Algae-Based Protective Coatings for Sustainable Infrastructure: A Novel Framework Linking Material Chemistry, Techno-Economics, and Environmental Functionality
Abstract
1. Introduction
2. Surface Degradation Mechanisms in Infrastructure Materials
2.1. Abiotic Degradation Mechanisms
2.1.1. UV Radiation Induced Degradation
2.1.2. Thermal Degradation and Temperature Cycling
2.1.3. Moisture, Humidity, and Water Penetration
2.1.4. Chemical Attack from Acidic, Alkaline, and Saline Environments
2.1.5. Mechanical Wear and Abrasion
2.1.6. Synergistic Interactions Among Abiotic Factors
2.2. Biotic Degradation Mechanisms (Microbiologically Influenced Corrosion)
2.2.1. Bacterial Induced Deterioration
2.2.2. Fungal Degradation
2.2.3. Algal and Lichen Colonization
2.3. Extent and Severity of Degradation
2.4. Implications for Protective Coating Design and Relevance to Algae Based Coatings
| Degradation Factor | Mechanism | Resulting Damage | References |
|---|---|---|---|
| UV radiation | Polymer chain scission, oxidation, photodegradation | Embrittlement, discoloration, reduced adhesion | [16,17] |
| Thermal cycling | Expansion–contraction mismatch; freeze–thaw | Cracking, delamination, interface failure | [18] |
| Moisture & humidity | Hydrolysis, leaching, ion transport | Blistering, corrosion, concrete dissolution | [16,19,20] |
| Chemical pollutants | Acid/alkali attack, chloride ingress | Corrosion, mineral dissolution, ASR expansion | [21] |
| Abrasion | Mechanical erosion and friction | Surface wear, loss of coating thickness | [17] |
| Aerobic bacteria | Organic acid secretion | Mineral dissolution, pitting | [16,22] |
| Anaerobic bacteria | Sulfate reduction producing H2S | Deep pitting, steel corrosion | [16,21] |
| Silicate bacteria | Active dissolution of silicate phases | Concrete surface weakening | [16,19] |
| Fungi | Enzyme-mediated degradation, hyphal penetration | Severe softening, discoloration, loss of adhesion | [17,18,23] |
| Algae & lichens | Biofilm formation, organic acid secretion | Increased porosity, roughness, moisture retention | [16,24] |
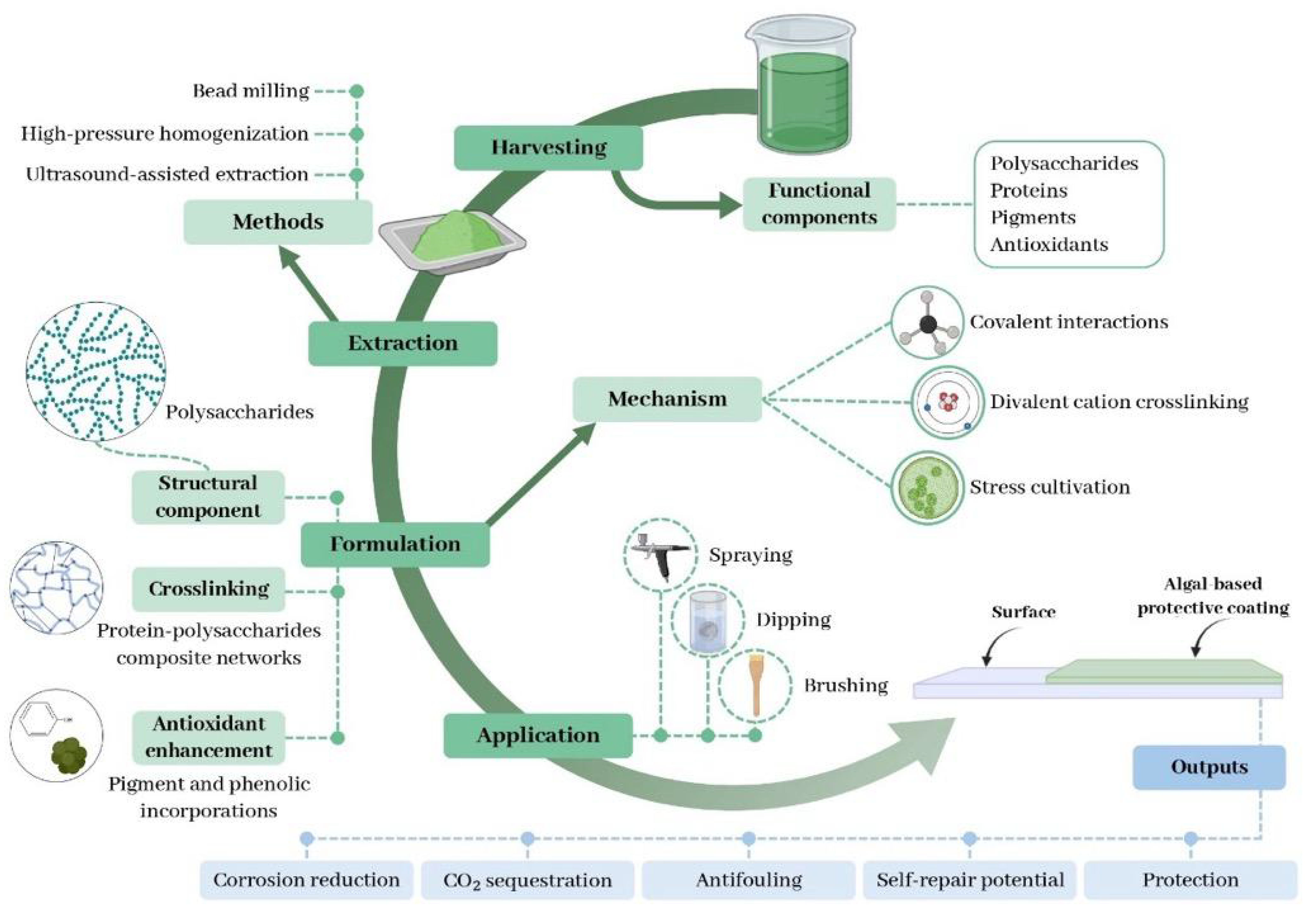
3. Fundamentals of Algae-Based Coatings
3.1. Chemistry and Functional Compounds
3.2. Coating Formulation and Application
3.3. Advantages over Conventional Coatings
3.4. Comparison with Other Bio-Based Coating Systems
3.4.1. Lignin-Based Coatings
3.4.2. Nanocellulose-Based Coatings
3.4.3. Chitosan-Based Coatings
3.4.4. Shellac-Based Natural Resin Coatings
3.4.5. Vegetable-Oil-Based Coatings and Polyols
3.4.6. Bacterial Biopolymer-Based Coatings
4. Production & Scalability Challenges
4.1. Cultivation Systems
4.2. Processing & Extraction Challenges
4.3. Scale-Up & Manufacturing Barriers
4.4. Sensitivity Analysis
4.4.1. Cultivation Sensitivity
4.4.2. Processing Sensitivity
4.4.3. Environmental Sensitivity: Moisture, Temperature, and Oxygen
4.4.4. Cost and Scalability Sensitivity
4.5. Technical Feasibility Outlook
5. Multi-Functional Performance Analysis
5.1. Mechanical Properties
5.2. Protective Properties
5.3. Environmental Impact
6. Economic Viability & Industrial Adoption
6.1. Cost Analysis vs. Petroleum-Based Coatings
6.2. Sensitivity Analysis of Key Economic Drivers for Industrial-Scale Adoption
| Parameter | Reported Values/Ranges | Economic Sensitivity | System Type/Operational Context | References |
|---|---|---|---|---|
| Biomass Productivity | 0.1–0.75 g·L−1·day−1 (derived from 20–90 g·m−2·day−1) | Very High: Directly determines OPEX per kg biomass | Raceway ponds, thin-layer cascades, PBRs | [104,105] |
| Energy Cost (Mixing/Aeration) | 10–20 W·m−3 (raceway); 248.64 kWh/day (large-scale PBR) | High: Strongly dependent on electricity price | Raceway ponds, PBRs (AL/GH) | [106,107] |
| Solvent Extraction Efficiency | 2% (unruptured) to 70%+ (ruptured); 94–100% (ETBE, limonene, hexane) | High: Reduces biomass & energy demand; lowers solvent recovery cost | Lipid-based and pigment-extraction downstream processes | [108,109] |
| Carbon Credit Price | $17–261 ton−1 CO2 (modeled SCC scenarios) | Moderate–High: Can offset 10–20% OPEX | CO2-utilizing cultivation systems | [112] |
| CAPEX | €50–100·m−3 (ponds); €8000–28,000·m−3 (PBRs); $26–28 M (extraction plants); $739M+ (full systems) | Very High: Largest barrier to industrial adoption | PBRs, raceways, biorefinery systems | [106,107] |
| OPEX | 60–82% of total cost; 1.1–2.5 €/kg labor; $2.06–3.79/kg lipid extraction | Very High: Dominant cost driver | All cultivation + downstream systems | [106,109] |
6.3. Market Readiness & Commercial Barriers
6.4. Regulatory Standards & Future Implementation Strategies
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, N.; Phelan, P.E.; Harris, C.; Langevin, J.; Nelson, B.; Sawyer, K. Past visions, current trends, and future context: A review of building energy, carbon, and sustainability. Renew. Sustain. Energy Rev. 2018, 82, 976–993. [Google Scholar] [CrossRef]
- Liu, J.L.; Wang, K.; Xiahou, Q.R.; Liu, F.M.; Zou, J.; Kong, Y. China’s long-term low carbon transition pathway under the urbanization process. Adv. Clim. Change Res. 2019, 10, 240–249. [Google Scholar] [CrossRef]
- Wang, N.; Pei, H.; Xiang, W.; Li, T.; Lin, S.; Wu, J.; Chen, Z.; Wu, H.; Li, C.; Wu, H. Rapid Screening of Microalgae as Potential Sources of Natural Antioxidants. Foods 2023, 12, 2652. [Google Scholar] [CrossRef]
- Maung, T.Z.; Bishop, J.E.; Holt, E.; Turner, A.M.; Pfrang, C. Indoor Air Pollution and the Health of Vulnerable Groups: A Systematic Review Focused on Particulate Matter (PM), Volatile Organic Compounds (VOCs) and Their Effects on Children and People with Pre-Existing Lung Disease. Int. J. Environ. Res. Public Health 2022, 19, 8752. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnan, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Toughening of Petroleum Based (DGEBA) Epoxy Resins with Various Renewable Resources Based Flexible Chains for High Performance Applications: A Review. Ind. Eng. Chem. Res. 2018, 57, 2711–2726. [Google Scholar] [CrossRef]
- Chandra, R.; Iqbal, H.M.N.; Vishal, G.; Lee, H.S.; Nagra, S. Algal biorefinery: A sustainable approach to valorize algal-based biomass towards multiple product recovery. Bioresour. Technol. 2019, 278, 346–359. [Google Scholar] [CrossRef]
- Calovi, M.; Zanardi, A.; Rossi, S. Recent Advances in Bio-Based Wood Protective Systems: A Comprehensive Review. Appl. Sci. 2024, 14, 736. [Google Scholar] [CrossRef]
- Fabris, M.; Abbriano, R.M.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiuparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef]
- Prathiksha, K.; Priyadharshini, S.J.E.; Jacob, J.; Jayakumar, A.V.; Rao, P.H. Microalgal pigments as natural hues in environmentally-sustainable and commercially-prospective biopaints. J. Appl. Phycol. 2024, 36, 191–204. [Google Scholar] [CrossRef]
- Natarajan, S.; Lakshmi, D.S.; Thiagarajan, V.; Mrudula, P.; Chandrasekaran, N.; Mukherjee, A. Antifouling and anti-algal effects of chitosan nanocomposite (TiO2/Ag) and pristine (TiO2 and Ag) films on marine microalgae Dunaliella salina. J. Environ. Chem. Eng. 2018, 6, 6870–6880. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, H.; Chen, X.; McDonald, A.; Li, H. Effect of Chlorella vulgaris Biofilm Adhesion on Electrochemical Behaviors of Wire Arc-Sprayed Aluminum Coatings. J. Therm. Spray Technol. 2020, 29, 1991–2000. [Google Scholar] [CrossRef]
- Tang, J.; Liu, B.; Gao, L.; Wang, W.; Liu, T.; Su, G. Impacts of surface wettability and roughness of styrene-acrylic resin films on adhesion behavior of microalgae Chlorella sp. Colloids Surf. B Biointerfaces 2021, 199, 111522. [Google Scholar] [CrossRef]
- Caldwell, G.S.; In-Na, P.; Hart, R.; Sharp, E.; Stefanova, A.; Pickersgill, M.; Walker, M.; Unthank, M.; Perry, J.; Lee, J.G.M. Immobilising microalgae and cyanobacteria as biocomposites: New opportunities to intensify algae biotechnology and bioprocessing. Energies 2021, 14, 2566. [Google Scholar] [CrossRef]
- Tong, C.Y.; Chua, M.X.; Tan, W.H.; Derek, C.J.C. Microalgal extract as bio-coating to enhance biofilm growth of marine microalgae on microporous membranes. Chemosphere 2023, 315, 137712. [Google Scholar] [CrossRef]
- Martins, R.; Sales, H.; Pontes, R.; Nunes, J.; Gouveia, I. Food Wastes and Microalgae as Sources of Bioactive Compounds and Pigments in a Modern Biorefinery: A Review. Antioxidants 2023, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L.; Heikkilä, A.M.; Pandey, K.K.; Bruckman, L.S.; White, C.C.; Zhu, M.; Zhu, L. Effects of UV radiation on natural and synthetic materials. Photochem. Photobiol. Sci. 2023, 22, 1177–1202. [Google Scholar] [CrossRef]
- Zhao, J.; Cai, G.; Cui, L.; Si Larbi, A.; Tsavdaridis, K.D. Deterioration of basic properties of the materials in FRP-strengthening RC structures under ultraviolet exposure. Polymers 2017, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Riahinezhad, M.; Hallman, M.; Masson, J.F. Critical review of polymeric building envelope materials: Degradation, durability and service life prediction. Buildings 2021, 11, 299. [Google Scholar] [CrossRef]
- Videla, H.A.; Herrera, L.K. Microbiologically influenced corrosion: Looking to the future. Int. Microbiol. 2005, 8, 169. [Google Scholar] [PubMed]
- Shkromada, O.; Paliy, A.; Nechyporenko, O.; Naumenko, O.; Nechyporenko, V.; Burlaka, O.; Reshetnichenko, A.; Tsereniuk, O.; Shvets, O.; Paliy, A. Improvement of functional performance of concrete in livestock buildings through the use of complex admixtures. East.-Eur. J. Enterp. Technol. 2019, 5, 14–23. [Google Scholar] [CrossRef]
- Pletincx, S.; Fockaert, L.L.I.; Mol, J.M.C.; Hauffman, T.; Terryn, H. Probing the formation and degradation of chemical interactions from model molecule/metal oxide to buried polymer/metal oxide interfaces. npj Mater. Degrad. 2019, 3, 23. [Google Scholar] [CrossRef]
- Grantham, M.C.; Dove, P.M.; Dichristina, T.J. Microbially catalyzed dissolution of iron and aluminum oxyhydroxide mineral surface coatings. Geochim. Cosmochim. Acta 1997, 61, 4467–4477. [Google Scholar] [CrossRef]
- Tyagi, P.; Verma, R.K.; Jain, N. Fungal degradation of cultural heritage monuments and management options. Curr. Sci. 2021, 121, 1553–1560. [Google Scholar] [CrossRef]
- Khan, M.S.; Yang, K.; Liu, Z.; Zhou, L.; Liu, W.; Lin, S.; Wang, X.; Shang, C. Microorganisms Involved in the Biodegradation and Microbiological Corrosion of Structural Materials. Coatings 2023, 13, 1683. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, Y.J.; Kwon, O.N.; Cha, K.H.; Um, B.H.; Chung, D.; Pan, C.H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Lourenço-Lopes, C.; Garcia-Oliveira, P.; Carpena, M.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Scientific approaches on extraction, purification and stability for the commercialization of fucoxanthin recovered from brown algae. Foods 2020, 9, 1113. [Google Scholar] [CrossRef]
- Reyes, F.A.; Mendiola, J.A.; Ibañez, E.; Del Valle, J.M. Astaxanthin extraction from Haematococcus pluvialis using CO2-expanded ethanol. J. Supercrit. Fluids 2014, 92, 75–83. [Google Scholar] [CrossRef]
- Pappou, S.; Dardavila, M.M.; Savvidou, M.G.; Louli, V.; Magoulas, K.; Voutsas, E. Extraction of Bioactive Compounds from Ulva lactuca. Appl. Sci. 2022, 12, 2117. [Google Scholar] [CrossRef]
- Bedoux, G.; Pliego-Cortés, H.; Dufau, C.; Hardouin, K.; Boulho, R.; Freile-Pelegrín, Y.; Robledo, D.; Bourgougnon, N. Production and properties of mycosporine-like amino acids isolated from seaweeds. Adv. Bot. Res. 2020, 95, 213–245. [Google Scholar] [CrossRef]
- Saluri, M.; Kaldmäe, M.; Tuvikene, R. Extraction and quantification of phycobiliproteins from the red alga Furcellaria lumbricalis. Algal Res. 2019, 37, 115–123. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Ribeiro, A.R.; Patinha, C.; Silva, A.M.S.; Cardoso, S.M.; Costa, R. Water Extraction Kinetics of Bioactive Compounds of Fucus vesiculosus. Molecules 2019, 24, 3408. [Google Scholar] [CrossRef] [PubMed]
- Sirmerova, M.; Prochazkova, G.; Siristova, L.; Kolska, Z.; Branyik, T. Adhesion of Chlorella vulgaris to solid surfaces, as mediated by physicochemical interactions. J. Appl. Phycol. 2013, 25, 1687–1695. [Google Scholar] [CrossRef]
- Saji, S.; Hebden, A.; Goswami, P.; Du, C. A Brief Review on the Development of Alginate Extraction Process and Its Sustainability. Sustainability 2022, 14, 5181. [Google Scholar] [CrossRef]
- Jan, M.; Kazik, P. Nannochloropsis: Biology, Biotechnological Potential and Challenges; Nova Science Publishers, Inc.: New York, NY, USA, 2017. [Google Scholar]
- Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. A green approach for alginate extraction from Sargassum muticum brown seaweed using ultrasound-assisted technique. Int. J. Biol. Macromol. 2019, 124, 451–459. [Google Scholar] [CrossRef]
- Gómez de Saravia, S.G.; Rastelli, S.E.; Blustein, G.; Viera, M.R. Natural compounds as potential algaecides for waterborne paints. J. Coat. Technol. Res. 2018, 15, 1191–1200. [Google Scholar] [CrossRef]
- Demarco, M.; de Moraes, J.O.; Ferrari, M.C.; de Farias Neves, F.; Laurindo, J.B.; Tribuzi, G. Production of Spirulina (Arthrospira platensis) powder by innovative and traditional drying techniques. J. Food Process Eng. 2022, 45, e13919. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. Comparative Analysis of the Advantages and Disadvantages of Utilizing Spirulina-Derived Pigment as a Bio-Based Colorant for Wood Impregnator. Coatings 2023, 13, 1158. [Google Scholar] [CrossRef]
- Thevarajah, B.; Nishshanka, G.K.S.H.; Premaratne, M.; Nimarshana, P.H.V.; Nagarajan, D.; Chang, J.S.; Ariyadasa, T.U. Large-scale production of Spirulina-based proteins and c-phycocyanin: A biorefinery approach. Biochem. Eng. J. 2022, 185, 108541. [Google Scholar] [CrossRef]
- Pocha, C.K.R.; Chia, W.Y.; Chew, K.W.; Munawaroh, H.S.H.; Show, P.L. Current advances in recovery and biorefinery of fucoxanthin from Phaeodactylum tricornutum. Algal Res. 2022, 65, 102735. [Google Scholar] [CrossRef]
- Thanh, T.T.T.; Quach, T.M.T.; Nguyen, T.N.; Vu Luong, D.; Bui, M.L.; Tran, T.T. Van Structure and cytotoxic activity of ulvan extracted from green seaweed Ulva lactuca. Int. J. Biol. Macromol. 2016, 93, 695–702. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and drawbacks of ultrasound-assisted extraction for the recovery of bioactive compounds from marine algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef]
- Sousa, V.; Pereira, R.N.; Vicente, A.A.; Dias, O.; Geada, P. Microalgae biomass as an alternative source of biocompounds: New insights and future perspectives of extraction methodologies. Food Res. Int. 2023, 173, 113282. [Google Scholar] [CrossRef]
- Rodrigues, R.D.P.; de Castro, F.C.; de Santiago-Aguiar, R.S.; Rocha, M.V.P. Ultrasound-assisted extraction of phycobiliproteins from Spirulina (Arthrospira) platensis using protic ionic liquids as solvent. Algal Res. 2018, 31, 454–462. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Kovačević, D.B.; Garofulić, I.E.; Dragović-Uzelac, V. Advanced technologies for the extraction of marine brown algal polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef]
- Gomez, C.G.; Pérez Lambrecht, M.V.; Lozano, J.E.; Rinaudo, M.; Villar, M.A. Influence of the extraction-purification conditions on final properties of alginates obtained from brown algae (Macrocystis pyrifera). Int. J. Biol. Macromol. 2009, 44, 365–371. [Google Scholar] [CrossRef]
- Kartik, A.; Akhil, D.; Lakshmi, D.; Panchamoorthy Gopinath, K.; Arun, J.; Sivaramakrishnan, R.; Pugazhendhi, A. A critical review on production of biopolymers from algae biomass and their applications. Bioresour. Technol. 2021, 329, 124868. [Google Scholar] [CrossRef] [PubMed]
- Noreen, A.; Mahmood, S.; Khalid, A.; Takriff, S.; Anjum, M.; Riaz, L.; Ditta, A.; Mahmood, T. Synthesis and characterization of bio-based UV curable polyurethane coatings from algal biomass residue. Biomass Convers. Biorefinery 2024, 14, 11505–11521. [Google Scholar] [CrossRef]
- Kim, B.; Youn Lee, S.; Lakshmi Narasimhan, A.; Kim, S.; Oh, Y.K. Cell disruption and astaxanthin extraction from Haematococcus pluvialis: Recent advances. Bioresour. Technol. 2022, 343, 126124. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.R.; Senhorinho, G.N.A.; Scott, J.A. Microalgae under environmental stress as a source of antioxidants. Algal Res. 2020, 52, 102104. [Google Scholar] [CrossRef]
- Shin, W.; Hong, J.S.; Kim, D.Y.; Kim, S.Y.; Hyun, K.; Park, J.D.; Ahn, K.H. Effect of cellulose nanocrystals on the emulsion stability and rheological properties of microalgal Pickering emulsions. Algal Res. 2024, 83, 103731. [Google Scholar] [CrossRef]
- Eyssautier-Chuine, S.; Franco-Castillo, I.; Misra, A.; Hubert, J.; Vaillant-Gaveau, N.; Streb, C.; Mitchell, S.G. Evaluating the durability and performance of polyoxometalate-ionic liquid coatings on calcareous stones: Preventing biocolonisation in outdoor environments. Sci. Total Environ. 2023, 884, 163739. [Google Scholar] [CrossRef] [PubMed]
- Cheah, Y.T.; Chan, D.J.C. Physiology of microalgal biofilm: A review on prediction of adhesion on substrates. Bioengineered 2021, 12, 7577–7599. [Google Scholar] [CrossRef] [PubMed]
- Mari, A.; Fafalis, C.; Krokida, M. Evaluation of Edible Coatings from Components from Chlorella vulgaris and Comparison with Conventional Coatings. Coatings 2024, 14, 621. [Google Scholar] [CrossRef]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef]
- Patil, C.K.; Jirimali, H.D.; Paradeshi, J.S.; Chaudhari, B.L.; Gite, V.V. Functional antimicrobial and anticorrosive polyurethane composite coatings from algae oil and silver doped egg shell hydroxyapatite for sustainable development. Prog. Org. Coat. 2019, 128, 127–136. [Google Scholar] [CrossRef]
- Brannum, D.J.; Price, E.J.; Villamil, D.; Kozawa, S.; Brannum, M.; Berry, C.; Semco, R.; Wnek, G.E. Flame-retardant polyurethane foams: One-pot, bioinspired silica nanoparticle coating. ACS Appl. Polym. Mater. 2019, 1, 2015–2022. [Google Scholar] [CrossRef]
- Sarwer, A.; Hamed, S.M.; Osman, A.I.; Jamil, F.; Al-Muhtaseb, A.H.; Alhajeri, N.S.; Rooney, D.W. Algal biomass valorization for biofuel production and carbon sequestration: A review. Environ. Chem. Lett. 2022, 20, 2797–2851. [Google Scholar] [CrossRef]
- Sreejaya, M.M.; Jeevan Sankar, R.; K, R.; Pillai, N.P.; Ramkumar, K.; Anuvinda, P.; Meenakshi, V.S.; Sadanandan, S. Lignin-based organic coatings and their applications: A review. Mater. Today Proc. 2022, 60, 494–501. [Google Scholar] [CrossRef]
- Kerch, G. Chitosan films and coatings prevent losses of fresh fruit nutritional quality: A review. Trends Food Sci. Technol. 2015, 46, 159–166. [Google Scholar] [CrossRef]
- Yan, X.; Wang, L. Preparation of shellac resin microcapsules coated with urea formaldehyde resin and properties of waterborne paint films for Tilia amurensis Rupr. Membranes 2020, 10, 278. [Google Scholar] [CrossRef]
- Stradolini, P.; Gryczak, M.; Petzhold, C.L. Polyols from castor oil (Ricinus communis) and epoxidized soybean oil (Glycine max) for application as a lubricant base. JAOCS J. Am. Oil Chem. Soc. 2024, 101, 321–334. [Google Scholar] [CrossRef]
- Ghosh, S.; Lahiri, D.; Nag, M.; Dey, A.; Sarkar, T.; Pathak, S.K.; Edinur, H.A.; Pati, S.; Ray, R.R. Bacterial biopolymer: Its role in pathogenesis to effective biomaterials. Polymers 2021, 13, 1242. [Google Scholar] [CrossRef]
- Oruganti, R.K.; Biji, A.P.; Lanuyanger, T.; Show, P.L.; Sriariyanun, M.; Upadhyayula, V.K.K.; Gadhamshetty, V.; Bhattacharyya, D. Artificial intelligence and machine learning tools for high-performance microalgal wastewater treatment and algal biorefinery: A critical review. Sci. Total Environ. 2023, 876, 162797. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, H.; Cheng, P.; Chang, T.; Chen, P.; Zhou, C.; Ruan, R. Development of integrated culture systems and harvesting methods for improved algal biomass productivity and wastewater resource recovery—A review. Sci. Total Environ. 2020, 746, 141039. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Duan, C.; Yi, S.; Gao, Z.; Xiao, C.; Agathos, S.N.; Wang, G.; Li, J. Biotechnological production of astaxanthin from the microalga Haematococcus pluvialis. Biotechnol. Adv. 2020, 43, 107602. [Google Scholar] [CrossRef]
- Zhu, J.; Wakisaka, M.; Omura, T.; Yang, Z.; Yin, Y.; Fang, W. Advances in industrial harvesting techniques for edible microalgae: Recent insights into sustainable, efficient methods and future directions. J. Clean. Prod. 2024, 436, 140626. [Google Scholar] [CrossRef]
- Koller, M. Design of closed photobioreactors for algal cultivation. In Algal Biorefineries: Volume 2: Products and Refinery Design; Springer: Berlin/Heidelberg, Germany, 2015; pp. 133–186. [Google Scholar]
- Wahlström, N.; Harrysson, H.; Undeland, I.; Edlund, U. A Strategy for the Sequential Recovery of Biomacromolecules from Red Macroalgae Porphyra umbilicalis Kützing. Ind. Eng. Chem. Res. 2018, 57, 42–53. [Google Scholar] [CrossRef]
- Esquivel-Hernández, D.A.; López, V.H.; Rodríguez-Rodríguez, J.; Alemán-Nava, G.S.; Cuéllar-Bermúdez, S.P.; Rostro-Alanis, M.; Parra-Saldívar, R. Supercritical carbon dioxide and microwave-assisted extraction of functional lipophilic compounds from Arthrospira platensis. Int. J. Mol. Sci. 2016, 17, 658. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Schuur, B.; Kersten, S.R.A.; Brilman, D.W.F. Opportunities for switchable solvents for lipid extraction from wet algal biomass: An energy evaluation. Algal Res. 2015, 11, 271–283. [Google Scholar] [CrossRef]
- Figueroa, F.L. Mycosporine-Like Amino Acids from Marine Resource. Mar. Drugs 2021, 19, 18. [Google Scholar] [CrossRef]
- Peng, S.; Hu, A.; Ai, J.; Zhang, W.; Wang, D. Changes in molecular structure of extracellular polymeric substances (EPS) with temperature in relation to sludge macro-physical properties. Water Res. 2021, 201, 117316. [Google Scholar] [CrossRef]
- Jurić, S.; Jurić, M.; Król-Kilińska, Ż.; Vlahoviček-Kahlina, K.; Vinceković, M.; Dragović-Uzelac, V.; Donsì, F. Sources, stability, encapsulation and application of natural pigments in foods. Food Rev. Int. 2022, 38, 1735–1790. [Google Scholar] [CrossRef]
- Randhawa, K.S. Synthesis, Properties, and Environmental Impact of Hybrid Pigments. Sci. World J. 2024, 2024, 2773950. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Feng, Y.; Shi, J. Carbon Dots as Multifunctional Nanofillers in Sustainable Food Packaging: A Comprehensive Review. Foods 2025, 14, 3082. [Google Scholar] [CrossRef]
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: A review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef]
- Linares, G.; Rojas, M.L. Ultrasound-assisted extraction of natural pigments from food processing by-products: A review. Front. Nutr. 2022, 9, 891462. [Google Scholar] [CrossRef] [PubMed]
- Nonglait, D.L.; Gokhale, J.S. Review insights on the demand for natural pigments and their recovery by emerging microwave-assisted extraction (MAE). Food Bioprocess Technol. 2024, 17, 1681–1705. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.D.; Albahnasawi, A.; Eyvaz, M.; Al Maskari, T.; Nassani, D.E.; Abu Amr, S.S.; Abujazar, M.S.S.; Bashir, M.J.K. An overview of green bioprocessing of algae-derived biochar and biopolymers: Synthesis, preparation, and potential applications. Energies 2023, 16, 791. [Google Scholar] [CrossRef]
- Dai, K.; Cao, S.; Yuan, J.; Wang, Z.; Li, H.; Yuan, C.; Yan, X.; Xing, R. Recent Advances of Sustainable UV Shielding Materials: Mechanisms and Applications. ACS Appl. Mater. Interfaces 2025, 17, 30402–30422. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Yeh, K.-L.; Aisyah, R.; Lee, D.-J.; Chang, J.-S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef]
- Penloglou, G.; Pavlou, A.; Kiparissides, C. Recent advancements in photo-bioreactors for microalgae cultivation: A brief overview. Processes 2024, 12, 1104. [Google Scholar] [CrossRef]
- Barajas-Ferreira, C.; Contreras-Ropero, J.E.; García-Martínez, J.B.; Barajas-Solano, A.F.; Zuorro, A. Large-Scale Microalgae Drying. In Microalgae Horizons: Fundamentals, Innovations, and Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2025; pp. 401–423. [Google Scholar]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Kampa, Ł.; Sadowski, Ł.; Królicka, A. The use of synthetic and natural fibers in epoxy coatings: A comparative mechanical and economic analysis. Int. J. Adhes. Adhes. 2022, 117, 103017. [Google Scholar] [CrossRef]
- Habib-ur-Rahman, M.; Ahmad, A.; Raza, A.; Hasnain, M.U.; Alharby, H.F.; Alzahrani, Y.M.; Bamagoos, A.A.; Hakeem, K.R.; Ahmad, S.; Nasim, W. Impact of climate change on agricultural production; Issues, challenges, and opportunities in Asia. Front. Plant Sci. 2022, 13, 925548. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef]
- Alvira, D.; Antorán, D.; Manyà, J.J. Plant-derived hard carbon as anode for sodium-ion batteries: A comprehensive review to guide interdisciplinary research. Chem. Eng. J. 2022, 447, 137468. [Google Scholar] [CrossRef]
- Wong, G.K.Y.; Chiu, A.T. Gene therapy, gene targeting and induced pluripotent stem cells: Applications in monogenic disease treatment. Biotechnol. Adv. 2011, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dec, R.; Guza, M.; Dzwolak, W. Reduction of a disulfide-constrained oligo-glutamate peptide triggers self-assembly of β2-type amyloid fibrils with the chiroptical properties determined by supramolecular chirality. Int. J. Biol. Macromol. 2020, 162, 866–872. [Google Scholar] [CrossRef]
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, J.; Figueroa, F.L. Mycosporine-like amino acids from red macroalgae: UV-photoprotectors with potential cosmeceutical applications. Appl. Sci. 2021, 11, 5112. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef]
- Cole, G.M.; Greene, J.M.; Quinn, J.C.; McDaniel, B.; Kemp, L.; Simmons, D.; Hodges, T.; Nobles, D.; Weiss, T.L.; McGowen, J.; et al. Integrated techno-economic and life cycle assessment of a novel algae-based coating for direct air carbon capture and sequestration. J. CO2 Util. 2023, 69, 102421. [Google Scholar] [CrossRef]
- Patil, C.K.; Jirimali, H.D.; Paradeshi, J.S.; Chaudhari, B.L.; Alagi, P.K.; Mahulikar, P.P.; Hong, S.C.; Gite, V.V. Chemical transformation of renewable algae oil to polyetheramide polyols for polyurethane coatings. Prog. Org. Coat. 2021, 151, 106084. [Google Scholar] [CrossRef]
- Cruz, C.G.; da Silveira, J.T.; Ferrari, F.M.; Costa, J.A.V.; da Rosa, A.P.C. The use of poly(3-hydroxybutyrate), C-phycocyanin, and phenolic compounds extracted from Spirulina sp. LEB 18 in latex paint formulations. Prog. Org. Coat. 2019, 135, 100–104. [Google Scholar] [CrossRef]
- Kumar, B.R.; Mathimani, T.; Sudhakar, M.P.; Rajendran, K.; Nizami, A.S.; Brindhadevi, K.; Pugazhendhi, A. A state of the art review on the cultivation of algae for energy and other valuable products: Application, challenges, and opportunities. Renew. Sustain. Energy Rev. 2021, 138, 110649. [Google Scholar] [CrossRef]
- Zuber, M.; Zia, K.M.; Noreen, A.; Bukhari, S.A.; Aslam, N.; Sultan, N.; Jabeen, M.; Shi, B. Algae Based Polymers, Blends, and Composites: Chemistry, Biotechnology and Materials Science; Elsevier: Amsterdam, The Netherlands, 2017; pp. 499–529. [Google Scholar] [CrossRef]
- Sosa-Hernández, J.E.; Romero-Castillo, K.D.; Parra-Arroyo, L.; Aguilar-Aguila-Isaías, M.A.; García-Reyes, I.E.; Ahmed, I.; Parra-Saldivar, R.; Bilal, M.; Iqbal, H.M.N. Mexican microalgae biodiversity and state-of-the-art extraction strategies to meet sustainable circular economy challenges: High-value compounds and their applied perspectives. Mar. Drugs 2019, 17, 174. [Google Scholar] [CrossRef]
- Gieroba, B.; Kalisz, G.; Krysa, M.; Khalavka, M.; Przekora, A. Application of Vibrational Spectroscopic Techniques in the Study of the Natural Polysaccharides and Their Cross-Linking Process. Int. J. Mol. Sci. 2023, 24, 2630. [Google Scholar] [CrossRef]
- Verma, J.; Nigam, S.; Sinha, S.; Bhattacharya, A. Development of polyurethane based anti-scratch and anti-algal coating formulation with silica-titania core-shell nanoparticles. Vacuum 2018, 153, 24–34. [Google Scholar] [CrossRef]
- Mouga, T.; Fernandes, I.B. The Red Seaweed Giant Gelidium (Gelidium corneum) for New Bio-Based Materials in a Circular Economy Framework. Earth 2022, 3, 788–813. [Google Scholar] [CrossRef]
- Wilson, M.H.; Shea, A.; Groppo, J.; Crofcheck, C.; Quiroz, D.; Quinn, J.C.; Crocker, M. Algae-Based Beneficial Re-use of Carbon Emissions Using a Novel Photobioreactor: A Techno-Economic and Life Cycle Analysis. Bioenergy Res. 2021, 14, 292–302. [Google Scholar] [CrossRef]
- Kiran Kumar, A.; Sharma, S.; Dixit, G.; Shah, E.; Patel, A. Techno-economic analysis of microalgae production with simultaneous dairy effluent treatment using a pilot-scale High Volume V-shape Pond system. Renew. Energy 2020, 145, 1620–1632. [Google Scholar] [CrossRef]
- Acién Fernández, F.G.; Fernández Sevilla, J.M.; Molina Grima, E. Costs analysis of microalgae production. In Biomass, Biofuels, Biochemicals: Biofuels from Algae, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 551–566. [Google Scholar] [CrossRef]
- Oostlander, P.C.; van Houcke, J.; Wijffels, R.H.; Barbosa, M.J. Microalgae production cost in aquaculture hatcheries. Aquaculture 2020, 525, 735310. [Google Scholar] [CrossRef]
- Silva, C.; Soliman, E.; Cameron, G.; Fabiano, L.A.; Seider, W.D.; Dunlop, E.H.; Coaldrake, A.K. Commercial-scale biodiesel production from algae. Ind. Eng. Chem. Res. 2014, 53, 5311–5324. [Google Scholar] [CrossRef]
- Yap, B.H.J.; Crawford, S.A.; Dumsday, G.J.; Scales, P.J.; Martin, G.J.O. A mechanistic study of algal cell disruption and its effect on lipid recovery by solvent extraction. Algal Res. 2014, 5, 112–120. [Google Scholar] [CrossRef]
- Zapata-Boada, S.; Gonzalez-Miquel, M.; Jobson, M.; Cuéllar-Franca, R.M. Integrating Technoeconomic, Environmental, and Safety Criteria in Solvent Screening for Extraction Processes: The Case of Algae Lipid Extraction. ACS Sustain. Chem. Eng. 2022, 10, 472–485. [Google Scholar] [CrossRef]
- Marrone, B.L.; Lacey, R.E.; Anderson, D.B.; Bonner, J.; Coons, J.; Dale, T.; Downes, C.M.; Fernando, S.; Fuller, C.; Goodall, B. Review of the harvesting and extraction program within the National Alliance for Advanced Biofuels and Bioproducts. Algal Res. 2018, 33, 470–485. [Google Scholar] [CrossRef]
- Zapata-Boada, S.; Gonzalez-Miquel, M.; Jobson, M.; Cuéllar-Franca, R.M. Techno-economic and Environmental Analysis of Algae Biodiesel Production via Lipid Extraction Using Alternative Solvents. Ind. Eng. Chem. Res. 2022, 61, 18030–18044. [Google Scholar] [CrossRef]
- Cruce, J.R.; Quinn, J.C. Economic viability of multiple algal biorefining pathways and the impact of public policies. Appl. Energy 2018, 233–234, 735–746. [Google Scholar] [CrossRef]
- Sahu, S.; Sharma, S.; Kaur, A.; Singh, G.; Khatri, M.; Arya, S.K. Algal carbohydrate polymers: Catalytic innovations for sustainable development. Carbohydr. Polym. 2024, 327, 121691. [Google Scholar] [CrossRef]
- Di Fazio, M.; Fratello, C.; Paglialunga, G.; Mignardi, S.; Vergelli, L.; Frasca, F.; Rigon, C.; Ioele, M.; Gioventù, E.; Antonacci, A.; et al. The NYMPHA Algae Extract as a New Consolidant for the Restoration of Cultural Heritage: Studies and Considerations on Its Effectiveness on Painted Marble. Sustainability 2024, 16, 6868. [Google Scholar] [CrossRef]
- Kim, D.; Kang, S.M. Red Algae-Derived Carrageenan Coatings for Marine Antifouling Applications. Biomacromolecules 2020, 21, 5086–5092. [Google Scholar] [CrossRef]
- Bin Abu Sofian, A.D.A.; Lim, H.R.; Manickam, S.; Ang, W.L.; Show, P.L. Towards a Sustainable Circular Economy: Algae-Based Bioplastics and the Role of Internet-of-Things and Machine Learning. ChemBioEng Rev. 2024, 11, 39–59. [Google Scholar] [CrossRef]
- Markou, G.; Eliopoulos, C.; Argyri, A.; Arapoglou, D. Production of Arthrospira (Spirulina) platensis enriched in β-glucans through phosphorus limitation. Appl. Sci. 2021, 11, 8121. [Google Scholar] [CrossRef]
- Abdelhamid, F.M.; Elshopakey, G.E.; Aziza, A.E. Ameliorative effects of dietary Chlorella vulgaris and β-glucan against diazinon-induced toxicity in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 96, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, A.; Holdt, S.L.; De Francisci, D.; Alvarado-Morales, M.; Mishra, H.N.; Angelidaki, I. Extraction of alginate from Sargassum muticum: Process optimization and study of its functional activities. J. Appl. Phycol. 2016, 28, 3625–3634. [Google Scholar] [CrossRef]
- Patil, G.; Chethana, S.; Madhusudhan, M.C.; Raghavarao, K.S.M.S. Fractionation and purification of the phycobiliproteins from Spirulina platensis. Bioresour. Technol. 2008, 99, 7393–7396. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.; Cotas, J.; Gutiérrez, I.B.; Gonçalves, A.M.M.; Critchley, A.T.; Hinaloc, L.A.R.; Roleda, M.Y.; Pereira, L. Advanced Extraction Techniques and Physicochemical Properties of Carrageenan from a Novel Kappaphycus alvarezii Cultivar. Mar. Drugs 2024, 22, 491. [Google Scholar] [CrossRef]
- Ha, H.T.; Cuong, D.X.; Thuy, L.H.; Thuan, P.T.; Tuyen, D.T.T.; Mo, V.T.; Dong, D.H. Carrageenan of Red Algae Eucheuma gelatinae: Extraction, Antioxidant Activity, Rheology Characteristics, and Physicochemistry Characterization. Molecules 2022, 27, 1268. [Google Scholar] [CrossRef]
- Barakat, K.M.; Ismail, M.M.; Abou El Hassayeb, H.E.; El Sersy, N.A.; Elshobary, M.E. Chemical characterization and biological activities of ulvan extracted from Ulva fasciata (Chlorophyta). Rend. Lincei 2022, 33, 829–841. [Google Scholar] [CrossRef]
- Cabral, E.M.; Mondala, J.R.M.; Oliveira, M.; Przyborska, J.; Fitzpatrick, S.; Rai, D.K.; Sivagnanam, S.P.; Garcia-Vaquero, M.; O’Shea, D.; Devereux, M.; et al. Influence of molecular weight fractionation on the antimicrobial and anticancer properties of a fucoidan rich-extract from the macroalgae Fucus vesiculosus. Int. J. Biol. Macromol. 2021, 186, 994–1002. [Google Scholar] [CrossRef]
- January, G.G.; Naidoo, R.K.; Kirby-McCullough, B.; Bauer, R. Assessing methodologies for fucoidan extraction from South African brown algae. Algal Res. 2019, 40, 101517. [Google Scholar] [CrossRef]
- Qiu, Y.; Jiang, H.; Fu, L.; Ci, F.; Mao, X. Porphyran and oligo-porphyran originating from red algae Porphyra: Preparation, biological activities, and potential applications. Food Chem. 2021, 349, 129209. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Algae-Based Coatings | Traditional Coatings (Epoxy, Polyurethane) |
|---|---|---|
| Adhesion | Moderate to high, highly dependent on biopolymer composition (e.g., polysaccharides, protein binders). Experimental variability is higher due to natural biomass sources. | High and well-characterized; synthetic polymers are engineered for strong substrate adhesion. |
| UV Resistance | Excellent when rich in natural UV-absorbing pigments (e.g., carotenoids, scytonemin, MAAs). Provides inherent photoprotection. | Good to excellent depending on additives; UV stabilizers must be incorporated artificially. |
| Water Resistance | Moderate; hydrophilicity of algal polymers may reduce water resistance unless crosslinked or blended with hydrophobic components. | Excellent; engineered hydrophobicity and crosslinking ensure strong water barrier properties. |
| Corrosion Resistance | Promising anti-corrosive behavior via metal chelation, antioxidant pigments, and bio-based film formation; requires scaling validation. | Excellent corrosion protection; long-term performance widely documented in industry. |
| Biocorrosion Resistance | Very high potential due to antimicrobial pigments (phycocyanin, phenolics), bioactive metabolites, and biocide-free defense. More evidence needed for industrial conditions. | Moderate; often requires added biocides. Susceptible to microbial degradation if formulations are not biocide-reinforced. |
| CO2 Sequestration (Active Functionality) | Unique advantage—pigments and biomolecules originate from CO2-assimilating biomass. Potential for active CO2 uptake if living coatings are developed. | None. Fully fossil-derived systems with zero environmental functionality. |
| Production Cost | Moderate but highly variable; depends on biomass yield, extraction cost, energy input, cultivation mode, and downstream processing. Economies of scale still emerging. | Moderate to high but stable; mature petrochemical manufacturing keeps costs predictable. |
| Application Method | Can be applied via brushing, spraying, or dip-coating; rheology often needs modification using natural or synthetic binders. | Highly optimized for spraying, brushing, rolling; viscosity and curing behavior are well-controlled. |
| Durability | Moderate; current limitation due to biodegradability and environmental sensitivity. Stability enhanced using crosslinkers or hybrid formulations. | High durability; designed for long-term stability under harsh weather, chemical exposure, and mechanical stress. |
| Criterion | Algae-Based Coatings (This Study) | Lignin-Based Coatings | Cellulose/Nanocellulose Coatings | Chitosan Films | Shellac Coatings | Plant-Oil-Based Coatings | Bacterial Biopolymer Coatings |
|---|---|---|---|---|---|---|---|
| 1. Sustainability | High sustainability; renewable biomass; CO2 sequestration during cultivation | Renewable & abundant polymer; reduces petroleum dependency; low environmental footprint; uses industrial waste streams | Sustainable materials for packaging; designed for sustainable barrier systems | Biodegradable and edible materials | Natural resin; non-toxic; low VOCs; eco-friendly waterborne systems | Uses only renewable raw materials; supports global bioeconomy trends; environmentally friendly synthesis | Renewable materials that reduce carbon footprint; substitutes oil-based plastics |
| 2. UV Resistance | Strong UV-blocking due to pigments like carotenoids and phycobiliproteins | Strong natural UV-absorption; colloids enhance UV blocking; lignin-chitosan films show high UV absorbance | No explicit UV-resistance data (only UV irradiation treatments mentioned) | No UV tests; only thermal aging | No UV-resistance data | HB (PHB degradation product) considered for radiation exposure, but no material UV resistance data | |
| 3. Barrier Properties | Moisture-sensitive; requires modification for gas/water resistance | Improved barriers vs. conventional coatings; esterified kraft lignin improves WV & O2 barrier; nanoparticles reduce ion intrusion | Strong barrier properties: reduced WVTR, reduced water absorption, high air resistance, oil/grease resistance | Variable; pure films need improvement; additives reduce WVP by up to 65% | Hydrophilicity limits barrier quality; nanofillers improve barriers | ||
| 4. Adhesion | Good adhesion potential due to EPS and polysaccharides | Improved adhesion in polyurethane, epoxy-acrylate, and metals via silane | Hydrogen bonding improves stiffness; soy protein binder enhances adhesion | No direct adhesion measurements | Excellent adhesion (Grade 1 across all tested concentrations) | Fatty acid amides strongly adhere to metal surfaces | Adhesion mediated by polypeptides, EPS; soil/implant adhesion applications |
| 5. Biodegradability | Highly biodegradable biomass components | Lignin used for biodegradable product; improves PU biodegradability | CNC/CNF and soy protein are biodegradable | Films explicitly biodegradable | Natural resin; implied biodegradability | Oils and fatty amides show natural biodegradability | PHB and PLA entirely biodegradable; used in many biodegradable products |
| 6. Cost | Moderate to high (cultivation + extraction) | Low-cost resource; largely burned as cheap fuel; value-added conversion feasible | Low-cost additives | Low-cost microcapsule materials | Low-cost renewable oils; abundant; low-cost ecotox testing | High cost for cellulose and cell factory production; cost reduced using waste substrates | |
| 7. Industrial Scalability | Cultivation & extraction still early stage; sensitive to process variability | Used in agriculture, petroleum, cement, concrete; sourced via industrial pulping | Previously limited by viscosity; additives enable higher-solids coatings | Synthesis method simple; provides basis for industrial wood coatings | High scalability due to agricultural production; supports oleochemical sector | Large-scale fermenters used; xanthan first industrial biopolymer; cell factories advancing | |
| 8. Stability | Sensitive to oxygen, heat, moisture; pigment degradation | Strong photostability; strong thermal stability; hydrolytic stability | Moisture-sensitive; defects reduced using clay | Storage-dependent WVP changes | High thermal stability; stable UF resin; good aging stability | High oxidative & thermal stability (e.g., TACO, TE-ESO) | High thermal stability; structural stability via EPS and eDNA |
| 9. CO2 Sequestration | Strongest CO2 sequestration capacity (during growth) | Biopolymers reduce emissions vs. cement but do not sequester CO2 | |||||
| Reference | [20] | [58] | [59] | [61] | [62] | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dodangodage, C.A.; Premarathne, H.; Kasturiarachchi, J.C.; Perera, T.A.; Rajapakshe, D.; Halwatura, R.U. Algae-Based Protective Coatings for Sustainable Infrastructure: A Novel Framework Linking Material Chemistry, Techno-Economics, and Environmental Functionality. Phycology 2025, 5, 84. https://doi.org/10.3390/phycology5040084
Dodangodage CA, Premarathne H, Kasturiarachchi JC, Perera TA, Rajapakshe D, Halwatura RU. Algae-Based Protective Coatings for Sustainable Infrastructure: A Novel Framework Linking Material Chemistry, Techno-Economics, and Environmental Functionality. Phycology. 2025; 5(4):84. https://doi.org/10.3390/phycology5040084
Chicago/Turabian StyleDodangodage, Charith Akalanka, Hirasha Premarathne, Jagath C. Kasturiarachchi, Thilini A. Perera, Dilan Rajapakshe, and Rangika Umesh Halwatura. 2025. "Algae-Based Protective Coatings for Sustainable Infrastructure: A Novel Framework Linking Material Chemistry, Techno-Economics, and Environmental Functionality" Phycology 5, no. 4: 84. https://doi.org/10.3390/phycology5040084
APA StyleDodangodage, C. A., Premarathne, H., Kasturiarachchi, J. C., Perera, T. A., Rajapakshe, D., & Halwatura, R. U. (2025). Algae-Based Protective Coatings for Sustainable Infrastructure: A Novel Framework Linking Material Chemistry, Techno-Economics, and Environmental Functionality. Phycology, 5(4), 84. https://doi.org/10.3390/phycology5040084







