Providing Sterile Orthopedic Implants: Challenges Associated with Multiple Reprocessing of Orthopedic Surgical Trays
Abstract
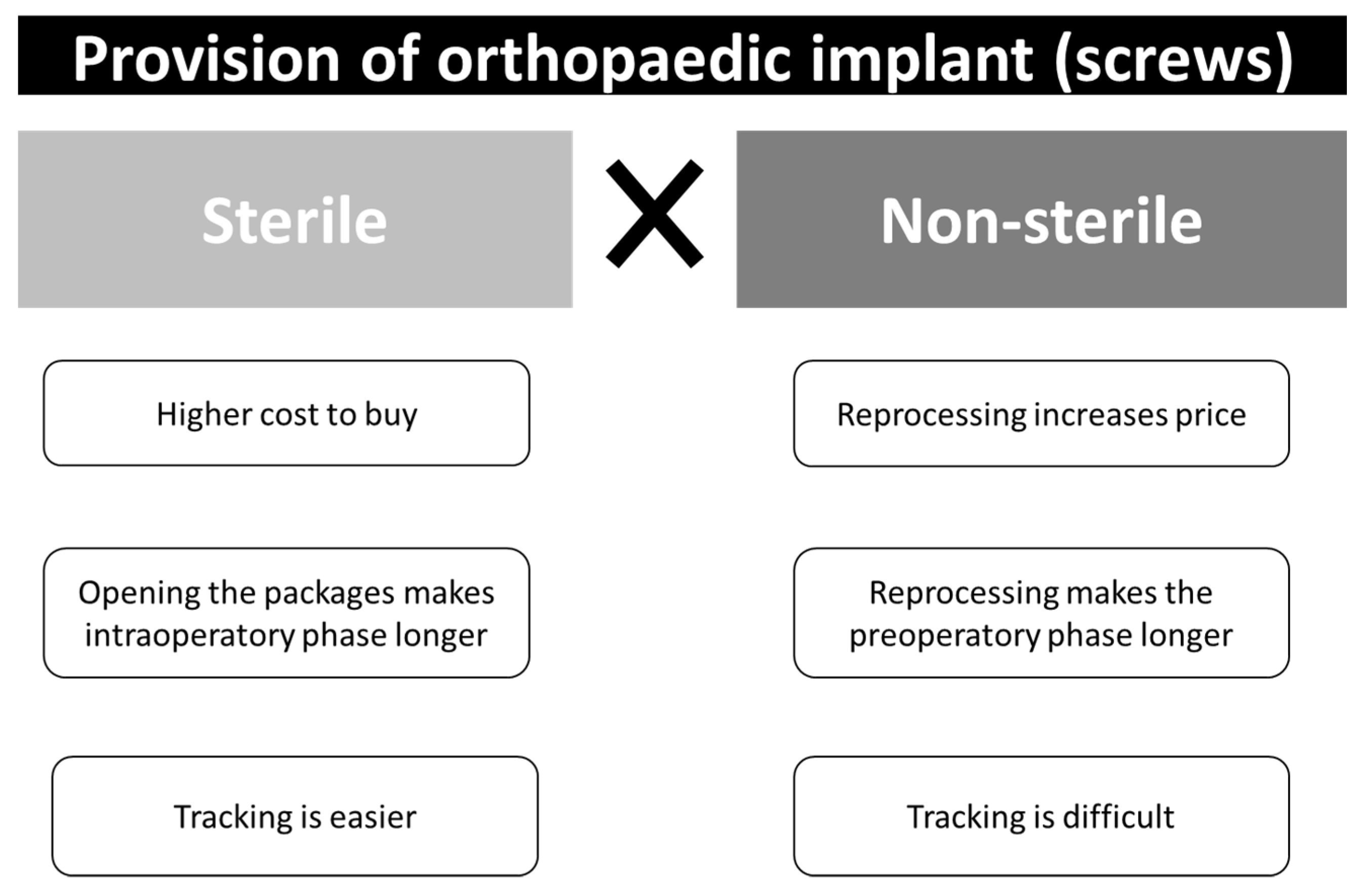
:1. Introduction
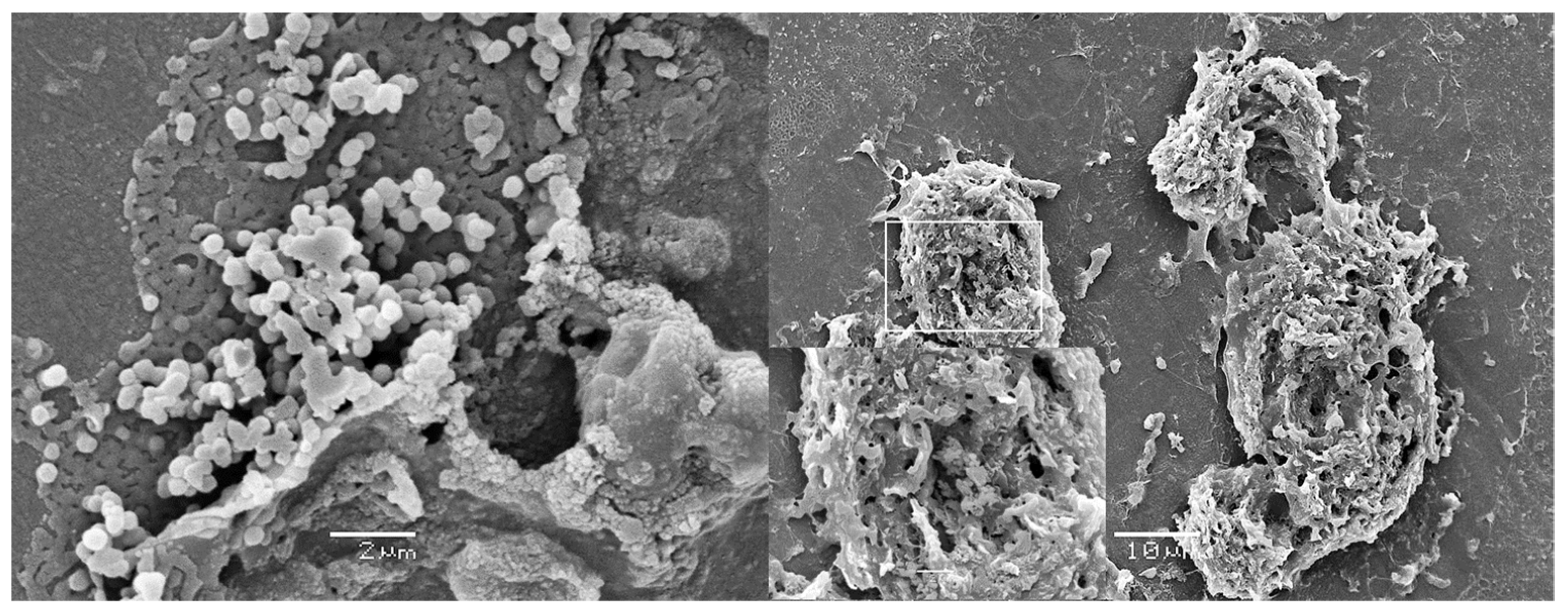
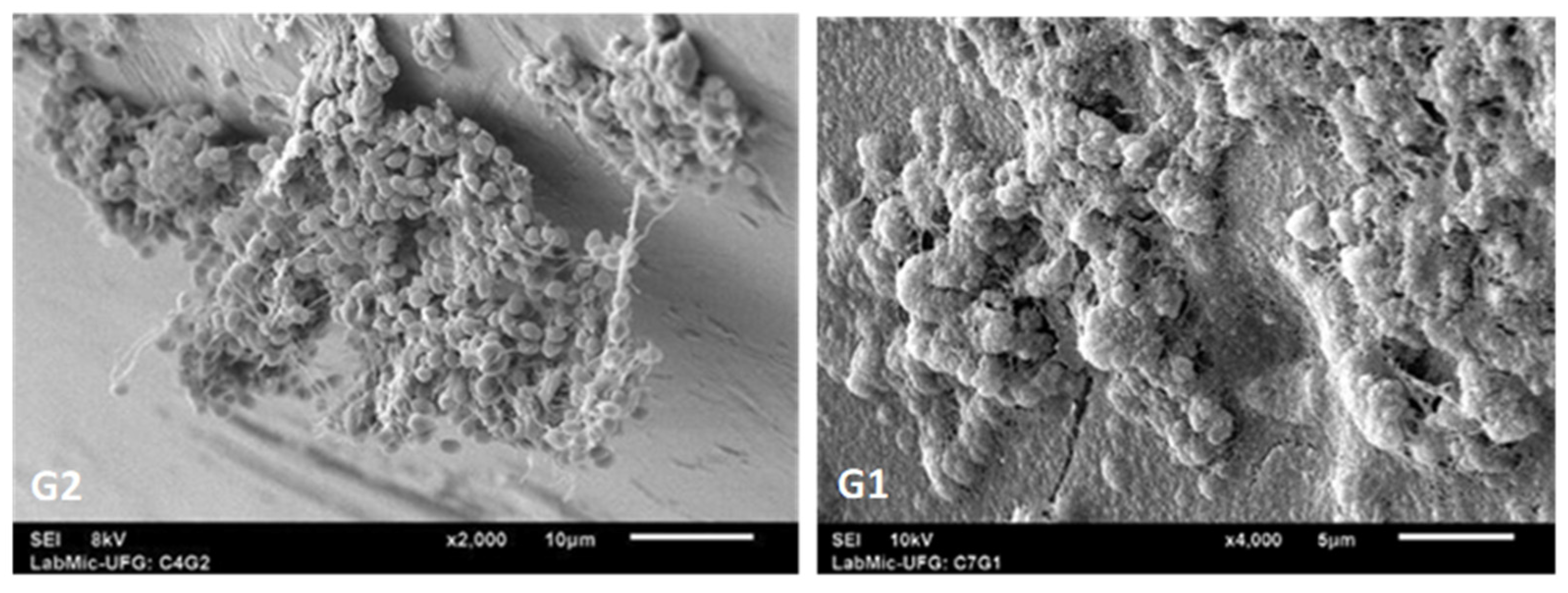
2. Microbiological Contamination: Biofilm
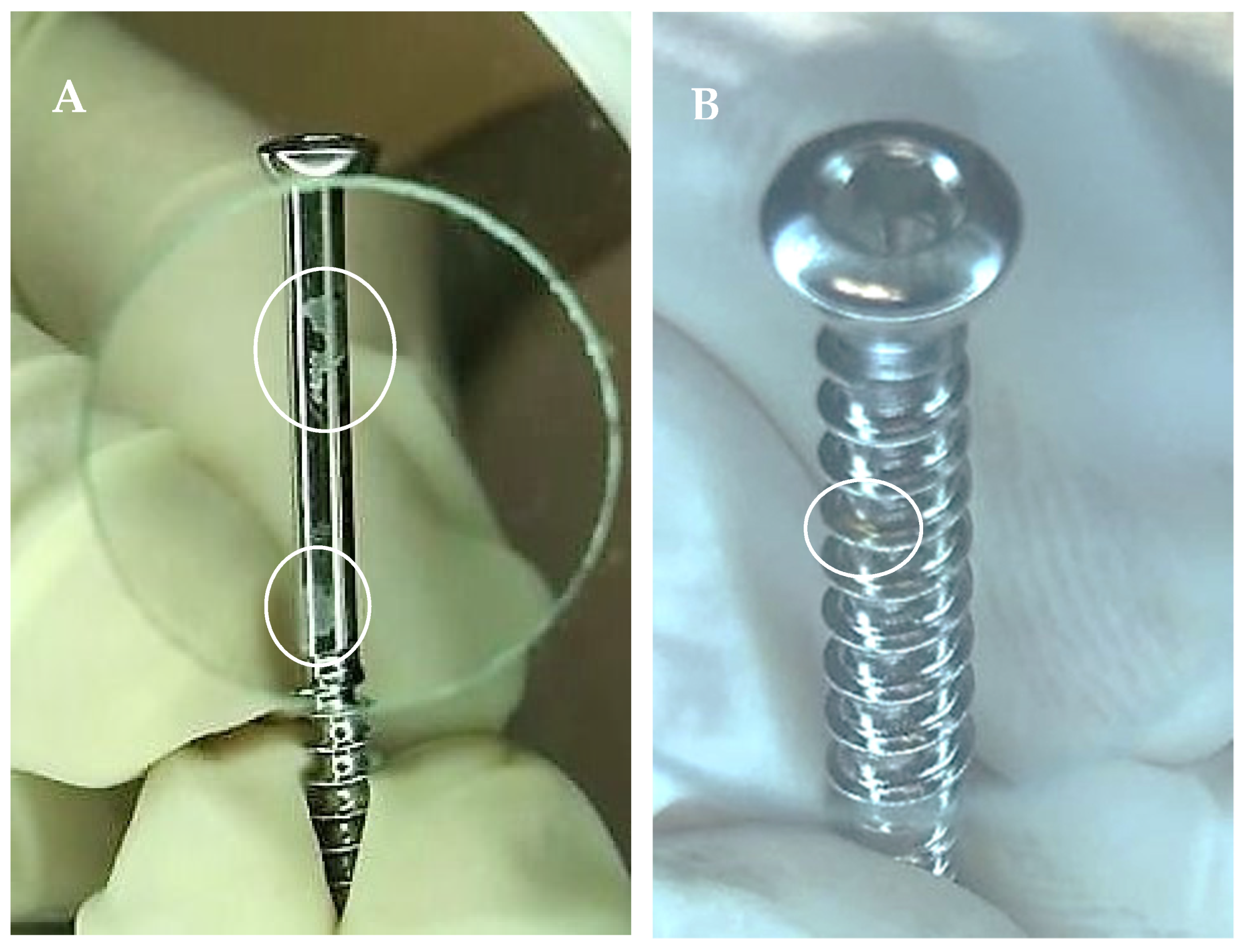
3. Non-Microbiological Contamination and Surface Damage
4. Acquisition through Loaner Companies
5. Initiatives to Solve This Issue and Further Challenges
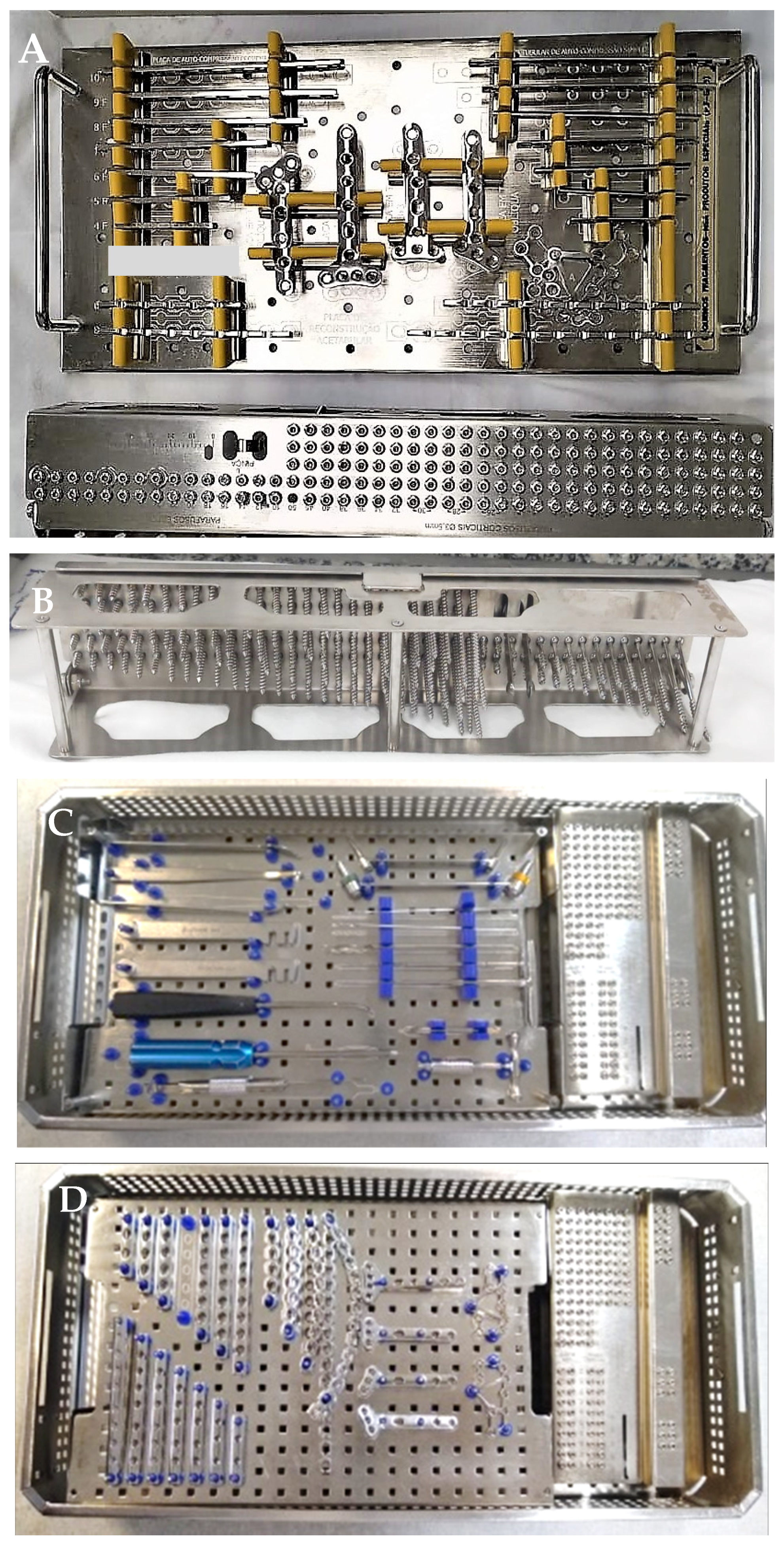
- The impossibility to remove some racks which hold the implants/screws from the surgical tray for reprocessing in automated equipment, such as ultrasonic washers;
- The screw rack is not designed for cleaning. The screws sitting on the rack limit the cleaning fluid access, thus cleaning and rinsing of each individual screws become challenging [33];
- Absence of clear criteria for a safe number of times the implant can be reprocessed;
- Absence of test to guarantee the biocompatibility and stability of an implant after multiple reprocessing;
- The impossibility to guarantee the quality of loaner implants reprocessed in several different healthcare services. This is particularly important in those situations where the loaner kits are returned to the company cleaned but not sterilized;
- The impossibility to guarantee the quality and safety of implants not used in surgery, but reprocessed together with contaminated surgical instruments; and those implants have been tried in the patient, but not implanted, and returned to the surgical tray;
- Who to “blame” in the event of an adverse effect related to these implants?
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seavey, R. Reducing the risks associated with loaner instrumentation and implants. AORN J. 2010, 92, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Decontamination: Migration to Single—Use Pre-Sterilized Individually Wrapped Small Orthopedic Implants. 29 January 2007. Available online: www.sehd.scot.nhs.uk/mels/hdl2007_04.pdf (accessed on 15 July 2021).
- Winthrop, T.G.; Sion, B.A.; Gaines, C. Loaner instrumentation: Processing the unknown. AORN J. 2007, 85, 566–573. [Google Scholar] [CrossRef]
- Amann, B.; Bröcheler, P.; Carter, A.; Diedrich, D.; Fiedler, S.; Forster, A.; Jones, A.; Kamer, M.; Krüger, S.; Thomann, R.; et al. Reprocessing Implants, Supplied in an Unsterile state, for Orthopaedics and Traumatology—Part 2. Injury 2015, 5, 374–376. [Google Scholar]
- Costa, D.M.; Lopes, L.K.O.; Vickery, K.; Watanabe, E.; Vasconcelos, L.S.N.O.L.; de Paula, M.C.; Melo, D.S.; Hu, H.; Deva, A.K.; Tipple, A.F.V. Reprocessing safety issues associated with complex-design orthopaedic loaned surgical instruments and implants. Injury 2018, 49, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Tipple, A.F.V.; Costa, D.M.; Lopes, L.K.O.; Veloso, T.R.; Pereira, L.A.; Hu, H.; Melo, D.D.S.; de Trindade, J.P.A.; Vickery, K. Reprocessing of loaned surgical instruments/implants in Australia and Brazil: A survey of those at the coalface. Infect. Dis. Health 2022, 27, 23–30. [Google Scholar] [CrossRef]
- Vickery, K.; Hu, H.; Jacombs, A.S.; Bradshaw, D.A.; Deva, A.K. A review of bacterial biofilms and their role in device-associated infection. Infect. Dis. Health 2013, 18, 61–66. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Costerton, J.W.; Ellis, B.; Lam, K.; Johnson, F.; Khoury, A.E. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob. Agents Chemother. 1994, 38, 2803–2809. [Google Scholar] [CrossRef]
- Lopes, L.K.O.; Costa, D.M.; Tipple, A.F.V.; Watanabe, E.; Castillo, R.B.; Hu, H.; Deva, A.; Vickery, K. Surgical instruments complex design as barrier for cleaning effectiveness, favouring biofilm formation. J. Hosp. Infect. 2019, 103, e53–e60. [Google Scholar] [CrossRef]
- Primo, M.G.B.; Tipple, A.F.V.; Costa, D.M.; Guadagnin, S.V.T.; Azevedo, A.S.; Leão-Vasconcelos, L.S.N.D.O.; Alfa, M.; Vickery, K. Biofilm accumulation in new flexible gastroscope channels in clinical use. Infect. Control Hosp. Epidemiol. 2022, 43, 174–180. [Google Scholar] [CrossRef]
- Vickery, K. Special Issue: Microbial Biofilms in Healthcare: Formation, Prevention and Treatment. Materiais 2019, 12, 2001. [Google Scholar] [CrossRef]
- Alfa, M.J.; Singh, H. Impact of wet storage and other factors on biofilm formation and contamination of patient-ready endoscopes: A narrative review. Gastrointest. Endosc. 2020, 91, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Alfa, M.; Howie, R.; Zelenitksy, S. Simulation of cyclic reprocessing buildup on reused medical devices. Comput. Biol. Med. 2009, 39, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Alfa, M.; Howie, R. Modeling microbial survival in buildup biofilm for complex medical devices. BMC Infect. Dis. 2009, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.A. Parafusos Corticais Ortopédicos em Sistema de Consignação: Análise da Integridade e Contaminação Microbiológica. Master’ Thesis, Universidade Federal de Goiás, Goiás, Brazil, 2020. [Google Scholar]
- Moraes, C.; Bruna, C.Q.M.; Lope, C.L.B.C.; Graziano, K.U. Research: Recovery of Microorganisms in Nonsterile, Reusable, Loaned Orthopedic Implants. Biomed. Instrum. Technol. 2019, 53, 351–354. [Google Scholar] [CrossRef]
- Almatroudi, A.; Tahir, S.; Hu, H.; Chowdhury, D.; Gosbell, I.B.; Jensen, S.O.; Whiteley, G.; Deva, A.; Glasbey, T.; Vickery, K. Staphylococcus aureus dry-surface biofilms are more resistant to heat treatment than traditional hydrated biofilms. J. Hosp. Infect. 2018, 98, 161–167. [Google Scholar] [CrossRef]
- Hermann, P.R.S.; Tipple, A.F.V.; Costa, D.M.; Watanabe, E.; Lopes, L.K.O.; Camargos, T.S.; Oliveira, V.C.; Nóbrega, M.M. Biofilmes na perspectiva das infecções relacionadas à assistência à saúde. In Infecção Relacionada à Assistência à Saúde: Subsídios para Assistência Segura, 1st ed.; Pedroso, C.F., Navarro, F.K.S.P., Andrade, G., Eds.; Editora Athena: Ponta Grossa, Brazil, 2021. [Google Scholar] [CrossRef]
- Zimmerli, W.; Sendi, P. Orthopaedic biofilm infections. APMIS 2017, 125, 353–364. [Google Scholar] [CrossRef]
- Agarwal, A.; Mooney, M.; Agarwal, A.G.; Jayaswal, D.; Saakyan, G.; Goel, V.; Wang, J.C.; Anand, N.; Garfin, S.; Shendge, V.; et al. High Prevalence of Biofilms on Retrieved Implants from Aseptic Pseudarthrosis Cases. Spine Surg. Relat. Res. 2020, 5, 104–108. [Google Scholar] [CrossRef]
- Agarwal, A.; Schultz, C.; Agarwal, A.K.; Wang, J.C.; Garfin, S.F.; Anand, N. Harboring Contaminants in Repeatedly Reprocessed Pedicle Screws. Glob. Spine J. 2019, 9, 173–178. [Google Scholar] [CrossRef]
- United Kingdom Department of Health. Health Technical Memorandum (HTM) 01-01: Management and Decontamination of Surgical Instruments (Medical Devices) Used in Acute Care. Part C: Steam Sterilization. UK: Department of Health. 2016. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/545863/HTM0101PartC.pdf (accessed on 30 July 2021).
- Bonsignore, L.A.; Colbrunn, R.W.; Tatro, J.M.; Messerschmitt, P.J.; Hernandez, C.J.; Goldberg, V.M.; Stewart, M.C.; Greenfield, E.M. Surface contaminants inhibit osseointegration in a novel murine model. Bone 2011, 49, 923–930. [Google Scholar] [CrossRef]
- Bonsignore, L.A.; Anderson, J.R.; Lee, Z.; Goldberg, V.M.; Greenfield, E.M. Adherent lipopolysaccharide inhibits the osseointegration of orthopedic implants by impairing osteoblast differentiation. Bone 2013, 52, 93–101. [Google Scholar] [CrossRef]
- Bonsignore, L.A.; Goldberg, V.M.; Greenfield, E.M. Machine oil inhibits the osseointegration of orthopaedic implants by impairing osteoblast attachment and spreading. J. Orthop. Res. 2015, 33, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Prior, F.; Fernie, K.; Renfrew, A.; Heneaghan, G. Alcoholic fixation of blood to surgical instruments-a possible factor in the surgical transmission of CJD? J. Hosp. Infect. 2004, 58, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.M.; Lopes, L.K.O.; Hu, H.; Tipple, A.F.V.; Vickery, K. Alcohol fixation of bacteria to surgical instruments increases cleaning difficulty and may contribute to sterilization inefficacy. Am. J. Infect. Control 2017, 45, e81–e86. [Google Scholar] [CrossRef] [PubMed]
- McAuley, T. Reprocessing of ‘Single-Use’ Orthopaedic Implants. A Study on the Effects of Repeated Reprocessing. In Proceedings of the 17th World Sterilization Congress, Brisbane, Australia, 26–29 October 2016. [Google Scholar]
- Duro, M. New IAHCSMM loaner instrumentation position paper and policy template. AORN J. 2011, 94, 287. [Google Scholar] [CrossRef]
- Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução da Diretoria Colegiada—RDC No 15, de 15 de Março de 2012. Dispõe Sobre Requisitos de Boas Práticas para o Processamento de Produtos para Saúde e dá Outras Providências. Brasília. 2012. Available online: https://www20.anvisa.gov.br/segurancadopaciente/index.php/legislacao/item/rdc-15-de-15-de-marco-de-2012 (accessed on 20 July 2021).
- World Health Organization. Decontamination and Reprocessing of Medical Devices for Health-Care Facilities. 2016. Available online: https://apps.who.int/iris/bitstream/handle/10665/250232/9789241549851-eng.pdf (accessed on 10 February 2022).
- Alfa, M.J. The “Pandora’s box” dilemma: Reprocessing of implantable screws and plates in orthopedic tray sets. Biomed. Instrum. Technol. 2012, 46, 55–59. [Google Scholar] [CrossRef]
- Alfred, M.; Catchpole, K.; Huffer, E.; Fredendall, L.; Taaffe, K.M. Work systems analysis of sterile processing: Decontamination. BMJ Qual. Saf. 2020, 29, 320–328. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2017/745 of the European Parliament and of the Council on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC. 2017. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0745 (accessed on 13 July 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, D.d.M.; Vickery, K.; Tipple, A.F.V.; Hu, H. Providing Sterile Orthopedic Implants: Challenges Associated with Multiple Reprocessing of Orthopedic Surgical Trays. Hygiene 2022, 2, 63-71. https://doi.org/10.3390/hygiene2010005
Costa DdM, Vickery K, Tipple AFV, Hu H. Providing Sterile Orthopedic Implants: Challenges Associated with Multiple Reprocessing of Orthopedic Surgical Trays. Hygiene. 2022; 2(1):63-71. https://doi.org/10.3390/hygiene2010005
Chicago/Turabian StyleCosta, Dayane de Melo, Karen Vickery, Anaclara Ferreira Veiga Tipple, and Honghua Hu. 2022. "Providing Sterile Orthopedic Implants: Challenges Associated with Multiple Reprocessing of Orthopedic Surgical Trays" Hygiene 2, no. 1: 63-71. https://doi.org/10.3390/hygiene2010005
APA StyleCosta, D. d. M., Vickery, K., Tipple, A. F. V., & Hu, H. (2022). Providing Sterile Orthopedic Implants: Challenges Associated with Multiple Reprocessing of Orthopedic Surgical Trays. Hygiene, 2(1), 63-71. https://doi.org/10.3390/hygiene2010005








