Evaluation of Prepacked Bone Cement Mixing Systems in Arthroplasty: Implications for Intraoperative Hygiene and Contamination Risk
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistic
2.2. Test of Doughing Time (ISO 5833)
2.3. Test of Intrusion (ISO 5833)
2.4. Test of Setting Time and Setting Temperature (ISO 5833)
2.5. Test of Compressive Strength (ISO 5833)
2.6. Test of Bending Modulus and Bending Strength (ISO 5833)
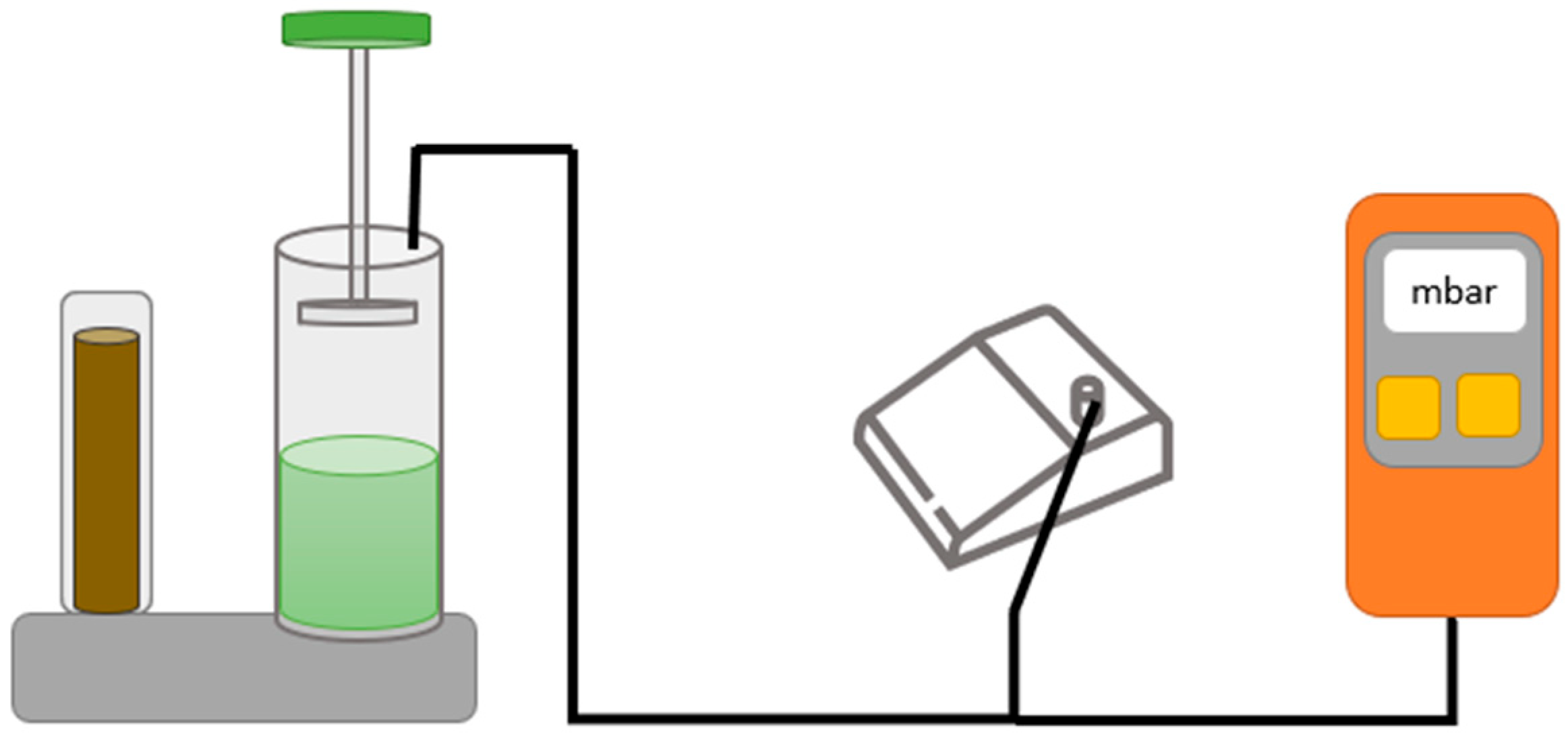
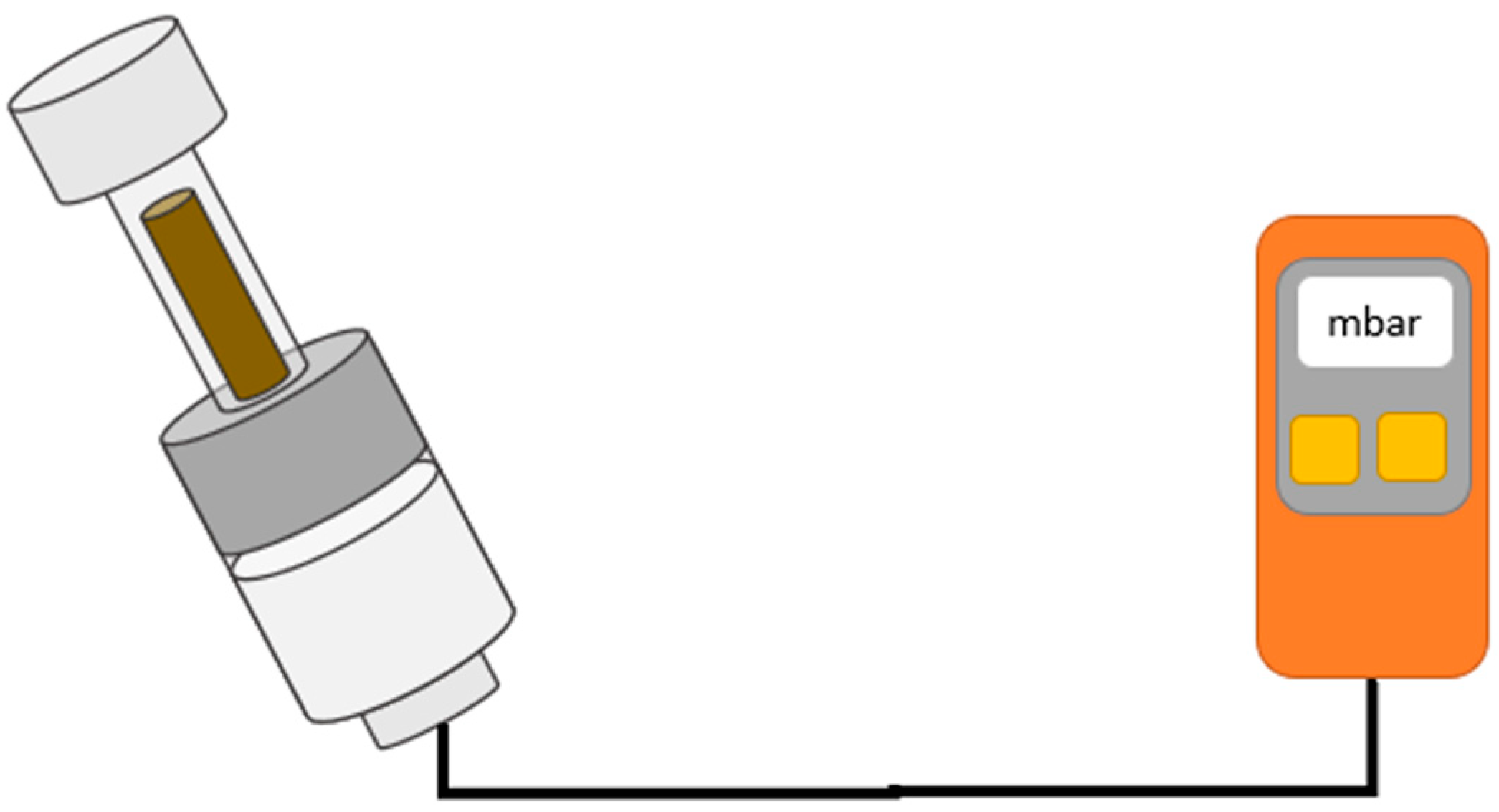
2.7. Test of Vacuum Tightness and Internal Pressure of the Mixing Systems
3. Results
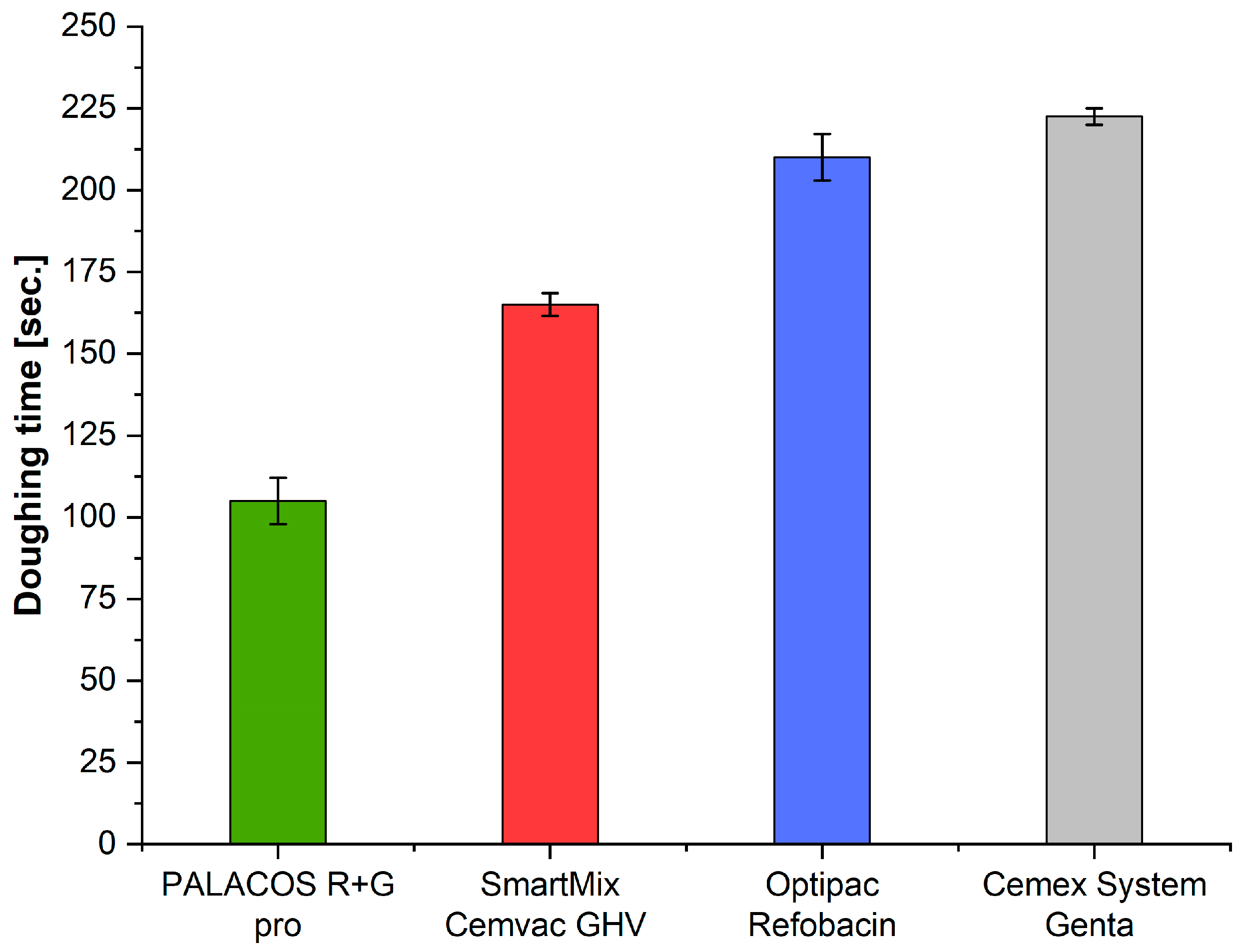
3.1. Doughing Time (ISO 5833)
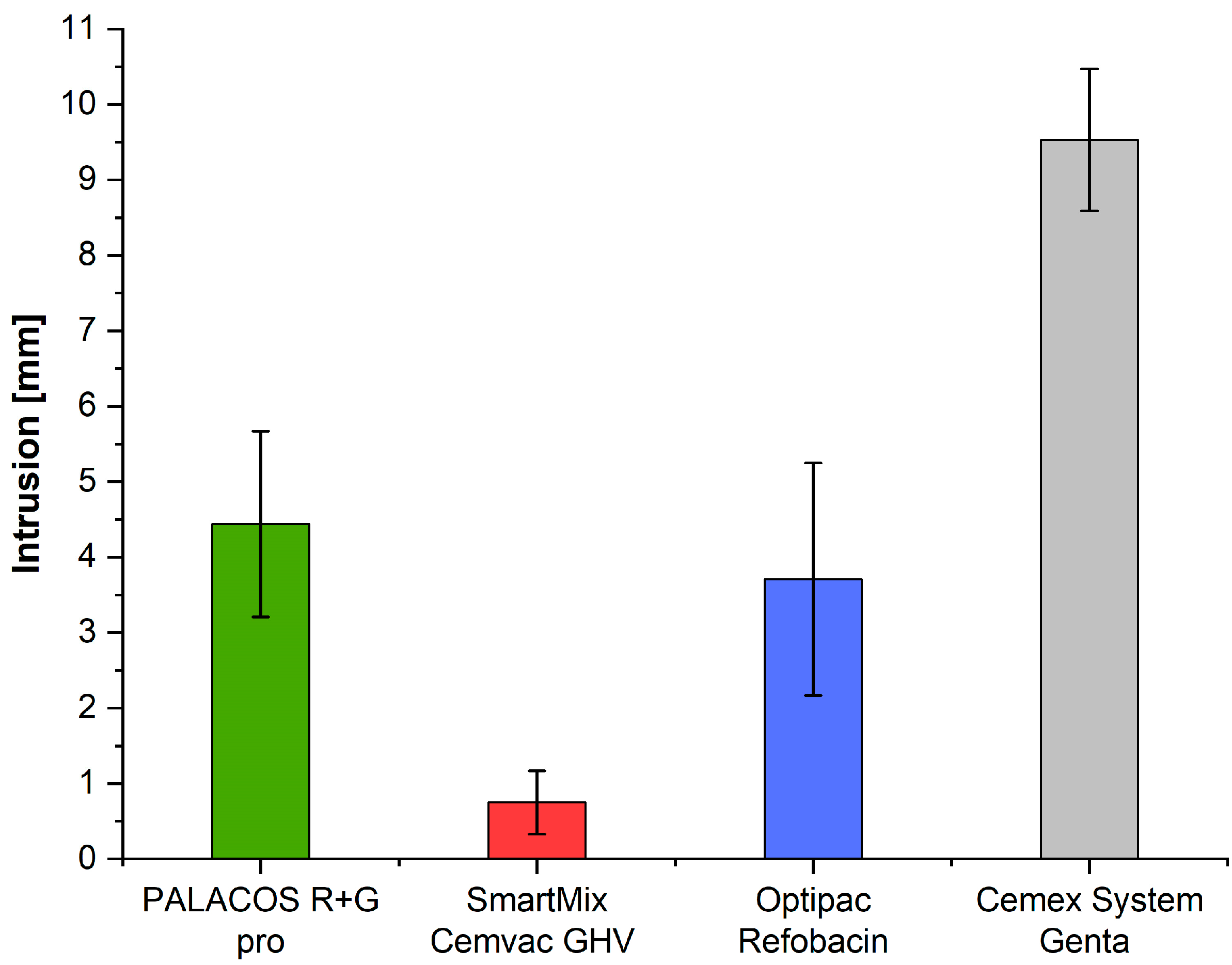
3.2. Intrusion (ISO 5833)
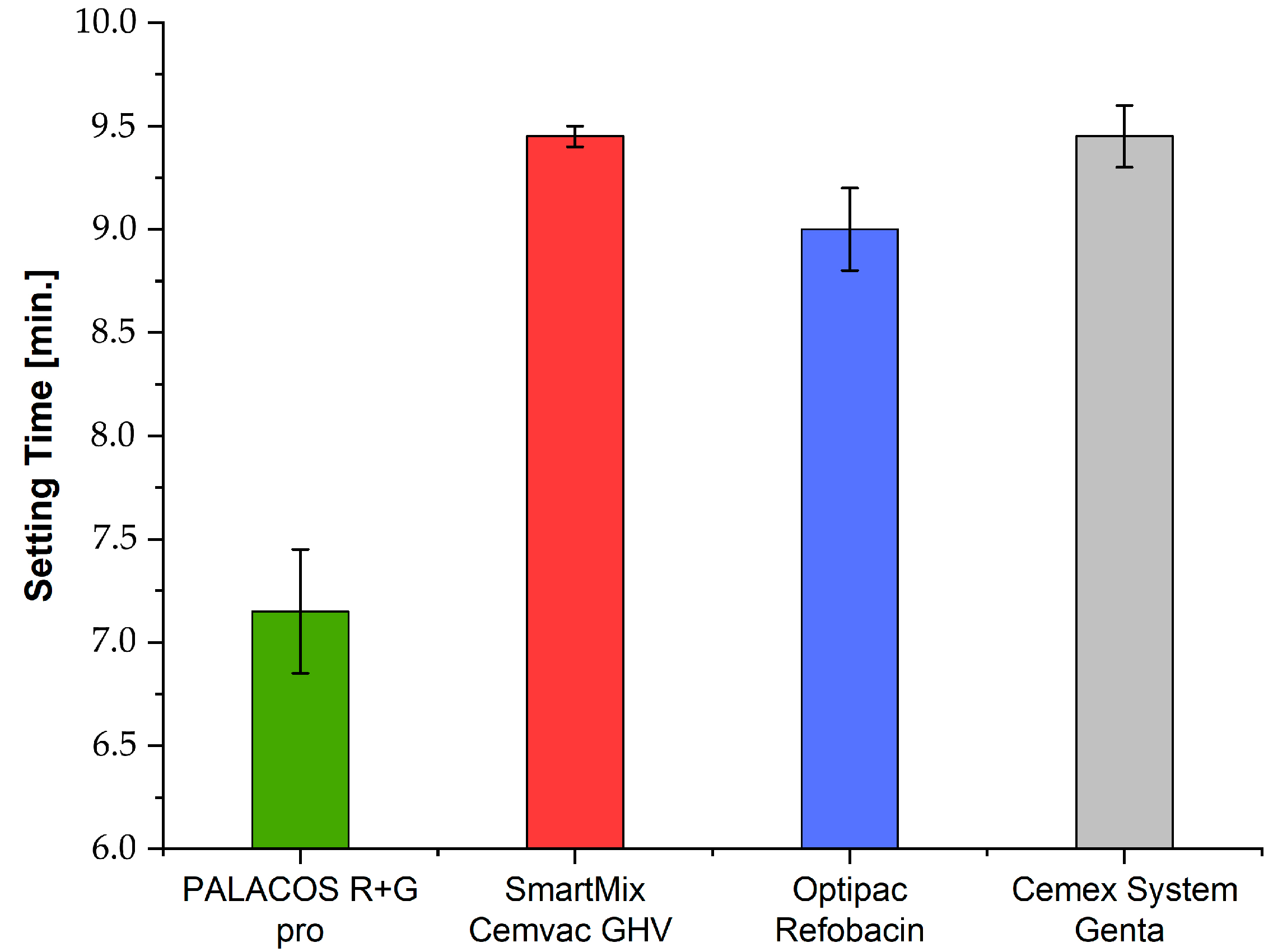
3.3. Setting Time (ISO 5833)
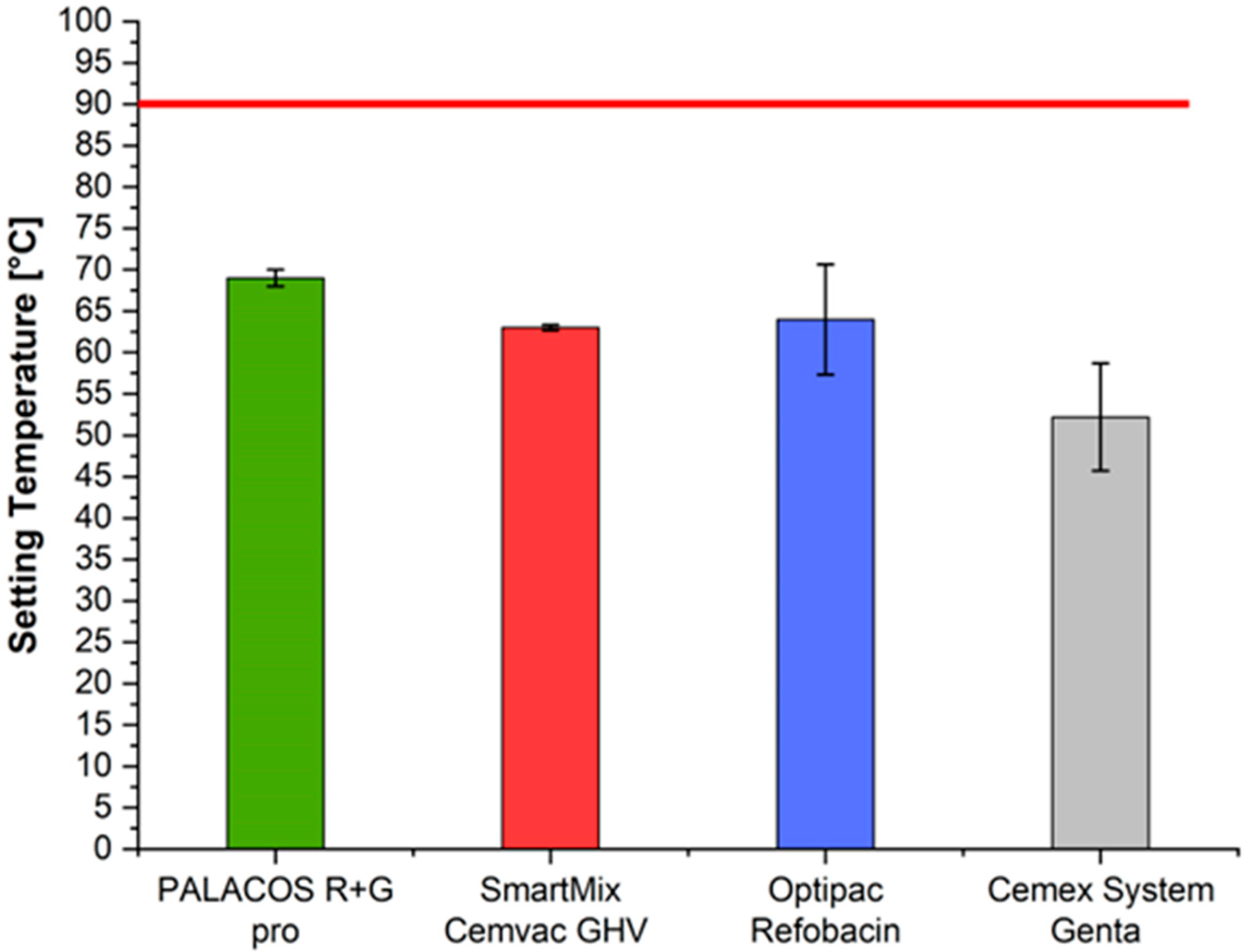
3.4. Setting Temperature (ISO 5833)
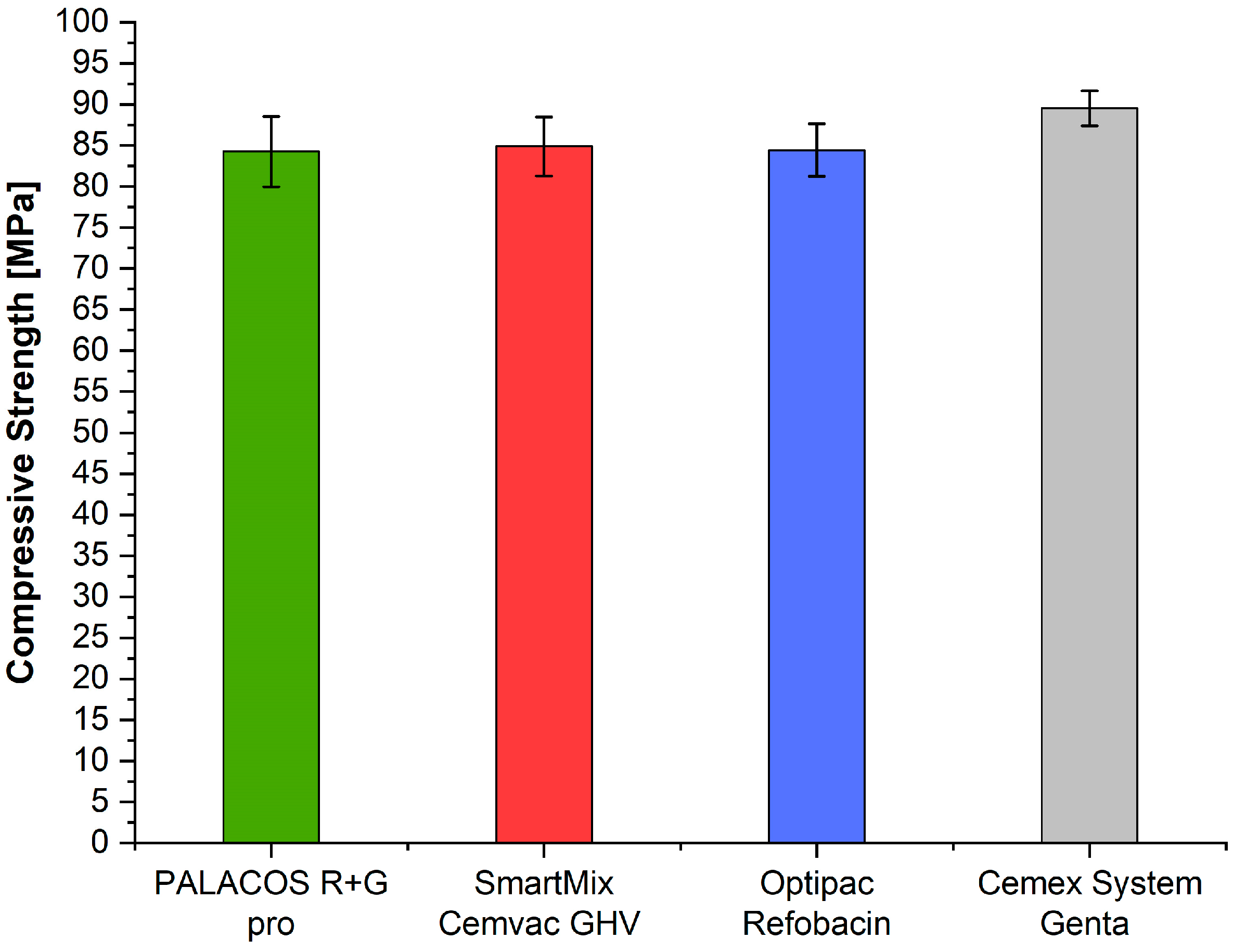
3.5. Compressive Strength (ISO 5833)
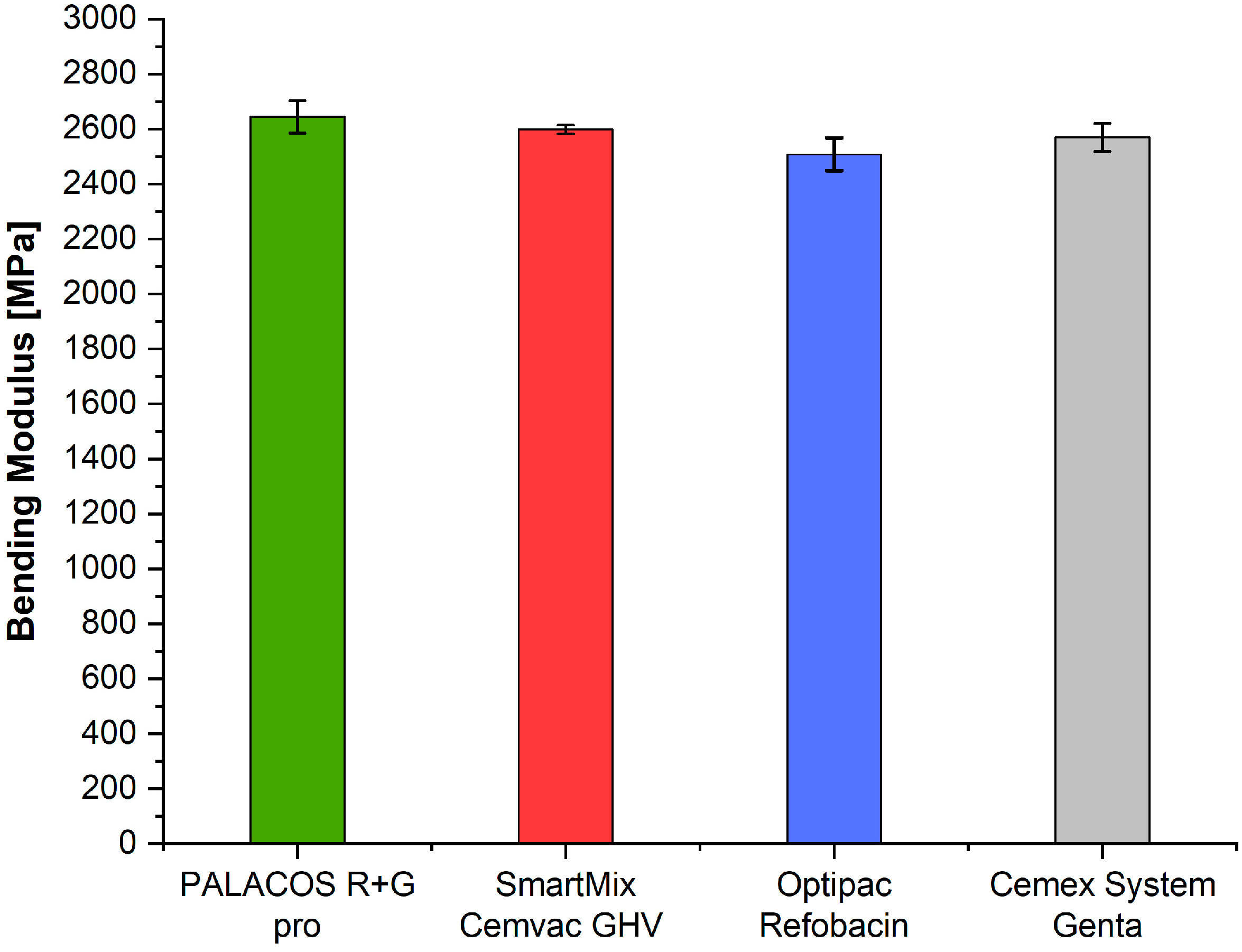
3.6. Bending Modulus (ISO 5833)
3.7. Bending Strength (ISO 5833)
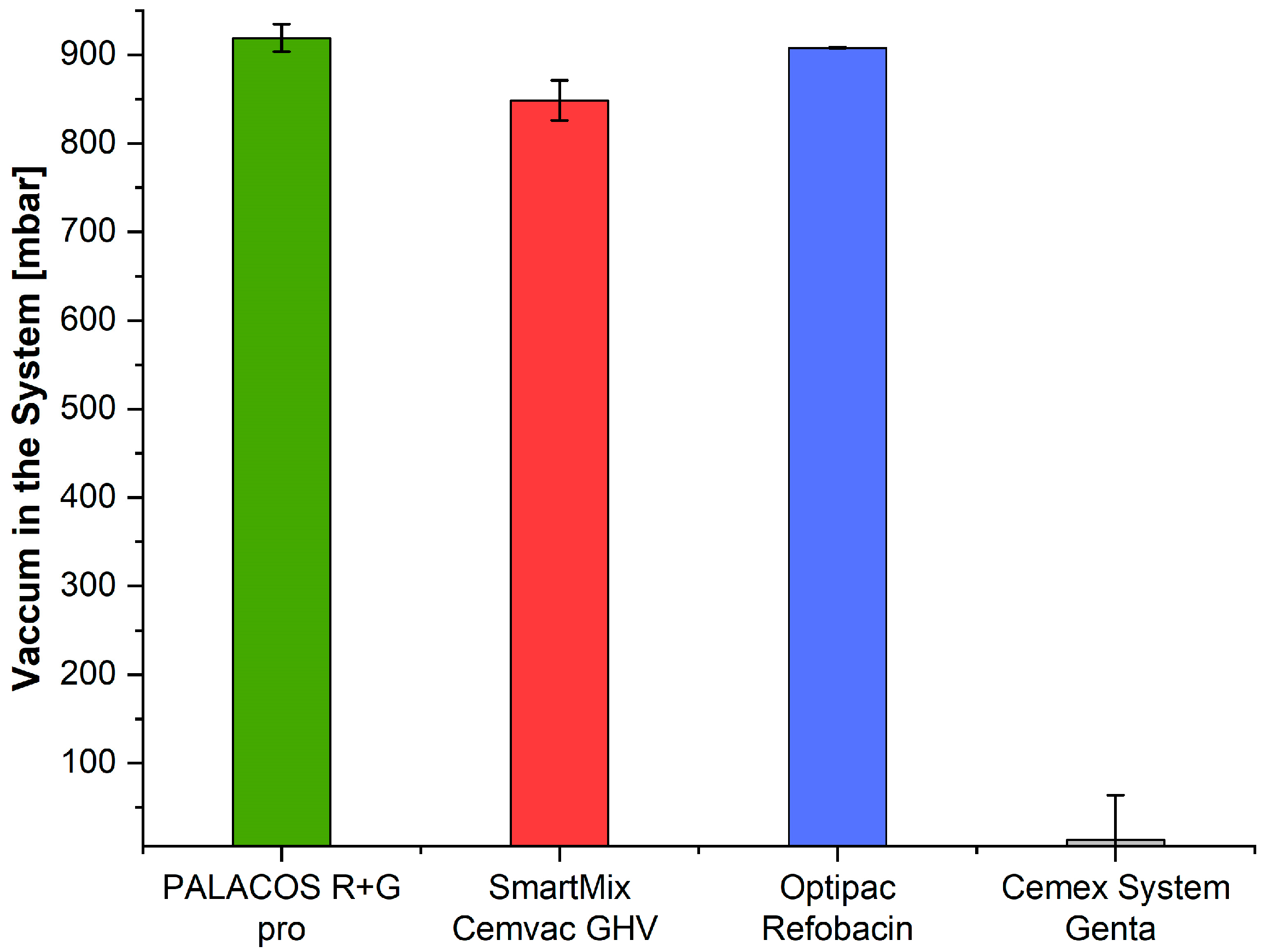
3.8. Vacuum Level
4. Discussion
4.1. System Design
4.2. Processing and Working Times
4.3. Intrusion Depth
4.4. Setting Temperature
4.5. Mechanical Properties
4.6. Vacuum Level
5. Conclusions
- Use safe, standard-compliant and approved systems.
- Use closed systems that are safe from a hygiene perspective.
- Closed prepacked systems are mainly recommended for primary surgery.
- When selecting systems, ensure that the cement contained is ready for use quickly (short doughing time) and has a comparatively fast setting time.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MCT | Modern Cementing Technique |
| PMMA | Polymethylmethacrylate |
| MMA | Methylmethacrylate |
| EO | Ethylene Oxide |
| ISO | International Organization for Standardization |
References
- Deb, S. Orthopaedic Bone Cements; Elsevier Science: Burlington, VT, USA; Cambridge UK, 2008; ISBN 978-1-84569-376-3. [Google Scholar]
- Kühn, K.-D. PMMA Cements: Are We Aware What We Are Using? Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-41535-7. [Google Scholar]
- Humez, M.; Fröschen, F.S.; Wirtz, D.C.; Kühn, K.-D. The third-generation modern cementing technique in hip and knee arthroplasty. Die Orthopädie 2023, 52, 968–980. [Google Scholar] [CrossRef]
- Humez, M.; Kötter, K.; Skripitz, R.; Kühn, K.-D. Evidence for cemented TKA and THA based on a comparison of international register data. Die Orthopädie 2024, 53, 597–607. [Google Scholar] [CrossRef]
- Kühn, K.-D. Bone Cements: Up-to-Date Comparison of Physical and Chemical Properties of Commercial Materials; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Bökeler, U.; Bühler, A.; Eschbach, D.; Ilies, C.; Liener, U.; Knauf, T. The Influence of a Modified 3rd Generation Cementation Technique and Vaccum Mixing of Bone Cement on the Bone Cement Implantation Syndrome (BCIS) in Geriatric Patients with Cemented Hemiarthroplasty for Femoral Neck Fractures. Medicina 2022, 58, 1587. [Google Scholar] [CrossRef] [PubMed]
- Lindén, U.; Gillquist, J. Air inclusion in bone cement. Importance of the mixing technique. Clin. Orthop. Relat. Res. 1989, 247, 148–151. [Google Scholar] [CrossRef]
- Evans, S.L. Effects of porosity on the fatigue performance of polymethyl methacrylate bone cement: An analytical investigation. Proc. Inst. Mech. Eng. H 2006, 220, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Geiger, M.H.; Keating, E.M.; Ritter, M.A.; Ginther, J.A.; Faris, P.M.; Meding, J.B. The clinical significance of vacuum mixing bone cement. Clin. Orthop. Relat. Res. 2001, 382, 258–266. [Google Scholar] [CrossRef]
- Breusch, S.; Malchau, H. The Well-Cemented Total Hip Arthroplasty: Theory and Practice; Springer: Berlin/Heidelberg, Germany, 2005; ISBN 978-3-540-28924-1. [Google Scholar]
- Karpiński, R.; Szabelski, J.; Maksymiuk, J. Seasoning Polymethyl Methacrylate (PMMA) Bone Cements with Incorrect Mix Ratio. Materials 2019, 12, 3073. [Google Scholar] [CrossRef] [PubMed]
- Dunne, N.J.; Orr, J.F. Influence of mixing techniques on the physical properties of acrylic bone cement. Biomaterials 2001, 22, 1819–1826. [Google Scholar] [CrossRef]
- Mulroy, R.D.; Harris, W.H. The effect of improved cementing techniques on component loosening in total hip replacement. An 11-year radiographic review. J. Bone Jt. Surg. Br. Vol. 1990, 72, 757–760. [Google Scholar] [CrossRef]
- Jamison, M.P.; Hunt, E.R.; West, M.C.; Chen, A.F. Bone Cement Fumes Generated in Laminar Airflow Versus Conventionally Ventilated Operating Rooms: Does the Mixing System Matter? J. Bone Jt. Surg. Am. 2023, 105, 1676–1685. [Google Scholar] [CrossRef]
- Karpiński, R.; Szabelski, J.; Maksymiuk, J. Effect of Physiological Fluids Contamination on Selected Mechanical Properties of Acrylate Bone Cement. Materials 2019, 12, 3963. [Google Scholar] [CrossRef]
- Schlegel, U.J.; Sturm, M.; Eysel, P.; Breusch, S.J. Pre-packed vacuum bone cement mixing systems. A further step in reducing methylmethacrylate exposure in surgery. Ann. Occup. Hyg. 2010, 54, 955–961. [Google Scholar] [CrossRef][Green Version]
- Quint, U.; Benen, T. Mögliche Instrumentenkontamination im Operationssaal während der Implantation von Hüft- und Kniegelenkendoprothesen. Z. Orthop. Unfall. 2016, 154, 157–162. [Google Scholar] [CrossRef]
- Wang, J.-S.; Toksvig-Larsen, S.; Müller-Wille, P.; Franzén, H. Is there any difference between vacuum mixing systems in reducing bone cement porosity? J. Biomed. Mater. Res. 1996, 33, 115–119. [Google Scholar] [CrossRef]
- Kock, H.-J.; Huber, F.-X.; Hillmeier, J.; Jäger, R.; Volkmann, R.; Handschin, A.E.; Letsch, R.; Meeder, P.-J. In vitro studies on various PMMA bone cements: A first comparison of new materials for arthroplasty. Z. Orthop. Unfall. 2008, 146, 108–113. [Google Scholar] [CrossRef]
- Verdonschot, N.; Huiskes, R. Creep properties of three low temperature-curing bone cements: A preclinical assessment. J. Biomed. Mater. Res. 2000, 53, 498–504. [Google Scholar] [CrossRef] [PubMed]
- ISO 5833; Implants for Surgery—Acrylic Resin Cements. Internationale Organisation für Normung: Geneva, Switzerland, 2002.
- Tecres, S.p.A. Cemex® System Genta. Available online: https://www.tecres.com/product_details/cemex-system-genta (accessed on 22 September 2024).
- Heraues Medical GmbH. PALACOS® R+G pro—All-in-One Fixation System™. Available online: https://www.heraeus-medical.com/de/healthcare-professionals/products/palacos-rg-pro/ (accessed on 22 September 2024).
- Zimmer Biomet. Optipac® Vacuum Mixing System. Available online: https://www.zimmerbiomet.eu/en/products/optipac-vacuum-mixing-system (accessed on 22 September 2024).
- Johnson & Johnson Med Tech. SmartMix CemVac Zement. Available online: https://www.jnjmedtech.com/de-DE/service-details/zement (accessed on 22 September 2024).
- Jelecevic, J.; Maidanjuk, S.; Leithner, A.; Loewe, K.; Kuehn, K.-D. Methyl methacrylate levels in orthopedic surgery: Comparison of two conventional vacuum mixing systems. Ann. Occup. Hyg. 2014, 58, 493–500. [Google Scholar] [CrossRef] [PubMed][Green Version]
- James, S.P.; Jasty, M.; Davies, J.; Piehler, H.; Harris, W.H. A fractographic investigation of PMMA bone cement focusing on the relationship between porosity reduction and increased fatigue life. J. Biomed. Mater. Res. 1992, 26, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Breusch, S.J.; Lukoschek, M.; Schneider, U.; Ewerbeck, V. “State of the art” in cemented hip arthroplasty: The quality of the cementing technique is crucial. Dtsch Arztebl 2000, 97, A-2030–2033 [Heft 30]. [Google Scholar]
- Fölsch, C.; Schirmer, J.; Glameanu, C.; Ishaque, B.; Fonseca Ulloa, C.A.; Harz, T.; Rickert, M.; Martin, J.R.; Scherberich, J.; Steinbart, J.; et al. Cement Viscosity and Application Time Lead to Significant Changes in Cement Penetration and Contact Surface Area. Arthroplast. Today 2024, 30, 101476. [Google Scholar] [CrossRef]
- Malchau, H.; Herberts, P.; Eisler, T.; Garellick, G.; Söderman, P. The Swedish Total Hip Replacement Register. J. Bone Jt. Surg. Am. 2002, 84 (Suppl. 2), 2–20. [Google Scholar] [CrossRef]
- Szoradi, G.T.; Feier, A.M.; Zuh, S.G.; Russu, O.M.; Pop, T.S. Polymethyl Methacrylate Bone Cement Polymerization Induced Thermal Necrosis at the Cement–Bone Interface: A Narrative Review. Appl. Sci. 2024, 14, 11651. [Google Scholar] [CrossRef]
- Biehl, G.; Harms, J.; Hanser, U. Experimental studies on heat development in bone during polymerization of bone cement. Intraoperative measurement of temperature in normal blood circulation and in bloodlessness. Arch. Orthop. Unfallchir. 1974, 78, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Humez, M.; Domann, E.; Thormann, K.M.; Fölsch, C.; Strathausen, R.; Vogt, S.; Alt, V.; Kühn, K.-D. Daptomycin-Impregnated PMMA Cement against Vancomycin-Resistant Germs: Dosage, Handling, Elution, Mechanical Stability, and Effectiveness. Antibiotics 2023, 12, 1567. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.W.; Abramowicz, M.; Kühn, K.D.; Foelsch, C.; Hansen, E.N. High and Low Dosage of Vancomycin in Polymethylmethacrylate Cements: Efficacy and Mechanical Properties. Antibiotics 2024, 13, 818. [Google Scholar] [CrossRef]
- Dunne, N.; Buchanan, F.; Hill, J.; Newe, C.; Tunney, M.; Brady, A.; Walker, G. In vitro testing of chitosan in gentamicin-loaded bone cement: No antimicrobial effect and reduced mechanical performance. Acta Orthop. 2008, 79, 851–860. [Google Scholar] [CrossRef]
- Al Thaher, Y.; Yang, L.; Jones, S.A.; Perni, S.; Prokopovich, P. LbL-assembled gentamicin delivery system for PMMA bone cements to prolong antimicrobial activity. PLoS ONE 2018, 13, e0207753. [Google Scholar] [CrossRef]
- Lewis, G.; Mladsi, S. Correlation between impact strength and fracture toughness of PMMA-based bone cements. Biomaterials 2000, 21, 775–781. [Google Scholar] [CrossRef]
- Karpiński, R.; Szabelski, J.; Krakowski, P.; Jonak, J.; Falkowicz, K.; Jojczuk, M.; Nogalski, A.; Przekora, A. Effect of various admixtures on selected mechanical properties of medium viscosity bone cements: Part 1, Part 2 and Part 3. Compos. Struct. 2024, 343, 118306. [Google Scholar] [CrossRef]
- Eyerer, P.; Jin, R. Influence of the Mixing Technique on the Properties of PMMA Bone Cement Part 2: Analysis of the Mixing Procedure. Biomed. Tech. Biomed. Eng. 1986, 31, 11–18. [Google Scholar] [CrossRef]
- Paul, C.; Kühn, K.-D. Chemical and physical properties of PMMA bone cements. Die Orthopädie 2023, 52, 943–956. [Google Scholar] [CrossRef] [PubMed]



| Test Method | Sample Number | Cemex® System Genta | Palacos® R+G Pro | Optipac® Refobacin | SmartMix™ Cemvac™ GHV | F-Statistic | p-Value |
|---|---|---|---|---|---|---|---|
| Visual inspection | − | + | + | + | + | − | − |
| ISO doughing time [s] | n = 2 | 225.0 | 100.0 | 215.0 | 162.5 | 234.19 | 0.0001 |
| ISO intrusion [mm] | n = 4 | 9.53 | 4.44 | 3.71 | 0.75 | 42.84 | 0.0000 |
| ISO setting time [s] | n = 2 | 582.5 | 440.0 | 535.0 | 587.5 | 48.28 | 0.0013 |
| ISO maximum temperature [°C] | n = 2 | 52.20 | 70.95 | 64.18 | 63.45 | 5.46 | 0.0672 |
| ISO compressive strength [MPa]) | n = 5 | 89.5 | 84.2 | 84.4 | 84.9 | 2.77 | 0.0755 |
| ISO bending modulus [MPa] | n = 5 | 2569.2 | 2644.0 | 2508.2 | 2598.8 | 6.52 | 0.0044 |
| ISO bending strength [MPa] | n = 5 | 64.8 | 65.3 | 63.3 | 63.4 | 1.82 | 0.1843 |
| Internal pressure during mixing [mbar] | n = 3 | 999.5 | 94.0 | 164.5 | 105.0 | 1420.41 | 0.0000 |
| Test Method | Significant Group Difference Comparisons (p < 0.05) |
|---|---|
| ISO doughing time | Cemex–Palacos (p = 0.0001), Cemex–SmartMix (p = 0.0010), Optipac–Palacos (p = 0.0001), Optipac–SmartMix (p = 0.0020), Palacos–SmartMix (p = 0.0010) |
| ISO intrusion | Cemex–Optipac (p = 0.0000), Cemex–Palacos (p = 0.0002), Cemex–SmartMix (p = 0.0000), Optipac–SmartMix (p = 0.0128), Palacos–SmartMix (p = 0.0026) |
| ISO setting time | Cemex–Palacos (p = 0.0018), Optipac–Palacos (p = 0.0083), Palacos–SmartMix (p = 0.0016) |
| ISO maximum temperature | None—(all p > 0.05) |
| ISO compressive strength | None—(all p > 0.05) |
| ISO bending modulus | Optipac–Palacos (p = 0.0028), Optipac–SmartMix (p = 0.0489) |
| ISO bending strength | None—(all p > 0.05) |
| Internal pressure during mixing | Cemex–Optipac (p = 0.0000), Cemex–Palacos (p = 0.0000), Cemex–SmartMix (p = 0.0000), Optipac–Palacos (p = 0.0117), Optipac–SmartMix (p = 0.0286) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, C.; Ruiz, P.S.; Zeneli, M.; Kühn, K.-D. Evaluation of Prepacked Bone Cement Mixing Systems in Arthroplasty: Implications for Intraoperative Hygiene and Contamination Risk. Hygiene 2025, 5, 40. https://doi.org/10.3390/hygiene5030040
Paul C, Ruiz PS, Zeneli M, Kühn K-D. Evaluation of Prepacked Bone Cement Mixing Systems in Arthroplasty: Implications for Intraoperative Hygiene and Contamination Risk. Hygiene. 2025; 5(3):40. https://doi.org/10.3390/hygiene5030040
Chicago/Turabian StylePaul, Christian, Pablo Sanz Ruiz, Muhamed Zeneli, and Klaus-Dieter Kühn. 2025. "Evaluation of Prepacked Bone Cement Mixing Systems in Arthroplasty: Implications for Intraoperative Hygiene and Contamination Risk" Hygiene 5, no. 3: 40. https://doi.org/10.3390/hygiene5030040
APA StylePaul, C., Ruiz, P. S., Zeneli, M., & Kühn, K.-D. (2025). Evaluation of Prepacked Bone Cement Mixing Systems in Arthroplasty: Implications for Intraoperative Hygiene and Contamination Risk. Hygiene, 5(3), 40. https://doi.org/10.3390/hygiene5030040







