Abstract
Orthopedic implants, such as screws, are provided in a non-sterile state and must be reprocessed before each use, therefore they may be subjected to multiple reprocessing cycles until they are implanted in the patient. The effect of these various reprocessing cycles on the quality and safety of these implants has been a subject of concern and discussion around the world. In this narrative review, we discuss the four main challenges associated with supplying these non-sterile implants to the same standard, with respect to their quality and safety, as implants that are provided sterile: microbiological contamination (focusing on biofilm), non-microbiological contamination, surface damage, and their acquisition in surgical trays from loaner companies.
1. Introduction
Orthopedic implants are single-use medical devices and are an essential resource for bone fracture-related treatment. These devices may be provided by the manufacturer sterile, such as hip implants, or non-sterile, including plates, intramedullary nails, and screws. Screws are usually provided in racks inside surgical trays (Figure 1), along with the instruments necessary for the surgical procedure.
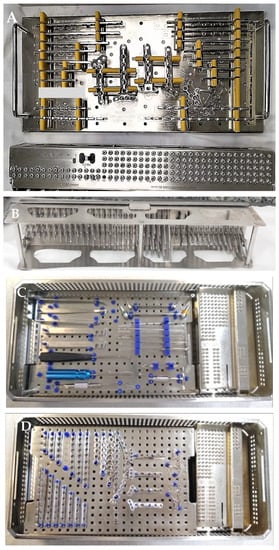
Figure 1.
Orthopedic surgical tray with implantable plates ((A,D)—view from above), screws ((A,C,D)—view from above and (B)—view from below, showing screws of different sizes) and surgical instruments ((C)—view from above) provided non-sterile by the manufacturer. Images (C,D) show the racks, which hold the screws, are not removable from the surgical box.
As these implants are not supplied sterile, they must be reprocessed before each use. The racks contain many screws, often numbering in the hundreds, however as few implants are used during a single surgical procedure, most of them remain in the racks, and are therefore subjected to multiple reprocessing. The provided screws vary greatly in size, with some being very small, measuring only millimeters, while other screws are of a size that is infrequently used, and thus remain in the tray for an extensive period and are subjected to reprocessing. Additionally, some implants have been tried in the patient but are found to be the incorrect size and therefore returned to the surgical tray for reprocessing, while unused screws may become contaminated during the procedure due to splashing of patients’ body fluids/tissue and handling by the surgical team. During reprocessing, these implants are exposed to chemical (cleaning products), physical (brushing/friction, temperature, pressure), and biological (patient fluids, microorganisms, biofilm formation) agents. The effect of these various contamination opportunities and exposure to reprocessing agents on the quality and safety of these implants has been of concern around the world [1,2,3,4,5,6]. The challenge is to supply these non-sterile implants to the same standard, with respect to their quality and safety, as implants that are provided sterile, and this is discussed below.
2. Microbiological Contamination: Biofilm
Biofilm consists of a three-dimensional aggregation of microorganisms adhered to a substrate, interface, or each other, embedded in a matrix of extracellular polymeric substances, which exhibits phenotypic and genotypic changes [7]. The biofilm structure offers various advantages to microorganisms, such as protection against the action of the immune system [8], antimicrobials [9], and cleaning, disinfection, and sterilizing agents [5,10,11]. In the healthcare context, biofilms can be classified as traditional biofilm, which forms under constantly humid conditions, such as in the lumen of a venous catheter; dry surface biofilm, such as the one that forms on countertops, on door handles, and equipment; build-up biofilm, that forms on reusable medical devices that is formed over time by the accumulation of organic matter and microorganisms, throughout the multiple cycles of use and reprocessing [12,13]. Studies have shown that build-up biofilms are more compact, adherent [14], and resistant to biocidal agents’ action [15] than traditional biofilms.
We evaluated orthopedic screws provided by three companies in Goiânia, Brazil [5]. Surgical boxes for femoral fracture fixation with an intramedullary nail that had been for clinical use for more than one year were included in the study. The longest screws from the surgical set were selected, as they are generally the least used and, therefore, undergo a greater number of reprocessing cycles before use. The implants evaluated were patient-ready after reprocessing and steam sterilization, and extensive biofilms (Figure 2) were detected on these implants.
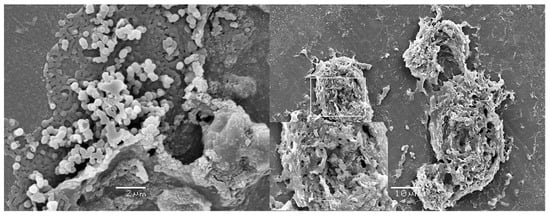
Figure 2.
Biofilms on ready-to-use orthopedic screws provided by a loaner company in Brazil. Adapted from ref. [5]. Copyright 2022, Injury.
Biofilm on patient-ready orthopedic screws was also found in a similar study (Figure 3) [16]. However, in this study biofilm was found on both the most commonly used screws (Group 1), which means that they more likely remain in the surgical box for a shorter period, and the least commonly used screw (Group 2), which means they are more likely to stay longer in the boxes. Additionally, viable bacteria (Micrococcus luteus; Kocuria rhizophila, and Staphylococcus hominis resistant to rifampicin) were also isolated from both groups. Detection of viable bacteria on steam sterilized implants is worrying but was confirmed by Moraes et al. [17]. This study was performed in response to an increase in orthopedic surgical site infection (38 patients) in a hospital in the Southeast region of Brazil and found that 30% of ready-to-use screws were contaminated with antibiotic resistant bacteria. Almatroudi et al. [18] demonstrated that between 62% to 74% of bacteria in Staphylococcus aureus dry surface biofilm survives steam sterilization at 121 °C. These findings demonstrate that biofilm can form on heat-sterilized implants subjected to multiple cycles of reprocessing, and that once formed the biofilm protects the incorporated bacteria, which poses a risk to patients.

Figure 3.
Biofilms on ready-to-use orthopedic screws provided by a loaner company in Brazil. G2—the most used screws, which remain in the surgical box for less time; G1—the least used screws, which remain in the surgical box for longer time. G2 adapted from ref. [19], Copyright 2022, Hermann et al.
Biofilm on implants may cause clinical infection [20] or sub-clinical chronic infection [20]. Interestingly, a high prevalence of biofilm was found on orthopedic screws explanted from patients with pseudarthrosis [21]. In general, pseudarthrosis and screw/implant loosening are reported as aseptic loosening, due to the absence of signs of clinical infection. However, in 70% of the cases [21] biofilm was found on the loosened implant, and in 50% of these patients the implant procedure had occurred more than 2 years previously, demonstrating the sub-clinical effect of biofilm infection. Mineralization of the bone-to-implant contact site did not occur in the region of the screws where the biofilms were, whereas in those regions without biofilm, the mineralization process occurred normally [21]. Once formed, biofilms are more resistant to the host immune system and antibiotics. Additionally, the surgical process itself compromises the blood supply, which decreases access of immune cells and antibiotics. Taken together, in many cases, the only successful treatment is to remove the implant, which poses major risks to the patient.
3. Non-Microbiological Contamination and Surface Damage
Organic and inorganic matter and surface damage have been reported on orthopedic screws [5,16,22] (Figure 4).
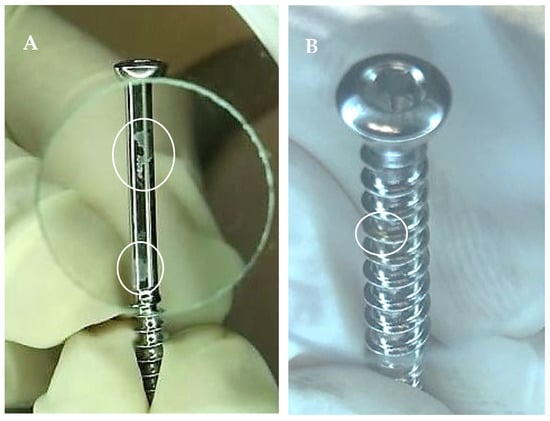
Figure 4.
Orthopedic screws in clinical use with stains ((A)—white circles) and corrosion ((B)—white circles).
Agarwal et al. [22] detected carbohydrate, lipids, residual soap, and salt on pedicle orthopedic screws, which were ready to use. Protein was detected on 96.3% of patient-ready orthopedic screws, with the majority being contaminated with more than 10 µg of protein [16]. This is double the cutoff recommended by the UK guidelines, which is 5 µg per side of the device [23]. Costa et al. [5] detected blood on one of eight loaner orthopedic surgical boxes upon delivery to a Brazilian hospital. The organic matter was probably a result of inadequate cleaning carried out by the hospital that had previously used the set. Similar findings were reported in a study carried out in the US [3]. Surgical sets can also become contaminated with debris if transported incorrectly, such as on an open trolley. The presence of organic matter on loaner surgical sets during the storage at the company, favors biofilm formation. Additionally, surface contaminants, such as lipopolysaccharide and machine oil, on orthopedic screws have been shown to inhibit the osseointegration process (in vitro) [24,25,26].
While not common, the practice of wiping surgical instruments with alcohol to remove excess organic matter can occur prior to cleaning [6]. Alcohol was found to fix haemoglobin and increase cleaning difficulty [27]. A similar effect occurred when arterial forceps were contaminated with S. aureus and treated with alcohol [28]. Additionally, forceps contaminated with blood and S. aureus or Pseudomonas aeruginosa and treated with 70% ethanol by using (a) one alcohol moistened wipe for each forceps, or (b) alcohol moistened wipe for every 4 forceps, or (c) spray on each forceps showed a significant increase in the amount of residual protein compared to those forceps treated with water only. These results lead to the conclusion that the use of alcohol on the surgical sets increases the cleaning difficulty, which may favor biofilm formation later.
Surface damage on orthopedic screws has also been verified [16,22]. McAuley [29] showed the correlation between the increase in the number of reprocessing of implants (screws) and the occurrence of corrosion and deterioration. Damage on implants favors biofilm formation, as imperfections provide shelter for microorganisms and organic matter. In addition, the degradation of the implant also results in the release of products that can cause an inflammatory reaction [22], which can culminate in the aseptic loss of the implant, or even a fracture of the implanted site.
4. Acquisition through Loaner Companies
The system of orthopedic implant acquisition, through loaner companies, is a growing phenomenon worldwide. Using this system means that healthcare services do not need to purchase instruments. Given the rapid technological advances occurring this results in a cost saving to the service as infrequently used, expensive instruments need not be purchased [1]. However, delayed delivery of loaner kits challenges reprocessing of instruments/implants and can affect their quality and safety [1,5,10].
Delayed delivery and insufficient reprocessing time can be a result of lack of communication and planning [1,6]. Unacceptable late loaner surgical kit delivery, i.e., less than 24 h before the surgical procedure occurred in approximately 60% of Brazilian and Australian hospitals [6]. This shortened delivery time may interfere with cleaning, which is the most important reprocessing step, and is fundamental for minimizing/avoiding biofilm formation. According to the International Association of Healthcare Central Service Material Management [30], the delivery of loaner instrumentation to the hospital must occur 48 h before the surgical procedure, or 72 h ahead, if this is the first use of that service. In countries such as Brazil, the current legislation on reprocessing of reusable medical devices [31] does not dictate a minimum deadline for delivering loaner surgical sets and leaves this responsibility to the sterilizing service unit manager.
Agarwal et al. [22] found that 19 h would be theoretically necessary to reprocess pedicle screws if the manufacturers’ instructions for use were followed, making this a barrier to proper reprocessing. In contrast, direct observation in a Sterilization Service Unit found reprocessing took less than 2 h and this possibly represents the reality in many sterilizing units worldwide.
Ultrasonic cleaners are effective and recommended for cleaning hard-to-reach parts of surgical instruments/medical devices, including orthopedic screws [32], however, huge variations and challenges are still presented. A major challenge is the absence of instructions for reprocessing these implants, as highlighted by Alfa [33], who assessed seven orthopedic screw manufacturers’ instructions for use. Alfa found that the instructions for use do not clearly state how these implants should be reprocessed, for example, only two manufacturers presented validated cleaning protocols, and only two manufacturers recommended that the screw racks should be removed from the surgical box for cleaning. The author also highlighted that screw racks are not designed for cleaning, but for presentation and easy removal by the surgeon. This underscores how emphasis on instrument design for ease of use can increase the difficulty and quality of reprocessing.
A recent decontamination work systems analysis of sterile processing by Alfred et al. reported about 2% of surgical trays were spotted as instant failures in sterile reprocessing and returned for re-cleaning decontamination [34]. The authors identified 21 different performance shaping factors, 30 potential failures, 16 types of process variance, and 10 outcome variances in decontamination [34], highlighting the huge challenges presented.
5. Initiatives to Solve This Issue and Further Challenges
In summary, many studies have shown that implants, such as orthopedic screws subjected to multiple reprocessing cycles, readily become contaminated by biofilm [5], viable bacteria, protein [16,17], and other organic and inorganic matter. Multiple cycles of processing also damage their surfaces [16,22], which increases the risk of additional contamination. In addition, it is difficult to reprocessing these devices adequately [22]. Therefore, the concern is: how to guarantee the quality and the safety of these implants?
In Scotland [3] and Germany [4], the answer to this issue had been to use only implants that are provided sterilized, which eliminates the problems arising from the reprocessing. In Scotland, the use of sterile supplied implants was recommended about 15 years ago [3], and one of the factors considered for this change is the negative effect of multiple reprocessing on the implant quality.
European Union regulation 2017/745 [35] requires that whoever reprocesses a single-use medical device, which includes orthopedic implants, must assume the obligations incumbent on the manufacturer. Furthermore, the safety and performance of reprocessed device must be equivalent to a new device when first supplied by the manufacturer. Moreover, this regulation states that the medical devices must be identified with a “unique identification code” to allow tracking, and that the deadline for adapting services to this regulation is 2023 for those classified in the class II, in which orthopedic screws belong.
In addition to patient safety, which is paramount, other factors must be considered when discussing the acquisition of implants delivered sterile or non-sterile, as both types of implant delivery have advantages and disadvantages, such as the cost, the time, and the traceability (Figure 5).
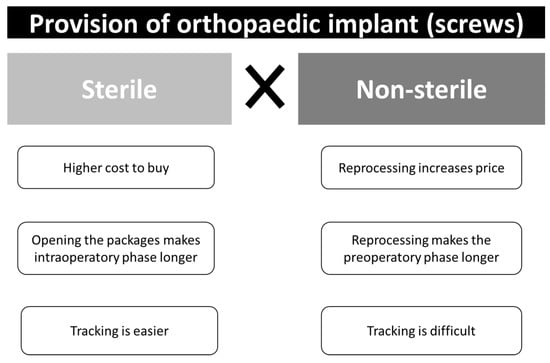
Figure 5.
Factors related cost, time, and the traceability of orthopedic implants delivered sterile or non-sterile.
Additional issues inherent to the use of implants that are subjected to reprocessing include:
- The impossibility to remove some racks which hold the implants/screws from the surgical tray for reprocessing in automated equipment, such as ultrasonic washers;
- The screw rack is not designed for cleaning. The screws sitting on the rack limit the cleaning fluid access, thus cleaning and rinsing of each individual screws become challenging [33];
- Absence of clear criteria for a safe number of times the implant can be reprocessed;
- Absence of test to guarantee the biocompatibility and stability of an implant after multiple reprocessing;
- The impossibility to guarantee the quality of loaner implants reprocessed in several different healthcare services. This is particularly important in those situations where the loaner kits are returned to the company cleaned but not sterilized;
- The impossibility to guarantee the quality and safety of implants not used in surgery, but reprocessed together with contaminated surgical instruments; and those implants have been tried in the patient, but not implanted, and returned to the surgical tray;
- Who to “blame” in the event of an adverse effect related to these implants?
These issues require a partnership discussion with all involved in this process, including sanitary authorities, manufacturers, loaner companies’ managers and workers, sterilizing service unit’s manager and workers, hospital managers, and surgical theater personnel.
6. Conclusions
The use of implants provided as non-sterile should be phased out, as multiple reprocessing increases the risk of surgical infections, which incur increased social and economic costs. Investment on individual sterile screw package, strategies to improve aseptic opening of sterile screw packages, and helping surgeons to precisely estimate the screw size to use would be valuable and decrease both patients’ surgical infection risks and costs related to screw reprocessing and acquisition.
Author Contributions
Conceptualization, H.H. and D.d.M.C.; writing—original draft preparation, D.d.M.C., K.V., A.F.V.T., H.H.; writing—review and editing, D.d.M.C., K.V., A.F.V.T., H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
Conflicts of Interest
The authors declare no conflict of interest.
References
- Seavey, R. Reducing the risks associated with loaner instrumentation and implants. AORN J. 2010, 92, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Decontamination: Migration to Single—Use Pre-Sterilized Individually Wrapped Small Orthopedic Implants. 29 January 2007. Available online: www.sehd.scot.nhs.uk/mels/hdl2007_04.pdf (accessed on 15 July 2021).
- Winthrop, T.G.; Sion, B.A.; Gaines, C. Loaner instrumentation: Processing the unknown. AORN J. 2007, 85, 566–573. [Google Scholar] [CrossRef]
- Amann, B.; Bröcheler, P.; Carter, A.; Diedrich, D.; Fiedler, S.; Forster, A.; Jones, A.; Kamer, M.; Krüger, S.; Thomann, R.; et al. Reprocessing Implants, Supplied in an Unsterile state, for Orthopaedics and Traumatology—Part 2. Injury 2015, 5, 374–376. [Google Scholar]
- Costa, D.M.; Lopes, L.K.O.; Vickery, K.; Watanabe, E.; Vasconcelos, L.S.N.O.L.; de Paula, M.C.; Melo, D.S.; Hu, H.; Deva, A.K.; Tipple, A.F.V. Reprocessing safety issues associated with complex-design orthopaedic loaned surgical instruments and implants. Injury 2018, 49, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Tipple, A.F.V.; Costa, D.M.; Lopes, L.K.O.; Veloso, T.R.; Pereira, L.A.; Hu, H.; Melo, D.D.S.; de Trindade, J.P.A.; Vickery, K. Reprocessing of loaned surgical instruments/implants in Australia and Brazil: A survey of those at the coalface. Infect. Dis. Health 2022, 27, 23–30. [Google Scholar] [CrossRef]
- Vickery, K.; Hu, H.; Jacombs, A.S.; Bradshaw, D.A.; Deva, A.K. A review of bacterial biofilms and their role in device-associated infection. Infect. Dis. Health 2013, 18, 61–66. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Costerton, J.W.; Ellis, B.; Lam, K.; Johnson, F.; Khoury, A.E. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob. Agents Chemother. 1994, 38, 2803–2809. [Google Scholar] [CrossRef]
- Lopes, L.K.O.; Costa, D.M.; Tipple, A.F.V.; Watanabe, E.; Castillo, R.B.; Hu, H.; Deva, A.; Vickery, K. Surgical instruments complex design as barrier for cleaning effectiveness, favouring biofilm formation. J. Hosp. Infect. 2019, 103, e53–e60. [Google Scholar] [CrossRef]
- Primo, M.G.B.; Tipple, A.F.V.; Costa, D.M.; Guadagnin, S.V.T.; Azevedo, A.S.; Leão-Vasconcelos, L.S.N.D.O.; Alfa, M.; Vickery, K. Biofilm accumulation in new flexible gastroscope channels in clinical use. Infect. Control Hosp. Epidemiol. 2022, 43, 174–180. [Google Scholar] [CrossRef]
- Vickery, K. Special Issue: Microbial Biofilms in Healthcare: Formation, Prevention and Treatment. Materiais 2019, 12, 2001. [Google Scholar] [CrossRef]
- Alfa, M.J.; Singh, H. Impact of wet storage and other factors on biofilm formation and contamination of patient-ready endoscopes: A narrative review. Gastrointest. Endosc. 2020, 91, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Alfa, M.; Howie, R.; Zelenitksy, S. Simulation of cyclic reprocessing buildup on reused medical devices. Comput. Biol. Med. 2009, 39, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Alfa, M.; Howie, R. Modeling microbial survival in buildup biofilm for complex medical devices. BMC Infect. Dis. 2009, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.A. Parafusos Corticais Ortopédicos em Sistema de Consignação: Análise da Integridade e Contaminação Microbiológica. Master’ Thesis, Universidade Federal de Goiás, Goiás, Brazil, 2020. [Google Scholar]
- Moraes, C.; Bruna, C.Q.M.; Lope, C.L.B.C.; Graziano, K.U. Research: Recovery of Microorganisms in Nonsterile, Reusable, Loaned Orthopedic Implants. Biomed. Instrum. Technol. 2019, 53, 351–354. [Google Scholar] [CrossRef]
- Almatroudi, A.; Tahir, S.; Hu, H.; Chowdhury, D.; Gosbell, I.B.; Jensen, S.O.; Whiteley, G.; Deva, A.; Glasbey, T.; Vickery, K. Staphylococcus aureus dry-surface biofilms are more resistant to heat treatment than traditional hydrated biofilms. J. Hosp. Infect. 2018, 98, 161–167. [Google Scholar] [CrossRef]
- Hermann, P.R.S.; Tipple, A.F.V.; Costa, D.M.; Watanabe, E.; Lopes, L.K.O.; Camargos, T.S.; Oliveira, V.C.; Nóbrega, M.M. Biofilmes na perspectiva das infecções relacionadas à assistência à saúde. In Infecção Relacionada à Assistência à Saúde: Subsídios para Assistência Segura, 1st ed.; Pedroso, C.F., Navarro, F.K.S.P., Andrade, G., Eds.; Editora Athena: Ponta Grossa, Brazil, 2021. [Google Scholar] [CrossRef]
- Zimmerli, W.; Sendi, P. Orthopaedic biofilm infections. APMIS 2017, 125, 353–364. [Google Scholar] [CrossRef]
- Agarwal, A.; Mooney, M.; Agarwal, A.G.; Jayaswal, D.; Saakyan, G.; Goel, V.; Wang, J.C.; Anand, N.; Garfin, S.; Shendge, V.; et al. High Prevalence of Biofilms on Retrieved Implants from Aseptic Pseudarthrosis Cases. Spine Surg. Relat. Res. 2020, 5, 104–108. [Google Scholar] [CrossRef]
- Agarwal, A.; Schultz, C.; Agarwal, A.K.; Wang, J.C.; Garfin, S.F.; Anand, N. Harboring Contaminants in Repeatedly Reprocessed Pedicle Screws. Glob. Spine J. 2019, 9, 173–178. [Google Scholar] [CrossRef]
- United Kingdom Department of Health. Health Technical Memorandum (HTM) 01-01: Management and Decontamination of Surgical Instruments (Medical Devices) Used in Acute Care. Part C: Steam Sterilization. UK: Department of Health. 2016. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/545863/HTM0101PartC.pdf (accessed on 30 July 2021).
- Bonsignore, L.A.; Colbrunn, R.W.; Tatro, J.M.; Messerschmitt, P.J.; Hernandez, C.J.; Goldberg, V.M.; Stewart, M.C.; Greenfield, E.M. Surface contaminants inhibit osseointegration in a novel murine model. Bone 2011, 49, 923–930. [Google Scholar] [CrossRef]
- Bonsignore, L.A.; Anderson, J.R.; Lee, Z.; Goldberg, V.M.; Greenfield, E.M. Adherent lipopolysaccharide inhibits the osseointegration of orthopedic implants by impairing osteoblast differentiation. Bone 2013, 52, 93–101. [Google Scholar] [CrossRef]
- Bonsignore, L.A.; Goldberg, V.M.; Greenfield, E.M. Machine oil inhibits the osseointegration of orthopaedic implants by impairing osteoblast attachment and spreading. J. Orthop. Res. 2015, 33, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Prior, F.; Fernie, K.; Renfrew, A.; Heneaghan, G. Alcoholic fixation of blood to surgical instruments-a possible factor in the surgical transmission of CJD? J. Hosp. Infect. 2004, 58, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.M.; Lopes, L.K.O.; Hu, H.; Tipple, A.F.V.; Vickery, K. Alcohol fixation of bacteria to surgical instruments increases cleaning difficulty and may contribute to sterilization inefficacy. Am. J. Infect. Control 2017, 45, e81–e86. [Google Scholar] [CrossRef] [PubMed]
- McAuley, T. Reprocessing of ‘Single-Use’ Orthopaedic Implants. A Study on the Effects of Repeated Reprocessing. In Proceedings of the 17th World Sterilization Congress, Brisbane, Australia, 26–29 October 2016. [Google Scholar]
- Duro, M. New IAHCSMM loaner instrumentation position paper and policy template. AORN J. 2011, 94, 287. [Google Scholar] [CrossRef]
- Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução da Diretoria Colegiada—RDC No 15, de 15 de Março de 2012. Dispõe Sobre Requisitos de Boas Práticas para o Processamento de Produtos para Saúde e dá Outras Providências. Brasília. 2012. Available online: https://www20.anvisa.gov.br/segurancadopaciente/index.php/legislacao/item/rdc-15-de-15-de-marco-de-2012 (accessed on 20 July 2021).
- World Health Organization. Decontamination and Reprocessing of Medical Devices for Health-Care Facilities. 2016. Available online: https://apps.who.int/iris/bitstream/handle/10665/250232/9789241549851-eng.pdf (accessed on 10 February 2022).
- Alfa, M.J. The “Pandora’s box” dilemma: Reprocessing of implantable screws and plates in orthopedic tray sets. Biomed. Instrum. Technol. 2012, 46, 55–59. [Google Scholar] [CrossRef]
- Alfred, M.; Catchpole, K.; Huffer, E.; Fredendall, L.; Taaffe, K.M. Work systems analysis of sterile processing: Decontamination. BMJ Qual. Saf. 2020, 29, 320–328. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2017/745 of the European Parliament and of the Council on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC. 2017. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0745 (accessed on 13 July 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).