Bacterial Contamination of Antiseptics, Disinfectants and Hand Hygiene Products in Healthcare Facilities in High-Income Countries: A Scoping Review
Abstract
1. Introduction
2. Objective, Focus and Scope of this Review
3. Materials and Methods
3.1. Terms and Definitions
- 1.
- Antiseptics: Antiseptics are used to prepare the skin or mucosa for invasive procedures and surgery, surgical hand preparation, oral care for intubated patients and (in community settings with high neonatal mortality) umbilical care in newborns and topical wound care [7,28,29,30,31,32], e.g., alcohol, chlorhexidine and iodine compounds.
- 2.
- Disinfectants: products that inactivate microorganisms or inhibit their growth and are applied to inanimate objects and surfaces [42]
- ○
- Disinfectants with high-level effectiveness inactivate all microorganisms except bacterial spores. Examples are glutaraldehyde and ortho-phthalaldehyde, used for disinfection of endoscopes [30]; they are not subject to this review.
- ○
- Low-level disinfectants act on most bacteria and some viruses and fungi, but most have no activity on mycobacteria and spores (Table 1). Examples are sodium hypochlorite, quaternary ammonium compounds and chloroxylenol. They are used for:
- 3.
- Products used for Hand Hygiene:
- 3.1.
- Alcohol-based handrub: an alcohol-containing product that inactivates microorganisms or inhibits their growth. It is applied to the hands without use of water; after application, hands are rubbed until they are dry. Water and towels to dry the hands are not needed [7].
- ○
- ○
- Alcohol-based handrub is available as a liquid (solution), gel or foam [7].
- ○
- Alcohol-based handrub can be combined with antiseptics such as chlorhexidine (0.5–1%) [7].
- ○
- ○
- The term Hand Sanitizer is a general term referring to alcohol-based handrub used in the community setting [47]. It is not used in this review.
- 3.2.
- Soaps: although soaps, strictly defined, are naturally occurring (anionic) detergents, the term “soap” in this review indicates both natural and synthetic detergents.
- ○
- Detergents are surfactants i.e., products that allow suspension of fats in water. Detergents may be anionic, cationic, amphoteric or nonionic, i.e., having positive, negative, both positive and negative, or no electrical charge [48].
- ○
- Cationic detergents have antimicrobial activity (e.g., quaternary ammonium compounds).
- ○
- Soaps can contain antiseptic agents (antiseptic soap, antimicrobial soap, medicated soap) or not (plain soap, unmedicated or nonmedicated soap).
- ○
- ○
- ○
- Some soaps contain antiseptics with a sustained activity (synonym: residual, remnant) effect, i.e., they have an effect that extends beyond the application, e.g., chlorhexidine.
| Product | Characteristics and Indications |
|---|---|
| Products for hand hygiene | |
| Alcohol-based handrub Ethanol 80% vol/vol * Isopropyl alcohol 75% vol/vol * | Procured as ready-to-use products or diluted with water (from a 96% solution) See also Box 1 Formulations combined with chlorhexidine or quaternary ammonium compounds are available |
| Antiseptic soap | Examples of antiseptics added to soap are (concentrations according to reference (WHO, 2009):
|
| Antiseptics | |
| Chlorhexidine (Chlorhexidine digluconate: CHG) 5% digluconate solution for dilution * Class: biguanides Examples: Hibiclens, Hibiscrub, Hibitane | Dilutions made in water or alcohol: 0.5% up to 4% Has detergent activity and residual activity (4–6 h) Good activity against Gram-positives Gram-negatives may be intrinsically resistant Vulnerable to contamination with Gram-negative bacteria |
| Ethanol and Isopropyl-alcohol (60–90%) | Ethanol 70% (denatured) solution * |
| Povidone iodine 7.5–10% * solution (water-based) Class: iodophors Example: Betadine | 10% povidone iodine is equivalent to 1% available iodine Procured as ready-to-use product, water-based Can be applied on intact skin but also on mucosa and wounds Note: Iodophors have largely replaced 1% iodine in alcohol (iodine tincture) (Weber et al., 2007; WHO, 2018a) |
| Cetrimide, cetrimonium Class: Quaternary Ammonium Compounds | Used as antiseptic soap or as water-based solution Procured as ready-to-use product or product for dilution See disinfectants |
| Chloramine Class: Chlorine compounds Example: Dakin, a stabilized chlorine product | Product which provides slow release of chlorine Less irritating and longer acting than chlorine compounds (see Disinfectants) Mostly used in French-speaking countries |
| Low-level disinfectants (note chlorine is sometimes categorized as an intermediate-level disinfectant) | |
| Chlorine (sodium hypochlorite) Powder 0.1% concentration of available chlorine * Household Bleach (concentration 5%) Eau de Javel (concentration 8–15°) Sodium dichloroisocyanurate granules Calcium hypochlorite tabs Chloramine-T tablets Class: Chlorine compounds | Chlorine solutions are made in water by dilution up to 0.5% or 1.0% final concentration of hypochloric acid (HOCl) Household bleach contains 5.25–6.15% sodium hypochlorite Eau de Javel mostly used in French speaking countries (1° (“degree chlorométrique”) equals 0.317% HOCl) Chloramine-T releases chlorine very slowly, resulting in a more prolonged effect, is (also) used as antiseptic |
| Chloroxylenol * Therapeutic alternatives: 4th level ATC chemical subgroup (D08AE Phenol and derivatives) PCMX (para-chloro-meta-xylenol) solution 4.8% DCMX (Dichloro-meta-xylenol) solution 2.5% Class: PhenolicsExample: Dettol | Ready-to-use product, water-based Residual activity Products are diluted in water Also marketed as product for hand hygiene (water-based) and antiseptic (wound cleansing) |
| Cetrimide (Cetrimonium bromide) Didecyl dimethyl ammonium bromide Dioctyl dimethyl ammonium bromide Benzalkonium chloride Class: Quaternary Ammonium Compounds (QUAT) Example: Zephiran | Water-based, procured ready-to-use or product for dilution Detergent activity High water hardness, cotton and gauze diminish activity Most active against Gram-positives. Some Gram-negatives (e.g., Pseudomonas aeruginosa) are intrinsically resistant Vulnerable to contamination with Gram-negatives, in particular Benzalkonium chloride Formulations combining QUAT and chlorhexidine: Examples: Savlon, HAC (hospital antiseptic concentrate) |
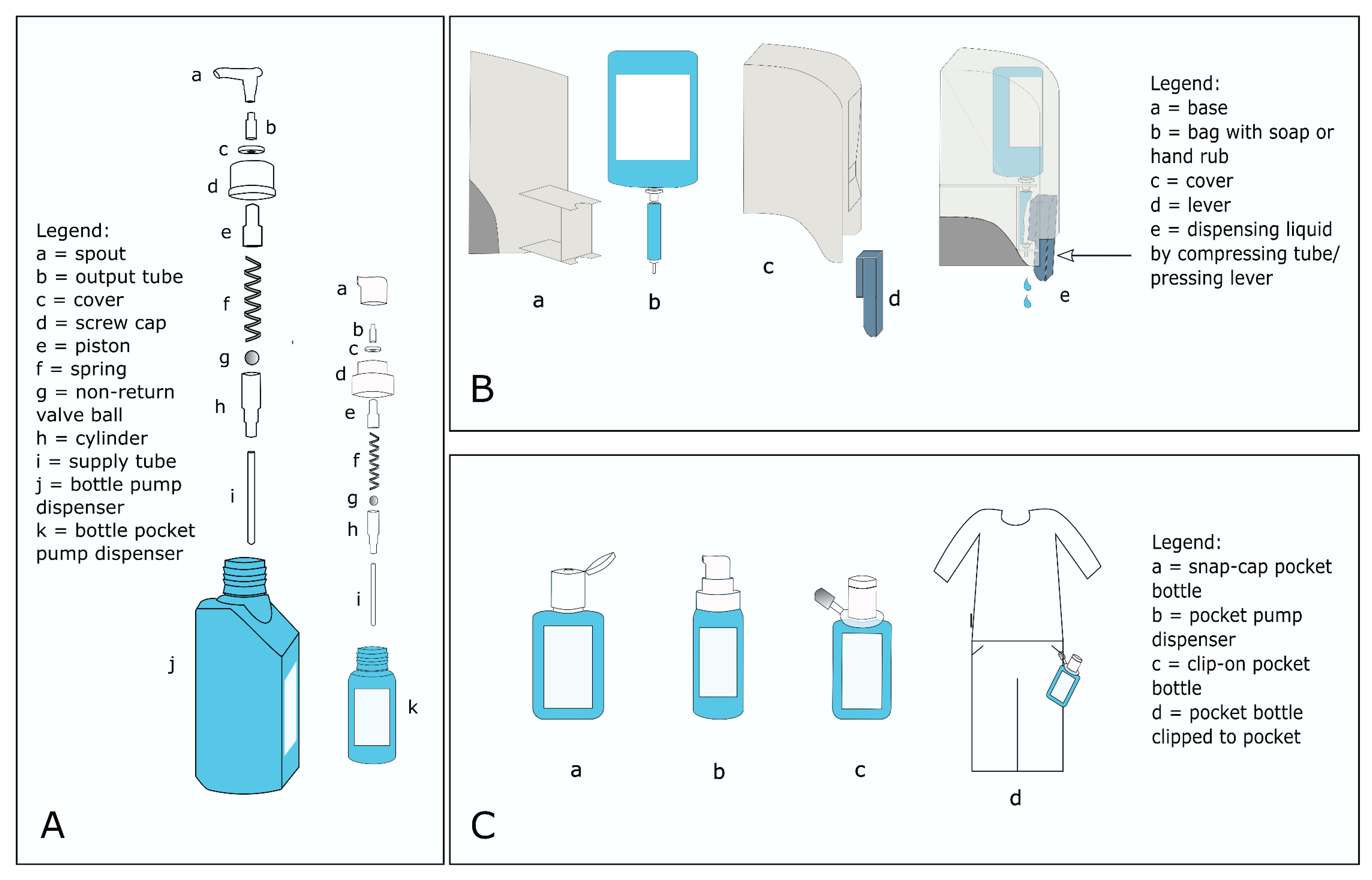
3.2. Search Strategy
3.3. Data Extraction
4. Results and Discussion
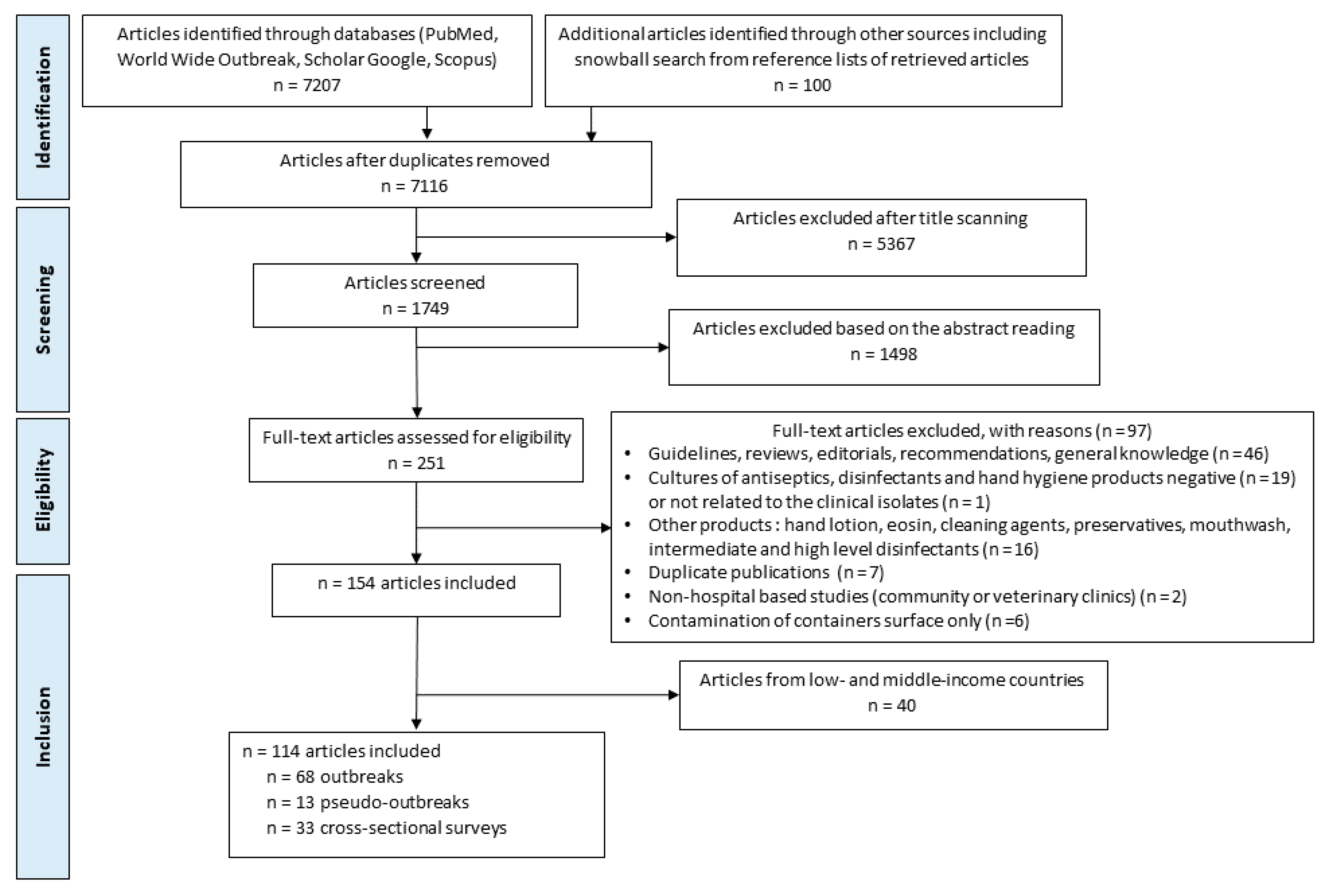
4.1. (Pseudo-) Outbreak Reports and Cross-Sectional Surveys: Overview
| United Nations Geoscheme/Countries | Cross-Sectional | Outbreak | Pseudo-Outbreak | Total |
|---|---|---|---|---|
| Northern Europe | 9 | 7 | - | 16 |
| Iceland | 1 | - | - | 1 |
| Norway | 1 | - | - | 1 |
| United Kingdom of Great Britain and Northern Ireland | 7 | 7 | - | 14 |
| Western Europe | 8 | 8 | 4 | 20 |
| Belgium | 1 | - | 1 | 2 |
| France | - | 8 | 2 | 10 |
| Germany | 6 | - | 1 | 7 |
| Switzerland | 1 | - | - | 1 |
| Southern Europe | 2 | 13 | - | 15 |
| Italy | 1 | 4 | - | 5 |
| Spain a | 1 | 9 | - | 10 |
| North America | 7 | 26 | 3 | 36 |
| Canada | 1 | 2 | - | 3 |
| United States of America a | 6 | 24 | 3 | 33 |
| Latin America and the Caribbean | - | 1 | - | 1 |
| Chile | - | 1 | - | 1 |
| Oceania: Australia and New Zealand | 4 | 4 | 1 | 9 |
| Australia | 3 | 3 | 1 | 7 |
| New Zealand | 1 | 1 | - | 2 |
| Eastern Asia | 3 | 7 | 3 | 13 |
| Hong Kong, Special Administrative Region, China | - | 1 | - | 1 |
| Japan | 3 | 3 | 1 | 7 |
| Republic of Korea | - | 3 | 2 | 5 |
| Taiwan b | - | - | 1 | 1 |
| Western Asia | - | 2 | 1 | 3 |
| Cyprus | - | 1 | - | 1 |
| Israel | - | 1 | 1 | 2 |
| Total | 33 | 68 | 13 | 114 |
| Decades | 1950s | 1960s | 1970s | 1980s | 1990s | 2000s | 2010s | 2020s | Total Outbreak/Pseudo-Outbreak |
|---|---|---|---|---|---|---|---|---|---|
| Outbreaks and pseudo-outbreaks (n = 81) | 2/0 | 5/0 | 12/1 | 10/5 | 7/1 | 16/3 | 14/3 | 2/0 | 68/13 |
| Alcohol | - | - | - | 1 | 1 | - | 2 | - | 2/2 |
| CHG | - | 2 | 4 | 6 | 2 | 6 | 9 | 2 | 26/5 |
| QUAT | 2 | 3 | 4 | 3 | 2 | 6 | 4 | - | 20/4 |
| CHG–QUAT | - | - | 4 | - | - | - | - | - | 4/0 |
| Iodophor | - | - | - | 4 | 1 | - | - | - | 3/2 |
| Phenol | - | - | 1 | 1 | - | - | - | - | 2/0 |
| Liquid Soap a | - | - | - | - | 2 | 7 | 2 | - | 11/0 |
| Cross-sectional surveys (n = 33) | 2 | 5 | 7 | 6 | 2 | 2 | 8 | 1 | 33 |
| CHG | - | 1 | - | 2 | - | - | - | - | 3 |
| QUAT | 1 | 1 | 2 | 1 | 2 | - | 3 | 1 | 11 |
| Phenol b | - | 2 | 3 | 1 | - | - | - | - | 6 |
| Liquid Soap c | 1 | - | 1 | 2 | - | 2 | 4 | - | 5/1/4 c |
| Bar Soap | - | 1 | 1 | 1 | - | - | - | - | 3 |
4.2. Products Involved
4.3. Epidemic and Microbiological Methods Used
4.4. Microorganisms Involved
| Contaminating Bacteria | Alcohol | CHG | QUAT | CHG–QUAT | Iodophor | Phenol | Liquid Soap a | TotalOutbreak/Pseudo-Outbreak |
|---|---|---|---|---|---|---|---|---|
| Enterobacterales | - | 9 | 7 | - | - | - | 8 | 21/3 |
| Serratia spp. b | - | 9 | 4 | - | - | - | 8 | 20/1 |
| Enterobacter cloacae | - | - | 2 | - | - | - | - | 1/1 |
| Pantoea agglomerans | - | - | 1 | - | - | - | - | 0/1 |
| Nonfermentative Gram-negative rods | 1 | 28 | 28 | 4 | 5 | 2 | 5 | 56/17 |
| Burkholderia cepacia complex c | - | 14 | 10 | 3 | 3 | 1 | 1 | 24/8 |
| Achromobacter spp. d | - | 6 | 6 | - | - | - | - | 10/2 |
| Pseudomonas aeruginosa | - | 1 | 5 | - | 2 | 1 | 3 | 11/1 |
| Ralstonia pickettii | - | 4 | - | - | - | - | - | 2/2 |
| Pseudomonas spp. | - | 2 | 1 | - | - | - | - | 3/0 |
| Pseudomonas fluorescens | 1 | - | 2 | - | - | - | - | 2/1 |
| Stenotrophomonas maltophilia | - | - | 1 | 1 | - | - | - | 1/1 |
| Comamonas testosteroni | - | - | 1 | - | - | - | - | 0/1 |
| Elizabethkingia meningoseptica | - | 1 | - | - | - | - | - | 1/0 |
| Pseudomonas putida | - | - | 1 | - | - | - | - | 1/0 |
| Pseudomonas stutzeri | - | - | - | - | - | - | 1 | 1/0 |
| Sphingomonas paucimobilis | - | - | 1 | - | - | - | - | 0/1 |
| Gram-positive rods | 6 | - | - | - | - | - | - | 4/2 |
| Bacillus cereus | 3 | - | - | - | - | - | - | 2/1 |
| Bacillus spp. | 3 | - | - | - | - | - | - | 2/1 |
| Mycobacterium | - | - | 2 | - | - | - | - | 2/0 |
| Mycobacterium abscessus | - | - | 2 | - | - | - | - | 2/0 |
| Total | 7 | 37 | 37 | 4 | 5 | 2 | 13 | 83/22 |
| Contaminating Bacteria | CHG Aqueous/Alcohol | QUAT/CHG–QUAT | Iodophor/ Iodine Tincture | Phenol a | Liquid Soap Antiseptic/Plain/ No Information | Bar Soap Antiseptic/ Plain | Total |
|---|---|---|---|---|---|---|---|
| Enterobacterales | 1/0 | 3/0 | 5 | 6/1/1 | 2/3 | 22 | |
| Enterobacter spp. | - | - | - | - | 1 | - | 1 |
| Escherichia coli | - | - | - | 2 | 1 | 2 | 5 |
| Klebsiella spp. b | - | - | - | 1 | 5 | 2 | 8 |
| Serratia spp. c | 1 | 2 | - | 2 | 1 | 1 | 7 |
| Non-lactose-fermenting coliforms | - | 1 | - | - | - | - | 1 |
| Nonfermentative Gram-negative rods | 4/0 | 20/1 | - | 9 | 2/1/2 | 5/1 | 45 |
| Achromobacter spp. d | - | 5 | - | 1 | - | - | 6 |
| Acinetobacter calcoaceticus | - | - | - | - | - | 1 | 1 |
| Aeromonas spp. | - | 1 | - | - | - | - | 1 |
| Burkholderia cepacia complex | 1 | 3 | - | - | 2 | - | 6 |
| Flavobacterium spp. | 1 | - | - | - | - | 1 | 2 |
| Myroides odoratus | - | - | - | - | - | 1 | 1 |
| Pseudomonas aeruginosa | - | 4 | - | 4 | 3 | 1 | 12 |
| Pseudomonas spp. e | 2 | 5 | - | 3 | - | 2 | 12 |
| Stenotrophomonas maltophilia | - | 1 | - | - | - | - | 1 |
| Others | - | 2 | - | 1 | - | - | 3 |
| Gram-positive bacteria f | 0/1 | 0/1 | 2/2 | - | 9/0/0 | 7/7 | 29 |
| CNS/Micrococcus spp. g | 1 | 1 | 4 | - | 4 | 5 | 15 |
| Staphylococcus aureus | - | - | - | - | 2 | 2 | 4 |
| Gram-positive rods h | - | - | - | - | 3 | 7 | 10 |
| Yeast | - | - | - | - | 1/0/0 | - | 1 |
| Candida parapsilosis | - | - | - | - | 1/0/0 | - | 1 |
| Total | 5/1 | 23/2 | 2/2 | 14 | 18/2/3 | 14/11 | 97 |
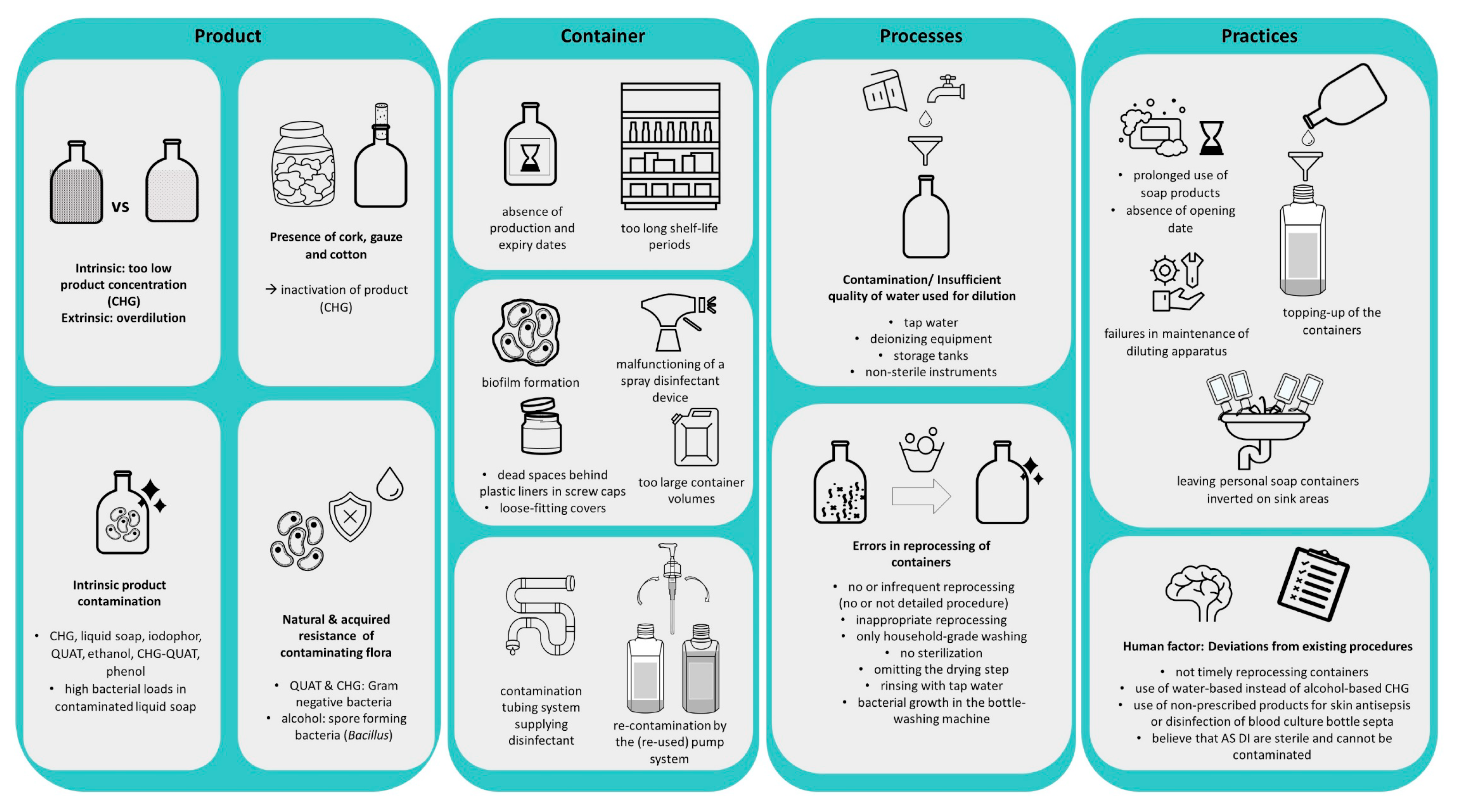
4.5. Factors Associated with Contamination
4.6. Attribution and Transmission
4.7. Interventions
4.8. Outstanding Issues, Research Questions and Recommendations
| Section | Outstanding Issues, Research Questions and Recommendations |
|---|---|
| Setting and overview of studies | What is the risk and impact of contamination of AS, DI and HH products? How to monitor frequency and characteristics?
|
| Products involved and assessed | Which products are most vulnerable to contamination? What is the actual risk for contamination of AS, DI and HH products during use?
|
| Epidemiological and microbiological methods used | Need to adhere to ORION guidelines [112,218] Need for additional guidance and criteria: environmental investigation
|
| Microorganisms, antimicrobial resistance and typing | Need for accelerated/feasible phenotypic identification/typing of environmental bacteria [198]
|
| Factors associated with contamination | Which type of container provides the best mitigation of contamination? [145,199]
Which quality/grade is needed for water and how can it be assured? [7] What is the role of single-dose containers?
|
| Attribution and transmission | How to optimize (pseudo-)outbreak investigations
|
| Interventions | Are active surveillance cultures of AS, DI and HH products useful?
|
4.9. Limitations and Strengths, Generalizability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APIC | Association for Professionals in Infection Control and Epidemiology |
| AS | Antiseptics |
| CDC | Center for Diseases Control |
| CFU/mL | Colony-Forming Unit per milliliter |
| CHG | Chlorhexidine gluconate |
| DI | Disinfectants |
| EML | Model List of Essential Medicines |
| EQUATOR | Enhancing the QUAlity and Transparency Of health Research |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| FDA | Food and Drug Administration |
| HH | Hand hygiene |
| HIC | High-income countries |
| IPC | Infection Prevention and Control |
| LMIC | Low- and middle-income countries |
| ORION | Outbreak Reports and Intervention studies Of Nosocomial infection |
| PCMX | Chloroxylenol |
| QUAT | Quaternary ammonium compounds |
References
- World Health Organization (WHO). Member States Information Session on Infection Prevention and Control (IPC). Available online: https://apps.who.int/iris/handle/10665/80135 (accessed on 28 August 2022).
- Boyce, J.M. Modern Technologies for Improving Cleaning and Disinfection of Environmental Surfaces in Hospitals. Antimicrob. Resist. Infect. Control 2016, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Chemaly, R.F.; Ghantoji, S.S.; Simmons, S.; Dale, C.; Rodriguez, M.; Gubb, J.; Stachowiak, J.; Stibich, M. The Role of the Healthcare Environment in the Spread of Multidrug-Resistant Organisms: Update on Current Best Practices for Containment. Ther. Adv. Infect. Dis. 2014, 2, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, H.; Rutala, W.A.; Weber, D.J. The Role of Patient Care Items as a Fomite in Healthcare-Associated Outbreaks and Infection Prevention. Clin. Infect. Dis. 2017, 65, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. Best Practices for Disinfection of Noncritical Environmental Surfaces and Equipment in Health Care Facilities: A Bundle Approach. Am. J. Infect. Control 2019, 47, A96–A105. [Google Scholar] [CrossRef] [PubMed]
- Otter, J.A.; Yezli, S.; French, G.L. The Role Played by Contaminated Surfaces in the Transmission of Nosocomial Pathogens. Infect. Control Hosp. Epidemiol. 2011, 32, 687–699. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Guidelines on Hand Hygiene in Health Care. Available online: https://www.who.int/publications/i/item/9789241597906 (accessed on 1 June 2022).
- Weber, D.J.; Rutala, W.A.; Sickbert-Bennett, E.E. Outbreaks Associated with Contaminated Antiseptics and Disinfectants. Antimicrob. Agents Chemother. 2007, 51, 4217–4224. [Google Scholar] [CrossRef]
- Lowbury, E.J.L. Contamination of Cetrimide and Other Fluids with Pseudomonas Pyocyanea. Br. J. Ind. Med. 1951, 8, 22–25. [Google Scholar] [CrossRef]
- Annotations. Bacteria in Antiseptic Solutions. Br. Med. J. 1958, 2, 436. [Google Scholar]
- Annotations. Failure of Detergents to Disinfect. Lancet 1958, 272, 306. [Google Scholar] [CrossRef]
- Bassett, D.C.J.; Stokes, K.J.; Thomas, W.R.G. Wound Infection with Pseudomonas Multivorans: A Water-Borne Contaminant of Disinfection Solutions. Lancet 1970, 1, 1188–1191. [Google Scholar] [CrossRef]
- Dixon, R.E.; Kaslow, R.A.; Mackel, D.C.; Fulkerson, C.C.; Mallison, G.F. Aqueous Quaternary Ammoniums Antiseptics and Disinfectants Use and Misuse. JAMA 1976, 236, 2415–2417. [Google Scholar] [CrossRef]
- Rutala, W.A.; Cole, E.C. Antiseptics and Disinfectants. Safe and Effective? Infect. Control 1984, 5, 215–218. [Google Scholar] [CrossRef]
- Sanford, J.P. Disinfectants That Don’t.Pdf. Ann. Intern. Med. 1970, 72, 282–283. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Model List of Essential Medicine. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02 (accessed on 1 June 2022).
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic Review or Scoping Review? Guidance for Authors When Choosing between a Systematic or Scoping Review Approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Dancer, S.J. Controlling Hospital-Acquired Infection: Focus on the Role of the Environment and New Technologies for Decontamination. Clin. Microbiol. Rev. 2014, 27, 665–690. [Google Scholar] [CrossRef]
- Dancer, S.J.; King, M.F. Systematic Review on Use, Cost and Clinical Efficacy of Automated Decontamination Devices. Antimicrob. Resist. Infect. Control 2021, 10, 34. [Google Scholar] [CrossRef]
- Curran, E.T. Outbreak Column 3: Outbreaks of Pseudomonas spp. from Hospital Water. J. Infect. Prev. 2012, 13, 125–127. [Google Scholar] [CrossRef]
- Kanamori, H.; Weber, D.J.; Rutala, W.A. Healthcare Outbreaks Associated with a Water Reservoir and Infection Prevention Strategies. Clin. Infect. Dis. 2016, 62, 1423–1435. [Google Scholar] [CrossRef]
- Weinbren, M.J.; Collins, M.; Heathcote, R.; Umar, M.; Nisar, M.; Ainger, C.; Masters, P. Optimization of the Blood Culture Pathway: A Template for Improved Sepsis Management and Diagnostic Antimicrobial Stewardship. J. Hosp. Infect. 2018, 98, 232–235. [Google Scholar] [CrossRef]
- Becker, S.L.; Berger, F.K.; Feldner, S.K.; Karliova, I.; Haber, M.; Mellmann, A.; Schäfers, H.J.; Gärtner, B. Outbreak of Burkholderia Cepacia Complex Infections Associated with Contaminated Octenidine Mouthwash Solution, Germany, August to September 2018. Eurosurveillance 2018, 23, 1800540. [Google Scholar] [CrossRef]
- Becks, V.E.; Lorenzoni, N.M. Pseudomonas Aeruginosa Outbreak in Neonatal Intensive Care Unit: A Possible Link to Contaminated Hand Lotion. Am. J. I 1995, 23, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Leong, L.E.X.; Lagana, D.; Carter, G.P.; Wang, Q.; Smith, K.; Stinear, T.P.; Shaw, D.; Sintchenko, V.; Wesselingh, S.L.; Bastian, I.; et al. Burkholderia Lata Infections from Intrinsically Contaminated Chlorhexidine Mouthwash, Australia, 2016. Emerg. Infect. Dis. 2018, 24, 2109–2111. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Sullivan, L.; Booker, A.; Baker, J. Quaternary Ammonium Disinfectant Issues Encountered in an Environmental Services Department. Infect. Control Hosp. Epidemiol. 2016, 37, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Lompo, P.; Agbobli, E.; Heroes, A.-S.; vanden Poel, B.; Kühne, V.; Kpossou, G.; Zida, A.; Halidou, T.; Dissou, A.; Jacobs, J. Bacterial Contamination of Antiseptics, Disinfectants and Hand Hygiene Products Used in Healthcare Settings in Low- and Middle Income Countries—A Systematic Review. Hygiene 2023. submitted. [Google Scholar] [CrossRef]
- Dumville, J.; Mcfarlane, E.; Edwards, P.; Lipp, A.; Holmes, A.; Liu, Z. Preoperative Skin Antiseptics for Preventing Surgical Wound Infections After Clean Surgery (Review). Cochrane Database Syst. Rev. 2015, CD003949. [Google Scholar] [CrossRef]
- Hadiati, D.R.; Hakimi, M.; Nurdiati, D.S.; Masuzawa, Y.; da Silva Lopes, K.; Ota, E. Skin Preparation for Preventing Infection Following Caesarean Section. Cochrane Database Syst. Rev. 2018, 6, CD007462. [Google Scholar] [CrossRef]
- Weber, D.J.; Sickbert-Bennett, E.E.; Kanamori, H.; Rutala, W.A. New and Emerging Infectious Diseases (Ebola, Middle Eastern Respiratory Syndrome Coronavirus, Carbapenem-Resistant Enterobacteriaceae, Candida Auris): Focus on Environmental Survival and Germicide Susceptibility. Am. J. Infect. Control 2019, 47, A29–A38. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Evidence of Hand Hygiene as the Building Block for Infection Prevention and Control An Extract from the Systematic Literature Reviews Undertaken as the Background for the WHO Guidelines on Core Components. Available online: https://apps.who.int/iris/bitstream/handle/10665/330079/WHO-HIS-SDS-2017.7-eng.pdf?sequence=1&isAllowed=y (accessed on 1 June 2022).
- World Health Organization (WHO). Global Guidelines for the Prevention of Surgical Site Infection, Second Edition. Available online: https://www.who.int/publications/i/item/global-guidelines-for-the-prevention-of-surgical-site-infection-2nd-ed (accessed on 1 June 2022).
- Centers for Disease Control and Prevention (CDC). Guideline for Hand Hygiene in Health-Care Settings Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Available online: https://www.cdc.gov/mmwr/indrr_2002.html (accessed on 1 July 2022).
- Curran, E.T. Outbreak Column 7: Pseudo-Outbreaks (Part 1). J. Infect. Prev. 2013, 14, 69–74. [Google Scholar] [CrossRef]
- Curran, E.T. Pseudo Outbreaks and No-Infection Outbreaks (Part 2). J. Infect. Prev. 2013, 14, 108–113. [Google Scholar] [CrossRef]
- List of Prokaryotic Names with Standing in Nomenclature (LPSN). List of Prokaryotic Names with Standing in Nomenclature. Available online: https://www.dsmz.de/services/online-tools/prokaryotic-nomenclature-up-to-date (accessed on 20 June 2022).
- Clara, L.; Staneloni, M.I.; Salazar, E.; Greco, G.; Visus, M.; Lizzi, A.; Alexander, V.; Gutkind, G.; Radice, M.; Papalia, M. Report of Two Events of Nosocomial Outbreak and Pseudo-Outbreak Due to Contamination with Achromobacter spp. Rev. Argent. Microbiol. 2021, 54, 175–180. [Google Scholar] [CrossRef]
- Wong, S.C.Y.; Wong, S.; Chen, J.H.K.; Poon, R.W.S.; Hung, D.L.L.; Chiu, K.H.Y.; So, S.Y.C.; Leung, W.S.; Chan, T.M.; Yap, D.Y.H.; et al. Complex Outbreak in Peritoneal Dialysis Patients Caused by Contaminated Aqueous Chlorhexidine. Emerg. Infect. Dis. 2020, 26, 1987–1997. [Google Scholar] [CrossRef]
- Coenye, T.; Vandamme, P.; Govan, J.R.W.; Lipuma, J.J. Taxonomy and Identification of the Burkholderia Cepacia Complex. J. Clin. Microbiol. 2001, 39, 3427–3436. [Google Scholar] [CrossRef]
- Sfeir, M.M. Burkholderia Cepacia Complex Infections: More Complex than the Bacterium Name Suggest. J. Infect. 2018, 77, 166–170. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Expected Resistant Phenotypes. Available online: https://www.eucast.org/ (accessed on 1 May 2022).
- World Health Organizatio (WHO). Guidelines on Core Components of Infection Prevention and Control Programmes at the National and Acute Health Care Facility Level. Available online: http://apps.who.int/bookorders (accessed on 1 June 2022).
- World Health Organization (WHO). Minimum Requirements for Infection Prevention and Control Programmes. Available online: https://www.who.int/publications/i/item/9789241516945 (accessed on 1 June 2022).
- Centers for Disease Control and Prevention (CDC). The Hand Hygiene in Healthcare Settings. Available online: https://www.cdc.gov/handhygiene/index.html (accessed on 12 July 2022).
- Bauer-Savage, J.; Pittet, D.; Kim, E.; Allegranzi, B. Local Production of WHO-Recommended Alcohol-Based Handrubs: Feasibility, Advantages, Barriers and Costs. Bull. World Health Organ. 2013, 91, 963–969. [Google Scholar] [CrossRef]
- Gerbens-Leenes, W.; Hoekstra, A.Y. The Water Footprint of Sweeteners and Bio-Ethanol. Environ. Int. 2012, 40, 202–211. [Google Scholar] [CrossRef]
- United States Food and Drug Administration (FDA). Topical Antiseptic Products: Hand Sanitizers and Antibacterial Soaps. Available online: https://www.fda.gov/drugs/information-drug-class/topical-antiseptic-products-hand-sanitizers-and-antibacterial-soaps (accessed on 28 August 2022).
- Nix, D.H. Factors to Consider When Selecting Skin Cleansing Products. J. Wound Ostomy Cont. Nurs. 2000, 27, 260–268. [Google Scholar] [CrossRef]
- Brooks, S.E.; Walczak, M.A.; Malcom, S.; Hameed, R. Intrinsic Klebsiella Pneumoniae Contamination of Liquid Germicidal Hand Soap Containing Chlorhexidine. Infect. Control Hosp. Epidemiol. 2004, 25, 883–885. [Google Scholar] [CrossRef]
- McBride, M.E. Microbial Flora of In-Use Soap Products. Appl. Environ. Microbiol. 1984, 48, 338–341. [Google Scholar] [CrossRef]
- EngenderHealth. Infection Prevention: A Reference Booklet for Health Care Providers, 2nd ed.; EngenderHealth: New York, NY, USA, 2011. [Google Scholar]
- EngenderHealth Technical Publications & Resources. Available online: https://www.engenderhealth.org/ (accessed on 1 June 2022).
- Drugbank Drugbank Online. Available online: https://go.drugbank.com/ (accessed on 6 September 2021).
- Rutala, W.A.; Weber, D.J. Disinfection, Sterilization, and Antisepsis: An Overview. Am. J. Infect. Control 2019, 47, A3–A9. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO 8th Essential Medicines List for Children 2021. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.03 (accessed on 1 June 2022).
- Rutala, W.A.; Weber, D.J. Disinfection and Sterilization in Health Care Facilities: An Overview and Current Issues. Infect. Dis. Clin. N. Am. 2016, 30, 609–637. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Best Practices for Environmental Cleaning in Healthcare Facilities: In Resource-Limited Settings. Available online: http://www.icanetwork.co.za/icanguideline2019/ (accessed on 12 July 2022).
- Bánsághi, S.; Soule, H.; Guitart, C.; Pittet, D.; Haidegger, T. Critical Reliability Issues of Common Type Alcohol-Based Handrub Dispensers. Antimicrob. Resist. Infect. Control 2020, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Institute for Hygiene and Environmental Medicine Charité—University Medicine Berlin Worldwide Database for Nosocomial Outbreaks. Available online: https://www.outbreak-database.com/Home.aspx (accessed on 31 May 2022).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile APP for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- World Bank. World Bank Country Classification. Available online: https://databank.worldbank.org/home.aspx (accessed on 1 May 2022).
- Johnson, J.; Milstone, A.M. Hospital-Onset Neonatal Sepsis and Mortality in Low-Resource Settings: Will Bundles Save the Day? Clin. Infect. Dis. 2019, 69, 1368–1369. [Google Scholar] [CrossRef] [PubMed]
- Loftus, M.J.; Guitart, C.; Tartari, E.; Stewardson, A.J.; Amer, F.; Bellissimo-Rodrigues, F.; Lee, Y.F.; Mehtar, S.; Sithole, B.L.; Pittet, D. Hand Hygiene in Low- and Middle-Income Countries. Int. J. Infect. Dis. 2019, 86, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Zingg, W.; Storr, J.; Park, B.J.; Jernigan, J.A.; Harbarth, S.; Grayson, M.L.; Tacconelli, E.; Allegranzi, B.; Cardo, D.; Pittet, D.; et al. Broadening the Infection Prevention and Control Network Globally; 2017 Geneva IPC-Think Tank (Part 3). Antimicrob. Resist. Infect. Control 2019, 8, 74. [Google Scholar] [CrossRef]
- Berkelman, R.L.; Lewin, S.; Allen, J.R.; Anderson, R.L.; Budnick, L.D.; Shapiro, S.; Friedman, S.M.; Nicholas, P.; Holizman, R.S.; Haley, R.W. Pseudobacteremia Attributed to Contamination of Povidone-Iodine with Pseudomonas Cepacia. Ann. Intern. Med. 1981, 95, 32–36. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Epidemiologic Notes and Reports Pseudomonas Aeruginosa Peritonitis Attributed to a Contaminated Iodophor Solution—Georgia. Morb. Mortal. Wkly. Rep. 1982, 31, 197–198. [Google Scholar]
- Craven, D.E.; Moody, B.; Connolly, M.G.; Kollisch, N.R.; Stottmeier, K.D.; McCabe, W.R. Pseudobacteremia Caused by Povidone-Iodine Solution Contaminated with Pseudomonas Cepacia. Syria Stud. 1981, 305, 621–623. [Google Scholar] [CrossRef]
- Panlilio, A.L.; Beck-Sague, C.M.; Siegel, J.D.; Anderson, R.L.; Yetts, S.Y.; Clark, N.C.; Duer, P.N.; Thomassen, K.A.; Vess, R.W.; Hill, B.C.; et al. Infections and Pseudoinfections Due to Povidone-Iodine Solution Contaminated with Pseudomonas Cepacia. Clin. Infect. Dis. 1992, 14, 1078–1083. [Google Scholar] [CrossRef]
- Parrott, P.L.; Terry, P.M.; Whitworth, E.N.; Frawley, L.W.; Coble, R.S. Pseudomonas Aeruginosa Peritonitis Associated with Contaminated Poloxamer-Iodine Solution. Lancet 1982, 25, 683–685. [Google Scholar] [CrossRef]
- de Frutos, M.; Lopez-Urrutia, L.; Dominguez-Gil, M.; Arias, M.; Munoz-Bellido, J.L.; Eiros, J.M.; Ramos, C. Serratia Marcescens Outbreak Due to Contaminated 2% Aqueous Chlorhexidine. Enferm. Infecc. Microbiol. Clin. 2017, 35, 624–629. [Google Scholar] [CrossRef]
- Fernandez, A.L.; Adrio, B.; Cereijo, J.M.M.; Monzonis, M.A.M.; El-Diasty, M.M.; Escudero, J.A. Clinical Study of an Outbreak of Postoperative Mediastinitis Caused by Serratia Marcescens in Adult Cardiac Surgery. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 523–527. [Google Scholar] [CrossRef]
- Grupo de Estudio del Brote. Brote Por Serratia Marcescens Asociado a La Utilización de Un Antiséptico de Clorhexidina Contaminado. Bol. Epidemiol. Semenal 2016, 24, 85–101. [Google Scholar]
- Molina-Cabrillana, J.; Santana-Reyes, C.; González-García, A.; Bordes-Benítez, A.; Horcajada, I. Outbreak of Achromobacter Xylosoxidans Pseudobacteremia in a Neonatal Care Unit Related to Contaminated Chlorhexidine Solution. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 435–437. [Google Scholar] [CrossRef]
- Morillo, A.; Torres, M.J.; Salas, M.T.A.; Conde, M.; Aznar, J.Y. Implicación de Un Brote Nacional de Infección Por Serratia Marcescens Asociado a Clorhexidina Contaminada En Un Hospital Pediátrico Implication of a National Outbreak of Serratia Marcescens Associated with a Contaminated Solution of Chlorhexidine in a Pae. Cart. Cient. 2017, 88, 171–172. [Google Scholar] [CrossRef]
- Lehours, P.; Rogues, A.M.; Occhialini, A.; Boulestreau, H.; Gachie, J.P.; Mégraud, F. Investigation of an Outbreak Due to Alcaligenes Xylosoxydans Subspecies Xylosoxydans by Random Amplified Polymorphic DNA Analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 108–113. [Google Scholar] [CrossRef]
- Malizia, W.F.; Gangarosa, E.J.; Goley, A.F. Benzalkonium Chloride as a Source of Infection. N. Engl. J. Med. 1960, 263, 800–802. [Google Scholar] [CrossRef]
- Sartor, C.; Jacomo, V.; Duvivier, C.; Tissot-Dupont, H.; Sambuc, R.; Drancourt, M. Nosocomial Serratia Marcescens Infections Associated with Extrinsic Contamination of a Liquid Nonmedicated Soap. Infect. Control Hosp. Epidemiol. 2000, 21, 196–199. [Google Scholar] [CrossRef]
- Vigeant, P.; Loo, V.G.; Bertrand, C.; Dixon, C.; Hollis, R.; Pfaller, M.A.; McLean, P.A.H.; Briedis, D.J.; Perl, T.M.; Robson, H.G. An Outbreak of Serratia Marcescens Infections Related to Contaminated Chlorhexidine. Infect. Control Hosp. Epidemiol. 1998, 19, 791–794. [Google Scholar] [CrossRef]
- Wishart, M.M.; Riley, T.V. Infection with Pseudomonas Matophilia Hospital Outbreak Due to Contaminated Disinfectant. Med. J. Aust. 1976, 2, 710–712. [Google Scholar] [CrossRef]
- De Smet, B.; Veng, C.; Kruy, L.; Kham, C.; van Griensven, J.; Peeters, C.; Ieng, S.; Phe, T.; Vlieghe, E.; Vandamme, P.; et al. Outbreak of Burkholderia Cepacia Bloodstream Infections Traced to the Use of Ringer Lactate Solution as Multiple-Dose Vial for Catheter Flushing, Phnom Penh, Cambodia. Clin. Microbiol. Infect. 2012, 19, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Lee, H.B.; Cho, Y.G.; Park, J.H.; Lee, H.S. Hospital-Acquired Burkholderia Cepacia Infection Related to Contaminated Benzalkonium Chloride. Hosp. Infect. Soc. 2008, 68, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Kaslow, R.A.; Mackel, D.C.; Mallison, G.F. Nosocomial Pseudobacteremia: Positive Blood Cultures Due to Contaminated Benzalkonium Antiseptic. JAMA 1976, 236, 2407–2409. [Google Scholar] [CrossRef] [PubMed]
- Tena, D.; Carranza, R.; Barberá, J.R.; Valdezate, S.; Garrancho, J.M.; Arranz, M.; Sáez-Nieto, J.A. Outbreak of Long-Term Intravascular Catheter-Related Bacteremia Due to Achromobacter Xylosoxidans Subspecies Xylosoxidans in a Hemodialysis Unit. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 727–732. [Google Scholar] [CrossRef]
- Vu-Thien, H.; Darbord, J.C.; Moissenet, D.; Dulot, C.; Dufourcq, J.B.; Marsol, P.; Garbarg-Chenon, A. Investigation of an Outbreak of Wound Infections Due to Alcaligenes Xylosoxidans Transmitted by Chlorhexidine in a Burns Unit. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 724–726. [Google Scholar] [CrossRef]
- Verschraegen, G.; Claeys, G.; Meeus, G.; Delanghe, M. Pseudomonas Pickettii as a Cause of Pseudobacteremia. J. Clin. Microbiol. 1985, 21, 278–279. [Google Scholar] [CrossRef]
- Merino, J.L.; Bouarich, H.; Pita, M.J.; Martínez, P.; Bueno, B.; Caldés, S.; Corchete, E.; Jaldo, M.T.; Espejo, B.; Paraíso, V. Serratia Marcescens Bacteraemia Outbreak in Haemodialysis Patients with Tunnelled Catheters Due to Colonisation of Antiseptic Solution. Experience at 4 Hospitals. Nefrologia 2016, 36, 667–673. [Google Scholar] [CrossRef]
- Lee, S.; Han, S.W.; Kim, G.; Song, D.Y.; Lee, J.C.; Kwon, K.T. An Outbreak of Burkholderia Cenocepacia Associated with Contaminated Chlorhexidine Solutions Prepared in the Hospital. Am. J. Infect. Control 2013, 41, 93–96. [Google Scholar] [CrossRef]
- Vonberg, R.P.; Eckmanns, T.; Welte, T.; Gastmeier, P. Impact of the Suctioning System (Open vs. Closed) on the Incidence of Ventilation-Associated Pneumonia: Meta-Analysis of Randomized Controlled Trials. Intensive Care Med. 2006, 32, 1329–1335. [Google Scholar] [CrossRef]
- Boyce, J.M.; Havill, N.L. In-Use Contamination of a Hospital-Grade Disinfectant. Am. J. Infect. Control 2022, 50, 1296–1301. [Google Scholar] [CrossRef]
- D’Errico, M.M.; Savini, S.; Prospero, E.; Annino, I. Report on a Packaged Handwashing Antiseptic Contaminated With Pseudomonas Aeruginosa. Infect. Control Hosp. Epidemiol. 2000, 21, 302. [Google Scholar] [CrossRef]
- Coyle-Gilchrist, M.M.; Crewe, P.; Roberts, G. Flavobacterium Meningosepticum in the Hospital Environment. J. Clin. Pathol. 1976, 29, 824–826. [Google Scholar] [CrossRef]
- Dulake, C.; Kidd, E. Contaminated Irrigating Fluid. Lancet 1966, 287, 980. [Google Scholar] [CrossRef]
- Guinness, M.; Levey, J. Contamination of Aqueous Dilutions of Resiguard Disinfectant with Pseudomonas. Med. J. Aust. 1976, 2, 392. [Google Scholar]
- Lanini, S.; D’Arezzo, S.; Puro, V.; Martini, L.; Imperi, F.; Piselli, P.; Montanaro, M.; Paoletti, S.; Visca, P.; Ippolito, G.; et al. Molecular Epidemiology of a Pseudomonas Aeruginosa Hospital Outbreak Driven by a Contaminated Disinfectant-Soap Dispenser. PLoS ONE 2011, 6, e17064. [Google Scholar] [CrossRef]
- McNaughton, M.; Mazinke, N.; Thomas, E. Newborn Conjuctivitis Associated with Triclosan 0.5% Antiseptic Intrinsically Contaminated with Serratia Marcescens. Can. J. Infect. Control 1995, 10, 7–8. [Google Scholar]
- Shigeta, S.; Yasunaga, Y.; Honzumi, K.; Okamura, H.; Kumata, R.; Endo, S. Cerebral Ventriculitis Associated with Achromobacter Xylosoxidans. J. Clin. Pathol. 1978, 31, 156–161. [Google Scholar] [CrossRef]
- Hervé, B.; Chomali, M.; Gutiérrez, C.; Luna, M.; Rivas, J.; Blamey, R. Brote de Infección Nosocomial Por Serratia Marcescens Asociado a Contaminación Intrínseca de Clorhexidina Acuosa. Rev. Chil. Infectol. 2015, 32, 517–522. [Google Scholar] [CrossRef]
- United Nations (UN). Countries or Areas /Geographical Regions. Available online: https://unstats.un.org/unsd/methodology/m49/#geo-regions (accessed on 1 June 2022).
- European Committee for Standardization. Chemical Disinfectants and Antiseptics—Application of European Standards for Chemical Disinfectants and Antiseptics. Available online: https://standards.iteh.ai/catalog/standards/cen/37a9a967-990c-437b-979a-68f121bf4679/en-14885-2022 (accessed on 20 October 2022).
- United States Food and Drug Administration (FDA). Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use. U.S. Food Drug Adm. Fed. Regist. 2016, 81, 61106–61129. [Google Scholar]
- Milanović, M.; Đurić, L.; Milošević, N.; Milić, N. Comprehensive Insight into Triclosan—From Widespread Occurence to Health Outcomes. Environ. Sci. Pollut. Res. 2021, 30, 25119–25140. [Google Scholar] [CrossRef]
- Gleeson, S.; Mulroy, E.; Bryce, E.; Fox, S.; Taylor, S.L.; Talreja, H. Burkholderia Cepacia: An Outbreak in the Peritoneal Dialysis Unit. Perit. Dial. Int. 2019, 39, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Contamination of Alcohol Prep Pads with Bacillus Cereus Group and Bacillus Species—Colorado, 2010. Morb. Mortal. Wkly. Rep. 2011, 60, 347. [Google Scholar]
- Dolan, S.A.; Littlehorn, C.; Glodé, M.P.; Dowell, E.; Xavier, K.; Nyquist, A.-C.; Todd, J.K. Association of Bacillus Cereus Infection with Contaminated Alcohol Prep Pads. Infect. Control Hosp. Epidemiol. 2012, 33, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.A. Pseudobacteremia Due to Contaminated Alcohol Swabs. J. Clin. Microbiol. 1983, 18, 974–975. [Google Scholar] [CrossRef]
- Hsueh, P.; Teng, L.; Yang, P.; Pan, H.; Ho, S. Nosocomial Pseudoepidemic Caused by Bacillus Cereus Traced to Contaminated Ethyl Alcohol from a Liquor Factory. J. Clin. Microbiol. 1999, 37, 2280–2284. [Google Scholar] [CrossRef]
- Kampf, G. Acquired Resistance to Chlorhexidine—Is It Time to Establish an ‘Antiseptic Stewardship’ Initiative? J. Hosp. Infect. 2016, 94, 213–227. [Google Scholar] [CrossRef]
- Song, J.E.; Kwak, Y.G.; Um, T.H.; Cho, C.R.; Kim, S.; Park, I.S.; Hwang, J.H.; Kim, N.; Oh, G.-B. Outbreak of Burkholderia Cepacia Pseudobacteraemia Caused by Intrinsically Contaminated Commercial 0.5% Chlorhexidine Solution in Neonatal Intensive Care Units. J. Hosp. Infect. 2018, 98, 295–299. [Google Scholar] [CrossRef]
- Eiref, S.D.; Leitman, I.M.; Riley, W. Hand Sanitizer Dispensers and Associated Hospital-Acquired Infections: Friend or Fomite? Surg. Infect. 2012, 13, 137–140. [Google Scholar] [CrossRef]
- Maciel, A.L.P.; De Assis, D.B.; Madalosso, G.; Padoveze, M.C. Evaluating the Quality of Outbreak Reports on Health Care-Associated Infections in São Paulo, Brazil, during 2000–2010 Using the ORION Statement Findings and Recommendations. Am. J. Infect. Control 2014, 42, 47–53. [Google Scholar] [CrossRef]
- Enhancing the Quality and Transparency of health Research (EQUATOR Network). Enhancing the Quality and Transparency of Health Research. Available online: https://www.equator-network.org/ (accessed on 22 August 2022).
- Stone, S.P.; Cooper, B.S.; Kibbler, C.C.; Cookson, B.D.; Roberts, J.A.; Medley, G.F.; Duckworth, G.; Lai, R.; Ebrahim, S.; Brown, E.M.; et al. The ORION Statement: Guidelines for Transparent Reporting of Outbreak Reports and Intervention Studies of Nosocomial Infection. Lancet Infect. Dis. 2007, 7, 282–288. [Google Scholar] [CrossRef]
- Fanci, R.; Bartolozzi, B.; Sergi, S.; Casalone, E.; Pecile, P.; Cecconi, D.; Mannino, R.; Donnarumma, F.; Leon, A.G.; Guidi, S.; et al. Molecular Epidemiological Investigation of an Outbreak of Pseudomonas Aeruginosa Infection in an SCT Unit. Bone Marrow Transplant. 2009, 43, 335–338. [Google Scholar] [CrossRef]
- Villari, P.; Crispino, M.; Salvadori, A.; Scarcella, A. Molecular Epidemiology of an Outbreak of Serratia Marcescens in a Neonatal Intensive Care Unit. Infect. Control Hosp. Epidemiol. 2001, 22, 630–634. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Centers for Disease Control & Prevention (CDC) Contaminated Detergent Solution. Morb. Mortal. Wkly. Rep. 1969, 18, 366. [Google Scholar]
- Hocevar, S.N.; Meites, E.; Williams, M.; Pascoe, N.; O’Connell, H.; Jensen, B.; Hatch, M.; MacCannell, T. Allergy Injection-Associated Mycobacterium Abscessus Outbreak, Texas, 2009. Infect. Dis. Soc. Am. 2010, 48, 109–110. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Guidelines for Environmental Infection Control in Health-Care Facilities. In Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Available online: https://www.cdc.gov/infectioncontrol/guidelines/environmental/index.html (accessed on 12 July 2022).
- Torbeck, L.; Raccasi, D.; Guilfoyle, D.E.; Friedman, R.L.; Hussong, D. Burkholderia Cepacia: This Decision Is Overdue. PDA J. Pharm. Sci. Technol. 2011, 65, 535–543. [Google Scholar] [CrossRef]
- Wiemken, T.L. Skin Antiseptics in Healthcare Facilities: Is a Targeted Approach Necessary? BMC Public Health 2019, 19, 10–13. [Google Scholar] [CrossRef]
- Kelsey, J.C.; Maurer, I.M. An In-Use Test for Hospital Disinfectants. Mon. Bull. Minist. Hlth. Lab. Serv. 1966, 25, 180–184. [Google Scholar]
- Hardy, P.C.; Ederer, G.M.; Mastsen, J.M. Contamination of Commercially Packaged Urinary Catheder Kits with Pseudomonas EO-1. N. Engl. J. Med. 1970, 282, 33–35. [Google Scholar] [CrossRef]
- Rabier, V.; Bataillon, S.; Jolivet-Gougeon, A.; Chapplain, J.M.; Beuchée, A.; Bétrémieux, P. Hand Washing Soap as a Source of Neonatal Serratia Marcescens Outbreak. Acta Paediatr. 2008, 97, 1381–1385. [Google Scholar] [CrossRef]
- Stoesser, N.; Sheppard, A.E.; Shakya, M.; Sthapit, B.; Thorson, S.; Giess, A.; Kelly, D.; Pollard, A.J.; Peto, T.E.A.; Walker, A.S.; et al. Dynamics of MDR Enterobacter Cloacae Outbreaks in a Neonatal Unit in Nepal: Insights Using Wider Sampling Frames and next-Generation Sequencing. J. Antimicrob. Chemother. 2015, 70, 1008–1015. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. C.M.E. Principles and Procedures for Blood Cultures. 2nd Edition. Available online: https://www.clsi.org/standards/products/microbiology/documents/m47/ (accessed on 6 October 2022).
- Bruun, J.N.; Digranes, A. Survival of Gram-Negative Bacicilli and Candida Albicans in Hexachlorophene Preparations and Other Disinfectants. Scand. J. Infect. Dis. 1971, 3, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, R.; Hammerl, J.A.; Hahmann, H.; Wicke, A.; Kleta, S.; Dabrowski, P.W.; Nitsche, A.; Stämmler, M.; Al Dahouk, S.; Lasch, P. Rapid Characterisation of: Klebsiella Oxytoca Isolates from Contaminated Liquid Hand Soap Using Mass Spectrometry, FTIR and Raman Spectroscopy. R. Soc. Chem. 2016, 187, 353–375. [Google Scholar] [CrossRef] [PubMed]
- Nkibiassala, S.; Devleeschouwer, M.; Ganssbeke, V.B.; Rost, F.; Dony, J. Disinfectants Prepared in a Hospital Pharmacy—Assessment of Their Microbiological Purity and Antimicrobial Effectiveness. J. Clin. Pharm. Ther. 1989, 14, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Exner, M.; Bhattacharya, S.; Christiansen, B.; Gebel, J.; Goroncy-Bermes, P.; Hartemann, P.; Heeg, P.; Ilschner, C.; Kramer, A.; Larson, E.; et al. Antibiotic Resistance: What Is so Special about Multidrug-Resistant Gram-Negative Bacteria? GMS Hyg. Infect. Control 2017, 12, Doc05. [Google Scholar] [CrossRef] [PubMed]
- Sanz-García, F.; Gil-Gil, T.; Laborda, P.; Ochoa-Sánchez, L.E.; Martínez, J.L.; Hernando-Amado, S. Coming from the Wild: Multidrug Resistant Opportunistic Pathogens Presenting a Primary, Not Human-Linked, Environmental Habitat. Int. J. Mol. Sci. 2021, 22, 8080. [Google Scholar] [CrossRef]
- Shaban, R.Z.; Sotomayor-Castillo, C.; Nahidi, S.; Li, C.; MacBeth, D.; Mitchell, B.G.; Russo, P.L. Global Burden, Point Sources, and Outbreak Management of Healthcare-Associated Burkholderia Cepacia Infections: An Integrative Review. Infect. Control Hosp. Epidemiol. 2020, 41, 777–783. [Google Scholar] [CrossRef]
- Tavares, M.; Kozak, M.; Balola, A.; Sá-Correia, I. Burkholderia Cepacia Complex Bacteria: A Feared Contamination Risk in Water-Based Pharmaceutical Products. Clin. Microbiol. Rev. 2020, 33, e00139-19. [Google Scholar] [CrossRef]
- Hugon, E.; Marchandin, H.; Poirée, M.; Fosse, T.; Sirvent, N. Achromobacter Bacteraemia Outbreak in a Paediatric Onco-Haematology Department Related to Strain with High Surviving Ability in Contaminated Disinfectant Atomizers. J. Hosp. Infect. 2015, 89, 116–122. [Google Scholar] [CrossRef]
- Oie, S.; Arakawa, J.; Furukawa, H.; Matsumoto, S.; Matsuda, N.; Wakamatsu, H. Microbial Contamination of a Disinfectant-Soaked Unwoven Cleaning Cloth. J. Hosp. Infect. 2012, 82, 61–63. [Google Scholar] [CrossRef]
- Siebor, E.; Llanes, C.; Lafon, I.; Ogier-Desserrey, A.; Duez, J.M.; Pechinot, A.; Caillot, D.; Grandjean, M.; Sixt, N.; Neuwirth, C. Presumed Pseudobacteremia Outbreak Resulting from Contamination of Proportional Disinfectant Dispenser. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 26, 195–198. [Google Scholar] [CrossRef]
- McDonnell, G.E. Antisepsis, Disinfection, and Sterilization: Types, Action, and Resistance, 2nd ed.; ASM Press: Washington, DC, USA, 2017; ISBN 9772081415. [Google Scholar]
- Rose, H.; Baldwin, A.; Dowson, C.G.; Mahenthiralingam, E. Biocide Susceptibility of the Burkholderia Cepacia Complex. J. Antimicrob. Chemother. 2009, 63, 502–510. [Google Scholar] [CrossRef]
- Espinosa De Los Monteros, L.E.; Silva-Sanchez, J.; Jiménez, L.V.; Rojas, T.; Garza-Ramos, U.; Valverde, V. Outbreak of Infection by Extended-Spectrum β-Lactamase SHV-5-Producing Serratia Marcescens in a Mexican Hospital. J. Chemother. 2008, 20, 586–592. [Google Scholar] [CrossRef]
- Gastmeier, P.; Balderjahn, S.S.; Hansen, S.; Tiemann, N.; Zuschneid, I.; Groneberg, K.; Rüden, H.; Of, A.N.; Utbreaks, O. How Outbreaks Can Contribute to Prevention of Nosocomial Infections: Analysis of 1022 Outbreaks. Infect. Control Hosp. Epidemiol. 2005, 26, 357–361. [Google Scholar] [CrossRef]
- Gastmeier, P.; Loui, A.; Stamm-Balderjahn, S.; Hansen, S.; Zuschneid, I.; Sohr, D.; Behnke, M.; Obladen, M.; Vonberg, R.P.; Rüden, H. Outbreaks in Neonatal Intensive Care Units-They Are Not like Others. Am. J. Infect. Control 2007, 35, 172–176. [Google Scholar] [CrossRef]
- Kampf, G.; McDonald, C.; Ostermeyer, C. Bacterial In-Use Contamination of an Alcohol-Based Hand Rub under Accelerated Test Condition. J. Hosp. Infect. 2004, 59, 269–271. [Google Scholar] [CrossRef]
- Steinhauer, K.; Meyer, B.; Ostermeyer, C.; Rödger, H.-J.; Hintzpeter, M. Hygienic Safety of Alcohol-Based Hand Disinfectants and Skin Antiseptics. GMS Hyg. Infect. Control 2013, 8, Doc19. [Google Scholar] [CrossRef]
- Kampf, G.; Degenhardt, S.; Lackner, S.; Jesse, K.; von Baum, H.; Ostermeyer, C. Poorly Processed Reusable Surface Disinfection Tissue Dispensers May Be a Source of Infection. BMC Infect. Dis. 2014, 14, 37. [Google Scholar] [CrossRef]
- Hakuno, H.; Yamamoto, M.; Oie, S.; Kamiya, A. Microbial Contamination of Disinfectants Used for Intermittent Self Catheterization. Jpn. J. Infect. Dis. 2010, 63, 277–279. [Google Scholar] [CrossRef]
- Oie, S.; Kamiya, A. Bacterial Contamination of Commercially Available Ethacridine Lactate (Acrinol) Products. J. Hosp. Infect. 1996, 34, 51–58. [Google Scholar] [CrossRef]
- Gräf, W.; Kersch, D.; Scherzer, G. Microbial Contamination of Liquid-Soap Wall Dispensers with One-Way Bottles. Zentralbl. Bakteriol. Mikrobiol. Hyg. B Umwelthyg. Krankenhaushyg. Arbeitshyg. Prav. Med. 1988, 186, 166–179. [Google Scholar]
- Momeni, S.S.; Tomlin, N.; Ruby, J.D. Isolation of Raoultella Planticola from Refillable Antimicrobial Liquid Soap Dispensers in a Dental Setting. J. Am. Dent. Assoc. 2015, 146, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.D.; Wynne, C.D.; Enwright, L.; Williams, J.D. Handwashing and Antiseptic-Containing Soaps in Hospital. J. Clin. Pathol. 1979, 32, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Falkiner, F.R.; Jacoby, G.A.; Keane, C.T.; Mccann, S.R. Amikacin, Gentamicin and Tobramycin Resistant Pseudomonas Aeruginosa in a Leukaemic Ward Epidemiology and Genetic Studies. J. Hosp. Infect. 1982, 3, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Naparstek, L.; Carmeli, Y.; Chmelnitsky, I.; Banin, E.; Navon-Venezia, S. Reduced Susceptibility to Chlorhexidine among Extremely-Drug-Resistant Strains of Klebsiella Pneumoniae. J. Hosp. Infect. 2012, 81, 15–19. [Google Scholar] [CrossRef]
- Anderson, K.; Keynes, R. Infected Cork Closures and the Apparent Survival of Organisms in Antiseptic Solutions. Br. Med. J. 1958, 2, 274–275. [Google Scholar] [CrossRef]
- Marrie, T.J.; Costerton, J.W. Prolonged Survival of Serratia Marcescens in Chlorhexidine. Appl. Environ. Microbiol. 1981, 42, 1093–1102. [Google Scholar] [CrossRef]
- Otter, J.A.; Vickery, K.; Walker, J.T.; deLancey Pulcini, E.; Stoodley, P.; Goldenberg, S.D.; Salkeld, J.A.G.; Chewins, J.; Yezli, S.; Edgeworth, J.D. Surface-Attached Cells, Biofilms and Biocide Susceptibility: Implications for Hospital Cleaning Anddisinfection. J. Hosp. Infect. 2015, 89, 16–27. [Google Scholar] [CrossRef]
- Günther, F.; Merle, U.; Frank, U.; Gaida, M.M.; Mutters, N.T. Pseudobacteremia Outbreak of Biofilm-Forming Achromobacter Xylosoxidans—Environmental Transmission. BMC Infect. Dis. 2016, 16, 584. [Google Scholar] [CrossRef]
- Simmons, N.A.; Gardner, D.A. Bacterial Contamination of a Phenolic Disinfectant. Br. Med. J. 1969, 2, 668–669. [Google Scholar] [CrossRef]
- Tiwari, T.S.P.; Ray, B.; Jost, K.C.; Rathod, M.K.; Zhang, Y.; Brown-Elliott, B.A.; Hendricks, K.; Wallace, R.J. Forty Years of Disinfectant Failure: Outbreak of Postinjection Mycobacterium Abscessus Infection Caused by Contamination of Benzalkonium Chloride. Clin. Infect. Dis. 2003, 36, 954–962. [Google Scholar] [CrossRef]
- Rudnick, J.R.; Beck-Sague, C.M.; Anderson, R.L.; Schable, B.; Miller, M.J. Gram-Negative Bacteremia in Open-Heart-Surgery Patients Traced to Probable Tap-Water Contamination of Pressure-Monitoring Equipment. Infect. Control Hosp. Epidemiol. 1996, 17, 281–285. [Google Scholar]
- Newman, K.A.; Tenney, J.H.; Oken, H.A.; Moody, M.R.; Wharton, R.; Schimpff, S.C. Persistent Isolation of an Unusual Pseudomonas Species From a Phenolic Disinfectant System. Infect. Control 1984, 5, 219–222. [Google Scholar] [CrossRef]
- Chattman, M.; Maxwell, S.L.; Gerba, C.P. Occurrence of Heterotrophic and Coliform Bacteria in Liquid Hand Soaps from Bulk Refillable Dispensers in Public Facilities. J. Environ. Health 2011, 73, 26–29. [Google Scholar]
- Spainhour, S. Serratia Marcescens Outbreak Associated with Extrinsic Contamination of 1% Chloroxylenol Soap. Infect. Control Hosp. Epidemiol. 1998, 19, 476. [Google Scholar] [CrossRef]
- Burdon, D.W.; Whitby, J.L.; Wmitbyt, J.L. Contamination of Hospital Disinfectants with Pseudomonas Species. Br. Med. J. 1967, 2, 153–155. [Google Scholar] [CrossRef]
- Plotkin, S.; Austrian, R. Bacteremia Caused by Pseudomonas sp. Following the Use of Materials Stored in Solutions of a Cationic Surface-Active Agent. Am. J. Med. Sci. 1958, 235, 621–627. [Google Scholar] [CrossRef]
- Kahan, A.; Philippon, A.; Paul, G.; Weber, S.; Richard, C.; Hazebroucq, G.; Degeorges, M. Nosocomial Infection by Chlorhexidine Solution Contaminated with Pseudomonas Pickettii (Biovar VA-I). J. Infect. 1983, 7, 256–263. [Google Scholar] [CrossRef]
- Poty, F.; Denis, C.; Baufine-Ducrocq, H. Infection Nosocomiale à Pseudomonas Pickettii. Danger de l’utilisation Des Résines Échangeuses d’ions. Presse Med. 2008, 16, 1185–1187. [Google Scholar]
- Sobel, J.D.; Hashman, N.; Reinherz, G.; Merzbach, D. Nosocomial Pseudomonas Cepacia Infection Associated with Chlorhexidine Contamination. Am. J. Med. 1982, 73, 183–186. [Google Scholar] [CrossRef]
- Maroye, P.; Doermann, H.P.; Rogues, A.M.; Gachie, J.P.; Mégraud, F. Investigation of an Outbreak of Ralstonia Pickettii in a Paediatric Hospital by RAPD. J. Hosp. Infect. 2000, 44, 267–272. [Google Scholar] [CrossRef]
- Ehrenkranz, J.N.; Bolyard, E.A.; Wiener, M.; Clearry, T. Antibiotic-Sensitive Serratia Marcescens Infection Complicating Cardiopulmonary Operations: Contaminated Disinfectant as a Reservoir. Lancet 1980, 2, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Grohskopf, L.; Roth, V.; Feikin, D.; Arduino, M.; Carson, L.; JI, T.; Holt, S.; Jensen, B.; Hoffman, R.; Jarvis, W. Serratia Liquefaciens Bloodstream Infections from Contamination of Epoetin Alfa at a Hemodialysis Center. N. Engl. J. Med. 2001, 344, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Archibald, L.K.; Shah, B.; Schulte, M.; Arduino, M.J.; Aguero, S.; Fisher, D.J.; Stechenberg, B.W.; Banerjee, S.N.; Jarvis, W.R. Serratia Marcescens Outbreak Associated with Extrinsic Contamination of 1% Chlorxylenol Soap. Infect. Control Hosp. Epidemiol. 1997, 18, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Gosden, P.; Norman, P. Pseudobacteremia Associated with Contaminated Skin Cleaning Agent. Lancet 1985, 2, 671–672. [Google Scholar] [CrossRef]
- Ko, S.; Rn, H.A.; Hwan, J.; Park, S. American Journal of Infection Control An Outbreak of Burkholderia Cepacia Complex Pseudobacteremia Associated with Intrinsically Contaminated Commercial 0.5% Chlorhexidine Solution. Am. J. Infect. Control 2015, 43, 266–268. [Google Scholar] [CrossRef]
- Kupfahl, C.; Walter, M.; Wendt, C.; von Baum, H.; Kupfah, C.; Walther, M.; Wendt, C.; von Baum, H. Identical Achromobacter Strain in Reusable Surface Disinfection Tissue Dispensers and a Clinical Isolate. Infect. Control Hosp. Epidemiol. 2015, 36, 1362–1364. [Google Scholar] [CrossRef]
- Anderson, R.L.; Vess, R.W.; Panlilio, A.L.; Favero, M.S. Prolonged Survival of Pseudomonas Cepacia in Commercially Manufactured Povidone-Iodine. Appl. Environ. Microbiol. 1990, 56, 3598–3600. [Google Scholar] [CrossRef]
- Oie, S.; Kamiya, A. Microbial Contamination of Antiseptics and Disinfectants. Am. J. Infect. Control 1996, 24, 389–395. [Google Scholar] [CrossRef]
- Serikawa, T.; Kobayashi, S.; Tamura, T.; Uchiyama, M.; Tsukada, H.; Takakuwa, K.; Tanaka, K.; Ito, M. Pseudo Outbreak of Burkholderia Cepacia in Vaginal Cultures and Intervention by Infection Control Team. J. Hosp. Infect. 2010, 75, 242–243. [Google Scholar] [CrossRef]
- Blanc, D.S.; Magalhaes, G.B.; Abdelbary, M.; Prod’hom, G.; Greub, G.; Wasserfallen, J.B.; Genoud, P.; Zanetti, G.; Senn, L. Hand Soap Contamination by Pseudomonas Aeruginosa in a Tertiary Care Hospital: No Evidence of Impact on Patients. J. Hosp. Infect. 2016, 93, 63–67. [Google Scholar] [CrossRef]
- Anderson, R.L. Iodophor Antiseptics: Intrinsic Microbial Contamination with Resistant Bacteria. Infect. Control Hosp. Epidemiol. 1989, 10, 443–446. [Google Scholar] [CrossRef]
- United States Pharmacopeia (USP). Bioburden Control of Nonsterile Drug Substances and Products; USP-NF: Rockville, MD, USA, 2019; Volume 1115. [Google Scholar]
- Takahashi, H.; Kramer, M.H.; Yasui, Y.; Fujii, H.; Nakase, K.; Ikeda, K.; Imai, T.; Okazawa, A.; Tanaka, T.; Ohyanna, T.; et al. Nosocomial Serratia Marcescens Outbreak in Osaka, Japan, From 1999 to 2000. Infect. Control Hosp. Epidemiol. 2004, 25, 156–161. [Google Scholar] [CrossRef]
- McAllister, T.A.; Lucas, C.E.; Mocan, H.; Liddell, R.H.A.; Gibson, B.E.S.; Hann, I.M.; Platt, D.J. Serratia Marcescens Outbreak in a Paediatric Oncology Unit Traced to Contaminated Chlorhexidine. Scott. Med. J. 1989, 34, 525–528. [Google Scholar] [CrossRef]
- Shickman, M.D.; Guze, L.B.; Pearge, M.L. Bacteremia Following Cardiac Catheterization: Report of a Case and Studies on the Source. N. Engl. J. Med. 1959, 260, 1164–1166. [Google Scholar] [CrossRef]
- Nakashima, A.K.; Highsmith, A.K.; Martone, W.J. Survival of Serratia Marcescens in Benzalkonium Chloride and in Multiple-Dose Medication Vials: Relationship to Epidemic Septic Arthritis. J. Clin. Microbiol. 1987, 25, 1019–1021. [Google Scholar] [CrossRef]
- Olson, R.K.; Voorhees, R.E.; Eitzen, H.E.; Rolka, H.; Sewell, C.M. Cluster of Postinjection Abscesses Related to Corticosteroid Injections and Use of Benzalkonium Chloride. West. J. Med. 1999, 170, 143–147. [Google Scholar]
- Buffet-Bataillon, S.; Rabier, V.; Bétrémieux, P.; Beuchée, A.; Bauer, M.; Pladys, P.; Le Gall, E.; Cormier, M.; Jolivet-Gougeon, A. Outbreak of Serratia Marcescens in a Neonatal Intensive Care Unit: Contaminated Unmedicated Liquid Soap and Risk Factors. J. Hosp. Infect. 2009, 72, 17–22. [Google Scholar] [CrossRef]
- Barry, M.A.; Craven, D.E.; Goularte, T.A.; Lichtenberg, D.A. Serratia Marcescens Contamination of Antiseptic Soap Containing Triclosan: Implicatons for Nosocomial Infection. Infect. Control 1984, 5, 427–430. [Google Scholar] [CrossRef]
- Baird, R.M.; Shooter, R.A. Pseudomonas Aeruginosa Infections Associated with Use of Contaminated Medicaments. Br. Med. J. 1976, 2, 349–350. [Google Scholar] [CrossRef]
- Speller, D.C.; Stephens, M.E.; Viant, A.C. Hospital Infection by Pseudomonas Cepacia. Lancet 1971, 1, 798–799. [Google Scholar] [CrossRef]
- Nakashima, A.K.; McCarthy, M.A.; Martone, W.J.; Anderson, R.L. Epidemic Septic Arthritis Caused by Serratia Marcescens and Associated with a Benzalkonium Chloride Antiseptic. J. Clin. Microbiol. 1987, 25, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.J.; Schaffner, W. Contaminated Aqueous Benzalkonium Chloride An Unnecessary Hospital Infection Hazard. JAMA J. Am. Med. Assoc. 1976, 236, 2418–2419. [Google Scholar] [CrossRef]
- Tacconelli, E.; Cataldo, M.A.; Dancer, S.J.; De Angelis, G.; Falcone, M.; Frank, U.; Kahlmeter, G.; Pan, A.; Petrosillo, N.; Rodríguez-Baño, J.; et al. ESCMID Guidelines for the Management of the Infection Control Measures to Reduce Transmission of Multidrug-Resistant Gram-Negative Bacteria in Hospitalized Patients. Clin. Microbiol. Infect. 2014, 20, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Oie, S.; Kamiya, A. Microbial Contamination of Antiseptic-Soaked Cotton Balls. Biol. Pharm. Bull. 1997, 20, 667–669. [Google Scholar] [CrossRef]
- Gastmeier, P.; Stamm-Balderjahn, S.; Hansen, S.; Zuschneid, I.; Sohr, D.; Behnke, M.; Vonberg, R.P.; Rüden, H. Where Should One Search When Confronted with Outbreaks of Nosocomial Infection? Am. J. Infect. Control 2006, 34, 603–605. [Google Scholar] [CrossRef]
- Chang, C.Y.; Furlong, L.-A. Microbial Stowaways in Topical Antiseptic Products Christina. N. Engl. J. Med. 2012, 367, 2170–2173. [Google Scholar] [CrossRef]
- HAI-Net European Healthcare-Associated Infections Surveillance Network (HAI-Net). Available online: https://www.ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/hai-net (accessed on 28 August 2022).
- Curran, E.T.; Dalziel, C.E. Outbreak Column 18: The Undervalued Work of Outbreak: Prevention, Preparedness, Detection and Management. J. Infect. Prev. 2015, 16, 266–272. [Google Scholar] [CrossRef]
- Tsutsui, A.; Yahara, K.; Clark, A.; Fujimoto, K.; Kawakami, S.; Chikumi, H.; Iguchi, M.; Yagi, T.; Baker, M.A.; O’Brien, T.; et al. Automated Detection of Outbreaks of Antimicrobial-Resistant Bacteria in Japan. J. Hosp. Infect. 2019, 102, 226–233. [Google Scholar] [CrossRef]
- Farthing, K.; Wares, K.D.; Siani, H. When 2% Chlorhexidine Isn’t 2%! Implications on MRSA Decolonisation Guidelines. J. Hosp. Infect. 2022, 127, 133–134. [Google Scholar] [CrossRef]
- Turner, P.; Fox-Lewis, A.; Shrestha, P.; Dance, D.A.B.; Wangrangsimakul, T.; Cusack, T.P.; Ling, C.L.; Hopkins, J.; Roberts, T.; Limmathurotsakul, D.; et al. Microbiology Investigation Criteria for Reporting Objectively (MICRO): A Framework for the Reporting and Interpretation of Clinical Microbiology Data. BMC Med. 2019, 17, 70. [Google Scholar] [CrossRef]
- Cundell, T. USP <1111> Microbial Contamination Risk Factors Re-Visited. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/583957-USP-1111-Microbial-Contamination-Risk-Factors-Re-Visited/ (accessed on 6 October 2022).
- Assadian, O.; Kramer, A.; Christiansen, B.; Exner, M.; Martiny, H.; Sorger, A.; Suchomel, M. Recommendations and Requirements for Soap and Hand Rub Dispensers in Healthcare Facilities. GMS Krankenhhyg. Interdiszip. 2012, 7, Doc03. [Google Scholar] [CrossRef]
- Association for Professionals in Infection Control and Epidemiology (APIC). Re: Docket No. FDA-2012-N-1040, Comments to FDA on Antiseptic Patient Preoperative Skin Preparation Products. Available online: www.apic.org (accessed on 12 November 2022).
- United States Food and Drug Administration (FDA). FDA Drug Safety Communication: FDA Requests Label Changes and Single-Use Packaging for Some over-the-Counter Topical Antiseptic Products to Decrease Risk of Infection Safety. In FDA 2013. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-requests-label-changes-and-single-use-packaging-some-over-counter (accessed on 5 October 2022).
- United States Food and Drug Administration (FDA). Sterility of Antiseptic Skin Prep Products: FDA Hearing Stirs Debate. Available online: https://www.infectioncontroltoday.com/view/sterility-antiseptic-skin-prep-products-fda-hearing-stirs-debate (accessed on 28 August 2022).
- United States Food and Drug Administration (FDA). Class 2 Device Recall Triad Alcohol Prep Pads. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRes/res.cfm?id=100988 (accessed on 28 August 2022).
- United States Food and Drug Administration (FDA). Federal Register. Available online: https://www.govinfo.gov/content/pkg/FR-2012-11-21/pdf/2012-28321.pdf (accessed on 28 August 2022).
- United States Food and Drug Administration (FDA). Questions and Answers: FDA Requests Label Changes and Single-Use Packaging for Some over-the-Counter Topical Antiseptic Products to Decrease Risk of Infection. Available online: https://www.fda.gov/Drugs/DrugSafety/ucm374838.htm (accessed on 28 August 2022).
- United States Food and Drug Administration (FDA). Microbiological Quality Considerations in Non-Sterile Drug Manufacturing Guidance for Industry (Draft Guidance). Available online: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm (accessed on 28 August 2022).
- United States Food and Drug Administration (FDA). FDA Advises Drug Manufacturers That Burkholderia Cepacia Complex Poses a Contamination Risk in Non-Sterile, Water-Based Drug Products. FDA, 2021. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-advises-drug-manufacturers-burkholderia-cepacia-complex-poses-contamination-risk-non-sterile (accessed on 24 August 2022).
- United States Pharmacopeia (USP). Essentials of Testing and Control of Microbial Quality of Nonsterile Drug Substances and Products. Available online: https://www.usp.org/events-training/course/essentials-testing-and-control-microbial-quality-nonsterile-drug-substances (accessed on 20 June 2022).
- Kramer, A.; Kampf, G. Ist Die Anwendung Steriler Antiseptika zur Präoperativen Hautantiseptik Erforderlich? Eine Nutzen-Risiko-Bewertung. Available online: https://www.krankenhauspharmazie.de/heftarchiv/2017/12/ist-die-anwendung-steriler-antiseptika-zur-praoperativen-hautantiseptik-erforderlich-eine-nutzen-risiko-bewertung.html (accessed on 28 August 2022).
- Becton Dickinson (BD). Shouldn’t Your Skin Antiseptic Be Completely Sterile ? With Sterile SolutionTM Contaminated Antiseptics Have Harmed Patients. Available online: https://www.bd.com/en-us/products-and-solutions/products/product-page.930715#overview (accessed on 6 October 2022).
- Kuczewski, E.; Henaff, L.; Regard, A.; Argaud, L.; Lukaszewicz, A.; Rimmel, T.; Cassier, P.; Fredenucci, I.; Loeffert-fr, S.; Khanafer, N.; et al. Bacterial Cross-Transmission between Inanimate Surfaces and Patients in Intensive Care Units under Real-World Conditions: A Repeated Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 9401. [Google Scholar] [CrossRef]
- Zapka, C.A.; Campbell, E.J.; Maxwell, S.L.; Gerba, C.P.; Dolan, M.J.; Arbogast, J.W.; Macinga, D.R. Bacterial Hand Contamination and Transfer after Use of Contaminated Bulk-Soap-Refillable Dispensers. Appl. Environ. Microbiol. 2011, 77, 2898–2904. [Google Scholar] [CrossRef]
- European Parliament (Gavecelt). Optimising Skin Antisepsis for an Enhanced Prevention of Healthcare-Associated Infections in the EU. Available online: https://gavecelt.xn--itsitesdefaultfilesuploads-v92pfahf/ (accessed on 6 September 2022).
- European Commission. Guidance Document on the Demarcation between the Cosmetic Products Directive 76/768 and the Medicinal Products Directive 2001/83 as Agreed between the Commission Services and the Competent Authorities of Member States; European Commission: Brussels, Belgium, 2015; Available online: https://ec.europa.eu/docsroom/documents/13032/attachments/1/translations (accessed on 15 September 2022).
- Centers for Disease Control and Prevention (CDC). The Regulatory Framework for Disinfectants and Sterilants: Guideline for Disinfection and Sterilization in Healthcare Facilities. 2008. Available online: https://www.cdc.gov/infectioncontrol/guidelines/ (accessed on 12 July 2022).
- Johnson, J.; Bracken, R.; Tamma, P.D.; Aucott, S.W.; Bearer, C.; Milstone, A.M. Trends in Chlorhexidine Use in US Neonatal Intensive Care Units: Results from a Follow-Up National Survey. Infect. Control Hosp. Epidemiol. 2016, 37, 1116–1118. [Google Scholar] [CrossRef]
- United States Food and Drug Administration (FDA). Medical Device Recalls. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRes/resCollection_2.cfm?ID=98876&CREATE_DT=2011-05-03 (accessed on 28 August 2022).
- ORION. Outbreak Reports and Intervention Studies of Nosocomial Infection. Available online: https://www.ucl.ac.uk/amr/Reporting_Guidelines/ORION (accessed on 28 August 2022).
- International Health Facility Guidelines Hand Hygiene. Available online: https://www.healthfacilityguidelines.com/ (accessed on 28 August 2022).
- Clinical and Laboratory Standards Institute. C.M.E. Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data, 5th Edition. Available online: https://infostore.saiglobal.com/en-us/standards/clsi-m39-ed5-2022-1299841_saig_clsi_clsi_3143074/ (accessed on 6 October 2022).
- Anderson, R.L.; Holland, B.W.; Carr, J.K.; Bond, W.W.; Favero, M.S. Effect of Disinfectants on Pseudomonads Colonized on the Interior Surface of the PVC Pipes. Am. J. Public Health 1990, 80, 17–21. [Google Scholar] [CrossRef]
- Garcı’a-San Miguel, L.; Saez-Nieto, J.; Medina, M.J.; Lopez Hernandez, S.; Sanchez-Romero, I.; Ganga, B.; Asensio, A. Contamination of Liquid Soap for Hospital Use with Raoultella Planticola. J. Hosp. Infect. 2014, 86, 219–220. [Google Scholar] [CrossRef]
- European Union (EU). Safety Gate: The EU Rapid Alert System for Dangerous Non-Food Products. Available online: https://ec.europa.eu/safety-gate/#/screen/home (accessed on 6 September 2022).
- Fox, J.G.; Beaucage, C.M.; Folta, C.A.; Thornton, G.W. Nosocomial Transmission of Serratia Marcescens in a Veterinary Hospital Due to Contamination by Benzalkonium Chloride. J. Clin. Microbiol. 1981, 14, 157–160. [Google Scholar] [CrossRef]
- Schaffner, D.W.; Jensen, D.; Gerba, C.P.; Shumaker, D.; Arbogast, J.W. Influence of Soap Characteristics and Food Service Facility Type on the Degree of Bacterial Contamination of Open, Refillable Bulk Soaps. J. Food Prot. 2018, 81, 218–225. [Google Scholar] [CrossRef]
- Hayward, C.; Ross, K.E.; Brown, M.H.; Whiley, H. Water as a Source of Antimicrobial Resistance and Healthcare-Associated Infections. Pathogens 2020, 9, 667. [Google Scholar] [CrossRef]
- Chapman, P.; Forde, B.M.; Roberts, L.W.; Bergh, H.; Vesey, D.; Jennison, A.V.; Moss, S.; Paterson, D.L.; Beatson, S.A.; Harris, P.N.A. Genomic Investigation Reveals Contaminated Detergent as the Source of an Extended-Spectrum-β-Lactamase-Producing Klebsiella Michiganensis Outbreak in a Neonatal Unit. J. Clin. Microbiol. 2020, 58, e01980-19. [Google Scholar] [CrossRef]
- Shimono, N.; Takuma, T.; Tsuchimochi, N.; Shiose, A.; Murata, M.; Kanamoto, Y.; Uchida, Y.; Morita, S.; Matsumoto, H.; Hayashi, J. An Outbreak of Pseudomonas Aeruginosa Infections Following Thoracic Surgeries Occurring via the Contamination of Bronchoscopes and an Automatic Endoscope Reprocessor. J. Infect. Chemother. 2008, 14, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Lerma, F.; Maull, E.; Terradas, R.; Segura, C.; Planells, I.; Coll, P.; Knobel, H.; Vázquez, A. Moisturizing Body Milk as a Reservoir of Burkholderia Cepacia: Outbreak of Nosocomial Infection in a Multidisciplinary Intensive Care Unit. Crit. Care 2008, 12, R10. [Google Scholar] [CrossRef] [PubMed]
- Morse, L.J.; Schonbeck, L.E. Hand Lotions—A Potential Nosocomial Hazard. N. Engl. J. Med. 1968, 278, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Roshan, R.; Feroz, A.S.; Rafique, Z.; Virani, N. Rigorous Hand Hygiene Practices Among Health Care Workers Reduce Hospital-Associated Infections during the COVID-19 Pandemic. J. Prim. Care Community Health 2020, 11, 2150132720943331. [Google Scholar] [CrossRef]
- Mengato, D.; Di Spazio, L. Hand Hygiene for Healthcare Workers: Did We Need COVID-19 to Raise Awareness of Proper Disinfection Practice? Eur. J. Hosp. Pharm. 2022, 29, 302. [Google Scholar] [CrossRef]
- Founou, R.C.; Blocker, A.J.; Noubom, M.; Tsayem, C.; Choukem, S.P.; Van Dongen, M.; Founou, L.L. The COVID-19 Pandemic: A Threat to Antimicrobial Resistance Containment. Future Sci. OA 2021, 7, FSO736. [Google Scholar] [CrossRef]
- Fortune Business Insights. Impact of COVID-19 on the Global Hand Sanitizer Market. Available online: https://www.fortunebusinessinsights.com/infographics/impact-of-covid-19-on-hand-sanitizer-market-102719 (accessed on 6 December 2022).
- UP MARKET RESEARCH (UMR). Global Antiseptics and Disinfectants Market—Global Industry Analysis 2017–2019 and Forecast 2020–2027; Up Market Research: Pune, India, 2020. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lompo, P.; Heroes, A.-S.; Agbobli, E.; Kühne, V.; Tinto, H.; Affolabi, D.; Jacobs, J. Bacterial Contamination of Antiseptics, Disinfectants and Hand Hygiene Products in Healthcare Facilities in High-Income Countries: A Scoping Review. Hygiene 2023, 3, 136-175. https://doi.org/10.3390/hygiene3020012
Lompo P, Heroes A-S, Agbobli E, Kühne V, Tinto H, Affolabi D, Jacobs J. Bacterial Contamination of Antiseptics, Disinfectants and Hand Hygiene Products in Healthcare Facilities in High-Income Countries: A Scoping Review. Hygiene. 2023; 3(2):136-175. https://doi.org/10.3390/hygiene3020012
Chicago/Turabian StyleLompo, Palpouguini, Anne-Sophie Heroes, Esenam Agbobli, Vera Kühne, Halidou Tinto, Dissou Affolabi, and Jan Jacobs. 2023. "Bacterial Contamination of Antiseptics, Disinfectants and Hand Hygiene Products in Healthcare Facilities in High-Income Countries: A Scoping Review" Hygiene 3, no. 2: 136-175. https://doi.org/10.3390/hygiene3020012
APA StyleLompo, P., Heroes, A.-S., Agbobli, E., Kühne, V., Tinto, H., Affolabi, D., & Jacobs, J. (2023). Bacterial Contamination of Antiseptics, Disinfectants and Hand Hygiene Products in Healthcare Facilities in High-Income Countries: A Scoping Review. Hygiene, 3(2), 136-175. https://doi.org/10.3390/hygiene3020012







