented the transcriptional results in two independent manuscripts [44,45]. In the first study, the authors examined susceptibility to insulin resistance using skeletal muscle RNA-seq, among other methods, in a mixed population of young and older adults (18–75 years) stratified into groups with high and low susceptibility to unloading-induced insulin resistance [44]. Pathway analysis showed that lipid metabolism and lipid storage pathways were among the top 10 biological processes that were altered only in the high susceptibility group. There was also a remarkable increase in the expression of inducible 6-phosphofructo-2-kinase (PFKFB3) and fatty acid synthase gene (FASN). Thus, it appears that the muscles of adults at higher risk for developing insulin resistance experience a metabolic fuel shift characterized by increased lipogenesis and glycolysis after bed rest. Increased glycolysis after prolonged unloading (i.e., 5 weeks of ULLS or 90 days of bed rest) has already been noted in healthy middle-aged adults [58,59]. Therefore, the response reported by Mahmassani et al. [44] for the high-susceptibility group may not be a singular event occurring in a specific population but rather may represent an accelerated process in a specific population of a general signature of skeletal muscle disuse.
The same authors, using the same cohort, performed a comparison of the transcriptional response to 5 days of bed rest in young (23 years) and older (68 years) adults [45]. It is noteworthy that only the older adult’s lost leg lean mass (3.4%). Despite some significant differences in gene expression between age groups at baseline (i.e., before bed rest), ingenuity pathway analysis revealed common downregulated pathways in young and older adults after short-term bed rest: actin cytoskeleton signalling, integrin-linked kinase (ILK) signalling, and Ras homolog family member A (RhoA) signalling. However, the extent of downregulation was greater in the older adults, suggesting greater changes in the mechano-sensing machinery with aging. The most highly regulated pathway in older muscle was the hepatic fibrosis/hepatic stellate cell activation pathway, which includes fibrotic and inflammatory genes (e.g., NFkB, IL-6, CTGF, and VEGFA). Thus, it appears that the model of disuse used here elicits a stronger fibrotic and inflammatory response in older versus younger muscle. An interesting finding of the study was that there were 51 genes that changed after bed rest only in the young adults. Most of these genes had group differences at baseline, and the differential expression induced by bed rest in the young caused these genes to resemble the baseline condition in the older adults.
Another study of bed rest in older adults (>65 years), using RNA-seq in skeletal muscle, showed that genes related to mitochondria, ribosomes and oxidative metabolism were downregulated after 10 days of disuse, with concomitant upregulation of genes involved in extracellular matrix, focal adhesion and collagen [46]. These are changes typical of a disuse signature in skeletal muscle [15,17]. This study also tested a nutritional countermeasure based on β-hydroxy-β-methyl-buturate (HMB) that was able to offset some of the transcriptional changes induced by bed rest [46].
Moving away from total unloading models of disuse, Mahmassani et al. [47] used a step reduction model (from ~11,000 to 2200 steps/day) to assess the effects of disuse in the transcriptome-translatome response to leucine intake in older adults. In addition to skeletal muscle RNA-seq, ribosomal profiling or ribosomal sequencing, which allows translational activity to be measured in a transcript-specific manner [60], was also employed. The authors reported a clear disuse effect when analysing the response to leucine stimulation and showed an altered response for several transcripts (e.g., PFKFB3, GADD45A, NMRK2), supporting previous reports of bed rest [45]. Interestingly, there were leucine-induced changes at the translational level that were not mirrored by transcriptional alterations (i.e., “uncoupled translation”). This was the case for the key muscle mass regulator mTORC1 pathway. Although the disuse intervention applied in this study can be considered rather mild compared with other forms of unloading, it was strong enough to cause a reduction in translation of mRNAs encoding ribosomal proteins and to alter circadian regulators [47], a response that has already been described after long-term bed rest in middle-aged adults [15].
4. Analytical Considerations
With RNA-seq becoming a more prevalent feature of human RET/disuse studies, some key considerations are required when performing whole-transcriptome analyses in these contexts. Whilst the following section is not exhaustive, several technical aspects may be generalisable to other transcriptomic technologies that have been/are still used in human RET/disuse studies (e.g., microarray), and it includes considerations that are just as valid for transcriptomic analyses in physiological/biological settings outside the scope of this review.
Firstly, it is important that the analytical intent of a RET/disuse RNA-seq study is considered when generating reads, as parameters such as read length, depth and coverage can all have an influence. For example, 50 bp single-end reads are generally sufficient for standard gene expression profiling, whereas identification of novel variants or splice sites typically benefits from longer (75–100 bp) paired-end reads [61]. Further, RNA-seq-based quantification of gene expression commonly involves either (i) counting the number of reads that align to a genomic feature of a reference genome or (ii) inferring from transcript-level abundances that have been estimated via pseudoalignment of reads to a reference transcriptome [62]. Either way, feature annotations defined in the context of the genome are required [63], for which several genome annotation databases can serve as a source (e.g., Ensembl, NCBI, etc.). It is not the intent of this review to make firm recommendations on annotation data source, but rather highlight that, while considerable overlap often prevails between/across them, methodological subtleties do exist [63], which may impact on which features are included in downstream analyses (e.g., the Ensembl database contains a substantial number of non-coding RNAs that are not present in the Entrez database).
The vast majority of RNA-seq (and microarray) studies in human RET/disuse research have centred on the application of traditional differential gene analysis—i.e., testing each gene in the dataset individually to determine if it is differentially expressed [62]. Nevertheless, while its utility has helped generate many new insights and hypotheses on the molecular mechanisms of muscle adaption to RET/disuse (e.g., [31]), traditional differential gene analysis is not without limitations. Indeed, focusing on genes in detachment vastly overlooks the molecular complexity of muscle and is not wholly sensitive to the fact that key molecular drivers of adaptation do not always display evidence of differential regulation in isolation [20,64,65,66]—limiting the extent of information that can be delineated from a muscle RET/disuse RNA-seq dataset. To overcome this, network analysis can be leveraged as a useful complementary/alternative analysis. Network modelling of underlying patterns in the expression data itself (i.e., ‘data-driven’ network analysis) is an effective approach to unearth new interacting gene pathways involved in regulating human muscle with RET/disuse [22,40,67]. A common tool for this in the systems biology community is weighted gene co-expression network analysis (WGCNA), which focuses on groups of co-regulated genes (‘modules’) and their most highly connected ‘hub’ components [68,69]. Such module-centric analysis manifests as a powerful form of biologically motivated data reduction, especially benefiting human RET/disuse RNA-seq studies, by offering a strong framework for linking molecules to end-point physiology and, in turn, pinpointing key candidates of individual responsiveness to RET/disuse for guiding personalised intervention development [20,38,40]. When sample size is insufficient for true data-driven network modelling (e.g., WGCNA recommendations are ≥15 samples [70]), several databases/tools can be used to infer gene–gene interactions based on existing knowledge (i.e., ‘knowledge-based’ network analysis) (e.g., [71,72,73]), though this is naturally impeded by possible information loss and a suboptimal ability to leverage on new molecular inferences.
Whether derived via gene-centric or via network-driven analysis, it is also important to decipher coherent biological roles (if any) among genes associated with RET/disuse. Accordingly, several databases exist which ‘functionally characterise’ genes. Largest is Gene Ontology (GO), which groups genes into logically organised ‘terms’ across the domains of biological process (BP), cellular component (CC), and molecular function (MF) [74]. Other popular database choices such as the Kyoto Encyclopaedia of Genes and Genomes (KEGG) and Reactome databases categorise genes into pathways [75,76]. GO generally provides greater human gene annotation coverage than KEGG or Reactome but perhaps at the expense of a larger number of ‘inferred’ annotations. Utilising a compendium of annotation databases may therefore be fruitful when elucidating functional characteristics of a given muscle RET/disuse gene list [77]. From a ‘statistical’ standpoint, the gene list could be directly tested to see if it is more ‘enriched’ with genes that map to a specific functional term than expected by chance (i.e., relative to an appropriate background list, which is typically all genes that were utilised throughout RNA-seq analysis [78,79]), a process termed ‘overrepresentation analysis’ [80]. Alternatively, every gene in the data set can be ranked in a biologically motivated manner (e.g., by log2 fold-change) and ‘gene set enrichment analysis’ employed to determine if genes in a given term tend to display concordant expression change based on their ranked position [80]. This is especially beneficial if, e.g., the number of genes significantly regulated by a RET/disuse intervention is too small to make sensible or definable functional assignments using overrepresentation analysis. Regardless, numerous programmatic or online software tools provide a means for overrepresentation an/or gene set enrichment analysis [81,82,83,84,85,86]. Naturally, a trade-off often exists in terms of ease-of-use, statistical constraints/assumptions, ‘up-to-date-ness’, parameter flexibility, and specific database incorporation, and one should therefore carefully consider the relative merits of a given tool when utilising it.
The growing use of RNA-seq in human muscle RET/disuse research should also translate into an increase in the total number of topical whole-transcriptome datasets (RNA-seq or microarray) that are available in public data archives (e.g., the Gene Expression Omnibus [87]). Importantly, this facilitates re-use and integration of multiple transcriptomic RET/disuse datasets, to enhance statistical power towards identifying robust gene signatures of RET/disuse muscle adaptations and/or help answer entirely new research questions within these contexts [17,88]. An obvious methodological constraint is dealing with how to aggregate within and/or across technologies and platforms. Upstream (i.e., pre-analysis) merging of different datasets into one large, singular dataset (‘early-stage integration’ [89]) may make downstream analyses less arduous, but properly accounting for non-biological noise is and remains a difficult challenge [90]. One could instead focus on correctly processing and analysing each dataset separately, then aggregate across primary (e.g., p-value, effect-size, etc) or secondary (e.g., gene ranking) test statistics (‘meta-analysis’ [89]), or other useful downstream analytical features such as enriched terms, to obtain a more unified cross-dataset molecular picture. Nevertheless, while this may enable more flexibility when it comes to integrating data from different technologies/platforms [90], there remains a need to ensure that RET/disuse study designs are sufficiently homogenous in order to maximise analytical performance [91].
In regard to data outcomes, we highlight that certain transcriptional themes (e.g., energy metabolism, extracellular matrix, muscle development) significantly appear in the majority, if not all, muscle transcriptomic datasets pertaining to ageing, exercise and/or disuse interventions, which is supported from the results of studies included in this review. It is important to note that while these themes reoccur, they are not always underpinned by the same genes. This raises the question of whether these themes/signatures truly represent major, dominating responses/adaptations and therefore should be the mainstay of future targeted mechanistic and interventional work, or whether these responses are simply so large that they overwhelm smaller, more subtle molecular networks representing important gene changes (e.g., ion channels, calcium regulation/signalling) that may (also) be functionally important. Whether this represents an artefact of the informatics pipelines/bias in gene annotations applied or simply a lack of deep diving into individual transcriptional datasets requires more attention.
Finally, it is not the intent of this review to act as an RNA-seq best practice guideline or to provide an RNA-seq pipeline and for this we refer the readers to other more comprehensive resources (e.g., [62]). Nonetheless, we provide a non-exhaustive summary of some common tools utilized in RNA-seq analysis pipelines, particularly of muscle disuse/RET datasets (Table 2).

Table 2.
A summary of some common tools utilized in RNA-seq analysis pipelines.
5. Conclusions
Studies using RNA-seq to investigate RET- and/or disuse-induced transcriptional changes in skeletal muscle are starting to emerge (Figure 1). While the weight of these studies is still very small compared to microarray studies, current evidence demonstrates that the transcriptomic signatures obtained with RNA-seq are generally consistent with those obtained with microarrays and could provide further complementary molecular insight via the detection of (e.g.,) novel transcripts and/or low abundance transcripts. It is imperative that, when performing whole-transcriptome analyses in these contexts, researchers consider the most appropriate: study design parameters (e.g., read length, depth and coverage), bioinformatic analysis (differential expression, correlation network analysis) and appropriate statistical analysis (e.g., choice of background gene list) (Figure 1). By doing so, RNA-seq coupled with powerful bioinformatic analysis stands to significantly further our understanding into the precise regulators of skeletal muscle plasticity in response to increased (e.g., RET) or decreased (e.g., unloading/disuse) use.
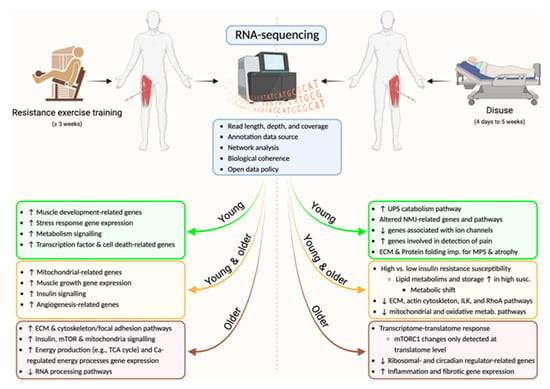
Figure 1.
Schematic representation of the skeletal muscle transcriptional changes assessed by RNA-sequencing after resistance exercise training or disuse in young, young and older, and older volunteers. Ca; calcium; ECM; extracellular matrix, mTOR(C1); mechanistic target of rapamycin (complex 1), TCA; tricarboxylic acid, UPS; ubiquitin-proteosome system, NMJ; neuromuscular junction, MPS; muscle protein synthesis, ILK; integrin-linked kinase, RhoA; Ras homolog family member A. Created with BioRender.com (accessed on 27 September 22).
Author Contributions
Conceptualization, R.F.-G. and C.S.D.; writing—original draft preparation, R.F.-G., C.R.G.W. and C.S.D.; writing—review and editing, R.F.-G., C.R.G.W., T.E. and C.S.D.; visualization, R.F.-G., C.R.G.W. and C.S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. R.F.G. is supported by a career grant from the Swedish National Space Agency (2021-00159).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ivy, J.L.; Katz, A.L.; Cutler, C.L.; Sherman, W.M.; Coyle, E.F. Muscle glycogen synthesis after exercise: Effect of time of carbohydrate ingestion. J. Appl. Physiol. 1988, 64, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Burguera, B.; Jensen, M.D. Kinetics of intramuscular triglyceride fatty acids in exercising humans. J. Appl. Physiol. 2000, 89, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef]
- Wall, B.T.; Dirks, M.L.; Snijders, T.; Senden, J.M.; Dolmans, J.; van Loon, L.J. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol. 2014, 210, 600–611. [Google Scholar] [CrossRef]
- Alkner, B.A.; Tesch, P.A. Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. Eur. J. Appl. Physiol. 2004, 93, 294–305. [Google Scholar] [CrossRef]
- Berg, H.E.; Larsson, L.; Tesch, P.A. Lower limb skeletal muscle function after 6 wk of bed rest. J. Appl. Physiol. 1997, 82, 182–188. [Google Scholar] [CrossRef]
- Haus, J.M.; Carrithers, J.A.; Carroll, C.C.; Tesch, P.A.; Trappe, T.A. Contractile and connective tissue protein content of human skeletal muscle: Effects of 35 and 90 days of simulated microgravity and exercise countermeasures. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 293, R1722–R1727. [Google Scholar] [CrossRef]
- Brook, M.S.; Wilkinson, D.J.; Mitchell, W.K.; Lund, J.N.; Szewczyk, N.J.; Greenhaff, P.L.; Smith, K.; Atherton, P.J. Skeletal muscle hypertrophy adaptations predominate in the early stages of resistance exercise training, matching deuterium oxide-derived measures of muscle protein synthesis and mechanistic target of rapamycin complex 1 signaling. FASEB J. 2015, 29, 4485–4496. [Google Scholar] [CrossRef]
- Phillips, B.E.; Williams, J.P.; Greenhaff, P.L.; Smith, K.; Atherton, P.J. Physiological adaptations to resistance exercise as a function of age. JCI Insight 2017, 2, e95581. [Google Scholar] [CrossRef]
- Brook, M.S.; Wilkinson, D.J.; Mitchell, W.K.; Lund, J.N.; Phillips, B.E.; Szewczyk, N.J.; Greenhaff, P.L.; Smith, K.; Atherton, P.J. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J. Physiol. 2016, 594, 7399–7417. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.M.; Dasari, S.; Konopka, A.R.; Johnson, M.L.; Manjunatha, S.; Esponda, R.R.; Carter, R.E.; Lanza, I.R.; Nair, K.S. Enhanced Protein Translation Underlies Improved Metabolic and Physical Adaptations to Different Exercise Training Modes in Young and Old Humans. Cell Metab. 2017, 25, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Reidy, P.T.; Bhattarai, N.; Sidossis, L.S.; Rasmussen, B.B. Resistance Exercise Training Alters Mitochondrial Function in Human Skeletal Muscle. Med. Sci. Sports Exerc. 2015, 47, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Hvid, L.; Suetta, C.; Nielsen, J.; Jensen, M.; Frandsen, U.; Ørtenblad, N.; Kjaer, M.; Aagaard, P. Aging impairs the recovery in mechanical muscle function following 4days of disuse. Exp. Gerontol. 2014, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Timmons, J.A. Variability in training-induced skeletal muscle adaptation. J. Appl. Physiol. 2011, 110, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gonzalo, R.; Tesch, P.A.; Lundberg, T.R.; Alkner, B.A.; Rullman, E.; Gustafsson, T. Three months of bed rest induce a residual transcriptomic signature resilient to resistance exercise countermeasures. FASEB J. 2020, 34, 7958–7969. [Google Scholar] [CrossRef]
- Rullman, E.; Fernandez-Gonzalo, R.; Mekjavić, I.B.; Gustafsson, T.; Eiken, O. MEF2 as upstream regulator of the transcriptome signature in human skeletal muscle during unloading. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2018, 315, R799–R809. [Google Scholar] [CrossRef]
- Deane, C.S.; Willis, C.R.G.; Phillips, B.E.; Atherton, P.J.; Harries, L.W.; Ames, R.M.; Szewczyk, N.J.; Etheridge, T. Transcriptomic meta-analysis of disuse muscle atrophy vs. resistance exercise-induced hypertrophy in young and older humans. J. Cachexia Sarcopenia Muscle 2021, 12, 629–645. [Google Scholar] [CrossRef]
- Mahoney, D.J.; Tarnopolsky, M.A. Understanding skeletal muscle adaptation to exercise training in humans: Contributions from microarray studies. Phys. Med. Rehabil. Clin. N. Am. 2005, 16, 859–873. [Google Scholar] [CrossRef]
- van Dam, S.; Vosa, U.; van der Graaf, A.; Franke, L.; de Magalhaes, J.P. Gene co-expression analysis for functional classification and gene-disease predictions. Brief. Bioinform. 2018, 19, 575–592. [Google Scholar] [CrossRef] [PubMed]
- Willis, C.R.G.; Ames, R.M.; Deane, C.S.; Phillips, B.E.; Boereboom, C.L.; Abdulla, H.; Bukhari, S.S.I.; Lund, J.N.; Williams, J.P.; Wilkinson, D.J.; et al. Network analysis of human muscle adaptation to aging and contraction. Aging 2020, 12, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Song, W.M.; Zhang, B. Multiscale Embedded Gene Co-expression Network Analysis. PLoS Comput. Biol. 2015, 11, e1004574. [Google Scholar] [CrossRef] [PubMed]
- Stokes, T.; Timmons, J.A.; Crossland, H.; Tripp, T.R.; Murphy, K.; McGlory, C.; Mitchell, C.J.; Oikawa, S.Y.; Morton, R.W.; Phillips, B.E.; et al. Molecular Transducers of Human Skeletal Muscle Remodeling under Different Loading States. Cell Rep. 2020, 32, 107980. [Google Scholar] [CrossRef]
- Giorgi, F.M.; Del Fabbro, C.; Licausi, F. Comparative study of RNA-seq- and microarray-derived coexpression networks in Arabidopsis thaliana. Bioinformatics 2013, 29, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.S.; Van Vleet, T.R.; Ciurlionis, R.; Buck, W.R.; Mittelstadt, S.W.; Blomme, E.A.G.; Liguori, M.J. Comparison of RNA-Seq and Microarray Gene Expression Platforms for the Toxicogenomic Evaluation of Liver From Short-Term Rat Toxicity Studies. Front. Genet 2018, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, B.T.; Landry, J.R. RNA-Seq-quantitative measurement of expression through massively parallel RNA-sequencing. Methods 2009, 48, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, Y.; Hertwig, F.; Thierry-Mieg, J.; Zhang, W.; Thierry-Mieg, D.; Wang, J.; Furlanello, C.; Devanarayan, V.; Cheng, J.; et al. Comparison of RNA-seq and microarray-based models for clinical endpoint prediction. Genome Biol. 2015, 16, 133. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.F.; Tycksen, E.D.; Sandell, L.J.; Brophy, R.H. Advantages of RNA-seq compared to RNA microarrays for transcriptome profiling of anterior cruciate ligament tears. J. Orthop. Res. 2018, 36, 484–497. [Google Scholar] [CrossRef]
- Tumasian, R.A., 3rd; Harish, A.; Kundu, G.; Yang, J.H.; Ubaida-Mohien, C.; Gonzalez-Freire, M.; Kaileh, M.; Zukley, L.M.; Chia, C.W.; Lyashkov, A.; et al. Skeletal muscle transcriptome in healthy aging. Nat. Commun. 2021, 12, 2014. [Google Scholar] [CrossRef]
- Lindholm, M.E.; Huss, M.; Solnestam, B.W.; Kjellqvist, S.; Lundeberg, J.; Sundberg, C.J. The human skeletal muscle transcriptome: Sex differences, alternative splicing, and tissue homogeneity assessed with RNA sequencing. FASEB J. 2014, 28, 4571–4581. [Google Scholar] [CrossRef]
- Deane, C.S.; Ames, R.M.; Phillips, B.E.; Weedon, M.N.; Willis, C.R.G.; Boereboom, C.; Abdulla, H.; Bukhari, S.S.I.; Lund, J.N.; Williams, J.P.; et al. The acute transcriptional response to resistance exercise: Impact of age and contraction mode. Aging 2019, 11, 2111–2126. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.M.; D’Lugos, A.C.; Naymik, M.A.; Siniard, A.L.; Wolfe, A.J.; Curtis, D.R.; Huentelman, M.J.; Carroll, C.C. Transcriptome response of human skeletal muscle to divergent exercise stimuli. J. Appl. Physiol. 2018, 124, 1529–1540. [Google Scholar] [CrossRef]
- Lim, C.; Shimizu, J.; Kawano, F.; Kim, H.J.; Kim, C.K. Adaptive responses of histone modifications to resistance exercise in human skeletal muscle. PLoS ONE 2020, 15, e0231321. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Kim, H.J.; Morton, R.W.; Harris, R.; Phillips, S.M.; Jeong, T.S.; Kim, C.K. Resistance Exercise-induced Changes in Muscle Phenotype Are Load Dependent. Med. Sci. Sports Exerc. 2019, 51, 2578–2585. [Google Scholar] [CrossRef]
- Chapman, M.A.; Arif, M.; Emanuelsson, E.B.; Reitzner, S.M.; Lindholm, M.E.; Mardinoglu, A.; Sundberg, C.J. Skeletal Muscle Transcriptomic Comparison between Long-Term Trained and Untrained Men and Women. Cell Rep. 2020, 31, 107808. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.S.; Peck, B.D.; Walton, R.G.; Kern, P.A.; Mar, J.C.; Windham, S.T.; Bamman, M.M.; Barzilai, N.; Peterson, C.A. Metformin alters skeletal muscle transcriptome adaptations to resistance training in older adults. Aging 2020, 12, 19852–19866. [Google Scholar] [CrossRef]
- Walton, R.G.; Dungan, C.M.; Long, D.E.; Tuggle, S.C.; Kosmac, K.; Peck, B.D.; Bush, H.M.; Villasante Tezanos, A.G.; McGwin, G.; Windham, S.T.; et al. Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: A randomized, double-blind, placebo-controlled, multicenter trial: The MASTERS trial. Aging Cell 2019, 18, e13039. [Google Scholar] [CrossRef]
- Lavin, K.M.; Bell, M.B.; McAdam, J.S.; Peck, B.D.; Walton, R.G.; Windham, S.T.; Tuggle, S.C.; Long, D.E.; Kern, P.A.; Peterson, C.A.; et al. Muscle transcriptional networks linked to resistance exercise training hypertrophic response heterogeneity. Physiol. Genom. 2021, 53, 206–221. [Google Scholar] [CrossRef]
- Bolotta, A.; Filardo, G.; Abruzzo, P.M.; Astolfi, A.; De Sanctis, P.; Di Martino, A.; Hofer, C.; Indio, V.; Kern, H.; Lofler, S.; et al. Skeletal Muscle Gene Expression in Long-Term Endurance and Resistance Trained Elderly. Int. J. Mol. Sci. 2020, 21, 3988. [Google Scholar] [CrossRef]
- Willis, C.R.G.; Gallagher, I.J.; Wilkinson, D.J.; Brook, M.S.; Bass, J.J.; Phillips, B.E.; Smith, K.; Etheridge, T.; Stokes, T.; McGlory, C.; et al. Transcriptomic links to muscle mass loss and declines in cumulative muscle protein synthesis during short-term disuse in healthy younger humans. FASEB J. 2021, 35, e21830. [Google Scholar] [CrossRef]
- Sarto, F.; Stashuk, D.W.; Franchi, M.V.; Monti, E.; Zampieri, S.; Valli, G.; Sirago, G.; Candia, J.; Hartnell, L.M.; Paganini, M.; et al. Effects of short-term unloading and active recovery on human motor unit properties, neuromuscular junction transmission and transcriptomic profile. J. Physiol. 2022, 600, 4731–4751. [Google Scholar] [CrossRef] [PubMed]
- McFarland, A.J.; Ray, P.R.; Bhai, S.; Levine, B.D.; Price, T.J. RNA sequencing on muscle biopsy from a 5-week bed rest study reveals the effect of exercise and potential interactions with dorsal root ganglion neurons. Physiol. Rep. 2022, 10, e15176. [Google Scholar] [CrossRef] [PubMed]
- Krainski, F.; Hastings, J.L.; Heinicke, K.; Romain, N.; Pacini, E.L.; Snell, P.G.; Wyrick, P.; Palmer, M.D.; Haller, R.G.; Levine, B.D. The effect of rowing ergometry and resistive exercise on skeletal muscle structure and function during bed rest. J. Appl. Physiol. 2014, 116, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Mahmassani, Z.S.; Reidy, P.T.; McKenzie, A.I.; Stubben, C.; Howard, M.T.; Drummond, M.J. Disuse-induced insulin resistance susceptibility coincides with a dysregulated skeletal muscle metabolic transcriptome. J. Appl. Physiol. 2019, 126, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Mahmassani, Z.S.; Reidy, P.T.; McKenzie, A.I.; Stubben, C.; Howard, M.T.; Drummond, M.J. Age-dependent skeletal muscle transcriptome response to bed rest-induced atrophy. J. Appl. Physiol. 2019, 126, 894–902. [Google Scholar] [CrossRef]
- Standley, R.A.; Distefano, G.; Trevino, M.B.; Chen, E.; Narain, N.R.; Greenwood, B.; Kondakci, G.; Tolstikov, V.V.; Kiebish, M.A.; Yu, G.; et al. Skeletal Muscle Energetics and Mitochondrial Function Are Impaired Following 10 Days of Bed Rest in Older Adults. J. Gerontol. Ser. A 2020, 75, 1744–1753. [Google Scholar] [CrossRef]
- Mahmassani, Z.S.; McKenzie, A.I.; Petrocelli, J.J.; de Hart, N.M.; Fix, D.K.; Kelly, J.J.; Baird, L.M.; Howard, M.T.; Drummond, M.J. Reduced Physical Activity Alters the Leucine-Stimulated Translatome in Aged Skeletal Muscle. J. Gerontol. Ser. A 2021, 76, 2112–2121. [Google Scholar] [CrossRef]
- Kumar, V.; Selby, A.; Rankin, D.; Patel, R.; Atherton, P.; Hildebrandt, W.; Williams, J.; Smith, K.; Seynnes, O.; Hiscock, N.; et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J. Physiol. 2009, 587, 211–217. [Google Scholar] [CrossRef]
- Raue, U.; Trappe, T.A.; Estrem, S.T.; Qian, H.R.; Helvering, L.M.; Smith, R.C.; Trappe, S. Transcriptome signature of resistance exercise adaptations: Mixed muscle and fiber type specific profiles in young and old adults. J. Appl. Physiol. 2012, 112, 1625–1636. [Google Scholar] [CrossRef]
- Deane, C.S.; Phillips, B.E.; Willis, C.R.G.; Wilkinson, D.J.; Smith, K.; Higashitani, N.; Williams, J.P.; Szewczyk, N.J.; Atherton, P.J.; Higashitani, A.; et al. Proteomic features of skeletal muscle adaptation to resistance exercise training as a function of age. Geroscience 2022. [Google Scholar] [CrossRef]
- Suetta, C.; Frandsen, U.; Jensen, L.; Jensen, M.M.; Jespersen, J.G.; Hvid, L.G.; Bayer, M.; Petersson, S.J.; Schrøder, H.D.; Andersen, J.L.; et al. Aging affects the transcriptional regulation of human skeletal muscle disuse atrophy. PLoS ONE 2012, 7, e51238. [Google Scholar] [CrossRef] [PubMed]
- Kjær, M. Role of Extracellular Matrix in Adaptation of Tendon and Skeletal Muscle to Mechanical Loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Carland, C.R.; Guo, Y.; Bernstein, S.I. Getting Folded: Chaperone Proteins in Muscle Development, Maintenance and Disease. Anat. Rec. 2014, 297, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- Inns, T.B.; Bass, J.J.; Hardy, E.J.O.; Wilkinson, D.J.; Stashuk, D.W.; Atherton, P.J.; Phillips, B.E.; Piasecki, M. Motor unit dysregulation following 15 days of unilateral lower limb immobilisation. J. Physiol. 2022, 600, 4753–4769. [Google Scholar] [CrossRef]
- Desaphy, J.F.; Pierno, S.; Léoty, C.; George, A.L.; De Luca, A.; Camerino, D.C. Skeletal muscle disuse induces fibre type-dependent enhancement of Na+ channel expression. Brain J. Neurol. 2001, 124, 1100–1113. [Google Scholar] [CrossRef]
- Neubauer, O.; Sabapathy, S.; Ashton, K.J.; Desbrow, B.; Peake, J.M.; Lazarus, R.; Wessner, B.; Cameron-Smith, D.; Wagner, K.-H.; Haseler, L.J.; et al. Time course-dependent changes in the transcriptome of human skeletal muscle during recovery from endurance exercise: From inflammation to adaptive remodeling. J. Appl. Physiol. 2014, 116, 274–287. [Google Scholar] [CrossRef]
- Pillon, N.J.; Smith, J.A.B.; Alm, P.S.; Chibalin, A.V.; Alhusen, J.; Arner, E.; Carninci, P.; Fritz, T.; Otten, J.; Olsson, T.; et al. Distinctive exercise-induced inflammatory response and exerkine induction in skeletal muscle of people with type 2 diabetes. Sci. Adv. 2022, 8, eabo3192. [Google Scholar] [CrossRef]
- Fernandez-Gonzalo, R.; Irimia, J.M.; Cusso, R.; Gustafsson, T.; Linné, A.; Tesch, P.A. Flywheel Resistance Exercise to Maintain Muscle Oxidative Potential During Unloading. Aviat. Space Environ. Med. 2014, 85, 694–699. [Google Scholar] [CrossRef]
- Irimia, J.M.; Guerrero, M.; Rodriguez-Miguelez, P.; Cadefau, J.A.; Tesch, P.A.; Cussó, R.; Fernandez-Gonzalo, R. Metabolic adaptations in skeletal muscle after 84 days of bed rest with and without concurrent flywheel resistance exercise. J. Appl. Physiol. 2017, 122, 96–103. [Google Scholar] [CrossRef]
- Ingolia, N.T.; Ghaemmaghami, S.; Newman, J.R.S.; Weissman, J.S. Genome-Wide Analysis in Vivo of Translation with Nucleotide Resolution Using Ribosome Profiling. Science 2009, 324, 218–223. [Google Scholar] [CrossRef]
- Illumina. Considerations for RNA-Seq read length and coverage. Available online: https://emea.support.illumina.com/bulletins/2017/04/considerations-for-rna-seq-read-length-and-coverage-.html (accessed on 4 October 2022).
- Conesa, A.; Madrigal, P.; Tarazona, S.; Gomez-Cabrero, D.; Cervera, A.; McPherson, A.; Szczesniak, M.W.; Gaffney, D.J.; Elo, L.L.; Zhang, X.; et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016, 17, 13. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, B. A comprehensive evaluation of ensembl, RefSeq, and UCSC annotations in the context of RNA-seq read mapping and gene quantification. BMC Genom. 2015, 16, 97. [Google Scholar] [CrossRef] [PubMed]
- Oldham, M. Transcriptomics: From Differential Expression to Coexpression. In The OMICs: Applications in Neuroscience; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Oldham, M.C.; Konopka, G.; Iwamoto, K.; Langfelder, P.; Kato, T.; Horvath, S.; Geschwind, D.H. Functional organization of the transcriptome in human brain. Nat. Neurosci. 2008, 11, 1271–1282. [Google Scholar] [CrossRef]
- Ramsey, S.A.; Gold, E.S.; Aderem, A. A systems biology approach to understanding atherosclerosis. EMBO Mol. Med. 2010, 2, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Joanisse, S.; Lim, C.; McKendry, J.; McLeod, J.C.; Stokes, T.; Phillips, S.M. Recent advances in understanding resistance exercise training-induced skeletal muscle hypertrophy in humans. F1000Research 2020, 9, 141. [Google Scholar] [CrossRef]
- Langfelder, P.; Zhang, B.; Horvath, S. Defining clusters from a hierarchical cluster tree: The Dynamic Tree Cut package for R. Bioinformatics 2008, 24, 719–720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Horvath, S. A general framework for weighted gene co-expression network analysis. Stat. Appl. Genet Mol. Biol. 2005, 4, 17. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA Package FAQ. Available online: https://horvath.genetics.ucla.edu/html/CoexpressionNetwork/Rpackages/WGCNA/faq.html (accessed on 4 October 2022).
- Kramer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Zhou, G.; Soufan, O.; Ewald, J.; Hancock, R.E.W.; Basu, N.; Xia, J. NetworkAnalyst 3.0: A visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019, 47, W234–W241. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020, 48, D498–D503. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, S.; Hoyt, C.T.; Gemund, A.; Hofmann-Apitius, M.; Frohlich, H.; Domingo-Fernandez, D. The Impact of Pathway Database Choice on Statistical Enrichment Analysis and Predictive Modeling. Front. Genet. 2019, 10, 1203. [Google Scholar] [CrossRef]
- Timmons, J.A.; Szkop, K.J.; Gallagher, I.J. Multiple sources of bias confound functional enrichment analysis of global-omics data. Genome Biol. 2015, 16, 186. [Google Scholar] [CrossRef] [PubMed]
- Wijesooriya, K.; Jadaan, S.A.; Perera, K.L.; Kaur, T.; Ziemann, M. Urgent need for consistent standards in functional enrichment analysis. PLoS Comput Biol 2022, 18, e1009935. [Google Scholar] [CrossRef]
- Soneson, C.; Delorenzi, M. A comparison of methods for differential expression analysis of RNA-seq data. BMC Bioinform. 2013, 14, 91. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Dennis, G., Jr.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, P3. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. WebGestalt 2019: Gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019, 47, W199–W205. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [PubMed]
- Clough, E.; Barrett, T. The Gene Expression Omnibus Database. Methods Mol. Biol. 2016, 1418, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Pillon, N.J.; Gabriel, B.M.; Dollet, L.; Smith, J.A.B.; Sardon Puig, L.; Botella, J.; Bishop, D.J.; Krook, A.; Zierath, J.R. Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. Nat. Commun. 2020, 11, 470. [Google Scholar] [CrossRef]
- Walsh, C.J.; Hu, P.; Batt, J.; Santos, C.C. Microarray Meta-Analysis and Cross-Platform Normalization: Integrative Genomics for Robust Biomarker Discovery. Microarrays 2015, 4, 389–406. [Google Scholar] [CrossRef]
- Rung, J.; Brazma, A. Reuse of public genome-wide gene expression data. Nat. Rev. Genet 2013, 14, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.C.; Lin, H.M.; Sibille, E.; Tseng, G.C. Meta-analysis methods for combining multiple expression profiles: Comparisons, statistical characterization and an application guideline. BMC Bioinform. 2013, 14, 368. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/A (accessed on 4 October 2022).
- 9Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 3. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; et al. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Patro, R.; Mount, S.M.; Kingsford, C. Sailfish enables alignment-free isoform quantification from RNA-seq reads using lightweight algorithms. Nat. Biotechnol. 2014, 32, 462–464. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Soneson, C.; Love, M.I.; Robinson, M.D. Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences. F1000Research 2015, 4, 1521. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).