Comparative Phycoremediation Performance of Two Green Microalgal Strains Under Four Biomass Conditions for Industrial Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Sampling, Isolation, Purification, and Identification of Microalgae Species
2.2. Industrial Wastewater Collection
2.3. Physicochemical Analysis
2.4. Biomass Preparation Techniques
2.5. Phycoremediation Experimental Designs
2.5.1. Phycoremediation by Free-Living Algal Culture
2.5.2. Phycoremediation by Alginate-Immobilized Microalgae (Algal Beads)
2.5.3. Phycoremediation by Raw Algal Biomass
2.5.4. Phycoremediation by Acid-Treated Algal Biomass
2.6. Principal Component Analysis (PCA)
2.7. Statistical Analysis
3. Results
3.1. Identification of Microalgae Species
3.2. Industrial Wastewater Analysis
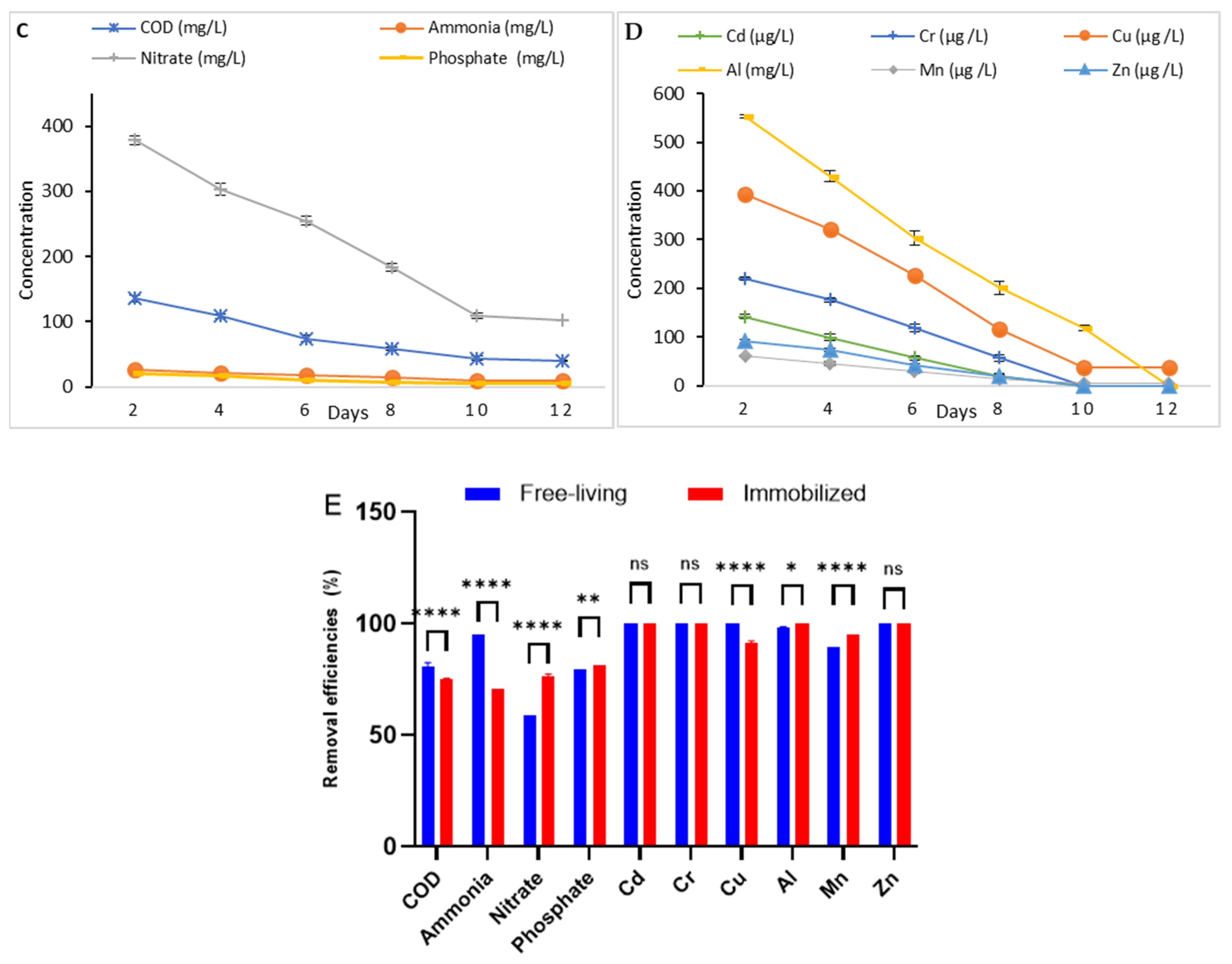
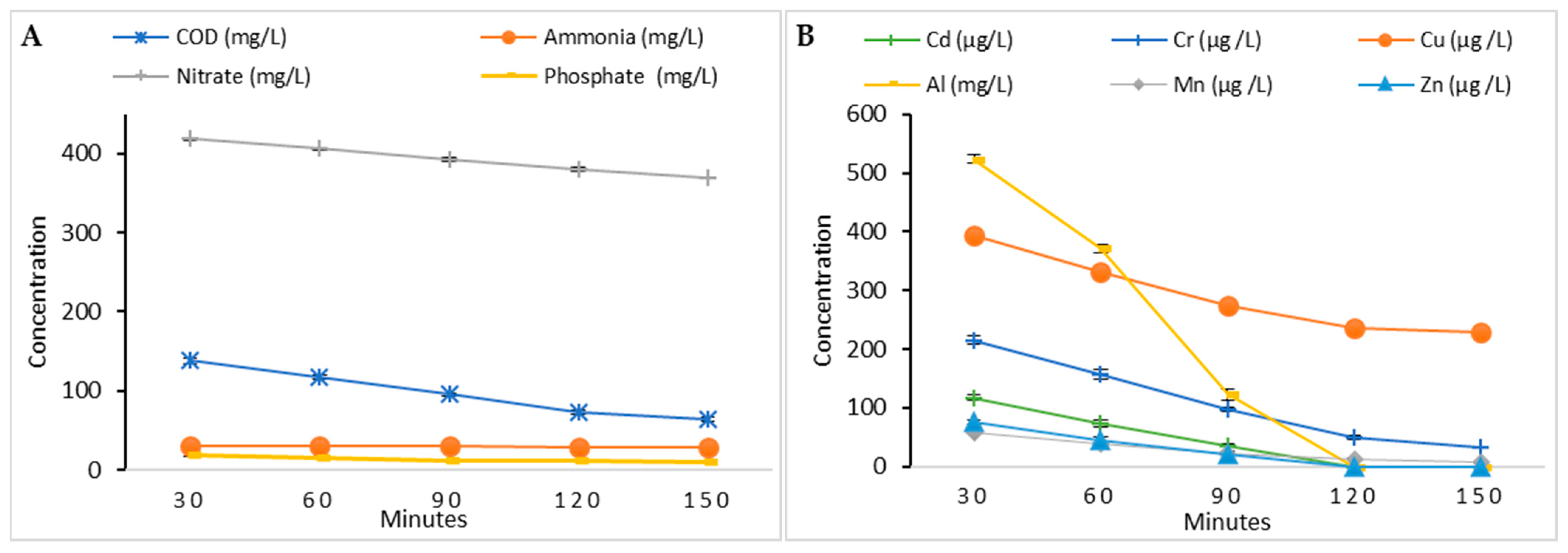
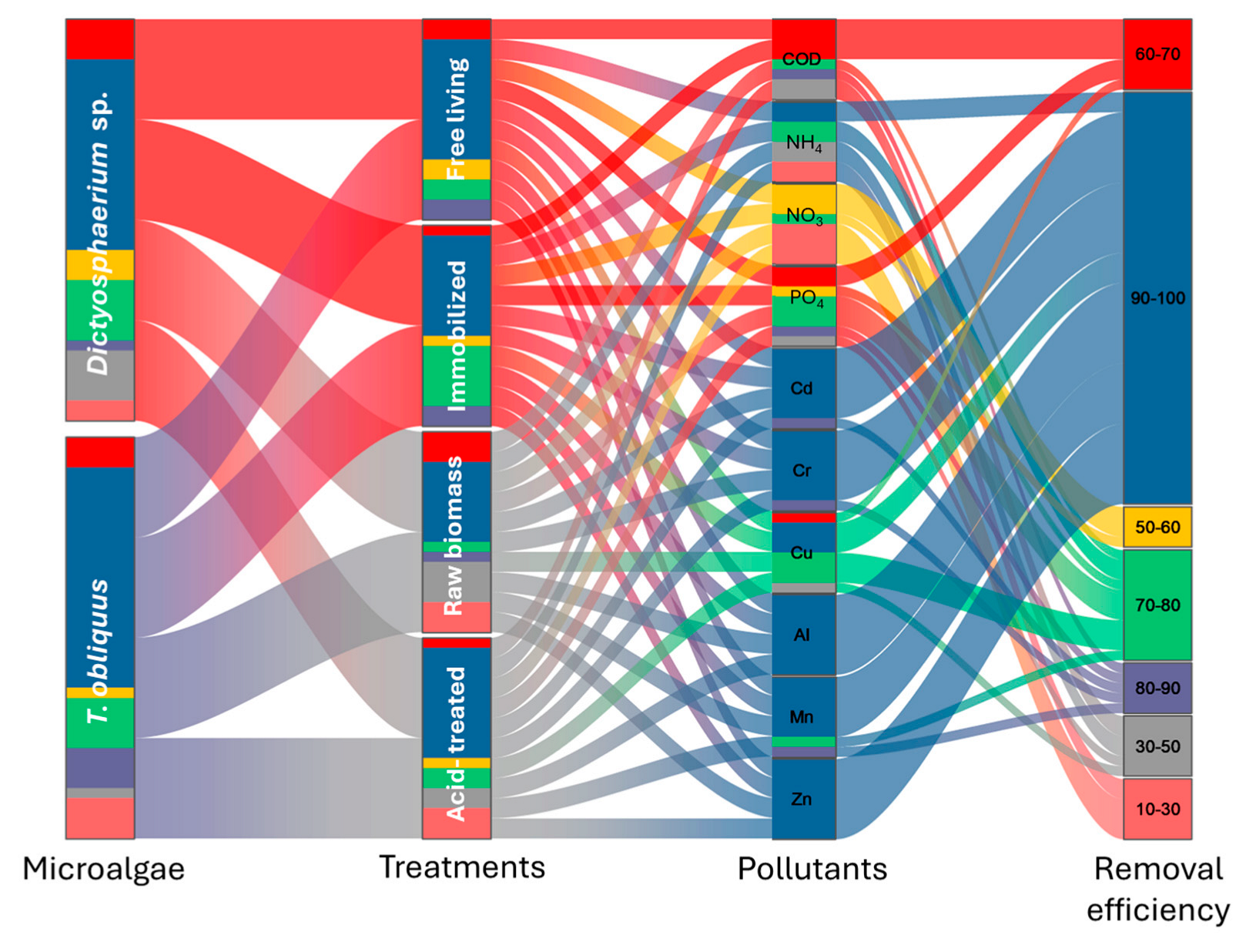
3.3. Evaluation of Industrial Wastewater Treatment (Phycoremediation)
3.3.1. Phycoremediation Using Dictyosphaerium sp.
3.3.2. Phycoremediation Using Tetradesmus obliquus
3.3.3. Comparison Between the Two Microalgae Phycoremediation Rates
3.3.4. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahim, E.G.; Gube-Ibrahim, M.A. Heavy Metals Assessment of Some Selected Packaged Drinking Water in Nasarawa State, Nigeria. Int. J. Adv. Res. Chem. Sci. 2015, 2, 30–35. [Google Scholar]
- Hosny, S.; Elshobary, M.E.; El-Sheekh, M.M. Unleashing the power of microalgae: A pioneering path to sustainability and achieving the sustainable development goals. Environ. Sci. Pollut. Res. 2025, 32, 17312–17342. [Google Scholar] [CrossRef]
- Shen, L.; Wang, J.; Li, Z.; Fan, L.; Chen, R.; Wu, X.; Li, J.; Zeng, W. A high-efficiency Fe2O3@ Microalgae composite for heavy metal removal from aqueous solution. J. Water Process Eng. 2020, 33, 101026. [Google Scholar] [CrossRef]
- Fang, H.; Wang, X.; Xia, D.; Zhu, J.; Yu, W.; Su, Y.; Zeng, J.; Zhang, Y.; Lin, X.; Lei, Y.; et al. Improvement of Ecological Risk Considering Heavy Metal in Soil and Groundwater Surrounding Electroplating Factories. Processes 2022, 10, 1267. [Google Scholar] [CrossRef]
- Oladimeji, T.E.; Oyedemi, M.; Emetere, M.E.; Agboola, E.; Adeoye, J.B.; Odunlami, O.A. Review on the impact of heavy metals from industrial wastewater effluent and removal technologies. Heliyon 2024, 10, e40370. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Zahmatkesh, S.; Bokhari, A.; Karimian, M.; Zahra, M.M.A.; Sillanpää, M.; Panchal, H.; Alrubaie, A.J.; Rezakhani, Y. A comprehensive review of various approaches for treatment of tertiary wastewater with emerging contaminants: What do we know? Environ. Monit. Assess. 2022, 194, 884. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Ahmad, F.; Ashraf, N.; Deng, X.; Yin, D.C. Crystallizing bacterial extracellular protein reveals paths of silver mineralization for recovery. J. Environ. Chem. Eng. 2025, 10, 119215. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Nayak, R.; Swain, S.S.; Jit, B.P.; Behera, C.; Ragusa, A.; Ki, J.-S.; Jena, M. Low-dose priming of gamma radiation enhanced cadmium tolerance in Chlamydomonas reinhardtii by modulating physio-biochemical pathways. Environ. Sci. Pollut. Res. 2022, 29, 80383–80398. [Google Scholar] [CrossRef]
- Bădescu, I.S.; Bulgariu, D.; Bulgariu, L. Alternative utilization of algal biomass (Ulva sp.) loaded with Zn(II) ions for improving of soil quality. J. Appl. Phycol. 2017, 29, 1069–1079. [Google Scholar] [CrossRef]
- Bădescu, I.S.; Bulgariu, D.; Ahmad, I.; Bulgariu, L. Valorisation possibilities of exhausted biosorbents loaded with metal ions—A Review. J. Environ. Manag. 2018, 224, 288–297. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.-S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Singh, D.V.; Bhat, R.A.; Upadhyay, A.K.; Singh, R.; Singh, D.P. Microalgae in aquatic environs: A sustainable approach for remediation of heavy metals and emerging contaminants. Environ. Technol. Innov. 2021, 21, 101340. [Google Scholar] [CrossRef]
- Osman, M.E.H.; Abo-shady, A.M.; Gheda, S.F.; Desoki, S.M.; Elshobary, M.E. Unlocking the potential of microalgae cultivated on wastewater combined with salinity stress to improve biodiesel production. Environ. Sci. Pollut. Res. 2023, 30, 114610–114624. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.M.; Saber, A.A.; Kafafy, M.M.; Moghazy, R.M. Unlocking the Intertwined Effects of CO2 Concentrations on Microalgae Structure in Wastewater Treatment. Egypt. J. Aquat. Biol. Fish. 2025, 29, 363–390. [Google Scholar] [CrossRef]
- Moghazy, R.M.; Abdalla, S.B. Boosting wastewater treatment and CO2 bioremediation with Nile River microalgae: Resilience to simulated smoke and enhanced biomass production. Bioresour. Technol. Rep. 2024, 25, 101809. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Kumar, P.S.; Jaikumar, V.; Vardhan, K.H.; SundarRajan, P.S. Microalgae for biofuel production and removal of heavy metals: A review. Environ. Chem. Lett. 2020, 18, 1905–1923. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dahms, H.U.; Won, E.J.; Lee, J.S.; Shin, K.H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef]
- Chen, Z.; Osman, A.I.; Rooney, D.W.; Oh, W.-D.; Yap, P.-S. Remediation of heavy metals in polluted water by immobilized algae: Current applications and future perspectives. Sustainability 2023, 15, 5128. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.R. Bio-sorption of toxic metals from industrial wastewater by algae strains Spirulina platensis and Chlorella vulgaris: Application of isotherm, kinetic models and process optimization. Sci. Total Environ. 2021, 755, 142654. [Google Scholar] [CrossRef]
- Prescott, G.W. Algae of the Western Great Lakes Area; Otto Koeltz Science Publishers: Königstein, Germany, 2008. [Google Scholar]
- John, D.M.; Whitton, B.A.; Brook, A.J. The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae; Cambridge University Press: Cambridge, UK, 2002; ISBN 0521770513. [Google Scholar]
- Stadtländer, C.T.-H.; Bellinger, E.G.; Sigee, D.C. Freshwater Algae: Identification and Use as Bioindicators; Wiley-Blackwell: Chichester, UK, 2013; ISBN 978-0-470-05814-5. [Google Scholar]
- Guiry, M.D. AlgaeBase; World-Wide Electronic Publication, National University of Ireland: Galway, Ireland, 2010; Available online: http://www.algaebase.org (accessed on 15 January 2025).
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Hernandez, J.P.; Bashan, Y. Interaction of Azospirillum spp. with microalgae: A basic eukaryotic–prokaryotic model and its biotechnological applications. In Handbook for Azospirillum: Technical Issues and Protocols; Springer: Berlin/Heidelberg, Germany, 2015; pp. 367–388. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Oliveira, C.D.L.; Prasad, R.; Ong, H.C.; Araujo, E.S.; Shabnam, N.; Gálvez, A.O. A multidisciplinary review of Tetradesmus obliquus: A microalga suitable for large-scale biomass production and emerging environmental applications. Rev. Aquac. 2021, 13, 1594–1618. [Google Scholar] [CrossRef]
- dos Santos, L.M.; da Silva, J.C.B.; de Farias Silva, C.E.; da Gama, B.M.V.; Medeiros, J.A.; Markou, G.; Almeida, R.M.R.G.; de Souza Abud, A.K. Co-Cultivation between the Microalga Tetradesmus obliquus and Filamentous Fungus Cunninghamella echinulata Improves Tertiary Treatment of Cheese Whey Effluent in Semicontinuous Mode. Processes 2024, 12, 1573. [Google Scholar] [CrossRef]
- Verma, V.K.; Tewari, S.; Rai, J.P.N. Ion exchange during heavy metal bio-sorption from aqueous solution by dried biomass of macrophytes. Bioresour. Technol. 2008, 99, 1932–1938. [Google Scholar] [CrossRef]
- Hashim, M.A.; Chu, K.H. Biosorption of cadmium by brown, green, and red seaweeds. Chem. Eng. J. 2004, 97, 249–255. [Google Scholar] [CrossRef]
- Krivina, E.S.; Temraleeva, A.D. Identification problems and cryptic diversity of Chlorella-clade microalgae (Chlorophyta). Microbiology 2020, 89, 720–732. [Google Scholar] [CrossRef]
- Celewicz, S.; Kozak, A.; Kuczyńska-Kippen, N. Chlorophytes response to habitat complexity and human disturbance in the catchment of small and shallow aquatic systems. Sci. Rep. 2022, 12, 13050. [Google Scholar] [CrossRef]
- Essa, D.I.; Elshobary, M.E.; Attiah, A.M.; Salem, Z.E.; Keshta, A.E.; Edokpayi, J.N. Assessing phytoplankton populations and their relation to water parameters as early alerts and biological indicators of the aquatic pollution. Ecol. Indic. 2024, 159, 111721. [Google Scholar] [CrossRef]
- Wang, H.; Hu, X.; Shao, C.; Elshobary, M.; Zhu, F.; Cui, Y.; Zhang, C.; Ni, J.; Huo, S. Optimizing mixotrophic cultivation of oil-rich Tribonema minus using volatile fatty acids and glycerin: A promising approach for pH-controlling and enhancing lipid productivity. J. Clean. Prod. 2023, 402, 136733. [Google Scholar] [CrossRef]
- Wang, H.; Hu, X.; Elshobary, M.; Sobhi, M.; Zhu, F.; Cui, Y.; Xu, X.; Ni, J.; El-Sheekh, M.; Huo, S. Integrated partial nitrification and Tribonema minus cultivation for cost-effective ammonia recovery and lipid production from slaughterhouse wastewater. Chem. Eng. J. 2024, 492, 152199. [Google Scholar] [CrossRef]
- Liang, P.; Wang, H.; Hu, X.; Elshobary, M.; Cui, Y.; Zou, B.; Zhu, F.; Schagerl, M.; El-Sheekh, M.; Huo, S. Impact of the NH4+/NO3− ratio on growth of oil-rich filamentous microalgae Tribonema minus in simulated nitrogen-rich wastewater. J. Water Process Eng. 2024, 68, 106378. [Google Scholar] [CrossRef]
- Talha, M.S.; Elshobary, M.E.; Khairy, H.M.; Alprol, A.E. Phycoremediation of dairy industry wastewater using Chlorella sorokiniana: A cost-effective strategy for biodiesel production. Environ. Sci. Pollut. Res. 2025, 32, 17407–17424. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, F.P.; De Farias Silva, C.E.; dos Santos, J.; Ribeiro, T.R.M.; Medeiros, J.A.; do Nascimento, M.A.A.; Santos, G.K.S.; dos Santos Carneiro, W.; Almeida, R.M.R.G.; de Oliveira, A.M.M. Dairy wastewater treatment by Tetradesmus sp. in open system: Molecular identification and the effect of light intensity and organic load in the process. Energy Ecol. Environ. 2023, 8, 356–369. [Google Scholar] [CrossRef]
- Ferreira, A.; Ribeiro, B.; Ferreira, A.F.; Tavares, M.L.A.; Vladic, J.; Vidović, S.; Cvetkovic, D.; Melkonyan, L.; Avetisova, G.; Goginyan, V. Scenedesmus obliquus microalga-based biorefinery–from brewery effluent to bioactive compounds, biofuels and biofertilizers–aiming at a circular bioeconomy. Biofuels Bioprod. Biorefin. 2019, 13, 1169–1186. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, L.; Song, S.; Farías, M.E.; Li, Y.; Tang, C. Cadmium removal from diluted wastewater by using high-phosphorus-culture modified microalgae. Chem. Phys. Lett. 2021, 771, 138561. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, F.; Cao, Y.; Xing, L.; Liu, W.; Mei, S.; Li, S. Cultivation of microalgae in dairy farm wastewater without sterilization. Int. J. Phytoremediat. 2015, 17, 222–227. [Google Scholar] [CrossRef]
- Ji, L.; Ge, Q.; Li, Y.; Gao, Y.; Xie, S. A comparative study of the growth and nutrient removal effects of five green microalgae in simulated domestic sewage. Water 2021, 13, 3613. [Google Scholar] [CrossRef]
- Pham, T.L.; Bui, M.H. Removal of nutrients from fertilizer plant wastewater using Scenedesmus sp.: Formation of bioflocculation and enhancement of removal efficiency. J. Chem. 2020, 2020, 8094272. [Google Scholar] [CrossRef]
- Shaker, S.; Nemati, A.; Montazeri-Najafabady, N.; Mobasher, M.A.; Morowvat, M.H.; Ghasemi, Y. Treating urban wastewater: Nutrient removal by using immobilized green algae in batch cultures. Int. J. Phytoremediat. 2015, 17, 1177–1182. [Google Scholar] [CrossRef]
- Mollamohammada, S.; Aly Hassan, A.; Dahab, M. Nitrate removal from groundwater using immobilized heterotrophic algae. Water Air Soil Pollut. 2020, 231, 26. [Google Scholar] [CrossRef]
- Alhumairi, A.; Hamouda, R.; Saddiq, A. Comparative study between immobilized and suspended Chlorella sp in treatment of pollutant sites in Dhiba port Kingdom of Saudi Arabia. Heliyon 2022, 8, e10766. [Google Scholar] [CrossRef]
- Severo, A.I.; Azevedo, O.G.D.A.; da Silva, P.A.S.; Jacob-Furlan, B.; Mariano, A.B.; Ordonez, J.C.; Vargas, J.V.C. Wastewater treatment process using immobilized microalgae. Water Sci. Technol. 2024, 90, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Ravindran, B.; Gupta, S.K.; Nasr, M.; Rawat, I.; Bux, F. Techno-economic estimation of wastewater phycoremediation and environmental benefits using Scenedesmus obliquus microalgae. J. Environ. Manag. 2019, 240, 293–302. [Google Scholar] [CrossRef] [PubMed]
- El Bouzidi, I.; Krimech, A.; Hejjaj, A.; Bouterfass, R.; Cherifi, O.; Mandi, L. Enhancing domestic wastewater treatment through four chlorophyta strains-based phycoremediation: Nutrient removal efficiency and algal physiology. Int. J. Phytoremediat. 2025, 27, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Faruque, M.O.; Uddin, S.; Hossain, M.M.; Hossain, S.M.Z.; Shafiquzzaman, M.; Razzak, S.A. A comprehensive review on microalgae-driven heavy metals removal from industrial wastewater using living and nonliving microalgae. J. Hazard. Mater. Adv. 2024, 16, 100492. [Google Scholar] [CrossRef]
- Edo, G.I.; Samuel, P.O.; Oloni, G.O.; Ezekiel, G.O.; Ikpekoro, V.O.; Obasohan, P.; Ongulu, J.; Otunuya, C.F.; Opiti, A.R.; Ajakaye, R.S. Environmental persistence, bioaccumulation, and ecotoxicology of heavy metals. Chem. Ecol. 2024, 40, 322–349. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- EPA. National Primary Drinking Water Regulations; U.S. Environmental Protection Agency: Washington, DC, USA, 2023.
- Xu, P.; Tu, X.; An, Z.; Mi, W.; Wan, D.; Bi, Y.; Song, G. Cadmium-induced physiological responses, biosorption and bioaccumulation in Scenedesmus obliquus. Toxics 2024, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Thi Nguyen, K.C.; Truong, P.H.; Ho, C.T.; Le, C.T.; Tran, K.D.; Nguyen, T.L.; Nguyen, M.T.; Nguyen, P. Van Copper tolerance of novel Rhodotorula sp. yeast isolated from gold mining ore in Gia Lai, Vietnam. Mycobiology 2023, 51, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Koshariya, A.K.; Kumar, S.; Kulkarni, M.; Madhu, P.; Kumar, P.S.; Nguyen, V.D.; Alarifi, S.; Ahamed, A.; Chang, S.W.; Ravindran, B. Effect of Acidic Treatment for Conventional Processing and Recent Advances on Lignocellulosic Ricinus Communis: A Comparative Evaluation on Decomposition of Biomass for Environmental Sustainability. Waste Biomass Valorization 2024, 15, 6051–6064. [Google Scholar] [CrossRef]
- Spain, O.; Plöhn, M.; Funk, C. The cell wall of green microalgae and its role in heavy metal removal. Physiol. Plant. 2021, 173, 526–535. [Google Scholar] [CrossRef]
- Kaloudas, D.; Pavlova, N.; Penchovsky, R. Phycoremediation of wastewater by microalgae: A review. Environ. Chem. Lett. 2021, 19, 2905–2920. [Google Scholar] [CrossRef]
- Bartolomé, M.C.; Cortés, A.A.; Sánchez-Fortún, A.; Garnica-Romo, M.G.; Sánchez-Carrillo, S.; Sánchez-Fortún, S. Morphological and physiological changes exhibited by a Cd-resistant Dictyosphaerium chlorelloides strain and its cadmium removal capacity. Int. J. Phytoremediat. 2016, 18, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Sun, H.; Qi, H.; Wang, C.; Yang, G.; Ma, X. Enhancement of Tetradesmus obliquus Adsorption for Heavy Metals Through Lysine Addition: Optimization and Competitive Study. Water 2025, 17, 935. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Yang, X.; Zhang, L. Preparation of a novel hydrogel of sodium alginate using rural waste bone meal for efficient adsorption of heavy metals cadmium ion. Sci. Total Environ. 2023, 863, 160969. [Google Scholar] [CrossRef]
- Balzano, S.; Sardo, A.; Blasio, M.; Chahine, T.B.; Dell’Anno, F.; Sansone, C.; Brunet, C. Microalgal Metallothioneins and Phytochelatins and Their Potential Use in Bioremediation. Front. Microbiol. 2020, 11, 517. [Google Scholar] [CrossRef]
- Nyika, J.; Dinka, M.O. Factors and mechanisms regulating heavy metal phycoremediation in polluted water. Discov. Water 2023, 3, 14. [Google Scholar] [CrossRef]
- El-Sheekh, M.; El Sabagh, S.; El-Souod, G.A.; Elbeltagy, A. Biosorption of Cadmium from Aqueous Solution by Free and Immobilized Dry Biomass of Chlorella vulgaris. Int. J. Environ. Res. 2019, 13, 511–521. [Google Scholar] [CrossRef]
- El-Sheekh, M.; Galal, H.R.; Mousa, A.S.H.; Farghl, A.M. Coupling Wastewarter Treatment, Biomass, Lipids, and Biodesiel Production of Some Green Microalgae. Environ. Sci. Pollut. Res Int. 2023, 30, 35492–35504. [Google Scholar] [CrossRef] [PubMed]


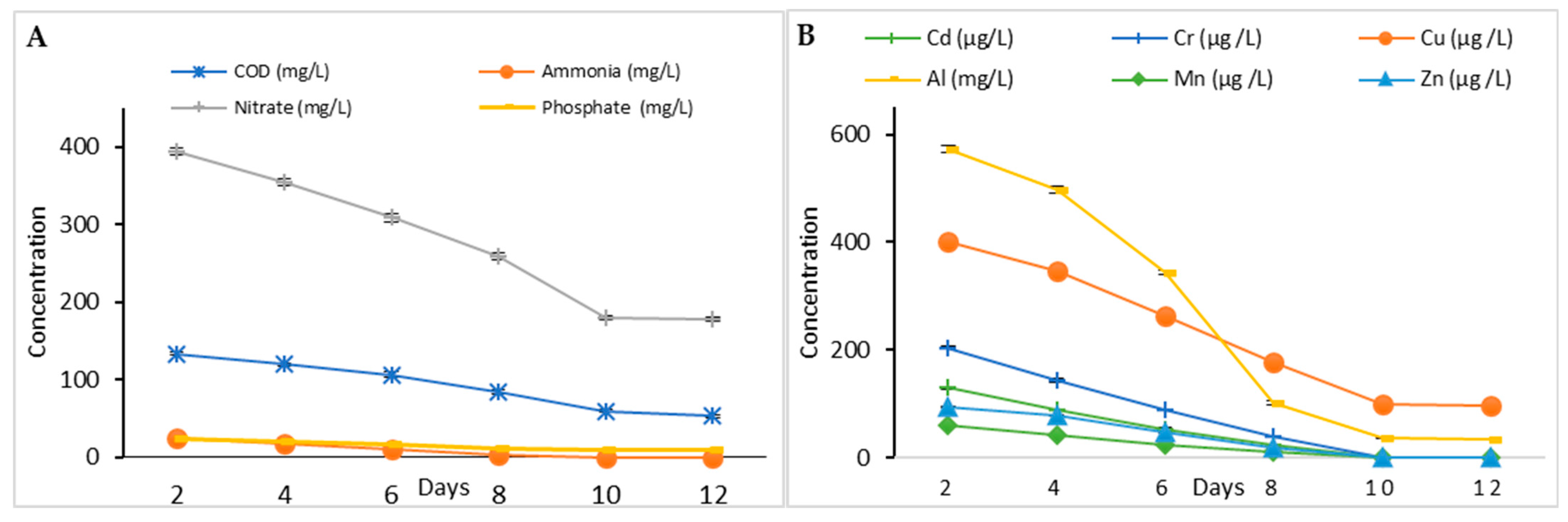
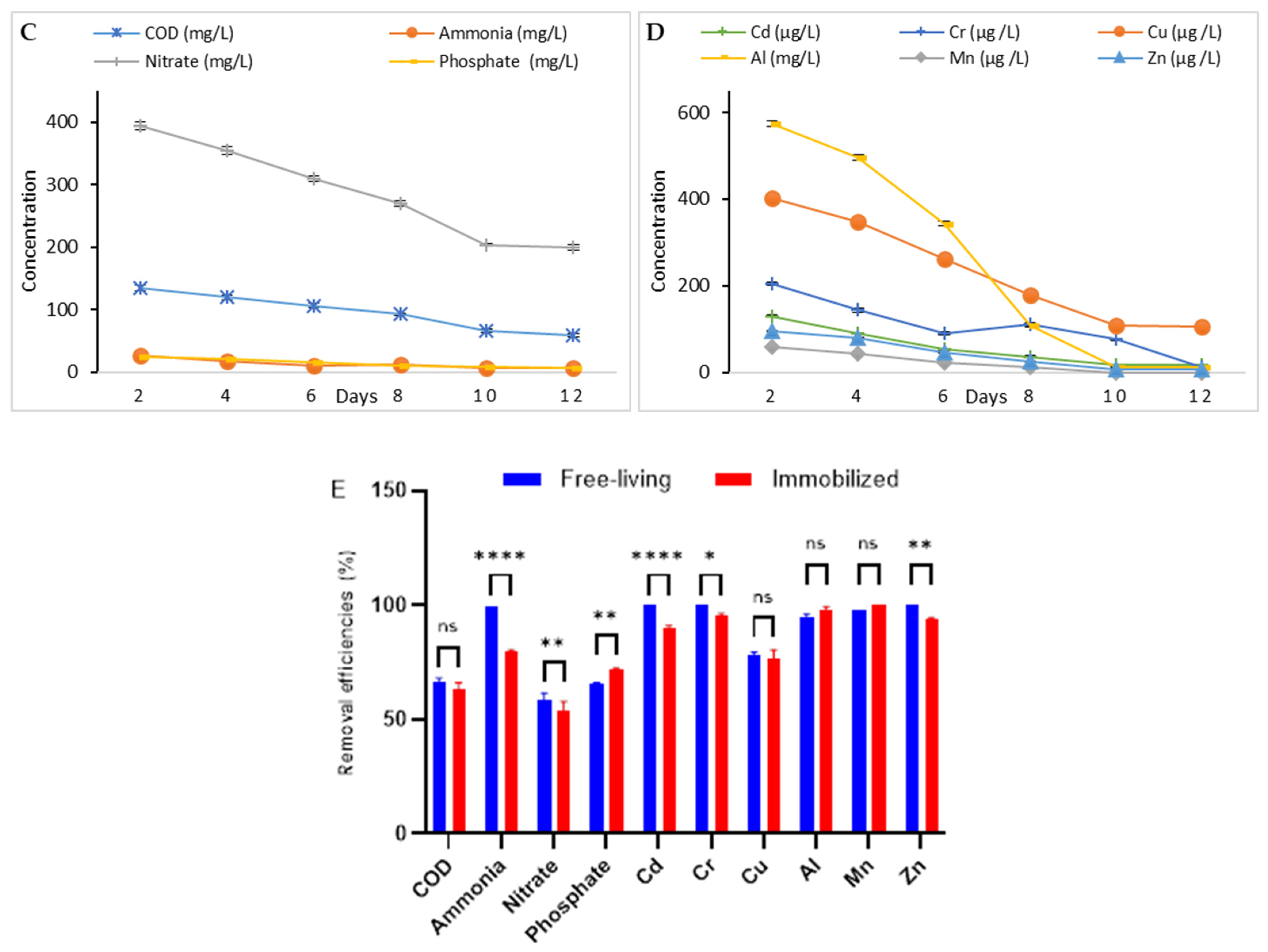
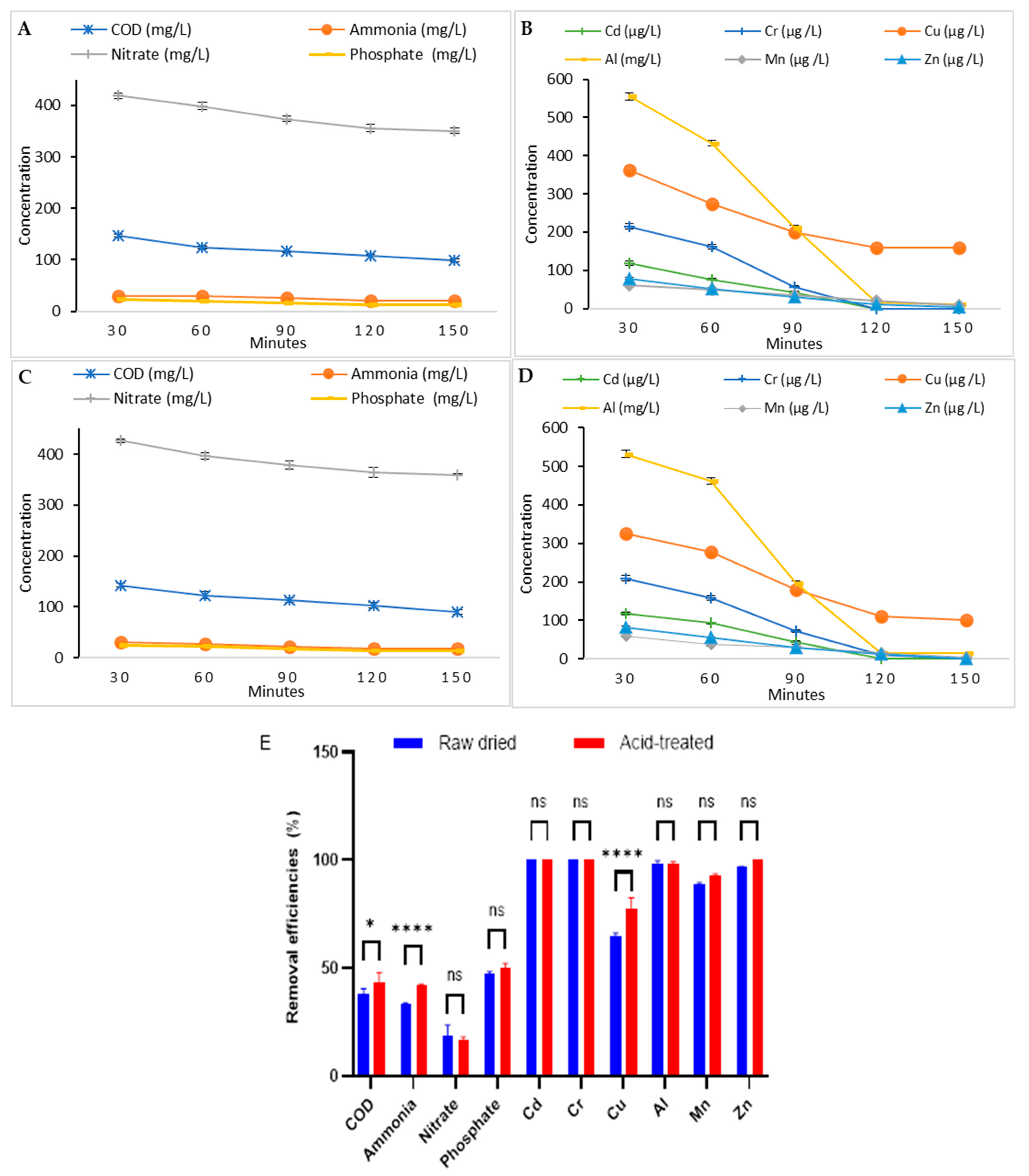
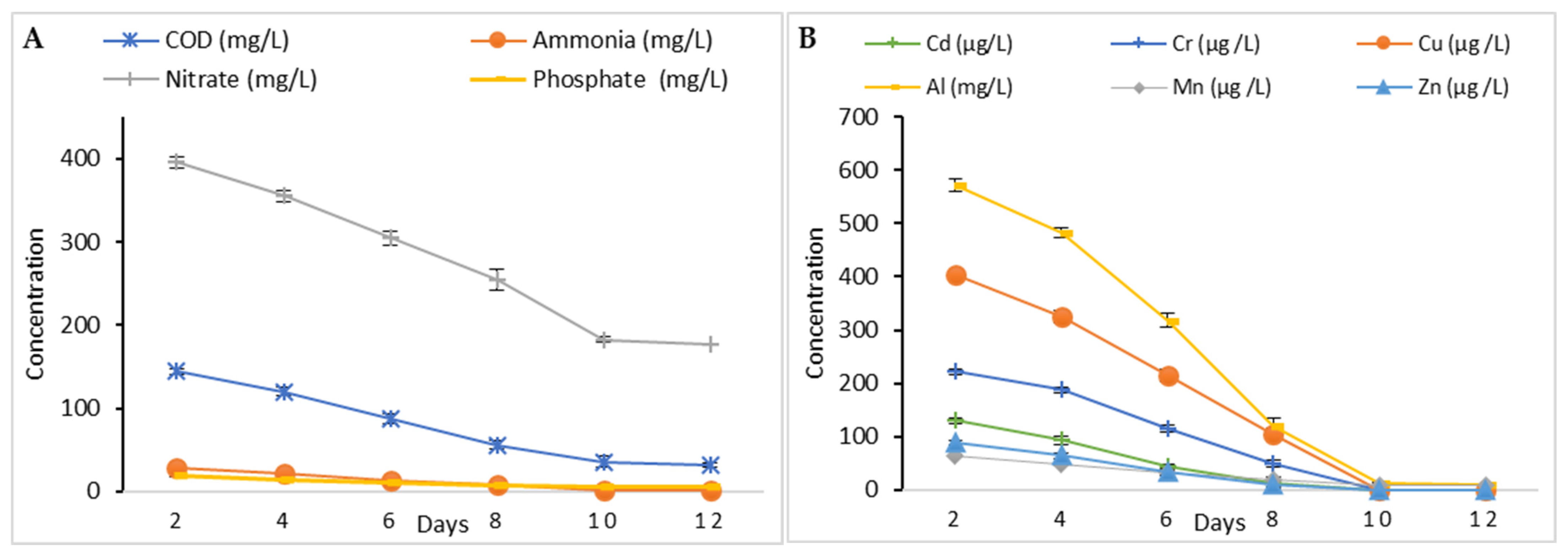


| Parameter | Value |
|---|---|
| Temp (°C) | 24 ± 0.5 |
| pH | 12.07 ± 0.4 |
| COD (mg/L) | 160 ± 3.1 |
| Ammonia (mg/L) | 31.2 ± 1.4 |
| Nitrate (mg/L) | 430.38 ± 5.3 |
| Phosphate (mg/L) | 26.01 ± 0.82 |
| Cd2+ (µg/L) | 165 ± 2.2 |
| Cr3+ (µg/L) | 260 ± 3.1 |
| Cu2+ (µg/L) | 448 ± 6.2 |
| Ni2+ (mg/L) | ND |
| Pb2+ (mg/L) | ND |
| Al3+ (mg/L) | 646.1 ± 6.5 |
| Mn2+ (µg/L) | 75 ± 1.5 |
| Zn2+ (µg/L) | 106 ± 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sheekh, M.M.; Moghazy, R.M.; Hamoud, M.M.; Elshobary, M.E. Comparative Phycoremediation Performance of Two Green Microalgal Strains Under Four Biomass Conditions for Industrial Wastewater Treatment. Phycology 2025, 5, 53. https://doi.org/10.3390/phycology5040053
El-Sheekh MM, Moghazy RM, Hamoud MM, Elshobary ME. Comparative Phycoremediation Performance of Two Green Microalgal Strains Under Four Biomass Conditions for Industrial Wastewater Treatment. Phycology. 2025; 5(4):53. https://doi.org/10.3390/phycology5040053
Chicago/Turabian StyleEl-Sheekh, Mostafa M., Reda M. Moghazy, Mai M. Hamoud, and Mostafa E. Elshobary. 2025. "Comparative Phycoremediation Performance of Two Green Microalgal Strains Under Four Biomass Conditions for Industrial Wastewater Treatment" Phycology 5, no. 4: 53. https://doi.org/10.3390/phycology5040053
APA StyleEl-Sheekh, M. M., Moghazy, R. M., Hamoud, M. M., & Elshobary, M. E. (2025). Comparative Phycoremediation Performance of Two Green Microalgal Strains Under Four Biomass Conditions for Industrial Wastewater Treatment. Phycology, 5(4), 53. https://doi.org/10.3390/phycology5040053







