Biomass Production of Chlorella vulgaris var. vulgaris TISTR 8261 During Cultivation in Modified Food Industry Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Wastewater Samples
2.2. Analysis of Wastewater
2.3. Green Algal Strain and Cultivation
2.4. Growth, Total Cell Concentration and Dry Weight Determination
2.5. Total Chlorophyll Concentration Determination
2.6. Growth Optimization
2.7. Biomass Characteristics of C. vulgaris var. vulgaris TISTR 8261
2.8. Statistical Analysis
3. Results
3.1. Chemical Characteristics of Wastewater from Food Industry Factories
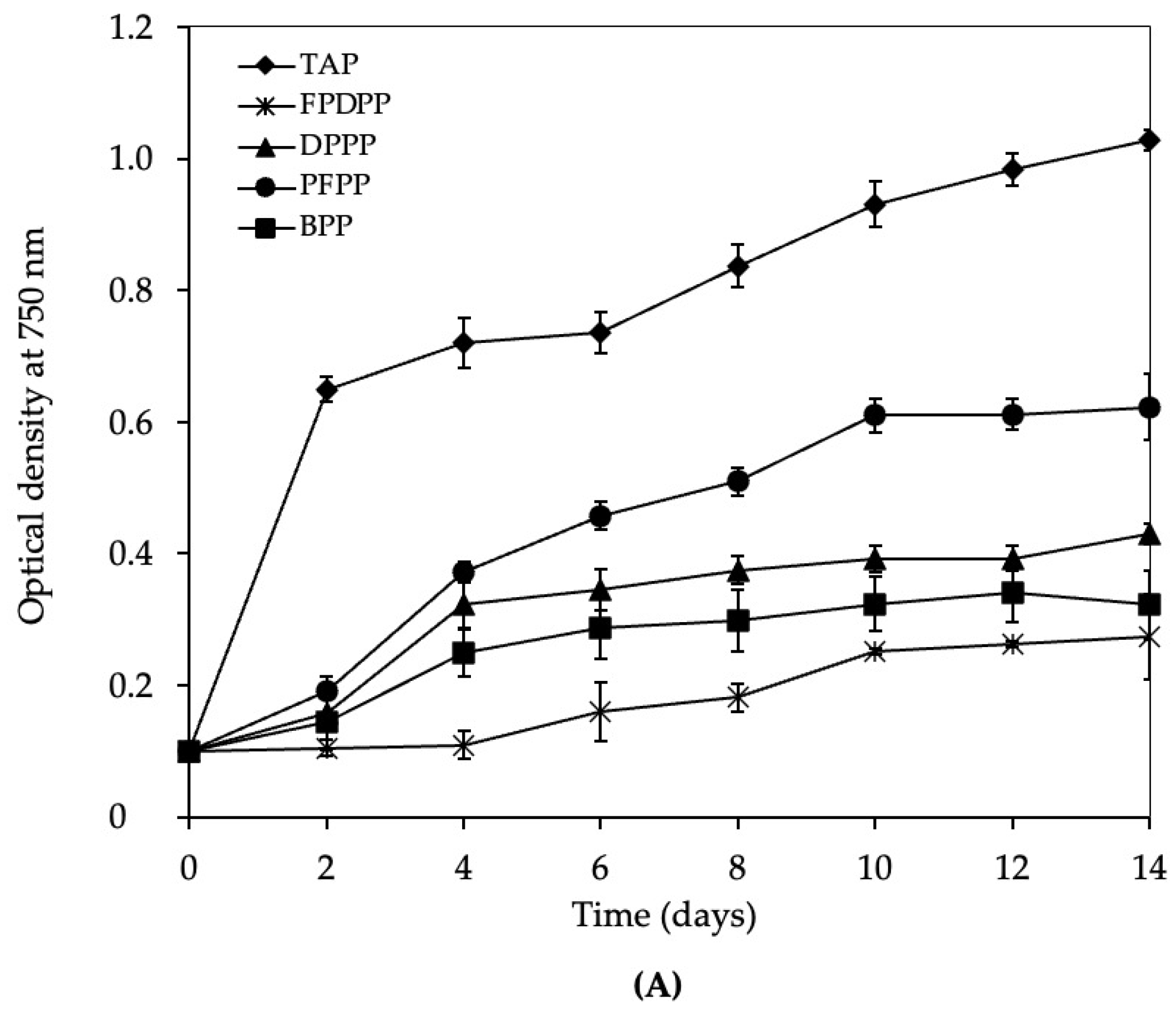
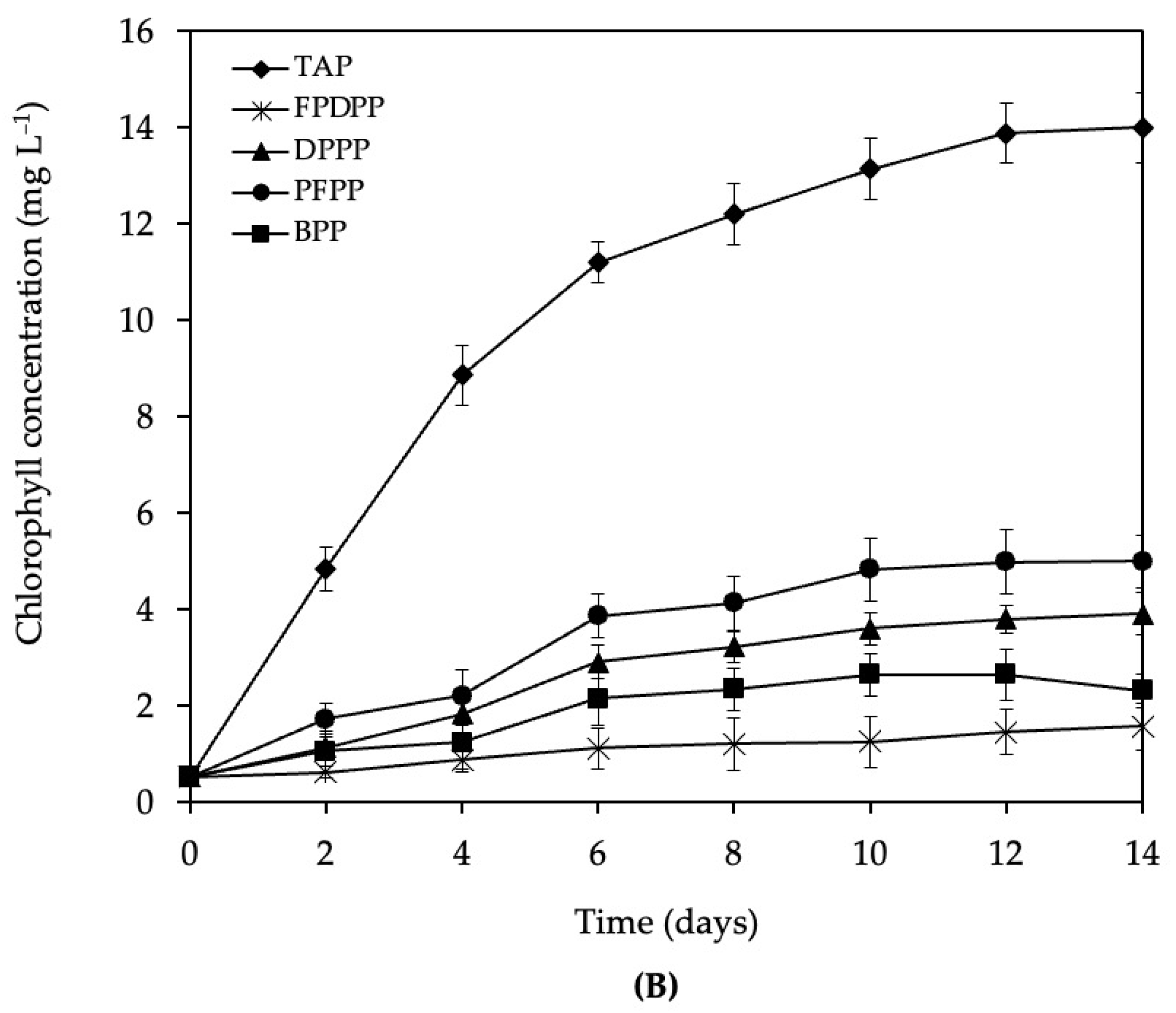
3.2. Growth of C. vulgaris var. vulgaris TISTR 8261 in Wastewater
3.3. Growth of C. vulgaris var. vulgaris TISTR 8261 Cultivated in PFPP Wastewater Supplemented with Different Sodium Acetate Concentrations
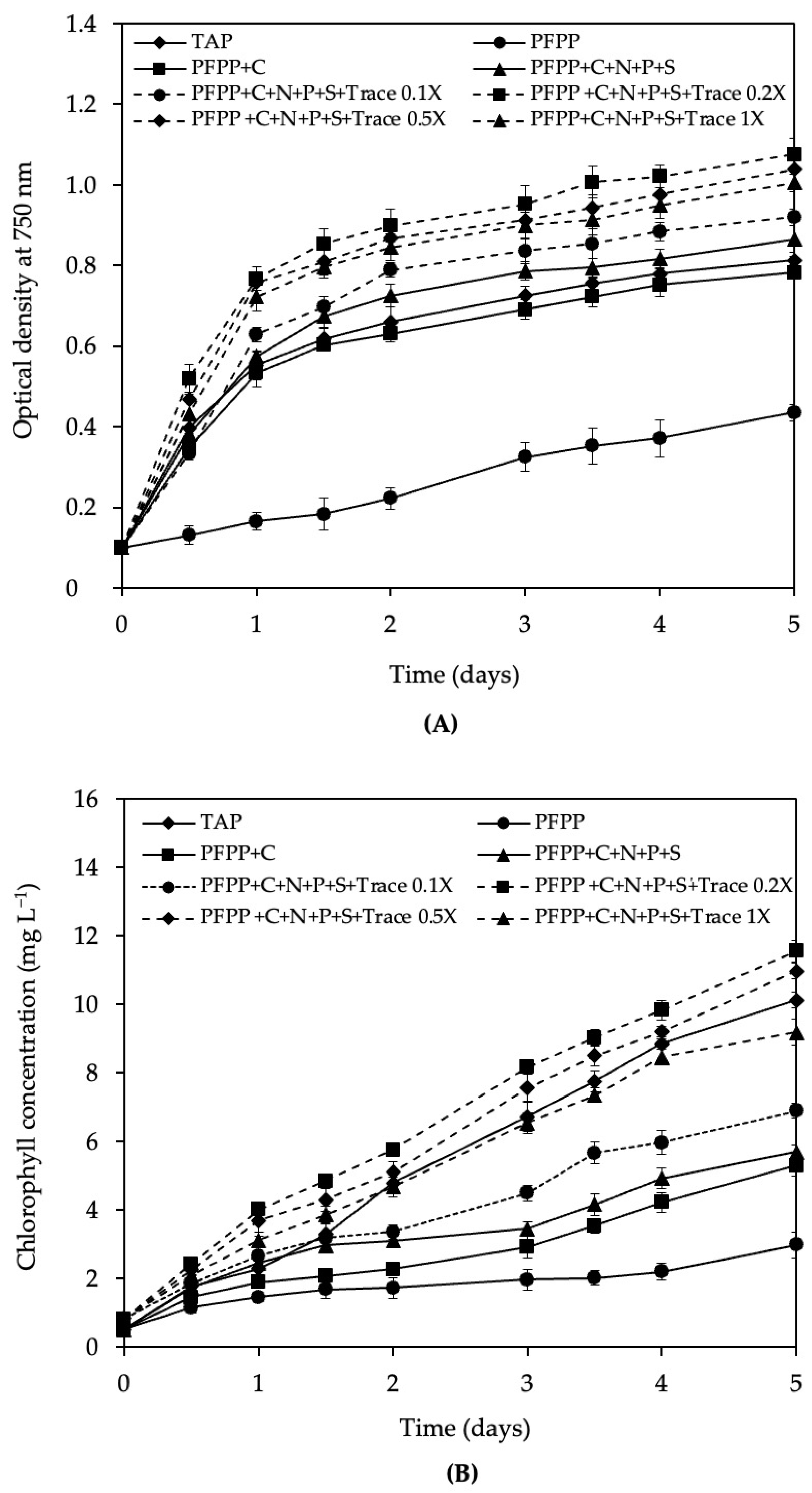
3.4. Growth of C. vulgaris var. vulgaris TISTR 8261 Cultivated in PFPP Wastewater Supplemented with Macronutrients
3.5. Growth of C. vulgaris var. vulgaris TISTR 8261 Cultivated in PFPP Wastewater Supplemented with Different Trace Mineral Concentrations
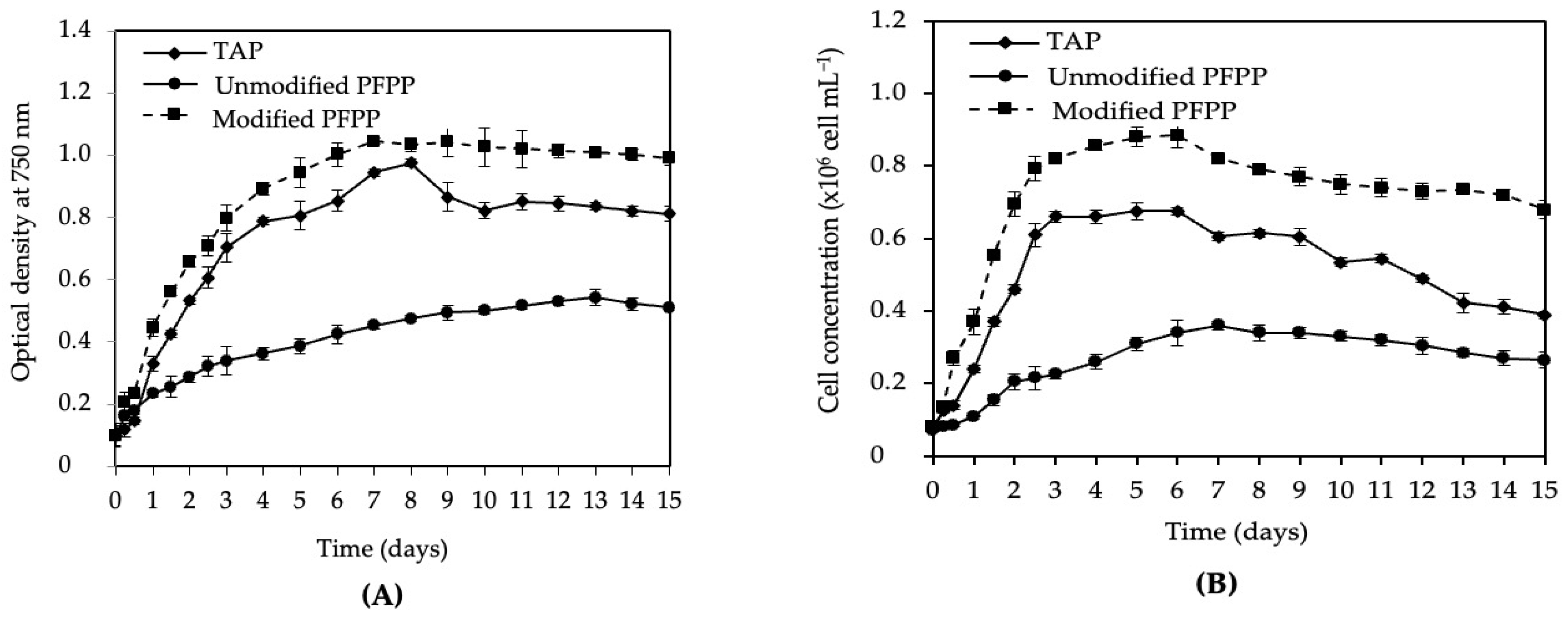
3.6. Scaled-Up Cultivation of C. vulgaris var. vulgaris TISTR 8261 in 3.5 L Culture Bottles
3.7. Nutritional Composition of C. vulgaris var. vulgaris TISTR 8261 Cultivated in Different Media
4. Discussion
4.1. Wastewater Characteristics and Implications for Algal Cultivation
4.2. Growth of C. vulgaris var. vulgaris TISTR 8261 in Different Wastewater Sources
4.3. Effect of Sodium Acetate Supplementation on the Growth of C. vulgaris var. vulgaris TISTR 8261 in PFPP Wastewater
4.4. Effect of Macronutrients and Trace Mineral Supplementation on Growth of C. vulgaris var. vulgaris TISTR 8261 in PFPP Wastewater
4.5. Scaled-Up Cultivation of C. vulgaris var. vulgaris TISTR 8261 Using Optimized PFPP Wastewater
4.6. Nutritional Quality of Biomass of C. vulgaris var. vulgaris TISTR 8261 Biomass Cultivated in Modified PFPP Wastewater
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Carvalho, J.C.; Molina-Aulestia, D.T.; Martinez-Burgos, W.J.; Karp, S.G.; Manzoki, M.C.; Medeiros, A.B.P.; Rodrigues, C.; Scapini, T.; Vandenberghe, L.P.d.S.; Vieira, S.; et al. Agro-Industrial Wastewaters for Algal Biomass Production, Bio-Based Products, and Biofuels in a Circular Bioeconomy. Fermentation 2022, 8, 728. [Google Scholar] [CrossRef]
- Pervez, M.N.; Mishu, M.R.; Stylios, G.K.; Hasan, S.W.; Zhao, Y.; Cai, Y.; Zarra, T.; Belgiorno, V.; Naddeo, V. Sustainable Treatment of Food Industry Wastewater Using Membrane Technology: A Short Review. Water 2021, 13, 3450. [Google Scholar] [CrossRef]
- Haske-Cornelius, O.; Gierlinger, S.; Vielnascher, R.; Gabauer, W.; Prall, K.; Pellis, A.; Guebitz, G.M. Cultivation of Heterotrophic Algae on Paper Waste Material and Digestate. Algal Res. 2021, 54, 102193. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, R.; Zhang, Y.; Liu, Y.; Chen, J. Microalgae-Based Wastewater Treatment for Nutrient Recovery: A Review. Waste Biomass Valorization 2021, 12, 1991–1999. [Google Scholar] [CrossRef]
- Liu, S.; Gifuni, I.; Mear, H.; Frappart, M.; Couallier, E. Recovery of soluble proteins from Chlorella vulgaris by bead-milling and microfiltration: Impact of the concentration and the physicochemical conditions during the cell disruption on the whole process. Process Biochem. 2021, 108, 34–47. [Google Scholar] [CrossRef]
- Taikhao, S.; Phunpruch, S. Biomass and Biohydrogen Production by Unicellular Green Alga Chlorella vulgaris var. vulgaris TISTR 8261 Using Frozen Food Industrial Wastewater. Asia-Pac. J. Sci. Technol. 2022, 27, 9-Jan. [Google Scholar] [CrossRef]
- Nápoles-Armenta, J.; Romero-Soto, I.C.; Samaniego-Moreno, L.; Díaz-Tenorio, L.M.; Soto, L.A.L.; Mora-Orozco, C.D.L.; Pérez, R.G.; Martínez-Orozco, E.; García-Gómez, C.; Pérez-Valencia, L.I. Advanced Municipal Wastewater Treatment and Bioproduct Generation via Optimized Autotrophic and Mixotrophic Microalgal Cultivation. Sustainability 2025, 17, 6539. [Google Scholar] [CrossRef]
- Faraloni, C.; Ena, A.; Pintucci, C.; Torzillo, G. Enhanced Hydrogen Production by Means of Sulfur-Deprived Chlamydomonas reinhardtii Cultures Grown in Pretreated Olive Mill Wastewater. Int. J. Hydrogen Energy 2011, 36, 5920–5931. [Google Scholar] [CrossRef]
- Kothari, R.; Prasad, R.; Kumar, V.; Singh, D.P. Production of biodiesel from microalgae Chlamydomonas polypyrenoideum grown on dairy industry wastewater. Bioresour. Technol. 2013, 144, 499–503. [Google Scholar] [CrossRef]
- Terán Hilares, R.; Sánchez Vera, F.P.; Colina Andrade, G.J.; Tejada Meza, K.; García, J.C.; Pacheco Tanaka, D.A. Continuous Cultivation of Microalgae in Cattle Slaughterhouse Wastewater Treated with Hydrodynamic Cavitation. Water 2022, 14, 1288. [Google Scholar] [CrossRef]
- Chawla, P.; Gola, D.; Dalvi, V.; Sreekrishnan, T.R.; Ariyadasa, T.U.; Malik, A. Design and development of mini-photobioreactor system for strategic high throughput selection of optimum microalgae-wastewater combination. Bioresour. Technol. Rep. 2022, 17, 100967. [Google Scholar] [CrossRef]
- Ogbonna, I.O.; Okpozu, O.O.; Ikwebe, J.; Ogbonna, J.C. Utilisation of Desmodesmus subspicatus LC172266 for simultaneous remediation of cassava wastewater and accumulation of lipids for biodiesel production. Biofuels 2018, 9, 657–664. [Google Scholar] [CrossRef]
- Okpozu, O.O.; Ogbonna, I.O.; Ikwebe, J.; Ogbonna, J.C. Phycoremediation of cassava wastewater by Desmodesmus armatus and the concomitant accumulation of lipids for biodiesel production. Bioresour. Technol. Rep. 2019, 7, 100255. [Google Scholar] [CrossRef]
- Vadiveloo, A.; Foster, L.; Kwambai, C.; Bahri, P.A.; Moheimani, N.R. Microalgae cultivation for the treatment of anaerobically digested municipal centrate (ADMC) and anaerobically digested abattoir effluent (ADAE). Sci. Total Environ. 2021, 775, 145853. [Google Scholar] [CrossRef] [PubMed]
- Shayesteh, H.; Vadiveloo, A.; Bahri, P.A.; Moheimani, N.R. Long term outdoor microalgal phycoremediation of anaerobically digested abattoir effluent. J. Environ. Manag. 2022, 323, 116322. [Google Scholar] [CrossRef]
- Viegas, C.; Gouveia, L.; Gonçalves, M. Evaluation of microalgae as bioremediation agent for poultry effluent and biostimulant for germination. Environ. Technol. Innov. 2021, 24, 102048. [Google Scholar] [CrossRef]
- Safafar, H.; Uldall Nørregaard, P.; Ljubic, A.; Møller, P.; Løvstad Holdt, S.; Jacobsen, C. Enhancement of protein and pigment content in two Chlorella species cultivated on industrial process water. J. Mar. Sci. Eng. 2016, 4, 84. [Google Scholar] [CrossRef]
- Lai, Y.C.; Chang, C.H.; Chen, C.Y.; Chang, J.S.; Ng, I.S. Towards protein production and application by using Chlorella species as circular economy. Bioresour. Technol. 2019, 289, 121625. [Google Scholar] [CrossRef]
- Xu, Q.; Hou, G.; Chen, J.; Wang, H.; Yuan, L.; Han, D.; Hu, Q.; Jin, H. Heterotrophically ultrahigh-cell-density cultivation of a high protein-yielding unicellular alga Chlorella with a novel nitrogen-supply strategy. Front. Bioeng. Biotechnol. 2021, 9, 774854. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Dragone, G. Challenges and opportunities to increase economic feasibility and sustainability of mixotrophic cultivation of green microalgae of the genus Chlorella. Renew. Sustain. Energy Rev. 2022, 160, 112284. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Zhao, X.-Q.; Yen, H.-W.; Ho, S.-H.; Cheng, C.-L.; Lee, D.-J.; Bai, F.-W.; Chang, J.-S. Microalgae-based carbohydrates for biofuel production. Biochem. Eng. J. 2013, 78, 1–10. [Google Scholar] [CrossRef]
- Ho, S.-H.; Huang, S.-W.; Chen, C.-Y.; Hasunuma, T.; Kondo, A.; Chang, J.-S. Characterization and optimization of carbohydrate production from an indigenous microalga Chlorella vulgaris FSP-E. Bioresour. Technol. 2013, 135, 157–165. [Google Scholar] [CrossRef]
- Ali, H.E.A.; El-Fayoumy, E.A.; Rasmy, W.E.; Soliman, R.M.; Abdullah, M.A. Two-stage cultivation of Chlorella vulgaris using light and salt stress conditions for simultaneous production of lipid, carotenoids, and antioxidants. J. Appl. Phycol. 2021, 33, 227–239. [Google Scholar] [CrossRef]
- Wang, W.; Sheng, Y. Ampicillin used in aseptic processing influences the production of pigments and fatty acids in Chlorella sorokiniana. World J. Microbiol. Biotechnol. 2021, 37, 3. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.A.; Cardoso, L.G.; Silva, J.S.J.; Souza, C.O.; Lemos, P.V.F.; Almeida, P.F.; Ferreira, E.S.; Lombardi, A.T.; Druzian, J.I. Strategy for the cultivation of Chlorella vulgaris with high biomass production and biofuel potential in wastewater from the oil industry. Environ. Technol. Innov. 2022, 25, 102204. [Google Scholar] [CrossRef]
- Baldev, E.; MubarakAli, D.; Sivasubramanian, V.; Pugazhendhi, A.; Thajuddin, N. Unveiling the induced lipid production in Chlorella vulgaris under pulsed magnetic field treatment. Chemosphere 2021, 279, 130673. [Google Scholar] [CrossRef] [PubMed]
- Rautenberger, R.; Détain, A.; Skjånes, K.; Schulze, P.S.C.; Kiron, V.; Morales-Sánchez, D. Growth strategies of Chlorella vulgaris in seawater for a high production of biomass and lipids suitable for biodiesel. Algal Res. 2024, 77, 103360. [Google Scholar] [CrossRef]
- Kondzior, P.; Butarewicz, A. Influence of walls in a container on the growth of the Chlorella vulgaris algae. J. Ecol. Eng. 2021, 22, 98–108. [Google Scholar] [CrossRef]
- Supakriangkrai, T.; Phunpruch, S. Screening and optimization of high-efficiency H2-producing Chlorella strains. J. Appl. Biol. Biotechnol. 2025, 13, 71–81. [Google Scholar] [CrossRef]
- Abuhasheesh, Y.; Ghazal, A.; Tang, D.Y.Y.; Banat, F.; Hasan, S.W.; Show, P.L. Advances in Chlorella microalgae for sustainable wastewater treatment and bioproduction. Chem. Eng. J. Adv. 2025, 22, 100715. [Google Scholar] [CrossRef]
- Farooq, W.; Lee, Y.C.; Ryu, B.G.; Kim, B.H.; Kim, H.S.; Choi, Y.E.; Yang, J.W. Two-stage cultivation of two Chlorella sp. strains by simultaneous treatment of brewery wastewater and maximizing lipid productivity. Bioresour. Technol. 2013, 132, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Sudhanthiran, M.C.; Perumalsamy, M. Bioremediation of dairy industry wastewater and assessment of nutrient removal potential of Chlorella vulgaris. Biomass Convers. Biorefin. 2024, 14, 10335–10346. [Google Scholar] [CrossRef]
- Silva, G.; Cerqueira, K.; Rodrigues, J.; Silva, K.; Coelho, D.; Souza, R. Cultivation of microalgae Chlorella vulgaris in open reactor for bioethanol production. Phycology 2023, 3, 325–336. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Arroyo, T.; Wei, W.; Ruan, R.; Hu, B. Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. Biomass Bioenergy 2011, 35, 2245–2253. [Google Scholar] [CrossRef]
- Saberi, A.; Taherizadeh, M.; Amrollahi Biuki, N.; Fathurrahman, L.; Lavajoo, F. Mg, Sn, Cd, Zn and Fe accumulation in unicellular green alga Chlorella vulgaris and its effects on growth, content of photosynthetic pigments and protein. Thai J. Agric. Sci. 2022, 55, 135–145. Available online: https://li01.tci-thaijo.org/index.php/TJAS (accessed on 1 May 2025).
- Procházková, G.; Brányiková, I.; Zachleder, V.; Brányik, T. Effect of nutrient supply status on biomass composition of eukaryotic green microalgae. J. Appl. Phycol. 2014, 26, 1359–1377. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Ziganshin, A.M. Influence of nutrient medium composition on the redistribution of valuable metabolites in the freshwater green alga Tetradesmus obliquus (Chlorophyta) under photoautotrophic growth conditions. BioTech 2025, 14, 60. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA: Washington, DC, USA, 1998. [Google Scholar]
- Harris, E.H. The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use, 1st ed.; Academic Press: San Diego, CA, USA, 1989. [Google Scholar] [CrossRef]
- Sekine, M.; Yoshida, A.; Akizuki, S.; Kishi, M.; Toda, T. Microalgae cultivation using undiluted anaerobic digestate by introducing aerobic nitrification–desulfurization treatment. Water Sci. Technol. 2020, 82, 1070–1080. [Google Scholar] [CrossRef]
- Jafarpour, R.; Vahdat, S.; Asadi, F.; Dardashti, H.K.; Zarei, B.; Dadkhah, A. Investigating the growth rate of Dunaliella tertiolecta in the optimum N:P ratio in mono and mixed cultures. J. Appl. Phycol. 2023, 35, 1553–1563. [Google Scholar] [CrossRef]
- Elshobary, M.E.; Zabed, H.M.; Qi, X.; El-Shenody, R.A. Enhancing biomass and lipid productivity of a green microalga Parachlorella kessleri for biodiesel production using rapid mutation of atmospheric and room temperature plasma. Biotechnol. Biofuels Bioprod. 2022, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-K.; Shen, H. Basic culturing techniques. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Science: Oxford, UK, 2004; Chapter 3. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the AOAC, 16th ed.; AOAC: Washington, DC, USA, 2005; p. 1298. [Google Scholar]
- Anagnostopoulou, C.; Papachristou, I.; Kontogiannopoulos, K.N.; Mourtzinos, I.; Kougias, P.G. Optimization of microalgae cultivation in food industry wastewater using microplates. Sustain. Chem. Pharm. 2024, 39, 101510. [Google Scholar] [CrossRef]
- Talha, M.S.; Elshobary, M.E.; Khairy, H.M.; Alprol, A.E. Phycoremediation of dairy industry wastewater using Chlorella sorokiniana: A cost-effective strategy for biodiesel production. Environ. Sci. Pollut. Res. 2025, 32, 1–18. [Google Scholar] [CrossRef]
- Sahoo, A. Wastewater treatment by inverse fluidization technology. In Innovative Technologies for the Treatment of Industrial Wastewater, 1st ed.; Apple Academic Press: Palm Bay, FL, USA, 2017; p. 42. [Google Scholar]
- Zhu, L.; Wang, Z.; Takala, J.; Hiltunen, E.; Qin, L.; Xu, Z.; Qin, X.; Yuan, Z. Scale-up potential of cultivating Chlorella zofingiensis in piggery wastewater for biodiesel production. Bioresour. Technol. 2013, 137, 318–325. [Google Scholar] [CrossRef]
- Sarpal, A.S.; Teixeira, C.M.L.L.; Costa, I.C.R. Cultivation of Chlorella vulgaris in wastewater: Biodiesel potential and wastewater remediation. Environ. Sci. Pollut. Res. 2024, 31, 48795–48810. [Google Scholar] [CrossRef]
- Lopez Ponte, W.M.; Zamora Talaverano, N.; Oscanoa Huaynate, A.; Cafferata, E.A.; Cervantes Gallegos, M. Efficiency of microalgae cultures for nutrient removal from domestic wastewater. Adv. Environ. Technol. 2022, 1, 73–81. [Google Scholar] [CrossRef]
- Abate, R.; Oon, Y.S.; Oon, Y.L.; Bi, Y. Microalgae-Bacteria Nexus for Environmental Remediation and Renewable Energy Resources: Advances, Mechanisms and Biotechnological Applications. Heliyon 2024, 10, e31170. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Min, M.; Hu, B.; Chen, P.; Ruan, R. Local Bioprospecting for High-Lipid Producing Microalgal Strains to Be Grown on Concentrated Municipal Wastewater for Biofuel Production. Bioresour. Technol. 2011, 102, 6909–6919. [Google Scholar] [CrossRef]
- Hongyang, S.; Yalei, Z.; Chunmin, Z.; Xuefei, Z.; Jinpeng, L. Cultivation of Chlorella pyrenoidosa in Soybean Processing Wastewater. Bioresour. Technol. 2011, 102, 9884–9890. [Google Scholar] [CrossRef]
- Norena-Caro, D.A.; Malone, T.M.; Benton, M.G. Nitrogen Sources and Iron Availability Affect Pigment Biosynthesis and Nutrient Consumption in Anabaena sp. UTEX 2576. Microorganisms 2021, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Aguda, R.; Stelly, C.; Fonseca, L.; LeBoeuf, S.; Massiha, S.; Chistoserdov, A.; Holmes, W.E.; Hernandez, R.; Zappi, M.E.; Revellame, E.D. Effect of Macronutrient Levels on Chlorella vulgaris Cultivation for Long Duration Spaceflights and Space Settlements. Acta Astronaut. 2023, 206, 206–217. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Xu, H.; Wells, M.; Tefsen, B.; Qin, B. Effect of Micronutrients on Algae in Different Regions of Taihu, a Large, Spatially Diverse, Hypereutrophic Lake. Water Res. 2019, 151, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.U.; Abdullah, M.A. Effects of Macro/Micronutrients on Green and Brown Microalgal Cell Growth and Fatty Acids in Photobioreactor and Open-Tank Systems. Biocatal. Agric. Biotechnol. 2018, 14, 10–17. [Google Scholar] [CrossRef]
- Shang, H.; Shao, R.; Pan, X. Advancements in Understanding Oxygen-Evolving Complex through Structural Models in Photosystem II. Innov. Life 2024, 2, 100068. [Google Scholar] [CrossRef]
- Peers, G.; Price, N. Copper-Containing Plastocyanin Used for Electron Transport by an Oceanic Diatom. Nature 2006, 441, 341–344. [Google Scholar] [CrossRef]
- Manic, D.C.; Redil, R.D.; Rodriguez, I.B. Trace Metals in Phytoplankton: Requirements, Function, and Composition in Harmful Algal Blooms. Sustainability 2024, 16, 4876. [Google Scholar] [CrossRef]
- Nowicka, B. Heavy metal–induced stress in eukaryotic algae—Mechanisms of heavy metal toxicity and tolerance with particular emphasis on oxidative stress in exposed cells and the role of antioxidant response. Environ. Sci. Pollut. Res. 2022, 29, 16860–16911. [Google Scholar] [CrossRef]
- Ibarruri, J.; Manso, M.; Cebrián, M. Enhancement of Biomass and Protein Production of Chlorella protothecoides in Heterotrophic Cultivation Using Expired Juices as Alternative Source of Nutrients for an Added-Value Biorefinery Scheme. Fermentation 2023, 9, 360. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Mohanty, K. Valorization of Chlorella thermophila Biomass Cultivated in Dairy Wastewater for Biopesticide Production against Bacterial Rice Blight: A Circular Biorefinery Approach. BMC Plant Biol. 2023, 23, 644. [Google Scholar] [CrossRef]
- Qurat-ul-Ain; Javid, A.; Ali, S.; Hasan, A.; Senthilkumar, N.; Ranjitha, J.; Hussain, A. Coupling Wastewater Valorization with Sustainable Biofuel Production: Comparison of Lab- and Pilot-Scale Biomass Yields of Chlorella sorokiniana Grown in Wastewater Under Photoautotrophic and Mixotrophic Conditions. Chemosphere 2022, 301, 134703. [Google Scholar] [CrossRef]
- Lois-Milevicich, J.; Casá, N.; Alvarez, P.; González, J.; Montechiari, J. Chlorella vulgaris Biomass Production Using Brewery Wastewater with High Chemical Oxygen Demand. J. Appl. Phycol. 2020, 32, 2773–2783. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Koniuszy, A.; Gałczyńska, M.; Zając, G.; Szyszlak-Bargłowicz, J. Production of Microalgal Biomass Using Aquaculture Wastewater as Growth Medium. Water 2020, 12, 106. [Google Scholar] [CrossRef]
- He, Z.; Fan, X.; Qu, L.; Zhou, X.; Jin, W.; Hatshan, M.R.; Li, X.; Liu, H.; Jiang, G.; Wang, Q. Cultivation of Chlorella pyrenoidosa and Scenedesmus obliquus in Swine Wastewater: Nitrogen and Phosphorus Removal and Microalgal Growth. Process Saf. Environ. Prot. 2023, 179, 887–895. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Q.; Yu, H.; Du, X.; Zhang, T.; Sun, T.; Song, W. Assessing the Potential of Chlorella sp. Phycoremediation of Liquid Digestates from Brewery Wastes Mixture Integrated with Bioproduct Production. Front. Bioeng. Biotechnol. 2023, 11, 1199472. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, W.; Min, M.; Ma, X.; Chandra, C.; Doan, Y.T.T.; Ma, Y.; Zheng, H.; Cheng, S.; Griffith, R.; et al. Growing Chlorella sp. on Meat Processing Wastewater for Nutrient Removal and Biomass Production. Bioresour. Technol. 2015, 198, 189–197. [Google Scholar] [CrossRef]
- Saejung, C.; Ektasaeng, T. Evaluation of Chlorella vulgaris Grown in Sugar Industry Wastewater for Use as Aquaculture Feed. Int. J. Environ. Sci. Technol. 2023, 20, 5957–5964. [Google Scholar] [CrossRef]
- Mosibo, O.K.; Ferrentino, G.; Udenigwe, C.C. Microalgae Proteins as Sustainable Ingredients in Novel Foods: Recent Developments and Challenges. Foods 2024, 13, 733. [Google Scholar] [CrossRef]
- Abinandan, S.; Shanthakumar, S. Challenges and Opportunities in Application of Microalgae (Chlorophyta) for Wastewater Treatment: A Review. Renew. Sustain. Energy Rev. 2015, 52, 123–132. [Google Scholar] [CrossRef]
- Costa, M.M.; Spínola, M.P.; Prates, J.A.M. Microalgae as an Alternative Mineral Source in Poultry Nutrition. Vet. Sci. 2024, 11, 44. [Google Scholar] [CrossRef]
- Romanowska-Duda, Z.; Janas, R.; Grzesik, M.; van Duijn, B. Valorization of sorghum ash with digestate and biopreparations in the development biomass of plants in a closed production system of energy. Sci. Rep. 2023, 13, 18604. [Google Scholar] [CrossRef]
- Romanowska-Duda, Z.; Piotrowski, K.; Stępiński, D.; Popłońska, K. A promising ash supplementation strategy in the cultivation of Spirodela polyrrhiza plants. Cells 2023, 12, 289. [Google Scholar] [CrossRef]
- Nicholls, J.W.F.; Chin, J.P.; Williams, T.A.; Lenton, T.M.; O’Flaherty, V.; McGrath, J.W. On the Potential Roles of Phosphorus in the Early Evolution of Energy Metabolism. Front. Microbiol. 2023, 14, 1239189. [Google Scholar] [CrossRef]
- Prates, J.A.M. The Role of Microalgae in Providing Essential Minerals for Sustainable Swine Nutrition. Front. Anim. Sci. 2025, 6, 1526433. [Google Scholar] [CrossRef]




| Parameters | Wastewater Sources | TAP | |||
|---|---|---|---|---|---|
| Frozen Pizza Dough Production Plant (FPDPP) | Dairy Product Production Plant (DPPP) | Processed Food Production Plant (PFPP) | Beverage Production Plant (BPP) | ||
| pH | 6.87 ± 0.03 b | 7.38 ± 0.02 a | 7.32 ± 0.01 a | 4.81 ± 0.01 c | 7.20 |
| DO (mgO2 L−1) | 0.080 ± 0.01 b | 0.07 ± 0.01 b | 0.07 ± 0.01 b | 5.88 ± 0.11 a | N/A ** |
| BOD (mgO2 L−1) | 693.00 ± 4.90 b | 575.00 ± 3.50 c | 880.00 ± 1.10 a | 605.00 ± 4.70 bc | N/A ** |
| COD (mgO2 L−1) | 1536.00 ± 60.30 c | 1868.80 ± 11.10 b | 2227.20 ± 168.10 ab | 2739.20 ± 314.60 a | N/A ** |
| TC (mg L−1) | 148.30 ± 5.82 c | 132.80 ± 4.12 c | 176.90 ± 1.23 b | 751.34 ± 6.78 a | 417.50 |
| TIC (mg L−1) | 1.55 ± 0.52 b | 1.62 ± 0.21 b | 3.67 ± 0.75 ab | 7.14 ± 0.59 a | N/A ** |
| TOC (mg L−1) | 146.80 ± 3.67 c | 131.20 ± 4.34 c | 173.20 ± 7.25 b | 744.20 ± 9.87 a | 417.50 |
| TN (mg L−1) | 17.45 ± 0.24 b | 9.44 ± 0.12 c | 27.88 ± 0.02 a | 4.86 ± 0.02 d | 104.80 |
| TKN (mg L−1) | 15.53 ± 0.11 b | 8.49 ± 0.21 c | 25.01 ± 0.15 a | 3.06 ± 0.07 d | N/A ** |
| TP (mg L−1) | 4.45 ± 0.01 b | 4.81 ± 0.01 a | 4.99 ± 0.01 a | 4.86 ± 0.01 a | 15.47 |
| TS (mg L−1) | 3.58 ± 0.01 b | 2.77 ± 0.01 c | 4.17 ± 0.01 a | 1.11 ± 0.01 d | 16.06 |
| NO3− (mg L−1) | 1.66 ± 0.37 b | 0.59 ± 0.29 c | 2.72 ± 0.25 a | 1.62 ± 0.37 b | N/A ** |
| NO2− (mg L−1) | 0.26 ± 0.02 a | 0.36 ± 0.10 a | 0.15 ± 0.07 b | ND * | N/A ** |
| PO43− (mg L−1) | 4.16 ± 0.25 a | 4.02 ± 0.38 a | 4.29 ± 0.10 a | 3.92 ± 0.05 a | 47.47 |
| SO42− (mg L−1) | 2.96 ± 0.07 b | 1.97 ± 0.29 bc | 3.34 ± 0.04 a | 0.63 ± 0.07 d | 48.18 |
| Ca2+ (mg L−1) | 38.78 ± 0.13 a | 17.43 ± 0.11 c | 28.62 ± 0.22 b | 12.06 ± 0.05 d | 16.64 |
| Cu2+ (mg L−1) | 0.013 ± 0.01 ab | 0.02 ± 0.01 a | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.04 |
| TDFe (mg L−1) | 0.18 ± 0.01 d | 0.42 ± 0.01 a | 0.36 ± 0.01 b | 0.24 ± 0.01 c | 1.00 |
| K+ (mg L−1) | 15.61 ± 0.19 d | 17.62 ± 0.23 b | 16.80 ± 0.01 c | 22.06 ± 0.12 a | 31.25 |
| Mg2+ (mg L−1) | 8.31 ± 0.06 a | 4.44 ± 0.02 c | 8.60 ± 0.03 a | 5.13 ± 0.02 b | 9.87 |
| Mn2+ (mg L−1) | 0.04 ± 0.01 b | 0.02 ± 0.01 c | 0.04 ± 0.01 b | 0.13 ± 0.01 a | 1.41 |
| Na+ (mg L−1) | 250.20 ± 4.31 b | 159.37 ± 0.03 d | 197.07 ± 1.90 c | 319.50 ± 2.82 a | 0.08 |
| Zn2+ (mg L−1) | 0.14 ± 0.07 a | 0.11 ± 0.01 ab | 0.09 ± 0.01 b | 0.09 ± 0.01 b | 5.02 |
| Culture Media | Specific Growth Rate (Day−1) | Doubling Time (Day) |
|---|---|---|
| TAP | 0.549 ± 0.012 a | 1.262 ± 0.012 a |
| FPDPP | 0.109 ± 0.013 e | 6.387 ± 0.114 d |
| DPPP | 0.196 ± 0.021 c | 3.530 ± 0.023 c |
| PFPP | 0.329 ± 0.022 b | 2.104 ± 0.130 b |
| BPP | 0.171 ± 0.010 d | 4.047 ± 0.029 cd |
| Culture Media | Sodium Acetate Concentrations (mM) | Specific Growth Rate (Day−1) | Doubling Time (Day) |
|---|---|---|---|
| TAP | 17.40 | 0.548 ± 0.011 a | 1.265 ± 0.012 a |
| PFPP | 0.00 | 0.330 ± 0.014 d | 2.098 ± 0.051 d |
| PFPP | 1.74 | 0.398 ± 0.022 c | 1.743 ± 0.019 c |
| PFPP | 8.70 | 0.439 ± 0.011 b | 1.578 ± 0.013 b |
| PFPP | 17.40 | 0.544 ± 0.021 a | 1.274 ± 0.017 a |
| PFPP | 34.80 | 0.392 ± 0.031 c | 1.770 ± 0.013 c |
| Culture Media | Trace Mineral Concentration (×) | Specific Growth Rate (Day−1) | Doubling Time (Day) |
|---|---|---|---|
| TAP | 1.0 | 0.546 ± 0.021 c | 1.269 ± 0.011 c |
| PFPP | 0.0 | 0.338 ± 0.024 d | 2.050 ± 0.043 d |
| PFPP + Sodium acetate | 0.0 | 0.550 ± 0.022 bc | 1.261 ± 0.021 c |
| PFPP + Sodium acetate + NH4Cl + KH2PO4 + MgSO4 | 0.0 | 0.570 ± 0.025 bc | 1.215 ± 0.015 bc |
| PFPP + Sodium acetate + NH4Cl + KH2PO4 + MgSO4 | 0.1 | 0.738 ± 0.021 ab | 0.939 ± 0.012 ab |
| PFPP + Sodium acetate + NH4Cl + KH2PO4 + MgSO4 | 0.2 | 0.778 ± 0.035 a | 0.891 ± 0.021 a |
| PFPP + Sodium acetate + NH4Cl + KH2PO4 + MgSO4 | 0.5 | 0.732 ± 0.019 ab | 0.947 ± 0.017 ab |
| PFPP + Sodium acetate + NH4Cl + KH2PO4 + MgSO4 | 1.0 | 0.611 ± 0.010 bc | 1.134 ± 0.019 b |
| Culture Media | Specific Growth Rate (Day−1) | Doubling Time (Day) | Maximum Biomass Yield (g L−1) | Biomass Productivity (g L−1 Day−1) |
|---|---|---|---|---|
| TAP | 0.540 ± 0.028 b | 1.281 ± 0.018 b | 6.498 ± 0.436 b | 0.985 ± 0.032 b |
| Unmodified PFPP | 0.316 ± 0.015 c | 2.189 ± 0.093 c | 1.240 ± 0.245 c | 0.107 ± 0.021 c |
| Modified PFPP | 0.746 ± 0.070 a | 0.928 ± 0.014 a | 8.436 ± 0.378 a | 1.154 ± 0.043 a |
| Cell Content (% (w/w)) | Culture Media | |
|---|---|---|
| TAP | Modified PFPP | |
| Moisture | 10.60 ± 1.20 | 14.40 ± 0.11 |
| Crude protein | 46.70 ± 1.30 | 42.70 ± 1.40 |
| Crude fat | 2.20 ± 0.10 | 1.90 ± 0.10 |
| Crude fiber | 15.70 ± 1.40 | 11.90 ± 1.30 |
| Ash | 3.50 ± 0.40 | 11.70 ± 1.60 |
| Carbohydrate | 20.90 ± 1.60 | 17.10 ± 1.20 |
| Calcium | 1.00 ± 0.10 | 2.70 ± 0.20 |
| Phosphorus | 0.80 ± 0.10 | 2.40 ± 0.30 |
| Species | Substrate | Biomass Yield (g L−1) | Biomass Productivity (g L−1 Day−1) | Reference |
|---|---|---|---|---|
| Chlorella vulgaris var. vulgaris TISTR 8261 | Wastewater from a processed food production plant | 8.436 | 1.154 | This study |
| Chlorella thermophila | Simulated dairy wastewater | 2.1 | 0.175 | [65] |
| Chlorella sorokiniana | Sugarcane molasses, lab-scale | - | 0.128 | [66] |
| Chlorella protothecoides | Expired fruit juices, fed-batch bioreactor | 27.0 | 2.94 | [64] |
| Chlorella pyrenoidosa | Swine wastewater | 0.83 | - | [69] |
| Chlorella pyrenoidosa | Domestic wastewater | 1.44 | - | [69] |
| Chlorella sp. | Brewery wastewater digestate | 1.36 | - | [70] |
| Chlorella minutissima | Saline aquaculture wastewater | 4.77 | 0.55 | [68] |
| Chlorella vulgaris | Oil industry produced water | 1.69 | - | [26] |
| Chlorella vulgaris | Municipal wastewater (seasonal variation study) | - | 0.0215–0.0281 | [51] |
| Chlorella vulgaris | Meat-processing wastewater | 0.675–1.538 | - | [71] |
| Chlorella vulgaris | Brewery wastewater | - | 0.47 | [67] |
| Chlorella vulgaris | Sugar industry wastewater | 0.35 | - | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taikhao, S.; Phunpruch, S. Biomass Production of Chlorella vulgaris var. vulgaris TISTR 8261 During Cultivation in Modified Food Industry Wastewater. Phycology 2025, 5, 56. https://doi.org/10.3390/phycology5040056
Taikhao S, Phunpruch S. Biomass Production of Chlorella vulgaris var. vulgaris TISTR 8261 During Cultivation in Modified Food Industry Wastewater. Phycology. 2025; 5(4):56. https://doi.org/10.3390/phycology5040056
Chicago/Turabian StyleTaikhao, Samart, and Saranya Phunpruch. 2025. "Biomass Production of Chlorella vulgaris var. vulgaris TISTR 8261 During Cultivation in Modified Food Industry Wastewater" Phycology 5, no. 4: 56. https://doi.org/10.3390/phycology5040056
APA StyleTaikhao, S., & Phunpruch, S. (2025). Biomass Production of Chlorella vulgaris var. vulgaris TISTR 8261 During Cultivation in Modified Food Industry Wastewater. Phycology, 5(4), 56. https://doi.org/10.3390/phycology5040056








