In Vitro Evaluation of the Effectiveness of a Commercial Enzymatic Product Against Pseudomonas aeruginosa Biofilms According to the Parameters of Use
Abstract
1. Introduction
2. Materials and Methods
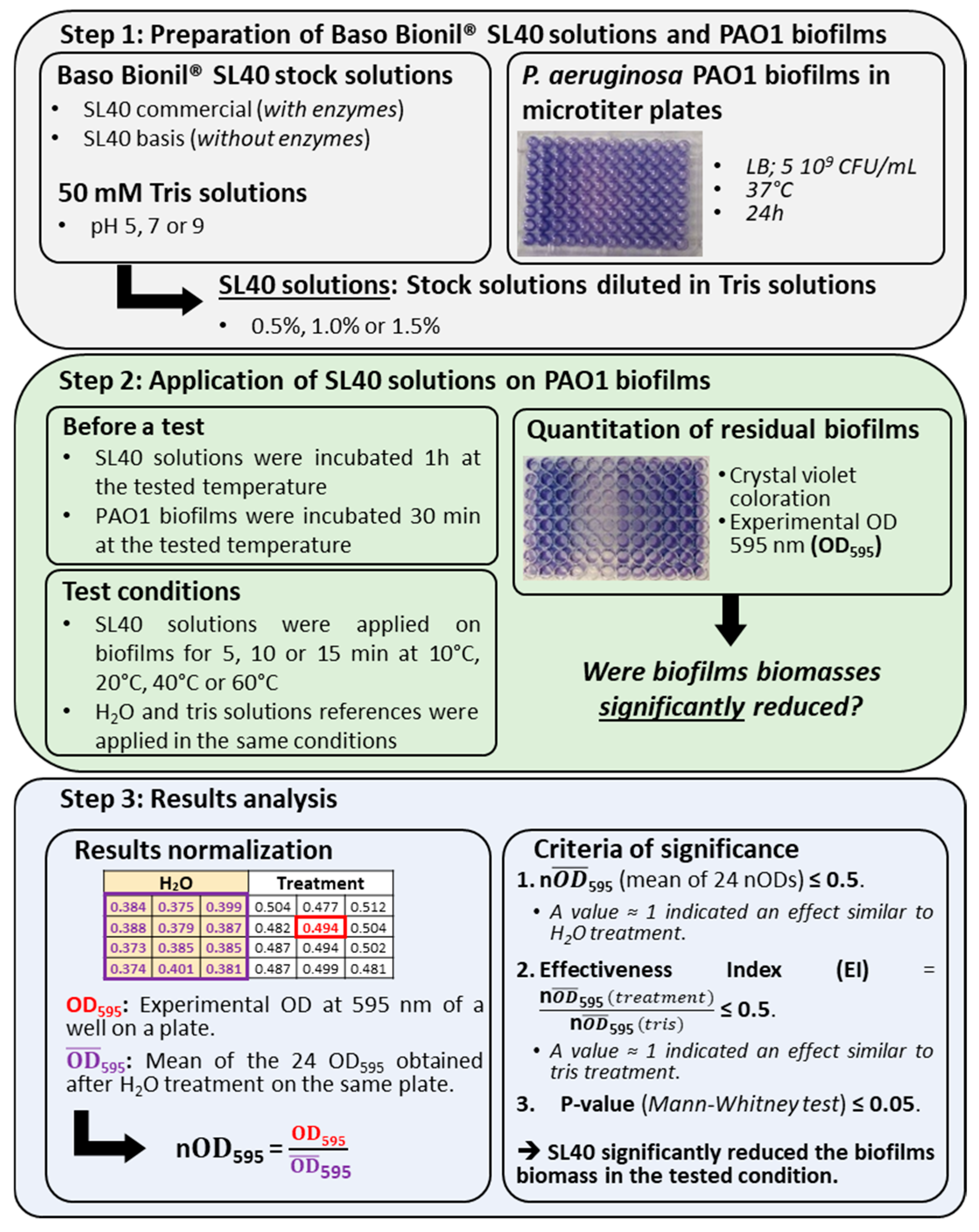
2.1. Bacterial Strain, Growth Medium, and Biofilms Preparation
2.2. Preparation of Baso Bionil SL40® Solutions and Biofilm Treatment
2.3. Data Analysis Methods
3. Results
3.1. Description of the Dataset, Variability of the Results, and Criteria to Qualify SL40 Effectiveness Against Pseudomonas aeruginosa Biofilms
3.1.1. A Dataset of 8640 Values
3.1.2. Variability of the Data Obtained from Microtiter Plates
3.1.3. Three Criteria to Qualify the Effectiveness of SL40 Against P. aeruginosa Biofilms
3.2. Effectiveness of the Di-Enzymatic Detergent SL40 to Remove P. aeruginosa Biofilms: Role of the Enzymes and Influence of the Temperature, Concentration, pH, and Incubation Time
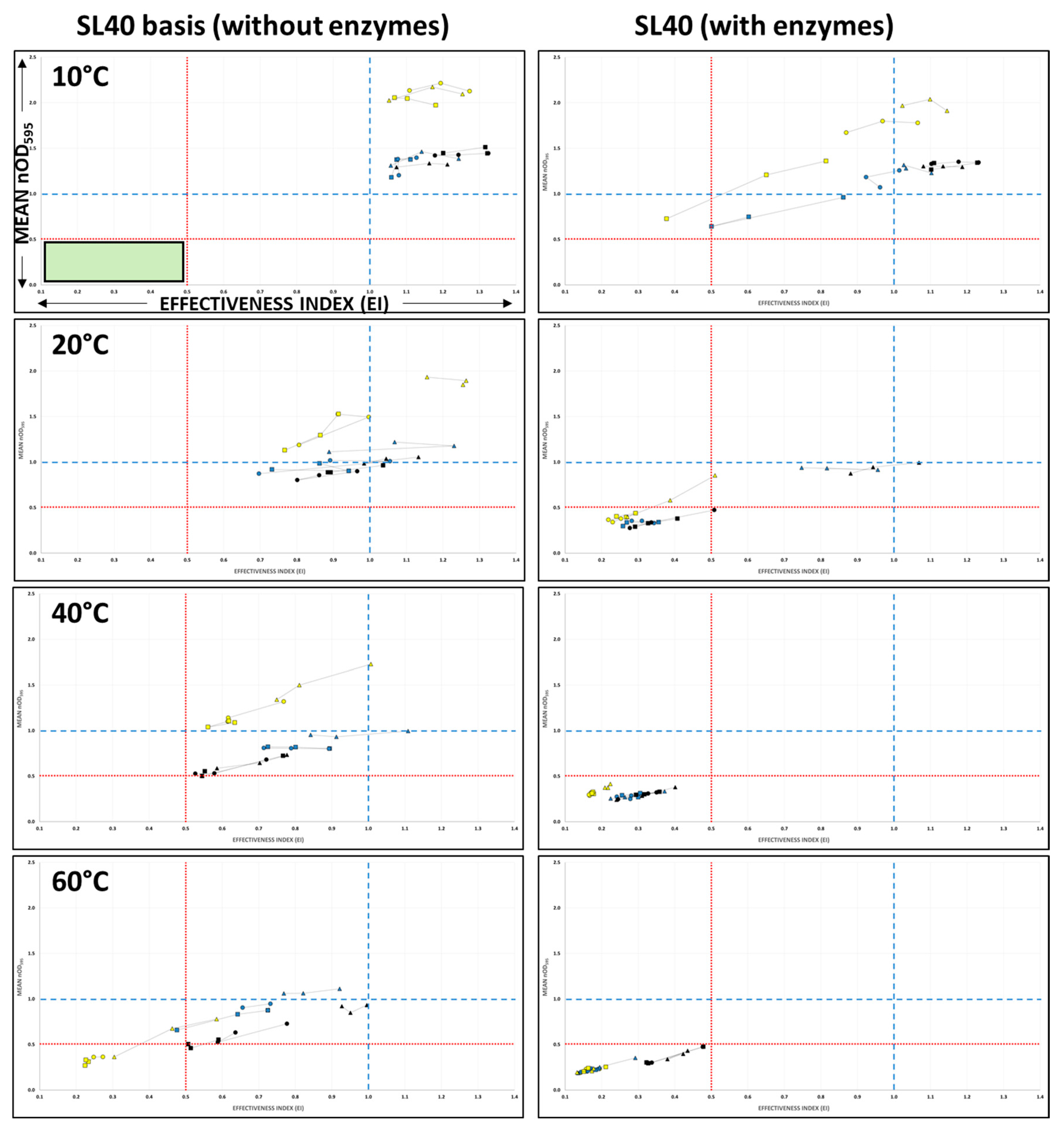
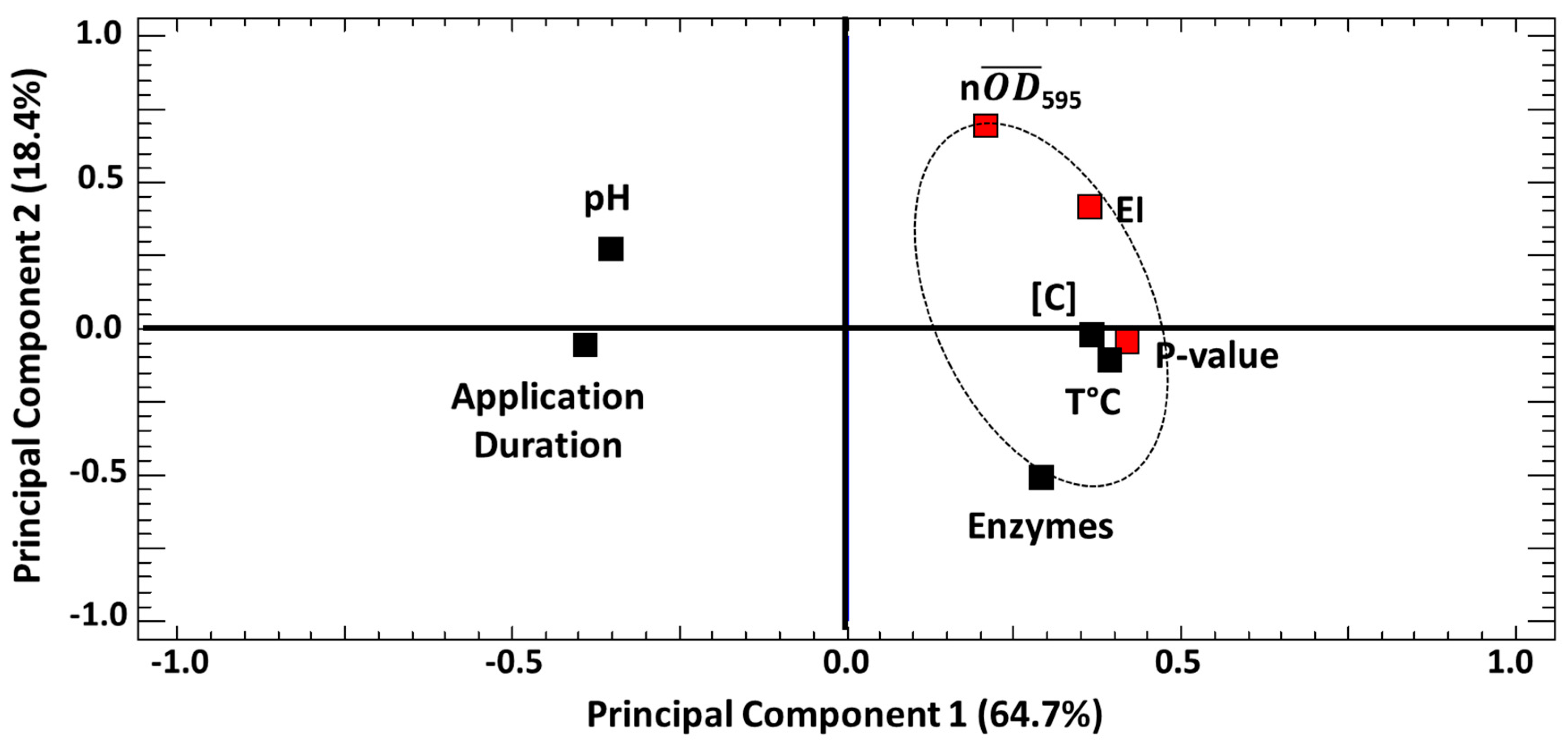
3.2.1. Global Analysis of the Influence of the Tested Parameters on SL40 Activity
3.2.2. Activity of SL40/SL40 Basis on P. aeruginosa Biofilms at 10 °C
3.2.3. Activity of SL40/SL40 Basis on P. aeruginosa Biofilms at 20 °C
3.2.4. Activity of SL40/SL40 Basis on P. aeruginosa Biofilms at 40 °C
3.2.5. Activity of SL40/SL40 Basis on P. aeruginosa Biofilms at 60 °C
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CV | Cristal Violet |
| EI | Effectiveness Index |
| PCA | Principal Component Analysis |
| RSD | Relative Standard Deviation |
| SL40 | Baso Bionil SL40® with enzymes |
| SL40 basis | Baso Bionil SL40® without enzymes |
References
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Flemming, H.C.; Baveye, P.; Neu, T.R.; Stoodley, P.; Szewzyk, U.; Wingender, J.; Wuertz, S. Who put the film in biofilm? The migration of a term from wastewater engineering to medicine and beyond. NPJ Biofilms Microbiomes 2021, 7, 10. [Google Scholar] [CrossRef]
- Tong, C.; Hu, H.; Chen, G.; Li, Z.; Li, A.; Zhang, J. Disinfectant resistance in bacteria: Mechanisms, spread, and resolution strategies. Environ. Res. 2021, 195, 110897. [Google Scholar] [CrossRef]
- Otter, J.A.; Vickery, K.; Walker, J.T.; deLancey Pulcini, E.; Stoodley, P.; Goldenberg, S.D.; Salkeld, J.A.G.; Chewins, J.; Yezli, S.; Edgeworth, J.D. Surface-attached cells, biofilms and biocide susceptibility: Implications for hospital cleaning and disinfection. J. Hosp. Infect. 2015, 89, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Cámara, M.; Green, W.; MacPhee, C.E.; Rakowska, P.D.; Raval, R.; Richardson, M.C.; Slater-Jefferies, J.; Steventon, K.; Webb, J.S. Economic significance of biofilms: A multidisciplinary and cross-sectoral challenge. NPJ Biofilms Microbiomes 2022, 8, 42. [Google Scholar] [CrossRef]
- Hofer, U. The cost of biofilms. Nat. Rev. Microbiol. 2022, 20, 445. [Google Scholar] [CrossRef]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: A data synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef]
- Zhu, T.; Yang, C.; Bao, X.; Chen, F.; Guo, X. Strategies for controlling biofilm formation in food industry. Grain Oil Sci. Technol. 2022, 5, 179–186. [Google Scholar] [CrossRef]
- Jee, S.C.; Kim, M.; Sung, J.S.; Kadam, A.A. Efficient biofilms eradication by enzymatic-cocktail of pancreatic protease type-I and bacterial α-amylase. Polymers 2020, 12, 3032. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Singh, A.K.; Singh, S.; Chakravortty, D.; Das, D. Enzymatic dispersion of biofilms: An emerging biocatalytic avenue to combat biofilm-mediated microbial infections. J. Biol. Chem. 2022, 298, 102352. [Google Scholar] [CrossRef]
- Di Martino, P. Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol. 2018, 4, 274–288. [Google Scholar] [CrossRef]
- Gohara, D.W.; Di Cera, E. Molecular mechanisms of enzyme activation by monovalent cations. J. Biol. Chem. 2016, 291, 20840–20848. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Weiser, R.; Green, A.E.; Bull, M.J.; Cunningham-Oakes, E.; Jolley, K.A.; Maiden, M.C.J.; Hall, A.J.; Winstanley, C.; Weightman, A.J.; Donoghue, D.; et al. Not all Pseudomonas aeruginosa are equal: Strains from industrial sources possess uniquely large multireplicon genomes. Microb. Genom. 2019, 5, e000276. [Google Scholar] [CrossRef] [PubMed]
- Rybtke, M.; Hultqvist, L.D.; Givskov, M.; Tolker-Nielsen, T. Pseudomonas aeruginosa biofilm infections: Community structure, antimicrobial tolerance and immune response. J. Mol. Biol. 2015, 427, 3628–3645. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A.; Pratt, L.A.; Watnick, P.I.; Newman, D.K.; Weaver, V.B.; Kolter, R. Genetic approaches to study of biofilms. Methods Enzymol. 1999, 310, 91–109. [Google Scholar] [CrossRef]
- Knisz, J.; Eckert, R.; Gieg, L.M.; Koerdt, A.; Lee, J.S.; Silva, E.R.; Skovhus, T.L.; Stepec, B.A.A.; A Wade, S. Microbiologically influenced corrosion-more than just microorganisms. FEMS Microbiol Rev. 2023, 47, fuad041. [Google Scholar] [CrossRef]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Bridier, A.; Sanchez-Vizuete, P.; Guilbaud, M.; Piard, J.C.; Naïtali, M.; Briandet, R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Han, Q.; Song, X.; Zhang, Z.; Fu, J.; Wang, X.; Malakar, P.K.; Liu, H.; Pan, Y.; Zhao, Y. Removal of foodborne pathogen biofilms by acidic electrolyzed water. Front. Microbiol. 2017, 8, 988. [Google Scholar] [CrossRef]
- Panlilio, A.L.; Beck-Sague, C.M.; Siegel, J.D.; Anderson, R.L.; Yetts, S.Y.; Clark, N.C.; Duer, P.N.; Thomassen, K.A.; Vess, R.W.; Hill, B.C.; et al. Infections and pseudoinfections due to povidone-iodine solution contaminated with Pseudomonas cepacia. Clin. Infect. Dis. 1992, 14, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Verified Market Research. Biofilms Treatment Market Size and Forecast. Available online: www.verifiedmarketresearch.com/product/biofilms-treatment-market/ (accessed on 3 June 2025).
- Allkja, J.; Bjarnsholt, T.; Coenye, T.; Cos, P.; Fallarero, A.; Harrison, J.J.; Lopes, S.P.; Oliver, A.; Pereira, M.O.; Ramage, G.; et al. Minimum information guideline for spectrophotometric and fluorometric methods to assess biofilm formation in microplates. Biofilm 2019, 2, 100010. [Google Scholar] [CrossRef]
- Allkja, J.; van Charante, F.; Aizawa, J.; Reigada, I.; Guarch-Pérez, C.; Vazquez-Rodriguez, J.A.; Cos, P.; Coenye, T.; Fallarero, A.; Zaat, S.A.J.; et al. Interlaboratory study for the evaluation of three microtiter plate-based biofilm quantification methods. Sci. Rep. 2021, 11, 13779. [Google Scholar] [CrossRef]
- Azevedo, N.F.; Allkja, J.; Goeres, D.M. Biofilms vs. cities and humans vs. aliens—A tale of reproducibility in biofilms. Trends Microbiol. 2021, 29, 1062–1071. [Google Scholar] [CrossRef]
- European Medecines Agency. ICH Guideline M10 on Bioanalytical Method Validation and Study Sample Analysis. Available online: www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m10-bioanalytical-method-validation-step-5_en.pdf (accessed on 3 June 2025).
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef]
- Kang, X.; Yang, X.; He, Y.; Guo, C.; Li, Y.; Ji, H.; Qin, Y.; Wu, L. Strategies and materials for the prevention and treatment of biofilms. Mater Today Bio. 2023, 23, 100827. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, R.; Sand, W.; Mathivanan, K.; Zhang, Y.; Wang, N.; Duan, J.; Hou, B. Comprehensive review on the use of biocides in microbiologically influenced corrosion. Microorganisms 2023, 11, 2194. [Google Scholar] [CrossRef]
- Redfern, J.; Cunliffe, A.J.; Goeres, D.M.; Azevedo, N.F.; Verran, J. Critical analysis of methods to determine growth, control and analysis of biofilms for potential non-submerged antibiofilm surfaces and coatings. Biofilm 2024, 7, 100187. [Google Scholar] [CrossRef] [PubMed]
- Paluch, E.; Rewak-Soroczyńska, J.; Jędrusik, I.; Mazurkiewicz, E.; Jermakow, K. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Böhning, J.; Tarafder, A.K.; Bharat, T.A.M. The role of filamentous matrix molecules in shaping the architecture and emergent properties of bacterial biofilms. Biochem. J. 2024, 481, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.; Nag, M.; Banerjee, R.; Mukherjee, D.; Garai, S.; Sarkar, T.; Dey, A.; Sheikh, H.I.; Pathak, S.K.; Edinur, H.A.; et al. Amylases: Biofilm inducer or biofilm inhibitor? Front. Cell. Infect. Microbiol. 2021, 11, 660048. [Google Scholar] [CrossRef]
- Bott, R.; Crissman, J.; Kollar, C.; Saldajeno, M.; Ganshaw, G.; Thomas, X.; Lane, T.H.; Klykken, P.; Davidson, J.M.; Nanney, L.B. A silicone-based controlled-release device for accelerated proteolytic debridement of wounds. Wound Repair Regen. 2007, 15, 227–235. [Google Scholar] [CrossRef]
- Kimijima, M.; Narisawa, N.; Hori, E.; Mandokoro, K.; Ito, T.; Ota, Y.; Sashida, M.; Kawai, Y.; Takenaga, F. Nattokinase, a subtilisin-like alkaline-serine protease, reduces mutacin activity by inactivating the competence-stimulating peptide in Streptococcus mutans. Pathogens 2024, 13, 286. [Google Scholar] [CrossRef]
- Solanki, P.; Putatunda, C.; Kumar, A.; Bhatia, R.; Walia, A. Microbial proteases: Ubiquitous enzymes with innumerable uses. 3 Biotech 2021, 11, 428. [Google Scholar] [CrossRef]
- Zacharis, E.; Halling, P.J.; Rees, D.G. Volatile buffers can override the “pH memory” of subtilisin catalysis in organic media. Proc. Natl. Acad. Sci. USA 1999, 96, 1201–1205. [Google Scholar] [CrossRef]
- Eser, A.; Aydemir, T. Subtilisin Carlsberg immobilization and its application for eco-friendly leather processing. J. Clean. Prod. 2022, 377, 134296. [Google Scholar] [CrossRef]
- Liu, J.; Madec, J.Y.; Bousquet-Mélou, A.; Haenni, M.; Ferran, A.A. Destruction of Staphylococcus aureus biofilms by combining an antibiotic with subtilisin A or calcium gluconate. Sci. Rep. 2021, 11, 6225. [Google Scholar] [CrossRef] [PubMed]
- Payen, A.; Persoz, J.F. Memoir on diastase, the principal products of its reactions, and their applications to the industrial arts. Ann. Chim. Phys. 1833, 53, 73–92. [Google Scholar]
- Fleming, D.; Chahin, L.; Rumbaugh, K. Glycoside hydrolases degrade polymicrobial bacterial biofilms in wounds. Antimicrob. Agents Chemother. 2017, 61, e01998-16. [Google Scholar] [CrossRef]
- Gurung, N.; Ray, S.; Bose, S.; Rai, V. A broader view: Microbial enzymes and their relevance in industries, medicine, and beyond. Biomed. Res. Int. 2013, 2013, 329121. [Google Scholar] [CrossRef]
- Ju, L.; Pan, Z.; Zhang, H.; Li, Q.; Liang, J.; Deng, G.; Yu, M.; Long, H. New insights into the origin and evolution of α-amylase genes in green plants. Sci. Rep. 2019, 9, 4929. [Google Scholar] [CrossRef]
- Pekgenc, E.; Gul, B.Y.; Vatanpour, V.; Koyuncu, I. Biocatalytic membranes in anti-fouling and emerging pollutant degradation applications: Current state and perspectives. Separ. Purif. Technol. 2022, 282, 120098. [Google Scholar] [CrossRef]
- Yadav, J.K.; Prakash, V. Stabilization of α-amylase, the key enzyme in carbohydrates properties alterations, at low pH. Int. J. Food Prop. 2011, 14, 1182–1196. [Google Scholar] [CrossRef]
- Mondal, S.; Mondal, K.; Halder, S.K.; Thakur, N.; Mondal, K.C. Microbial amylase: Old but still at the forefront of all major industrial enzymes. Biocatal. Agric. Biotech 2022, 45, 102509. [Google Scholar]
- Nowak-Lange, M.; Niedziałkowska, K.; Bernat, P.; Lisowska, K. In vitro study of the ecotoxicological risk of methylisothiazolinone and chloroxylenol towards soil bacteria. Sci. Rep. 2022, 12, 19068. [Google Scholar] [CrossRef] [PubMed]
- Hostacká, A.; Ciznár, I.; Stefkovicová, M. Temperature and pH affect the production of bacterial biofilm. Folia Microbiol. 2010, 55, 75–78. [Google Scholar] [CrossRef]

| Experimental Conditions | 595 Values Obtained with SL40 Basis (Without Enzymes) | 595 Values Obtained with SL40 (with Enzymes) | ||||||
|---|---|---|---|---|---|---|---|---|
| Temperature | pH | Time (min) | 0.5% | 1.0% | 1.5% | 0.5% | 1.0% | 1.5% |
| 10 °C | 5 | 5 | 1.21/1.32 | 1.32/1.45 | 1.32/1.44 | 1.19/1.30 | 1.23/1.34 | 1.23/1.34 |
| 10 | 1.16/1.34 | 1.24/1.43 | 1.32/1.51 | 1.13/1.30 | 1.18/1.35 | 1.10/1.27 | ||
| 15 | 1.07/1.29 | 1.18/1.42 | 1.20/1.45 | 1.08/1.30 | 1.10/1.33 | 1.11/1.34 | ||
| 7 | 5 | 1.24/1.39 | 1.08/1.20 | 1.06/1.18 | 1.10/1.23 | 0.96/1.07 | 0.86/0.96 | |
| 10 | 1.14/1.46 | 1.08/1.38 | 1.07/1.38 | 1.03/1.32 | 0.92/1.18 | 0.50/0.64 | ||
| 15 | 1.06/1.31 | 1.13/1.40 | 1.11/1.38 | 1.03/1.28 | 1.01/1.26 | 0.60/0.75 | ||
| 9 | 5 | 1.25/2.09 | 1.27/2.13 | 1.18/1.97 | 1.15/1.91 | 1.07/1.78 | 0.81/1.36 | |
| 10 | 1.17/2.17 | 1.19/2.21 | 1.10/2.05 | 1.10/2.04 | 0.97/1.80 | 0.65/1.21 | ||
| 15 | 1.05/2.02 | 1.11/2.13 | 1.07/2.05 | 1.02/1.97 | 0.87/1.67 | 0.38/0.73 | ||
| 20 °C | 5 | 5 | 1.13/1.05 | 0.97/0.90 | 1.04/0.96 | 1.07/0.99 | 0.51/0.47 | 0.41/0.38 |
| 10 | 0.98/0.99 | 0.80/0.80 | 0.89/0.89 | 0.94/0.94 | 0.34/0.34 | 0.33/0.33 | ||
| 15 | 1.04/1.04 | 0.86/0.86 | 0.89/0.89 | 0.88/0.87 | 0.28/0.28 | 0.29/0.29 | ||
| 7 | 5 | 1.07/1.22 | 0.89/1.02 | 0.86/0.98 | 0.82/0.93 | 0.31/0.35 | 0.26/0.30 | |
| 10 | 1.23/1.18 | 1.05/1.01 | 0.94/0.90 | 0.96/0.92 | 0.34/0.33 | 0.36/0.34 | ||
| 15 | 0.89/1.11 | 0.70/0.87 | 0.73/0.92 | 0.75/0.94 | 0.28/0.35 | 0.27/0.34 | ||
| 9 | 5 | 1.16/1.93 | 0.91/1.53 | 0.91/1.53 | 0.51/0.85 | 0.22/0.37 | 0.24/0.40 | |
| 10 | 1.26/1.89 | 1.00/1.49 | 0.86/1.30 | 0.39/0.58 | 0.25/0.38 | 0.29/0.44 | ||
| 15 | 1.25/1.85 | 0.81/1.19 | 0.77/1.13 | 0.27/0.40 | 0.23/0.34 | 0.27/0.39 | ||
| 40 °C | 5 | 5 | 0.78/0.73 | 0.72/0.68 | 0.77/0.72 | 0.40/0.38 | 0.33/0.31 | 0.32/0.30 |
| 10 | 0.70/0.65 | 0.58/0.53 | 0.54/0.50 | 0.31/0.29 | 0.35/0.32 | 0.36/0.33 | ||
| 15 | 0.59/0.59 | 0.53/0.53 | 0.55/0.55 | 0.24/0.24 | 0.24/0.25 | 0.29/0.30 | ||
| 7 | 5 | 1.11/0.99 | 0.89/0.80 | 0.89/0.80 | 0.37/0.33 | 0.28/0.25 | 0.30/0.27 | |
| 10 | 0.91/0.93 | 0.79/0.81 | 0.80/0.82 | 0.26/0.27 | 0.28/0.29 | 0.31/0.31 | ||
| 15 | 0.84/0.95 | 0.71/0.81 | 0.73/0.82 | 0.22/0.25 | 0.24/0.27 | 0.26/0.29 | ||
| 9 | 5 | 1.01/1.73 | 0.77/1.32 | 0.63/1.09 | 0.22/0.37 | 0.17/0.29 | 0.18/0.31 | |
| 10 | 0.81/1.50 | 0.62/1.14 | 0.56/1.04 | 0.22/0.41 | 0.17/0.32 | 0.18/0.33 | ||
| 15 | 0.75/1.34 | 0.62/1.10 | 0.62/1.11 | 0.21/0.37 | 0.17/0.30 | 0.17/0.31 | ||
| 60 °C | 5 | 5 | 1.00/0.93 | 0.78/0.73 | 0.59/0.55 | 0.42/0.40 | 0.32/0.30 | 0.32/0.30 |
| 10 | 0.95/0.85 | 0.59/0.52 | 0.51/0.46 | 0.38/0.34 | 0.34/0.30 | 0.33/0.29 | ||
| 15 | 0.93/0.92 | 0.64/0.63 | 0.51/0.50 | 0.43/0.43 | 0.48/0.47 | 0.48/0.47 | ||
| 7 | 5 | 0.92/1.11 | 0.73/0.87 | 0.73/0.87 | 0.29/0.35 | 0.19/0.23 | 0.18/0.22 | |
| 10 | 0.82/1.06 | 0.73/0.95 | 0.64/0.83 | 0.19/0.25 | 0.16/0.21 | 0.16/0.20 | ||
| 15 | 0.77/1.06 | 0.66/0.90 | 0.48/0.66 | 0.14/0.19 | 0.14/0.20 | 0.17/0.23 | ||
| 9 | 5 | 0.58/0.78 | 0.27/0.36 | 0.23/0.31 | 0.18/0.24 | 0.14/0.19 | 0.15/0.20 | |
| 10 | 0.46/0.67 | 0.25/0.36 | 0.23/0.33 | 0.13/0.19 | 0.16/0.23 | 0.16/0.24 | ||
| 15 | 0.30/0.36 | 0.22/0.27 | 0.22/0.27 | 0.17/0.21 | 0.19/0.23 | 0.21/0.25 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Sénéchal, C.; Fautras, Y.; Tokarski, C.; Vilain, S. In Vitro Evaluation of the Effectiveness of a Commercial Enzymatic Product Against Pseudomonas aeruginosa Biofilms According to the Parameters of Use. Hygiene 2025, 5, 41. https://doi.org/10.3390/hygiene5030041
Le Sénéchal C, Fautras Y, Tokarski C, Vilain S. In Vitro Evaluation of the Effectiveness of a Commercial Enzymatic Product Against Pseudomonas aeruginosa Biofilms According to the Parameters of Use. Hygiene. 2025; 5(3):41. https://doi.org/10.3390/hygiene5030041
Chicago/Turabian StyleLe Sénéchal, Caroline, Yoann Fautras, Caroline Tokarski, and Sébastien Vilain. 2025. "In Vitro Evaluation of the Effectiveness of a Commercial Enzymatic Product Against Pseudomonas aeruginosa Biofilms According to the Parameters of Use" Hygiene 5, no. 3: 41. https://doi.org/10.3390/hygiene5030041
APA StyleLe Sénéchal, C., Fautras, Y., Tokarski, C., & Vilain, S. (2025). In Vitro Evaluation of the Effectiveness of a Commercial Enzymatic Product Against Pseudomonas aeruginosa Biofilms According to the Parameters of Use. Hygiene, 5(3), 41. https://doi.org/10.3390/hygiene5030041






