1. Introduction
Osteoarthritis (OA) is the most common joint disease amongst adults in the world [
1]. Currently, there are 33 million adults in the United States living with osteoarthritis, and that number is expected to rise to 67 million by 2032 [
2,
3]. While OA disease primarily affects the elderly, with over half of the cases of those aged over 65 years old, it is estimated that working-aged adults (45–64 years old) will represent one-third of new cases in the coming years [
4]. Osteoarthritis results in inflammation with degeneration of surrounding joint cartilage and the underlying bone. Related symptoms of this degeneration include decreased range of motion, instability, chronic pain, and severe disability [
5]. Knee osteoarthritis has been ranked the 11th highest contributor to global disability [
6].
In the knee, articular cartilage encases the lower end of the femur, the upper end of the tibia, and the undersurface of the patella. The primary function of articular cartilage is to provide a smooth, lubricated surface for joint movement [
7]. Defects in articular cartilage are especially detrimental given that articular cartilage has extremely poor self-renewal capabilities [
2]. Adult articular cartilage is not innervated or vascularized and has a tightly condensed extracellular matrix [
2,
7,
8]. The unique structure of articular cartilage significantly complicates the treatment of these defects for the surgeon, the physical therapists, and the patient [
7]. The preservation of articular cartilage is highly dependent on conserving the architecture of the cartilage [
7]. Untreated defects significantly limit the knee’s active range of motion. These defects often progress into osteoarthritis and may eventually require surgical interventions from arthroscopy to a total knee replacement [
2,
9]. Early diagnosis and treatment of articular cartilage defects have recently taken precedence due to national and international cost burdens associated with peri- and post-surgical care.
Current trends in cartilage replacement therapies are both invasive and require lengthy, painful recovery times. Moreover, procedures such as drilling, microfracture, and osteochondral autographs have low success rates [
8]. Physical therapy, activity modification, and non-steroidal anti-inflammatory drugs (NSAIDs) have demonstrated only temporary benefits and are bereft with operator-dependent limitations and medication side effects. Both pharmacological agents have a long list of side effects, including gastritis, nephritis, dysfunction of the liver, increased mean arterial blood pressure, increase in bleeding time, and cardiovascular events [
10]. Additionally, the incorporation of dry needling into exercise recovery programs proved ineffective in reducing pain levels associated with osteoarthritis [
11].
The most common non-surgical procedures for knee osteoarthritis include cortisone injections, platelet-rich plasma (PRP) therapy, and hyaluronic acid (HA) injections. Corticosteroid injections are often used as an initial salvo; however, they rarely prove to be a long-term solution. Repeated injections can result in joint swelling, tendon rupture, stiffness, hyperglycemia, and arthralgia [
10]. PRP therapy utilizes patient-harvested blood typically by an antecubital approach, which is spun in a semi-sterile manner in a centrifuge. While some benefit has been shown when treating articular cartilage degeneration in younger patients, older patients do not respond as favorably. After the age of 50, the blood platelet count decreases by 20,000 platelets per µL, continuing to steadily decrease with more advanced age [
12]. As patients receiving such therapies are typically 60 years and older, the declining platelet count in these patients may limit the effectiveness of PRP treatment. While PRP may be efficacious for a younger patient demographic, there is a growing need for interventions readily accessible for patients 60 years and older.
Hyaluronic acid injections are utilized as a visco-supplement, ideally replacing the depleted HA in patients with knee osteoarthritis. While effective in affording temporary pain relief, visco-supplementation therapies lack durability. Repeated injections on a monthly basis or in a series of shots are required for more lasting benefit. Additionally, visco-supplementation has an increased risk of adverse effects [
13,
14]. Some may conclude the lack of statistically relevant literature and paucity of clinically significant benefits outweigh the potential adverse events associated with a series of HA injections.
Overall, the treatment of articular cartilage defects in patients with symptomatic osteoarthritis of the knee remains a clinical challenge. Most clinicians agree that non-surgical intervention is the standard of care, yet current treatments lack reproducible and lasting benefits for patients. In that knowledge, we present study results for the use of Wharton’s jelly allograft applications as a non-surgical alternative, with statistically significant patient outcomes in the treatment of patients with symptomatic, structural tissue defects of the knee joint.
Wharton’s jelly, first discovered by Thomas Wharton in 1656, is a gelatinous-like tissue that encompasses two arteries and one vein of the umbilical cord [
15]. Wharton’s jelly (WJ) functions to protect the vessels of the umbilical cord from external forces and simultaneously allow for umbilical arterial and venous blood flow [
8,
15]. WJ has been reported to contain robust amounts of growth factors, HA, and extracellular vesicles that could potentially reduce inflammation and promote a regenerative microenvironment to aid in healing musculoskeletal injuries [
3,
10,
16]. Clinical applications of WJ as a tissue supplement are increasing in popularity due to recent successful outcomes [
17]. Supplementation with WJ centers on repairing the structural tissue defects in articular cartilage scaffolding. When the scaffold of articular cartilage is combined with WJ, significant amelioration of defects was observed [
18].
WJ is primarily comprised of collagen and glycosaminoglycans, mirroring articular cartilage composition [
19]. WJ is similar to articular cartilage in scaffold architecture and bio function, making WJ an ideal homologous allograft to supplement articular cartilage defects in patients with symptomatic knee osteoarthritis. Umbilical cord allografts have shown improvements in WOMAC scores for up to one year in patients with diffuse knee pain due to osteoarthritis [
4]. When compared to HA injections, umbilical cord allografts had significantly higher WOMAC improvements [
4].
In this study, we examined the effectiveness of umbilical cord tissue allografts in supplementing structural tissue defects in articular cartilage. We examined this through improvement in patient-reported outcomes of both Numeric Pain Rating Scale (NPRS) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) values. We hypothesize that patients receiving umbilical cord allograft supplementation will show an improvement in the NPRS and WOMAC scores over a 90-day period when compared to the initial administration date.
2. Materials and Methods
All methods were completed in compliance with the FDA and American Association of Tissue Banks (AATB) standards. This study was conducted under an Institute of Regenerative and Cellular Medicine IRB-approved protocol (RL-UCT-001) and informed consent was obtained from each study participant.
Human umbilical cords were obtained from consenting mothers following full-term Caesarian section deliveries. Prior to delivery, birth mothers underwent comprehensive medical, social, and blood testing. An independent certified laboratory tested all of the donations for infectious disease in accordance with Clinical Laboratory Improvement Amendments (CLIA) of 1988, 42 CFR part 493, and FDA regulations. Each birth mother was tested for hepatitis B core antibody (HBcAb), hepatitis B surface antigen (HBsAg), hepatitis C antibody (HCV), human immunodeficiency virus antibody (HIV-1/HIV-2 Plus O), human T-lymphotropic virus antibody (HLTV-I/11), syphilis (RPR), HIV-1/HCV/HBV, NAT, and West Nile virus (WNV). Each test was performed with an FDA-approved testing kit (see
Appendix A). All test results were required to be negative or non-reactive before processing the umbilical cord tissue.
Wharton’s jelly was aseptically dissociated from the rinsed umbilical cord. After dissociation, 150 mg of Wharton’s Jelly was suspended in approximately 2 mL of sterile sodium chloride 0.9% solution (normal saline). Dimethyl sulfoxide (DMSO) was added to the suspension as a cryoprotectant. The volume of DMSO was calculated as 5% of the total suspension volume. The cryoprotectant functions to preserve the integrity of the fibroblasts, pericytes, immune cells, and growth factors of the allograft while being stored in −40 °C freezers. [
20] The sample was not combined with cells, tissues, or articles other than the exceptions outlined in 21 CFR Part 1271.10 (a) (3) (Human Cells, Tissues, and Cellular and Tissue-Based Product Regulation).
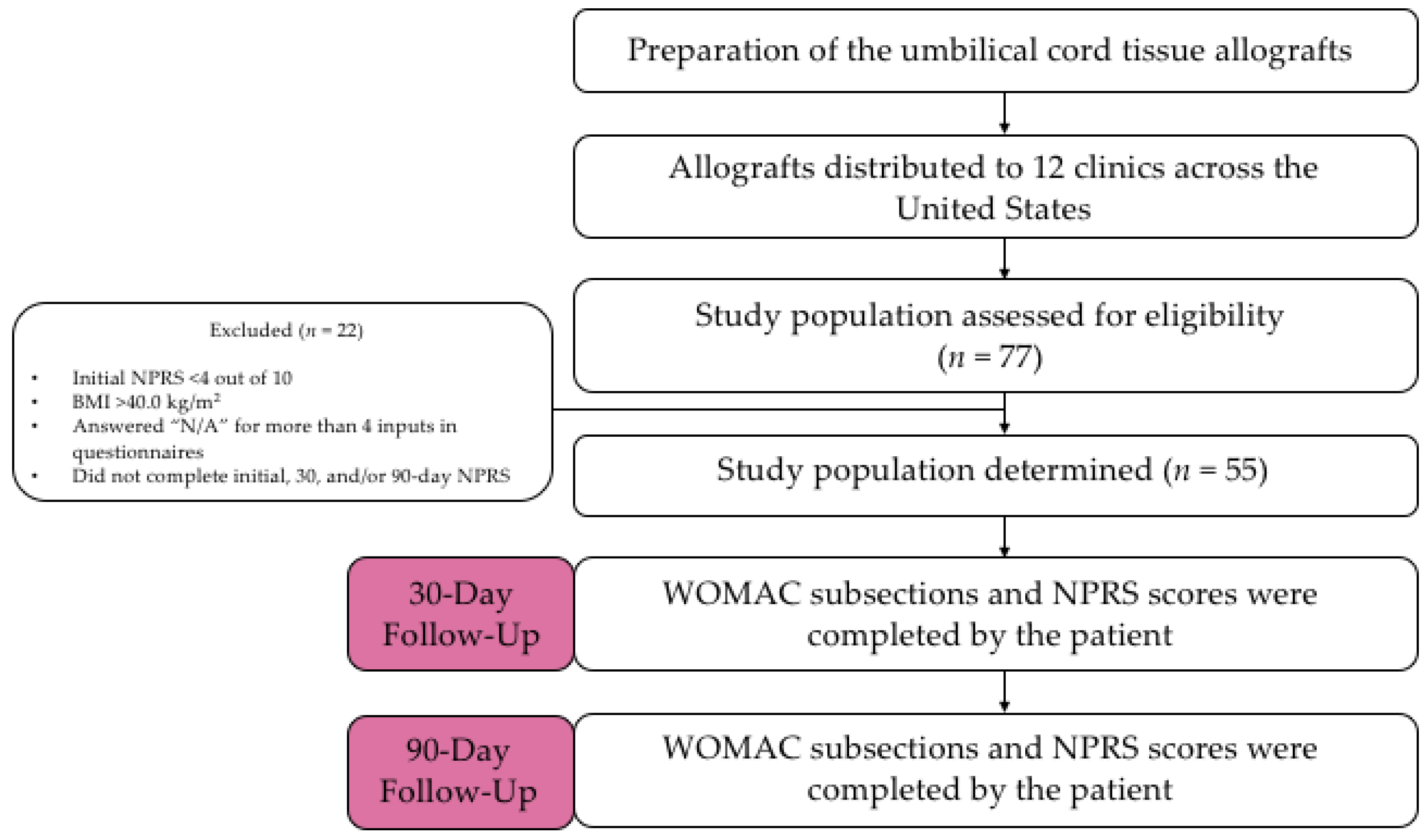
This observational data collection study analyzed 55 adult patients who met predetermined inclusion and exclusion criteria at 12 clinics throughout the United States. Eligible patients included adults older than 18 years of age with a body mass index (BMI) less than 40 kg/m2 and an initial NPRS pain score of at least 4 on a scale of 1–10. Participants were required to be classified a minimum of grade 2 on the Kellgren–Lawrence scale. Kellgren–Lawrence scale grades were determined by AP knee radiographs. Post-application radiographic imaging was only completed due to medical necessity and was not required. Upon physical examination, subjects were excluded if there were visible signs of joint infection including, but not limited to, redness of the skin surrounding the joint, swelling, and elevated body temperature. Subjects were also excluded from the study if they were currently taking blood thinners, received a recent diagnosis of cancer, or suffered recent trauma or injury to the joint. Patients who had not already received at least six weeks of conventional treatment (i.e., PRP, HA supplementation, or corticosteroids) were excluded from this study. Subjects who had completed six weeks of initial treatment and reported it to be unsuccessful then became eligible for umbilical cord allograft supplementation in this study. Subjects were not limited to allograft administration in one knee if both knees were determined to have structural tissue defects. Subjects were permitted to take pain medication in the event of excessive pain. Each clinic was required to report a medication review for patients at each visit. Of the 55 active participants, there were no participants actively taking pain medication.
Umbilical cord tissue allografts were obtained by 12 clinics. The allograft application was administered as an intra-articular knee application by anteromedial approach under ultrasound by a qualified health professional in a private medical setting. Patients were asked to fill out an initial questionnaire consisting of NPRS and WOMAC. Patients answered this same questionnaire at 30 days and 90 days after the initial allograft application. A qualitative flow diagram of the study procedures can be referenced in
Figure 1.
NPRS is a numerical pain scale employed as a subjective measurement of 0–10 for patients to rate their pain. A measurement of 0 indicates no pain, and 10 indicates the worst pain possible. WOMAC is a combination of three questionnaires measuring the pain, stiffness, and function of the knee and/or hip affected by osteoarthritis. The WOMAC questionnaire consists of 23 questions where patients rate their ability to perform the function from 0 (performs with ease) to 4 (extreme difficulty performing the function). The scores of these three categories were analyzed individually to allow for greater examination of physical mobility of the affected joint.
Patient-reported outcomes were statistically analyzed for significance in improvement. When comparing the difference between baseline and 90-day data, the average and standard deviation of each patient-reported outcome subsection was calculated. These values were then utilized to run a two-sample Z-test. The p-value of each patient-reported outcome subsection was calculated from the resulting z-score. p-values were determined at a 95% confidence level. A p-value less than or equal to 0.05 was considered to be statistically significant. Percent of change analysis was also conducted between the baseline, 30-day, and 90-day examinations as another statistical endpoint for comparison. The minimal clinically importance difference (MCID) was determined for NPRS and all three WOMAC subsections. MCID was calculated for baseline, 30-day, and 90-day questionnaires. Sample standard deviation and the number of subjects (n = 55) was used to calculate each MCID. MCID determined the minimum improvement in each subsection for the improvement to be considered clinically important. MCID was used as a secondary endpoint to determine the significance of improvement in patient-reported outcomes. Patients were excluded from the study if they answered more than four “N/A” responses in the questionnaires. Patients were also excluded from the study if they failed to complete the initial, 30-day, or 90-day questionnaires. These participants refused to complete the questionnaires due to their inability to return or resistance when asked to complete the forms. These patients were not included in the 55 patients examined in this study.
4. Discussion
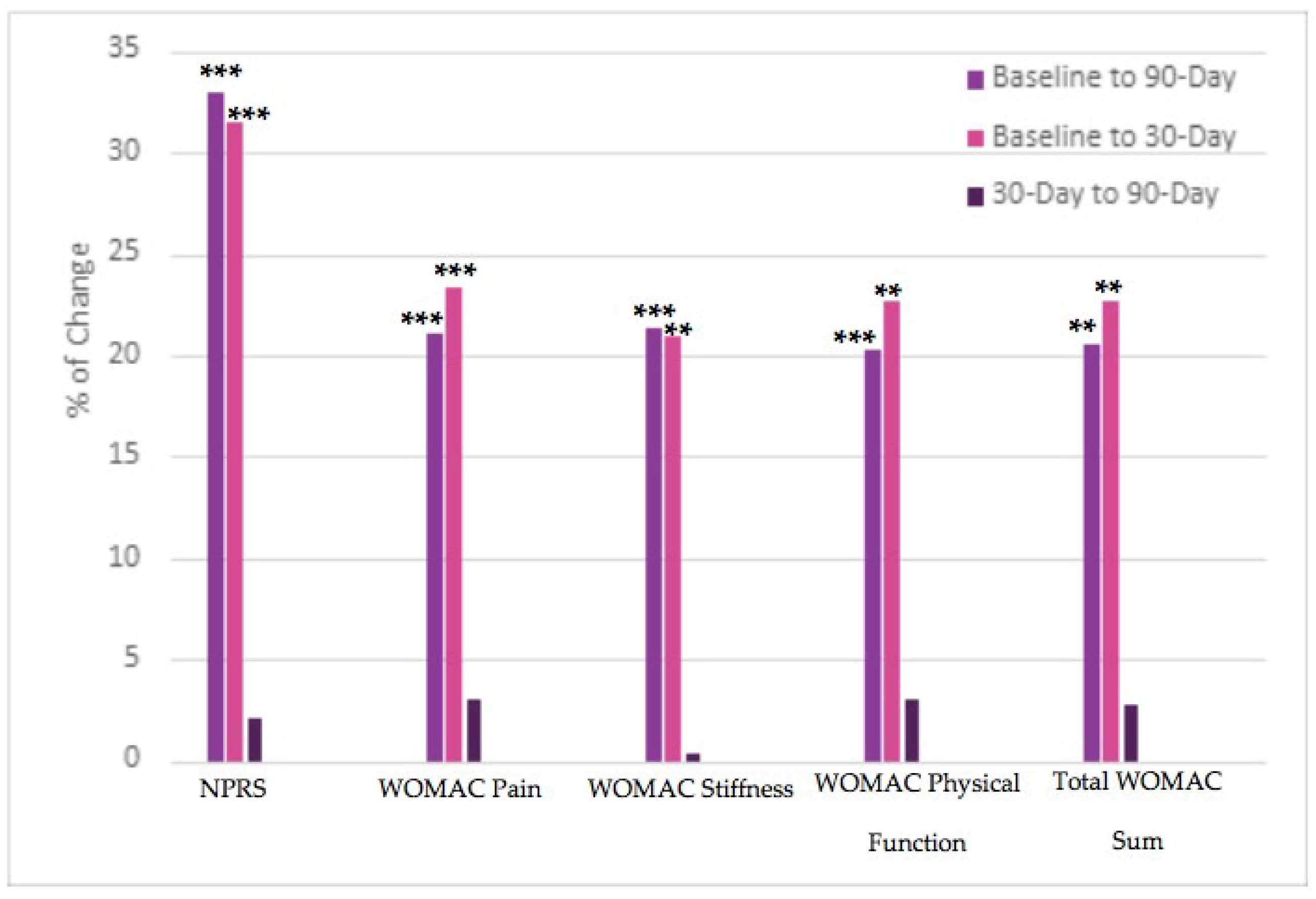
This observational data collection study found that the results of supplementation with umbilical cord tissue allograft significantly improved pain alleviation, stiffness, and physical mobility of the affected joint. We observed statistically significant differences between baseline and 90-day reported scores in NPRS and WOMAC scores. In addition, all three subsections reported improvement by the 30-day follow-up visit as well. Percentage of change between initial, 30-day, and 90-day visit are reported in
Table 2. A graphical comparison of the percentage of change is presented in
Figure 3.
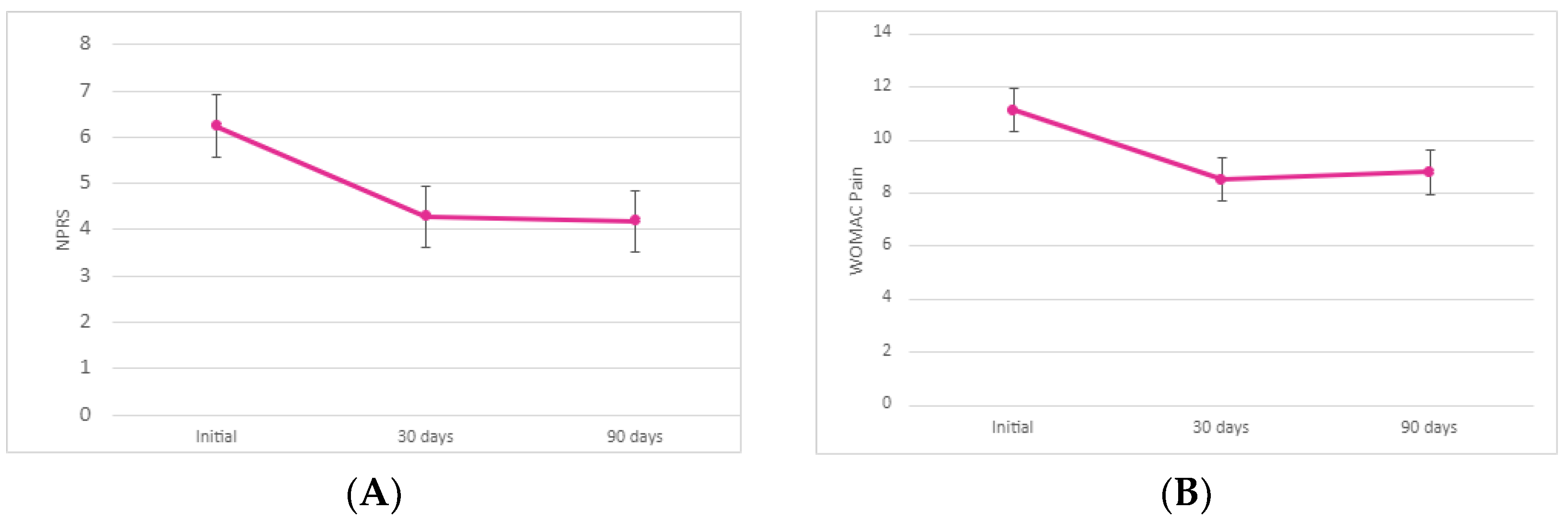
Comparison of the 30-day NPRS to the baseline data showed a significantly greater improvement in pain alleviation (decrease in pain by 31.48%). The 31.48% decrease (σ = 0.31) was statistically significant at a 95% confidence interval (p < 0.00001). We noted a slight decrease (−2.13%) change in pain alleviation between the 30- and 90-day NPRS values. This decrease (σ = 0.36) was not statistically significant (p = 0.39). There was an overall decrease in pain by 32.94% between the initial allograft application and the 90-day follow-up (σ = 0.35). This decrease was statistically significant at a 95% confidence interval (p < 0.00001). Here, 9 out of the 55 patients reported no change in improvement between initial and 90-day visits and 4 out of the 55 patients reported an increase in pain from the 30-day to 90-day visit. Since allograft application is performed according to medical necessity, it is likely that a majority of these patients required a second application. However, there is an insufficient amount of literature to presume that patients will benefit from a second allograft application. Future studies with two-dose allograft applications are required to warrant patient improvement upon a second dose administration.
When examining the pain subsect of the WOMAC scores, there is a significant improvement in pain alleviation between the baseline and 30-day follow-up (decrease in pain by 23.37%). With a standard deviation of 0.4 and a p-value less than 0.00001, this initial decrease was statistically significant. Comparison of the 90-day and 30-day average sums revealed a 2.99% increase in pain (σ = 0.49). This decrease was not significant at a 95% confidence interval (p = 0.31). Again, we contribute this to the additional need for supplementation. Regardless of this slight increase, there was an overall 21.07% decrease in the average sum of WOMAC pain scores (σ = 0.7) between the initial and 90-day examination. The decrease in pain between the initial application and 90-day examination was statistically significant (p < 0.001). Again, a larger standard deviation (σ = 4.01) was noted in the 90-day average WOMAC pain sum which therefore indicates larger variability in patient pain alleviation at 90 days post-application.
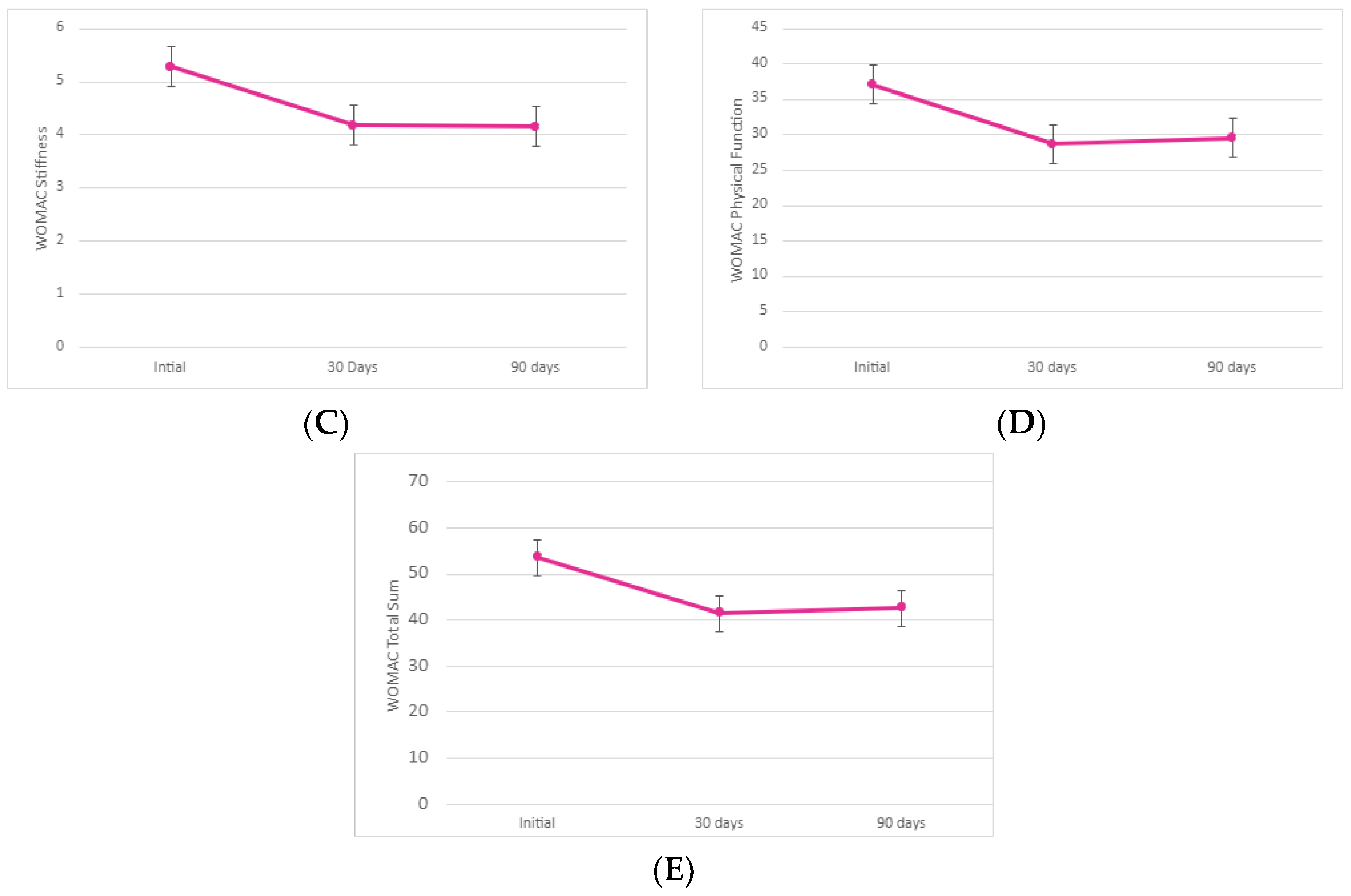
The stiffness subsection of the WOMAC scores revealed a 20.96% improvement in the average sum between initial application and 30-day follow-up (σ = 0.09). At a 95% confidence interval, the initial improvement was significant (p < 0.001). We observed a slight decrease in stiffness (0.43% decrease) between the 30-day and 90-day examinations (σ = 0.3). This slight decrease was not statistically significant (p = 0.48). However, the overall 21.30% decrease in stiffness of the knee between the initial and 90-day visit average sums indicates that the allograft application was largely successful in alleviating joint stiffness (σ = 0.3). The total improvement in patient-reported stiffness from the initial allograft application to the 90-day examination was significant (p < 0.001).
The final subsection of WOMAC, physical function, demonstrated a 22.64% improvement in joint physical function between the initial allograft application and 30-day follow-up. This initial improvement (σ = 2.23) was significant at a 95% confidence interval (p < 0.0001). From the 30-day to the 90-day follow-up, we observed a 3.10% decrease in the overall physical function of the affected knee (σ = 2.44). Again, we contribute this decrease in mobility to the absence of a second allograft application. The 3.10% decrease was not statistically significant (p = 0.36). From the initial application to the 90-day visit, patients reported an overall 20.23% improvement in the function and mobility of the affected knee (σ = 2.34). The overall patient-reported functionality improvement was significant (p < 0.001). We noted an unusually high standard deviation (σ = 13.36) in the 90-day average sum again, indicating a larger variation in each patient’s mobility 90 days after allograft application. However, the notable decrease in all WOMAC subsections between the initial application and 90-day visit indicates a continuous improvement in pain, stiffness, and physical mobility after allograft application.
The total sum of all WOMAC subsections demonstrated a 22.62% improvement in pain, stiffness, and physical function collectively between the initial allograft application and the 30-day follow-up (σ = 2.9). The initial improvement is significant at a 95% confidence interval (p < 0.0001). From the 30-day to the 90-day follow-up, we observed a 2.72% decrease in the subsections (σ = 3.34). Given the larger dataset and increased standard deviation at the 90-day examination (σ = 18.48), this was expected. This decrease was not statistically significant (p = 0.37). From the initial application to the 90-day visit, participants reported an overall 20.52% improvement in pain, joint stiffness, and physical function of the knee (σ = 3.29). The overall patient-reported outcome improvement was statistically significant (p < 0.001).
The results demonstrated a significant minimal clinically important difference (MCID) observed in NPRS and all WOMAC subsection improvements. We believe that the absence of a second application was detrimental to a larger improvement in patient-reported scores. However, changes in the NPRS and WOMAC subsection average sums were greater than the determined MCID and therefore illustrated improvements that were clinically significant in patients after the initial allograft application.
In addition to physical improvement, human umbilical cord tissue allografts are advantageous in that they do not elicit an immune response from the host. Coupled with clinical advantages, WJ also demonstrates structural similarities to articular cartilage. WJ is partially comprised of glycosaminoglycans and collagen, similar to articular cartilage [
18]. WJ exhibits similar functions to articular cartilage as well. WJ functions to protect the vessels of the umbilical cord from external forces while also allowing for umbilical arterial and venous blood flow [
7,
14]. These primary functions are similar to those of articular cartilage, which supports and distributes external forces in the knee. The structural and functional similarities between WJ and articular cartilage, combined with significant improvement of NPRS and WOMAC indicate that umbilical cord tissue allografts are an effective supplementation for osteoarthritis cartilage degeneration. These results are consistent with a study in 2022 that found that 30 patients who received umbilical cord tissue (UCT) applications in the knee had decreased pain, decreased medication use (opiates and NSAIDs), and an improvement in physical function lasting over 24 weeks [
19].
Our observational study has limitations; the design was an analysis of unblinded data collection. However, the primary outcomes of NPRS and WOMAC were patient-reported, which eliminates the influence of an unblinded investigator. We plan to perform a double-blinded placebo-controlled study in the fall of 2022. In addition, we were limited in patient improvement due to the restriction of only one application. In future studies, we plan to analyze NPRS and WOMAC improvements and compare them to the MCID in patients with structural tissue defects of the knee after two allograft applications. The insufficient amount of radiographic data regarding joint structural changes warrants an additional single allograft application study with the inclusion of HA supplementation, manual therapy, analgesic agents, and glucocorticosteroids. We plan to utilize magnetic resonance imaging (MRIs) and X-rays to analyze the improvement in patient-reported pain levels when umbilical cord tissue allografts are combined with different standard of care measures.