Adaptation of Microalgae for the Production of Settling Flocs, Carotenoids, and Mineral Recovery from Municipal Secondary Effluents
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgal Consortium Propagation
2.2. Determination of Flocculation in Microalgae
2.3. Secondary Wastewater Effluent Used for Microalgal Cultivation
2.4. Preadaptation and Cultivation of Microalgae in Secondary Municipal Effluents
2.5. Flocculent Microalgal Biomass Production Using Secondary Effluents Under Outdoor Conditions
2.6. Analysis of Mineral and Carotenoid Content in Microalgal Biomass
3. Results
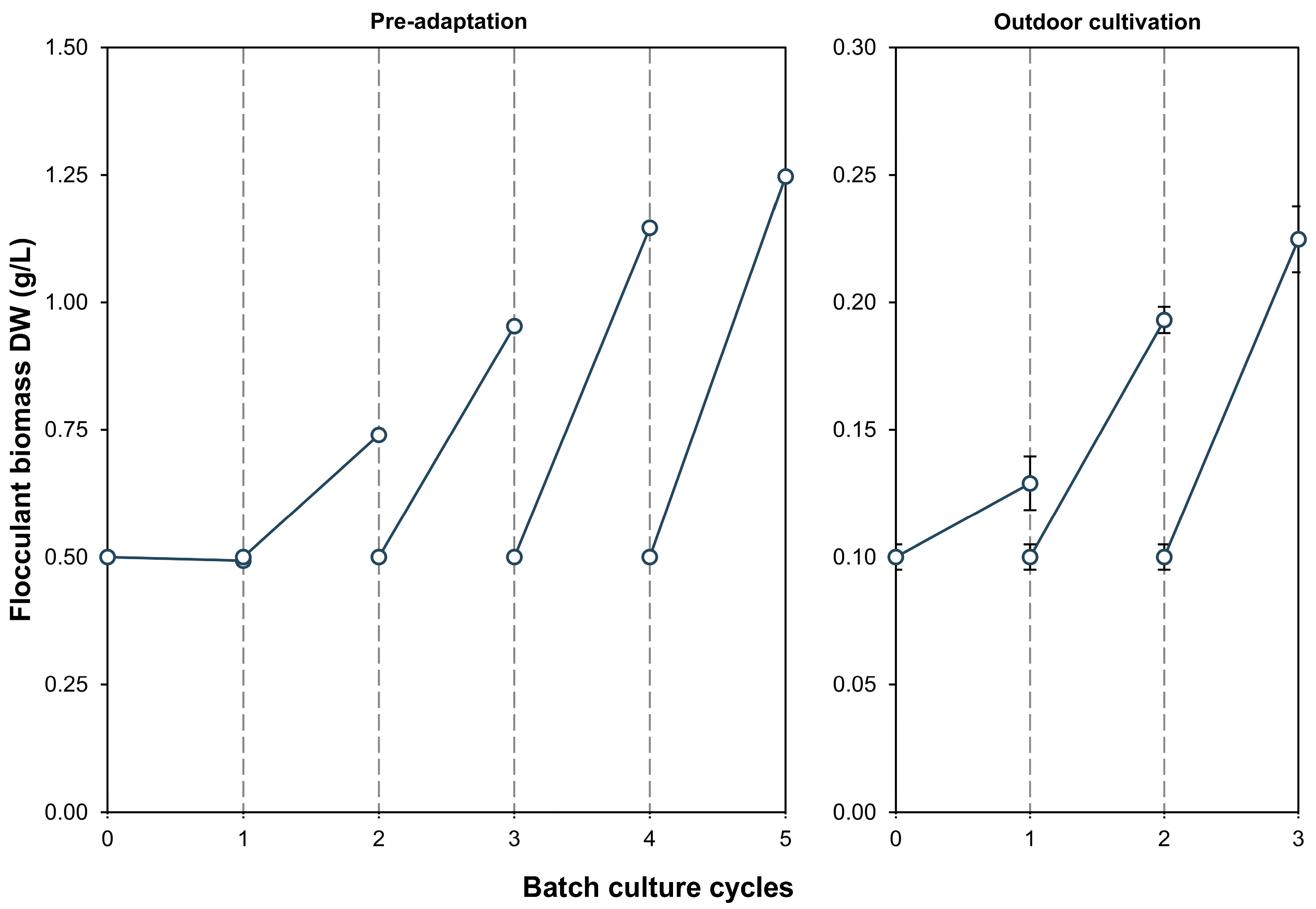
3.1. Flocculent Biomass Production
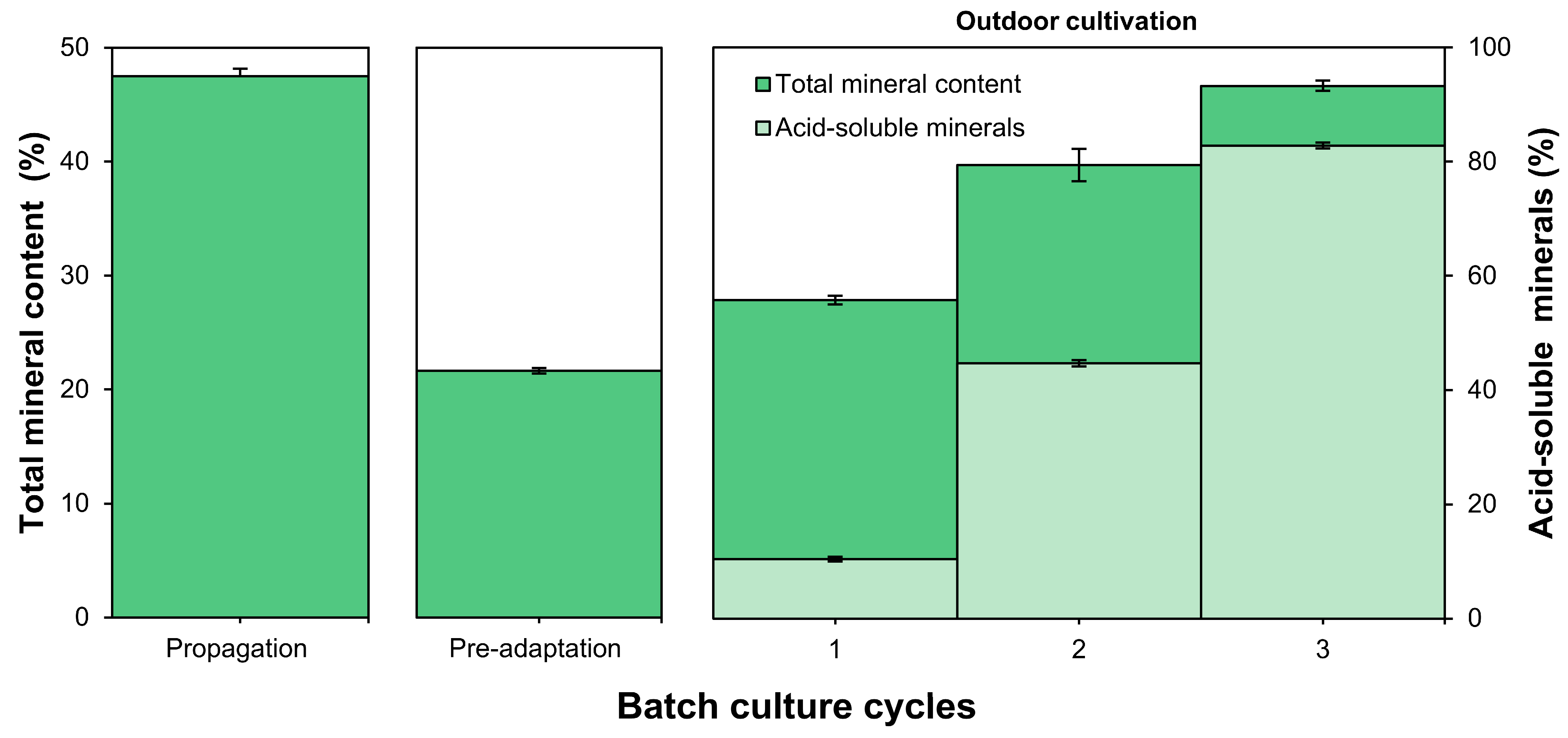
3.2. Mineral and Carotenoid Content in Biomass
4. Discussion
4.1. Flocculent Biomass Production and Microalgal Adaptation in Secondary Effluents
4.2. Adaptive Responses, Mineral Composition, and Carotenoid Content in Microalgae
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Razzak, S.A. Recent advances in sustainable biological nutrient removal from municipal wastewater. Clean. Water 2024, 2, 100047. [Google Scholar] [CrossRef]
- Wilkinson, G.M.; Johnson, R.A. Eutrophication of freshwater and coastal ecosystems. In Encyclopedia of Sustainable Technologies, 2nd ed.; Abraham, M.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 710–721. [Google Scholar] [CrossRef]
- Akter, M.M.; Hosen, M.A.; Gilroyed, B.H.; Akter Eti, S.; Sarker, P.; Kabir, M.M.; Mohinuzzaman, M. Nutrient recovery for fertilizer production and wastewater treatment for a circular economy. ACS Sustain. Resour. Manag. 2024, 1, 787–798. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Verma, P. Sequential two stage cultivation system using novel microalga consortia for treatment of municipal wastewater and simultaneous biomass production: Sustainable environmental management. J. Environ. Manag. 2024, 366, 121711. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.A.; Machado, C.A.; Esteves, A.F.; Salgado, E.M.; Dias, J.M.; Vilaça, J.S.; Pires, J.C.M. Microalgae based wastewater remediation: Linking N:P ratio and nitrogen sources to treatment performance by Chlorella vulgaris and biomass valorisation. Chem. Eng. J. 2025, 518, 164701. [Google Scholar] [CrossRef]
- Guo, H.; Hong, C.; Zheng, B.; Lu, F.; Jiang, D.; Qin, W. Bioflocculants’ production in a biomass-degrading bacterium using untreated corn stover as carbon source and use of bioflocculants for microalgae harvest. Biotechnol. Biofuels 2017, 10, 306. [Google Scholar] [CrossRef]
- Kumar, N.; Banerjee, C.; Negi, S.; Shukla, P. Microalgae harvesting techniques: Updates and recent technological interventions. Crit. Rev. Biotechnol. 2023, 43, 342–368. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, D.H.; Ki, M.R.; Pack, S.P. Recent progress in flocculation, dewatering, and drying technologies for microalgae utilization: Scalable and low-cost harvesting process development. Bioresour. Technol. 2022, 344, 126404. [Google Scholar] [CrossRef] [PubMed]
- Koyande, A.K.; Show, P.L. Microalgae harvest technology. In Handbook of Biorefinery Research and Technology: Production of Biofuels and Biochemicals; Bisaria, V., Ed.; Springer Nature: Singapore, 2024; pp. 705–730. [Google Scholar] [CrossRef]
- Zhu, X.; Yin, T.; Yin, L.; Jia, X.; Wang, J. Efficient microalgae harvesting: Synergistic flocculation and flotation with polyacrylamide and graphite electrolysis. Environ. Technol. Innov. 2025, 39, 104281. [Google Scholar] [CrossRef]
- Wan, C.; Alam, M.A.; Zhao, X.Q.; Zhang, X.Y.; Guo, S.L.; Ho, S.H.; Chang, J.S.; Bai, F.W. Current progress and future prospect of microalgal biomass harvest using various flocculation technologies. Bioresour. Technol. 2015, 184, 251–257. [Google Scholar] [CrossRef]
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Einarsson, H. Overview and challenges of large-scale cultivation of photosynthetic microalgae and cyanobacteria. Mar. Drugs 2023, 21, 445. [Google Scholar] [CrossRef]
- de Morais, E.G.; Sampaio, I.C.F.; González-Flo, E.; Ferrer, I.; Uggetti, E.; García, J. Microalgae harvesting for wastewater treatment and resources recovery: A review. N. Biotechnol. 2023, 78, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.P.; Frappart, M.; Jaouen, P.; Pruvost, J.; Bourseau, P. Harvesting Chlorella vulgaris by natural increase in pH: Effect of medium composition. Environ. Technol. 2014, 35, 1378–1388. [Google Scholar] [CrossRef]
- Galán-González, J.; Quintero-Zapata, I.; Elías-Santos, M.; Galán-Wong, L.J.; López-Chuken, U.J.; Guajardo-Barbosa, C.; Beltrán-Rocha, J.C. Harvest by autoflocculation, biomass, and carotenoid production in sequential batch culture of Haematococcus pluvialis under high ionic strength and macroelements content. J. Mar. Sci. Technol. Taiw. 2024, 32, 6. [Google Scholar] [CrossRef]
- Demir, I.; Besson, A.; Guiraud, P.; Formosa-Dague, C. Towards a better understanding of microalgae natural flocculation mechanisms to enhance flotation harvesting efficiency. Water Sci. Technol. 2020, 82, 1009–1024. [Google Scholar] [CrossRef]
- Bonnefond, H.; Lie, Y.; Lacour, T.; Saint-Jean, B.; Carrier, G.; Pruvost, E.; Talec, A.; Bernard, O.; Sciandra, A. Dynamical Darwinian selection of a more productive strain of Tisochrysis lutea. Algal Res. 2022, 65, 102743. [Google Scholar] [CrossRef]
- Li, D.; Wang, L.; Zhao, Q.; Wei, W.; Sun, Y. Improving high carbon dioxide tolerance and carbon dioxide fixation capability of Chlorella sp. by adaptive laboratory evolution. Bioresour. Technol. 2015, 185, 269–275. [Google Scholar] [CrossRef]
- He, Z.; Wang, J.; Li, Y. Recent advances in microalgae-driven carbon capture, utilization, and storage: Strain engineering through adaptive laboratory evolution and microbiome optimization. Green Carbon 2024, 3, 74–99. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.H.; Sun, J. Microalgae-based wastewater treatment: Mechanisms, challenges, recent advances, and future prospects. Environ. Sci. Ecotechnol. 2023, 13, 100205. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.R.; Deprá, M.C.; de Menezes, C.R.; Zepka, L.Q.; Jacob-Lopes, E. Microalgae cultivation in wastewater: How realistic is this approach for value-added product production? Processes 2025, 13, 2052. [Google Scholar] [CrossRef]
- Osman, M.E.; Abo-Shady, A.M.; Gheda, S.F.; Desoki, S.M.; Elshobary, M.E. Unlocking the potential of microalgae cultivated on wastewater combined with salinity stress to improve biodiesel production. Environ. Sci. Pollut. Res. 2023, 30, 114610–114624. [Google Scholar] [CrossRef] [PubMed]
- Nur, M.M.A.; Murni, S.W.; Setyoningrum, T.M.; Hadi, F.; Widayati, T.W.; Jaya, D.; Sulistyawati, R.R.E.; Puspitaningrum, D.A.; Dewi, R.N.; Hadiyantoi; et al. Innovative strategies for utilizing microalgae as dual-purpose biofertilizers and phycoremediators in agroecosystems. Biotechnol. Rep. 2024, 45, e00870. [Google Scholar] [CrossRef]
- Beltrán Rocha, J.C.; Barceló Quintal, I.D.; García Martínez, M.; Osornio Berthet, L.; Saavedra Villarreal, N.; Villarreal Chiu, J.; López Chuken, U.J. Polishing of municipal secondary effluent using native microalgae consortia. Water Sci. Technol. 2017, 75, 1693–1701. [Google Scholar] [CrossRef]
- Korek, M.; Marzec, M. Strigolactones and abscisic acid interactions affect plant development and response to abiotic stresses. BMC Plant Biol. 2023, 23, 314. [Google Scholar] [CrossRef]
- Parmar, P.; Kumar, R.; Neha, Y.; Srivatsan, V. Microalgae as next generation plant growth additives: Functions, applications, challenges and circular bioeconomy based solutions. Front. Plant Sci. 2023, 14, 1073546. [Google Scholar] [CrossRef]
- Geng, Y.; Shaukat, A.; Azhar, W.; Raza, Q.U.A.; Tahir, A.; Abideen, M.Z.U.; Rehim, A. Microalgal biorefineries: A systematic review of technological trade-offs and innovation pathways. Biotechnol. Biofuels Bioprod. 2025, 18, 93. [Google Scholar] [CrossRef]
- Selvaraj, D.; Sanjiv, R.; Banu, S.S.; Arivazhagan, M. Recent advances in microalgae harvesting and dewatering: A techno-economic evaluation perspective. Bioresour. Technol. Rep. 2025, 31, 102201. [Google Scholar] [CrossRef]
- López Chuken, U.J.; Young, S.D. Modelling sulphate enhanced cadmium uptake by Zea mays from nutrient solution under conditions of constant free Cd2⁺ ion activity. J. Environ. Sci. 2010, 22, 1080–1085. [Google Scholar] [CrossRef]
- Pathom Aree, W.; Sensupa, S.; Wichaphian, A.; Sriket, N.; Kitwetch, B.; Pekkoh, J.; Sattayawat, P.; Lomakool, S.; Chromkaew, Y.; Srinuanpan, S. An innovative co cultivation of microalgae and actinomycete inoculated lettuce in a hydroponic deep water culture system for the sustainable development of a food–agriculture–energy nexus. Horticulturae 2024, 10, 70. [Google Scholar] [CrossRef]
- ISO 5667–10; Water Quality—Sampling. Part 10: Guidance on Sampling of Wastewaters. ISO: Geneva, Switzerland, 1992.
- AOAC International. Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Gaithersburg, MD, USA, 1997. [Google Scholar]
- New, M.B. Feed and Feeding of Fish and Shrimp; UNDP/FAO: Rome, Italy, 1987; ADCP/REP/87/26. [Google Scholar]
- Vo, T.; Tran, S.; Nguyen, P.; Mai, T. Growth, carotenoid production, antioxidant capacity and lipid accumulation of Haematococcus sp. under different light intensities. Am. J. Plant Biol. 2017, 2, 142–147. [Google Scholar]
- Matter, I.A.; Bui, V.K.H.; Jung, M.; Seo, J.Y.; Kim, Y.E.; Lee, Y.C.; Oh, Y.K. Flocculation harvesting techniques for microalgae: A review. Appl. Sci. 2019, 9, 3069. [Google Scholar] [CrossRef]
- Lipsman, V.; Shlakhter, O.; Rocha, J.; Segev, E. Bacteria contribute exopolysaccharides to an algal-bacterial joint extracellular matrix. Npj Biofilms Microbiomes 2024, 10, 36. [Google Scholar] [CrossRef]
- Yuan, Y.; Lu, J.; Wang, Q. Evaluating bioflocculation harvesting of freshwater and marine microalgae using exopolysaccharides (EPSs) from Klebsiella sp. Separations 2024, 11, 355. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, X.; Wu, B.; Wang, Z.; Liu, Y.; Ren, T.; Rittmann, B.E. Microalgal extracellular polymeric substances (EPS) and their roles in cultivation, biomass harvesting, and bioproducts extraction. Bioresour. Technol. 2024, 406, 131054. [Google Scholar] [CrossRef]
- Stratigakis, N.C.; Nazos, T.T.; Goumenaki, M.; Tsolakidi, A.; Spantidaki, M.; Lagouvardou-Spantidaki, A.; Ghanotakis, D.F. Growth performance and adaptability of an EPS-producing Chlorella strain in cheese whey with high and low salinity: Prospects for the sustainable production of microalgal biomass. J. Appl. Phycol. 2025, 37, 1777–1794. [Google Scholar] [CrossRef]
- Xiu, J.; Ow, Z.; Zhao, Y.; Bai, X. Mechanisms of microalgae-induced flocculation for urban water carbon-negative clarification. J. Environ. Chem. Eng. 2025, 13, 116841. [Google Scholar] [CrossRef]
- Muir, E.; Grossman, A.R.; Chisti, Y.; Fedrizzi, B.; Guieysse, B.; Plouviez, M. Self-aggregation for sustainable harvesting of microalgae. Algal Res. 2024, 83, 103685. [Google Scholar] [CrossRef]
- Shaikh, S.M.; Quadir, M.A.; Nasser, M.S.; Rekik, H.; Hassan, M.K.; Ayesh, A.I.; Sayadi, S. Investigation of flocculation and rheological properties of microalgae suspensions cultivated in industrial process wastewater. Sep. Purif. Technol. 2024, 328, 125016. [Google Scholar] [CrossRef]
- Van Den Hende, S.; Carré, E.; Cocaud, E.; Beelen, V.; Boon, N.; Vervaeren, H. Treatment of industrial wastewaters by microalgal bacterial flocs in sequencing batch reactors. Bioresour. Technol. 2014, 161, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Gojkovic, Z.; Skrobonja, A.; Radojicic, V.; Mattei, B. The use of flocculation as a preconcentration step in the microalgae harvesting process. Physiol. Plant 2025, 177, e70366. [Google Scholar] [CrossRef]
- Keshinro, T.A.; Keshinro, O.M.; Titilawo, Y.; Cowan, A.K. Aggregation/disaggregation of microalgal–bacterial flocs in high-rate oxidation ponds is a response to biotic/abiotic-induced changes in microbial community structure. J. Appl. Phycol. 2024, 36, 1311–1325. [Google Scholar] [CrossRef]
- Satiro, J.; dos Santos Neto, A.G.; Marinho, T.; Sales, M.; Marinho, I.; Kato, M.T.; Florencio, L. The role of the microalgae–bacteria consortium in biomass formation and its application in wastewater treatment systems: A comprehensive review. Appl. Sci. 2024, 14, 6083. [Google Scholar] [CrossRef]
- Mahlangu, D.; Mphahlele, K.; De Paola, F.; Mthombeni, N.H. Microalgae-mediated biosorption for effective heavy metals removal from wastewater: A review. Water 2024, 16, 718. [Google Scholar] [CrossRef]
- Levine, I.A.; Fleurence, J. Microalgae. In Health and Disease Prevention; Fox, J.M., Zimba, P.V., Levine, I.A., Fleurence, J., Eds.; Academic Press: London, UK, 2018; pp. 177–193. [Google Scholar]
- Ciani, M.; Adessi, A. Cyanoremediation and phyconanotechnology: Cyanobacteria for metal biosorption toward a circular economy. Front. Microbiol. 2023, 14, 1166612. [Google Scholar] [CrossRef]
- Paper, M.; Jung, P.; Koch, M.; Lakatos, M.; Nilges, T.; Brück, T.B. Stripped: Contribution of cyanobacterial extracellular polymeric substances to the adsorption of rare earth elements from aqueous solutions. Front. Bioeng. Biotechnol. 2023, 11, 1299349. [Google Scholar] [CrossRef]
- Dudeja, C.; Masroor, S.; Mishra, V.; Kumar, K.; Sansar, S.; Yadav, P.; Kumar, A. Cyanobacteria-based bioremediation of environmental contaminants: Advances and computational insights. Discov. Agric. 2025, 3, 42. [Google Scholar] [CrossRef]
- Hamai-Amara, H.; Saadaoui, I.; Cherif, M.; Da’ana, D.A.; Soubra, L.; Al-Ghouti, M.A. Evidencing nickel biosorption capacity of cyanobacteria Chroococcidiopsis sp.: Potential metallo-protective agents. BMC Chem. 2025, 19, 59. [Google Scholar] [CrossRef]
- Mishra, A.; Mandoli, A.; Jha, B. Physiological characterization and stress-induced metabolic responses of Dunaliella salina isolated from salt pan. J. Ind. Microbiol. Biotechnol. 2008, 35, 1093–1100. [Google Scholar] [CrossRef]
- Pan, Y.; Amenorfenyo, D.K.; Dong, M.; Zhang, N.; Huang, X.; Li, C.; Li, F. Effects of salinity on the growth, physiological and biochemical components of microalga Euchlorocystis marina. Front. Mar. Sci. 2024, 11, 1402071. [Google Scholar] [CrossRef]
- Pearson, C.J.; Norman, D.W.; Dixon, J. Sustainable Dryland Cropping in Relation to Soil Productivity; Food & Agriculture Org: Rome, Italy, 1995; Volume 72. [Google Scholar]
- Najdenko, E.; Lorenz, F.; Dittert, K.; Olfs, H.W. Rapid in-field soil analysis of plant-available nutrients and pH for precision agriculture—A review. Precis. Agric. 2024, 25, 3189–3218. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Wei, M.; Gao, Z.; Tian, M.; He, F. Comparisons of acid and water solubilities of rice straw ash together with its major ash-forming elements at different ashing temperatures: An experimental study. Sustainability 2019, 11, 1989. [Google Scholar] [CrossRef]
- Liu, K. New and improved methods for measuring acid insoluble ash. Anim. Feed Sci. Technol. 2022, 288, 115282. [Google Scholar] [CrossRef]
- Shaviv, A. Advances in controlled-release fertilizers. Adv. Agron. 2001, 71, 1–49. [Google Scholar]
- Díaz, L.E.; Gonzalez, J.D.; Morales-Gonzalez, M.P.; Garzón-Castro, C.L. Harnessing the power of microalgae consortia for sustainable crop production: Case study on lettuce (Lactuca sativa L.). J. Appl. Phycol. 2024, 36, 3273–3286. [Google Scholar] [CrossRef]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, C.; Ghosh, T.; George, B.; Pancha, I.; Maurya, R.; Chokshi, K.; Ghosh, A.; Mishra, S. Microalgal carotenoids: Potential nutraceutical compounds with chemotaxonomic importance. Algal Res. 2016, 15, 24–31. [Google Scholar] [CrossRef]


| Compound | Final Concentration |
|---|---|
| KH2PO4 | 1 mM |
| MgSO4 × 7H2O | 2 mM |
| KNO3 | 5 mM |
| Ca(NO3)2 × 4H2O | 6.25 mM |
| MnCl2 × 4H2O | 9.15 μM |
| FeSO4 × 7H2O | 20 μM |
| Na2EDTA (C10H14N2Na2O8) | 20 μM |
| H3BO3 | 46 μM |
| (NH4)6Mo7O24 × 4H2O | 15 nM |
| CuSO4 × 5H2O | 320 nM |
| ZnSO4 × 7H2O | 765 nM |
| Parameters | Value |
|---|---|
| Total alkalinity (H2CO3, HCO3−, CO32−) | 209.3 ± 2.5 mg/L as CaCO3 |
| Nitrate (NO3−) | 77.9 ± 0.6 mg/L |
| Phosphate (PO43−) | 11.7 ± 0.2 mg/L |
| pH | 7.6 ± 0.1 |
| Electrical conductivity | 110.7 ± 0.5 mS/m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guajardo-Barbosa, C.; Guajardo-Rodríguez, T.; López-Chuken, U.J.; Barceló-Quintal, I.D.; Cruz-Chávez, D.; Beltrán-Rocha, J.C. Adaptation of Microalgae for the Production of Settling Flocs, Carotenoids, and Mineral Recovery from Municipal Secondary Effluents. Phycology 2025, 5, 57. https://doi.org/10.3390/phycology5040057
Guajardo-Barbosa C, Guajardo-Rodríguez T, López-Chuken UJ, Barceló-Quintal ID, Cruz-Chávez D, Beltrán-Rocha JC. Adaptation of Microalgae for the Production of Settling Flocs, Carotenoids, and Mineral Recovery from Municipal Secondary Effluents. Phycology. 2025; 5(4):57. https://doi.org/10.3390/phycology5040057
Chicago/Turabian StyleGuajardo-Barbosa, Claudio, Tomás Guajardo-Rodríguez, Ulrico Javier López-Chuken, Icela Dagmar Barceló-Quintal, David Cruz-Chávez, and Julio César Beltrán-Rocha. 2025. "Adaptation of Microalgae for the Production of Settling Flocs, Carotenoids, and Mineral Recovery from Municipal Secondary Effluents" Phycology 5, no. 4: 57. https://doi.org/10.3390/phycology5040057
APA StyleGuajardo-Barbosa, C., Guajardo-Rodríguez, T., López-Chuken, U. J., Barceló-Quintal, I. D., Cruz-Chávez, D., & Beltrán-Rocha, J. C. (2025). Adaptation of Microalgae for the Production of Settling Flocs, Carotenoids, and Mineral Recovery from Municipal Secondary Effluents. Phycology, 5(4), 57. https://doi.org/10.3390/phycology5040057






